Abstract
Background
Triple‐negative breast cancer (TNBC) is one subtype of breast cancer, which is characterized by an aggressive disease. It is commonly accompanied with extremely poor prognosis because of no available molecularly targeted therapy. Thus, understanding the detailed molecular mechanisms of TNBC is urgently needed.
Methods
The levels of Axis inhibition protein 1 (Axin1), Cyclin D1, c‐Myc, and miR‐124‐3p.1 were measured by quantitative real‐time PCR (qRT‐PCR). Furthermore, the breast cancer cell proliferation was measured by CCK‐8 assay, colony formation assays, and EdU staining. Xenograft model was used to show the tumor genesis of breast cancer cells. The regulatory function of miR‐124‐3p.1 on Wnt/β‐catenin signaling activation through directly targeting Axin1 was proven using qRT‐PCR, Western blot analysis, and dual‐luciferase reporter assay. To further assess the clinical significance of miR‐124‐3p.1 in the prognosis of breast cancer patients, we performed Kaplan‐Meier survival analysis and log‐rank tests.
Results
miR‐124‐3p.1 expression was elevated in advanced TNBC patients, and high miR‐124‐3p.1 predicts poor overall survival in TNBC patients. Further data showed that miR‐124‐3p.1 downregulation diminished, while miR‐124‐3p.1 upregulation increased the growth of TNBC cells in vitro and in vivo. Finally, we proved that miR‐124‐3p.1 exerted its function via targeting tumor suppressor gene Axin1 and activating the Wnt signaling pathway.
Conclusion
In summary, all the results demonstrate that miR‐124‐3p.1 promotes TNBC cell growth by controlling Axin1, suggesting that targeting miR‐124‐3p.1 might offer an effective therapeutic strategy for TNBC in the future.
Keywords: Axin1, cell proliferation, miR‐124‐3p.1, poor prognosis
Abbreviations
- 3′‐UTRs
3′‐untranslated regions
- Axin1
Axis inhibition protein 1
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- qRT‐PCR
quantitative real‐time PCR
- TNBC
triple‐negative breast cancer
1. INTRODUCTION
Breast cancer is the most frequently diagnosed cancer in women worldwide, accounting for approximately one million novel cancer cases.1 It contains various types, such as estrogen receptor (ER)‐positive, human epidermal growth factor receptor 2 (HER2)‐positive types, and triple‐negative breast cancer (TNBC). Among all kinds of breast cancer, TNBC, characterized by the absence of receptors for ER, HER2, and progesterone receptor, is one subtype with the worst 5‐year overall survival rate.2 Targeted therapy was widely used for the treatment of breast cancer carrying ER and HER2.3 Thus, understanding the detailed molecular mechanisms of TNBC is urgently needed, and it will no doubt to speed up new drug development.
Wnt/β‐catenin signaling is required for both embryonic development and adult tissue homeostasis, and this pathway is often deregulated in various kinds of cancers.4 Briefly, Wnt/β‐catenin signaling is activated by canonical Wnt legends, which in turn lead to inhibition of β‐catenin degradation by the destruction complex including the AXIN, APC, and GSK3β proteins.5 As a consequence, stabilized β‐catenin translocates to the nucleus to activate transcription of downstream genes, thereby tightly regulating cell proliferation and tumor genesis. Considering Wnt/β‐catenin pathway is frequently activated in many cancer types; thus, revealing these factors that modulate the Wnt/β‐catenin signaling pathway is of biological and clinical importance for the future development of more efficacious therapeutic strategies.
MicroRNAs (miRNA) are small non‐coding RNAs which downregulate the expression of target genes, related to a broad field of physiological and pathological processes.6, 7 Various studies have demonstrated that miRNA deregulation emerged as the main contributor to tumor genesis in breast cancers.8, 9 Recently, miR‑124‐3p.1 has been reported that it participates in neurodegenerative diseases10, 11 and various cancer development, including astrocytoma,12 pancreatic ductal adenocarcinoma,13 and bladder cancer.14 However, the accurate function of miR‑124‐3p.1 in TNBC remains poorly understood.
Our present study aimed to investigate the effect of miR‐124‐3p.1 in TNBC. The expression of miR‑124‐3p.1 in TNBC tissue samples was detected, and the increased level of miR‐124‐3p.1 was correlated with the pathology status. Kaplan‐Meier survival analysis was performed and found that high miR‑124‐3p.1 expression predicted poor overall survival. To further illustrate the function of miR‑124‐3p.1 in TNBC, BT20 cells were transected with miR‑124‐3p.1 mimics and inhibitor to increase and reduce the level of miR‑124‐3p.1. The changes in cell growth were then explored in vitro and in vivo. To identify the underlying mechanism, we examined the impact of miR‐124‐3p.1 on its target gene Axis inhibition protein 1 (Axin1), and further role of Axin1 was also investigated in our study.
2. MATERIALS AND METHODS
2.1. Cell lines and culture
The human TNBC cell lines BT20 and HCC70, as well as 293T cells, were both obtained from Cell Bank of the Chinese Academy of Science. BT20 and HCC70 were cultured in RPMI 1640 medium, while 293T was cultured in DMEM (Gibco), both added with 10% fetal calf serum (FBS) (Invitrogen) at 37°C with 5% CO2.
2.2. Human subjects
The study was approved by the Ethics Committee of the Shiyan No.1 People's Hospital, Hubei, China, and written informed consent was obtained from each patient prior to participation.
Triple‐negative breast cancer was diagnosed via ultrasound, pathological examinations, magnetic resonance imaging, or cytological examination. Demographics, as well as therapy protocol, imaging data, laboratory data, and follow‐up records of 76 patients with TNBC from August 1, 2014, to January 31, 2016, were collected. The relevant clinical information on the recruited patients is presented in Table 1. The exclusion criteria include (a) unrespectable primary tumor, (b) overt double primary malignancy, (c) loss of follow‐up after surgery, and (d) presence of the prior history of cancer. After surgery, all tumor tissue samples and adjacent noncancerous tissue samples were immediately frozen in liquid nitrogen and stored at −80°C. Follow‐up was performed and ended on April 30, 2019. Death and dates of recurrence were verified by hospital records or by phone contact with the patients or their relatives.
Table 1.
The relationship between miR‐124‐3p.1 expression and clinicopathological characteristics in triple‐negative breast cancer patients
| Clinicopathological features | n | miR‐124‐3p.1 | miR‐124‐3p.1 | x2 | P |
|---|---|---|---|---|---|
| High | Low | ||||
| Age(y) | |||||
| <55 | 40 | 24 | 16 | 0.402 | .526 |
| ≥55 | 36 | 19 | 17 | ||
| Menstrual | |||||
| Menopause | 43 | 19 | 14 | 0.746 | .388 |
| Non‐menopause | 33 | 15 | 17 | ||
| Tumor size | |||||
| ≤2cm | 21 | 6 | 15 | 9.448 | .002 |
| >2cm | 45 | 31 | 14 | ||
| Distant metastasis | |||||
| Present | 41 | 29 | 12 | 7.259 | .007 |
| Absent | 35 | 14 | 21 | ||
| TNM staging | |||||
| Ⅰ+Ⅱ | 26 | 8 | 18 | 11.924 | .001 |
| Ⅲ | 50 | 36 | 14 | ||
| Histological grade | |||||
| Ⅰ+Ⅱ | 30 | 12 | 18 | 7.754 | .006 |
| Ⅲ | 46 | 33 | 13 | ||
2.3. Cell transfection
The miR‐124‐3p.1 mimic and/or inhibitor, as well as matching control, were purchased from the RiboBio Company. miR‐124‐3p.1 mimic/inhibitor or the matching control (150 nmol/L) was transiently transfected into TNBC cells using RNAi Max (Invitrogen). The cells were collected and treated after 48‐h transfection. PCR production of Axin1 amplification was cloned into the pcDNA3.1‐FLAG vector. siAxin1 was obtained from GenePharma Co., Ltd. The transfection assay was conducted with Lipofectamine 2000 (Invitrogen) according to the manufacturers' protocol.
2.4. RNA isolation and quantitative real‐time PCR (qRT‐PCR)
miRNAs were extracted by a miRNA Extraction Kit obtained from Tiangen. After adding the Poly (A) firstly, 1 μg RNA containing miRNAs was then reversely transcribed into cDNA. To detect the level of miR‐124‐3p.1, we got primers of miR‐124‐3p.1 and U6 from Sangon Biotech. Moreover, total RNA was isolated using TRIzol (Invitrogen). Then, total RNA was reversely transcribed into cDNA by a Promega Reverse Transcription Kit (USA) for the detection of Axin1 expression. The expression level of miRNA or Axin1 was analyzed using SYBR Premix Ex Taq from Takara in an ABI QS6 system (ABI). The endogenous control was U6 or GAPDH. The qRT‐PCR primers used were as follows: Axin1‐F 5′‐GGTTTCCCCTTGGACCTCG‐3′, Axin1‐R 5′‐CCGTCGAAGTCTCACCTTTAATG‐3′; Cyclin D1‐F 5′‐AGGAACAGAAGTGCGAGGAGG‐3′, Cyclin D1‐R 5′‐GGATGGAGTTGTCGGTGTAGATG‐3′; c‐Myc‐F 5′‐TACCCTCTCAACGACAGCAG‐3′, c‐Myc‐R 5′‐TCTTGACATTCTCCTCGGTG‐3′; GAPDH‐F 5′‐G GTGGTCTCCTCTGACTTCAACA‐3′, and GAPDH‐R 5′‐GTTGCTG TAGCCAAATTCGTTGT‐3′.
2.5. Western blot analysis
Cells were harvested after 48‐h transfection and were lysed for total protein extraction in 2X SDS Sample Buffer (100 mmol/L Tris‐HCl (pH 6.8), 4% SDS, 10% Glycine, 10 mmol/L EDTA). Primary antibodies to Axin1 (ab93119), Bax (ab53154), Bcl‐2 (ab196495), β‐catenin (ab6302) and p‐β‐catenin (ab75777) were purchased from Abcam. Primary antibodies to β‐actin (#4967) were purchased from Cell Signaling Technology. Secondary antibodies containing anti‐rabbit HRP (sc‐2004) and anti‐mouse HRP (sc‐2380) were obtained from Santa Cruz Biotechnology.
2.6. CCK‐8 viability assay
The growth of cells was assessed by the CCK‐8 assay (Sigma). Equal numbers of cells were seeded into 96‐well plates and cultured for various durations after siRNA transfection and/or miR‐124‐3p.1 mimics/inhibitor treatments. At indicated time point (24, 48, 72, 96, and 120 hours), CCK‐8 reagent was added to the cell medium and incubated for another 1hour at 37°C. The 450‐nm absorbance was then measured quantitatively.
2.7. Colony formation assay
Equal numbers of BT20 cells transected with miR‐124‐3p.1 mimics and inhibitor (500 cells/well) were inoculated in 6‐well plates and cultured for 8 days, respectively. Then, colonies were stained by crystal violet and counted.
2.8. EdU staining assay
To label proliferating cancer cells, EdU (5‐ethynyl‐2′‐deoxyuridine, 1 mg/mL) (Molecular probes, Invitrogen) was added to the culture medium before analysis. After 3‐hour culture, the cells were stained with the reaction cocktail according to the manufacturers' instruction. At least three independent experiments were performed. The values of EdU‐positive cells were analyzed by ImageJ software.
2.9. Mouse xenograft model
Mouse xenograft model was performed as previously reported.15 Briefly, the BALB/c (6‐8 weeks old, n = 15) athymic nude mice were randomly divided into three groups and subcutaneously injected in the flank regions with 1.0 × 106 cells in 0.1 mL of PBS. The tumor size was measured every 5 days with calipers, and the tumor volume was calculated with the formula: (Length × Width2)/2. Twenty days following implantation, mice were euthanized by asphyxiation in a CO2 chamber, and tumors were excised immediately for taking the photograph. All procedures were conducted in accordance with Animal Care and Use Committee guidelines of Wuhan University.
2.10. Luciferase reporter assays
The wild‐type (WT) or mutated human Axin1 3′‐untranslated region (3′UTR) sequence, including potential binding sites, was amplified and cloned into the pGL3‐Basic vector (Promega). A mixture of 20 ng pGL3‐Basic‐Axin1 and 150 nmol/L miR‐124‐3p.1 mimic was together transiently transected into 293T cells. After transfection for 48 hours, firefly luciferase activity and relative Renilla expression were detected using the Dual‐Luciferase Reporter Assay System (Promega) according to a protocol provided by the manufacturer.
For measurements of β‐catenin transcriptional activity, the reporter plasmid containing the DNA binding site of TCF/LEF (CCTTTGATC; TOP‐Flash) (Addgene) was used. Luciferase and Renilla signals were measured 48 hours after transfection using the Dual‐Luciferase Reporter Assay System.
2.11. Statistical analysis
All statistical analyses were performed by Prism 6 software. All the data were presented as the mean ± SEM The difference was analyzed with Student's t test. When P < .05, the differences between groups were considered significant.
3. RESULTS
3.1. miR‐124‐3p.1 correlated with pathology status, and increased miR‐124‐3p.1 level predicts poor overall survival
To determine whether miR‐124‐3p.1 is involved in breast cancer development, we collected fresh breast cancer tumor tissues (n = 76) at different disease status and then performed qRT‐PCR to measure the relative expression levels of miR‐124‐3p.1. Interestingly, the relative expression level of miR‐124‐3p.1 in the breast cancer tissues was significantly increased as the disease pathology progress (Figure 1A), which suggested that miR‐124‐3p.1 may be involved in the development of breast cancer. To further assess the clinical significance of miR‐124‐3p.1 in the prognosis of TNBC cancer patients, we performed Kaplan‐Meier survival analysis and log‐rank tests. We separated the samples into high (above the median, n = 38) and low (below the median, n = 38) miR‐124‐3p.1 expression groups according to the mean value of the miR‐124‐3p.1 level. The data demonstrated that increased miR‐124‐3p.1 level was associated with poor overall survival (Figure 1B). To further confirm the prognostic role of miR‐124‐3p.1 in TNBC patients, we performed the chi‐square test. Chi‐square test revealed that miR‐124‐3p.1 expression was associated with tumor size (P = .002), metastasis (P = .007), TNM stage (P = .001), and histological grade (P = .006) (Table 1). However, the other clinic pathological characteristics, such as age (≤55, >55) and menstrual (menopause, non‐menopause), were found not to be statistically significantly related to miR‐124‐3p.1 expression (Table 1). Therefore, increased miR‐124‐3p.1 seemed to play an important role in the development of TNBC.
Figure 1.
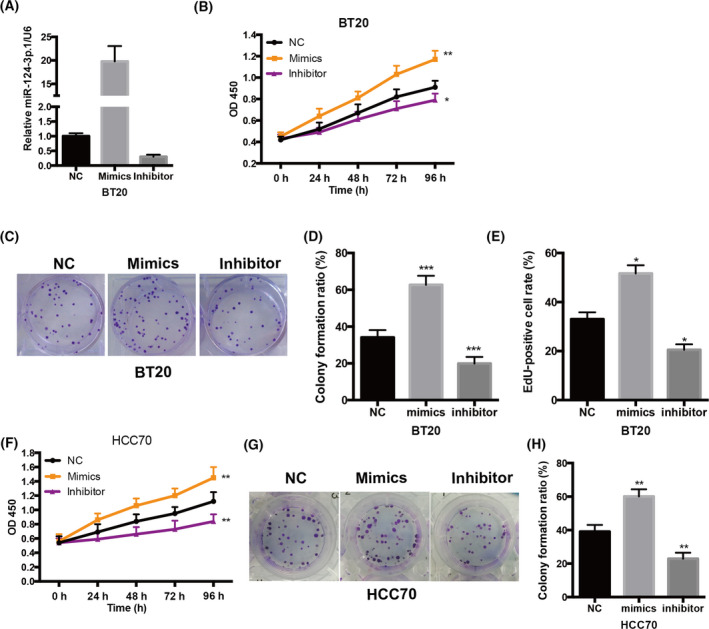
miR‐124‐3p.1 is upregulated in breast cancer tissues and promotes breast cancer cell growth in vitro. A, Increased level of miR‐124‐3p.1 was positively correlated with pathology status by RT‐qPCR. (r 2 = .83, P = .000, n = 76). Pathology status 1‐9 represents Stage 0, Stage IA, Stage IB, Stage IIA, Stage IIB, Stage IIIA, Stage IIIB, Stage IIIC, and Stage IV, respectively. B, The expression level of miR‐124‐3p.1 in BT20 cells that were treated with miR‐124‐3p mimic or miR‐124‐3p inhibitor. C, The effect of miR‐124‐3p.1 on BT20 cell growth was measured by CCK‐8. BT20 cells were treated with miR‐124‐3p mimic or miR‐124‐3p inhibitor. D, E, The effect of miR‐124‐3p.1 on the cell proliferation of BT20 cells was investigated using colony formation assay. The representative results of colony formation of BT20 cells (D) and the quantitative result (E) were shown. F, The effect of miR‐124‐3p.1 on the cell proliferation of BT20 cells was further analyzed by EdU staining. BT20 cells were incubated with EdU for 2 h; then, EdU‐positive cells were detected and counted. (G) The effect of miR‐124‐3p.1 on HCC70 cell growth was measured by CCK‐8. HCC70 cells were treated with miR‐124‐3p mimic or miR‐124‐3p inhibitor. H, I, The effect of miR‐124‐3p.1 on the cell proliferation of HCC70 cells was investigated using colony formation assay. The representative results of colony formation of HCC70 cells (H) and the quantitative result (I) were shown. *P < .05, **P < .01, ***P < .001
3.2. miR‐124‐3p.1 promotes breast cancer cell growth in vitro
To further explore the role of miR‐124‐3p.1 in TNBC development, TNBC cell BT20 was transected with a particular miR‐124‐3p.1 inhibitor and mimics to decrease and increase the level of miR‐124‐3p.1 (Figure 2A). To measure the cell growth of BT20 cells, CCK‐8 assay was performed. These results revealed that miR‐124‐3p.1 deficiency in BT20 cells leads to diminished cell growth, while miR‐124‐3p.1 overexpression increased cell growth (Figure 2B), implying that miR‐124‐3p.1 promotes breast cancer cell growth in vitro. We also performed the colony formation assay to assess the effect of miR‐124‐3p.1 on proliferation. Intriguingly, we found that miR‐124‐3p.1 inhibition caused decreased, while miR‐124‐3p.1 overexpression increased cell proliferation significantly in BT20 cells (Figure 2C,D), indicating that miR‐124‐3p.1 has a role in promoting the proliferation of BT20 cells. Furthermore, the EdU incorporation experiment showed that the EdU incorporation was significantly increased after miR‐124‐3p.1 mimics treatment, while it was reduced after miR‐124‐3p.1 inhibitor treatment (Figure 2E), confirming the role of miR‐124‐3p.1 in regulating TNBC cell proliferation. Most importantly, to verify our findings, we repeated all the experiments in another TNBC cell line, HCC70; the data were similar (Figure 2F‐H). All results suggested that oncogenic miR‐124‐3p.1 promoted TNBC cell growth via promoting cell proliferation in vitro.
Figure 2.
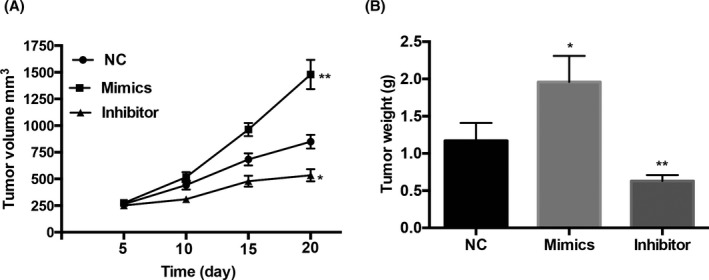
miR‐124‐3p.1 promotes BT20 cell growth in vivo. A, miR‐124‐3p.1 mimics and miR‐124‐3p.1 inhibitor cells were subcutaneously injected into nude mice. Tumor volumes were calculated every 5 d after injection. B, After injected for 20 d, the tumors were excised. Tumor weights of excised tumors were represented as means of tumor weights. *P < .05, **P < .01
3.3. miR‐124‐3p.1 directly inhibits the expression of Axin1 via targeting its 3′‐UTR
To identify the molecular mechanism, we used the miRNA target analysis tool TargetScan 6.2 to screen the potential target of miR‐124‐3p.1. As Figure 3A shows, Axin1 was predicted to be a target of miR‐124‐3p.1 (Figure 3A). To confirm Axin1 is the target by miR‐124‐3p.1, we performed luciferase activity assay in 293T cells. The wild‐type (WT) or the mutated (Mut) Axin1 3′UTR luciferase reporter plasmids were cotransfected with the control mimic or miR‐124‐3p.1 mimic. The co‐transfection of miR‐124‐3p.1 mimic with WT luciferase reporter plasmids in 293T cells displayed significantly decreased luciferase activity compared to the control mimic group, while Mut luciferase activity was not altered (Figure 3B). The mRNA and protein levels of Axin1 were significantly increased after transfection of miR‐124‐3p.1 inhibitor into BT20 cells (Figure 3C,D), while transfection with a miR‐124‐3p.1 mimic in BT20 cells obviously decreased those levels compared to NC group (Figure 3C,D). In conclusion, these results suggested that Axin1 is the target of miR‐124‐3p.1 in TNBC cells.
Figure 3.
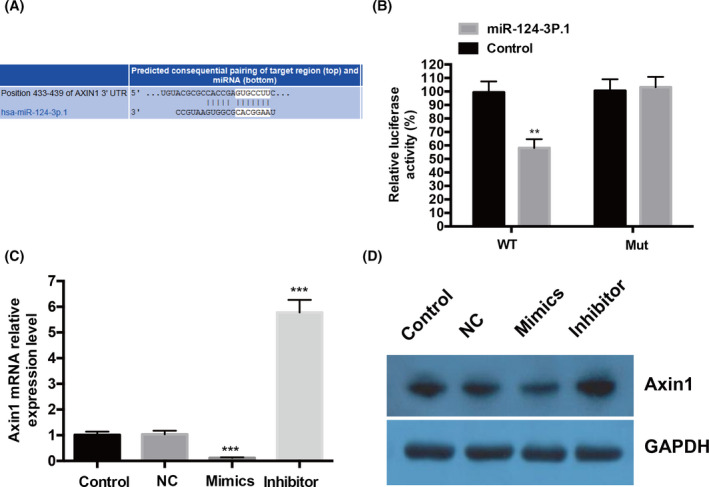
miR‐124‐3p.1 directly inhibits the expression of Axis inhibition protein 1 (Axin1) via targeting its 3′‐untranslated region (3′‐UTR). A, Sequence alignment between miR‐124‐3p.1 and the 3′‐UTR of Axin1 mRNA predicted by TargetScan. B, The effect of miR‐124‐3p.1 on the activity of luciferase reporter containing either wild‐type (WT) or the mutated (Mut) Axin1 3′UTR was examined by luciferase reporter gene assays. 293T cells were transected with WT or the Mut Axin1 3′UTR luciferase reporter plasmids, and the control mimic or miR‐124‐3p.1 mimic. After 24 h, the luciferase activity was detected. (C and D) The effect of miR‐124‐3p.1 on the endogenous expression levels of Axin1 was examined in BT20 cells by RT‐qPCR (C) and Western blotting (D). **P < .01, ***P < .001
3.4. miR‐124‐3p.1 promotes TNBC growth primarily by targeting tumor suppressor Axin1
To further dissect the role of Axin1 in TNBC, loss‐of‐function and gain‐of‐function experiments in vitro were performed. Firstly, we estimated the effect of Axin1 on TNBC cell growth in vitro by CCK‐8 assay. Intriguingly, we found that Axin1 knockdown caused increased cell growth significantly, while Axin1 overexpression caused decreased cell growth in BT20 cells among every time point, indicating that Axin1 has a role in inhibiting the growth of BT20 cells (Figure 4A). Furthermore, Western blotting data showed that apoptosis‐related protein Bax was decreased, while Bcl2 was increased after Axin1 silencing (Figure 4B). In contrast, Bax was increased, while Bcl2 was decreased after Axin1 overexpression (Figure 4B). As a negative regulator of Wnt/β‐catenin signaling, Axin1 knockdown led to decreased phosphorylation of β‐catenin and increased β‐catenin (Figure 4B). On the contrary, Axin1 overexpression resulted in upregulated phosphorylation of β‐catenin and diminished β‐catenin (Figure 4B). Besides, we overexpressed Axin1 in BT20 cells with the miR‐124‐3p.1 mimic treatment to study the effects. The data showed that Axin1 overexpression could partially rescue the increased cell viability driven by miR‐124‐3p.1 when compared to the control (Figure 4C). These influences seem to be triggered by altered cell proliferation, as Axin1 overexpression abolished miR‐124‐3p.1 mimic‐mediated cell proliferation through colony formation assay (Figure 4D). To evaluate the Wnt/β‐catenin signaling activity, we introduced the TOP‐Flash reporter plasmid into BT20 cells. We found that the luciferase activity was dramatically increased following miR‐124‐3p.1 mimics, while it was attenuated by Axin1 overexpression (Figure 4E). Accordingly, the targeting genes of Wnt/β‐catenin signaling, such as CyclinD1 and c‐Myc, were both induced by miR‐124‐3p.1 mimics but suppressed by Axin1 overexpression (Figure 4F). Further, we also found that phosphorylation of β‐catenin and apoptosis‐related protein Bax were both decreased, while β‐catenin and Bcl2 were both increased after miR‐124‐3p.1 mimic treatment (Figure 4G), mimicking the effect of Axin1 knockdown. However, these influences were both rescued by Axin1 overexpression (Figure 4G). Together, all these results proved that miR‐124‐3p.1 promotes TNBC growth primarily by targeting tumor suppressor Axin1 and then modulating Wnt/β‐catenin signaling.
Figure 4.
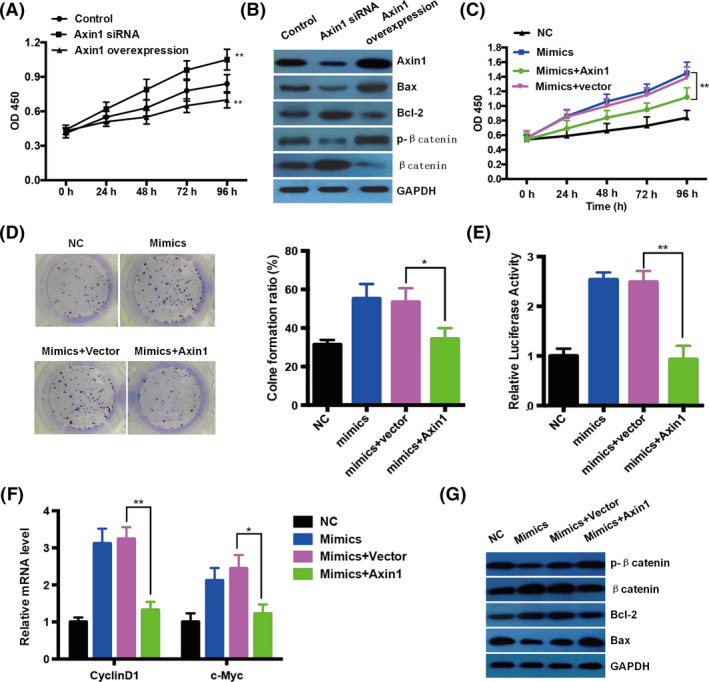
miR‐124‐3p.1 promotes triple‐negative breast cancer growth primarily by targeting tumor suppressor Axis inhibition protein 1 (Axin1). A, Cell growth of BT20 cells was investigated after transfection with empty vector, Axin1 siRNA, and Axin1 overexpression plasmid through CCK‐8 assay. B, Western blotting results showed that changes in Axin1 expression influenced the expression of Bax, Bcl‐2, p‐β‐catenin, and β‐catenin after 48 h of transfection. GAPDH was used as a loading control. C, Cell growth of BT20 cells was investigated after transfection with empty vector, and Axin1 overexpression plasmid with miR‐124‐3p.1 mimics through CCK‐8 assay. D, The representative results of colony formation and the quantitative result of BT20 cells were shown after transfection with empty vector and Axin1 overexpression plasmid with miR‐124‐3p.1 mimics. E, The effect of miR‐124‐3p.1 and Axin1 on the activity of TOP‐Flash reporter activity was examined by luciferase reporter assays. F, Transcriptional level of Wnt pathway targeting genes, CyclinD1, and c‐Myc, in BT20 cells after transfection with empty vector and Axin1 overexpression plasmid with miR‐124‐3p.1 mimics. G, The expression of Bax, Bcl‐2, p‐β‐catenin, and β‐catenin after transfection was analyzed by Western blotting. GAPDH was used as a loading control. *P < .05, **P < .01
3.5. miR‐124‐3p.1 promotes BT20 cell growth in vivo
We further confirmed the above results in the xenograft tumor model in vivo. BT20 cells transected with NC, miR‐124‐3p.1 inhibitor, and mimics were injected subcutaneously into three groups of nude mice randomly. As expected, the tumor volume of miR‐124‐3p.1 inhibitor group was less than the NC group, while the tumor volume of miR‐124‐3p.1 mimic group was bigger than the NC group among all the time points (Figure 5A). Besides, tumor weights of excised breast tumors derived from miR‐124‐3p.1 mimic group grew heavier than those from the NC group after 20 days implantation (Figure 5B). In contrast, the miR‐124‐3p.1 inhibitor group grew lighter than those from the NC group (Figure 5B). These results proved that miR‐124‐3p.1 promoted the growth of breast cancer cells in vivo.
Figure 5.
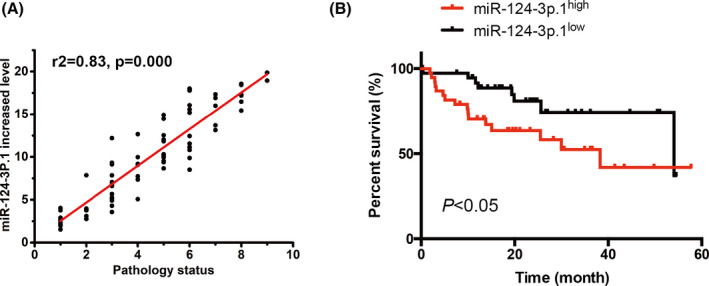
Increased miR‐124‐3p.1 level predicts poor overall survival. Kaplan‐Meier survival analysis of high (above the median, n = 38) and low (below the median, n = 38) miR‐124‐3p.1 expression patient groups. Patients with high levels of miR‐124‐3p.1 expression showed reduced overall survival times compared with patients with low levels of miR‐124‐3p.1. *P < .05
Together, our study discovered that miR‐124‐3p.1 was raised as the TNBC advances and high miR‐124‐3p.1 predicts poor overall survival in TNBC patients. Besides, the increased miR‐124‐3p.1 level promoted the growth of TNBC cells in vitro and in vivo. Furthermore, miR‐124‐3p.1 exerted its function via targeting tumor suppressor gene Axin1. This study provided a novel molecular mechanism for understanding TNBC development and might offer an effective therapeutic strategy for TNBC in the future.
4. DISCUSSION
Triple‐negative breast cancer is the most malignant subtype of breast cancer, with the worst prognosis. However, chemotherapy and radio‐therapy are still used for the treatment of advanced TNBC patients. The development of novel treatments is barred mainly because of less understanding of the molecular mechanism of TNBC development. In the last few years, miRNAs, one class of small non‐coding, single‐stranded RNAs with multiple regulatory functions, emerged as key regulators in the initiation and progression of various human cancers, including TNBC.16, 17, 18 Most importantly, since miRNAs can directly target oncogenes and/or tumor suppressors via modulating its translation and stability, which finally leads to deregulated cellular physiological processes, we believed that miRNAs indeed play a causal character in cancer etiology. As miR‐124‐3p.1 mainly enriched in the brain of mammals, previous studies verified that miR‐124‐3p.1 is related to brain development, neuronal function, and neurodegenerative diseases.19, 20 Recently, miR‐124‐3p.1 has also been identified as a tumor suppressor in certain cancers, including hepatocellular carcinoma,21 cervical cancer,22 and gastric cancer.23 However, the detailed role of miR‐124‐3p.1 in TNBC is largely unclear. Here, we discovered that miR‐124‐3p.1 expression was elevated in advanced TNBC patients and high miR‐124‐3p.1 predicts poor overall survival. Furthermore, we displayed that miR‐124‐3p.1 downregulation diminished, while miR‐124‐3p.1 upregulation increased the growth of TNBC cells in vitro and in vivo. Finally, we proved that miR‐124‐3p.1 exerted its function via targeting tumor suppressor gene Axin1. In summary, all the results demonstrate that miR‐124‐3p.1 promotes TNBC cell growth by controlling Axin1, implying that targeting miR‐124‐3p.1 may be effective. Recent study showed that miR‐124‐3p.1 suppressed cell proliferation and metastasis by targeting MGA5,24 ABCC4,25 and CBL,26 indicating that miR‐124‐3p.1 is a tumor suppressor. What's more, exosomal miR‐124‐3p.1 had been found upregulated in breast cancer patients with recurrence, seems that miR‐124‐3p.1 may promote breast cancer progression.27 The contrary data of this study that miR‐124‐3p promotes TNBC support that TNBC is fundamentally different from other breast cancer and miR‐124‐3p.1 maybe has a context‐dependent role in breast cancer. In the future, we need to illustrate the possibility of targeting miR‐124‐3p.1 in TNBC therapy and develop novel methods.
The two most crucial behaviors of cancer cells involve their ability to sustain uncontrolled proliferation and resist cell death.28 An essential finding of this study is the identification of miR‐124‐3p.1 as a tumor promoter by altering the proliferation. We found that the inhibition of miR‐124‐3p.1 could induce decreased colony formation, while miR‐124‐3p.1 overexpression increased EdU incorporation. Both these results are inconsistent with the previous study that miR‐124‐3p.1 functions as a tumor suppressor, suggesting the role of miR‐124‐3p.1 in different cancer types is relatively context‐dependent.
To better understand the molecular mechanism of miR‐124‐3p.1 in TNBC, we predicted the targets of miR‐124‐3p.1 and focused on Axin1. Axin family, containing Axin1 and Axin2, is a negative regulator of the Wnt/beta‐catenin signaling pathway via regulating the level of beta‐catenin and plays a key role in the developmental processes and pathogenesis of human diseases, including cancer.4, 29, 30 We confirmed miR‐124‐3p.1 targeting the 3′UTR of Axin1 mRNA through luciferase reporter assay, qRT‐PCR, and Western blotting results. Besides, we found that Axin1 overexpression in BT20 cells abrogated the increased cell growth caused by miR‐124‐3p.1 overexpression, implying that Axin1 is the direct target in TNBC. Therefore, Axin1 is considered an appealing target for clinical therapy for TNBC in the future. Based on these findings, it is interesting to examine whether miR‐124‐3p.1 and Axin1 can regulate other cell behavior such as invasion and metastasis.
Together, our study discovered that miR‐124‐3p.1 exerted its oncogenic function via targeting Axin1, which will help to understand the development of TNBC and might offer an effective therapeutic strategy for TNBC.
Yang W, Cui G, Ding M, Yang M, Dai D. MicroRNA‐124‐3p.1 promotes cell proliferation through Axin1‐dependent Wnt signaling pathway and predicts a poor prognosis of triple‐negative breast cancer. J Clin Lab Anal. 2020;34:e23266 10.1002/jcla.23266
Funding information
This work was supported by the guiding project of the scientific research plan of the Hubei education department (B2015479).
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol. 2008;26(8):1275‐1281. [DOI] [PubMed] [Google Scholar]
- 3. Gucalp A, Traina TA. Triple‐negative breast cancer: adjuvant therapeutic options. Chemother Res Pract. 2011;2011:696208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clevers H. Wnt/beta‐catenin signaling in development and disease. Cell. 2006;127(3):469‐480. [DOI] [PubMed] [Google Scholar]
- 5. Clevers H, Nusse R. Wnt/beta‐catenin signaling and disease. Cell. 2012;149(6):1192‐1205. [DOI] [PubMed] [Google Scholar]
- 6. Lee HC, Kim JG, Chae YS, et al. Prognostic impact of microRNA‐related gene polymorphisms on survival of patients with colorectal cancer. J Cancer Res Clin Oncol. 2010;136(7):1073‐1078. [DOI] [PubMed] [Google Scholar]
- 7. Yang H, Dinney CP, Ye Y, Zhu Y, Grossman HB, Wu X. Evaluation of genetic variants in microRNA‐related genes and risk of bladder cancer. Can Res. 2008;68(7):2530‐2537. [DOI] [PubMed] [Google Scholar]
- 8. Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Can Res. 2005;65(16):7065‐7070. [DOI] [PubMed] [Google Scholar]
- 10. Dong RF, Zhang B, Tai LW, Liu HM, Shi FK, Liu NN. The neuroprotective role of MiR‐124‐3p in a 6‐hydroxydopamine‐induced cell model of Parkinson's disease via the regulation of ANAX5. J Cell Biochem. 2018;119(1):269‐277. [DOI] [PubMed] [Google Scholar]
- 11. Kang Q, Xiang Y, Li D, et al. MiR‐124‐3p attenuates hyperphosphorylation of Tau protein‐induced apoptosis via caveolin‐1‐PI3K/Akt/GSK3beta pathway in N2a/APP695swe cells. Oncotarget. 2017;8(15):24314‐24326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng D, Wang L, Chen Y, et al. MicroRNA‐124‐3p regulates cell proliferation, invasion, apoptosis, and bioenergetics by targeting PIM1 in astrocytoma. Cancer Sci. 2016;107(7):899‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idichi T, Seki N, Kurahara H, et al. Involvement of anti‐tumor miR‐124‐3p and its targets in the pathogenesis of pancreatic ductal adenocarcinoma: direct regulation of ITGA3 and ITGB1 by miR‐124‐3p. Oncotarget. 2018;9(48):28849‐28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zo RB, Long Z. MiR‐124‐3p suppresses bladder cancer by targeting DNA methyltransferase 3B. J Cell Physiol. 2018;234(1):464‐474. [DOI] [PubMed] [Google Scholar]
- 15. Li Z, Tian T, Lv F, et al. Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PLoS ONE. 2013;8(3):e59203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu JH, Xu J, Wu YQ, et al. Identification of microRNA‐93 as a functional dysregulated miRNA in triple‐negative breast cancer. Tumor Biol. 2015;36(1):251‐258. [DOI] [PubMed] [Google Scholar]
- 17. D'Ippolito E, Iorio MV. MicroRNAs and triple negative breast cancer. Int J Mol Sci. 2013;14(11):22202‐22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koleckova M, Janikova M, Kolar Z. MicroRNAs in triple‐negative breast cancer. Neoplasma. 2018;65(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 19. Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell‐derived neurogenesis. Stem Cells. 2006;24(4):857‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9(10):1274‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR‐124 and miR‐203 are epigenetically silenced tumor‐suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31(5):766‐776. [DOI] [PubMed] [Google Scholar]
- 22. Wilting SM, van Boerdonk RA, Henken FE, et al. Methylation‐mediated silencing and tumour suppressive function of hsa‐miR‐124 in cervical cancer. Mol Cancer. 2010;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia JT, Wu ZQ, Yu CP, et al. miR‐124 inhibits cell proliferation in gastric cancer through down‐regulation of SPHK1. J Pathol. 2012;227(4):470‐480. [DOI] [PubMed] [Google Scholar]
- 24. Yan G, Li Y, Zhan L, et al. Decreased miR‐124‐3p promoted breast cancer proliferation and metastasis by targeting MGAT5. Am J Cancer Res. 2019;9(3):585‐596. [PMC free article] [PubMed] [Google Scholar]
- 25. Hu D, Li M, Su J, Miao K, Qiu X. Dual‐targeting of miR‐124‐3p and ABCC4 promotes sensitivity to adriamycin in breast cancer cells. Genet Test Mol Biomarkers. 2019;23(3):156‐165. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Chen L, Wu Z, et al. miR‐124‐3p functions as a tumor suppressor in breast cancer by targeting CBL. BMC Cancer. 2016;16(1):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sueta A, Yamamoto Y, Tomiguchi M, Takeshita T, Yamamoto‐Ibusuki M, Iwase H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget. 2017;8(41):69934‐69944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 29. Zeng L, Fagotto F, Zhang T, et al. The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181‐192. [DOI] [PubMed] [Google Scholar]
- 30. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781‐810. [DOI] [PubMed] [Google Scholar]


