Abstract
Background
Clinicians need to know timelines of requested laboratory tests to provide effective patient management. We developed a real‐time laboratory progress checking system and measured its effectiveness using appropriate indicators in an emergency room setting.
Methods
In our original in‐house health information system display, blank spaces, which were generated for test results when tests were ordered, remained empty until the final results reported. We upgraded the laboratory reporting system to show real‐time testing information. The stages included requests for test, label printing, sampling, laboratory receipts, performance of tests, verification of results, and interpretation of results and final report by laboratory physician. To assess the usefulness of the function, we measured the emergency department healthcare workers' satisfaction and compared the number of phone calls about test status before and after implementation.
Results
After the system upgrade, the healthcare workers' understanding of the testing process increased significantly as follows. More clinicians could estimate the time of final test results through the real‐time testing status information (61.9% and 85.7%, P = .002), and respondents reported that the upgraded system was more convenient than the original system (41.3% and 22.2%, respectively, P = .022). The number of phone calls about the test status decreased after implementation of the upgrade; however, the difference was not statistically significant (before, 0.13% [63 calls/48 637 tests] and after, 0.09% [42/46 666]; P = .066).
Conclusions
The real‐time display of laboratory testing status increased understanding of testing process among healthcare workers in emergency room, which ultimately may increase the usefulness and efficiency of the laboratory service use.
Keywords: computerized physician order entry (CPOE), laboratory management, laboratory test status, quality improvement, questionnaire
1. INTRODUCTION
Computerized physician order entry (CPOE) systems are computer applications used to enter diagnostic and therapeutic patient care orders (eg, laboratory test requests) and to view the test results on the network.1 Clinicians can enter their requests directly and work through a digital interface rather than handwriting.2 As hospitals have a mixture of various professionals and there is a need to provide medical services to patients in a timely manner, the transmission of hospital information through an CPOE is very important.3
In our hospital, the laboratory test results are displayed via the in‐house hospital information system (HIS), which includes a laboratory information system (LIS), CPOE, electronic medical records (EMR), and electronic nurse records (ENR). When samples are received by the laboratory, blank spaces are generated for the test results in the display and remain empty until the results are reported. Thus, medical staff must call the laboratory when they want to check the status of tests they have ordered, which is inconvenient, particularly for those who need test results urgently. Moreover, repeated calls to the laboratory interrupt the testing process, further delaying the reports. Although the usage rate of the HIS is high (71.3% in 2015) in Korea, about 70% of hospitals (and most of university hospitals) develop their own HIS according to their individual needs rather than buying the commercialized product.4 Therefore, it makes difficult to standardize procedure, use them interchangeably, and repair systems. Some functions (eg, TAT monitoring for emergency testing) required by the laboratory accreditation have been implemented similarly, but each HIS varies widely. As well as we know, there is no system in Korea that shows the status of the laboratory tests to clinicians in real time.
We hypothesized that a real‐time display of test progress would improve clinician satisfaction. Therefore, we developed a real‐time test progress checking system and assessed its effectiveness in an emergency room setting using appropriate indicators.
2. MATERIALS AND METHODS
The study was conducted between April and October in 2018. Our hospital is a 670‐bed secondary care university hospital in a metropolitan area of Korea. The study was divided into development and evaluation phases. The development phase included upgrading the result viewer to a real‐time display of test status and integrating it into the HIS in June 2018. The evaluation phase consisted of a user satisfaction survey and the measurement of changes in the number of inquiry calls to the laboratory.
2.1. Development of a real‐time laboratory data display
Our LIS was developed in 1999 as an in‐house system (programming language, Visual Basic.NET 2008). Information concerning the laboratory tests ordered by physicians and the final results were linked into and displayed via the HIS, which includes a LIS, CPOE, EMR, and ENR. When tests were ordered, blank spaces were generated in the HIS laboratory result viewer, which remained empty until the final results were reported.
The status of laboratory tests from clinician's request to the final report was sorted in chronological order as follows: request for the test, label printing, sampling, laboratory receipt, performance of the test, verification of the results, interpretation of the results, and final report by a laboratory physician. The laboratory result viewer was updated to enable real‐time display of the ongoing status of laboratory tests according to the sorted stages. When a clinician requests a test in the ward, the barcode of the requested test is automatically printed and “label printing” is shown in the test result viewer. After collection from the patient, samples are transferred to the clinical laboratory for reception. Laboratory personnel receive samples, and “laboratory receipt” is shown. When the sample passes through the barcode scanner in the automated instrument, “test in progress” is shown. During the verification process, “verification of the results” is shown. If the test requires interpretation, incomplete results are moved to another screen on which the laboratory physician can make comments. During this process, “interpretation of results” is shown in the laboratory results viewer. After interpretation, the final results are released and shown in the result viewer. In the case of blood culture, “test in progress” is shown when the barcode has been scanned just before adding the blood bottle to the blood culture incubation instrument. The technical aspects of the updates to the information system were handled by the medical information team of our hospital. To validate the updated HIS function, we created a test sample identification number (ID) that mimicked a patient ID. The test ID was processed using the same procedure as that for a patient sample from receipt to result report to determine whether the function was satisfactory.
2.2. Assessment of the real‐time display
2.2.1. Telephone inquiry investigation
The number of phone inquiries about the current status of a sample or expected result reporting time and the number of tests ordered in the 2‐week period before the CPOE upgrade (April 9‐22, 2018) were compared with those made in the 2‐week period after implementation of the upgrade (August 6‐19, 2018). The laboratory personnel who received the phone calls were asked to record the information on the list. The call/test ratios before and after implementation of the real‐time system were calculated.
2.2.2. Satisfaction survey
The satisfaction survey was checked for ambiguities by two emergency room nurses and one physician before it was distributed. After the questionnaire was validated, we made a few minor modifications. The questionnaire was composed of six questions graded on a five‐point Likert scale regarding users’ impressions and satisfaction with the laboratory results viewer (Table 1). Surveys before the display upgrade were conducted for 2 weeks from 10 to 20 April 2018 among 17 doctors (11 residents and six faculty members) and 46 nurses working in the emergency room. The survey was repeated after implementation of the updated result viewer for 2 weeks from 8 to 22 August 2018 among the same doctors and nurses.
Table 1.
The user satisfaction questionnaire
| Questions | Response options | |||||
|---|---|---|---|---|---|---|
| 1. The upgraded laboratory result viewer is satisfactory | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 2. I know the expected turnaround time. | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 3. I understand the laboratory testing process (from ordering to reporting the result) | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 4. After requesting a "routine chemistry" test | 4‐1) I know whether the sample has been collected | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree |
| 4‐2) I know whether the sample has arrived in the laboratory | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 4‐3) I know whether a test procedure has started | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 4‐4) I know when the laboratory results will be reported | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 4‐5) If the result report is delayed, I know the reason | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 5. I know the status of a test after ordering it | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
| 6. Communicating with the laboratory about the test progress was convenient | ① Strongly agree | ② Agree | ③ Neutral | ④ Disagree | ⑤ Strongly disagree | |
2.2.3. Statistical analyses
The “strongly agree” and “agree” and the “strongly disagree” and “disagree” categories were combined into nominal categories of “agree” and “disagree” to facilitate statistical analysis and interpretation. The chi‐squared test was used to test for differences between the groups.5 We compared the proportion of “yes” responses between the pre‐ and post‐implementation periods. Statistical analyses were performed using STATA/SE version 15.1 for Windows (Stata Corp. LLC, College Station, TX). In all analyses, P < .05 was taken to indicate statistical significance.
3. RESULTS
3.1. Real‐time display of the laboratory test status
The schematic workflow of laboratory testing and the corresponding status displayed in the laboratory results viewer are shown in Figure 1.
Figure 1.
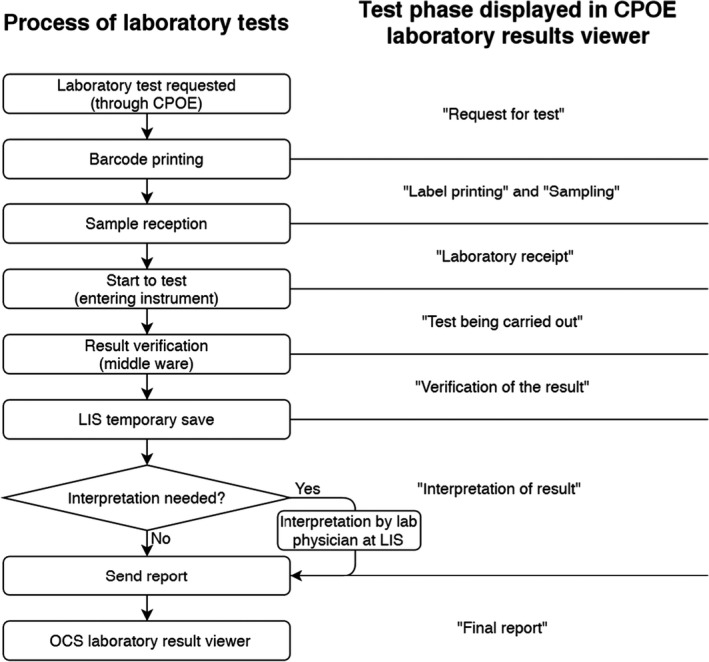
Flowchart of the workflow process for laboratory tests and the corresponding test status displayed in the laboratory result viewer. CPOE, computerized physician order entry; LIS, laboratory information system
In the original system, after a laboratory test was ordered and the barcode label printed, no further information was provided until the results were posted (Figure 2A). In the updated system, the real‐time test status is shown in the laboratory result viewer (Figure 2B).
Figure 2.
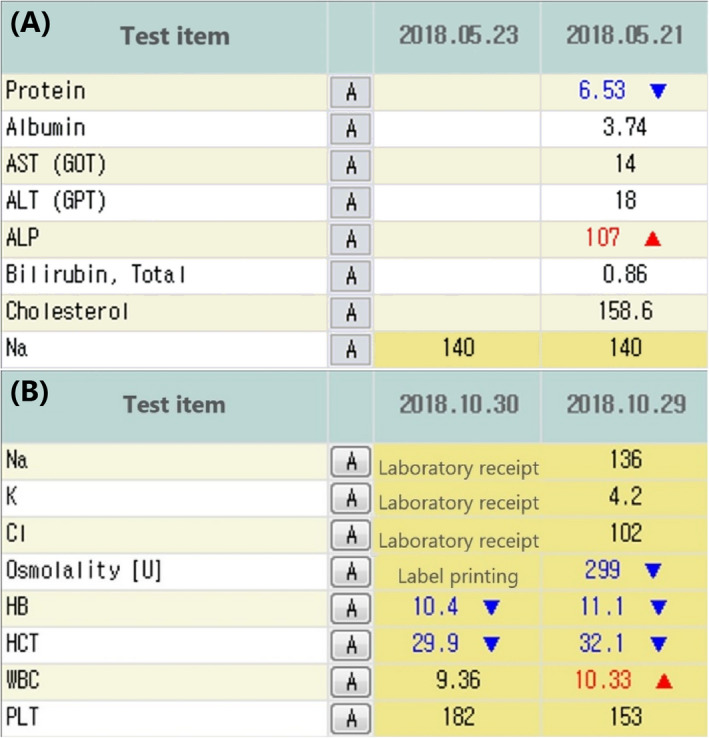
Laboratory information display before (A) and after (B) the upgrade showing the real‐time status of a test before the results are reported. The testing stages and results are displayed according to the receipt date
3.2. Telephone inquiries and tests ordered
The number of phone calls from emergency room staff after implementation of the updated system decreased by 33.3%, from 63 calls in the 2‐week period before to 42 calls after implementation of the system. However, the call/test ratios were not significantly different (before, 0.13% [63/48 637] vs after, 0.09% [42/46 666]; P = .066).
3.3. User satisfaction survey
All 63 healthcare workers invited to participate in the study completed the survey. The respondents reported general satisfaction with the updated system. The pre‐ and post‐implementation survey responses are shown in Table 2. The respondents reported that the real‐time information allowed them to estimate the time of final reports, and they indicated that the upgraded system was more convenient than the original system.
Table 2.
Responses to a user satisfaction survey conducted before and after the laboratory information system upgrade
| Survey item | Number (%) | P‐value * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strongly agree | Agree | Neutral | Disagree | Strongly disagree | ||||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | |||
| 1. The upgraded laboratory result viewer is satisfactory | 2 (3.2) | 6 (9.5) | 19 (25.4) | 24 (38.1) | 28 (44.4) | 26 (41.3) | 10 (15.9) | 4 (6.3) | 4 (6.3) | 3 (4.8) | .102 | |
| 2. I know the expected turnaround time | 2 (3.2) | 1 (1.6) | 37 (58.7) | 53 (84.1) | 18 (28.6) | 6 (9.5) | 6 (9.5) | 3 (4.8) | 0 (0.0) | 0 (0.0) | .002 | |
| 3. I understand the laboratory testing process (from ordering to reporting the result) | 1 (1.6) | 3 (4.8) | 13 (20.6) | 22 (34.9) | 20 (31.7) | 21 (33.3) | 23 (36.5) | 13 (20.6) | 6 (9.5) | 4 (6.3) | .034 | |
| 4. After requesting a "routine chemistry" test | 4‐1) I know whether the sample has been collected. | 9 (14.3) | 7 (11.1) | 33 (52.4) | 24 (38.1) | 7 (11.1) | 12 (19.0) | 13 (20.6) | 18 (28.6) | 1 (1.6) | 2 (3.2) | .047 |
| 4‐2) I know whether the sample has arrived in the laboratory. | 11 (17.5) | 16 (25.4) | 40 (63.5) | 39 (61.9) | 8 (12.7) | 6 (9.5) | 4 (6.3) | 2 (3.2) | 0 (0.0) | 0 (0.0) | .329 | |
| 4‐3) I know whether a test procedure has started. | 1 (1.6) | 7 (11.1) | 16 (25.4) | 27 (42.9) | 15 (23.8) | 10 (15.9) | 26 (41.3) | 19 (30.2) | 5 (7.9) | 0 (0.0) | .002 | |
| 4‐4) I know when the laboratory results will be reported. | 1 (1.6) | 1 (1.6) | 17 (27.0) | 20 (31.7) | 18 (28.6) | 24 (38.1) | 21 (33.3) | 17 (27.0) | 6 (9.5) | 1 (1.6) | .563 | |
| 4‐5) If the result report is delayed, I know the reason. | 0 (0.0) | 2 (3.2) | 4 (6.3) | 5 (7.9) | 17 (27.0) | 20 (31.7) | 31 (49.2) | 28 (44.4) | 11 (17.5) | 8 (12.7) | .344 | |
| 5. I know the status of a test after ordering it. | 0 (0.0) | 2 (3.2) | 8 (12.7) | 20 (31.7) | 22 (34.9) | 22 (34.9) | 23 (36.5) | 17 (27.0) | 10 (15.9) | 2 (3.2) | .003 | |
| 6. Communicating with the laboratory about the test progress was convenient. | 0 (0.0) | 5 (7.9) | 14 (22.2) | 21 (33.3) | 21 (33.3) | 17 (27.0) | 23 (36.5) | 16 (25.4) | 5 (7.9) | 4 (6.3) | .022 | |
Statistical analysis of “Agree” (combination of “Strongly agree” and “agree”) vs “Disagree” (combination of “Strongly disagree” and “Disagree”).
Questions 2, 3, 4.1, 4.3, 5, and 6 showed statistically significant increases in satisfaction after implementation of the changes. More respondents answered that they could predict when the results would be reported after implementation of the new real‐time display (question 2, 61.9% before development vs 85.7% after development, P = .002). Question 3 was related to the respondent's knowledge of the work process of laboratory tests, and question 5 was related to the status of the test after requesting the laboratory tests, and they both improved significantly after the development of this new system (question 3, 22.2% before development vs 39.7% after development, P = .034; question 5, 12.7% before development vs 34.9% after development, P = .003). Question 4 was composed of five subtopics related to routine chemistry tests. Among them, question 4.1 was related to the respondent's degree of understanding of whether a sample had been collected or not, and question 4.3 was related to knowledge of whether a test procedure had begun or not. In question 4.1, the number of “yes” answers decreased from 42 to 31 (question 4.1, 66.7% before development vs 49.2% after development, P = .047) and that in question 4.3 increased from 17 to 34 (question 4.3, 27.0% before development vs 54.0% after development, P = .002). Question 6 was related to the feeling of convenience when exchanging information about the test status with laboratory personnel, and the number of “yes” answers increased from 14 to 26 (question 6, 22.2% before development vs 41.3% after development, P = .022).
4. DISCUSSION
We developed and implemented a real‐time displaying function to monitor laboratory results in our HIS and conducted a survey to assess its usefulness. The system was designed to help clinicians and nurses track ordered tests and estimate the time of final results reporting. To our knowledge, this is the first study regarding a real‐time function for tracking the status of laboratory tests.
Real‐time information can help clinicians make medical decisions. Timely laboratory reports are crucial for emergency medical specialists who may need the result to make a diagnosis or discharge a patient. The advantages of displaying real‐time test status are similar to those of a package tracking system. By checking the result viewer, a clinician can see who has taken charge of the specimen, the stage of processing, and the expected turnaround time. For example, in our hospital, the routine chemistry test usually takes about 30 minutes to transport samples, 30 minutes to receive and pre‐process, 30 minutes to analyze in the instrument, and 5–10 minutes to verify and report results. Under these circumstances, if the status points to "labeling," the test will take at least an hour to report, and if the status points to "verification of the results," clinicians can roughly expect the examination to come within 10 minutes." Moreover, the real‐time system is useful for tracking tests that take a long time, such as microbial tests, or referring or sending‐away tests.6
Some laboratory tests, such as blood cultures, are complex and require considerable time. In our blood culture system, the test status changes to “test performed” when the blood culture bottles are placed in the blood culture incubator. Some laboratory tests are only performed on designated days of the week rather than daily. Changing the test status from “sample collection” to “received by the laboratory” (indicating that the laboratory has received the specimen but has not started the analysis) and “processing” (indicating that the laboratory has started analyzing the sample) provides valuable information for healthcare providers.
The user satisfaction survey revealed a generally positive response to the upgraded system. The survey items that showed significant improvement between the pre‐ and post‐implementation surveys were related to the time of result reporting, the current status of the laboratory tests, whether testing has started, convenience, and improved understanding of the testing process. Patients in the outpatient clinic usually provide samples a few hours before seeing the clinician, so the laboratory tests should be completed before the medical appointment. A real‐time display of the test status helps predict the time until the final result will be obtained and then providing information before deciding on further treatment.
Various result verification processes are used, including delta checking, critical value checking, and retesting for confirmation in the clinical laboratory.7, 8 Most clinicians did not recognize these processes and just waited for the test result report. The updated result viewer displays “verification of the result” during the verification process. It would be useful to emphasize quality improvement activities performed in the clinical laboratory, allowing the clinician to recognize that various tasks are needed for reliable reporting of test results.
Our study has some limitations. First, the evaluation period was relatively short. The 2‐week periods before and after the implementation of the real‐time displaying function may not have been long enough to establish the impact of the system. However, the assessment period could not be extended due to the time constraints of the project. Second, the user satisfaction survey was completed by a small sample of emergency medical staff, who may depend more on timely laboratory test results compared with medical staff in other areas of the hospital. Therefore, our findings were not representative of the healthcare providers in our hospital. Furthermore, we could not compare responses among respondent groups (eg, faculty vs residents vs nurses) because there were too few participants in each group to allow subgroup analyses. Further study with a larger sample and longer evaluation period is needed to demonstrate the true impact of the real‐time displaying system on the behavior and satisfaction of healthcare workers.
In conclusion, we implemented a real‐time display function in our HIS to monitor laboratory test status. The system increased the users' understanding of the laboratory testing process in the emergency room, which ultimately may increase the usefulness and efficiency of the laboratory service use.
AUTHOR CONTRIBUTIONS
SK, THU, and CRC designed the study; JC, DWS, and SJY conducted the research; SJY and SK performed the statistical analyses and data interpretation; JC and SK drafted the study; and THU, CRC, and SJY critically revised the study.
ACKNOWLEDGMENTS
We wish to thank Choong Choi from the medical informatics team, members of the emergency department, and the quality improvement team in the department of laboratory medicine at Ilsan Paik Hospital for help provided during the research.
Chang J, Kim S, Um TH, Cho CR, Shin DW, Yoo SJ. Real‐time display of laboratory testing status improves satisfaction levels in an emergency department: A pilot study. J Clin Lab Anal. 2020;34:e23290 10.1002/jcla.23290
Funding information
This work was supported by a grant for research year 2015 from Inje University (20150607).
REFERENCES
- 1. Main C, Moxham T, Wyatt JC, Kay J, Anderson R, Stein K. Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: systematic reviews of the effects and cost‐effectiveness of systems. Health Technol Assess. 2010;14(48):1‐227. [DOI] [PubMed] [Google Scholar]
- 2. Khanna R, Yen T. Computerized physician order entry: promise, perils, and experience. Neurohospitalist. 2014;4(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeon J. Current status and development of electronic medical record in Korea. Health Insurance Rev Assess Service Policy Trend. 2018;12(3):7‐16. [Google Scholar]
- 5. Clason DL, Dormody TJ. Analyzing data measured by individual Likert‐type items. J Agric Educ. 1994;35(4):31‐35. [Google Scholar]
- 6. Bruins MJ, Ruijs GJ, Wolfhagen MJ, Bloembergen P, Aarts JE. Does electronic clinical microbiology results reporting influence medical decision making: a pre‐ and post‐interview study of medical specialists. BMC Med Inform Decis Mak. 2011;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawkins R. Managing the pre‐ and post‐analytical phases of the total testing process. Ann Lab Med. 2012;32(1):5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih M‐C, Chang H‐M, Tien NI, Hsiao C‐T, Peng C‐T. Building and validating an autoverification system in the clinical chemistry laboratory. Lab Med. 2011;42(11):668‐673. [Google Scholar]


