Abstract
Objectives.
To report the clinical outcomes a cohort of men with high-risk prostate cancer treated with neoadjuvant docetaxel and mitoxantrone ten years after treatment. To identify pretreatment clinical parameters that maybe predictors of recurrence, and to describe tumor infiltrating leukocytes present in the radical prostatectomy specimens.
Methods.
We conducted a phase I/II study of neoadjuvant docetaxel and mitoxantrone prior to radical prostatectomy in high-risk localized prostate cancer to determine the feasibility of this combination and predictors of prostate cancer recurrence after cytotoxic chemotherapy.
Results.
After ten years of follow up, 34 (63%) of 54 participants had a recurrence. In univariate analysis, PSA density (p=0.01), pathological stage (p=0.03), lymph node status (p<0.0001), seminal vesicle invasion (p=0.003) and tissue VEGF expression (p=0.016) were significantly associated with recurrence. In multivariate analysis, only lymph node status, PSA density, and VEGF expression were significant predictors of disease recurrence. We used a tissue microarray for the first 50 participants to characterize the tumor infiltrating lymphocytes and evaluate them for association with recurrence. We measured CD3, CD4, CD8, FoxP3, CD20, CD15, CD68 and CD163 by immunohistochemistry in both tumor and normal prostate specimens, but did not find an association between immunophenotype and recurrence. There was a significantly different density of CD68+ and CD163+ cells between normal and tumor tissue.
Conclusions.
Lymph node status, PSA density, and tissue VEGF expression predict for recurrence after chemotherapy for high-risk prostate cancer. Additional studies are needed to determine the potential benefit of chemotherapy in the neoadjuvant setting.
Keywords: Prostate Cancer, Prostatic Neoplasms, Prostate-Specific Antigen, Immunohistochemistry, Neoadjuvant Therapy, Vascular Endothelial Growth Factor A
INTRODUCTION:
Prostate cancer is a common malignancy, with 220,800 new cases and 27,540 deaths predicted in the United States in 2015, making it the second leading cause of cancer-related death for US men [1]. Prostate cancer is a complex disease characterized by inherent heterogeneity and variable patient outcome as evident by treatment failure rates of 20% to 40% after radical prostatectomy or radiation therapy even in standard risk disease [2–6]. Recurrence may result from the presence of locally extensive disease or systemic disease at the time of treatment. Predictive models using readily available information such as PSA, clinical stage, and pathologic grade can identify patients at high risk of recurrence prior to surgery and allow researchers to target these patients for novel therapeutic strategies [7–9].
Radical prostatectomy remains an option for patients classified as high risk for recurrence, and treatment protocols to eradicate micrometastases with androgen-deprivation therapy following surgery or radiation in patients with high risk prostate cancer have resulted in improved outcomes [10–12]. Several randomized clinical trials have evaluated neoadjuvant androgen-deprivation therapy alone but this intervention has failed to reduce the risk of prostate cancer recurrence [13–16], possibly because of the presence of castration resistant clones at diagnosis. Our understanding of the presence of castration resistant cells early in the disease course was enhanced by the CHAARTED and STAMPEDE studies where men with newly diagnosed metastatic prostate cancer or biochemically recurrent prostate cancer were treated with docetaxel chemotherapy and androgen deprivation therapy had improved survival relative to ADT alone [17, 18]. Thus, docetaxel chemotherapy may be most beneficial in terms of prolonging survival in hormone naïve prostate cancer.
In a previously described phase I/II study we examined the short-term efficacy of neoadjuvant combination chemotherapy of weekly docetaxel and escalating doses mitoxantrone prior to prostatectomy in a cohort of fifty-seven patients [19, 20]. Here, we report long-term outcomes and examine new potential biomarkers. Using radical prostatectomy tissue from patients who participated in this clinical trial, we examined tumor-infiltrating lymphocytes post-chemotherapy to look for correlations between lymphocyte subsets and recurrence. Published reports characterizing the immune composition of prostate cancer at diagnosis in the context of meaningful clinical outcomes (e.g., survival) for prostate cancer have been limited. One group examined “inflammation” broadly in 161 radical prostatectomy specimens and found that higher inflammation was associated with higher likelihood of biochemical recurrence after radical prostatectomy [21]. Similarly, another group found the presence of tumor-infiltrating lymphocytes was associated with reduced survival in 188 patients [22], while others examined prostate cancer tissue from 80 patients and determined that increased CD4+ T-lymphocyte count was independently associated with a shorter cancer-specific survival [23]. These studies suggest the potential for immune cells to promote prostate cancer growth/recurrence and hint that there may be immune targets amenable to therapeutic targeting. Alternatively, the presence of tumor infiltrating lymphocytes may be an indicator of more aggressive tumor clones. However, the absence of definitive studies in human prostate cancer have left us without clarity about the types of immune cells present within tumors and the significance that immune cells have in mediating tumor progression, response to therapy, and survival.
Here, we report the clinical outcomes of this cohort of men with high-risk prostate cancer treated with neoadjuvant docetaxel and mitoxantrone ten years after treatment and describe the tumor infiltrating leukocytes present in the radical prostatectomy specimens. Pathological, biochemical and molecular variables were examined and assessed for their ability to predict progression-free survival.
MATERIAL and METHODS:
Patients:
The clinical trial design has been described previously [19, 20]. Briefly, 57 patients were recruited between 2001 and 2004 with a new histological diagnosis of adenocarcinoma of the prostate with a planned prostatectomy as the primary therapy and at least one of the following high- risk features: clinical stage T2c or surgically resectable T3a, serum prostate specific antigen (PSA) ≥15 ng/ml, or Gleason grade ≥4+3 for a multicenter phase I-II clinical trial of neoadjuvant chemotherapy.
Ethics Statement
The clinical trial protocol (NCT 00017563) was approved by the Institutional Review Boards of the Oregon Health and Science University, VA Portland Health Care System, Kaiser Permanente Northwest Region, Legacy Health System, and the University of Washington. All patients signed informed consent.
Treatment:
Docetaxel was administered at a fixed dose of 35 mg/m2 and the MTD of mitoxantrone was established in 10 patients to be 4 mg/m2 [20]. Subsequently, all patients received this dose of mitoxantrone. Patients received four 28-day cycles of chemotherapy with docetaxel and mitoxantrone administered weekly for 3 weeks followed by a 1-week break. Dexamethasone 4 mg was given orally 12 hours and 1 hour before and 12 hours after treatment. Four to six weeks after completing chemotherapy, patients underwent open radical prostatectomy at their respective institutions. Three of the 57 patients did not complete the 4 cycles of chemotherapy prior to surgery. Two patients developed grade 2 side effects before completing the full 4 cycles of therapy (one patient developed persistent grade 2 nausea and another developed grade 2 neuropathy). The third patient was lost to follow up because he was incarcerated.
Patient Monitoring:
During the first week of every 4-week cycle, a serum PSA and chemistry panel were obtained from each patient. A complete blood count was also obtained each week during the first three weeks of drug treatment. A physical exam was completed during each week, and a serum testosterone level was obtained prior to and after treatment. Surgical morbidities and pathological findings were recorded. After completion of chemotherapy and prostatectomy, patient follow-up, clinical evaluation and serum PSA levels were performed every 3 months and regular clinic visits for ten years or until evidence of recurrence as defined below.
Translational Studies
Pre- and post-chemotherapy plasma vascular endothelial growth factor (VEGF) were measured. Tumor expression of tissue VEGF was characterized by tissue microarray. Methods for these assays have been described [20]. Also, serum total testosterone levels were obtained prior to and after neoadjuvant chemotherapy.
Immune infiltration was examined via immunohistochemistry with a panel of 8 markers in primary prostatic tumors and adjacent normal tissue using a tissue microarray (TMA) of 50 patients treated neoadjuvantly with docetaxel and mitoxantrone. Heat mediated antigen retrieval was performed with citrate buffer (BioGenex, Fremont, CA), with the exception of retrieval prior to CD4 staining, which was achieved using 10 mM Tris/1 mM EDTA. Staining was performed with the Ultravision Detection System (Thermo Scientific, Waltham, MA), followed by counterstaining with methyl green. Antibodies CD3, CD4, CD8, CD15, and CD163 were from Thermo Scientific, CD68 (clone PG-M1) and CD20 were from AbCAM (Cambridge, UK), and FoxP3 was from eBioscience (San Diego, CA). Slides were digitally scanned with an Aperio ScanScope CT Slide Scanner at 40x objective and individual TMA cores were analyzed separately using TMALab (Aperio, Buffalo Grove, IL) and the nuclear stain detection algorithm to quantify the number of positive cells within each core. Data is displayed as cell density (cells/mm2).
Statistical Analysis
The primary endpoint for this analysis was to determine the ten-year recurrence-free survival following combination neoadjuvant chemotherapy and prostatectomy. Recurrence was defined as the detection and confirmation of a serum PSA > 0.4 ng/ml or any other clinical evidence of disease or initiation of any prostate-cancer directed therapy.
Variables investigated included: age, day 1 (at entry to study) PSA level, baseline PSA level, PSA level after chemotherapy, percent change in PSA following chemotherapy and PSA density (PSAD) which was calculated by dividing the day 1 PSA value by prostate volume as measured by transrectal ultrasound. Gleason scores were assigned into either three category scheme (6, 7, 8–10) or two category scheme (6–7 or 8–10). Pathologic and clinical T-stages were split into T1–2 and T3–4. Other variables included: clear pathological surgical margins, seminal vesicle involvement, extension to lymph nodes, serum total testosterone levels prior to and after neoadjuvant chemotherapy, serum VEGF prior to and after chemotherapy, percent change in serum VEGF and tissue VEGF expression. Sign Rank Test was used to compare means between pre and post-chemotherapy testosterone levels as well as VEGF levels.
Survival analyses were analyzed using the Kaplan-Meier method. Univariate and multivariate regression analyses were conducted to evaluate the effect of covariates on PFS using respectively the Log-Rank Test and the Cox Proportional Hazard Ratio. For the Kaplan-Meier, univariate and multivariate regression analysis the continuous variables: percent change in PSA level following chemotherapy, percent change in VEGF levels following chemotherapy and tissue VEGF expression were dichotomized into high and low groups based on median values. In addition to the analysis of serum testosterone levels as a continuous variable, the serum testosterone level variable was also dichotomized into two groups, patients with a serum testosterone levels < 200 ng/dL and ≥ 200 ng/dL. In order to determine whether physiological male hypogonadism affects recurrence of prostate cancer, this variable was included in the analyses since one criterion for male hypogonadism is a serum testosterone less than 200 ng/dL.
RESULTS
Patient characteristics
Of the 57 patients total patients enrolled, 3 did not complete the 4 cycles of chemotherapy prior to surgery. Two patients developed grade 2 side effects before completing the full 4 cycles of therapy (one patient developed persistent grade 2 nausea and the second patient developed grade 2 neuropathy). The third patient was lost to follow up because he was incarcerated. Only the 54 patients who received all cycles of chemotherapy and underwent surgery are described in this report. Patient characteristics are presented in Table 1 and were presented previously [20].
Table 1.
Patient characteristics
| No. of patients | % | Mean | Median | SD | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|---|
| Age | 54 | 62.1 | 62.5 | 6.3 | 49.5 | 74.3 | ||
| Day 1 PSA (ng/ml) | 54 | 18.4 | 12.0 | 15.1 | 1.4 | 58.6 | ||
| Prostate volume (cc) | 48 | 35.7 | 31.9 | 19.7 | 14.3 | 136.0 | ||
| PSA density | 48 | 0.61 | 0.36 | 0.66 | 0.05 | 3.68 | ||
| Serum testosterone levels pre chemotherapy (ng/dL) | 53 | 342.9 | 310.0 | 145.5 | 97.0 | 914.0 | ||
| Serum VEGF levels pre chemotherapy (pg/ml) | 49 | 78.6 | 44.8 | 80.7 | 10.6 | 314.7 | ||
| Tissue VEGF expression | 46 | 3.7 | 3.6 | 1.7 | 0.3 | 6.8 | ||
| Gleason Score at Biopsy | 6 | 5 | 9.3 | |||||
| 7 | 22 | 40.7 | ||||||
| 8 | 14 | 25.9 | ||||||
| 9 | 12 | 22.2 | ||||||
| 10 | 1 | 1.9 | ||||||
| Gleason Score at Surgery | 6 | 8 | 15.1 | |||||
| 7 | 25 | 47.2 | ||||||
| 8 | 8 | 15.1 | ||||||
| 9 | 11 | 20.8 | ||||||
| 10 | 1 | 1.9 | ||||||
| Surgical margins | Negative | 36 | 66.7 | |||||
| Positive | 18 | 33.3 | ||||||
| Clinical T Stage | T1c | 7 | 13.0 | |||||
| (AJCC 2002 criteria) | T2a | 6 | 11.1 | |||||
| T2b | 10 | 18.5 | ||||||
| T2c | 18 | 33.3 | ||||||
| T3a | 10 | 18.5 | ||||||
| T3b | 2 | 3.7 | ||||||
| T4 | 1 | 1.9 | ||||||
| Pathological T Stage | T2a | 4 | 7.4 | |||||
| (AJCC 2002 criteria) | T2b | 5 | 9.3 | |||||
| T2c | 16 | 29.6 | ||||||
| T3a | 10 | 18.5 | ||||||
| T3b | 9 | 16.7 | ||||||
| T3c | 7 | 13.0 | ||||||
| T4a | 3 | 5.6 | ||||||
| Node Status | Negative | 44 | 81.5 | |||||
| Positive | 10 | 18.5 | ||||||
| Seminal Vesical Involvement | Negative | 36 | 69.2 | |||||
| Positive | 16 | 30.8 |
Long-term outcome
At ten years, 34 (63%) of the 54 men had a PSA recurrence. The median time to recurrence was 51 months (range: 1–140 months). Six men were lost to follow up for a ten-year retention rate of 89%. The recurrence free survival at 2 years was 63% (95% CI: 49–74), at 5 years was 46% (95% CI: 32–59) and at 10 years was 29% (95% CI: 16–44).
Predictors of disease recurrence
The univariate Cox regression model identified five predictors of disease recurrence: PSAD (p=0.01), pathological stage (p=0.03), lymph node status (p<0.0001), seminal vesical invasion (p=0.003) and tissue VEGF expression (p=0.016) (Table 2).
Table 2:
Predictors of Disease Recurrence on Univariate Analysis
| Covariate | Comparison | Hazard Ratio | 95% Hazard Ratio Confidence Interval | P-value | |
|---|---|---|---|---|---|
| Age | n/a | 0.98 | 0.93 | 1.03 | 0.436 |
| PSA density | n/a | 2.51 | 1.31 | 4.81 | 0.005 Ɨ |
| Prostate volume | n/a | 1.00 | 0.98 | 1.02 | 0.987 |
| PSA baseline | n/a | 1.02 | 1.00 | 1.05 | 0.070 |
| Percent change in PSA | Low vs high | 1.22 | 0.61 | 2.45 | 0.576 |
| Gleason score at biopsy | 6 vs 8–10 | 0.77 | 0.26 | 2.27 | 0.620 |
| 7 vs 8–10 | 0.70 | 0.34 | 1.46 | ||
| Gleason score at surgery | 6 vs 8–10 | 0.85 | 0.30 | 2.38 | 0.949 |
| 7 vs 8–10 | 0.93 | 0.45 | 1.94 | ||
| Gleason score at biopsy | 6–7 vs 8–10 | 0.72 | 0.37 | 1.40 | 0.333 |
| Gleason score at surgery | 6–7 vs 8–10 | 0.91 | 0.45 | 1.82 | 0.788 |
| Surgical margin | Negative vs Positive | 0.70 | 0.35 | 1.41 | 0.315 |
| Clinical Stage | T1–2 vs T3–4 | 0.60 | 0.30 | 1.22 | 0.158 |
| Pathological Stage | T1–2 vs T3–4 | 0.47 | 0.24 | 0.94 | 0.032 Ɨ |
| Node Status | Negative vs. Positive | 0.19 | 0.09 | 0.41 | <.0001 Ɨ |
| Seminal Vesical involvement | Negative vs. Positive | 0.33 | 0.16 | 0.68 | 0.003 Ɨ |
| Percent change in VEGF | Low vs high | 0.93 | 0.46 | 1.90 | 0.848 |
| Serum testosterone level | <200 vs. ≥200 ng/dL | 1.12 | 0.43 | 2.89 | 0.822 |
| Tissue VEGF expression | Low vs high | 0.39 | 0.19 | 0.84 | 0.016 Ɨ |
Significant at 0.05 level.
n/a = continuous variable
At the five-year follow-up period, with multivariate analysis after stepwise procedure, 4 variables (lymph node status, Gleason score at biopsy, PSAD and tissue VEGF expression) were identified as independent predictors of disease recurrence [20]. The ten-year follow-up with multivariate analysis identified three predictors of disease recurrence (lymph node status, PSAD, and tissue VEGF expression) that at baseline were independently associated with risk of recurrence. (Table 3)
Table 3.
Multivariate analysis of predictors of recurrence at 5 and 10 years.
| Parameter | Comparison | Hazard Ratio | 95% Hazard Ratio Confidence Interval | P-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5 year PFS | 10 year PFS | 5 Year PFS | 10 Year PFS | 5 year PFS | 10 year PFS | ||||
| Lymph Node Status | Negative vs Positive | 9.70 | 0.23 | 2.80 | 33.40 | 0.84 | 0.64 | <.0001 | 0.005 Ɨ |
| Gleason score at biopsy | 6–7 vs 8–10 | 1.80 | 1.60 | 1.10 | 3.10 | 0.946 | 2.71 | 0.030 | 0.080 |
| PSA density | continuous | 6.40 | 4.09 | 1.20 | 10.40 | 1.645 | 10.14 | 0.004 | 0.002 Ɨ |
| Tissue VEGF expression | Low vs high | 3.50 | 0.31 | 1.20 | 12.00 | 0.125 | 0.78 | 0.023 | 0.013 Ɨ |
Significant at 0.05 level
Immunophenotype
Immunohistochemistry revealed prominent tissue infiltration of both tumors and adjacent normal tissue by T cells (CD3, CD4, CD8) and macrophages (CD68, CD163) (Figure 2A). Minimal presence of CD15+ neutrophils, CD20+ B cells, or FoxP3+ regulatory T cells were observed. A paired analysis between matching adjacent normal and tumor tissue revealed no significant differences in lymphocyte infiltration (Figure 2B), but did identify a small, yet significant (p<0.001) increase in the presence of CD68+ and CD163+ cells. This was also true in an unpaired analysis (Figure 2C) with an approximate 2-fold increase in CD68+ (87.68±8.872 versus 178.9±15.75) and CD163+ (101.0±8.852 versus 173.6±15.30) cells. Cellular density of CD68+ and CD163+ cells displayed a strong correlation (R=0.8946), suggesting that the majority of macrophages were positive for both markers (Figure 2C).
Figure 2.
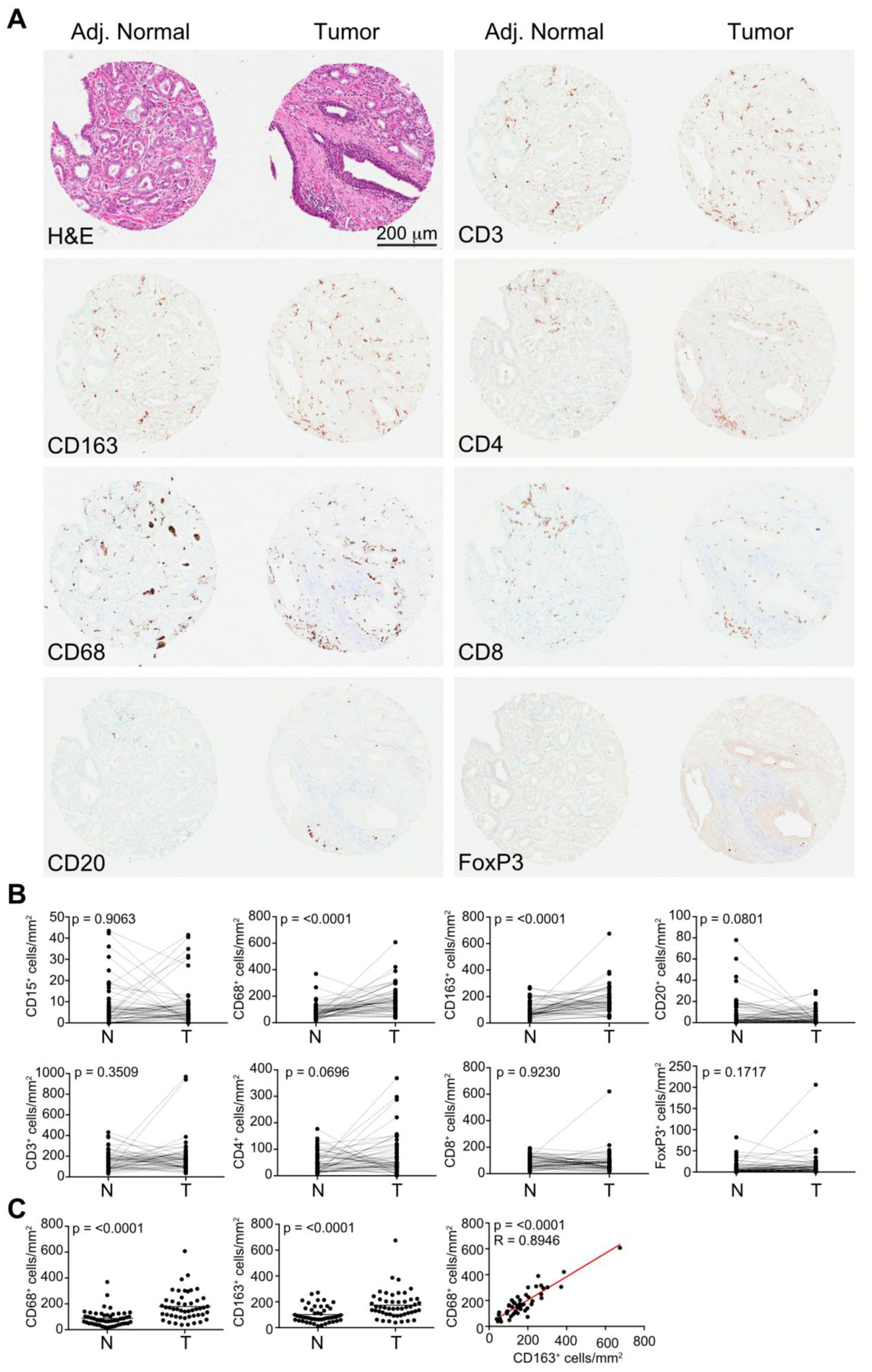
(A) Representative immunohistochemistry of the post-chemotherapy prostatectomy specimens stained for leukocyte markers. The adjacent normal and tumor samples displayed are neighboring samples on the TMA, but are not patient matched. (B) Paired sample analysis between the density of immune cells within tumors and adjacent normal tissue. (C) Unpaired sample analysis for the density of CD68+ (left panel) and CD163+ positive cells (center panel). Linear regression analysis of CD68 and CD163 cell density is shown in the right panel.
We next examined whether immune infiltration in either tumors or adjacent normal tissue was predicative of progression using a logistic regression model. None of the parameters examined displayed significance (p<0.05). A subgroup analysis of lymph node negative patients (n=41) also found no significant correlations. Finally, a multivariable analysis incorporating combined immune scores also failed to identify combinations that were predictive of progression.
DISCUSSION:
At the ten year follow-up, the progression-free survival for the entire study was 29%. Our results are similar to the recently published study of neoadjuvant docetaxel therapy in patients with locally advanced prostate cancer. In that study, 10 (36%) of the 28 patients were disease free clinically and biochemically, and the estimated ten-year biochemical recurrence-free survival was 33.5% [24]. It is encouraging that even with the most sensitive definition of recurrence (i.e. PSA or any cancer directed intervention), a third of patients with high risk disease remain free of disease a decade later.
Our study is unique in that we were able to identify predictors for recurrent prostate cancer after neoadjuvant therapy and radical prostatectomy. Other groups have identified PSAD as a predictor for adverse pathologic features in localized disease [25–27]. To our knowledge, this is the first demonstration that PSAD is a ten-year predictor of prostate cancer recurrence both in univariate and multivariate analyses. PSAD can be readily calculated prior to any therapy or surgery. Tissue VEGF expression is a measure of VEGF receptor status on the pathologic prostate cancer specimen. The finding that a higher tissue VEGF expression predicts the ten-year recurrence after neoadjuvant therapy and radical prostatectomy is provocative. In a recent review VEGF receptors have been implicated in the pathogenesis of metastatic prostate cancer to the skeleton [28]. The finding of elevated tissue VEGF expression suggests a possible targeted therapeutic intervention in those patients likely to recurrence.
Ours is the first study to characterize infiltrating lymphocytes in prostate cancer tissue post-chemotherapy. We observed both strong macrophage and T cell infiltration, with minimal detection of neutrophils, B cells, or regulatory T cells. Only an increase in macrophage infiltration was found between tumor and adjacent normal samples, possibly reflecting the use of adjacent normal, rather than truly normal prostate tissue. However, as these samples were all obtained post-chemotherapy, it is not possible to determine whether these findings are specific to post-treated tumors, or reflect the immunophenotype of prostate tumors broadly. For example, a reduction in B cells and CD4+ T cells, along with an increase in macrophages, has previously been noted within the tumors of breast cancer patients treated with neoadjuvant chemotherapy [29]. Similarly, the disappearance of FoxP3+ regulatory T cells has been observed in breast carcinoma patients that have a complete response to chemotherapy [30]. Regardless, in this small patient population, we did not find a population of lymphocytes that could predict recurrence. Whether infiltration by specific immune populations can predict recurrence prior to chemotherapy remains to be determined.
The study has several strengths. First, it is a prospective ten-year multicenter study, thus reducing biases that are inherent when studies are conducted at one institution. Second, it is relatively large for phase II trials, and there was a high retention rate for ten years (89%). Finally, we have a tissue microarray that contains the prostatectomy tissue from most of the participants (93%) that we can use for biological correlates. Unfortunately, this study is limited in that there is not a comparator group. Also, it may not have been large enough to define the predictive potential of tumor infiltrating lymphocytes post-chemotherapy.
In the 15 years since this study was designed, there have been no phase III data that reported the results of neoadjuvant treatment in high-risk localized prostate cancer. However, there is a phase III cooperative group study underway to test the combination of docetaxel and ADT given prior to surgery versus surgery alone in high-risk disease (NCT00430183). There is currently no indication for neoadjuvant chemotherapy outside of the setting of a clinical trial.
Figure 1.
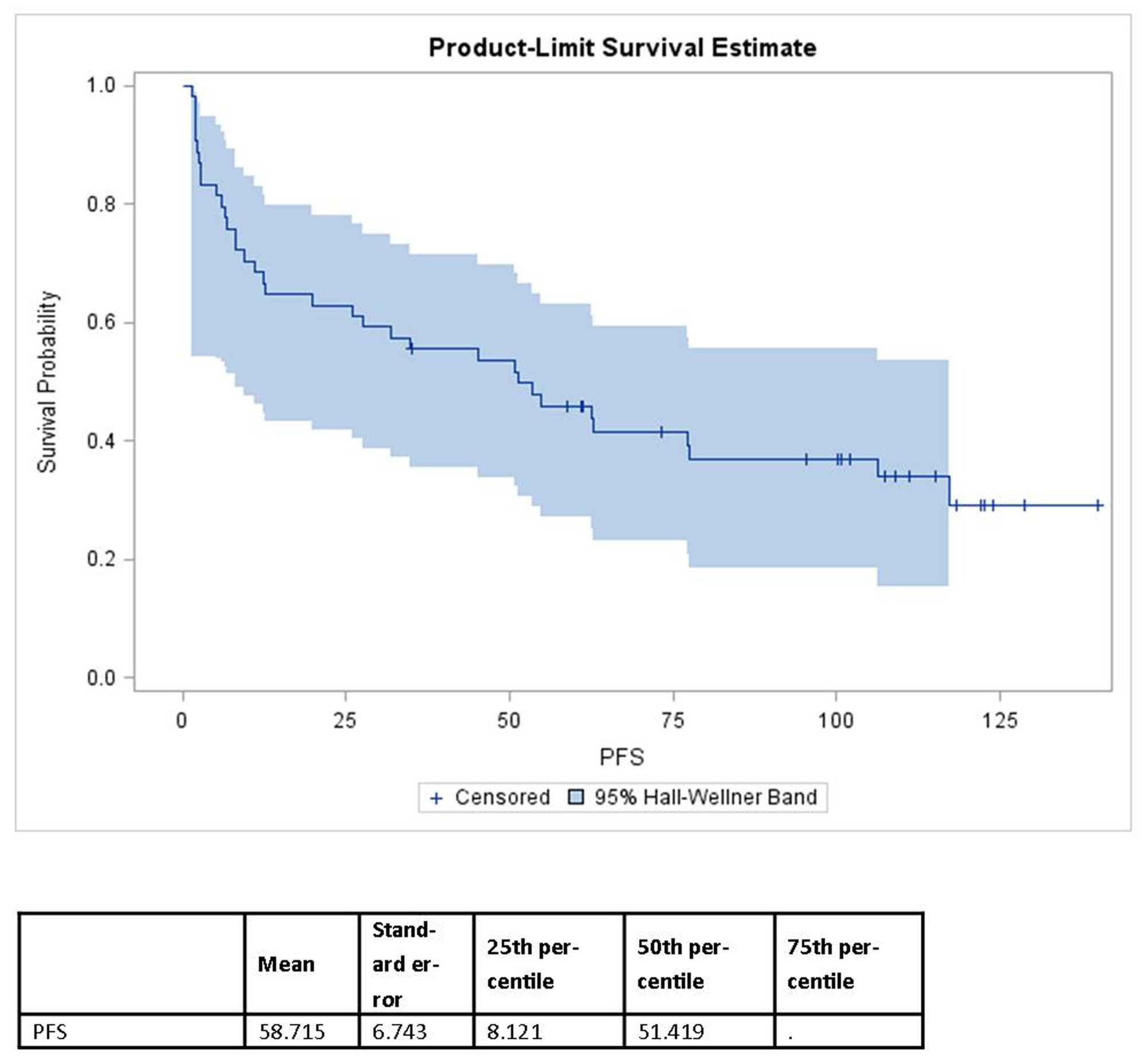
The Kaplan-Meier progression-free survival for entire study group.
Acknowledgements:
Funding by Aventis Pharmaceuticals Grant-in-Aid United States Grant 16080, Serono, Inc., Grant 031.G0008, and NIH Grant 3M01RR00334-33S2
Source of Funding: Aventis Pharmaceuticals Grant-in-Aid United States Grant 16080, Serono, Inc., Grant 031.G0008, and NIH Grant 3M01RR00334-33S2
Footnotes
Conflict of interest: None declared
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- [2].Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nature Clinical Practice Urology Nat Clin Pract Urol. 2005; 2: 174–82. [DOI] [PubMed] [Google Scholar]
- [3].Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys. 2003; 57: 915–928. [DOI] [PubMed] [Google Scholar]
- [4].Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001; 28: 555–565. [DOI] [PubMed] [Google Scholar]
- [5].Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010; 28: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer: studies on natural history and therapeutic considerations. J Natl Compr Canc Netw. 2004; 2: 249–256. [DOI] [PubMed] [Google Scholar]
- [7].Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007; 69: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006; 98: 715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999; 17: 168–172. [DOI] [PubMed] [Google Scholar]
- [10].Rosenthal SA, Sandler HM. Treatment strategies for high-risk locally advanced prostate cancer. Nat Rev Urol. 2010; 7: 31–38. [DOI] [PubMed] [Google Scholar]
- [11].Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014; 11: 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, Rolland F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015; 16: 787–794. [DOI] [PubMed] [Google Scholar]
- [13].Aus G, Abrahamsson P-A, Ahlgren G, Hugosson J, Lundberg S, Schain M, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002; 90: 561–566. [DOI] [PubMed] [Google Scholar]
- [14].Gleave ME, Goldenberg SL, Chin JL, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001; 166: 500–506; discussion 6–7. [PubMed] [Google Scholar]
- [15].Hurtado-coll A, Goldenberg SL, Klotz L, Gleave ME. Preoperative neoadjuvant androgen withdrawal therapy in prostate cancer: the Canadian experience. Urology. 2002; 60: 45–51. [DOI] [PubMed] [Google Scholar]
- [16].Soloway MS, Pareek K, Sharifi R, Wajsman Z, Mcleod D, Wood DP, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002; 167: 112–116. [PubMed] [Google Scholar]
- [17].Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015; 373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].James N, Sydes M, Mason M, Clarke N, Dearnaley D, Spears M, et al. Docetaxel and/or zoledronic acid for hormone-naive prostate cancer: First overall survival results from STAMPEDE (NCT00268476). J Clin Oncol 2015; suppl: 33 (abstr 5001). [Google Scholar]
- [19].Beer TM, Garzotto M, Lowe BA, Ellis WJ, Montalto MA, Lange PH, et al. Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2004; 10: 1306–1311. [DOI] [PubMed] [Google Scholar]
- [20].Garzotto M, Higano CS, O’brien C, Rademacher BLS, Janeba N, Fazli L, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010; 116: 1699–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Irani J, Goujon J-M, Ragni E, Peyrat L, Hubert J, Saint F, et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology. 1999; 54: 467–472. [DOI] [PubMed] [Google Scholar]
- [22].Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005; 25: 4435–4438. [PubMed] [Google Scholar]
- [23].McArdle PA, Canna K, McMillan DC, McNicol AM, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004; 91: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao B, Yerram NK, Gao T, Dreicer R, Klein EA. Long-term survival of patients with locally advanced prostate cancer managed with neoadjuvant docetaxel and radical prostatectomy. Urol Oncol. 2015; 33: 19–23. [DOI] [PubMed] [Google Scholar]
- [25].Sfoungaristos S, Perimenis P. PSA density is superior than PSA and Gleason score for adverse pathologic features prediction in patients with clinically localized prostate cancer. Can Urol Assoc J. 2012; 6: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kundu SD, Roehl KA, Yu X, Antenor JA, Suarez BK, Catalona WJ. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol. 2007; 177: 505–509. [DOI] [PubMed] [Google Scholar]
- [27].Radwan MH, Yan Y, Luly JR, et al. Prostate-specific antigen density predicts adverse pathology and increased risk of biochemical failure. Urology. 2007; 69: 1121–1127. [DOI] [PubMed] [Google Scholar]
- [28].Roberts E, Cossigny DAF, Quan GMY. The Role of Vascular Endothelial Growth Factor in Metastatic Prostate Cancer to the Skeleton. Prostate Cancer. 2013;2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012; 109: 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008; 14: 2413–2420. [DOI] [PubMed] [Google Scholar]


