Abstract
Liver fibrosis occurs in most cases of chronic liver disease, which are a somewhat common, but also a potentially deadly group of diseases. In vitro modeling of liver fibrosis relies primarily on the isolation of in vivo activated hepatic stellate cells (aHSCs) and studying them in standard tissue culture dishes (2D). In contrast, modeling of fibrosis in bio-fabricated three-dimensional (3D) construct allows us to study changes to the environment, such as extracellular matrix (ECM) composition and structure, and tissue rigidity. In the current study, we used aHSCs produced through sub-cultures in 2D and encapsulated them in a 3D collagen gel to form spherical constructs. In parallel, and as a comparison, we used an established HSC line, LX-2, representing early and less severe fibrosis. Compared with LX-2 cells, the aHSCs created a stiffer environment and expressed higher levels of TIMP1 and LOXL2, all of which are indicative of advanced liver fibrosis. Collectively, this study presents a fibrosis model that could be incorporated with multi-cellular models to more accurately reflect the effects of a severe fibrotic environment on liver function.
Keywords: tissue stiffness, collagen fiber analysis, ECM remodeling, liver fibrosis, activated hepatic stellate cell
Introduction:
Chronic liver disease (CLD) claims the lives of an estimated 40,000 lives per year (Wiegand & Berg, 2013). Symptomatically, CLD often begins with inflammation of the liver, progressing through ever more severe fibrosis, and in many cases, ending with cirrhosis or hepatocellular carcinoma (Manka, Zeller, & Syn, 2019; O’Rourke, Sagar, Shah, & Shetty, 2018). The fibrosis is progressive and the result of continuous and uncontrolled pathogenic remodeling of the liver stroma. This aberrant remodeling generally consists of excessive extracellular matrix (ECM) production, comprised mainly of fibrillar collagen, inhibition of metalloproteinases through tissue inhibitor of metalloproteinases (TIMP), increase of fibrillar collagen crosslinking through Lysyl oxidase (LOX), and the resultant increase in tissue rigidity (Baiocchini et al., 2016; Hemmann, Graf, Roderfeld, & Roeb, 2007; Saneyasu, Akhtar, & Sakai, 2016; Vaillant, Chiaramonte, Cheever, Soloway, & Wynn, 2001). Much research has been conducted on cellular and molecular elements during the fibrotic stages of these disorders, as the liver may still be able to recuperate sufficient function through means other than transplant. There are a multitude of etiologies that induce hepatic fibrosis. Viral hepatitis B and C infection, genetic mutations, drug toxicity, autoimmune disorders, excessive alcohol consumption, and dietary imbalance resulting in non-alcoholic fatty liver disease or non-alcoholic steato-hepatitis are a few of the many insults that can lead to hepatic fibrosis (Bottcher & Pinzani, 2017).
Though fibrosis is multifactorial, of all the cellular constituents, hepatic stellate cells (HSCs) have become a primary focus when studying the induction, propagation, and ultimate resolution of the fibrotic environment (Higashi, Friedman, & Hoshida, 2017; Yin, Evason, Asahina, & Stainier, 2013). HSCs are highly complex and plastic cells with a broad range of functions from retinol storage and wound repair to development (Yin et al., 2013). In adults HSCs generally exist in two states, quiescent and activated. In the quiescent state, HSCs serve as vitamin A reservoir, but when the cells become activated, they support wound healing and tissue repair through contraction, proliferation and synthesis of large amounts of ECM molecules (Friedman, 2008). HSC activation is a profound biochemical and morphological shift where cells effectively transdifferentiate into an activated hepatic stellate cell/myofibroblast (aHSC/MF), acquiring features such as elevated alpha-smooth muscle actin (αSMA) expression. When HSCs become acutely activated their presence is beneficial and essential to the reparation and regeneration of damaged tissue. However, when chronically activated their wound repair mechanisms turn pathogenic resulting in fibrosis (Zhang, Yuan, He, Lei, & Wang, 2016).
There are several in vivo models of hepatic fibrosis such as, murine models utilizing bile duct ligation (BDL) or carbon tetrachloride (CCL4), which have been used extensively in research for decades (Cameron & Karunaratne, 1936; Cameron & Oakley, 1932). In murine models of alcohol induced fibrosis it was found that exposure to ethanol induces oxidative stress in hepatocytes, resulting in the production of reactive oxygen species (ROS) and acetaldehyde through its metabolism. Both byproducts can directly induce HSC activation (Ceni, Mello, & Galli, 2014), but not necessarily in a chronic fashion. Most variations of alcohol induced fibrosis are not beneficial as they do not necessarily cause fibrosis without an additional second hit, such as CCL4, to induce a fibrotic response (Lamas-Paz et al., 2018). Additionally, animal models do not always accurately reflect mechanisms of fibrosis. Research indicates that there is only a ~12–18% overlap of differentially regulated genes when comparing human to murine models of HSC activation (El Taghdouini et al., 2015).
In vitro modeling of fibrosis relies primarily on the isolation of in vivo activated HSCs from mouse or rat induced via CCL4 or through BDL. Researchers also rely on the use of human immortalized cell lines such as LX-2, but analysis has indicated the basal phenotype exhibited is a low or partially activated state (Weiskirchen et al., 2013). For example, LX-2 produces low levels of TIMP1 as compared to its in vivo counterparts (Xu et al., 2005), which would not be reflective of chronic late stage fibrotic tissue since TIMP1 becomes more highly elevated over time (Busk et al., 2014). Much of this work has also been done on tissue culture plastic dishes (2D). In contrast, bio-fabricated 3D tissue constructs more accurately reproduce in vivo environments (van Grunsven, 2017) and allows for the analysis of multiple cellular phenotypes, such as ECM remodeling, which is not possible in 2D. In the current study, we compared activated HSCs (aHSCs) and LX-2 cell line cultures in 3D organoids by measuring tissue stiffness, remodeling capability, and gene expression. Additionally, we tested the cellular response to Methotrexate, an anti-metabolite used to ameliorate auto-immune disorders in adults (Conway & Carey, 2017). We found that aHSCs, compared with LX-2 cells, had higher capacity to remodel the ECM, they created a more rigid environment and had higher expression levels of genes associated with fibrosis. Altogether, we have created an in vitro model of fibrosis that reflects a gradient of liver fibrosis, from a moderate to a more severe, late stage chronic liver fibrosis.
Methods:
Cell Culture:
Primary cells were isolated from 18 to 22-week-old human fetal livers procured from Advanced Bioscience Resources (Alameda, CA). Hepatic stellate cells (HSCs) were isolated using Nycodenz gradient as previously described (Mederacke, Dapito, Affo, Uchinami, & Schwabe, 2015). The primary fetal HSCs were cultured and maintained in DMEM high glucose supplemented with 10% (v/v) fetal bovine serum, 1% antibiotics at 5% CO2 at 37°C. To ensure activation cells were passaged 2–3 times on standard tissue culture plates, and phenotype was verified by staining for αSMA and Desmin. LX-2 cells were provided by Dr. Scott Friedman (Icahn School of Medicine at Mount Sinai, New York, NY), and were cultured at 5% CO2 at 37°C in DMEM high glucose supplemented with 2% (v/v) fetal bovine serum (FBS), 1 % antibiotics.
HSC Organoid Construction:
Organoids were manufactured by encapsulating aHSCs or LX-2 within ~1.0 mg/ml Collagen I (from rat tail, BD Biosciences). Essentially as shown in Figure 1. First, cells were dissociated from the culture plates using 0.05% Trypsin-EDTA (ThermoFisher Scientific) and neutralized by each cell lines’ respective culture media. Cells were counted using a standard hemocytometer. Cells were then dispensed into a 15 ml centrifuge tube and centrifuged at 1,200 rpm for 5 minutes when the supernatant was removed. A standard solution of stock 1 mg/ml Collagen I, dH2O, and NaOH was prepared beforehand and mixed with the cell pellet such that the resulting mixture had a cellular concentration of 5.0X106/ml. 100 μl of this solution was dispensed into each of the six wells of the PDMS collagen mold. The cell-collagen solution was allowed to solidify by placing the 6-well tissue culture plate containing the PDMS mold into a standard cell culture incubator (37° C) for a period of 25 minutes.
Figure 1. Fabrication of HSC organoids.
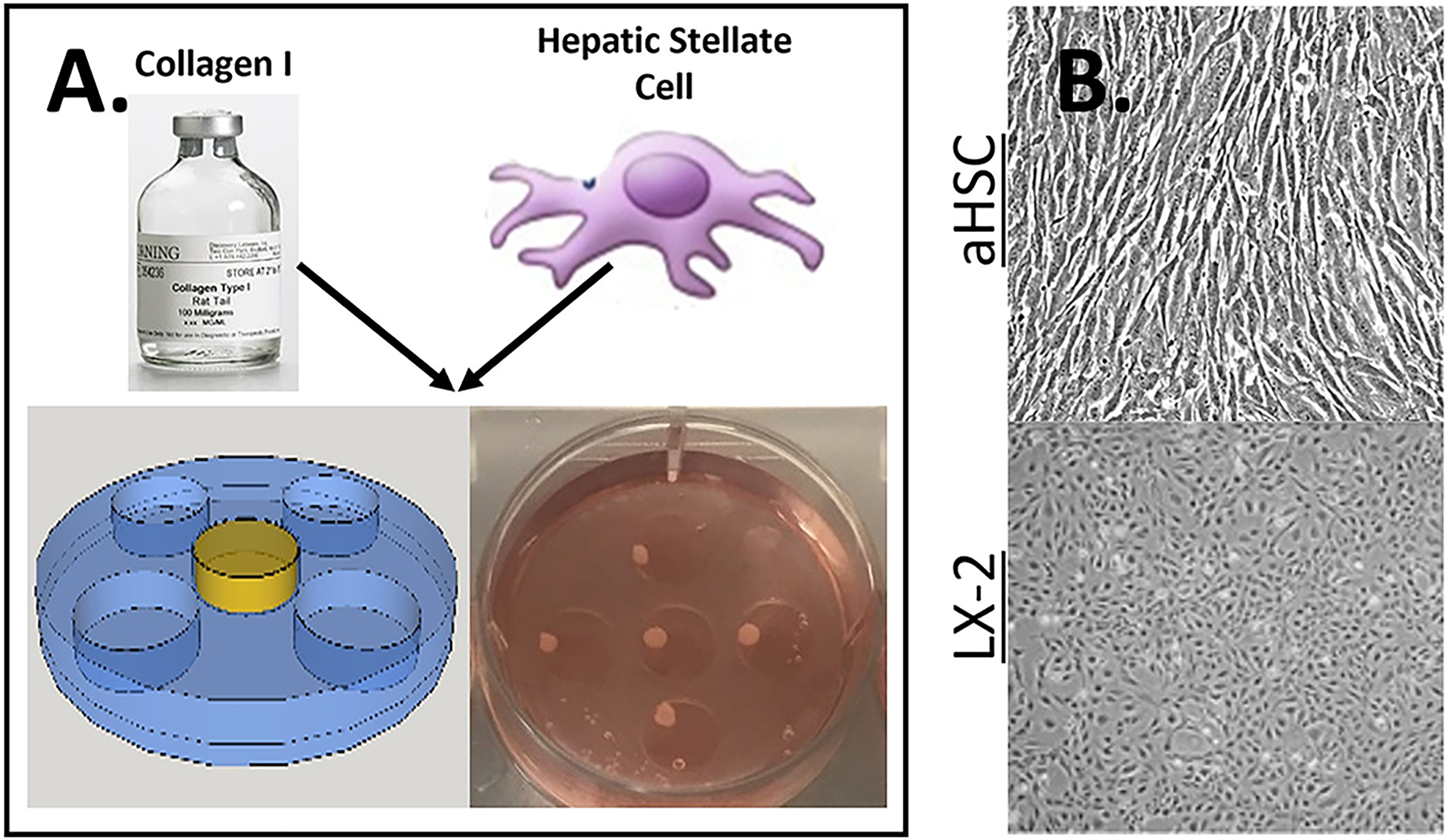
A) Schematics of organoid construction. Organoids were fabricated by mixing one of two types of HSC in collagen I and dispensing 100 μl of this solution (yellow) into a PDMS mold (blue) inside a 6 well culture dishes, as described in the Methods. After the collagen was solidify, organoids were cultured for up to 7 days (bottom right panel). B) Microscopic images of both aHSC (top) and LX-2 (bottom) in a standard 2D tissue culture plate, note the differences in morphology where aHSC is more spindle like.
2D and Organoid Experimental Culture:
For 2D conditions, cells were trypsinized, counted, and 2.5X104 aHSC or LX-2 cells were plated per well in a standard 6-well plate. Cells in 2D and organoids were cultured in Williams E Media supplemented with 1% glutamine and HepaRG Growth Media ADD710 procured from Lonza. All experiments were conducted for 7 days. Control groups were maintained in the base media described above. The experimental groups were treated in the following ways: TGF-β 1 was administered at a concentration of 10 ng/mL. The small molecule inhibitor SB431542 (ALK5i) (R&D Systems) was administered at a concentration of 94 nM and Methotrexate (Sigma Aldrich) was administered at a concentration of 1 μM. All conditions were maintained throughout the 7 days by refreshing media containing the drugs at the above stated concentrations on day 4.
Histology:
Samples were fixed in 10% neutral-buffered formalin (NBF) for a period of 1 hour then processed and paraffin embedded. Paraffin blocks were sectioned into 5 μm sections and stained with H&E using standard staining protocols.
Rheology and Elastic Modulus Calculation:
Elastic moduli of samples were determined through generation of a force-displacement curve through compression testing on a TA Instruments HR-2 Discovery rheometer equipped with a flat, 8mm, round geometry. Samples were briefly placed onto the center of the rheometer stage and excess liquid was removed. Initially, the geometry was positioned above the sample without contacting the sample surface. The geometry was set to compress the sample until terminating at a gap distance of 25 μm. Force and gap distance measurements were collected every 0.25 seconds of compression. Samples were discarded after compression. Stress values were generated by dividing force measurements by sample area (equation 1). Strain values were generated by subtracting the gap distance from the sample height and dividing the total sum by the height (equation 2). Stress and strain were then plotted against one another to yield a stress-strain curve consisting of two phases: an initial amorphous phase and a subsequent crystalline phase occurring after a curve elbow. Elastic modulus was calculated by finding the slope of the amorphous phase.
| (1) |
| (2) |
CT-Fire and CurveAlign analysis:
CT-Fire and CurveAlign are programs designed by the Laboratory for Optical and Computational Instrumentation at the University of Wisconsin, which uses quantifiable parameters of fiber alignment (Langhans, 2018). Fibers are isolated from images by identifying edges (curvelet transform [CT]) and fiber extraction (FIRE) algorithm and connecting those edges to segment total fibers. These segmented fibers are then analyzed to generate histograms of fiber parameters, such as angle, width, length, and straightness. Longer and/or thicker fibers may indicate crosslinking of the collagen chains, and a lower angle variance may indicate an increase in fiber alignment due to remodeling by the hepatic stellate cells. This software, used to analyze the collagen fibers, can be used to assess overall ECM remodeling capabilities of the HSCs. Formalin fixed paraffin embedded samples were cut and stained with Picrosirius red (PS-red), which were then imaged using polarized light microscopy with a linear polarizing filter. Images captured were exposed for the same amount of time for each group within a statistical test. Entire organoids were captured by use of motorized stage and image tiling. Resulting tiled TIFF images were imported into Adobe Photoshop. Images were converted to grayscale and a suitable black level was chosen and applied to all the other images for background normalization. Three 500×500 pixel ROIs were selected from each of the organoids resulting in a minimum of 9 ROI per group. CT-Fire batch processed each group using the default settings for thresholding. CurveAlign analysis was performed using the same method. Default settings were used save the sampling rate, which was changed from the default 0.001 to 0.06 to allow for higher levels of sampling.
RNA isolation and qPCR:
Organoids were collected and stored in RNALater (Qiagen™) at −20°C for RNA extraction. RNA was extracted using Fibrous Tissue RNA kit (Qiagen™). Steel beads (1–2) were used for each organoid when using the TissueLyser (Qiagen™) for a period of up to 5 minutes to break apart the collagen gel. The remaining steps of the RNA extraction were performed according to the manufacturer’s instructions. Equimolar concentrations of RNA were converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems by ThermoFisher Scientific™) according to the manufacturer’s instructions. qPCR was accomplished by use of PowerSYBR Green PCR Master Mix (Applied Biosystems by ThermoFisher Scientific™) in a 96- well plate using the QuantStudio 3 Real-Time PCR System™. Each condition was performed in triplicate. Cycling conditions were as follows: hot activation (50°C for 2 minutes followed by 95°C for 10 minutes), amplification (95°C for 15 seconds, 60°C for 1 minute) repeated 40 times, and quantification by SYBR Green fluorescence measurement. Automated thresholding was used to acquire CT of each sample. Primers were selected from the open source Harvard PrimerBank database. The following primer pairs were used in the study (Table 1).
Table 1. List of Primer Sequences Used for PCR Analysis of Gene Expression.
| β2M | (F) 5’-GAGGCTATCCAGCGTACTCCA-3’ and (R) 5’-CGGCAGGCATACTCATCTTTT-3’ |
| COL1A2 | (F) 5’-GGCCCTCAAGGTTTCCAAGG-3’ and (R) 5’-CACCCTGTGGTCCAACAACTC-3 |
| LOXL2 | (F) 5’-AGGACATTCGGATTCGAGCC-3’ and (R) 5’-CTTCCTCCGTGAGGCAAAC-3’ |
| MMP9 | (F) 5’-GGGACGCAGACATCGTCATC-3’ and (R) 5’-TCGTCATCGTCGAAATGGGC-3’ |
| TIMP1 | (F) 5’-ACCACCTTATACCAGCGTTATGA-3’ and (R) 5’-GGTGTAGACGAACCGGATGTC-3’ |
| TGFβR1 | (F) 5’-CACAGAGTGGGAACAAAAAGGT-3’ and (R) 5’-CCAATGGAACATCGTCGAGCA-3’ |
| TGFβR2 | (F) 5’-GTAGCTCTGATGAGTGCAATGAC-3’ and (R) 5’-CAGATATGGCAACTCCCAGTG-3’ |
| TGFβ1 | (F) 5’-CAATTCCTGGCGATACCTCAG-3’ and (R) 5’-GCACAACTCCGGTGACATCAA-3’ |
Beta-2-microglobulin (β2M), Collagen I alpha 2 (COL1α2),Lysyl-oxidase like 2 (LOXL2), Matrix metalloproteinase 9 (MMP9), Tissue inhibitor of metalloproteinase 1 (TIMP1),Transforming growth factor beta receptor 1 (TGFβR1),Transforming growth factor beta receptor 2 (TGFβR2), Transforming growth factor beta 1 (TGFβ1)
Statistics:
qPCR results were calculated by using the Livak method. Samples were normalized to their respective β2M value then normalized to either the control, the 2D counterpart or to LX-2. The resultant -ΔΔCt was transformed to fold change by the formula 2^-ΔΔCt, then the log base 2 was taken. Absolute log values greater than 0.59 represent a fold change greater than 1.5. Statistical analysis was performed through MATlab, Graphpad and Excel. One-way ANOVA and Students t-test with Holms-Sidak correction for multiple comparisons were used to analyze -ΔCt values. Statistics were evaluated and considered significant using a p-value of <0.05 for statistical significance.
Results:
Fibrotic organoid analysis- Histology and stiffness.
To better understand the differences of HSC driven ECM remodeling capabilities we cultured in vitro activated HSCs (aHSCs) within a collagen gel substrate and compared to that of an HSC cell line, LX-2. H&E staining (Figure 2A.) was used for gross observation of the organoids and to analyze cell distribution (hematoxylin, purple-cell nuclei) and ECM (eosin, pink color) in the organoids. The apparent difference between the aHSC and LX-2 organoids, cultured in standard media (control), is the spindle like morphology of the aHSCs when compared to that of the LX-2 organoids. Exposure of organoids to TGF-β resulted in increased ECM staining in the aHSC organoids compared with LX-2 organoids, while exposure to Alk5i, an inhibitor of TGF-β pathway, reduced ECM staining in the aHSC organoids and almost completely eliminated ECM staining in LX-2 organoids. Methotrexate (MTX), used for treatment of rheumatic and non-rheumatic diseases, could induce serious fibrotic side effects in the liver and lungs (Conway & Carey, 2017). Exposure of organoids to MTX had a similar effect as observed with TGF-β, resulting in increased ECM staining in aHSC organoids, and to a lesser extent in LX-2 organoids.
Figure 2. Organoid Histological and ECM analysis.
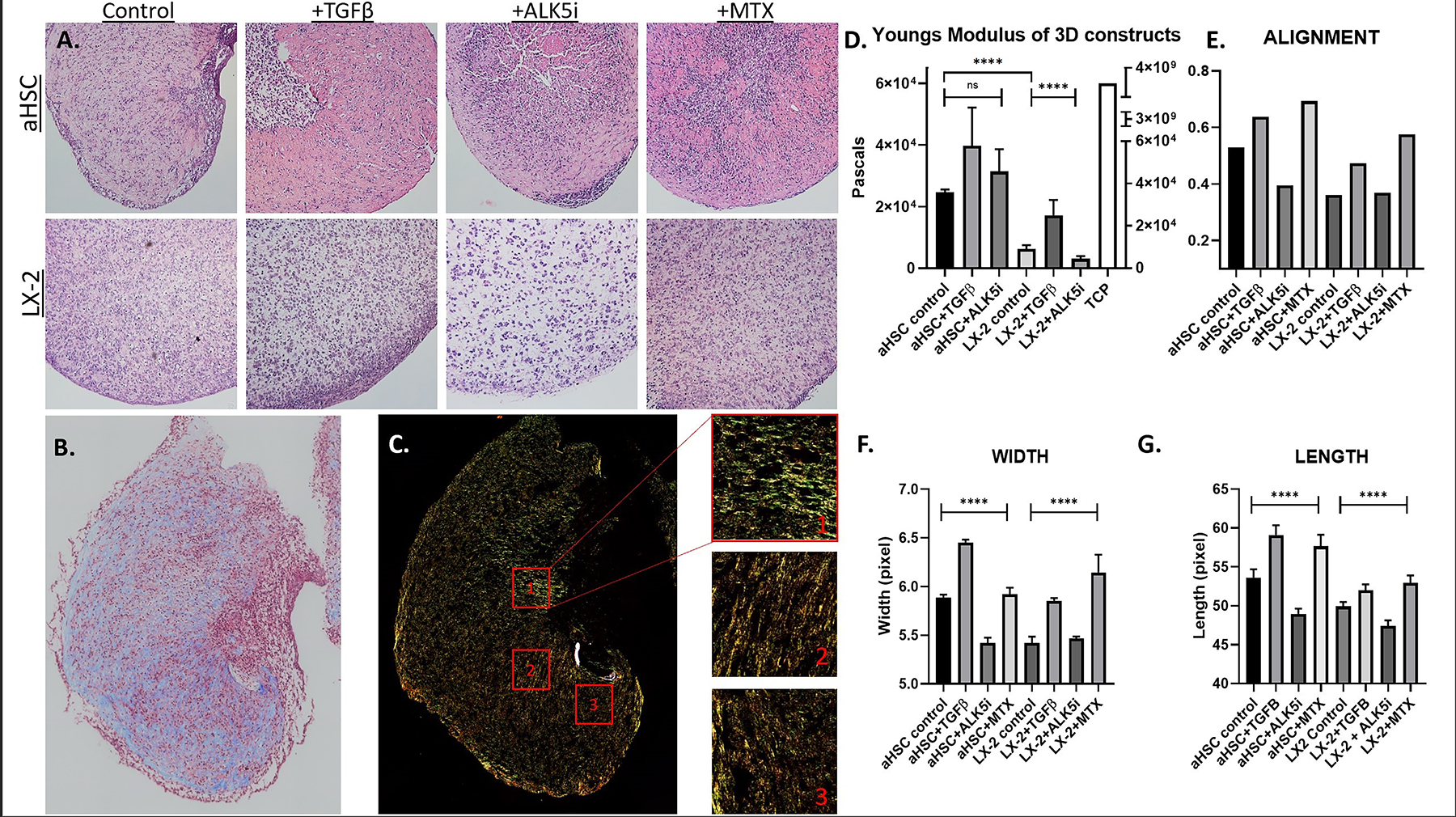
A) H&E staining of aHSC and LX-2 organoids cultured under control and experimental conditions, as described in the Methods. B) Masson’s Trichrome staining of aHSC organoids cultured under control conditions, denoting collagen in blue and cells in red. C) aHSC organoids cultured under control conditions were stained with Picrosirius red and imaged in polarized light. Left panels show 3 representative ROIs that were selected and analyzed using CT-FIRE and Curvealign. D) Stiffness of organoids cultured under control and experimental conditions, as indicated. E) Overall alignment of collagen fibers within organoid, cultured under control and experimental conditions, as indicated. Values closer to 1 represent complete alignment. F, G) Calculated width and length of collagen fibers within organoid, cultured under control and experimental conditions, as indicated. Statistical analysis using ANOVA shows significant impact of the different experimental conditions.
As fibrosis worsens, tissue rigidity increases (Wells, 2013). This stiffness can affect the state of healthy cells in surrounding tissue, can induce developmental changes, and/or may induce trans-differentiation from one cell type to another. Given that contractility and ECM modification are standard tenants of fibrosis, we tested if the organoids’ ECM configuration could be altered through TGF-β pathway manipulation. Rheometric analysis was used to test the rigidity of the organoids through the generation of a stress strain curve. Rheometry confirmed that aHSCs created an approximately 3-fold more rigid environment at 24.5 kPa as compared to LX-2 at 7.5 kPa, at a statistical significance of p-value<0.0001 (Figure 2D). Exposure of organoids to TGF-β increased the rigidity of the substrate in both aHSC and LX-2 organoids to 40.8 kPa and 18.5 kPa, respectively. On the other hand, exposure of LX-2 organoids to ALK5i resulted in a statistically significant decrease in rigidity, to 2.8 kPa (p-value=0.0063). While there was no statistical significance within aHSC organoids treated with TGF-β and ALK5i, similar rigidity trends were noted. Collectively, LX-2 organoids showed more significant response to substrate rigidity manipulation with TGF-β and ALK5i compared with a more variable response in aHSC organoids.
Collagen fiber analysis in fibrotic organoids.
Fibrotic tissue is characterized by a dense network of fibrillar collagen in the ECM. To obtain a preliminary gross picture of the collagenous component in the organoids, sections were stained with Masson’s trichrome (Figure 2B). Control aHSC organoids revealed a high collagen:cell ratio (blue: red stains), confirming the observations made based on the H&E images. A focused analysis and quantification of collagen fiber organization was achieved through Picrosirius red (PS-red) staining and polarized light microscopy (Figure 2C). The collagen fibers, revealed by the PS-red staining, informs us of the length, width, and direction of the fibers themselves. Here we can see there are highly organized areas within the organoid (Figure 2C) and areas around the stellate cells that show particularly high levels of alignment. The color variation of the fibers may give some indication as to the maturity (thicker, more highly cross-linked and contracted) of the fibrillar collagen. Green fibers tend to be finer, less mature fibers whereas yellow, orange, and red fibers tend to indicate more mature fibers. aHSC organoids tended to have more mature fibers as compared to LX-2 organoids (data not shown). The PS-red stained images were further analyzed with CT-FIRE and Curvealign software for length, width, and direction of collagen fibers. The entire organoid was imaged and 3 ROIs representative of the ECM within the organoid were selected for analysis (Figure 2C). Quantified with Curvealign, the X-axis represents the overall alignment, and is denoted by a circular statistic based on fiber vectors (Figure 2E). Zero is considered completely diffuse with no alignment and 1 indicates that all fibers are perfectly aligned in the same direction. We expected that a fibrotic microenvironment would contain larger fiber bundles equating to increased fiber length and width with higher levels of alignment. The results show that aHSC organoids tend to have greater overall alignment than LX-2 organoids and exposure to TGF-β and MTX increased overall alignment, while Alk5i reduced alignment. A similar pattern is seen in terms of fiber length and width. Specifically, when exposed to TGF-β, aHSC organoids demonstrated collagen fibers whose length and width (Figures 2F and 2G) were increased to a statistically significant degree (p <0.01 and p<0.0001, respectively). Conversely, ALK5i decreased the length and width of collagen fibers (p<0.01 and p<0.0001 respectively). MTX treatment increased the width of collagen fibers but not the length.
Further experiments with the anti-fibrotic agent β-aminopropionitrile (BAPN) were conducted on aHSC organoids in order to determine the role of ECM remodeling of the physical properties of the organoids BAPN blocks the crosslinking of collagen fibers through the inhibition of LOXL2 which should reduce ECM remodeling. Addition of BAPN in the culture media increased organoids’ size by reducing contraction from approximately 1.3 mm to approximately 2.7 mm (Supplemental Figure 1A). Picrosirius red staining indicates the levels of collagen fiber bundling; green colors being the least bundled and red colors being the most bundled. Using picrosirius red we observed that collagen fibers were more sparsely distributed in the presence of BAPN and appear more disorganized (Supplemental Figure 1B). Whole organoids, stained with Picrosirius red, were analyzed using a MATlab script that segments the pixel colors into green, yellow, orange, or red groups. Hue analysis of the organoids showed that the distribution of pixel colors shifted away from red and more towards yellow in the presence of BAPN, indicating less bundling of collagen fibers (Supplemental Figure 1C). CT-FIRE analysis of fiber width and length showed higher overall fiber width at approximately 5.6 pixels on average in the presence of BAPN compared to 5.9 pixels in control (Supplemental Figure 1D). Although, the fibers did not appear statistically shorter in the presence of BAPN compared to control, the fibers were trending in that fashion. Altogether, these results indicate that collagen remodeling via LOXL2 plays a role in regulating the physical properties of the ECM in aHSC organoids.
LX-2 organoids demonstrated similar results except that ALK5i did not reduce the fiber width or length to a significant degree. A comparison between aHSC and LX-2 organoids revealed statistically significant differences in the width and length of collagen fibers (Supplemental table 1). Specifically, aHSC vs. LX-2 organoids produced fibers with a length of 53.63 pixels vs 49.92 pixels and a width of 5.88 pixels vs 5.421 pixels, respectively. aHSC and LX-2 organoids exposed to TGF-β had fiber lengths of 59.08 and 51.98 pixels and widths of 6.453 pixels and 5.854 pixels, respectively. There was no significant difference between aHSC and LX-2 organoids exposed to ALK5i. Lastly, exposure to MTX resulted in a significant difference between aHSC and LX-2 organoids only in the fiber length. Collectively, these results indicate that aHSCs remodel the collagen gel more dramatically in comparison to LX-2 organoids, as indicated by the greater aligned fiber network with larger fibers in both measures. These findings suggest there is an inherent difference in ECM remodeling capabilities between these two cell types.
Molecular pathway analysis of fibrosis associated genes:
The substrate in which cells are cultured causes alterations in a multitude of signaling pathways. For example, LX-2 cells have been shown to become less fibrotic and have a reduced activation state on soft substrates (Wells, 2008). Comparing 2D and 3D culture conditions shows us the substrate’s effect on HSCs activation level. To our knowledge, a comparative analysis of the effect of collagen gel encapsulation on activated HSCs of different origins has not been done previously. The genes listed in Table 1 were chosen because they demonstrate activation level and fibrotic state severity (Zhang et al., 2016). COL1A2, LOXL2, and TIMP1 were analyzed for ECM modification activity, which increases with increased activation state. TGF-β1 also tends increase in more severe forms of fibrosis. Expression of TGF-β1 acts in both autocrine and paracrine manner - autocrine when maintaining an activated state and paracrine when affecting parenchymal cells in close proximity to aHSCs. Organoids were cultured for 7 days and RNA was extracted for qPCR analysis. The relative expression levels were calculated via the Livak method (Livak & Schmittgen, 2001) using β2-microglobulin expression for normalization. Relative gene expression was also normalized to the controls, which consisted of either untreated cells grown in 2D or 3D (organoids) substrates. ΔΔCT values were transformed for fold change and the log base 2 was taken. Statistical significance (Table 2.) occurs at p<0.05 and fold change of 1.5 is achieved when LOG2=0.59. Figure 3 describes the comparison between 2D and 3D culture systems for each cell type (LX-2 and aHSC), as well as comparison between LX-2 and aHSC for each culture system (2D and 3D).
Figure 3. Comparison of Fibrotic Gene Expression Between 2D and 3D Culture Conditions for aHSC and LX-2 cells.
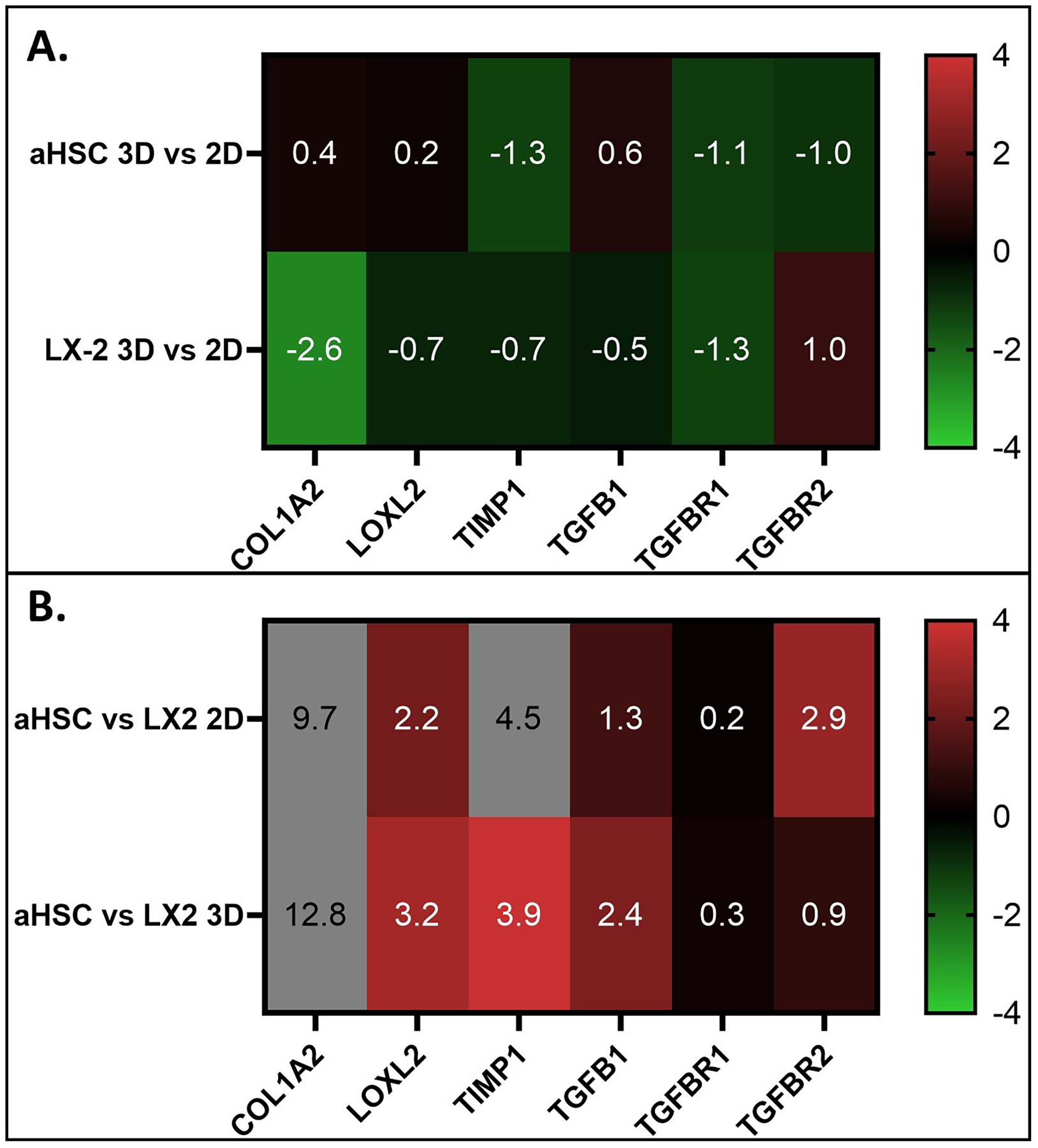
A) Comparison of the effects of culture conditions. Note the change in fibrotic genes expression between 3D and 2D that is generally higher in LX-2 cells as compared to aHSC. B) Comparison of fibrotic genes expression between aHSC and LX-2 cells. Note that aHSC has mostly higher levels of fibrotic gene expression in both 2D and in 3D culture conditions as compared to LX-2 cells.
aHSCs in 2D versus 3D culture systems showed statistically significant down regulation of TIMP1, TGF-βR1, and TGF-βR2 expression and relatively similar levels of LOXL2, COL1A2 and TGF-β1 expression (Figure 3A). LX-2 cells in 2D versus 3D showed a larger, statistically significant downregulation of COL1A2 and TGF-βR1 expression, with a LOG2 fold change of −2.6 and 1.3, respectively, and to a lesser extent downregulation of LOXL2, TIMP1 and TGF-β1. Greater differences were found in the comparisons between the aHSC and LX-2 cells for each culture system, except for the expression levels of TGF-βR1 that remained relatively equal in aHSC and LX-2 in both culture systems. (Figure 3B). All genes analyzed had higher levels of relative expression in aHSC as compared to LX-2 in both 2D and 3D. Collagen expression was much higher in aHSCs, with relative expression of LOG2=9.7 and 12.8 in 2D and 3D, respectively. LOXL2 and TGF-β1 was highly upregulated in aHSC vs LX-2 cells when both cell types were cultured in 3D, with relative expression of LOG2=3.2 (p<0.01) and 2.4 (p<0.00001), respectively (Figure 3B). Conversely, when aHSCs in a 3D culture system was incubated with BAPN, LOX2 inhibitor, we observed reduced the expression of αSMA, COL1A2 and TGF-β (Supplemental Figure 2), which may be the result of the relaxation of mechanical properties of the organoid.
Expression of pathways associated with fibrosis during induction and inhibition of TGF-β pathways:
LX-2 cells were previously shown to vary their fibrotic phenotype when exposed to fibrosis-inducing and inhibiting factors (Bollong et al., 2017; Xu et al., 2005). Our results, shown in Figure 2, confirm these observations and show that exposure of LX-2 organoids to exogenous TGF-β and MTX resulted in induction of a fibrotic phenotype including stiffness and collagen organization. LX-2 cells in both 2D and 3D culture systems showed an overall increase in the expression of fibrosis-related genes examined here. Specifically, exposure to TGF-β1 significantly increased the expression of COL1A2, TIMP1 and TGF-β1 in 2D cultures and of COL1A2 and LOXL2 in 3D organoids (Figure 4AB). Exposure to MTX had a more moderate increase in the expression of these genes (Figure 4AB). In contrast, exposure to TGF-β inhibitor, Alk5i, resulted in significant downregulation of these genes in 2D cultures and to a lesser extent in 3D organoids. Interestingly, LX-2 expression of TGF-βR1 was downregulated in response to both TGF-β1 and MTX treatment in 2D cultures, but not in 3D organoids. It is possible a negative feedback loop with regards to TGR-βR1 expression exists, which may be suppressed when cells are cultured on a less rigid substrate.
Figure 4. The Effects of Fibrosis Inducers and Inhibitors on Fibrotic Gene Expression.
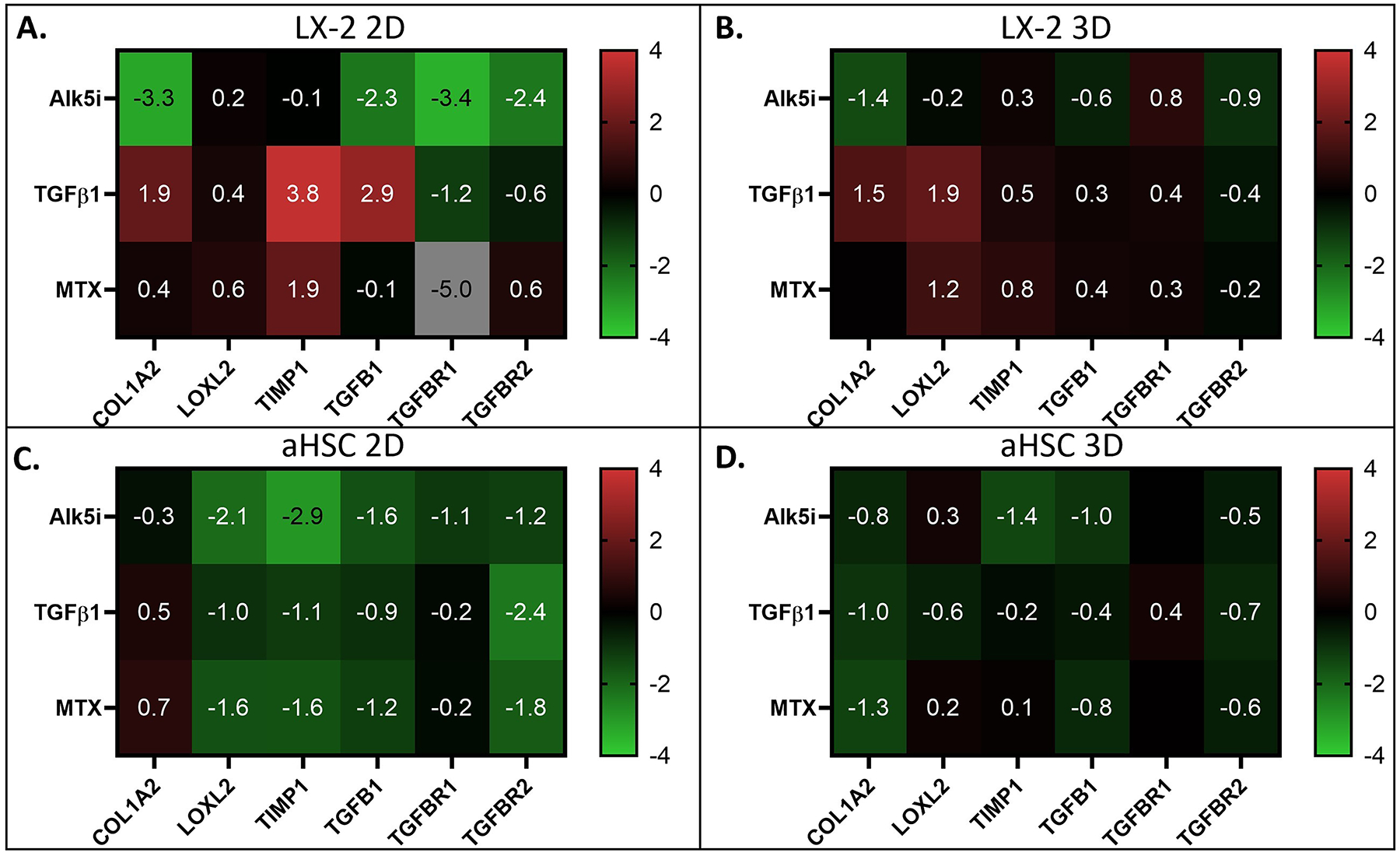
A, B) The effects of fibrosis inducers and inhibitors on fibrotic gene expression in LX-2 cells under 2D and 3D culture conditions, as indicated. Note the large increase in gene expression in response to TGF-β1 and large decrease in response to Alk5i in 2D (A), and moderate changes in 3D (B). C, D) The effects of fibrosis inducers and inhibitors on fibrotic gene expression in aHSCs under 2D and 3D culture conditions, as indicated. Note the decrease in gene expression in response to both TGF-β1 and the fibrosis inhibitors in both 2D and 3D culture conditions.
Conversely, aHSCs had an overall reduced expression of the fibrosis-related genes examined here, in response to TGF-β1 and MTX (Figure 4C, D). As expected, we also observed reduced expression of these genes in response to and Alk5i. Notably, aHSCs in 2D culture showed more reduced expression of these genes compared with 3D organoids (Figure 4C, D). This generalized downregulation could be an indication of pathway saturation or part of the complex nature of the TGF-β pathway, as it can act as either an inducer or inhibitor based on overall concentration (Wu, Bitzer, Ju, Mundel, & Bottinger, 2005).
We next compared the response of each cell type in 3D organoids to fibrosis inducing (TGF-β and MTX) and inhibiting (Alk5i) factors, relative to cells in 2D cultures (Figure 5). To do that, the qPCR results for cells in 3D organoids were normalized for cells in 2D cultures under similar experimental conditions. For LX-2 cells, exposure to Alk5i resulted in much higher relative expression of LOXL2, TGF-β1, TGF-βR1, and TGF-βR2 with LOG2=3.9, 1.1, 3.0, and 2.3 higher fold expression, respectively, in 3D organoids (Figure 5A). The reverse was generally true when exogenous TGF-β1 was applied, suggesting that that LX-2 cells in 3D cultures can better sense activation and inhibition of the TGF-β pathways compared with cells in 2D cultures. The response of LX-2 cells to MTX in 3D organoids, relative to 2D cultures, was variable for the expression of genes analyzed here, with increased induction of TGF-βR1 expression and decreased expression of COL1A2 and TIMP1 in 3D organoids.
Figure 5. The Effects of Culture Conditions and Fibrosis Inducers and Inhibitors on Fibrotic Gene Expression.
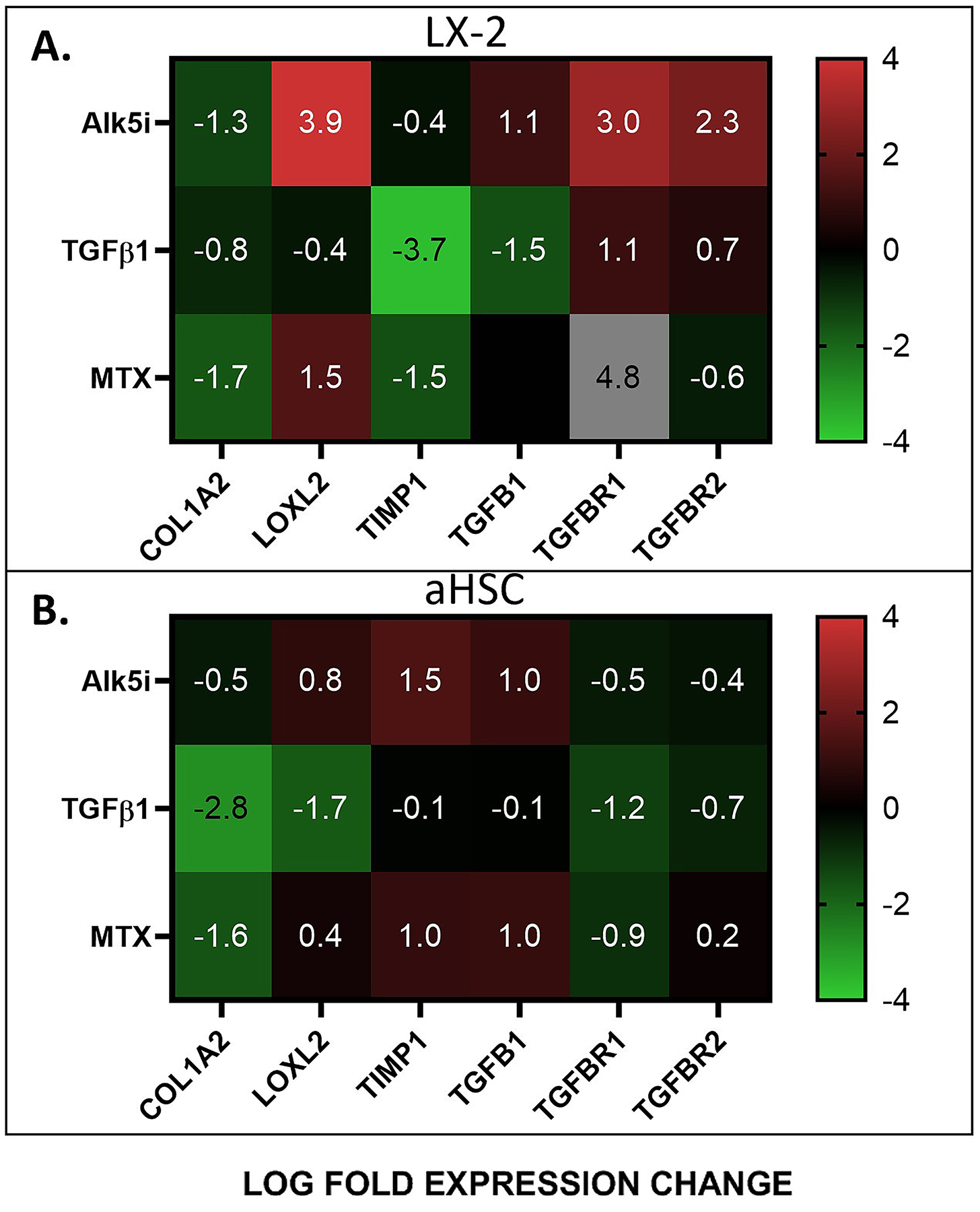
Fibrotic gene expression in LX-2 cells A) and aHSCs B) in 3D organoids, in response to fibrosis inducing (TGF-β) and inhibiting (Alk5i and MTX) factors, relative to cells in 2D cultures.
For aHSC cells, exposure to ALK5i and MTX resulted in moderate upregulation of LOXL2, TIMP1 and TGF-β1 expression and moderate downregulation of COL1A2, TGF-βR1 and TGF-βR2 in 3D organoids, relative to 2D cultures (Figure 5B). On the other hand, exposure to TGF-β resulted in decreased expression of all genes analyzed here, most significantly of COL1A2, in 3D organoids, relative to 2D cultures (Figure 5B). Together, these results further support the observations that aHSCs are less responsive to TGF-β pathway inhibition than LX-2 cells.
Finally, we compared the response of aHSCs to fibrosis inducing (TGF-β and MTX) and inhibiting (Alk5i) factors, in 2D and 3D culture conditions, relative to the response of LX-2 cells (Figure 6). Generally, we observed that in both 2D and 3D culture systems, exposure of aHSC cells to ALK5i and MTX showed higher expression of most genes analyzed here, relative to LX-2 cells. This was especially pronounced for the expression of COL1A2. The response of aHSC to TGF-β1 was similar when compared with LX-2 cells except for a robust increase in COL1A2, in 2D and 3D organoids in TIMP1 in 3D organoids. These results suggest that the differences between aHSC and LX-2 cells are more influential than the differences in the culture systems (2D vs 3D) in determining the response to fibrosis inducing and inhibiting factors.
Figure 6. The Differential Response of aHSCs and LX-2 cells to Culture Conditions and Fibrosis Inducers and Inhibitors.
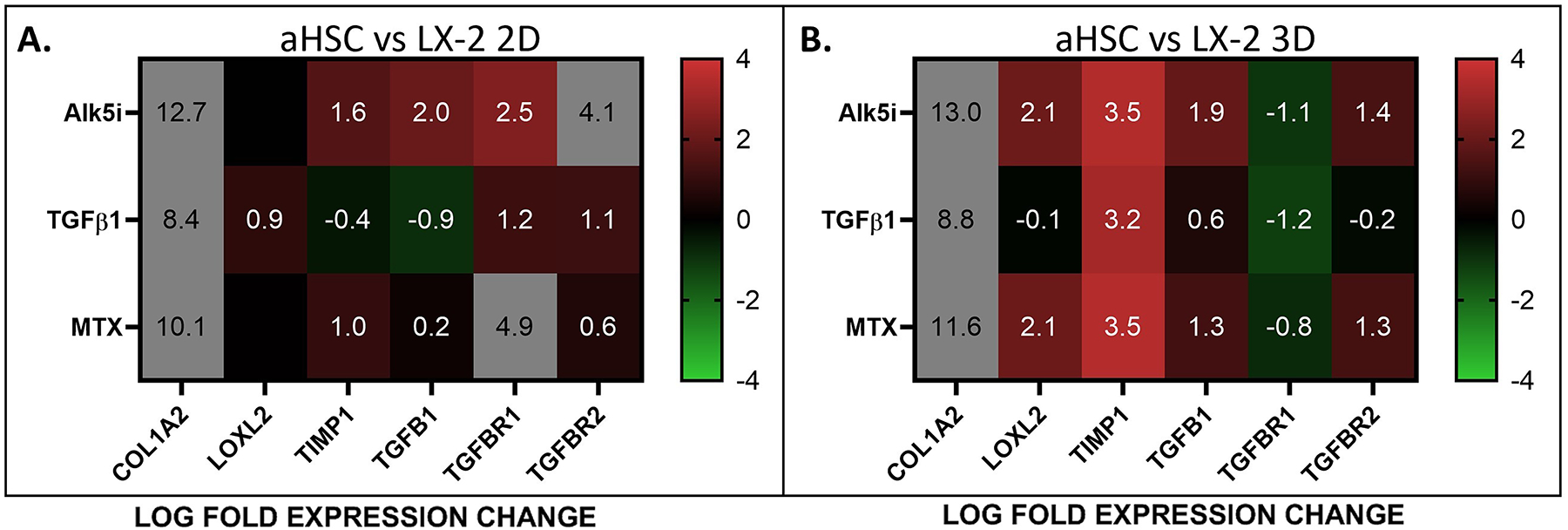
Fibrotic gene expression in aHSCs in 2D A) and B) 3D culture conditions, in response to fibrosis inducing (TGF-β) and inhibiting (Alk5i and MTX) factors, relative to LX-2 cells.
Discussion:
As the principal cellular agonist of fibrosis, hepatic stellate cells are of great interest. Immortalized HSC lines such as, LX-2, GREF-X, hTERT-HSC, and HSC-180, are mostly myofibroblast-like in morphology and are predominantly used in in vitro studies (Herrmann, Gressner, & Weiskirchen, 2007). Culture-activated hepatic stellate cells are also used to study liver fibrosis however, to our knowledge, comparative analysis of between cell lines and culture activated HSCs regarding their ECM remodeling capabilities have not been thoroughly conducted. We endeavor to compare these two major types of cellular models for their ability to create a fibrotic environment analogous to stages of liver fibrosis found in several types of chronic liver disease.
Standard tissue culture plastic (2D) models are still valuable, however, in the pursuit of more physiological relevance, 3D cell cultures have flourished. 3D models generally combine a hepatic stellate cell line with hydrogels of varying compositions and chemical manipulations to control environmental mechanics (Haycock, 2011; Langhans, 2018). The 3D models have allowed for more direct observation of the HSC’s fibrotic phenotype demonstrated by their ECM remodeling capabilities. The physio-mechanical properties of the ECM, such as stiffness and architecture, can have profound effects on cellular responses to paracrine and juxtacrine signaling (Wells, 2008).
Substrate rigidity can have immense impact on cellular function, proliferation, and differentiation (Wells, 2008). As fibrosis increases in severity, overall stiffness increases. For adult patients there are several non- invasive clinical platforms that can roughly estimate global stiffness of the liver tissue through the use of elastography. Fibroscan considers tissue healthy and normal at values <7 kPa and cirrhotic tissue at >14.5 kPA, though it can go as high as 75 kPa (Foucher et al., 2006). This is noteworthy, aHSCs used here generated a higher degree of stiffness than LX-2, at ~24 kPa vs ~7.5kPa respectively. Using these thresholds, the contraction and ECM modification from the LX-2 cells generated a relatively normal stiffness, whereas aHSCs would be considered cirrhotic. These basal stiffness differences could be due to a number of factors. First, the aHSCs have higher expression of LOXL2 which mediates the crosslinking of fibrillar collagen. Previous research shows that there is a relation of LOXL2 mediated crosslinking and tissue stiffness (Levental et al., 2009; Pfeiffer, Franklin, Hsieh, Bank, & Phillips, 2005) which can be remediated by the application of BAPN, a LOXL2 inhibitor (Supplemental Figure 1). Second, in preliminary studies aHSCs were shown to have higher contractility as compared to LX-2 cells in a gel contraction assay (data not shown).
Several studies have shown that the traction force generated by contractile cells can align collagen fibers (Abhilash, Baker, Trappmann, Chen, & Shenoy, 2014; Baiocchini et al., 2016). Our quantitative analysis of collagen fibers confirmed that aHSCs were able to modify the collagen substrate to a statistically higher degree than LX-2 in terms of length, width, and overall alignment of fibers. These numbers also correspond to stiffness levels though the relationship was not quantified. Other studies showed that higher alignment can reduce global stiffness (Taufalele, VanderBurgh, Munoz, Zanotelli, & Reinhart-King, 2019) however, these are complex environments and localized stiffness may be variable.
Exposure to ethanol induces oxidative stress in hepatocytes. Ethanol leads to the production of reactive oxygen species (ROS) and acetaldehyde through its metabolism. Both byproducts can directly induce HSC activation, and ROS was shown to directly induce higher COL1A2 expression in HSCs (Nieto et al., 2000; Svegliati-Baroni et al., 2001). This increased level of COL1A2 expression was found to be highly prevalent in cases of alcohol induced fibrosis. As shown in our study, aHSC have significantly higher expression of COL1A2 than that of LX-2. This may be more indicative of an alcohol-induced fibrotic state, which may underscore the utility of aHSCs when studying subsets of CLD like alcoholic liver disease (ALD).
High levels of TIMP1 expression is a major factor in the progression of fibrosis and has been correlated with increasing fibrotic scores in multiple studies. Using the METAVIR scoring system researchers have found a strong correlation with increasing TIMP1 expression at each stage of fibrosis, with an F4 stage fibrosis score having the highest TIMP1 expression level. (Hemmann et al., 2007; Kim et al., 2019; Zhang et al., 2016). This inhibition of MMP activity leads to the pathologic accumulation of collagen, which has been observed in patients with ALD and in animal models. The comparison of TIMP1 expression between LX-2 and aHSCs have found a log2-fold=3.9 higher levels of expression in aHSCs. This further substantiates the differences in the fibrotic severity achieved in culturing various cell lines in 3D culture. LX-2 cells demonstrating a lower fibrotic severity, maybe closer in relation to an a mild/moderate fibrosis score where as aHSCs more closely represent more severe fibrosis score. Similarly, TGF-β expression is also correlated with the stages of fibrosis progression (Abdelghany, BaSalamah, Idris, Ahmad, & Refaat, 2016; Neuman et al., 2012) and as inhibition of TGF-β pathways have been of keen interest in the amelioration of fibrosis a comparison is warranted (Meng, 2016). High levels of TGF-β expression leads to constant hepatic stellate cell stimulation and causes the cells to remain in an activated state. The aHSCs had a much higher TGF-β expression level compared to the LX-2 cells, which again may indicate the cell’s abilities to create a more severe fibrotic environment. However, when exogenous TGF-β was added it had the opposite effect on the aHSC compared with LX-2. This result may be due to oversaturation of the TGF-β pathway in the aHSC since it was included in the culture for long periods of time (7 days) at high doses. This hypothesis could be supported in that fibrotic livers have peak TGF-β expression at the latest stages of fibrosis as noted above. It is unclear from the literature if hepatic stellate cells within a late stage fibrotic liver should be responsive in vivo.
When comparing aHSCs directly to LX-2 we found that all fibrosis-associated gene studies here, except for TGF-βR1, had substantially higher expression in the aHSCs, and that aHSCs lacked response to TGF-β1, at least at the gene expression level. Activation of hepatic stellate cells in vivo is achieved by a multitude of factors, many of which coalesce through SMAD phosphorylation. LX-2 has been found to be responsive to TGF-β in vitro, responding with increased collagen production and proinflammatory cytokines (Bollong et al., 2017; Xu et al., 2005). In 2D and 3D cell culture, LX-2 responded in the expected manner with TGF-β inducing upregulation of most fibrosis related genes and ALK5i lowering the expression. Methotrexate has also been found to induce fibrosis in vivo in adults and induce a profibrotic response in vitro. Methotrexate induced a similar response to the TGF-β pathway induction with fibrotic gene upregulation. Conversely, aHSCs responded with a generalized downregulation of all pathways, however ALK5i still reduced the expression of all genes to a greater extent.
Summary and Conclusions:
Fibrosis is a complex process and modeling it in vitro is challenging. Many models studying liver diseases associated with fibrosis do not consider the physiological changes brought about by fibrosis. By creating a simple, yet robust model system comprised of readily available hepatic stellate cells and a substrate capable of being actively remodeled, we have overcome the shortcomings of animal models which lack fibrosis generation, and of in vitro models, which often utilize non-human cells. By comparing two lines of HSCs, we found that organoids containing aHSCs a stiffer environment, which is more physiologically relevant late stage fibrosis than those containing LX-2. aHSCs were also capable of remodeling the collagen substrate to a much higher degree than LX-2. aHSCs had higher relative levels of expression of fibrosis-related genes including COL1A2, TIMP1, LOXL2 and TGF-β. Conversely, application of exogenous TGF-β1 significantly increased the fibrotic phenotype in organoids containing LX-2 cells, compared with marginal effects in organoids containing aHSC cells. As expected, inhibition via the small molecule ALK5i reduced the fibrotic phenotype in both cell types. Collectively, these results suggest that this model creates a gradient of fibrotic phenotypes ranging from less severe (LX-2) to a more severe (aHSC) form of fibrosis. As up one third of individuals in the US have some form of liver disease, modeling liver diseases such as NAFLD or NASH should include stiffness, a critical aspect of fibrosis and tissue mechanics. Incorporating this model into more complex multi-cellular in vitro models of liver disease cells will pave the way for faster and more physiologically relevant pre-clinical modeling translating into improved therapeutics for disease amelioration.
Supplementary Material
Supplemental Figure 1. The effect of LOXL2 inhibition on collagen fiber organization of aHSC organoids BAPN treated organoids were larger than the control organoids A) and had differences in the distribution of fibers B). These fibers also had a different distribution of fiber hues, where BAPN treated groups shifted more towards the green, less bundled type fibers C). CT-FIRE analysis also corresponds with the bundling data in that BAPN treated groups had smaller fiber widths D).
Supplemental Figure 2. The effect of LOX2 inhibition of aHSC phenotype in 3D organoid Gene expression of aHSC organoids in the presence of BAPN were normalized to control aHSC organoids. The results indicate that inhibition of Collagen I cross linking with BAPN downregulated αSMA, COL1A2 and TGF-β expression and upregulated the expression of GFAP, suggesting a less fibrotic phenotype.
Funding information:
National Cancer Institute, Grant/Award numbers: R01CA180149 (S.S.), R33CA202822 (S.S.)
References:
- Abdelghany AH, BaSalamah MA, Idris S, Ahmad J, & Refaat B (2016). The fibrolytic potentials of vitamin D and thymoquinone remedial therapies: insights from liver fibrosis established by CCl4 in rats. J Transl Med, 14(1), 281. doi: 10.1186/s12967-016-1040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhilash AS, Baker BM, Trappmann B, Chen CS, & Shenoy VB (2014). Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. Biophys J, 107(8), 1829–1840. doi: 10.1016/j.bpj.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiocchini A, Montaldo C, Conigliaro A, Grimaldi A, Correani V, Mura F, Ciccosanti F, Rotiroti N, Brenna A, Montalbano M, D’Offizi G, Capobianchi MR, Alessandro R, Piacentini M, Schinina ME, Maras B, Del Nonno F, Tripodi M, & Mancone C (2016). Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS One, 11(3), e0151736. doi: 10.1371/journal.pone.0151736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollong MJ, Yang B, Vergani N, Beyer BA, Chin EN, Zambaldo C, Wang D, Chatterjee AK, Lairson LL, & Schultz PG (2017). Small molecule-mediated inhibition of myofibroblast transdifferentiation for the treatment of fibrosis. Proc Natl Acad Sci U S A, 114(18), 4679–4684. doi: 10.1073/pnas.1702750114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher K, & Pinzani M (2017). Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv Drug Deliv Rev, 121, 3–8. doi: 10.1016/j.addr.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Busk TM, Bendtsen F, Nielsen HJ, Jensen V, Brunner N, & Moller S (2014). TIMP-1 in patients with cirrhosis: relation to liver dysfunction, portal hypertension, and hemodynamic changes. Scand J Gastroenterol, 49(9), 1103–1110. doi: 10.3109/00365521.2014.934910 [DOI] [PubMed] [Google Scholar]
- Cameron GR, & Karunaratne WAE (1936). Carbon tetrachloride cirrhosis in relation to liver regeneration. 42(1), 1–21. doi: 10.1002/path.1700420104 [DOI] [Google Scholar]
- Cameron GR, & Oakley CL (1932). Ligation of the common bile duct. 35(5), 769–798. doi: 10.1002/path.1700350512 [DOI] [Google Scholar]
- Ceni E, Mello T, & Galli A (2014). Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol, 20(47), 17756–17772. doi: 10.3748/wjg.v20.i47.17756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway R, & Carey JJ (2017). Risk of liver disease in methotrexate treated patients. World J Hepatol, 9(26), 1092–1100. doi: 10.4254/wjh.v9.i26.1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Taghdouini A, Sorensen AL, Reiner AH, Coll M, Verhulst S, Mannaerts I, Oie CI, Smedsrod B, Najimi M, Sokal E, Luttun A, Sancho-Bru P, Collas P, & van Grunsven LA (2015). Genome-wide analysis of DNA methylation and gene expression patterns in purified, uncultured human liver cells and activated hepatic stellate cells. Oncotarget, 6(29), 26729–26745. doi: 10.18632/oncotarget.4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, Bertet J, Couzigou P, & de Ledinghen V (2006). Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut, 55(3), 403–408. doi: 10.1136/gut.2005.069153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL (2008). Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev, 88(1), 125–172. doi: 10.1152/physrev.00013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW (2011). 3D cell culture: a review of current approaches and techniques. Methods Mol Biol, 695, 1–15. doi: 10.1007/978-1-60761-984-0_1 [DOI] [PubMed] [Google Scholar]
- Hemmann S, Graf J, Roderfeld M, & Roeb E (2007). Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol, 46(5), 955–975. doi: 10.1016/j.jhep.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Herrmann J, Gressner AM, & Weiskirchen R (2007). Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J Cell Mol Med, 11(4), 704–722. doi: 10.1111/j.1582-4934.2007.00060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Friedman SL, & Hoshida Y (2017). Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev, 121, 27–42. doi: 10.1016/j.addr.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Li T, Hernandez J, Teeman C, Tamargo J, Sherman K, Rouster S, Abdel-Hameed E, Jasmin J, Seminario L, Liu Q, Zarini G, Martinez S, Piperato J, Campa A, & Baum M (2019). Association of TIMP1 Levels and Liver Disease Progression Among HIV/HCV Co-infected, HIV Mono-, HCV Mono-infected, and Healthy Groups from the MASH Cohort (FS09-07-19). Current Developments in Nutrition, 3(Supplement_1). doi: 10.1093/cdn/nzz044.FS09-07-19 [DOI] [Google Scholar]
- Lamas-Paz A, Hao F, Nelson LJ, Vazquez MT, Canals S, Gomez Del Moral M, Martinez-Naves E, Nevzorova YA, & Cubero FJ (2018). Alcoholic liver disease: Utility of animal models. World J Gastroenterol, 24(45), 5063–5075. doi: 10.3748/wjg.v24.i45.5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans SA (2018). Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol, 9, 6. doi: 10.3389/fphar.2018.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, & Weaver VM (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 139(5), 891–906. doi: 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Manka P, Zeller A, & Syn WK (2019). Fibrosis in Chronic Liver Disease: An Update on Diagnostic and Treatment Modalities. Drugs, 79(9), 903–927. doi: 10.1007/s40265-019-01126-9 [DOI] [PubMed] [Google Scholar]
- Mederacke I, Dapito DH, Affo S, Uchinami H, & Schwabe RF (2015). High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc, 10(2), 305–315. doi: 10.1038/nprot.2015.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, & Lan HY (2016). TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol, 12(6), 325–338. doi: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- Neuman MG, Schmilovitz-Weiss H, Hilzenrat N, Bourliere M, Marcellin P, Trepo C, Mazulli T, Moussa G, Patel A, Baig AA, & Cohen L (2012). Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int J Hepatol, 2012, 231210. doi: 10.1155/2012/231210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, & Cederbaum AI (2000). Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem, 275(26), 20136–20145. doi: 10.1074/jbc.M001422200 [DOI] [PubMed] [Google Scholar]
- O’Rourke JM, Sagar VM, Shah T, & Shetty S (2018). Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J Gastroenterol, 24(39), 4436–4447. doi: 10.3748/wjg.v24.i39.4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BJ, Franklin CL, Hsieh FH, Bank RA, & Phillips CL (2005). Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol, 24(7), 451–458. doi: 10.1016/j.matbio.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Saneyasu T, Akhtar R, & Sakai T (2016). Molecular Cues Guiding Matrix Stiffness in Liver Fibrosis. Biomed Res Int, 2016, 2646212. doi: 10.1155/2016/2646212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Ridolfi F, Di Sario A, Saccomanno S, Bendia E, Benedetti A, & Greenwel P (2001). Intracellular signaling pathways involved in acetaldehyde-induced collagen and fibronectin gene expression in human hepatic stellate cells. Hepatology, 33(5), 1130–1140. doi: 10.1053/jhep.2001.23788 [DOI] [PubMed] [Google Scholar]
- Taufalele PV, VanderBurgh JA, Munoz A, Zanotelli MR, & Reinhart-King CA (2019). Fiber alignment drives changes in architectural and mechanical features in collagen matrices. PLoS One, 14(5), e0216537. doi: 10.1371/journal.pone.0216537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, & Wynn TA (2001). Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol, 167(12), 7017–7026. doi: 10.4049/jimmunol.167.12.7017 [DOI] [PubMed] [Google Scholar]
- van Grunsven LA (2017). 3D in vitro models of liver fibrosis. Adv Drug Deliv Rev, 121, 133–146. doi: 10.1016/j.addr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Weiskirchen R, Weimer J, Meurer SK, Kron A, Seipel B, Vater I, Arnold N, Siebert R, Xu L, Friedman SL, & Bergmann C (2013). Genetic characteristics of the human hepatic stellate cell line LX-2. PLoS One, 8(10), e75692. doi: 10.1371/journal.pone.0075692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG (2008). The role of matrix stiffness in regulating cell behavior. Hepatology, 47(4), 1394–1400. doi: 10.1002/hep.22193 [DOI] [PubMed] [Google Scholar]
- Wells RG (2013). Tissue mechanics and fibrosis. Biochim Biophys Acta, 1832(7), 884–890. doi: 10.1016/j.bbadis.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand J, & Berg T (2013). The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int, 110(6), 85–91. doi: 10.3238/arztebl.2013.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DT, Bitzer M, Ju W, Mundel P, & Bottinger EP (2005). TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol, 16(11), 3211–3221. doi: 10.1681/ASN.2004121055 [DOI] [PubMed] [Google Scholar]
- Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, & Eng FJ (2005). Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut, 54(1), 142–151. doi: 10.1136/gut.2004.042127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Evason KJ, Asahina K, & Stainier DY (2013). Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest, 123(5), 1902–1910. doi: 10.1172/JCI66369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Yuan WG, He P, Lei JH, & Wang CX (2016). Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol, 22(48), 10512–10522. doi: 10.3748/wjg.v22.i48.10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The effect of LOXL2 inhibition on collagen fiber organization of aHSC organoids BAPN treated organoids were larger than the control organoids A) and had differences in the distribution of fibers B). These fibers also had a different distribution of fiber hues, where BAPN treated groups shifted more towards the green, less bundled type fibers C). CT-FIRE analysis also corresponds with the bundling data in that BAPN treated groups had smaller fiber widths D).
Supplemental Figure 2. The effect of LOX2 inhibition of aHSC phenotype in 3D organoid Gene expression of aHSC organoids in the presence of BAPN were normalized to control aHSC organoids. The results indicate that inhibition of Collagen I cross linking with BAPN downregulated αSMA, COL1A2 and TGF-β expression and upregulated the expression of GFAP, suggesting a less fibrotic phenotype.


