Background: Normally, regulatory T cells (also known as T regulatory cells or Tregs) migrate into inflamed tissues, dampening inflammatory responses and hastening tissue repair (1). Patients with coronavirus disease 2019 (COVID-19) and acute respiratory distress syndrome (ARDS) have protracted hospitalizations characterized by excessive systemic inflammation (cytokine storm) and delayed lung repair, which is partly due to reduced or defective Tregs (2).
Objective: To describe outcomes in 2 patients with COVID-19 and ARDS who were treated with Tregs.
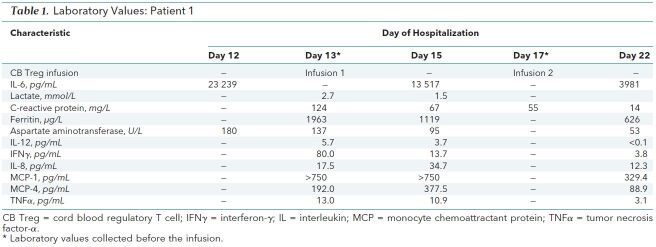
Case Reports: The first patient was a 69-year-old man with autism who was hospitalized from his nursing home with COVID-19–induced fever and dyspnea 1 week after initial symptom onset. Despite 5 days of receiving hydroxychloroquine and broad-spectrum antimicrobial agents, he progressed to ARDS and received tocilizumab on day 7 and mechanical ventilation beginning on day 8. Renal failure and shock developed, requiring continuous, venovenous hemofiltration and vasopressors. Refractory hypoxemia required prone positioning, neuromuscular paralysis, inhaled nitric oxide, and FIo 2 greater than 70%. We administered compassionate use, cryopreserved, allogeneic Tregs derived from cord blood (CB) and expanded ex vivo (Cellenkos) at 1 × 108 cells per dose intravenously on days 13 and 17. By day 17, he had returned to supine positioning, paralytics were withdrawn, inhaled nitric oxide was weaned to zero, FIo 2 was decreased to 50%, vasopressors were withdrawn, and inflammatory markers were reduced (Table 1). He was extubated on day 22. On day 25, he required a tracheostomy. He is currently receiving care in a weaning facility.
Table 1. Laboratory Values: Patient 1.
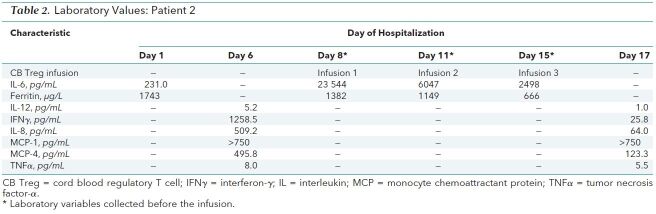
The second patient was a 47-year-old man who was hospitalized for COVID-19–induced fever and dyspnea 1 week after initial symptom onset. On day 2 of his hospitalization, he received tocilizumab, and hours later, he had increasing lactate levels and required mechanical ventilation and high-dose vasopressors. Despite prone positioning, neuromuscular paralysis, inhaled nitric oxide, and 100% FIo 2, he required venovenous, extracorporeal membrane oxygenation on day 7. We administered compassionate use of the same Treg treatment on days 8, 11, and 15. By day 9, vasopressors were withdrawn and inflammatory markers were reduced (Table 2). By day 13, tidal volumes improved despite no change in the pressure mode of ventilation or in the driving pressure of 10 cm H2O. He had a tracheostomy on day 19 and a pulmonary embolus on day 33. He was taken off extracorporeal membrane oxygenation support on day 34 and was weaned to room air by day 51. His tracheostomy was decannulated on day 53, and he was discharged home on day 54.
Table 2. Laboratory Values: Patient 2.
Discussion: There are several reasons to believe that Tregs may be effective in patients with COVID-19 and ARDS. Adoptive Treg therapy has been effective in multiple preclinical models of ARDS (1). In addition, phenotypic CD4+25+127loFOXP3hi, ex vivo–expanded CB Tregs express the lung homing markers CD49d, CCR4, and P-selectin glycoprotein ligand-1 (3). Moreover, compared with adult Tregs, CB Tregs are readily available, maintain suppressive function in inflammatory milieu, and have a low risk for converting into pathogenic RORγt-expressing T helper type 17 cells (4). In a xenogeneic model, CB Tregs can accumulate in lung tissue up to 14 days after intravenous infusion, with a reciprocal decrease in systemic and parenchymal effector T cells, which correlates with the resolution of inflammation (Lyu M, Parmar S. Adoptive therapy with cord blood regulatory T cells treats the inflammatory syndromes of lupus. In preparation.). In a phase I trial of CB Tregs manufactured by Cellenkos (CK0801) to treat inflammatory bone marrow failure, at 1 month there was a decrease in Janus kinase 2 mutant allele burden, significant improvement in myelofibrosis symptoms, and improvement in blood and platelet transfusion requirement even though circulating CB Tregs were undetectable (4).
To our knowledge, this is the first report of human therapy with Tregs for ARDS mediated by COVID-19. We emphasize that the Tregs used were allogeneic, off-the-shelf, and CB Tregs and that both patients became critically ill despite receiving tocilizumab. We planned 3 infusions for each patient, with the first infusion on day 1, the second on day 3, and the third on day 7. Neither patient had an infusion reaction, inflammatory rebound, or other adverse reaction.
We recognize that both patients received multiple interventions that could have contributed to their recovery, but we believe that the temporal relationship between Treg infusions and recovery cannot be ignored. Table 1 and Table 2 show that the infusions were rapidly followed by decreases in interleukin (IL)-6, tumor necrosis factor-α, and interferon-γ. Decreases also occurred in IL-8, which is a potent neutrophil attractant or activator and modulator of acute lung injury, and in IL-12, which is important for defining T helper type 1 or type 2 cell commitment in lung inflammatory responses induced by respiratory syncytial virus infection. Monocyte chemoattractant protein-1 and monocyte chemoattractant protein-4 levels, which are associated with mononuclear pulmonary cellular infiltration, decreased in the first patient, but only the monocyte chemoattractant protein-4 level decreased in the second patient.
We expect that future treatment of patients with COVID-19 and ARDS will be multifaceted (5), and we are planning a multicenter, randomized, double-blind, placebo-controlled trial of CB Tregs for ARDS associated with COVID-19.
Footnotes
This article was published at Annals.org on 6 July 2020.
References
- 1.D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898-913. [PMID: 19770521] doi:10.1172/JCI36498 [DOI] [PMC free article] [PubMed]
- 2.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036-1045.e9. [PMID: 32416070] doi:10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed]
- 3.Lyu M, Khoury JD, Nishimoto M, et al. Single injection of cord blood regulatory T cells can delay the manifestations of systemic lupus erythematosus. Blood. 2019;134:1938. doi:10.1182/blood-2019-131436
- 4.Kadia TM, Ma H, Zeng K, et al. Phase I clinical trial of CK0801 (cord blood regulatory T cells) in patients with bone marrow failure syndrome (BMF) including aplastic anemia, myelodysplasia and myelofibrosis. Blood. 2019;134:1221. doi:10.1182/blood-2019-127702
- 5.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:CD013600. [PMID: 32406927] doi:10.1002/14651858.CD013600 [DOI] [PMC free article] [PubMed]