Abstract
The autonomic nervous system plays a central role in the pathogenesis of multiple cardiac arrhythmias, including atrial fibrillation and ventricular tachycardia. As such, autonomic modulation represents an attractive therapeutic approach in these conditions. Notably, autonomic modulation exploits the plasticity of the neural tissue to induce neural remodeling and thus obtain therapeutic benefit. Different forms of autonomic modulation include vagus nerve stimulation, tragus stimulation, renal denervation, baroreceptor activation therapy, and cardiac sympathetic denervation. This review seeks to highlight these autonomic modulation therapeutic modalities, which have shown promise in early pre-clinical and clinical trials and represent exciting alternatives to standard arrhythmia treatment. We also present an overview of the various methods used to assess autonomic tone, including heart rate variability, skin sympathetic nerve activity, and alternans, which can be used as surrogate markers and predictors of the treatment effect. Although the use of autonomic modulation to treat cardiac arrhythmias is supported by strong preclinical data and preliminary studies in humans, in light of the disappointing results of a number of recent randomized clinical trials of autonomic modulation therapies in heart failure, the need for optimization of the stimulation parameters and rigorous patient selection based on appropriate biomarkers cannot be overemphasized.
Keywords: alternans, arrhythmias, autonomic modulation, autonomic nervous system, heart rate variability
The heart is innervated by both the sympathetic and the parasympathetic nervous system (1). The cardiac neural hierarchy includes the central nervous system (brain, spinal cord), the extrinsic intrathoracic ganglia (dorsal root ganglia, stellate ganglia, and sympathetic chain) and the intrinsic cardiac autonomic nervous system (CANS). The intrinsic CANS is comprised of an extensive epicardial neural network of nerve axons, interconnecting neurons, and clusters of autonomic ganglia, known as ganglionated plexi (GP), which contain from a few neurons to over 400 neurons (2,3). From a physiological point of view, the GP serve as the communication centers between the intrinsic and the extrinsic CANS, coordinating the response to afferent and efferent neural trafficking to control regional electrophysiological, vascular, and contractile function (4). Notably, the major atrial GP are identified clinically during an electrophysiological study by applying high-frequency stimulation (HFS) at the respective anatomical locations, which in turn results in slowing of the heart rate or atrioventricular conduction (5).
The CANS is regulated by autonomic and neurohormonal reflexes. Baroreceptors in the carotid arteries and large vessels sense changes in blood pressure, and activation of the baroreceptor reflex triggers an increase in parasympathetic activity and reflex inhibition of the sympathetic tone. The sympathetic-parasympathetic balance achieved by this baroreflex is important in maintaining blood pressure and afterload of the heart (6). Additionally, neurohormonal feedback from the renin-angiotensinaldosterone system is also important for maintaining CANS balance (7).
Complex feedback neural loops also occur at the level of the heart, with efferent limbs carrying sympathetic and parasympathetic signals from the brain to the heart and afferent limbs carrying sensory signals from the heart to the brain to achieve beat to beat regulation of cardiac function (8). Importantly, it has been recently shown that afferent neuronal transmission amplifies sympathoexcitation through central and intrathoracic sympathetic reflexes, while surgical decentralization or spinal cord stimulation (SCS), a form of autonomic modulation, attenuates this amplification of sympathoexcitation, thus preventing ventricular arrhythmias (9).
AUTONOMIC MODULATION: BASIC PRINCIPLES
Autonomic modulation has been used for over 20 years for neurological diseases such as epilepsy (10) and depression (11). In light of the prominent role of the autonomic nervous system in a variety of cardiovascular diseases, including heart failure and arrhythmias (1), there exists a growing interest for autonomic modulation approaches. A fascinating property of autonomic modulation is that its autonomic effects exhibit memory, whereby short durations of therapy result in long-lasting effects (12,13). This is akin to the well-known phenomenon of long-term potentiation or long-term depression, whereby the stimulatory or inhibitory effects of neuro-stimulation greatly outlast the duration of stimulation (14). Importantly, recent evidence suggests that synaptic plasticity, the ability of neurons to alter their strength of communication at synapses, which in turn results in long-term potentiation or depression, occurs not only in the brain, but also in the cardiac GP (15).
Different forms of autonomic modulation include vagus nerve stimulation (VNS), tragus stimulation, renal denervation (RDN), SCS, baroreceptor activation therapy (BAT), and cardiac sympathetic denervation (CSD) (Central Illustration). Although the exact mechanisms by which autonomic modulation exerts its beneficial effects on the heart have not been fully elucidated, it is likely that these include restoration of ANS balance, enhancement of nitric oxide, and inhibition of inflammation, fibrosis, and apoptosis, among others (9,16–20). A summary of prospective clinical trials of autonomic modulation in humans is shown in Table 1.
CENTRAL ILLUSTRATION.
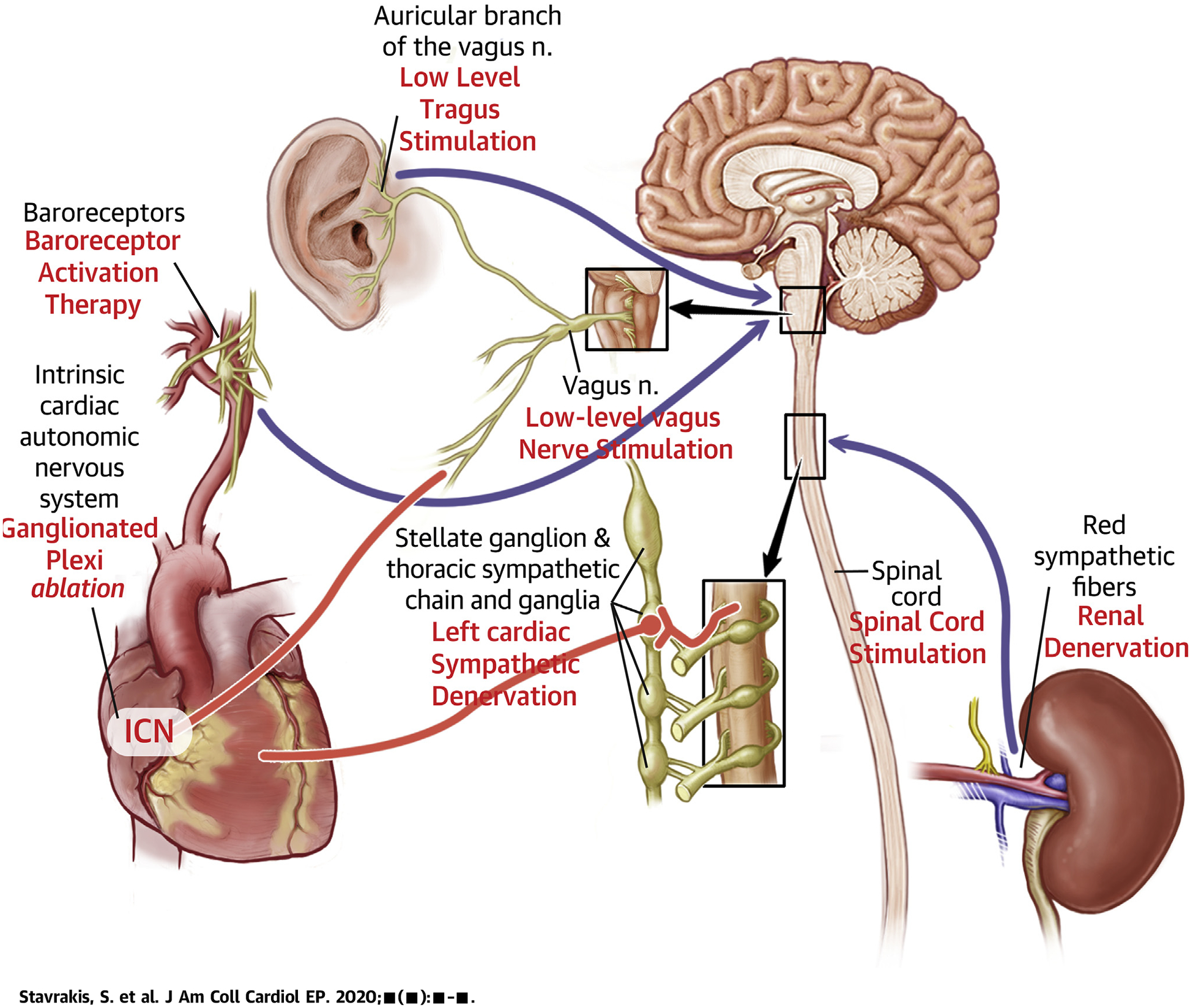
Schematic Representation of the Autonomic Nervous System and the Targets of Autonomic Modulation Therapy
Afferent and efferent nerves are depicted in black and red, respectively.
TABLE 1.
Summary of Prospective Clinical Trials of Autonomic Modulation in Humans
| Modality | Clinical Trial | Design | Patient Population | n | Outcome |
|---|---|---|---|---|---|
| Atrial arrhythmias | |||||
| Vagus nerve stimulation | Stavrakis et al. (51) | Randomized sham-controlled trial | Patients undergoing cardiac surgery | 54 | Decreased incidence of post-operative AF |
| Tragus stimulation | Stavrakis et al. (55) | Randomized sham-controlled trial | Patients with paroxysmal AF undergoing EP study | 40 | Decreased inducible AF duration during EP study |
| TREAT AF (56) | Randomized sham-controlled trial | Paroxysmal AF | 53 | Decreased AF burden at 6 months | |
| Renal denervation | Pokushalov et al. (63) | Randomized open-label trial | Paroxysmal or persistent AF and resistant hypertension | 27 | Decreased recurrence of any atrial tachyarrhythmia at 3 months |
| AFFORD (64) | Single-arm feasibility trial | Paroxysmal or persistent AF and hypertension | 20 | Decreased AF burden at 12 months | |
| ERADICATE AF (65) | Randomized open-label trial | Paroxysmal AF undergoing AF ablation | 302 | Increased freedom from AF, flutter, or tachycardia recurrence at 12 months | |
| Ventricular arrhythmias | |||||
| Vagus nerve stimulation | De Ferrari et al. (87) | Single-arm open-label trial | Systolic heart failure, NYHA functional class II or III, LVEF ≤35% | 32 | Significant improvement in NYHA functional class, quality of life, 6-min walk distance |
| ANTHEM HF (91) | Randomized open-label trial | Systolic heart failure, NYHA functional class II or III, LVEF ≤40% | 60 | Significant improvement in LVEF, HRV, NYHA functional class | |
| NECTAR HF (90) | Randomized single-blind trial | Systolic heart failure, NYHA functional class II or III, LVEF ≤35% | 96 | No significant change in LVESV or LVEF at 6 months | |
| INOVATE HF (89) | Randomized open-label trial | Systolic heart failure, NYHA functional class III, LVEF ≤40% | 707 | No significant difference in all-cause mortality or worsening heart failure | |
| Tragus stimulation | Yu et al. (97) | Randomized sham-controlled trial | Patients with ST-segment elevation myocardial infarction | 95 | Decreased ventricular arrhythmias within 24 h |
| Renal denervation | Symplicity HF (99) | Single-arm feasibility trial | Systolic heart failure, NYHA functional class III, LVEF <40%, renal impairment | 39 | Decreased NT-proBNP at 12 months |
| Spinal cord stimulation | SCS HEART (106) | Nonrandomized open-label trial | Systolic heart failure, NYHA functional class III, LVEF ≤35% | 22 | Improved NYHA functional class, LVEF, exercise capacity, quality of life |
| DEFEAT HF (107) | Randomized single-blind trial | Systolic heart failure, NYHA functional class III, LVEF ≤35% | 66 | No difference in incidence of ventricular arrhythmias | |
| Baroreceptor activation therapy | HOPE4HF (113) | Randomized open-label trial | Systolic heart failure, NYHA functional class III, LVEF ≤35% | 146 | Improved NYHA functional class, quality of life, exercise capacity, NT-proBNP |
AF = atrial fibtillation; AFFORD = Atrial fibrillation reduction by renal sympathetic denervation; ANTHEM HF = Autonomic Regulation Therapy for the Improvement of Left Ventricular Function and Heart Failure Symptoms; DEFEAT-HF = Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure; ERADICATE AF = Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension; HRV = heart rate variability; LVEF = left ventricular ejection fraction; LVESV = left ventricular end systolic volume; NECTAR HF = NEural Cardiac TherApy foR Heart Failure; HOPE4HF = Hope for Heart Failure; NT-proBNP=N-terminal B-type natriuretic peptide; NYHA = New York Heart Association; SCS HEART = Spinal Cord Stimulation for Heart Failure as a Restorative Treatment; Symplicity HF = Renal Denervation in Patients With Chronic Heart Failure & Renal Impairment; TREAT AF = Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation.
ROLE OF ANS IN ATRIAL ARRHYTHMIAS ARRHYTHMIAS.
A series of basic experiments provided many lines of evidence linking the intrinsic CANS with focal firing from the pulmonary veins (PVs). PV myocytes have distinctive cellular electrophysiological properties from the adjacent atrium, and particularly, a shorter action potential duration, which facilitates the initiation of atrial fibrillation (AF). Under conditions of local stimulation of both parasympathetic and sympathetic nerve endings (21) or simultaneous administration of acetylcholine plus norepinephrine (or isoproterenol) (22), early after depolarizations were induced, giving rise to rapid triggered firing from canine PVs. On the other hand, triggered firing from the PVs is suppressed by muscarinic cholinergic receptor blockade, betaadrenoceptor antagonism, inhibition of calcium transients, or sodium-calcium exchanger blockade (21). Furthermore, Scherlag et al. (23) demonstrated that stimuli applied to PVs would not induce AF unless there was simultaneous activation of the GP adjacent to that PV.
Variations in autonomic tone in humans (24) and hyperactivity of the GP in ambulatory dogs (25) have been documented before episodes of paroxysmal AF. Moreover, the autonomic neural activity recorded from the anterior right GP increased hour by hour during 6 hours of rapid atrial pacing, suggesting that GP may provide not only the trigger for AF initiation, but also the substrate for AF maintenance (26). These results support the notion that enhanced activity of the CANS and AF form a vicious cycle, in which high CANS activity initiates AF, while AF further augments CANS activity (26). In support of this notion, GP ablation reversed acute autonomic remodeling (shortening of atrial effective refractory period and increased inducibility of AF) induced by 6 h of rapid atrial pacing in a canine model of AF (27). Human studies showing that the addition of GP ablation to the standard pulmonary vein isolation (PVI) procedure enhances sinus rhythm maintenance corroborate the animal data (28,29). In fact, wide antrum circumferential PVI, using radiofrequency current (5) or cryoablation (30), results in partial GP ablation (transection at least 3 of 4 major atrial GP at the PVatrial junction), and this inadvertent form of autonomic modulation may contribute to the increased success of the current form of PVI procedures as compared with segmental isolation (5), further supporting the importance of assessing autonomic modulation for a wide range of patients with cardiac arrhythmias.
ROLE OF ANS IN VENTRICULAR ARRHYTHMIAS ARRHYTHMIAS.
The ANS plays a central role in the pathophysiology of ventricular arrhythmias leading to sudden cardiac death in the presence of structural heart disease (31). Patients with prior anterior wall myocardial infarction have a particularly increased risk of sudden cardiac death from ventricular arrhythmias (32). It has been demonstrated that myocardial infarction, which interrupts autonomic innervation in the area of the infarct, leads to sympathetic hypersensitivity, nerve sprouting, and heterogeneous gradients of sympathetic innervation around the infarct area (33,34). In a canine model of healed myocardial infarction, the presence of reduced baroreflex sensitivity was associated with a greater susceptibility to ventricular fibrillation (VF) (35). Moreover, bursts of sympathetic nerve activity in the stellate ganglia preceded ventricular arrhythmias in ambulatory dogs with ischemic cardiomyopathy (36). In addition, an increase in systemic and cardiac sympathetic activity induced by electrical stimulation of the left renal sympathetic nerves increased the incidence of ventricular arrhythmias (37).
Increased risk for ventricular arrhythmias and sudden death is also prevalent in cardiomyopathy and heart failure, even in the absence of ischemic heart disease (31). Heart failure is characterized by sympathetic hyperactivity, which increases the risk for ventricular arrhythmias (1,31). The fact that beta adrenergic receptor blockers increase survival and decrease sudden cardiac death in patients with heart failure corroborates the important role of sympathetic hyperactivity in the pathogenesis of adverse events in heart failure, irrespective of etiology (1,31).
In patients with congenital arrhythmia syndromes, including long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT), episodes of ventricular tachycardia are commonly triggered by increased sympathetic tone (38,39). In CPVT, beta-blockers are the first-line therapy, whereas lack of beta-blocker therapy is an independent predictor of adverse cardiac events including sudden cardiac death, highlighting the importance of elevated sympathetic tone as the basis of ventricular tachycardia in these patients (40). In patients with LQTS, triggers of arrhythmias differ by genotype, with LQTS type 1 being more sensitive to adrenergic stimuli, such as exercise (41). Beta-blockers are first-line therapy for LQTS, even though response to beta-blocker therapy is higher in LQTS type 1 (41,42).
AUTONOMIC MODULATION FOR ATRIAL ARRHYTHMIAS
1. VAGUS NERVE STIMULATION.
VNS to suppress AF may appear paradoxical, since this method has been used for decades to induce AF (43). However, a recent study by Zhang et al. (44) has indicated that whereas strong vagal nerve stimulation (>60% sinus rate slowing) promoted AF inducibility, mild to moderate levels (<40% sinus rate slowing) did not have any effect on AF inducibility. Several recent studies supported the hypothesis that low-level vagus nerve stimulation (LLVNS), at levels 10% or 50% lower than the bradycardia threshold, exerts a strong antiarrhythmic effect (45–48). Notably, LLVNS applied to the distal ends of the sectioned vagosympathetic trunks did not significantly alter the results, thus indicating that the inhibitory effects of LLVNS on AF inducibility involve efferent conduction to the heart (49). The effects of LLVNS were mediated by inhibition of GP activity, as indicated by LLVNS-mediated suppression of the ability of the anterior right GP stimulation to slow the heart rate, as well as the marked suppression of the frequency and amplitude of the neural activity recorded from the anterior right GP or superior left GP (46). Furthermore, LLVNS of the right vagus nerve alone was sufficient to exert a strong antiarrhythmic action by suppressing the neural activity of both the adrenergic and the cholinergic component of the intrinsic CANS (47).
The antiarrhythmic effects of LLVNS were also observed in ambulatory dogs. In this experimental model, left-sided LLVNS suppressed left stellate ganglion neural activity, especially in the morning, and decreased tyrosine-hydroxylase positive cells in the left stellate ganglion 1 week after cessation of LLVNS (48). Moreover, in the same study, LLVNS prevented paroxysmal AF induced by rapid atrial pacing (48). In a subsequent study, the same investigators showed that chronic LLVNS damaged the stellate ganglia, resulting in reduced stellate ganglion nerve activity (50).
The only application of LLVNS in humans so far has been for the treatment of post-operative AF (51). In this study, 54 patients undergoing cardiac surgery were randomized to active or sham LLVNS (20 Hz, 50% of the bradycardia threshold). LLVNS was accomplished using a bipolar wire that was sutured to the pre-ganglionic fibers of the vagus nerve alongside the lateral aspect of the superior vena cava and was delivered for an average of 61 h post-operatively. At 1- month follow-up, there was a significant reduction of the incidence of post-operative AF in the LLVNS group compared with control. In addition, there was a significant reduction of inflammatory cytokines, consistent with the well-characterized anti-inflammatory effects of VNS (52). These results support the emerging paradigm of autonomic modulation for the treatment of AF and possibly other conditions, where inflammation plays a central role.
2. TRAGUS STIMULATION.
Based on the observation that stimulation of the tragus of the ear, where the auricular branch of the vagus nerve is located, results in evoked potentials in the brainstem in humans (53), transcutaneous stimulation of the vagus nerve at this site was used as a method of noninvasive vagal stimulation. In a rapid atrial pacing canine model of AF, tragus stimulation at 80% below the threshold of sinus rate or AV conduction slowing (low-level tragus stimulation [LLTS]) significantly attenuated atrial effective refractory period shortening induced by rapid atrial pacing, suppressed AF inducibility, and decreased the amplitude and frequency of GP firing, as demonstrated by direct neural recordings from the anterior right GP (54). When the bilateral vagus nerves were transected distal to the site of stimulation, the ability of LLTS to reverse the effects of rapid atrial pacing on atrial effective refractory period and AF inducibility was abolished, suggesting that efferent vagus nerves are an essential component of this effect (54).
These promising results were recently translated in humans, in a study of 40 patients with paroxysmal AF who were randomized to either 1 h of LLTS (n = 20) or sham stimulation (n = 20). LLTS (20 Hz) in the right ear, 50% lower than the voltage slowing the sinus rate, was accomplished by attaching a flat metal clip onto the tragus. Under general anesthesia, AF was induced by burst atrial pacing at baseline and after 1 h of LLTS or sham stimulation. Pacing-induced AF duration decreased significantly compared with baseline in the LLTS group, but not in the sham control, whereas atrial effective refractory period increased in the LLTS group but not in the control group (55). In the same study, LLTS significantly decreased the serum levels of tumor necrosis (TNF)-α compared with sham control. Although in this study the exact mechanism of the antiarrhythmic effect was not examined, it was hypothesized that it was due to its antiadrenergic and possibly anti-inflammatory effects (55).
More recently, LLTS was tested in the ambulatory setting. In the TREAT-AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation) study, 53 patients with paroxysmal AF were randomized to LLTS or sham (ear clip applied to the earlobe, which is devoid of vagal innervation) for 1 h daily over a 6-month period. The primary outcome of the study was AF burden, as measured by a 14-day continuous electrocardiogram (ECG) performed at baseline, 3 months, and 6 months. After adjusting for baseline values, AF burden at 6 months was reduced by 85% in the stimulation group compared with sham, with a concomitant 23% reduction in TNF-α levels (56) (Figure 1). These results are particularly encouraging because they show that a self-administered treatment with essentially no significant risks can have a significant impact on AF burden and levels of systemic inflammation. From a physiological point of view, tragus stimulation, which preferentially activates afferent rather than efferent vagal fibers, may offer a therapeutic advantage (57). Specifically, tragus stimulation has been shown to activate central vagal projections in the brain in humans, leading to decreased sympathetic output (58). In addition, tragus stimulation, as opposed to cervical VNS, may avoid concomitant stimulation of sympathetic fibers, which are colocalized with vagal fibers in the vagus nerve and are being inadvertently stimulated during cervical VNS in humans (59).
FIGURE 1. Effect of Low-Level Tragus Stimulation on AF Burden and TNF-α Levels.
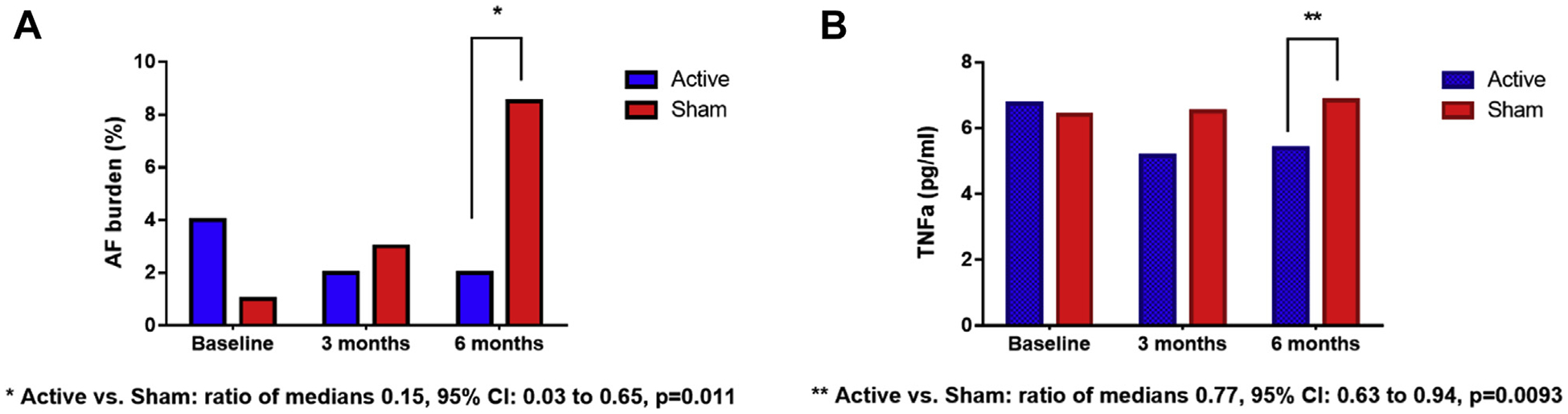
(A) Atrial fibrillation (AF) burden, and (B) tumor necrosis factor (TNF)-a levels. Data are presented as median values. AF burden and TNF-a levels at 6 months were significantly lower in the active compared with the sham group.
3. RENAL DENERVATION.
The proximal renal arteries are covered by a rich network of sympathetic ganglia and nerve fibers that provide afferent and efferent communication between the central nervous system and the kidney (60). Because renal afferent nerve activity has a strong direct effect on the sympathetic outflow to the kidneys, as well as the heart and the blood vessels, RDN has been proposed as a therapy for refractory hypertension.
Despite the disappointing effects of the SIMPLICITY-3 (A Controlled Trial of Renal Denervation for Resistant Hypertension) trial with regard to hypertension control, several studies demonstrated a beneficial effect of RDN on AF. Importantly, preclinical studies have suggested that the effect of RDN may be independent of changes in blood pressure (61,62). RDN reduced atrial sympathetic nerve sprouting and AF complexity in a goat model of pacing-induced chronic AF (61) and attenuated atrial effective refractory period shortening and AF inducibility in a porcine model of obstructive sleep apnea (62). These beneficial effects of RDN on AF are likely multifactorial and include attenuation of atrial electrical and structural remodeling related to a decrease in afferent sympathetic input to the central nervous system, which in turn decreases the efferent sympathetic output to the heart (60).
Small human studies, in which RDN was added to standard PVI, support the experimental data (63,64). Notably, effective RDN was assessed by performing HFS at the site of the aorticorenal ganglion, and deemed to be successful if the expected sudden hypertensive response was eliminated (63). Based on these promising preliminary results, the ERADICATE-AF (Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension) study was designed as the first large, multicenter trial to assess the efficacy of RDN when added to standard PVI in 302 patients with hypertension on at least 1 antihypertensive drug and paroxysmal AF. At 12-month follow-up, the addition of RDN resulted in a significantly greater freedom from AF off of antiarrhythmic drugs compared to standard PVI (71.4% vs. 57.8%; hazard ratio: 0.61 [Confidence Interval (CI): 0.41 to 0.90]; p = 0.011) (66). Complication rates were the same in both groups, and there was better blood pressure control in the RDN arm. Notably, only 57% of patients had RDN conclusively demonstrated by HFS response. Thus, it remains unclear if the beneficial effect of RDN on AF recurrence is related to autonomic modulation, improved blood pressure control, or a combination of these mechanisms.
4. SPINAL CORD STIMULATION.
SCS delivers electrical stimuli to the segments of spinal cord through implanted electrodes. SCS is currently used to treat patients with severe chronic pain or angina pectoris refractory to conventional therapeutic strategies (66,67). Notably, SCS can modulate the afferent and efferent connections between the heart and the CANS, thereby altering cardiac electrophysiology (68). In a canine AF model of chronic rapid atrial pacing, SCS prolonged the atrial effective refractory period and reduced the AF burden and inducibility (69). The underlying mechanism(s) by which SCS exerts its antiarrhythmic effects are related to suppression of the neural activity in atrial GP and the stellate ganglia (70,71). Moreover, SCS decreased the expression of c-fos and nerve growth factor as well as increased the expression of the small conductance calcium-activated potassium channels type 2 in the stellate ganglia neurons (70). Nonetheless, given its invasiveness and uncertain clinical benefits in humans, SCS as a therapy to treat AF is not likely to be widely adopted.
5. BARORECEPTOR STIMULATION.
Baroreceptors in the carotid sinus are activated by elevations in blood pressure, resulting in enhanced neural trafficking to the brain stem, eventually leading to a significant decrease of sympathetic nerve activity (72,73). Over the last 50 years, various medical devices for baroreceptor activation therapy (BAT) have been designed and used for treatment of drug-resistant hypertension by modulating autonomic nervous system (74,75). Studies have shown that BAT can reset ANS by sympathetic withdrawal and increasing vagal activation (76,77). Importantly, a recent canine study showed that low-level BAT, at 80% of the threshold for blood pressure reduction, could affect atrial electrophysiology, resulting in a progressive increase in AF inducibility and atrial effective refractory period (78). In the same study, the GP function was attenuated, suggesting that low-level BAT suppressed AF by inhibiting GP. In addition, low-level BAT was reported to attenuate atrial remodeling induced by rapid atrial pacing in canines (79). Collectively, these data support the notion that low-level BAT, even without any effects on blood pressure or heart rate, exhibits prominent antiarrhythmic effects, and further studies are warranted to examine the potential of this modality to serve as a novel therapeutic approach for AF. Nonetheless, the invasive nature of this approach may limit its applicability in human AF, unless a noninvasive option is developed.
AUTONOMIC MODULATION FOR VENTRICULAR ARRHYTHMIAS
1. VAGUS NERVE STIMULATION.
In the ventricle, a shift toward parasympathetic predominance exerts cardioprotective effects, whereas decreased vagal activity after myocardial infarction is associated with a higher risk of ventricular arrhythmias (80,81). Notably, VNS and cholinergic agonists prolong the ventricular effective refractory period in experimental models (82,83). Moreover, VNS favorably alters the vulnerability to VF, as evidenced by a decrease in the maximum slope of action potential duration restitution, attenuation of electrical alternans, and an increase in ventricular effective refractory period and VF thresholds in the presence of VNS (84).
Multiple animal studies have demonstrated the beneficial role of VNS in preventing adverse cardiac remodeling (16,85). Importantly, the favorable effects of VNS also extended to prevention of ventricular arrhythmias. In a rat model of acute myocardial infarction, VNS was shown to exert antiarrhythmic effects by preventing loss of phosphorylated connexin 43 (20). The favorable effects of VNS were confirmed in a canine model of high rate pacing-induced heart failure, where chronic VNS improved cardiac autonomic control and attenuated heart failure development along with pronounced anti-inflammatory effects (17). Similarly, Vanoli et al. (86) showed that VNS performed in the healed phase of myocardial infarction was effective in preventing sudden cardiac death due to ventricular arrhythmias. Interestingly, a reduction in heart rate does not appear to be an important determinant of the beneficial effect of VNS (87,88). These data formed the basis for the design of clinical trials examining the effect of VNS in humans.
Although a first-in-human, open-label, nonrandomized trial demonstrated that VNS resulted in significant improvement in functional class, quality of life, 6-min walk test, and LV end-systolic volume, in the absence of any major side effects (87), 3 subsequent randomized trials of VNS in heart failure showed either neutral effects (89,90) or only mild benefit (91). The rather disappointing results of these trials, despite the clear rationale for decreasing sympathovagal imbalance in heart failure, highlight the notion that optimizing patient selection and stimulation parameters is crucial to achieve a favorable effect (92). An important large clinical trial (ANTHEM-HFrEF [Autonomic Regulation Therapy to Enhance Myocardial Function and Reduce Progression of Heart Failure With Reduced Ejection Fraction] pivotal study) is underway and it will assess the cardiovascular mortality and heart failure hospitalizations in patients with symptomatic heart failure (NCT03425422) (93).
2. TRAGUS STIMULATION.
All major trials of VNS in heart failure required surgical placement of the electrodes in the neck around the cervical vagus nerve, which carries the risk for major complications, as illustrated by the death of 1 patient during implantation in ANTHEM-HF (91). Moreover, there is potential for long-term side effects, including infections and electrode malfunction, as well as the need for battery replacements (94). Additionally, direct stimulation of the vagus nerve may be associated with potential side effects, including tinnitus, dysphonia, cough, and nausea (94,95). Therefore, the use of tragus stimulation, which noninvasively stimulates the auricular branch of the vagus nerve (53) is an attractive alternative to cervical VNS.
Recent animal studies provided evidence for mitigation of adverse cardiac remodeling and arrhythmias by tragus stimulation. In a canine model of chronic myocardial infarction, chronic intermittent LLTS attenuated LV structural remodeling, fibrosis, and inflammation (85). In a similar model, chronic, intermittent LLTS (2 h daily for 2 months, 80% below the bradycardia threshold) suppressed left stellate ganglion activity, decreased cardiac sympathetic nerve sprouting, down-regulated protein levels of nerve growth factor, up-regulated small conductance calcium-activated potassium channels type 2, and suppressed ventricular arrhythmias with a flattening of the restitution curve slope (96). In a proof-of concept study in humans with ST-segment elevation myocardial infarction undergoing primary coronary intervention, LLTS for 2 h markedly reduced ventricular arrhythmias, biomarkers of myocardial injury, and inflammation, and preserved cardiac function, supporting the notion that this noninvasive autonomic modulation approach may become an adjunctive nonpharmacological therapy for the treatment of ST-segment elevation myocardial infarction (97).
3. RENAL DENERVATION.
The initial preclinical studies of RDN, which was achieved with low energy radiofrequency application along the renal arteries, showed improved cardiac output and reduction in angiotensin receptor density (98). In a single-arm feasibility study that enrolled 39 patients with mild to moderate symptoms, ejection fraction <40%, and renal impairment (SIMPLICITY HF), RDN was associated with reduction in N-terminal pro–B-type natriuretic peptide 12 months after treatment without any significant deterioration in cardiac and renal function (99). Moreover, RDN has been shown to be effective as an adjunctive therapy to catheter ablation in patients with cardiomyopathy and ventricular tachycardia resistant to standard interventions (100). Notably, RDN reduced implantable cardioverter-defibrillator (ICD) therapies in patients with cardiomyopathy who had ventricular tachycardia recurrence after catheter ablation and cardiac sympathetic denervation procedures (101).
4. SPINAL CORD STIMULATION.
Multiple preclinical studies have shown that SCS results in reduced ventricular arrhythmia episodes and recovery in left ventricular ejection fraction in canine models of ischemic cardiomyopathy (102–104). The antiarrhythmic effects of SCS were attributed to withdrawal of sympathetic tone and/or enhancement of vagal tone (103). The antiarrhythmic effects of SCS were also demonstrated in a recent case series of 2 patients with high ventricular arrhythmia burden (105). Nonetheless, 2 recent clinical trials designed to investigate SCS in patients with heart failure, including a randomized controlled trial (DEFEAT-HF [Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure]), produced conflicting results (106,107). Differences in the duty cycle, intensity of stimulation, electrode shape, and position between the 2 studies may account for the different outcome. The negative results of DEFEAT-HF highlight the importance of first systematically determining the optimal stimulation protocol in animal studies before embarking in a large human study.
5. BARORECEPTOR ACTIVATION THERAPY.
In canine models of heart failure, BAT resulted in favorable remodeling with improvement in interstitial fibrosis, myocyte hypertrophy, LV end-diastolic pressure, and survival (108,109). In addition, BAT prolonged the ventricular effective refractory period and reduced the maximum action potential duration restitution slope (Smax) in canines (110). Low-level BAT, at 80% below the voltage threshold for blood pressure lowering, suppressed premature ventricular contractions, ventricular tachycardia, and VF episodes after acute occlusion of the left anterior descending artery in canines, suggesting that low-level BAT may protect against ventricular arrhythmias by modulating ANS (111). A small, single-center, open-label clinical study demonstrated that in heart failure patients, carotid BAT was safe and improved patients’ quality of life and exercise capacity (112). Moreover, a small randomized clinical trial of BAT showed improvement in surrogate markers, including 6-min walk test, quality of life scores, functional class, and N-terminal pro–B-type natriuretic peptide at 6 months (113).
A large randomized clinical trial (BeAT-HF) with an adaptive sample size of up to 960 patients is underway that will assess the effect of BAT on heart failure morbidity and cardiovascular mortality (NCT02627196) (114). Through a unique collaboration with the Food and Drug Administration under the Expedited Access Pathway, which was designed to accelerate approval of novel therapies targeting unmet needs for life-threatening conditions, the BeAT-HF trial design may provide the opportunity for approval of BAT, initially for symptom relief and subsequently for improvement in hard clinical outcomes (114).
6. LEFT CARDIAC SYMPATHETIC DENERVATION.
Left cardiac sympathetic denervation (LCSD) for the treatment of patients with congenital LQTS refractory to beta-blockers was first introduced by Schwartz et al. (115) in 1991. A larger study demonstrated that LCSD was associated with a significant reduction in the incidence of aborted cardiac arrest and syncope in high-risk LQTS patients when compared with pre-LCSD events, but it did not completely abolish the risk of sudden cardiac death during long-term follow-up (116). Currently, LSCD is recommended as an adjunctive therapy in patients with LQTS who experience breakthrough events while on therapy with beta-blockers or ICD (117). LSCD has also been shown to be effective in the treatment of patients with CPVT (118). Importantly, recent advances in surgical techniques allow for LSCD to be performed safely and effectively using video-assisted thoracic surgery in patients with LQTS or CPVT (119).
Recently, CSD was performed in patients with structural heart disease and refractory ventricular arrhythmias. In this patient population, CSD decreased sustained ventricular tachycardia and defibrillator shock recurrence in patients with refractory ventricular tachycardia, with 1-year freedom from recurrent events of 58% (120). Notably, bilateral CSD was more effective than LCSD in preventing recurrent ventricular arrhythmias or ICD shocks (120). Bilateral CSD was also used successfully in patients with difficult-to-ablate ventricular arrhythmias (121) and in patients with cardiac sarcoidosis presenting with refractory ventricular tachycardia (122). A recent pooled analysis of all available studies showed that bilateral CSD in cases with structural heart disease resulted in freedom from events ranging from 58% to 100%, with only transient major complications (123). Epidural anesthesia at the C7-T2 level to temporarily block sympathetic output to the heart exerted similar beneficial effects in this patient population (124). Although randomized clinical trials to examine the effect of CSD in this population with structural heart disease are lacking at present, these promising results expand the armamentarium of available therapies in this high-risk group. These results illustrate the important role of the sympathetic nervous system in triggering ventricular arrhythmias leading to sudden cardiac death in patients with heart failure (31).
METHODS TO ASSESS AUTONOMIC TONE
1. HEART RATE VARIABILITY.
The time interval between consecutive heartbeats is never constant and varies stochastically due to the inherent HRV (125–127). Many physiological processes, such as the influence of circadian rhythms, temperature regulations, and changes in cardiac sympathetic and parasympathetic nerve activity, directly or indirectly modulate HRV. The sympathetic components of the autonomic nervous system increase heart rate by releasing catecholamines (epinephrine and norepinephrine), while the parasympathetic components decrease heart rate through the releasing of the neurohormone acetylcholine. Any small fluctuations in the levels of these neurohormones induce some degree of variability in the intervals between consecutive beats (128–130). HRV reflects the balance between sympathetic and parasympathetic mediators and can be computed based on the RR interval change over time.
Clinically, high HRV has been associated with healthy cardiac tissue (131,132), whereas low HRV has been correlated with increased risk of lethal cardiac arrhythmias and sudden death (35,133,134). Hence, evaluation of HRV has become an important method for assessing cardiac autonomic regulation. Several techniques have been used to evaluate HRV, but broadly, the measures for HRV can be grouped into 3 categories: 1) time domain measures; 2) frequency domain measures; and 3) nonlinear measures. It should be noted that changes in HRV reflect the effect of the autonomic nervous system on the sinus node, which may not correlate with clinical outcomes of interest, such as ventricular arrhythmias in patients with heart failure (135). Moreover, the dependency of HRV on the underlying heart rate and respiratory rate, as well as its interaction with exercise, diet, and medication use, should also be kept in mind when interpreting HRV data (136).
1A. Time domain measures.
The most straightforward and hence widely accepted techniques to evaluate HRV are based on computation of parameters from the time series of RR intervals. The standard deviation of RR intervals is a measure of long-term HRV and usually computed from 24-h recordings, whereas the root mean square of successive differences of the RR intervals is a common indicator of short-term HRV (125). Additionally, the percentage of consecutive RR intervals that differ by more than 50 ms is also used to gauge short-term HRV (125). Notably, HRV measures, including standard deviation of RR intervals, root mean square of successive differences of the RR intervals, and percentage of consecutive RR intervals that differ by more than 50 ms decrease with age, whereas preservation of autonomic function is associated with healthy longevity (137,138).
1B. Frequency domain measures.
The timedomain measurements of HRV are complemented by a frequency domain analysis using Fast Fourier transform and wavelet analysis, which can quantify the spectral and time-frequency content of HRV. Akselrod et al. (139) were the first to demonstrate that the low-frequency band (0.04 to 0.15 Hz) is related to both sympathetic and parasympathetic modulation, whereas the high-frequency band (0.15 to 0.40 Hz) is governed almost exclusively by parasympathetic effects. Since then, the ratio of low- to high-frequency power is considered a metric of autonomic balance (125). Clinically, frequency domain measures have been used to evaluate sympathovagal balance in various diseases, including sleep apnea (140), heart failure (134), and hypertension (141). Nonetheless, the accuracy of the low- to high-frequency ratio has been recently challenged, due to the fact that the low-frequency content is not a measure of sympathetic activity alone, but a combination of both sympathetic and parasympathetic inputs (128,142).
1C. NonLinear measures.
Given the complex nonlinear interactions between the sympathetic and parasympathetic nervous systems, many nonlinear measures of HRV have been developed to assess autonomic function the past decade (143–145). These measures quantify the degree of information, disorder or complexity of HRV, nonlinear measures such as entropy, detrended fluctuation analysis, and Poincare maps. SD1 and SD2 are measures of short- and long-term variability, respectively, calculated from Poincare maps and based on RR intervals.
Whereas SD1 measures instantaneous beat-to-beat variability and primarily signifies the parasympathetic influence on the heart, SD2 reflects the combined parasympathetic and sympathetic inputs (143). Hence, the ratio (SD1/SD2) denotes a change in autonomic tone and the interplay between both short- and long-term variability in the RR intervals. It has been demonstrated that intermittent VNS can cause an increase in the SD1/SD2 ratio, suggesting that VNS can help restore autonomic balance in heart failure patients (ANTHEM-HF trial) by improving the parasympathetic tone (144). Additionally, Poincare plot analysis of HRV was shown to be useful in risk stratification in patients with dilated cardiomyopathy (145). Approximate entropy was shown to be increased in patients with heart failure (146), indicating a more erratic heart rate and loss of autonomic control compared with healthy control subjects, whereas other complexity measures, such as detrended fluctuation analysis and sample entropy, were shown to successfully detect changes in autonomic drive and assess neural effects of HRV in healthy and spinal cord-injured subjects (147).
2. BAROREFLEX SENSITIVITY.
Baroreflex sensitivity (BRS), a measure of parasympathetic activity, has been traditionally calculated from measurement of the heart rate response to drugs that modify blood pressure (e.g., phenylephrine) (35). Notably, BRS can also be evaluated at the bedside as the slope between spontaneous changes in heart rate and changes in blood pressure, and this method is comparable to BRS derived from drug-induced changes in blood pressure (136). BRS has been shown to predict cardiovascular events in patients with myocardial infarction (35); however, its prognostic value in contemporary patients with heart failure receiving optimal medical therapy has been recently questioned (148).
3. IMAGING MODALITIES.
Direct imaging of cardiac sympathetic innervation can be accomplished by radiolabeled sympathomimetic amines, such as 123- iodine-metaiodobenzylguanidine (123-I MIBG) and 11-carbon-meta-hydroxyephedrine, that are actively taken up by sympathetic terminals (136). Cardiac sympathetic denervation, as assessed by decreased uptake of 123-I MIBG was demonstrated in patients with ventricular tachycardia in the absence of coronary artery disease (149) and predicted ventricular arrhythmias requiring ICD therapy in patients with heart failure (150). Moreover, 123-I MIBG imaging was recently shown to accurately identify the atrial GP, verified by HFS (151). Future studies should test the ability of these techniques to assess autonomic modulation therapies. It should be noted that a major limitation of imaging modalities in the assessment of cardiac autonomic function is that parasympathetic tracers are not yet available.
4. SYMPATHETIC NERVE ACTIVITY MEASUREMENT.
Direct muscle sympathetic nerve activity (MSNA) can be recorded from intraneural microelectrodes inserted percutaneously in a peripheral nerve (microneurography), usually at the level of the peroneal nerve (135,136). Compared with healthy individuals, MSNA is altered in neurological disorders, Parkinson’s disease and multiple systems atrophy, as well as in cardiovascular diseases such as hypertension and heart failure (135). Importantly, increased MSNA in patients with heart failure predicts mortality, whereas an increase in MSNA after cardiac resynchronization therapy differentiates responders from nonresponders in the same patient population (135,152). Although microneurography is a robust and reproducible technique, due its invasiveness and the time-consuming nature of the procedure, MSNA is not used for routine assessment of autonomic function in humans (136).
Recently, Doytchinova et al. (153) developed a novel noninvasive technique to quantify skin sympathetic nerve activity (SKNA) from ECG recordings. The raw signals were low-pass filtered to show the ECG (150 Hz) and high-pass filtered (500 Hz) to show nerve activity (153). SKNA increased in subjects undergoing cold water pressor test and Valsalva maneuver, which are known to increase sympathetic drive (153). Importantly, SKNA correlated well with stellate ganglion activity in ambulatory dogs (154). Using the same technique, increased SKNA has been shown to precede the onset and termination of paroxysmal atrial tachycardia and AF in humans (155), providing insight into the autonomic changes associated with these atrial tachyarrhythmias. Moreover, sustained increases in SKNA were associated with the temporal clustering of AF and spontaneous ventricular tachycardia and VF episodes in humans (156).
5. ALTERNANS.
An upsurge in the magnitude of ventricular repolarization alternans (VRA) has been reported during periods of elevated sympathetic activity in humans (157,158) and in an end-stage heart failure animal model (159). On the other hand, beta-blockers have been reported to reduce the amplitude of VRA (160,161), indicating that VRA can be modulated, at least partially, by sympathetic activity. The possible effect of beta-blockers on VRA is mediated by at least 2 factors: blunting the chronotropic response to exercise, which may prevent some patients from reaching the specific threshold heart rate to develop VRA (162), and directly reducing the magnitude of VRA (160). In basic science studies, VNS caused a decrease in VRA and a small increase in CL at which VF occurred (84).
Based on the notion that P-wave alternans (PWA) is likely to reflect the substrate that predisposes to AF (163), we examined the effect of autonomic modulation (LLTS for 1 h/day) on AF burden and PWA in patients with paroxysmal AF over a 6-month period relative to sham LLTS (164) (Figure 2). A 5-min ECG recording for PWA analysis was performed at each visit (baseline, 3 months, and 6 months). Immediately following the baseline ECG, an additional 5-min ECG was performed during active LLTS in all patients, irrespective of randomization group. We have used a previously described algorithm (165,166) that has been customized for estimation of PWA. In Figure 2A, PWA voltage and Kscore results are shown for a single sham and active patient during control (no stimulation) and LLTS. Delta values were calculated as the difference in mean parameter values at baseline and 3 months or 6 months. Reduction in both PWA voltage and Kscore, attributed to an acute effect of LLTS, is seen in the active patient compared with sham, at both 3 and 6 months. Diminished alternans voltage and Kscore values during control at 6 months compared with control at 3 months indicates a possible positive effect of chronic LLTS in the active patient. In Figure 2B, AF and PWA burden are shown for the same sham and active patients at baseline, 3 months, and 6 months. AF and PWA burden for the sham patient increased over time, whereas active LLTS over 6 months inhibited escalation of both AF and PWA burden.
FIGURE 2. Effect of Chronic Low-Level Tragus Stimulation on P- Wave Alternans (A) and AF Burden (B).
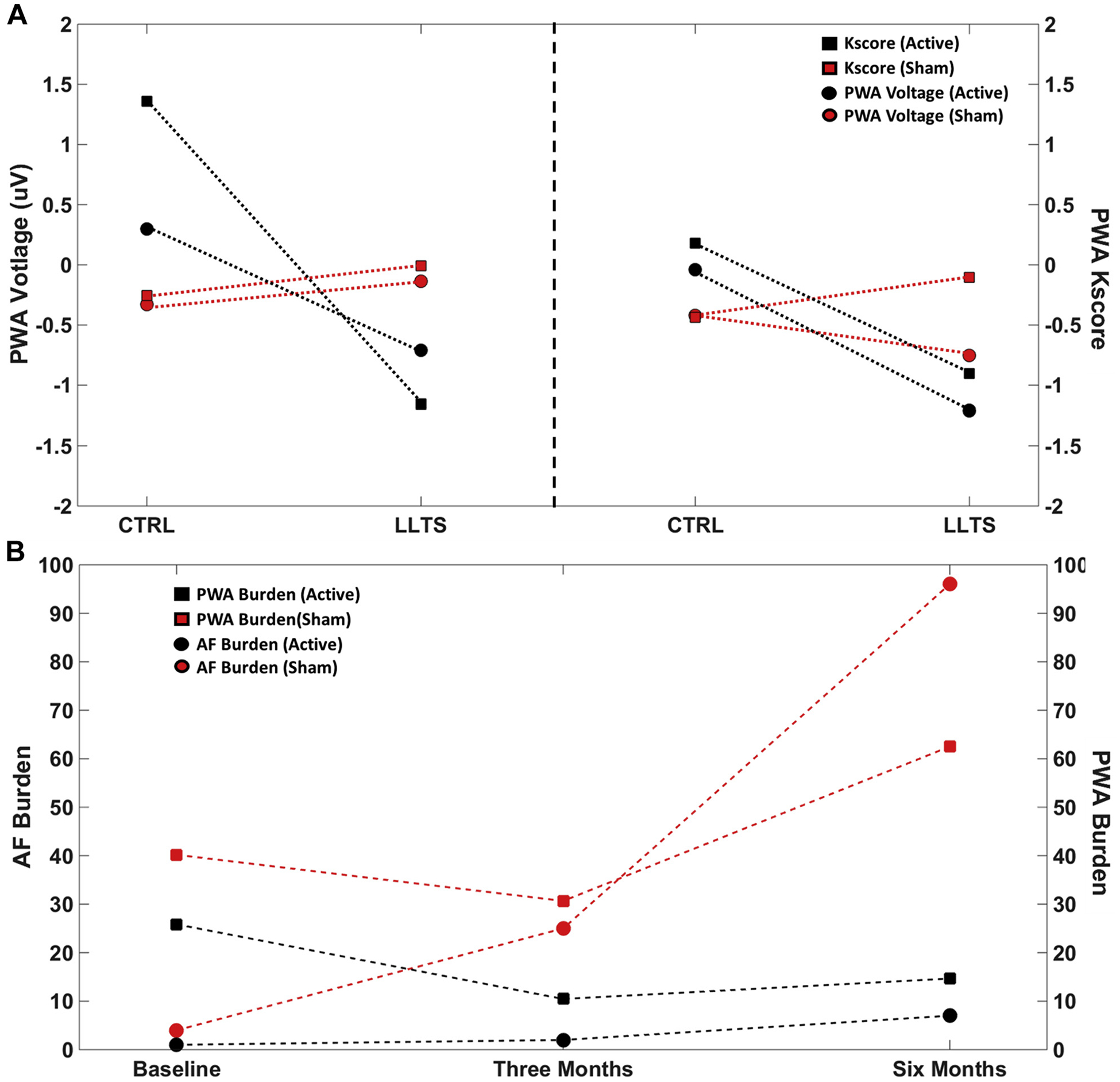
(A) Representative example depicting P-wave alternans (PWA) voltage and Kscore for a single sham and active patient during control (no stimulation) and low-level tragus stimulation (LLTS). Delta values were calculated as difference in mean parameter values at baseline and 3 or 6 months. Reduction in both PWA voltage and Kscore attributed to an acute effect of LLTS is seen in the active patient compared with sham at both 3 and 6 months. (B) Representative example depicting atrial fibrillation (AF) and PWA burden for a single sham and active patient at baseline, 3 months, and 6 months. AF and PWA burden for the sham patient increases with time, whereas active LLTS over 6 months inhibited escalation of both AF and PWA burden.
LIMITATIONS, CHALLENGES, AND FUTURE DIRECTIONS
The Achilles heel of autonomic modulation is that the optimal dosing and stimulation parameters have not been systematically determined. This notion is highlighted by the disappointing results of 2 of the recent trials of VNS in heart failure, despite the favorable preclinical data and the clear rationale for correcting the sympathovagal imbalance in this disease (92). It is thus imperative that future studies focus on defining the optimal stimulation parameters, including frequency and stimulation intensity, to maximize the effects of autonomic modulation therapies.
The use of biomarkers to optimize patient selection as a way to maximize the efficacy of autonomic modulation techniques is very attractive. This is akin to the use of customized, substrate-driven endpoints based on atrial physiology, rather than empiric ablation lesions, to improve outcomes of AF ablation procedures (163). A biomarker able to predict response to autonomic modulation therapy is lacking at present. Muscle sympathetic nerve activity (167), HRV (167,168), TNF-a (55), and global longitudinal strain (168) have been shown to change acutely with tragus stimulation in humans. However, the predictive value of such biomarkers for response to chronic treatment remains to be determined. Recently, the neurotrophic protein S100B, which is released from cardiac glial cells, has been shown to correlate with acute CANS damage in patients undergoing AF ablation (169). Importantly, higher levels of S100B in this patient population predicted sinus rhythm maintenance during follow-up. The role of this biomarker as a predictor of autonomic modulation therapies warrants further investigation.
CONCLUSIONS
The use autonomic modulation to treat cardiac arrhythmias is supported by strong preclinical data and preliminary studies in humans. The failure of large randomized trials to show a beneficial effect in patients with heart failure (INOVATE HF [INcrease Of VAgal TonE in CHF], NECTAR HF [Neural Cardiac TherApy foR Heart Failure], and DEFEAT HF) highlights the need for optimization of the stimulation parameters and rigorous patient selection based on appropriate biomarkers to maximize the efficacy of this novel treatment modality.
HIGHLIGHTS.
The autonomic nervous system plays a central role in the pathogenesis of multiple cardiac arrhythmias, including AF and ventricular tachycardia.
Autonomic modulation therapeutic modalities (such as VNS, tragus stimulation, RDN, BAT, and CSD) have shown promise in early preclinical studies and clinical trials as alternatives to standard arrhythmia treatment.
Various methods of assessment of the autonomic tone, including heart rate variability, SKNA, and alternans, can be used as surrogate markers and predictors of the autonomic modulation treatment effect.
Further research is warranted to optimize patient selection to maximize the efficacy of this novel therapeutic modality for cardiac arrhythmias.
Acknowledgments
This work was supported by a grant-inaid (#15GRNT23070001) and a mentored clinical/population research grant (#15MCPRP2579000) from the American Heart Association; the Institute of Precision Medicine (17UNPG33840017) from the American Heart Association; the RICBAC Foundation; and National Institutes of Health grants 1 R01 HL135335-01, 1 R21 HL137870-01, 1 R21EB026164-01, and 1U54GM10493. Dr. Singh has received consulting honoraria from Biotronik, Boston Scientific, Microport, EBR, Toray, Abbott, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Kalyanam Shivkumar, MD, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- 123-I MIBG
123-iodinemetaiodobenzylguanidine
- AF
atrial fibrillation
- BAT
baroreceptor activation therapy
- BRS
baroreflex sensitivity
- CANS
cardiac autonomic nervous system
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- CSD
cardiac sympathetic denervation
- ECG
electrocardiogram
- GP
ganglionated plexi
- HFS
high-frequency stimulation
- HRV
heart rate variability
- ICD
implantable cardioverter-defibrillator
- LCSD
left cardiac sympathetic denervation
- LLTS
low-level tragus stimulation
- LLVNS
low-level vagus nerve stimulation
- LQTS
long QT syndrome
- MSNA
muscle sympathetic nerve activity
- PV
pulmonary vein
- PVI
pulmonary vein isolation
- PWA
P-wave alternans
- RDN
renal denervation
- SCS
spinal cord stimulation
- SKNA
skin sympathetic nerve activity
- TNF
tumor necrosis factor
- VNS
vagus nerve stimulation
- VRA
ventricular repolarization alternans
REFERENCES
- 1.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014;114:1815–26. [DOI] [PubMed] [Google Scholar]
- 2.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289–98. [DOI] [PubMed] [Google Scholar]
- 3.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000;259:353–82. [DOI] [PubMed] [Google Scholar]
- 4.Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 2004;287:R262–71. [DOI] [PubMed] [Google Scholar]
- 5.Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. J Am Coll Cardiol EP 2015;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 2008;71:1733–8. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith SR. Interactions between the sympathetic nervous system and the RAAS in heart failure. Curr Heart Fail Rep 2004;1:45–50. [DOI] [PubMed] [Google Scholar]
- 8.Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 2016;594:3911–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardell JL, Foreman RD, Armour JA, Shivkumar K. Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation. JCI Insight 2019;4: e131648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Menachem E Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 2002;1: 477–82. [DOI] [PubMed] [Google Scholar]
- 11.Bajbouj M, Merkl A, Schlaepfer TE, et al. Two- year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol 2010;30:273–81. [DOI] [PubMed] [Google Scholar]
- 12.Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 2016;113:8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salavatian S, Beaumont E, Longpre JP, et al. Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. Am J Physiol Heart Circ Physiol 2016;311:H1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes-Osman J, Indahlastari A, Fried PJ, et al. Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain. Front Aging Neurosci 2018;10:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton JL, Burton RAB, Bub G, Smaill BH, Montgomery JM. Synaptic plasticity in cardiac innervation and its potential role in atrial fibrillation. Front Physiol 2018;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaumont E, Southerland EM, Hardwick JC, et al. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 2015;309:H1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2009;2:692–9. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Filiberti A, Humphrey MB, et al. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol 2019;104:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stavrakis S, Scherlag BJ, Fan Y, et al. Inhibition of atrial fibrillation by low-level vagus nerve stimulation: the role of the nitric oxide signaling pathway. J Interv Card Electrophysiol 2013;36: 199–208. [DOI] [PubMed] [Google Scholar]
- 20.Ando M, Katare RG, Kakinuma Y, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation 2005;112:164–70. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624–31. [DOI] [PubMed] [Google Scholar]
- 22.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2þ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol 2006;47:1196–206. [DOI] [PubMed] [Google Scholar]
- 23.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol 2005;45:1878–86. [DOI] [PubMed] [Google Scholar]
- 24.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–9. [DOI] [PubMed] [Google Scholar]
- 25.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 2010; 121:2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Scherlag BJ, Sha Y, et al. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm 2012;9:804–9. [DOI] [PubMed] [Google Scholar]
- 27.Lu Z, Scherlag BJ, Lin J, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 2008;1:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62: 2318–25. [DOI] [PubMed] [Google Scholar]
- 29.Pokushalov E, Romanov A, Katritsis DG, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm 2013;10:1280–6. [DOI] [PubMed] [Google Scholar]
- 30.Garabelli P, Stavrakis S, Kenney JFA, Po SS. Effect of 28-mm cryoballoon ablation on major atrial ganglionated plexi. J Am Coll Cardiol EP 2018;4:831–8. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 2015;116:2005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz PJ, Zaza A, Grazi S, et al. Effect of ventricular fibrillation complicating acute myocardial infarction on long-term prognosis: importance of the site of infarction. Am J Cardiol 1985;56:384–9. [DOI] [PubMed] [Google Scholar]
- 33.Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000;101: 1960–9. [DOI] [PubMed] [Google Scholar]
- 34.Zipes DP. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med 2008;75 Suppl 2:S94–6. [DOI] [PubMed] [Google Scholar]
- 35.La Rovere MT, Bigger JT Jr., Marcus FI, Mortara A, Schwartz PJ, for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998; 351:478–84. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Jung BC, Tan AY, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm 2008;5:131–9. [DOI] [PubMed] [Google Scholar]
- 37.Huang B, Yu L, Scherlag BJ, et al. Left renal nerves stimulation facilitates ischemia-induced ventricular arrhythmia by increasing nerve activity of left stellate ganglion. J Cardiovasc Electrophysiol 2014;25:1249–56. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz PJ, Zaza A, Locati E, Moss AJ. Stress and sudden death. The case of the long QT syndrome. Circulation 1991;83:II71–80. [PubMed] [Google Scholar]
- 39.Liu N, Colombi B, Memmi M, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res 2006; 99:292–8. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009;119:2426–34. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 2001;103: 89–95. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg I, Bradley J, Moss A, et al. Betablocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol 2010;21:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 1997; 273:H805–16. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ilsar I, Sabbah HN, Ben David T, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 2009;6:244–50. [DOI] [PubMed] [Google Scholar]
- 45.Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol 2011; 57:563–71. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Scherlag BJ, Li S, et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol 2011;22:455–63. [DOI] [PubMed] [Google Scholar]
- 47.Sha Y, Scherlag BJ, Yu L, et al. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J Cardiovasc Electrophysiol 2011;22:1147–53. [DOI] [PubMed] [Google Scholar]
- 48.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 2011;123:2204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S, Scherlag BJ, Yu L, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:645–51. [DOI] [PubMed] [Google Scholar]
- 50.Chinda K, Tsai WC, Chan YH, et al. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm 2016;13:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stavrakis S, Humphrey MB, Scherlag B, et al. Low-level vagus nerve stimulation suppresses post-operative atrial fibrillation and inflammation: a randomized study. J Am Coll Cardiol EP 2017;3: 929–38. [DOI] [PubMed] [Google Scholar]
- 52.Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol 2018;36:783–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fallgatter AJ, Neuhauser B, Herrmann MJ, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm 2003;110:1437–43. [DOI] [PubMed] [Google Scholar]
- 54.Yu L, Scherlag BJ, Li S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm 2013;10:428–35. [DOI] [PubMed] [Google Scholar]
- 55.Stavrakis S, Humphrey MB, Scherlag BJ, et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 2015;65:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stavrakis S, Stoner JA, Humphrey MB, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A randomized clinical trial. J Am Coll Cardiol EP 2020;6: 282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deuchars SA, Lall VK, Clancy J, et al. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol 2018;103:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 2015;8:624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verlinden TJ, Rijkers K, Hoogland G, Herrler A. Morphology of the human cervical vagus nerve: implications for vagus nerve stimulation treatment. Acta Neurol Scand 2016;133:173–82. [DOI] [PubMed] [Google Scholar]
- 60.Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol 2014;63:215–24. [DOI] [PubMed] [Google Scholar]
- 61.Linz D, van Hunnik A, Hohl M, et al. Catheterbased renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol 2015;8: 466–74. [DOI] [PubMed] [Google Scholar]
- 62.Linz D, Mahfoud F, Schotten U, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension 2012;60: 172–8. [DOI] [PubMed] [Google Scholar]
- 63.Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–70. [DOI] [PubMed] [Google Scholar]
- 64.Feyz L, Theuns DA, Bhagwandien R, et al. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol 2019;108:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinberg JS, Shabanov V, Ponomarev D, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 2020;323:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brodison A, Chauhan A. Spinal-cord stimulation in management of angina. Lancet 1999;354: 1748–9. [DOI] [PubMed] [Google Scholar]
- 67.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Spinal cord stimulation for chronic reflex sympathetic dystrophy—five-year follow-up. N Engl J Med 2006;354: 2394–6. [DOI] [PubMed] [Google Scholar]
- 68.Ardell JL. Heart failure: mechanisms of spinal cord neuromodulation for heart disease. Nat Rev Cardiol 2016;13:127–8. [DOI] [PubMed] [Google Scholar]
- 69.Bernstein SA, Wong B, Vasquez C, et al. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm 2012;9: 1426–33.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Zhou X, Huang B, et al. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm 2016; 13:274–81. [DOI] [PubMed] [Google Scholar]
- 71.Yu L, Huang B, He W, et al. Spinal cord stimulation suppresses focal rapid firing-induced atrial fibrillation by inhibiting atrial ganglionated plexus activity. J Cardiovasc Pharmacol 2014;64:554–9. [DOI] [PubMed] [Google Scholar]
- 72.Carlsten A, Folkow B, Grimby G, Hamberger CA, Thulesius O. Cardiovascular effects of direct stimulation of the carotid sinus nerve in man. Acta Physiol Scand 1958;44:138–45. [DOI] [PubMed] [Google Scholar]
- 73.Scheffers IJ, Kroon AA, de Leeuw PW. Carotid baroreflex activation: past, present, and future. Curr Hypertens Rep 2010;12:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohaupt MG, Schmidli J, Luft FC. Management of uncontrollable hypertension with a carotid sinus stimulation device. Hypertension 2007; 50:825–8. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz SI, Griffith LS, Neistadt A, Hagfors N. Chronic carotid sinus nerve stimulation in the treatment of essential hypertension. Am J Surg 1967;114:5–15. [DOI] [PubMed] [Google Scholar]
- 76.Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 2010;55:619–26. [DOI] [PubMed] [Google Scholar]
- 77.Linz D, Mahfoud F, Schotten U, et al. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol 2013;24:1028–33. [DOI] [PubMed] [Google Scholar]
- 78.Liao K, Yu L, Zhou X, et al. Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can J Cardiol 2015;31:767–74. [DOI] [PubMed] [Google Scholar]
- 79.Dai M, Bao M, Liao J, et al. Effects of low-level carotid baroreflex stimulation on atrial electrophysiology. J Interv Card Electrophysiol 2015;43: 111–9. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation 1984;69:790–800. [DOI] [PubMed] [Google Scholar]
- 81.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 2006;3:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res 1990;67:615–27. [DOI] [PubMed] [Google Scholar]
- 83.Martins JB, Zipes DP, Lund DD. Distribution of local repolarization changes produced by efferent vagal stimulation in the canine ventricles. J Am Coll Cardiol 1983;2:1191–9. [DOI] [PubMed] [Google Scholar]
- 84.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 2007;73:750–60. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z, Yu L, Wang S, et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail 2014;7:1014–21. [DOI] [PubMed] [Google Scholar]
- 86.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr., Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991;68:1471–81. [DOI] [PubMed] [Google Scholar]
- 87.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011;32:847–55. [DOI] [PubMed] [Google Scholar]
- 88.Ardell JL, Nier H, Hammer M, et al. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol 2017;595:6887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gold MR, Van Veldhuisen DJ, Hauptman PJ, et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol 2016;68:149–58. [DOI] [PubMed] [Google Scholar]
- 90.Zannad F, De Ferrari GM, Tuinenburg AE, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTARHF) randomized controlled trial. Eur Heart J 2015; 36:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Premchand RK, Sharma K, Mittal S, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 2014;20:808–16. [DOI] [PubMed] [Google Scholar]
- 92.Byku M, Mann DL. Neuromodulation of the failing heart: lost in translation? J Am Coll Cardiol Basic Trans Science 2016;1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindholm EE, Aune E, Seljeflot I, Otterstad JE, Kirkeboen KA. Biomarkers of inflammation in major vascular surgery: a prospective randomised trial. Acta Anaesthesiol Scand 2015;59:773–87. [DOI] [PubMed] [Google Scholar]
- 94.Ben-Menachem E Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol 2001;18:415–8. [DOI] [PubMed] [Google Scholar]
- 95.Spuck S, Tronnier V, Orosz I, et al. Operative and technical complications of vagus nerve stimulator implantation. Neurosurgery 2010;67: 489–94. [DOI] [PubMed] [Google Scholar]
- 96.Yu L, Wang S, Zhou X, et al. Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. J Am Coll Cardiol EP 2016;2:330–9. [DOI] [PubMed] [Google Scholar]
- 97.Yu L, Huang B, Po SS, et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of concept study. J Am Coll Cardiol Intv 2017;10: 1511–20. [DOI] [PubMed] [Google Scholar]
- 98.Bohm M, Ewen S, Linz D, et al. Therapeutic potential of renal sympathetic denervation in patients with chronic heart failure. Euro-Intervention 2013;9 Suppl R:R122–6. [DOI] [PubMed] [Google Scholar]
- 99.Hopper I, Gronda E, Hoppe UC, et al. Sympathetic response and outcomes following renal denervation in patients with chronic heart failure: 12-month outcomes from the Symplicity HF feasibility study. J Card Fail 2017;23:702–7. [DOI] [PubMed] [Google Scholar]
- 100.Remo BF, Preminger M, Bradfield J, et al. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm 2014; 11:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradfield JS, Hayase J, Liu K, et al. Renal denervation as adjunctive therapy to cardiac sympathetic denervation for ablation refractory ventricular tachycardia. Heart Rhythm 2020;17: 220–7. [DOI] [PubMed] [Google Scholar]
- 102.Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine post-infarction heart failure model. Circulation 2009; 120:286–94. [DOI] [PubMed] [Google Scholar]
- 103.Issa ZF, Zhou X, Ujhelyi MR, et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation 2005;111: 3217–20. [DOI] [PubMed] [Google Scholar]
- 104.Southerland EM, Milhorn DM, Foreman RD, et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 2007;292: H311–7. [DOI] [PubMed] [Google Scholar]
- 105.Grimaldi R, de Luca A, Kornet L, Castagno D, Gaita F. Can spinal cord stimulation reduce ventricular arrhythmias? Heart Rhythm 2012;9: 1884–7. [DOI] [PubMed] [Google Scholar]
- 106.Tse HF, Turner S, Sanders P, et al. Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): first-inman experience. Heart Rhythm 2015;12:588–95. [DOI] [PubMed] [Google Scholar]
- 107.Zipes DP, Neuzil P, Theres H, et al. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT-HF study. J Am Coll Cardiol HF 2016;4:129–36. [DOI] [PubMed] [Google Scholar]
- 108.Zucker IH, Hackley JF, Cornish KG, et al. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 2007;50:904–10. [DOI] [PubMed] [Google Scholar]
- 109.Sabbah HN, Gupta RC, Imai M, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail 2011;4: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liao K, Yu L, He B, et al. Carotid baroreceptor stimulation prevents arrhythmias induced by acute myocardial infarction through autonomic modulation. J Cardiovasc Pharmacol 2014;64:431–7. [DOI] [PubMed] [Google Scholar]
- 111.Liao K, Yu L, Yang K, et al. Low-level carotid baroreceptor stimulation suppresses ventricular arrhythmias during acute ischemia. PLoS One 2014;9:e109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, Lovett EG, Mancia G, Grassi G. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail 2014;16:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abraham WT, Zile MR, Weaver FA, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. J Am Coll Cardiol HF 2015;3:487–96. [DOI] [PubMed] [Google Scholar]
- 114.Zile MR, Abraham WT, Lindenfeld J, et al. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: BeAT-HF. Am Heart J 2018;204:139–50. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation 1991;84:503–11. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 2004;109:1826–33. [DOI] [PubMed] [Google Scholar]
- 117.Priori SG, Wilde AA, Horie M, et al. HRS/ EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 118.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 2008;358:2024–9. [DOI] [PubMed] [Google Scholar]
- 119.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm 2009;6:752–9. [DOI] [PubMed] [Google Scholar]
- 120.Vaseghi M, Barwad P, Malavassi Corrales FJ, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol 2017;69:3070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Richardson T, Lugo R, Saavedra P, et al. Cardiac sympathectomy for the management of ventricular arrhythmias refractory to catheter ablation. Heart Rhythm 2018;15:56–62. [DOI] [PubMed] [Google Scholar]
- 122.Okada DR, Assis FR, Gilotra NA, et al. Cardiac sympathectomy for refractory ventricular arrhythmias in cardiac sarcoidosis. Heart Rhythm 2019;16:1408–13. [DOI] [PubMed] [Google Scholar]
- 123.Shah R, Assis F, Alugubelli N, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: a systematic review. Heart Rhythm 2019;16: 1499–505. [DOI] [PubMed] [Google Scholar]
- 124.Bourke T, Vaseghi M, Michowitz Y, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 2010;121:2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93:1043–65. [PubMed] [Google Scholar]
- 126.Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol 2003;285: R927–31. [DOI] [PubMed] [Google Scholar]
- 127.Schwab JO, Eichner G, Schmitt H, Weber S, Coch M, Waldecker B. The relative contribution of the sinus and AV node to heart rate variability. Heart 2003;89:337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 2014;5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleiger RE, Stein PK, Bigger JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J Auton Nerv Syst 1985; 12:251–9. [DOI] [PubMed] [Google Scholar]
- 131.Jensen-Urstad K, Storck N, Bouvier F, Ericson M, Lindblad LE, Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol Scand 1997;160:235–41. [DOI] [PubMed] [Google Scholar]
- 132.Goldberger AL. Is the normal heartbeat chaotic or homeostatic? News Physiol Sci 1991;6: 87–91. [DOI] [PubMed] [Google Scholar]
- 133.Tsuji H, Larson MG, Venditti FJ Jr., et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–5. [DOI] [PubMed] [Google Scholar]
- 134.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003;107:565–70. [DOI] [PubMed] [Google Scholar]
- 135.Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous system dysfunction: JACC focus seminar. J Am Coll Cardiol 2019;73:1189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bernardi L, Spallone V, Stevens M, et al. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 2011;27:654–64. [DOI] [PubMed] [Google Scholar]
- 137.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 1998; 31:593–601. [DOI] [PubMed] [Google Scholar]
- 138.Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol 2010;105:1181–5. [DOI] [PubMed] [Google Scholar]
- 139.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981; 213:220–2. [DOI] [PubMed] [Google Scholar]
- 140.Vanninen E, Tuunainen A, Kansanen M, Uusitupa M, Lansimies E. Cardiac sympathovagal balance during sleep apnea episodes. Clin Physiol 1996;16:209–16. [DOI] [PubMed] [Google Scholar]
- 141.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl 1984;2: S383–5. [PubMed] [Google Scholar]
- 142.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng 2001;48:1342–7. [DOI] [PubMed] [Google Scholar]
- 144.Libbus I, Nearing BD, Amurthur B, KenKnight BH, Verrier RL. Quantitative evaluation of heartbeat interval time series using Poincare analysis reveals distinct patterns of heart rate dynamics during cycles of vagus nerve stimulation in patients with heart failure. J Electrocardiol 2017;50:898–903. [DOI] [PubMed] [Google Scholar]
- 145.Voss A, Fischer C, Schroeder R, Figulla HR, Goernig M. Segmented Poincare plot analysis for risk stratification in patients with dilated cardiomyopathy. Methods Inf Med 2010;49:511–5. [DOI] [PubMed] [Google Scholar]
- 146.Beckers F, Ramaekers D, Aubert AE. Approximate entropy of heart rate variability: validation of methods and application in heart failure. Cardiovascular Engineering: An International Journal 2001;1:177–82. [Google Scholar]
- 147.Merati G, Di Rienzo M, Parati G, Veicsteinas A, Castiglioni P. Assessment of the autonomic control of heart rate variability in healthy and spinal-cord injured subjects: contribution of different complexity-based estimators. IEEE Transactions on Biomedical Engineering 2005;53:43–52. [DOI] [PubMed] [Google Scholar]
- 148.Paleczny B, Olesinska-Mader M, Siennicka A, et al. Assessment of baroreflex sensitivity has no prognostic value in contemporary, optimally managed patients with mild-to-moderate heart failure with reduced ejection fraction: a retrospective analysis of 5-year survival. Eur J Heart Fail 2019;21:50–8. [DOI] [PubMed] [Google Scholar]
- 149.Mitrani RD, Klein LS, Miles WM, et al. Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease. J Am Coll Cardiol 1993; 22:1344–53. [DOI] [PubMed] [Google Scholar]
- 150.Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 2010;55:2769–77. [DOI] [PubMed] [Google Scholar]
- 151.Stirrup J, Gregg S, Baavour R, et al. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: Technique and validation in patients with atrial fibrillation. J Nucl Cardiol 2019. January 29 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 152.Najem B, Unger P, Preumont N, et al. Sympathetic control after cardiac resynchronization therapy: responders versus nonresponders. Am J Physiol Heart Circ Physiol 2006;291:H2647–52. [DOI] [PubMed] [Google Scholar]
- 153.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm 2015;12:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Uradu A, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 2017;14:964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kusayama T, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight 2019; 4:e125853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lampert R, Shusterman V, Burg MM, et al. Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol 2005;16:372–7. [DOI] [PubMed] [Google Scholar]
- 158.Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol 2004;19:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shusterman V, McTiernan CF, Goldberg A, Saba S, Salama G, London B. Adrenergic stimulation promotes T-wave alternans and arrhythmia inducibility in a TNF-alpha genetic mouse model of congestive heart failure. Am J Physiol Heart Circ Physiol 2010;298:H440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Klingenheben T, Gronefeld G, Li YG, Hohnloser SH. Effect of metoprolol and d,l-sotalol on microvolt-level T-wave alternans. Results of a prospective, double-blind, randomized study. J Am Coll Cardiol 2001;38:2013–9. [DOI] [PubMed] [Google Scholar]
- 161.Rashba EJ, Cooklin M, MacMurdy K, et al. Effects of selective autonomic blockade on T-wave alternans in humans. Circulation 2002;105: 837–42. [DOI] [PubMed] [Google Scholar]
- 162.Tapanainen JM, Still AM, Airaksinen KE, Huikuri HV. Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: results of a prospective follow-up study. J Cardiovasc Electrophysiol 2001;12:645–52. [DOI] [PubMed] [Google Scholar]
- 163.Katritsis D, Merchant FM, Mela T, Singh JP, Heist EK, Armoundas AA. Catheter ablation of atrial fibrillation the search for substrate-driven end points. J Am Coll Cardiol 2010;55:2293–8. [DOI] [PubMed] [Google Scholar]
- 164.Stavrakis S, Stoner JA, Humphrey MB, Scherlag BJ, Jackman WM, Po SS. Transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation (TREAT AF): a randomized clinical trial. Paper presented at: Heart Rhythm Society Annual Scientific Sessions; May 8–11, 2019; San Francisco, CA. [Google Scholar]
- 165.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–41. [DOI] [PubMed] [Google Scholar]
- 166.Smith JM, Rosenbaum DS, Cohen RJ. Variability in surface ECG morphology: signal or noise? Comput Cardiol 1988;14:257–60. [PubMed] [Google Scholar]
- 167.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014;7: 871–7. [DOI] [PubMed] [Google Scholar]
- 168.Tran N, Asad Z, Elkholey K, Scherlag BJ, Po SS, Stavrakis S. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res 2019;12:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scherschel K, Hedenus K, Jungen C, et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med 2019;11:eaav7770. [DOI] [PubMed] [Google Scholar]


