Summary
Type 2 immunity against pathogens is tightly regulated to ensure appropriate inflammatory responses that clear infection and prevent excessive tissue damage. Recent research has shown that type 2 innate lymphoid cells (ILC2s) contribute to steady-state tissue integrity and exert tissue-specific functions. However, upon exposure to inflammatory stimuli, they also initiate and amplify type 2 inflammation by inducing mucus production, eosinophilia, and Th2 differentiation. In this review, we discuss the regulation of ILC2 activation by transcription factors and metabolic pathways, as well as by extrinsic signals such as cytokines, lipid mediators, hormones, and neuropeptides. We also review recent discoveries about ILC2 plasticity and heterogeneity in different tissues, as revealed partly through single-cell RNA sequencing of transcriptional responses to various stimuli. Understanding the tissue-specific pathways that regulate ILC2 diversity and function is a critical step in the development of potential therapies for allergic diseases.
Keywords: disease resolution, innate immunity, innate lymphoid cells, mucosal immunology, neuroimmunology, type 2 inflammation
1 |. DISCOVERY AND FUNCTION OF ILC SUBSETS
Mucosal surfaces are exposed to an array of environmental stimuli, some of which are beneficial, such as commensal microbes or nutrients present in food, while others are potentially harmful, such as pathogenic bacteria, helminths, viruses, allergens, and other noxious substances. The resident innate immune system therefore constantly monitors mucosal environments to both promote epithelial barrier function and rapidly recognize potential pathogens. Recently, an innate immune cell type, termed innate lymphoid cells (ILCs), was discovered in mice and humans, and shown to play crucial roles in both tissue homeostasis and the coordination and amplification of innate immune responses.1–3
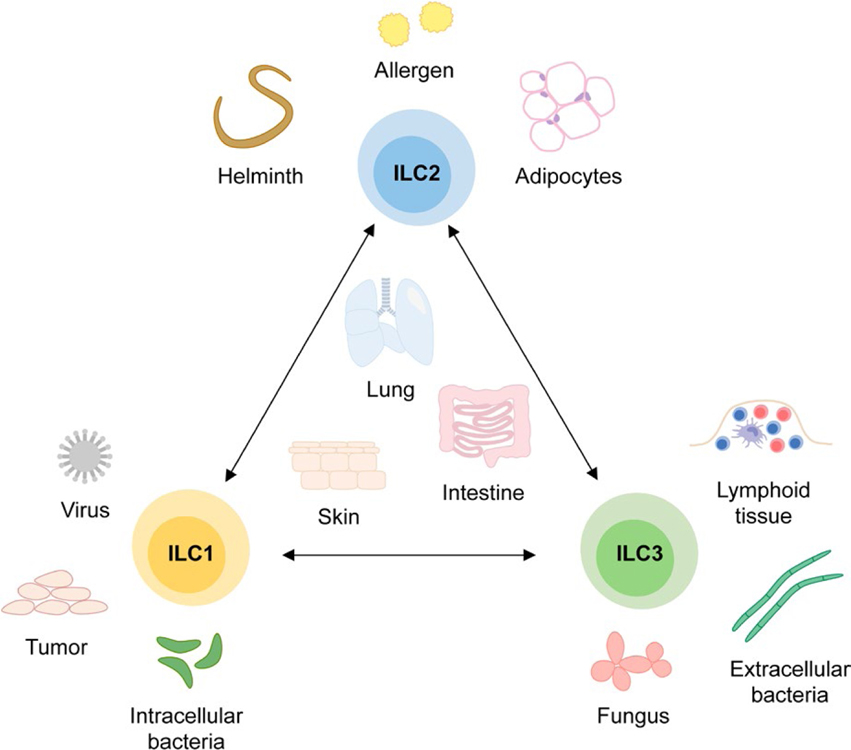
While ILCs develop from a common lymphoid progenitor and express the receptor for interleukin (IL) 7 (CD127), ILCs lack expression of common lineage markers, including those typical of T cells.1,4 In contrast to T cells, which express antigen-specific receptors, ILCs instead recognize tissue damage via inflammatory cytokines and mediators released by different cell types in response to stress or injury (Figure 1).1,5–7 Because they are similar to T-helper cells in terms of key transcription factors and effector cytokines, ILCs have been classified analogously into T-bet+ type 1 ILCs, GATA3+ type 2 ILCs, and RORγt+ type 3 ILCs.8,9 Type 1 ILCs, which consist of conventional NK cells and less cytotoxic ILC1s, produce IFNγ and are implicated in tumor and viral immunity, as well as the defense of intracellular pathogens.1,10 Type 2 ILCs, or ILC2s, produce IL-5 and IL-13 and are crucial for the expulsion of helminths, while also promoting the development of allergic inflammation.6,11,12 Type 3 ILCs, which include lymphoid tissue inducer cells (LTi cells)13,14 and ILC3s15–17, produce IL-17 and IL-22, and play a critical role in defense against extracellular bacteria and control of fungal infections.17–19
FIGURE 1.
Innate lymphoid cell (ILC) subsets and functions. ILCs consist of three subsets that play important roles in the immune response against distinct types of pathogens. While ILC1s are implicated in immunity against viral infections and intracellular bacteria as well as anti-tumor responses, ILC3s contribute to the development of lymphoid tissue and the defense of fungal infections and extracellular bacteria. ILC2s, however, are critical for the expulsion of helminths and play a protective role in the development of obesity but also contribute to the development of allergic inflammation. Importantly, this separation into different subsets is not stringent as each ILC subset can acquire phenotypic and functional features of other ILC subsets in response to the environment
Although ILCs are predominantly found at mucosal barriers, including the lung20,21, gut21–23, skin24–29 and uterus30, they are also present in other tissues, such as adipose tissue5,31–33, liver34 and meninges35–37. The relative proportions of different ILC types are distinct in each tissue, likely reflecting the unique homeostatic requirements of the tissue. In settings of inflammation and tissue damage, however, ILCs demonstrate a degree of plasticity, whereby ILCs of one subset preferentially expand,38 or partially adopt the phenotype of another subset (eg, ILC2s can become ILC1-like or ILC3-like cells).39–44 ILC plasticity may support a rapid, flexible immune response and promote tissue integrity, but it could also conceivably lead to alterations in tissue homeostasis, resulting in persistent organ dysfunction. By understanding the tissue-based cues that regulate ILC function, we can better assess the changes in ILCs that play a role in chronic disease.
While all three types of ILCs contribute to tissue homeostasis, for the purposes of this review, we focus on ILC2s, which play both protective and pathogenic roles in type 2 inflammatory responses in the lung and intestine. ILC2s were the first tissue-resident ILC subset to be described: a population of cells producing type 2 cytokines was noted in mice that lack T and B cells in studies examining the function of the cytokine IL-25.45–47 However, the cells were not further phenotypically characterized, and no function was shown. In 2010, several seminal studies identified ILC2s as critical for mounting an innate type 2 immune response against helminths.5,6,11 ILC2s are largely tissue-resident cells that do not migrate between organs,48,49 except for a subpopulation of IL-17-producing ILC2s, called “inflammatory ILC2s”, that appear to migrate from the gut to other organs during helminth infection.44,50
The tissue microenvironment is shaped by interactions between multiple cell types, including stromal cells, epithelial cells, immune cells, and neurons, as well as by the presence of cytokines, inflammatory mediators, and environmental exposures. Recent evidence has highlighted that ILC2s integrate both pro- and anti-inflammatory signals to promote host defense and epithelial barrier function.6,20 Moreover, although all ILC2s apparently arise from the same precursor and produce the signature type 2 cytokines IL-5 and IL-13,51 ILC2s from different tissues can differ significantly in terms of surface markers, resting transcriptional state, and response to certain stimuli.35,38,50,52,53 This suggests that the tissue context influences the function of ILC2s, which in turn adapt themselves to tissue-specific homeostatic requirements. Below, we highlight the signals that alter ILC2 function and phenotype, both at steady state and during inflammatory responses, and discuss the diversity and plasticity of ILC2s under homeostatic and inflammatory conditions in different tissues.
2 |. ACTIVATION OF ILC2S BY EXTRINSIC SIGNALS
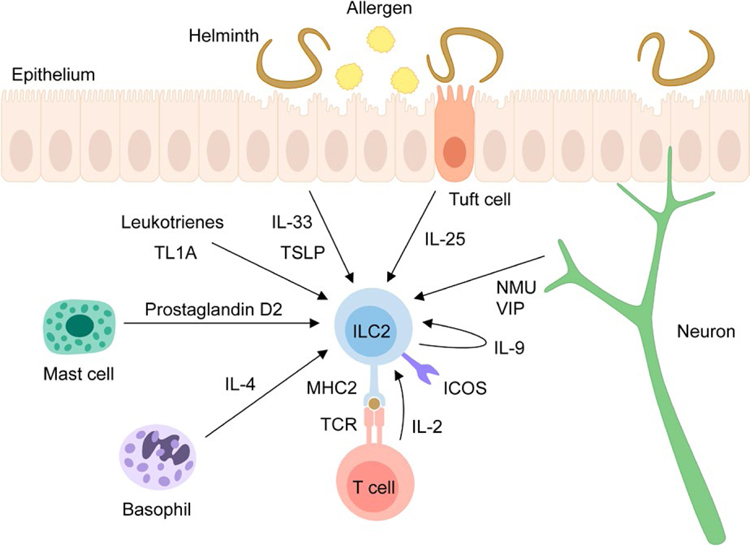
ILC2s are activated by the cytokines IL-33, IL-25, and thymic stromal lymphopoietin (TSLP), so-called alarmins, which are released into the local tissue environment by epithelial or other cells in response to cellular stress, damage or nonprogrammed cell death.54,55 In addition to alarmins, other mediators, such as neuropeptides and lipid mediators, and co-stimulatory molecules, including ICOS, can also activate ILC2s, and will be discussed in detail below (Figure 2).
FIGURE 2.
Pathways controlling ILC2 activation. ILC2s are activated by different cytokines, such as the alarmin cytokines IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) as well as IL-2, IL-4, IL-9 and TL1A. While IL-33 and TSLP are released by epithelial cells in response to stress or damage, IL-25 is produced by tuft cells, which undergo expansion during helminth infection. IL-2 is released by Th2 cells upon interaction with ILC2s via the TCR and MHC2 complex and IL-4 is produced by basophils whereas IL-9 can be produced by ILC2s themselves. Lipid mediators such as leukotrienes and mast cell-derived prostaglandin D2, as well as the neuropeptides Neuromedin U (NMU) and vasoactive intestinal peptide (VIP) play also important roles in the activation of ILC2s. Surface molecules including ICOS also promote ILC2 responses upon interaction with ICOS-L and expression of MHC2 enables the interaction with T cells, which subsequently activate ILC2s via production of IL-2
2.1 |. Activation of ILC2s by alarmins
IL-33 belongs to the IL-1 cytokine family and is localized at steady state in the nucleus of epithelial cells and other cell types, including endothelial and fibroblastic reticular cells.54 Upon release, IL-33 binds to its receptor ST2 (Il1rl1) and, in conjunction with the IL-1 receptor accessory protein (IL-1RAcP), activates intracellular signaling pathways that promote type 2 immune responses.54 In murine and human ILC2s in vitro, IL-33 signaling induces ILC2 expansion and promotes production of the type 2 cytokines IL-5 and IL-13.5,6,56–58 IL-33 is also a potent activator of ILC2s in vivo, as IL-33 promotes ILC2 responses in the liver in a mouse model of hepatic fibrosis,34 causes lung inflammation upon nasal administration,59 and induces atopic dermatitis in mice, with elevated frequencies of IL-5- and IL-13-producing ILC2s and eosinophilia.27
The alarmin IL-25 (IL-17E), expressed by epithelial cells and leukocytes, is part of the IL-17 family, with IL-17RA and IL-17RB forming the IL-25 receptor.60,61 Binding of IL-25 activates different signaling pathways, including that of the nuclear factor NF-κB.60,61 By promoting expression of the type 2 cytokines IL-4, IL-5, and IL-13 in vivo, IL-25 elicits eosinophilia, mucus production, and elevated serum levels of IgA, IgE, and IgG1.45,46 In the absence of IL-25, mice are less efficient in clearing the helminth Nippostrongylus brasiliensis (N. brasiliensis), indicating that endogenous IL-25 is important for rapid worm expulsion.47 Recently, tuft cells, an epithelial chemosensory cell type, were identified as the major IL-25 producers during helminth infection and following colonization with protozoa.62–64 Using IL-25-reporter mice, von Moltke et al62 also demonstrated that during N. brasiliensis infection, there is substantial expansion of tuft cells, which is reciprocally dependent on IL-13 produced by ILC2s. Indeed, IL-13 directly induces tuft cell hyperplasia, as shown by the increased frequency of tuft cells in epithelial organoid cultures in response to IL-4 or IL-13 stimulation.62–64 Other helminths, such as Heligmosomoides polygyrus and Trichinella spiralis, as well as the protozoa Tritrichomonas muris also promote tuft cell amplification.64
While IL-33 and IL-25 both activate ILC2s and promote type 2 cytokine production,56 these cytokines vary in their efficiency in inducing specific responses or expanding distinct cell populations. In the skin, both IL-33 and IL-25, as well as thymic stromal lymphopoietin (TSLP), were shown to be important for inducing ILC2 responses and inflammation, using a mouse model of atopic dermatitis induced by calcipotriol (MC 903).24,26 In a mouse model of ovalbumin (OVA)-induced airway inflammation, Il1rl1-deficient mice developed significantly less airway hyperreactivity in response to methacholine challenge than wildtype (WT) mice.65 In contrast, the airway hyper-reactivity of Il17rb-deficient mice was only slightly decreased compared to WT mice, suggesting that IL-33 signaling contributes more to the development of allergen-induced airway inflammation than IL-25 signaling.65 In accordance with those observations, more IL-13 expressing cells were detectable in the lungs of mice treated with IL-33 compared to IL-25.65
Moreover, it was recently shown that IL-33 and IL-25 promote the expansion of two distinct lung ILC2 populations, termed natural ILC2s (nILC2s) and inflammatory ILC2s (iILC2s), respectively.44 Both populations are lineage negative and positive for IL-7Rα, but nILC2s are ST2+ Thy1hi KLRG1int IL-17RBlo, while iILC2s are ST2− Thy1lo KLRG1hi IL-17RBhi and capable of jointly producing the cytokines IL-17 and IL-13, as well as participating in immune responses against the fungus Candida albicans.43,44 In vitro, iILCs develop into ST2+ cells with a surface phenotype similar to nILC2s and produce IL-5 and IL-13 after stimulation with IL-33 but not IL-25.44 In vivo, these cells also convert into ST2+ nILC2-like cells after adoptive transfer into RAG2−/−γc −/− mice and are able to reduce worm burden during N. brasiliensis infection.44 While these data point to the possibility that iILC2s may be a progenitor population of ILC2s that contributes to clearance of helminth infection, iILC2s could also represent an induced ILC3-like ILC2 population that reverts to a more classical type 2 phenotype without appropriate signaling. The latter scenario is consistent with the finding that Notch signaling is required for the emergence of iILC2s and induces the conversion of mature nILC2s into iILC2s, apparently by upregulating Il17rb mRNA expression and downregulating Il1rl1 mRNA expression.43
Subsequent to the identification of iILC2s, Huang et al50 used parabiotic mice to show that iILC2 progenitors are located in the intestinal lamina propria at steady state. Upon intraperitoneal injection of IL-25 or infection with N. brasiliensis, these progenitors give rise to proliferating iILC2s that migrate to other organs, including the lung and liver. In contrast, nILC2s do not circulate in response to IL-33. The authors observed that treatment with the inhibitor FTY720 prevented IL-25-induced migration of iILC2s, suggesting that their migration depends on chemotaxis mediated by sphingosine 1-phosphate (S1P). Consistent with this, the S1P receptors S1PR1 and S1PR4 were detected on IL-25-induced iILC2s. The study further shows that RAG1−/− mice were unable to contain N. brasiliensis infection when lymphocyte migration was inhibited by FTY720 treatment and succumbed to worm infection. However, adoptive transfer of iILC2s into these mice before FTY720 administration significantly increased the survival rate, suggesting that circulating iILC2s from the intestine play an important role in N. brasiliensis expulsion in the absence of adaptive immunity.50
Using massively parallel droplet-based single-cell RNA sequencing (scRNA-seq), we recently characterized the transcriptional landscape of lung-resident ILCs, both at steady state and in response to the alarmins IL-25 and IL-33.38 While both IL-25 and IL-33 upregulated production of type 2 effector cytokines in ILC2s, they also induced subpopulations with distinct profiles (discussed further below).38 For example, IL-33 induced a much more proliferative response, consistent with previous findings.38,56,57 We also identified a population of primarily IL-25 induced cells that were phenotypically similar to iILC2s, eg, expressing very high levels of Klrg1 but only low levels of Il1rl1.38 However, this population represented a minority of all IL-25-activated ILC2s,38 in contrast to other reports,44,50 apparently due to differing routes of IL-25 administration, as intranasal administration induces a much smaller population of iILC2s than intraperitoneal administration.50
The alarmin cytokine TSLP promotes type 2 inflammation, both by activating mouse and human ILC2 responses24,58 and by regulating the function of CD4 T cells, dendritic cells, basophils and mast cells.66 TSLP is expressed by epithelial cells and keratinocytes during inflammation and binds to a heterodimeric receptor consisting of the TSLP receptor chain and IL-7Rα.58,66,67 TSLP signaling phosphorylates signal transducer and activator of transcription 5 (STAT5) and induces expression of GATA3, the key transcription factor of ILC2s and Th2 cells.58 In vitro, human ILC2s isolated from blood or nasal polyps secreted type 2 cytokines in response to IL-2 and TSLP, though at lower levels than in response to the combination of IL-2 and IL-33.58 The combination of IL-2, TSLP, and IL-33 synergistically stimulated high levels of type 2 cytokine production.58 In mice, TSLP is sufficient to activate ILC2s in the skin, as demonstrated by intravenous injection of a TSLP-encoding cDNA plasmid, but TSLP induced fewer ILC2s in skin-draining lymph nodes (LNs) from IL-33-deficient mice than it did in WT mice, indicating again that IL-33 and TSLP synergize to activate ILC2s in vivo.24 Slightly contradictory findings were reported regarding the role of TSLP in a model of calcipotriol-induced atopic dermatitis-like skin inflammation. In this mouse model, ILCs contribute significantly to the development of skin inflammation, demonstrated by the fact that RAG1−/− mice treated with anti-CD25 or anti-CD90.2 to deplete ILCs develop less skin inflammation than isotype control-treated mice.24 One study reported that TSLPR-deficient mice, but not IL-33-deficient or IL-17RB-deficient mice, had lower frequencies of ILC2s in skin-draining LNs and a reduced severity of dermatitis compared to C57BL/6 wildtype mice, suggesting that activation of ILC2s in this model requires TSLP but not IL-33 or IL-25.24 However, another study reported that in Balb/c mice, IL-33 and IL-25 signaling contributed more than TSLP to the ILC2 response during skin inflammation in this model, and noted that in their hands IL-33 and IL-25 also seem to be important for the development of skin inflammation in C57BL/6 mice.26
In summary, IL-25 appears to preferentially promote intestinal ILC2 responses, while IL-33 promotes lung ILC2 responses and TSLP seems to facilitate skin ILC2 responses. There is clearly overlap, however, as recent data indicate that all three alarmins appear to have partially redundant roles in the induction of an immune response to N. brasiliensis infection.68 While WT mice are able to clear intestinal worms by day 10 after infection, a high worm burden is still detectable in ST2/IL-25/TSLPR-triple deficient KO mice.68 Mice singly deficient in ST2, IL-25 or TSLPR have persistent intestinal worms at day 10, but fewer than in the triple KO, indicating that the cytokines can compensate for the loss of each other to some extent.68 IL-33 and IL-25 signaling seemed to contribute more than TSLP signaling to worm clearance, supporting the idea that these alarmins are critical for lung and intestinal ILC2 activation.68 However, another study reported that after respiratory syncytial virus infection, lung-resident ILC2 responses were dependent on TSLP,69 suggesting that the type of pathogen, in addition to the tissue environment, influences the dependence of ILC2 activation on specific cytokines.
2.2 |. Activation of ILC2s by other cytokines
In addition to IL-33, IL-25 and TSLP, other cytokines also play important roles in regulating ILC2 responses. ILC2s express IL-2Rα (CD25), suggesting a role for IL-2 in their function.25,70 Treatment of RAG1−/− mice with IL-2 promotes ILC2 expansion, resulting in greatly elevated IL-5 and IL-13 levels in the lungs and skin and the concomitant development of type 2 inflammation in both tissues.25,70 IL-2 does not appear to directly induce IL-5 or IL-13 production, but rather, in combination with IL-33, it results in higher frequencies of IL-13-positive ILC2s and enhances IL-13 production on a per cell basis70, suggesting that IL-2 both directly induces ILC2 proliferation and functions as a co-stimulatory signal that promotes cytokine production by ILC2s.
IL-4 also promotes ILC2 responses in type 2 inflammation. In distinct mouse models of atopic dermatitis-like skin inflammation and papain-induced allergic airway inflammation, basophil-derived IL-4 is required for the expansion of ILC2s and development of type 2 inflammation in the skin or lung, respectively.71,72 In addition, IL-4 promotes ILC2 proliferation72 and induces the production of type 2 cytokines and CCL11, a chemokine implicated in eosinophil recruitment, in ILC2s.71 However, with the exception of iILC2s, which express little IL-5, ILC2s do not generally express IL-4, while highly expressing IL-13 and IL-5.6,38 Given the contrast with Th2 cells, which express all three type 2 cytokines, and the role of IL-4 in promoting ILC2 responses, the generally low production of IL-4 by ILC2s may represent a regulatory mechanism to prevent excessive ILC2 activation and expansion via autocrine or paracrine responses to IL-4.
Finally, ILC2s are also activated by TL1A (TNFSF15), a member of the tumor necrosis factor family.73,74 TL1A is expressed in the endothelium and myeloid cell compartment in response to inflammation and has been implicated in promoting CD4 T-cell function by inducing cytokine production and proliferation.73 Murine and human ILC2s express DR3, the receptor for TL1A, and stimulation with TL1A promotes type 2 cytokine production and ILC2 expansion.73,74 In the absence of TL1A signaling, ILC2 numbers are reduced in mice challenged with papain.73,74 In addition, DR3-deficient mice develop less airway inflammation, suggesting that TL1A signaling promotes type 2 immune responses through expansion of ILC2s.73,74
2.3 |. Activation of ILC2s by lipid mediators
Besides their activation by cytokines, ILC2s can also be activated by lipid mediators, often in synergy with alarmin cytokines. Leukotrienes are lipid mediators that are synthesized from arachidonic acid and have a well described role in promoting type 2 airway inflammation.75 Recent work suggests that leukotrienes mediate this pro-inflammatory effect in part by acting upon ILC2s in mice and humans.76–78 ILC2s express cysteinyl leukotriene receptor 1 (CysLT1R) and 2 (CysLT2R), which bind leukotriene C4 (LTC4) and leukotriene D4 (LTD4).76,77 Stimulation of ILC2s with LTC4 or LTD4 elicits type 2 cytokine production in a Cysltr1-dependent manner.76,77 In vivo, administration of LTD4 to Alternaria exposed RAG2−/− mice promotes IL-5 production and expansion of ILC2s, as well as eosinophilia.76 Moreover, lack of Cysltr1 signaling results in reduced frequencies of IL-13-producing ILCs during airway inflammation or helminth infection, indicating that leukotrienes contribute to ILC2 responses.77 While the transcription factor nuclear factor of activated T cells (NFAT) is observed in the cytoplasm of unstimulated ILC2s, it is detected in the nucleus after addition of LTC4, indicating that LTC4 signaling promotes nuclear translocation of NFAT.77 NFAT translocates into the nucleus upon dephosphorylation by calcineurin.79 Inhibition of calcineurin by cyclosporin A dampens LTC4-induced IL-13 expression, suggesting that LTC4 signaling promotes cytokine production via activation and translocation of NFAT.77 In T cells, TCR engagement activates different signaling pathways that result in activation of NFAT, NF-κB and activator protein 1 (AP-1).79 Von Moltke et al77 propose that in ILC2s, concomitant stimulation with leukotrienes and IL-33 mimics TCR signaling, as leukotrienes activate NFAT, whereas IL-33 activates NF-κB and AP-1.
Other lipid mediators have also been shown to modulate ILC2 responses. For instance, prostaglandin D2 (PDG2), produced by mast cells, is another derivate of arachidonic acid that activates ILC2s.80 Stimulation of human ILC2s with PDG2 and IL-2 induced expression of IL-13, which was dramatically amplified if IL-25 and IL-33 were added to the culture.81 PDG2 also promotes production of multiple cytokines, including IL-4, IL-5, and GM-CSF, as well as expression of ST2, and these effects are dependent on activation of CRTH2, a receptor for PDG2.80 PDG2 expression is elevated in patients with severe asthma, and PDG2 promotes migration of murine and human ILC2s in vitro suggesting that it might mediate the accumulation of CRTH2+ ILC2s at the site of inflammation.80,82–84 Consistent with this, significantly more wildtype ILC2s than CRTH2-deficient ILC2s were found in the lungs of mixed bone marrow chimeras several weeks after infection with N. brasiliensis.82 These findings suggest that lipid mediators can act in concert with alarmins to synergistically enhance ILC2 responses.
2.4 |. Activation of ILC2s by sex hormones
ILC2s are resident in the uterine mucosa and accumulate there in response to IL-33, but not IL-25, treatment, which also promotes their production of IL-5 and IL-13.30 ILC2s in the uterus express estrogen receptors α (ERα) and β (ERβ), and respond transcriptionally to in vitro activation with 17β-estradiol.30 Intriguingly, uterine ILC2s are greatly reduced after surgical removal of ovaries, while treatment with the combination of 17β-estradiol and progesterone is sufficient to restore their numbers, indicating that female sex hormones are required for ILC2 homeostasis in the uterus.30 On the other hand, removal of the ovaries and subsequent treatment with hormones did not affect ILC2 numbers in the lung, suggesting that the local tissue environment shapes ILC2 responsiveness to such signals.30
A recent study of experimental autoimmune encephalomyelitis (EAE) gave evidence that, partly through their effect on ILC2s, sex hormones modulate EAE pathogenesis.85 This study shows that while female mice from the SJL strain develop EAE in response to immunization with proteolipid peptide, male SJL mice develop no disease. After immunization, male mice have increased frequencies of ILC2s and Th2 cells and reduced frequencies of Th17 cells in their CNS, pointing to the possibility that an increased type 2 response might prevent EAE development. Mast cells from immunized male SJL mice express more IL-33 and in vitro stimulation of bone marrow derived macrophages with testosterone promotes IL-33 expression. Since IL-33 activates ILC2s, the authors suggest that IL-33 could prevent EAE in part via the induction of ILC2 responses. Supporting this hypothesis, treatment of female mice with IL-33 during EAE induces elevated ILC2 and Th2 frequencies and improves functional outcome, though additional work is needed to address the specific role of ILC2s.85
2.5 |. Activation of ILC2s by surface molecules
Besides soluble factors, ILC2 function is also regulated by cell-surface molecules. Inducible T cell costimulator (ICOS) and its ligand ICOS-L are both expressed on mouse and human ILC2s, and their interactions regulate ILC2 homeostasis and effector function.86–88 Notably, the frequency of ILC2s is reduced in both the lung and intestine of ICOS and ICOS-L deficient mice.87 Moreover, in vivo blockade of the ICOS/ICOS-L interaction with a monoclonal antibody against ICOS-L inhibited ILC2 proliferation at steady state.87 To study the function of ICOS during inflammation in vivo, WT or ICOS-deficient ILC2s were transferred into RAG2−/−γc −/− mice and challenged with IL-33.86 As suggested by the in vitro experiments, mice that received ICOS-deficient ILC2s had reduced ILC2 numbers and did not develop airway inflammation.86 In addition, culture of ILC2s on a cell line overexpressing ICOS-L promoted IL-2/IL-33-induced type 2 cytokine production and this effect was dependent on ICOS/ICOS-L signaling.88
ILC2s also express major histocompatibility complex 2 (MHC2), which seems to be needed to mount an optimal type 2 immune response, since transfer of wildtype, but not MHC2-deficient, ILC2s to IL-13-deficient mice restored the ability to clear helminth infection.89 The authors of that study suggest that MHC2-expressing ILC2s activate T cells to produce IL-2, which then enhances ILC2 expansion and IL-13 production, thus facilitating worm expulsion. Indeed, TCR-activated CD4 T cells produce IL-2 and induce ILC2 proliferation and type 2 cytokine production in vitro in an IL-2 dependent manner.89,90 Accordingly, treatment of RAG2−/− mice with IL-2 complex increases ILC2 numbers and restores the ability to expel worms after N. brasiliensis infection.89
2.6 |. Activation of ILC2s by neurons
Recent lines of inquiry support the idea that the nervous system may, in fact, directly respond to immune provocation. The sensory nervous system is well equipped to support the immune system, as it innervates surfaces that are in direct contact with the environment and responds very rapidly to danger signals.91,92 Noxious stimuli, such as heat, are recognized by sensory neurons, which in turn initiate reflexes to escape the harmful stimulus.91 However, sensory neurons also express receptors usually found on immune cells, such as danger-associated molecular pattern receptors, Toll-like receptors, and cytokine receptors, that enable them to directly and in-directly sense pathogens.91 In addition, nociceptor neurons can be directly stimulated by the bacterium Staphylococcus aureus via bacterial-derived pore-forming toxins or N-formylated peptides.93 The type 2 cytokines IL-4 and IL-13, as well as the alarmin cytokine TSLP, were shown to promote itch by binding to their respective receptors on sensory neurons,94,95 emphasizing the potential of sensory neurons to not only respond directly to bacteria but also to pathogen-or allergen-induced tissue inflammation. Upon activation, sensory neurons not only promote a muscular response, for example, coughing to remove the pathogenic stimulus, but also contribute to immune responses, for example, promoting skin inflammation, colitis, and OVA-induced airway inflammation.91,96–99 While multiple immune cell types, including mast cells, invariant NKT cells, and macrophages, are regulated by the nervous system,100–105 ILC2s appear to have particularly close interactions with the nervous system.
Neurons can also promote ILC2 responses through the production of neurotransmitters. Two studies found that vasoactive intestinal peptide (VIP), which is produced by sensory neurons, activates ILC2s.106,107 ILC2s express VPAC1 and VPAC2, the receptors for VIP, and stimulation with either VIP or a pharmacologic VPAC2 agonist promotes IL-5 production.106 Serum IL-5 levels and eosinophil frequencies were observed to correlate with circadian rhythm and the feeding cycle of the mice, suggesting that VIP secreted by neurons in response to caloric intake promotes ILC2 activation in vivo and thus regulates eosinophil homeostasis.106 VIP is also released by sensory neurons during allergic airway inflammation in response to IL-5, and induces ILC2 and Th2 cell activation.107 Treatment with an antagonist of VPAC2 during allergic airway inflammation diminishes IL-13 production and activation of ILC2s, as well as CD4 T-cell numbers in the lung, indicating that sensory neurons facilitate allergic airway inflammation, in part, via VIP/VPAC2 signaling.107
Three recent studies, including ours, identified another neuropeptide, Neuromedin U (NMU), as an amplifier of ILC2 responses that promotes allergic airway inflammation and helminth clearance.38,52,53 Neuromedin U receptor 1 (Nmur1), the receptor of NMU, is expressed by ILC2s and not other ILCs, and NMU/Nmur1 signaling induces production of IL-5 and IL-13 by ILC2s in vitro and in vivo.38,52,53 While NMU alone increases IL-5 and IL-13 expression dramatically in intestinal ILC2s, the effect is much smaller in lung-derived ILC2s.53 However, the combination of IL-25 and NMU synergistically induces significant IL-5 and IL-13 production in lung ILC2s,38 suggesting that the constitutive presence of IL-25 in the intestine might modulate the response of ILC2s to NMU. Indeed, type 2 cytokine responses to NMU were diminished in intestinal ILC2s from Il1rl1/Il17rb KO mice compared to WT mice.53 In addition, IL-25-producing tuft cells expand in response to colonization with Tritrichomonas muris, a protozoon that is often part of the microbiota.64 Thus, IL-25 from tuft cells in the intestine may pre-activate ILC2s, causing differential responses of lung-vs gut-derived ILC2s to NMU. Mechanistically, Nmur1 signaling activates Gaq proteins52 and induces ERK1/2 phosphorylation and calcium flux, which then activates calcineurin and promotes translocation of NFAT into the nucleus.53 NMU is upregulated after helminth infection,52 and purified N. brasiliensis excretory and secretory products or recombinant IL-33 stimulate NMU expression in neuronal organoids in a process dependent on MyD88, an adapter protein crucial for a number of innate immune receptor signaling pathways, including ST2.53 Furthermore, mice that lack MyD88 specifically in cholinergic neurons have reduced ILC2 activation in response to N. brasiliensis infection,53 indicating that both alarmins and helminth-derived factors can directly stimulate NMU expression in neurons, which then leads to ILC2 activation and facilitates immune responses to allergens and helminths. These findings are particularly exciting since they highlight a novel pathway by which the immune system and the nervous system communicate with one another at mucosal surfaces, to promote rapid detection and response to dangerous stimuli.
The crosstalk between mucosal ILCs and neurons potentially co-evolved since both systems constantly monitor the environment to identify potential dangers and both can respond quite rapidly. Moreover, if either neurons or immune cells recognize danger, it could be useful to have one system activate the other, potentially saving energy and time, as well as providing a means of amplifying responses, as seen with NMU and IL-25. Furthermore, the nervous system can learn to avoid potentially dangerous situations. Mouse studies showed that OVA-immunized mice avoid sweetened OVA solution compared to nonimmunized mice, indicating that the immune system can potentially teach the nervous system what is potentially beneficial or harmful, although this may also lead to the development of allergies.108 The communication of the nervous system and immune system has implications for the treatment of allergic diseases. Since the majority of therapies, such as corticosteroids or antihistamines, target the immune system, developing therapies that specifically target the nervous system might be of great benefit for asthmatics or patients with food allergies.
3 |. INHIBITION OF ILC2S BY EXTRINSIC SIGNALS
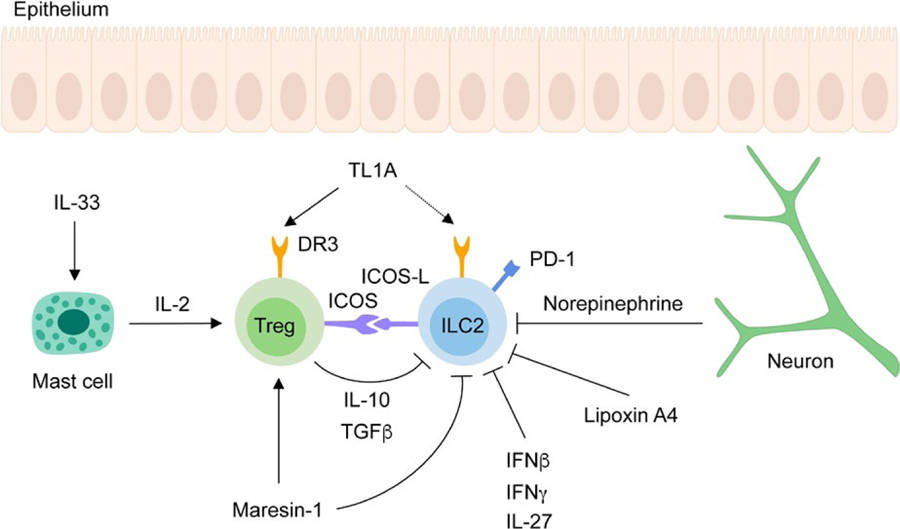
Without tight regulation, ILC2 responses can drive exaggerated tissue inflammation. Several cytokines and mediators have been identified that negatively regulate ILC2 function (Figure 3).
FIGURE 3.
Pathways inhibiting ILC2 activation. ILC2s are negatively regulated by several pathways. Neuron-derived norepinephrine, the lipid mediator lipoxin A4 and the cytokines IFNβ, IFNγ and IL-27 inhibit ILC2 responses. ILC2s express PD-1, which dampens ILC2 proliferation upon ligand binding. Tregs inhibit ILC2s via the production of IL-10 and TGFβ. The suppressive function of Tregs is promoted by maresin-1, mast cell-derived IL-2 and interaction of ICOS expressed on Tregs with ICOS-L expressed on ILC2s. Tregs also express DR3, the receptor for TL1A and compete for the binding of TL1A to DR3 expressed by ILC2s, thereby inhibiting their activation
3.1 |. Inhibition of ILC2s by interferons
Interferons boost immunity against viruses and bacteria through a variety of effects on different immune cells, including by promoting expression of antimicrobial mediators and pro-inflammatory cytokines and by enhancing the differentiation of effector T cells.109,110 However, interferons have also been implicated as immunosuppressive in certain settings.109 Three families of interferons exist: the large type I interferon family that includes IFNβ, the type II interferon family consisting only of IFNγ, and the type III interferon family of IFNλ molecules.109,110 The receptors for IFNβ and IFNγ are expressed on ILC2s at low levels at steady state and are upregulated upon activation.49,111 Stimulation of ILC2s with either IFNβ or IFNγ inhibited IL-33-induced proliferation and type 2 cytokine production in vitro, an inhibition that was dependent on the STAT 1 pathway.49,111,112 The inhibitory effect of INFβ and IFNγ was also observed in vivo, as the severity of IL-33-induced airway inflammation was significantly reduced in the presence of IFNβ or IFNγ.49,111,112 In addition, in mouse models of influenza or helminth infection, elevated ILC2 numbers and type 2 immune responses were observed in mice lacking either the type 1 interferon receptor or IFNγ receptor 1 compared to control mice, indicating that interferons control ILC2-mediated immune responses.49,111
Supporting this, NK cells can inhibit ILC2 expansion and type 2 cytokine production through secretion of IFNγ.113 In mice challenged with papain in the absence of NK cells, ILC2 expansion was enhanced, and increased frequencies of IL-5-and IL-13-producing ILC2s were observed, resulting in increased type 2 immunopathology.113 Moreover, in vivo, IL-33-induced ILC2 expansion was more pronounced in the presence of IFNγ-deficient NK cells compared to wildtype NK cells.113 IFNγ promotes expression of T-bet in ILC2s, which inhibits ILC2 expansion and IL-9 production during inflammatory responses, thereby limiting IL-33-induced lung inflammation.114 Similarly, a STAT1-dependent inhibitory function was also described for IL-27, which inhibited ILC2 responses in vitro and in vivo during Alternaria alternata-induced allergic airway inflammation and N. brasiliensis infection.49,111,115
3.2 |. Inhibition of ILC2s by lipid mediators
Lipid mediators are also able to negatively regulate ILC2 function. Human ILC2s express ALX/FPR2, the receptor for lipoxin A4, and lipoxin A4 inhibits IL-13 production by ILC2s in response to IL-2, IL-25, IL-33, and Prostaglandin D2.81 Another lipid mediator, Maresin-1, is upregulated in the lung during the sensitization and resolution phase of OVA-induced airway inflammation.116 In vitro, Maresin-1 inhibits ILC2 cytokine responses both directly and by stimulating TGFβ-induced Treg differentiation.116 In vivo, treatment with Maresin-1 during OVA-induced airway inflammation results in faster recovery from airway inflammation.116 The frequency of Tregs was elevated in these mice and ILC2s produced less IL-5 and IL-13,116 suggesting that Maresin-1 controls ILC2 responses both by directly modulating ILC2 function and also via induction of Tregs.
3.3 |. Inhibition of ILC2s by sex hormones
Laffont et al117 showed that male sex hormones can suppress ILC2 responses. In this study reduced ILC2 frequencies and less airway inflammation were observed in male mice compared to female mice in a model of IL-33-induced airway inflammation. Castration restored both ILC2 frequencies and airway inflammation to levels observed in female mice, pointing to negative regulation of ILC2s by male sex hormones. Interestingly, even the development of ILC2s seems to be controlled, as ILC2 progenitors were diminished in the bone marrow of male mice. ILC2 progenitors expressed transcripts of the androgen receptor, and their differentiation to mature ILC2s was impaired in the presence of dihydrotestosterone, suggesting that testosterone is responsible for the observed differences between male and female mice. Indeed, during IL-33-induced airway inflammation, the differences in ILC2 frequency and severity of airway inflammation seen between male and female mice were abolished in bone marrow chimeras lacking the androgen receptor, indicating that androgen signaling inhibits the generation of ILC2s, which accounts for the reduced airway inflammation in male mice.117
3.4 |. Inhibition of ILC2s by surface molecules
In mice, programmed death 1 (PD-1) is expressed on ILC2s and ILC3s and upregulated especially on ILC2s during inflammation, whereas in humans PD-1 is predominantly found on ILC2s.118,119 Treatment of RAG1−/− mice with PD-1 antibody led to the loss of almost all PD-1hi ILC2s and resulted in a greatly diminished type 2 immunopathology in response to papain challenge.118 Cell-intrinsic PD-1 signaling does not regulate ILC2 development but dampens IL-33-induced proliferation of mature KLRG1+ ILC2s in a STAT5-dependent manner.119 RAG2−/−γc −/− mice that received PD1-deficient ILC2s had elevated frequencies of cytokine-producing KLRG1+ ILC2s and were better in clearing worms than mice that received WT ILC2s.119 ILC2s also express elevated levels of the PD-1 ligand PD-L1 after administration of IL-33 both in vitro and in vivo.120 Mice lacking PD-L1 were less efficient in clearing worms than WT mice, although ILC2 proliferation and cytokine production were unchanged.120 Instead, ILC2s regulated the T-cell response via PD-L1 expression. Binding of PD-L1 to PD-1 expressed on T cells induced expression of GATA3 and IL-13 in Th2 cells, which facilitated worm clearance in a mouse model of helminth infection.120
3.5 |. Inhibition of ILC2s by Tregs
Regulatory T cells are crucial for the prevention of exaggerated immune responses and inhibition of autoimmunity.121 Several studies show that regulatory T cells (Tregs) can also inhibit ILC2 responses.116,122,123 Morita et al122 report that IL-33 activated mast cells to secrete IL-2, which increased the frequency of Tregs. Tregs, in turn, inhibited IL-33-induced ILC2 expansion via IL-10 production in vitro.122 In a mouse model of IL-33-induced airway inflammation, mice deficient in mast cells developed more eosinophilia than wildtype mice, and this was rescued by transfer of wildtype, but not IL-10-deficient, Tregs, indicating that Treg-derived IL-10 controls type 2 inflammation via inhibition of ILC2s.122
Two other studies report inhibition of IL-13 production of ILC2s by Tregs.116,124 As noted earlier, the pro-resolution lipid mediator Maresin-1 enhanced the inhibitory function of Tregs, which dampened the type 2 immune response in a model of OVA-induced allergic airway inflammation.116 A subsequent study found that induced Tregs (iTregs) limited type 2 cytokine production by ILC2s, whereas natural Tregs (nTregs) were unable to inhibit them.123 The suppressive effect was mediated by interaction of inducible T-cell costimulator ligand (ICOS-L) on ILC2s with ICOS expressed on Tregs and the Treg-derived cytokines IL-10 and TGFβ.123 Humanized mice that received human ILC2s developed airway hyperreactivity in response to IL-33 treatment, while simultaneous transfer of iTregs decreased airway hyperreactivity.123 However, if anti-ICOS-L antibody was added, the transferred Tregs were less suppressive, indicating that ICOS-L contributes to the inhibition of ILC2s by Tregs.123
Another study suggests a role for RORα-expressing Tregs in the regulation of ILC2 responses in atopic dermatitis-like inflammation.125 In Calcipotriol-or OVA-induced skin inflammation, Treg-specific deletion of Rorα resulted in more severe type 2 inflammation with increased frequencies of IL-5-producing ILC2s and Th2 cells.125 Interestingly, expression of the TL1A receptor death receptor 3 (DR3) was reduced on skin-resident Tregs from these mice.125 Previous studies showed that ILC2s also express DR3, and proliferate and produce cytokines in response to TL1A stimulation.73,74 Thus, the expression of DR3 on Tregs presumably decreases binding of TL1A to ILC2s, thereby limiting ILC2-driven skin inflammation.125 However, a definitive role of ILC2s as mediators of this effect has not yet been shown.
3.6 |. Inhibition of ILC2s by neurons
While we and others found that the nervous system promotes type 2 inflammation by positively regulating ILC2 responses,38,52,53,106,107 an inhibitory role of the nervous system has been described more recently.126 Moriyama et al126 report expression of β2AR (Adrb2), the receptor for norepinephrine, on ILC2s, as well as other ILC subsets, in both mouse and human, and used immunofluorescence microscopy to show that ILC2s were adjacent to adrenergic neurons. In this study, after helminth infection, mice treated with a β2AR agonist had difficulties mounting an efficient immune response, and this effect was independent of CD4 T cells. These mice also had lower frequencies of Ki67+ ILC2s and IL-5-and IL-13-producing ILC2s, suggesting that β2AR signaling impairs both proliferation and type 2 cytokine production by ILC2s. Consistent with this, Moriyama et al observed exaggerated ILC2 expansion in mice in which β2AR was conditionally deleted in IL-7R-positive cells, resulting in an increased efficiency of worm expulsion, even after depletion of CD4 T cells. The inhibitory effect of β2AR signaling on ILC2 proliferation and function was also seen in lung-resident ILC2s in airway inflammation and occurred in a cell autonomous manner. While the level of norepinephrine did not change, ILC2s downregulated Adrb2 expression in the small intestine during helminth infection, showing that ILC2s can decrease their responsiveness to norepinephrine during infection to promote type 2 inflammation. These findings highlight the importance of ILC2s in integrating neuroimmune signals and are particularly clinically relevant, given the widespread use of β-adrenergic agonists in the treatment of asthma. Further research is required to elucidate the pathways that regulate β2AR expression on ILC2s.
4 |. INTRINSIC REGULATION OF ILC2S
4.1 |. Development of ILC2s
All lymphoid lineage cells, including T cells, B cells, and ILCs, originate from a common lymphoid progenitor (CLP) in the mouse in fetal liver and bone marrow that expresses the cytokine receptor Flt3.127,128 From this CLP, development moves to the αLP stage, spurred by induction of the critical transcriptional regulator TOX by the transcription factor NFIL3.129,130 Increased expression of the integrin α4β7, followed by upregulation of the chemokine receptor CXCR6, is associated with loss of B-and T-cell potential, respectively.131 In mice, the NK lineage, dependent on Eomes, separates from that of the other ILCs.132 By directly regulating the transcriptional regulator Id2, NFIL3 then orchestrates the emergence of the IL-7Rα+ α4β7+ ID2+ CD25− Flt3− common helper-like innate lymphoid progenitor (CHILP) from which all IL-7Rα+ ILCs (T-bet+ Eomes− non-NK ILC1s, Gata3hi ILC2s, and RORγt+ ILC3s) are derived.132–134 GATA3 is indispensable for the development of these ILC subsets.135,136 After divergence of the RORγt+ lymphoid tissue inducer (LTi) lineage,133,137 transient, high expression of PLZF characterizes the GATA3+ TOX+ ID2+ PLZFhi ILC precursor (ILCP) of the remaining ILC lineages,133,138,139 which is also marked by high expression of PD-1.118,137 ILC lineage polarization initiates with multi-lineage priming characterized by co-expression in ILC precursors of genes associated with different ILC types.133 In immature ILC2s, the ILC3 potential is repressed by the lysine methyltransferase G9a, which, along with Bcl11b and Gfi1, maintain ILC2 identity and block expression of ILC3-associated genes in mature ILC2s,140–143 further supporting the concept that ILC polarization occurs via restriction of alternative programs. Robust ILC2 differentiation is specifically dependent on Notch signaling in the CHILP, as well as on the downstream transcription factor TCF-1.133,144–146 An early PD-1hiIL-25Rhi stage of development is specific to ILC2s,118 and depends critically on the transcription factors RORα144,147 and Ets1148, as well as the zinc-finger protein Bcl11b118,141,142,149.
4.2 |. Intrinsic regulation of ILC2s by transcription factors
The coordinate activity of a number of transcription factors is needed to support the differentiation and maintenance of ILC2s. Foremost among these is the transcription factor Gata3, which is important not only for ILC2 development but also for their function.58,135,136,150,151 ILC2s, but not ILC1s or ILC3s, disappear in mice after tamoxifen-induced deletion of Gata3, suggesting that Gata3 is crucial for both the generation of ILC2s and maintenance of fully differentiated ILC2s.151 The degree of Gata3 expression appears to determine whether common lymphoid precursors develop to ILC2s and also functionally regulates mature ILC2s.150 Moreover, Gata3 expression in human CRTH2− ILCs promotes differentiation into an ILC2 phenotype characterized by the expression of CRTH2, ST2, and TSLPR, and the ability to produce IL-5 and IL-13 upon IL-33 and TSLP stimulation.58
The transcription factor RORα is also important for the development of ILC2s from common lymphoid progenitors in a cell-intrinsic manner. Mice harboring a deletion of the ligand binding domain of RORα have no goblet cell hyperplasia following IL-25 administration, and this is associated with a lack of increase in ILC2 frequency in the mesenteric LNs.144 Moreover, both bone marrow chimeras reconstituted with RORα-deficient bone marrow and mice with lymphocyte-specific conditional deletion of RORα have impaired ILC2 development, resulting in a diminished ability to expel helminths and to develop allergen-induced airway inflammation.89,144,147
In contrast to GATA3 and RORα, which directly promote the ILC2 developmental program, other transcription factors, such as Bcl11b and Gfi1, work in part by repressing expression of genes associated with other ILC subsets.140,142,149 Chimeras generated with Bcl11b deficient fetal liver have severely decreased ILC2 progenitor frequencies and impaired clearance of helminths.142 Interestingly, while the population of mature ILC2s is significantly reduced in the small intestine in the absence of Bcl11b, ILC3 frequencies are elevated.142 Consistent with this, Bcl11b-deficient ILC2s produce Th17 cytokines and proliferate in response to IL-23.141 Chromatin immunoprecipitation and retroviral transduction of ILCs with Gfi1 indicate that Bcl11b controls the type 2 cell fate by inhibiting expression of aryl hydrocarbon receptor, which promotes ILC3 lineage identity, and by inducing expression of Gfi1.141 While Gfi1 promotes the development of ILC2s in the bone marrow, it also appears to regulate mature ILC2 responses by silencing genes associated with ILC3s, and enhancing ILC2 proliferation and promoting a type 2 transcriptional program, including expression of Gata3, ST2 and IL-5.140 Interestingly, while Gfi1-deficient cells lose their responsiveness to IL-33, they can still be activated by IL-25.140
While many transcription factors regulate both ILC2 development and maintenance of subset identity, alterations in hypoxia-inducible factor 1-alpha (HIF1α) levels by deletion of VHL appear to selectively impair the maturation of ILC2s in peripheral tissues.152 The transcription factor HIF1α is hydroxylated during steady state and subsequently labeled by VHL for proteasomal degradation.153 However, during hypoxia, HIF1α cannot be hydroxylated and translocates into the nucleus, where it induces expression of genes associated with cell metabolism, differentiation and function.153 This process is mimicked in mice that lack VHL in common innate lymphoid progenitor cells, resulting in accumulation of HIF1α.152 While accumulation of HIF1α did not influence ILC progenitor development, it did impair maturation of functional ILC2s, as VHL-deficient mice had a reduced capacity to mount an ILC2-dependent immune response after allergen challenge.152 This appears to be in part related to alterations in ILC2 metabolism, as VHL deficiency resulted in increased glycolysis, which in turn led to different epigenetic modifications that reduced ST2 expression.152
4.3 |. Intrinsic regulation of ILC2s by metabolic pathways
The role of metabolism in regulation of ILC2 function is just beginning to be investigated. In addition to HIF1α-mediated alterations in glycolysis, arginine metabolism also contributes to the regulation of ILC2 response. Arginase 1 (Arg1) is a metabolic enzyme that catalyzes the generation of ornithine and urea from arginine.154 ILC2 progenitors and mature ILC2s express Arg1 at steady state and during type 2 inflammation.154,155 While Arg1 appears to be dispensable for ILC2 development and homeostasis, it may be crucial for ILC2 expansion and effector cytokine responses. Conditional deletion of Arg1 in IL-7Rα-positive cells did not change ILC2 frequencies at baseline, but it did lead to reduced ILC2 frequencies and impaired IL-5 and IL-13 production after papain challenge, resulting in diminished allergic lung inflammation.154 This appeared to be, in part, due to decreased generation of the downstream metabolites of arginine in the absence of Arg1, many of which have been described to modulate cell proliferation and survival, while also dampening glycolytic function when Arg1 was pharmacologically inhibited.154 However, an earlier paper reported that ILC2 function was not altered when Arg1 was deleted in IL-5-expressing cells using an IL-5-specific Cre, raising the question of how to reconcile these findings.155
A recent study reports that the relative proportion of ILC2s to ILC3s in the gut is controlled by retinoic acid, a derivative of vitamin A.156 Mice on a vitamin-A-deficient diet, or treated with an inhibitor of the retinoic acid receptor, have elevated ILC2 frequencies and decreased ILC3 frequencies compared to control mice, an imbalance that is also observed in the absence of an adaptive immune system or microbiota. Mechanistically, retinoic acid compromises the generation of ILC2s, while promoting IL-22 production and increasing the number of ILC3s. The authors further show that infection of RAG1−/− mice with Citrobacter rodentium caused increased weight loss and colonic inflammation in the absence of retinoic acid signaling, suggesting that lack of vitamin A and the resulting decreased ILC3 frequency renders mice more susceptible to bacterial infection. In contrast, after helminth infection, inhibition of retinoic acid signaling resulted in more efficient clearance of Trichuris muris worms in an IL-13-and ILC2-dependent manner. These data indicate that an increase in ILC2s confers protection against helminth infection in times of limited access to nutrients, for example, in the absence of vitamin A.156 A subsequent study showed that ILC2s rely on fatty acids but not glucose during vitamin A deficiency, and that fatty acid oxidation contributes to IL-13 expression by ILC2s.157 While ILC2s are independent of fatty acid metabolism at steady state, they require fatty acids during helminth infection to efficiently clear worms.157
5 |. EFFECTOR FUNCTION OF ILC2S
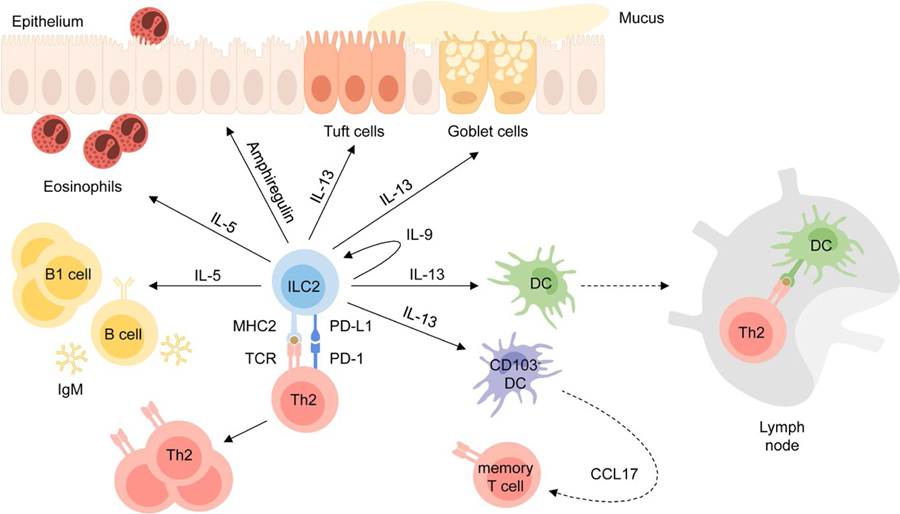
ILC2s are part of a complex interplay of different cell types that promote type 2 immune responses to helminth infection and allergens. Activated ILC2s produce an array of cytokines and factors with unique functions, while also regulating other cell types (Figure 4).
FIGURE 4.
ILC2 effector mechanisms. ILC2s have tissue-protective and pro-inflammatory effector functions. ILC2s produce IL-13 which promotes tuft cell and goblet cell hyperplasia, activates DCs to migrate to the lymph nodes, where they promote Th2 differentiation, and induces CCL17 production by CD103-DCs which recruits memory T cells to the site of inflammation. ILC2s also induce eosinophilia, B1 cell expansion and production of IgM by B cells via production of IL-5 and produce IL-9 which leads to autocrine activation. Interaction with Th2 cells via MHC2 and PD-L1 promotes Th2 differentiation and effector function. While these cytokines and surface molecules contribute to the development of tissue inflammation, the epidermal growth factor amphiregulin was shown to be important for tissue homeostasis and repair
5.1 |. Key effector program of ILC2s
Murine ILC2s express transcripts for the type 2 cytokines IL-4, IL-5, and IL-13.5,151 However, while activated ILC2s generally produce large amounts of IL-5 and IL-13,6,12,20,56,158 IL-4 is typically barely detectable on a protein level,6,56,158 except in specific inflammatory settings or disease models.25,38,44,120,124,159 ILC2s in airway inflammation induced by OVA or house dust mite produce IL-5 and IL-13, and less IL-4,56,57 and during helminth infection, ILC2s produce IL-13 but not IL-4.6,160 Similarly, human IL-25-or IL-33-activated ILC2s produce significant amounts of IL-5 and IL-13, while IL-4 is only expressed to a lesser extent.21,58
IL-5 binds to the IL-5R, a heterodimer formed by IL-5Rα and the common β-chain161 and induces eosinophilia by promoting eosinophilopoiesis in the bone marrow and peripheral eosinophil survival,162,163 and by recruiting eosinophils to the site of inflammation in conjunction with eotaxin.164,165 IL-13 binds to the type II IL-4 receptor, composed of IL-4Rα and IL-13Rα1, and the decoy receptor IL-13Rα2,166 and promotes mucus production by goblet cells independent of IL-5 signaling.164 IL-4 also binds to the type II IL-4 receptor, as well as to the type I IL-4 receptor, which is formed by IL-4Rα and the common γ-chain.166 Upon ligand binding, both IL-4 receptors signal via the signal transducer and activator of transcription 6 (STAT6) pathway.166 Notably, monoclonal antibodies targeting both IL-5 and IL-4Rα (thus inhibiting both IL-4 and IL-13) have recently been found to be effective in the treatment of eosinophilic and moderate to severe asthma, respectively, highlighting the importance of these pathways in allergic disease.167–169
Studies published before the discovery of ILCs show that IL-13 is crucial for the clearance of helminths170 and contributes to the development of airway inflammation, even in the absence of an adaptive immune system.171,172 The initial publications describing ILC2s reported type 2 cytokines and goblet cell hyperplasia in RAG2−/− but not ILC-deficient RAG2−/−γc −/− mice during N. brasiliensis infection.5 Transfer of ILCs in combination with IL-25 treatment restored the capability to induce eosinophilia and expel worms in RAG2−/−γc −/− mice after helminth infection.11 Moreover, transfer of wildtype but not IL-13-deficient ILC2s restored worm clearance in mice lacking the receptor for IL-25.6 These findings demonstrate that ILC2-derived IL-13 plays a crucial role in the defense against helminth infection. ILC2s are also sufficient to induce eosinophilia and mucus production in response to papain challenge in the absence of an adaptive immune system.12 After influenza virus infection, depletion of ILCs with anti-CD90.2 antibody in Rag2−/− mice impaired development of airway hyperreactivity.173 Furthermore, adoptive transfer of wildtype, but not IL-13-deficient, ILC2s restored airway hyperreactivity in IL-13-deficient recipient mice, indicating that ILC2s are sufficient to induce airway hyperreactivity, even in the absence of T and B cells.173
IL-9 production can be transiently induced in ILCs during airway inflammation and is dependent upon IL-2 produced by adaptive immune cells.174 IL-33, IL-25 and TSLP are unable to elicit IL-9 expression in ILC2s on their own, though the combination of IL-33 and TSLP can induce IL-9 production in ILC2s in an IRF4-dependent manner.174,175 In vitro stimulation of ILCs with IL-9 promoted IL-5 and IL-13 expression, while neutralization of IL-9 during lung inflammation inhibited it, indicating that IL-9 facilitates type 2 cytokine production in ILC2s.174 Following helminth infection, ILC2s are the major IL-9 producers and also express IL-9R, suggesting an autocrine loop.176 Analysis of helminth-infected IL-9R-deficient mice revealed that IL-9 signaling is important for the survival and effector function of ILC2s, and facilitates worm clearance and tissue repair, in part by promoting eosinophil accumulation in the lung and alternative macrophage activation.176 In a mouse model of cystic fibrosis, fungal infection induced the production of IL-9, which then activated mast cells to produce IL-2.177 In response to IL-2, ILC2s proliferated and further activated Th9 cells, resulting in exaggerated lung disease.177 In contrast, another study reports that in a mouse model of arthritis ILC2-derived IL-9 ameliorated inflammation by expanding ILC2s and promoting Treg function.178 In this study, Tregs from mice lacking IL-9 were less suppressive than control Tregs. However, when Tregs from IL-9-deficient mice were activated with recombinant ICOS-L and anti-GITR, they ameliorated joint inflammation in the resolution phase of antigen-induced arthritis. IL-9 induced proliferation and expression of ICOS-L and GITRL on ILC2s, suggesting that via production of IL-9 and subsequent upregulation of these co-stimulatory ligands, ILC2s promote the suppressive function of Tregs. Indeed, transfer of ILC2s in IL-9-deficient mice resulted in less inflammation in a model of antigen-induced arthritis. In line with these findings, the authors observed more IL-9-expressing ILC2s in rheumatoid arthritis patients in the remission phase compared to patients with active disease.178
Amphiregulin (encoded by Areg), a member of the epidermal growth factor family of cytokines, is produced by lung ILC2s during influenza virus infection and contributes to tissue repair.20 In lung ILC2s the expression of Areg seems to be regulated by IL-33 and IL-25.20,38 Amphiregulin-expressing ILC2s are also found in the mesenteric LNs and lamina propria of the colon and are expanded in DSS-induced colitis.179 Mice with impaired signaling of the epidermal growth factor receptor (EGFR), the receptor for amphiregulin, developed more severe colitis, similar to Areg-deficient mice, indicating that signaling of amphiregulin via EGFR induces tissue protective responses.179 ILC2-derived amphiregulin is important for maintaining barrier integrity by inducing tight junctions and mucus production during colonic inflammation.179 Areg expression is also detected in human skin ILC2s and increased upon IL-33-induced ILC2 activation.26
5.2 |. Interaction of ILC2s with CD4 T cells
Infection with parasites requires a fast and efficient type 2 response of the immune system to prevent dissemination and promote expulsion. As one of the main producers of IL-5 and IL-13, ILC2s are crucial for the initiation of type 2 inflammation. Along with inducing mucus hyperplasia and eosinophilia, ILC2s also communicate with other cell types to amplify inflammation and induce adaptive immunity and memory. Within hours of receiving alarmin signals, ILC2s induce type 2 inflammation via production of IL-5 and IL-13.12 In contrast, antigen-specific effector T cells need several days to respond, as they must be activated by antigen-presenting DCs in the draining LNs, differentiate into Th2 cells, and migrate to the site of tissue inflammation.180,181 Bridging the time until Th2 cells arrive, ILC2 activity goes beyond activation of innate immunity to communication with DCs and effector T cells. Th2 responses are diminished in mice lacking ILC2s, and, depending on the model of inflammation, this can be associated with reduced airway inflammation or impaired worm clearance.89,90,180,182,183 In a papain-induced airway inflammation model, elevated numbers of Th2 cells were found in the mediastinal lymph nodes (mLNs) of mice reconstituted with wildtype bone marrow.180 However, in RORα-deficient bone marrow chimeras, which lack ILC2s, Th2 cell numbers were severely reduced in mLNs after papain treatment, indicating that ILC2s promote the differentiation of Th2 cells.180 Subsequent experiments showed that during airway inflammation the frequency of activated DCs in the mLNs was also diminished in ILC2-deficient bone marrow chimeras and that administration of recombinant IL-13 restored DC numbers.180 Moreover, migration of DCs toward the chemokine CCL21 was reduced in lungs from IL-13-deficient mice compared to WT mice.180 These findings suggest that ILC2s enhance Th2 cell differentiation by production of IL-13, which facilitates migration of activated DCs to draining LNs.
ILC2s are also directly involved in the stimulation of effector CD4 T-cell responses. In vitro, ILC2s promote the proliferation of CD3/CD28-activated CD4 T cells.183 In the absence of CD3/CD28-induced activation, naïve ILC2s are not sufficient to induce T-cell proliferation.89,90 However, if ILC2s are pulsed with OVA peptide, they can induce proliferation of OVA-specific CD4 T cells.89,90,182 T-cell proliferation is inhibited if an anti-MHC2 antibody is added to the co-culture or if MHC2-deficient ILC2s are used.89,90,182 Curiously, if ILC2s were pulsed with OVA-protein instead of OVA peptide, they were not able to elicit CD4 T-cell proliferation, although ILC2s were able to process the protein.89,90,182
Besides MHC2, the co-stimulatory molecules CD80 and CD86 are also required for ILC2-induced T-cell proliferation.89 Co-culture of ILC2s and T cells resulted in increased type 2 cytokine production, which was dependent on the cytokine IL-4 as well as cell-cell contact via MHC2, CD80, CD86 and OX-40L.89,90,182,183 In vivo, OX40L expression on ILC2s was required for an efficient Th2 response during allergen-induced airway inflammation or N. brasiliensis infection.184 Another study found that interaction of programmed death-ligand 1 (PD-L1) on ILC2s with PD-1 on CD4 T cells promoted Th2 differentiation and effector function in the context of type 2 inflammation.120 PD-L1 is expressed by all ILC subsets, but is specifically induced in ILC2s during N. brasiliensis infection and also by IL-2 or IL-33.120 Worm clearance was enhanced in RAG2−/−γc −/− mice, and the type 2 immune response against N. brasiliensis was more robust, if CD4 T cells were transferred together with wildtype rather than PD-L1-deficient ILC2s.120 After Heligmosomoides polygyrus infection, ILC2s are an important source of IL-4 and contribute to Th2 differentiation, presumably via IL-4 secretion.159 ILC2s not only promote the differentiation of Th2 cells but also facilitate the recruitment of memory Th2 cells upon re-challenge to the site of allergen exposure.185 When ILC2s were deleted after sensitization with papain and 2W1S-peptide, the numbers of Th2 cells were decreased in the lung upon allergen re-challenge.185 The study further found that ILC2-derived IL-13 induced secretion of the chemokine CCL17 by CD11b+ CD103− DCs, and CCL17 in turn promoted migration of memory Th2 cells to the tissue.185
Collectively, these data suggest that the interaction of ILC2s and Th2 cells via direct contact and cytokines, or via the regulation of DC migration and activation, is important for the development and amplification of type 2 inflammation. Due to their powerful inflammatory capacity, ILC2s must be tightly regulated to prevent uncontrolled or excessive immune responses. As already discussed, several studies show that Tregs contribute to ILC2 inhibition. However, recent evidence suggests that ILC2s also influence Treg function. While OX40L expression on ILC2s leads to increased Treg numbers during type 2 inflammation184 and ILC2-derived IL-9 promotes the suppressive function of Tregs,178 ILC2-derived IL-4 inhibits Treg differentiation and thereby facilitates development of food allergy,124 indicating ILC2s can both promote and inhibit Treg function.
5.3 |. Interaction of ILC2s with other cell types
Along with regulating effector and regulatory T cells, ILC2s can also promote B cell responses. ILC2s stimulated B1 cell proliferation in an IL-5-dependent manner in vitro and in vivo.5,186 In addition, B cells activated with IL-4 and either anti-CD40 or LPS produced more IgM, IgG1, IgE, and IgA if they were cultured in the presence of ILC2s.5,186 The elevated production of IgM was not observed in the presence of IL-5-deficient ILC2s or after the administration of an anti-IL-5 antibody, indicating that ILC2-derived IL-5 positively regulates IgM production by B cells in vitro.186 Adoptive transfer of ILC2s into IL-7R-deficient mice increased antigen-specific IgM but not IgG production upon nasal administration of the protease bromelain and the thymus independent antigen NP-Ficoll.186
ILC2s also communicate with cells that are not of hematopoietic origin. Three-independent studies reported that ILC2-derived IL-13 promotes both Tuft cell differentiation and goblet cell hyperplasia, thus altering the intestinal and lung epithelial barrier.62–64 Moreover, IL-5 induces secretion of VIP from sensory neurons, which then activates ILC2s and Th2 cells, thereby driving airway inflammation.107 Although it is not shown that ILC2-derived IL-5 promotes VIP release, the findings suggest that ILC2s can regulate neurons since they produce high levels of IL-5 upon activation. ILC2s also potentially regulate neurons via other cytokines, as neurons express several cytokine receptors, including IL-13R, IL-33R, and IL-25R.107
6 |. TISSUE-SPECIFIC EFFECTOR FUNCTION OF ILC2S
6.1 |. Tissue-residency vs migration of ILC2s
ILC2s exert different functions depending on the tissue environment where they reside. To elucidate whether ILC2s are replenished by bone marrow progenitors or migrate between organs, experiments with parabiotic mice were performed. One month after parabiosis, almost all ILC2s in the lung, small intestine, salivary gland and mesenteric LNs were still host-derived, and 3 months later, only a small fraction of intestinal ILC2s were derived from the parabiotic partner.48 The frequency of this donor-derived ILC2 population increased very gradually over time, as 6–8 months after parabiosis, approximately 10% ILC2s in the small intestine and 20% of ILC2s in the mesenteric LNs were donor-derived.50 These experiments indicate that ILC2s are largely locally maintained, tissue-resident cells. To study whether ILC2s remain tissue resident during inflammation, different disease models were induced in parabiotic mice. During autoimmune disease and IL-33-induced airway inflammation, ILC2s did not enter the circulation, but during N. brasiliensis infection, a small portion of ILC2s exchanged between parabiotic partners.48,49 During acute worm infection, ILC2s remained largely tissue-resident, with donor-derived ILC2s only observed 15 days after infection.48 However, as mentioned previously, a recent study reported that KLRG1hi iILC2s, a population present in the lung during N. brasiliensis infection or after intraperitoneal injection of IL-25,44,50 migrated during helminth infection in an S1P-dependent manner from the small intestine to other organs, including the lung, where it effected an immune response.50 This study also reports a small percentage of donor-derived ILC2s in the lung 20 days after infection, though the authors suggest that these cells are former donor-derived iILC2s that became nILC2s.50
6.2 |. Tissue-specific effector function of ILC2s in adipose tissue
ILC2s are present at mucosal barriers but are also located in adipose tissue.5,33,187 Similar to ILC2s from other tissues, these ILC2s produce IL-5 and IL-13 in response to IL-33 treatment in vitro and in vivo.5,33 In addition, they contribute to the homeostasis of eosinophils and alternatively activated macrophages (AAM) in adipose tissue, demonstrated by the decrease in eosinophil and AAM numbers caused by a lack of ILC2s.33 IL-33 increases eosinophil and AAM frequencies in visceral adipose tissue in an ILC2-dependent manner, and eosinophil frequencies are also elevated in the presence of ILC2s in a model of helminth infection.33 In the absence of IL-5 signaling or eosinophils, mice fed a high fat diet (HFD) were heavier and developed increased glucose intolerance, while oxygen uptake and heat generation were reduced, indicating that ILC2s might help suppress development of obesity via their regulation of eosinophil accumulation in adipose tissue.33 Indeed, RAG1−/− mice lacking ILCs developed increased adiposity and glucose resistance on HFD, whereas adoptive transfer of ILC2s temporarily reduced weight in obese animals.187 Reduced ILC2 frequencies are also observed in adipose tissue from obese mice and humans.31,33
IL-33 induced accumulation of murine ILC2s in white adipose tissue (WAT), and, while body weight was not influenced, percentage of body fat decreased and caloric expenditure rose.31 Caloric expenditure is controlled by beige adipocytes, a special type of adipocytes that express uncoupling protein 1 (Ucp1).188 The protein Ucp1 disconnects the proton gradient generated by the mitochondrial electron transport chain from ATP synthesis, resulting in heat generation.31,188 Thus, beige adipocytes limit development of obesity by increasing caloric expenditure. Indeed, ILC2s promoted generation of beige adipocytes, indicating that they negatively regulate obesity.31 IL-33 treatment induced Ucp1 expression in white adipose tissue in an ILC2-dependent manner, as Ucp1 expression was only induced by IL-33 in RAG2−/−γc −/− mice after adoptive transfer of ILC2s.31 Formation of beige adipocytes and expression of Ucp1 is regulated by ILC2s via production of methionine-enkephalin, for which adipocytes express the receptors.31 Adipocytes upregulate Ucp1 expression in response to methionine-enkephalin, suggesting that ILC2s directly induce beiging of adipocytes.31 Consistent with this, another study showed that IL-33 induced Ucp1 expression and beige adipocyte formation.32 The authors of this study report that IL-33 promoted norepinephrine-induced oxygen consumption in an Ucp1-dependent manner. Adipocyte progenitors proliferated and expressed the beige adipocyte markers TMEM26 and CD137 upon IL-33 treatment in the presence of ILC2s. Eosinophils and the type 2 cytokines IL-4 and IL-13 are important for this proliferative response, as adipocyte progenitors proliferated less in mice lacking eosinophils or IL-4 and IL-13 signaling. Deficiency of IL-4Ra on adipocyte precursors, but not on myeloid cells, decreased the frequency of adipocytes precursors positive for the proliferation marker ki67, indicating that proliferation of adipocyte precursors depends on IL-4Ra signaling. Ucp1 expression and generation of beige adipocytes also depended on IL-4Ra signaling in adipocyte precursors, yet deficiency of IL-4Ra on mature adipocytes had no effect on the generation of beige adipocytes.32
A more recent study shows that, along with IL-13Rα1, the IL-13 decoy receptor IL-13Rα2 is also upregulated in WAT of mice placed on HFD.189 The authors hypothesize that increased expression of IL-13Rα2 reduces IL-13 levels in WAT of obese mice, thereby promoting obesity. While mice lacking IL-13Rα2 respond to HFD like control mice do, additional treatment with IL-33 leads them to have more pronounced weight loss and decreased blood glucose levels.189 However, IL-13Rα2-deficient mice on HFD treated with IL-33 also develop more diarrhea, thus, making it difficult to attribute the weight loss specifically to metabolic changes in response to IL-13Rα2 deficiency.189 As use of the IL-4Rα antagonist dupilumab becomes more widespread, it will be interesting to see if it is also associated with weight gain in asthmatics.167
6.3 |. Effector function of ILC2s in other tissues
ILC2s are also located in the brain and spinal cord meninges, where they expand in response to IL-33.35 Meningeal ILC2s are transcriptionally distinct from lung ILC2s.35 Spinal cord injury induced transcriptional changes in meningeal ILC2s, including the expression of IL-5 and IL-13, and IL-33 signaling contributed to ILC2 activation in the context of this injury model.35 While ILC2s were largely absent in the spinal cord parenchyma at steady state, spinal cord injury induced accumulation of ILC2s in the parenchyma at the site of inflammation.35 Locomotor dysfunction after spinal cord injury in ST2-deficient mice was ameliorated by the transfer of wildtype lung ILC2s into the cerebrospinal fluid.35 Moreover, these mice exhibited smaller lesion size, indicating that ILC2s may promote recovery from spinal cord injury.35
In a wound healing model, ILC2s were significantly increased at the site of skin injury compared to healthy skin, while mice lacking IL-33 had reduced ILC2 numbers and demonstrated slower closure of cutaneous wounds.190 In addition, diminished re-epithelialization and wound healing were also observed in Rag1−/− mice depleted of ILCs, suggesting that ILC2s contribute to wound closure.190 However, it remains to be clarified whether ILC2s also promote cutaneous wound healing in the presence of an adaptive immune system and how ILC2s exert their effect.
7 |. TISSUE SPECIFICITY AND PLASTICITY OF ILC2S
7.1 |. Transcriptional heterogeneity of ILCs
ILCs were initially characterized by flow cytometry using surface markers as well as subset-specific transcription factors and cytokines. However, this approach is limited by available antibodies and the limitations of flow cytometry, making it difficult to identify plasticity between ILC subsets, heterogeneity within each ILC subset, as well as ILC-specific functions that vary between tissues. To overcome these limitations, several studies have used RNA-sequencing (RNA-seq) to define the transcriptional landscape of ILCs.7,38,191,192
In addition to verifying expression patterns of genes previously associated with the major ILC subsets (eg, high levels of Gata3 in ILC2s and Ccl5 in ILC1s),7 transcriptional studies using population RNA-seq identified ILC subset-specific genes that indicate possible effector functions and novel genes associated with each ILC subset. For example, the first study to characterize murine ILC subsets in the lamina propria of the small intestine (siLP) via population RNA-seq observed in ILC2s (defined as Sca-1+ ST2+ KLRG1+ ILCs) unique expression of genes associated with lipid metabolism, such as Dgat2, Pparg, and Lpcat2, and of Bmp2, which is implicated in communication with enteric neurons7. Similarly, another study of intestinal ILC populations found specific expression of genes associated with sphingolipid and amino acid metabolism in ILC2s (defined as KLRG1+ RORγt− ILCs).192
Human tonsil-resident ILC2s analyzed by plate-based scRNA-seq, expressed common ILC2 marker genes, as well as genes associated with specific pathways, such as Notch signaling and prostaglandin generation and signaling.191 This study sampled in an unbiased way all CD127+ ILCs and also specifically gated on ILC1s and ILC2s to enrich these populations, which are rarer in the tonsils. Three clusters of ILC3s but only one cluster of ILC2s were identified, a finding that could stem from ILC2 homogeneity in that tissue, especially without a type 2 stimulus, or from a lack of statistical power due to the small number of cells sequenced.
Massively parallel scRNA-seq enabled a better powered, unbiased transcriptional assessment of murine CD127+ ILCs isolated from the siLP and the lung.38,192 Genes previously strongly associated with populations of ILCs from a single type turned out to have significant substructure in expression, depending on activation context and possibly on tissue. For example, in our study of murine lung ILCs, Dgat2 was upregulated specifically in ILC2s from IL-25 treated mice, while Lpcat2 and Bmp2 were observed primarily in resting ILCs from control mice.38 The single-cell data from the siLP ILCs suggests that, in addition to being expressed in ILC2s, Dgat2 is expressed in some ILC3s, particularly in a cluster with high MHC class II expression.192 Even Il1rl1 (ST2), Klrg1, and Ly6a (Sca-1), genes commonly used to identify murine ILC2s,7 have distinct expression patterns that suggest association with particular ILC activation states. Regardless of ILC type, activation appears to upregulate Ly6a in lung ILCs, while Il1rl1 (ST2) is broadly expressed in lung ILC2s, except in an IL-25-induced Klrg1-high group of iILCs that jointly produces Il13, Il4, Il17a, and some Gzma, but little Il5.38,44,50 The iILC2s are either not present among these siLP ILCs, or they are mixed with other ILCs in the clusters, possibly contributing cells to the “ILC2d” cluster, which has high Klrg1 and some Il17f expression, and the “ILC1a” cluster, which has expression of Gata3, Gzma, Il17a, and Klrg1 (and very low levels of Tbx21). The gradation of expression of both Ly6a and Klrg1, in addition to Gata3, Il5, Areg, and Csf2, across the ILC2 clusters in the siLP study suggests that a spectrum of activation may be partly driving the clustering.
There is increasing evidence that, at least in some contexts, ILCs may be better described by their positions on continuous spectra representing different aspects of immune response, rather than by discrete categories.41,150,193 Clustering does capture transcriptional programs that are unique to certain cell subpopulations, such as expression of MHC2 genes in distinct clusters of human tonsil ILC3s, murine siLP ILC3s, and murine lung ILC2s.38,191,192 However, responses to specific stimuli, changes in metabolism, and other biological processes can create convergent gene expression patterns in otherwise transcriptomically distinct groups of cells. For example, expression of Odc1, the rate-limiting enzyme in polyamine biosynthesis, is upregulated in certain clusters of siLP ILC3s, as well as in alarmin-induced clusters of lung ILC2s, which suggests that these groups may share specific metabolic regulation, despite differences in ILC type and tissue context. Similarly, treatment with antibiotics was shown to modulate the transcriptome and epigenome of ILC1 and ILC2 clusters in the siLP so they become transcriptionally and epigenetically more like ILC3s in a non-discrete way.192 While a functional role of these changes has not been shown yet, the findings point to the tissue environment, in this case, the microbiome, as a major influence on ILC plasticity. A potential mechanism underlying ILC plasticity was identified in a study of chromatin accessibility in ILCs, in which some regulatory elements at the loci of lineage-determining transcription factors were found to be accessible in multiple ILC lineages.194 For example, in ILC2s, the regulatory elements at the Rorc and Il17 loci are partially open, suggesting that inflammatory ILC2s are poised to produce IL-17 under specific conditions.44,194
On the other hand, despite functional plasticity, ILC lineages do apparently have distinct open-chromatin landscapes. Different types of ILCs from the same tissue had characteristic accessible regulatory elements that differed significantly more than the transcriptomes did, and which were not greatly changed by activation, especially compared to the activation-induced changes in T-cell chromatin.194 At steady state, lung ILC2s and naive CD4 T cells had very distinct open-chromatin landscapes, yet nearly three quarters of the regulatory elements that became accessible in Th2 cells after N. brasiliensis infection were already accessible in ILC2s, indicating the existence of shared regulatory networks for type 2 lymphocyte responses that are poised or functioning even before activation in ILC2s.194 While the comparison of transcriptional and epigenetic differences between ILC subsets suggests that the tissue environment primarily influences the transcriptome,194 this requires validation to be generalized to tissues outside the siLP. Future work is needed to directly compare ILC subsets from different tissues to determine how the tissue environment influences the transcriptome and effector functions of ILCs, ideally both at steady state as well in various models of tissue inflammation.
7.2 |. Transdifferentiation of ILC2s
Each tissue has a unique composition of different ILC subsets. The lung, for example, harbors mostly ILC2s in mice,38 which are poised to mount the type 2 immune response required to clear helminth infections. Yet, intracellular bacteria and viral infections provoke type 1 immune responses, which raise the question of how a type 1 immune response to a viral infection is mounted in the lung tissue when almost no ILC1s are present at steady state. This problem could be solved by either substantial expansion of a specific ILC subset, by conversion of one subset into another or by activation of another cell type with relevant effector function. Recent studies indicate that ILC2s can convert to ILC3-like ILC2s which express IL-17 along with IL-1343,44 and ILC1-like ILC2s which acquire ILC1-like features, including expression of T-bet and IFNγ production, while lowering expression of Gata3 and ST2.39,40,42,195 The cytokines IL-12 and IL-18 induce differentiation of murine ILC2s to ILC1s in vitro, and adoptively transferred ST2+ ILC2s seem to convert partly into ILC1-like ILC2s in Rag2−/−γc−/− mice upon influenza infection.42 Interestingly, ILC1-like ILC2s are not only induced by influenza virus but also by the bacterium Staphylococcus aureus, as well as long-term exposure to smoke, one of the major environmental stimuli underlying the development of chronic obstructive pulmonary disease (COPD).42 Increased frequencies of ILC1s are observed in patients with COPD compared to non-smoking healthy individuals, suggesting that ILC2s might convert to ILC1s in humans in response to smoke exposure.42
In vitro experiments indicate that human ILC2s can also demonstrate plasticity and switch between subsets. Human ILC2s cultured with IL-12 together with alarmin cytokines or IL-1β convert to ILC1-like ILC2s, characterized by expression of T-bet and production of IFNγ.39,40,42,195 The cytokine IL-1β signals through IL-1R1 and the NF-κB pathway and promotes type 2 cytokine production and ILC2 proliferation.39,40 Yet, IL-1β also contributes to the conversion of ILC2s into ILC1-like cells, as it triggers expression of IL-12Rβ2 and, together with IL-12, induces epigenetic modifications enabling the co-expression of type 1 and type 2 cytokines and transcription factors.40 In a mouse model, adoptive transfer of ILC1s induced by IL-12, IL-18 and IL-33, resulted in significantly more weight loss during influenza infection than did transfer of ILC2s, indicating that ILC1s play an important role during influenza infection and could contribute to post-viral exacerbations in COPD patients.42 The plasticity of ILC2s and ILC1s is bi-directional, as the cytokine IL-4 is able to switch ILC2-derived ILC1s back to ILC2s.39 In humans, IL-4 is expressed by eosinophils and may contribute to transdifferentiation back to ILC2s, as co-culture of activated eosinophils with ILC2s promoted IL-5 production by ILC2s in an IL-4-dependent manner.39
8 |. CONCLUSIONS AND FUTURE DIRECTIONS
Although ILC2s possess common core effector and transcriptional programs, they have diverse functions and phenotypes depending on the tissue environment. Their phenotype can be modified by inputs from a diverse array of cell types, including immune, epithelial, neuronal, and endocrine. Their ability to integrate signals from a wide spectrum of cell types in turn allows ILC2s to both take on unique, tissue-specific effector programs, such as modulating adipose tissue function, and coordinate responses to tissue injury or stress in a manner appropriate to the tissue in which they reside. The tissue-specific signals that confer these properties are just beginning to be uncovered. Notably, while epithelial-derived alarmins play a crucial role in ILC activation, these appear to be somewhat redundant between tissues.68 Rather, recent work has begun to suggest that signals derived from neurons and the endocrine system appear to be particularly important in inducing or modulating tissue-specific ILC phenotypes, although further work in this area is clearly needed.
The use of single-cell transcriptomics has begun to unravel the specific transcriptional programs of ILCs in different tissues, both in health and disease, as well as highlight the diversity of ILC2s both within and between tissues. Indeed, the work of our group and others has found that, while ILC2s within a given tissue are often largely transcriptionally homogeneous at steady state, inflammation can markedly alter ILCs within a tissue, resulting in transcriptionally distinct subgroups of cells, as well as expanded transcriptional heterogeneity along continuous dimensions. Crucial areas of future investigation include understanding the functional role of these changes, and determining whether induced transcriptional states are transient or represent discrete functional states.
Finally, the phenotype of ILC2s can change so dramatically in inflammatory settings that the cells appear to adopt some or all of the transcriptional profiles typical of ILC1s or ILC3s. Emerging data using ATAC-Seq and other methods to assay chromatin accessibility has highlighted the extent to which ILCs may be able to rapidly express effector cytokines typically thought of as characteristic of distinct ILC types. Understanding the mechanisms underlying disease-induced ILC plasticity can also help answer the question of which inflammation-induced alterations in ILC populations contribute to disease chronicity vs correlate with resolution. Indeed, given the degree to which ILCs integrate diverse signals, understanding the state of ILCs in a tissue may provide insight into the health of the tissue itself, while manipulating signals central to ILC regulation may offer a means to impact the course of chronic inflammatory disease.
ACKNOWLEDGEMENTS
A. W. is a PhD student jointly supervised by V. K. K. and Hans-Martin Jäck (Friedrich-Alexander University of Erlangen-Nürnberg, Erlangen, Germany). This work was supported by a Boehringer Ingelheim Fonds PhD fellowship (A. W.), NIH grants 1K08AI123516 (P. R. B.), 1R01 MH111502 (supporting S. J. R), R01 AI139536 (V. K. K.), the Klarman Cell Observatory and the Food Allergy Scientific Initiative at the Broad Institute.
Funding information Boehringer Ingelheim Fonds; NIH, Grant/Award Number: 1K08AI123516, 1R01 MH111502 and R01 AI139536; Klarman Cell Observatory and the Food Allergy Scientific Initiative
Footnotes
A. W., P. R. B., S. J. R., and V. K. K. have no financial or personal relationships that could be viewed as a conflict of interest.
REFERENCES
- 1.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. [DOI] [PubMed] [Google Scholar]
- 2.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. [DOI] [PubMed] [Google Scholar]
- 3.Maazi H, Akbari O. Type two innate lymphoid cells: The Janus cells in health and disease. Immunol Rev. 2017;278:192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mjosberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol. 2016;138:1265–1276. [DOI] [PubMed] [Google Scholar]
- 5.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. [DOI] [PubMed] [Google Scholar]
- 6.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinette ML, Fuchs A, Cortez VS, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells – A proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. [DOI] [PubMed] [Google Scholar]
- 9.Eberl G, Colonna M, Di SJ, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: A new paradigm in immunology. Science. 2015;348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: Partners in host defense. Nat Immunol. 2016;17:758–764. [DOI] [PubMed] [Google Scholar]
- 11.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. [DOI] [PubMed] [Google Scholar]
- 13.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. [DOI] [PubMed] [Google Scholar]
- 14.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. [DOI] [PubMed] [Google Scholar]
- 15.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. [DOI] [PubMed] [Google Scholar]
- 16.Sanos SL, Bui VL, Mortha A, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. [DOI] [PubMed] [Google Scholar]
- 18.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25-and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. [DOI] [PubMed] [Google Scholar]
- 22.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. [DOI] [PubMed] [Google Scholar]
- 24.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai Y, Yasuda K, Sakaguchi Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teunissen MBM, Munneke JM, Bernink JH, et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol. 2014;134:2351–2360. [DOI] [PubMed] [Google Scholar]
- 29.Villanova F, Flutter B, Tosi I, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartemes K, Chen CC, Iijima K, Drake L, Kita H. IL-33-responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus. J Immunol. 2018;200:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MW, Odegaard JI, Mukundan L, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med. 2017;214:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatfield JK, Brown MA. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell Immunol. 2015;297:69–79. [DOI] [PubMed] [Google Scholar]
- 37.Kwong B, Rua R, Gao Y, et al. T-bet-dependent NKp46(+) innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol. 2017;18:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallrapp A, Riesenfeld SJ, Burkett PR, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bal SM, Bernink JH, Nagasawa M, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. [DOI] [PubMed] [Google Scholar]
- 40.Ohne Y, Silver JS, Thompson-Snipes L, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17:646–655. [DOI] [PubMed] [Google Scholar]
- 41.Bernink JH, Krabbendam L, Germar K, et al. Interleukin-12 and −23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. [DOI] [PubMed] [Google Scholar]
- 42.Silver JS, Kearley J, Copenhaver AM, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K, Xu X, Pasha MA, et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J Immunol. 2017;198:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Guo L, Qiu J, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. [DOI] [PubMed] [Google Scholar]
- 46.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. [DOI] [PubMed] [Google Scholar]
- 47.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moro K, Kabata H, Tanabe M, et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17:76–86. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Mao K, Chen X, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klose CSN, Mahlakoiv T, Moeller JB, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardoso V, Chesne J, Ribeiro H, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. [DOI] [PubMed] [Google Scholar]
- 55.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 56.Klein Wolterink RG, Kleinjan A, van Nimwegen M, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. [DOI] [PubMed] [Google Scholar]
- 57.Barlow JL, Bellosi A, Hardman CS, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198 e191–194. [DOI] [PubMed] [Google Scholar]
- 58.Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. [DOI] [PubMed] [Google Scholar]
- 59.Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130:1159–1166 e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. [DOI] [PubMed] [Google Scholar]
- 62.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. [DOI] [PubMed] [Google Scholar]
- 66.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. [DOI] [PubMed] [Google Scholar]
- 68.Van Dyken SJ, Nussbaum JC, Lee J, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stier MT, Bloodworth MH, Toki S, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol. 2016;138:814–824 e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roediger B, Kyle R, Tay SS, et al. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol. 2015;136:1653–1663 e1657. [DOI] [PubMed] [Google Scholar]
- 71.Motomura Y, Morita H, Moro K, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–771. [DOI] [PubMed] [Google Scholar]
- 72.Kim BS, Wang K, Siracusa MC, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193:3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meylan F, Hawley ET, Barron L, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X, Pappu R, Ramirez-Carrozzi V, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochem Biophys Acta. 2011;1810:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med. 2017;214:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salimi M, Stoger L, Liu W, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol. 2017;140:1090–1100 e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macian F NFAT proteins: Key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. [DOI] [PubMed] [Google Scholar]
- 80.Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wojno ED, Monticelli LA, Tran SV, et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fajt ML, Gelhaus SL, Freeman B, et al. Prostaglandin D(2) pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901 e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Russi AE, Ebel ME, Yang Y, Brown MA. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc Natl Acad Sci USA. 2018;115:E1520–E1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maazi H, Patel N, Sankaranarayanan I, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paclik D, Stehle C, Lahmann A, Hutloff A, Romagnani C. ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur J Immunol. 2015;45:2766–2772. [DOI] [PubMed] [Google Scholar]
- 88.Kamachi F, Isshiki T, Harada N, Akiba H, Miyake S. ICOS promotes group 2 innate lymphoid cell activation in lungs. Biochem Biophys Res Comm. 2015;463:739–745. [DOI] [PubMed] [Google Scholar]
- 89.Oliphant CJ, Hwang YY, Walker JA, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. [DOI] [PubMed] [Google Scholar]
- 91.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baral P, Mills K, Pinho-Ribeiro FA, Chiu IM. Pain and itch: Beneficial or harmful to antimicrobial defense? Cell Host Microbe. 2016;19:755–759. [DOI] [PubMed] [Google Scholar]
- 93.Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217–228 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Engel MA, Leffler A, Niedermirtl F, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. [DOI] [PubMed] [Google Scholar]
- 98.Caceres AI, Brackmann M, Elia MD, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA. 2009;106:9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Riol-Blanco L, Ordovas-Montanes J, Perro M, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. Relevance of mast cell-nerve interactions in intestinal nociception. Biochem Biophys Acta. 2012;1822:74–84. [DOI] [PubMed] [Google Scholar]
- 101.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 103.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. [DOI] [PubMed] [Google Scholar]
- 104.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Talbot S, Abdulnour RE, Burkett PR, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basso AS, de Sa-Rocha LC, Palermo-Neto J. Immune-induced flavor aversion in mice: Modification by neonatal capsaicin treatment. NeuroImmunoModulation. 2001;9:88–94. [DOI] [PubMed] [Google Scholar]
- 109.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schoenborn JR, Wilson CB. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. [DOI] [PubMed] [Google Scholar]
- 111.Duerr CU, McCarthy CD, Mindt BC, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molofsky AB, Van Gool F, Liang HE, et al. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bi J, Cui L, Yu G, Yang X, Chen Y, Wan X. NK cells alleviate lung inflammation by negatively regulating group 2 innate lymphoid cells. J Immunol. 2017;198:3336–3344. [DOI] [PubMed] [Google Scholar]
- 114.Matsuki A, Takatori H, Makita S, et al. T-bet inhibits innate lymphoid cell-mediated eosinophilic airway inflammation by suppressing IL-9 production. J Allergy Clin Immunol. 2017;139:1355–1367 e1356. [DOI] [PubMed] [Google Scholar]
- 115.McHedlidze T, Kindermann M, Neves AT, Voehringer D, Neurath MF, Wirtz S. IL-27 suppresses type 2 immune responses in vivo via direct effects on group 2 innate lymphoid cells. Mucosal Immunol. 2016;9:1384–1394. [DOI] [PubMed] [Google Scholar]
- 116.Krishnamoorthy N, Burkett PR, Dalli J, et al. Cutting edge: Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laffont S, Blanquart E, Savignac M, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214:1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu Y, Tsang JC, Wang C, et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature. 2016;539:102–106. [DOI] [PubMed] [Google Scholar]
- 119.Taylor S, Huang Y, Mallett G, et al. PD-1 regulates KLRG1+ group 2 innate lymphoid cells. J Exp Med. 2017;214:1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwartz C, Khan AR, Floudas A, et al. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J Exp Med. 2017;214:2507–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morita H, Arae K, Unno H, et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015;43:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rigas D, Lewis G, Aron JL, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139:1468–1477 e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811 e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malhotra N, Leyva-Castillo JM, Jadhav U, et al. RORalpha-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol. 2018;3:eaao6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moriyama S, Brestoff JR, Flamar AL, et al. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. [DOI] [PubMed] [Google Scholar]
- 127.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mebius RE, Miyamoto T, Christensen J, et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J Immunol. 2001;166:6593–6601. [DOI] [PubMed] [Google Scholar]
- 129.Yu X, Wang Y, Deng M, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. ELife. 2014;3:e04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seehus CR, Aliahmad P, de la Torre B, et al. The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor. Nat Immunol. 2015;16:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Possot C, Schmutz S, Chea S, et al. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nat Immunol. 2011;12:949–958. [DOI] [PubMed] [Google Scholar]
- 132.Klose Christoph SN, Flach M, Möhle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. [DOI] [PubMed] [Google Scholar]
- 133.Ishizuka IE, Chea S, Gudjonson H, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu W, Domingues RG, Fonseca-Pereira D, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. 2015;10:2043–2054. [DOI] [PubMed] [Google Scholar]
- 135.Yagi R, Zhong C, Northrup DL, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serafini N, Klein Wolterink RG, Satoh-Takayama N, et al. Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. J Exp Med. 2014;211:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seillet C, Mielke LA, Amann-Zalcenstein DB, et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 2016;17:436–447. [DOI] [PubMed] [Google Scholar]
- 138.Constantinides MG, Gudjonson H, McDonald BD, et al. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci USA. 2015;112:5123–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spooner CJ, Lesch J, Yan D, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14:1229–1236. [DOI] [PubMed] [Google Scholar]
- 141.Califano D, Cho JJ, Uddin MN, et al. Transcription factor Bcl11b controls identity and function of mature type 2 innate lymphoid cells. Immunity. 2015;43:354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Walker JA, Oliphant CJ, Englezakis A, et al. Bcl11b is essential for group 2 innate lymphoid cell development. J Exp Med. 2015;212:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antignano F, Braam M, Hughes MR, et al. G9a regulates group 2 innate lymphoid cell development by repressing the group 3 innate lymphoid cell program. J Exp Med. 2016;213:1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Q, Monticelli LA, Saenz SA, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mielke LA, Groom JR, Rankin LC, et al. TCF-1 controls ILC2 and NKp46+RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191:4383–4391. [DOI] [PubMed] [Google Scholar]
- 147.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. [DOI] [PubMed] [Google Scholar]
- 148.Zook EC, Ramirez K, Guo X, et al. The ETS1 transcription factor is required for the development and cytokine-induced expansion of ILC2. J Exp Med. 2016;213:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu Y, Wang C, Clare S, et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J Exp Med. 2015;212:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Klein Wolterink RG, Serafini N, van Nimwegen M, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci USA. 2013;110:10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Q, Li D, Zhang X, et al. E3 Ligase VHL promotes group 2 innate lymphoid cell maturation and function via glycolysis inhibition and induction of interleukin-33 receptor. Immunity. 2018;48:258–270 e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monticelli LA, Buck MD, Flamar AL, et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol. 2016;17:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bando JK, Nussbaum JC, Liang HE, Locksley RM. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J Leukoc Biol. 2013;94:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spencer SP, Wilhelm C, Yang Q, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilhelm C, Harrison OJ, Schmitt V, et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J Exp Med. 2016;213:1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pelly VS, Kannan Y, Coomes SM, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016;9:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kouro T, Takatsu K. IL-5-and eosinophil-mediated inflammation: From discovery to therapy. Int Immunol. 2009;21:1303–1309. [DOI] [PubMed] [Google Scholar]
- 162.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–3908. [PubMed] [Google Scholar]
- 164.Pope SM, Brandt EB, Mishra A, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5-and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. [DOI] [PubMed] [Google Scholar]
- 165.Yang M, Hogan SP, Mahalingam S, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–943. [DOI] [PubMed] [Google Scholar]
- 166.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. [DOI] [PubMed] [Google Scholar]
- 168.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. [DOI] [PubMed] [Google Scholar]
- 169.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. [DOI] [PubMed] [Google Scholar]
- 170.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. [DOI] [PubMed] [Google Scholar]
- 171.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independentlyof IL-4 inexperimentalasthma. Science. 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 173.Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilhelm C, Hirota K, Stieglitz B, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2016;9:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turner JE, Morrison PJ, Wilhelm C, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moretti S, Renga G, Oikonomou V, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8:14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rauber S, Luber M, Weber S, et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med. 2017;23:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kubo M Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278:162–172. [DOI] [PubMed] [Google Scholar]
- 182.Liu B, Lee JB, Chen CY, Hershey GK, Wang YH. Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia. J Immunol. 2015;194:3583–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Halim TYF, Rana BMJ, Walker JA, et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48:1195–1207 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Halim TYF, Hwang YY, Scanlon ST, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2015;17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Drake LY, Iijima K, Bartemes K, Kita H. Group 2 innate lymphoid cells promote an early antibody response to a respiratory antigen in mice. J Immunol. 2016;197:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Duffen J, Zhang M, Masek-Hammerman K, et al. Modulation of the IL-33/IL-13 axis in obesity by IL-13Ralpha2. J Immunol. 2018;200:1347–1359. [DOI] [PubMed] [Google Scholar]
- 190.Rak GD, Osborne LC, Siracusa MC, et al. IL-33-Dependent group 2 innate lymphoid cells promote cutaneous wound healing. J Invest Dermatol. 2016;136:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bjorklund AK, Forkel M, Picelli S, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. [DOI] [PubMed] [Google Scholar]
- 192.Gury-BenAri M, Thaiss CA, Serafini N, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166:1231–1246 e1213. [DOI] [PubMed] [Google Scholar]
- 193.Klose CS, Kiss EA, Schwierzeck V, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. [DOI] [PubMed] [Google Scholar]
- 194.Shih HY, Sciume G, Mikami Y, et al. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell. 2016;165:1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lim AI, Menegatti S, Bustamante J, et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]