Abstract
Since 1967, researches have hunted for an etiology for Kawasaki Disease (KD). Meanwhile, the 2019 Coronavirus Disease (COVID-19) pandemic has produced a strange new illness termed multisystem inflammatory syndrome in children (MIS-C) and raised hopes that a cause for KD may be identified. This current review paper discusses KD and its potential connection to pediatric COVID-19 and MIS-C illness.
Keywords: COVID-19, Pediatric multisystem inflammatory syndrome (PMIS), Multisystem inflammatory syndrome in children (MIS-C), Kawasaki disease, Pediatric cardiology
Introduction
The 2019 Coronavirus Disease (COVID-19) pandemic will likely be looked back upon as a testament to the rapid and voracious nature of evidence-based medicine in the 21st century. For comparison, Kawasaki Disease (KD) is one of the most recognized pediatric inflammatory syndromes and took 6 years to be identified and reported by Dr. Tomisaku Kawasaki [1]. Meanwhile, the COVID-19 pandemic has generated more than 19,000 publications of case reports, molecular studies, and clinical trials in less than 3 months’ time.
Most have focused on COVID-19 in adults, as early data suggested that children were spared from severe disease [2]. However, since late April 2020, multiple American and European institutions have brought attention to an enigmatic phenomenon known as multisystem inflammatory syndrome in children (MIS-C) with seeming connections and/or overlapping phenotype to KD [3], [4], [5]. This current review paper will discuss KD and its potential connection to pediatric COVID-19 and MIS-C.
The history of Kawasaki disease
In 1967, Dr. Kawasaki published his detailed case series of 50 children that developed a condition he termed as “acute febrile mucocutaneous lymph node syndrome” [1]. The long term sequel of KD was not recognized until the 1970s, when surveys demonstrated a significant number of fatal cases secondary to coronary artery (CA) aneurysm (CAA) formation, with subsequent thrombosis and stenosis [6].
Nowadays, KD is recognized as the leading cause of acquired CA disease in the pediatric population [7]. Standards of care were established in 1984, following the introduction of high-dose intravenous immunoglobulin (IVIG) to reduce the prevalence of CA abnormalities [8]. Since 2004, the American Heart Association (AHA) has published guidelines describing the management, treatment and long-term management of KD [7]. To date, over 300,000 children have been diagnosed and treated for KD [9]. However, despite emerging treatment options, the precise etiology of KD has remained elusive. KD remains a clinical diagnosis - all diagnostic findings are non-specific.
Cardiac complications of Kawasaki disease
The most concerning complication of KD is CAAs that can be detected within 2 weeks during the convalescent stage of KD [10]. These abnormalities are typically identified by echocardiography. CAAs can be seen in 25% of untreated KD patients, and is reduced to 4% after the introduction of IVIG [7]. Current standard of care involves administering IVIG, along with high-dose oral aspirin, within 10 days (ideally 7 days) from onset of fever [7], [11].
The pathology of CA abnormalities involves three phases [12] - #1) necrotizing arteritis in the acute phase, with neutrophils destroying the wall (tunica media to tunica adventitia) leading to aneurysmal formation; #2) subacute/chronic vasculitis involving T cell lymphocytes, plasma B cells and macrophages infiltrating the vessel wall; and #3) luminal myofibroblastic proliferation, involving myofibroblasts and matrix deposition over months and years that contributes to arterial stenosis. Thus, untreated KD patients with severe CAAs (i.e. large/giant aneurysms) typically do not have any cardiac symptoms in the acute phase, and may present with late manifestations of myocardial ischemia and/or sudden cardiac death secondary to severe CA flow disturbance and/or thrombosis [12]. Some of these patients are missed in the acute phase and are not diagnosed until the therapeutic window has passed, presenting with findings of severe CAAs.
Myocarditis can also occur in the acute phase of KD. Myocardial edema has been found in KD patients before CAA develop [13]. Rarely, true myocardial cell necrosis or permanent cellular loss can develop [14]. Oftentimes there is transient left ventricular (LV) dysfunction and ventricular ectopy [15]. In a small subset of KD patients, this will manifest as KD shock syndrome, including cardiovascular shock and hypotension requiring the use of intravascular volume boluses and vasoactive medications [16].
Laboratory findings of Kawasaki disease
KD patients have characteristic serum lab findings of inflammation, albeit non-specific to other inflammatory disease [17]. Patients in acute phase of KD typically have leukocytosis with a predominance of neutrophils. Elevation of acute-phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) is always present and considered a key criterion particularly in cases of “incomplete” KD [7]. Thrombocytosis can be impressive and occur with peaks as high as 700 × 103/mL. Sterile pyuria is also common. N-terminal pro hormone B-type natriuretic peptide (NT-proBNP) is commonly elevated in KD patients [7] and is useful in diagnosis with a pooled sensitivity of 80–89% and positive likelihood ratio of 3.2 [18], [19]. NT-proBNP may also potentially serve as a prognostic marker for IVIG resistance [19].
Etiologies and epidemiology of Kawasaki disease
The etiology, immunology and pathophysiology of KD are still not fully understood, although elements of the immune system clearly play a role [20], [21]. There are several proposed components, best summarized by Marrani et al. [22].
#1) Response to a pathogen: KD is self-limited with low recurrence rate [23], in addition to having seasonal variation in North America [24]. Additionally, RNA-containing intracytoplasmic inclusion bodies [25] and IgA antibodies [26] can also be found in the bronchial epithelium of KD patients. These studies suggest a viral pathogen activating the immune response via molecular mimicry [22], and subsequent immunity against re-exposure. However, genetic profile has also identified that patients with acute KD have clear differences from other patients with viral disease [27].
#2) T-cell driven autoimmune process: Anti-endothelial antibodies can be found in KD patients [28], resembling other forms of vasculitis such as lupus or anti-phospholipid syndrome. KD patients after IVIG appear to have increased regulatory T cells that affect immunomodulation [21]. However, few studies show association of KD patients with other forms of autoimmune disease.
#3) Aberrant activation of proinflammatory cytokines: During the acute phase, KD patients demonstrate massive release of interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF) [29]. IL-1 in particular has been implicated in CAA formation, leading to current trials investigating the use of IL-1 antagonists (Anakinra) to treat IVIG resistant CAAs [30]. However, autoimmune diseases involving cytokines are usually associated with single gene defects, whereas the KD profile suggests more polygenic influence [31].
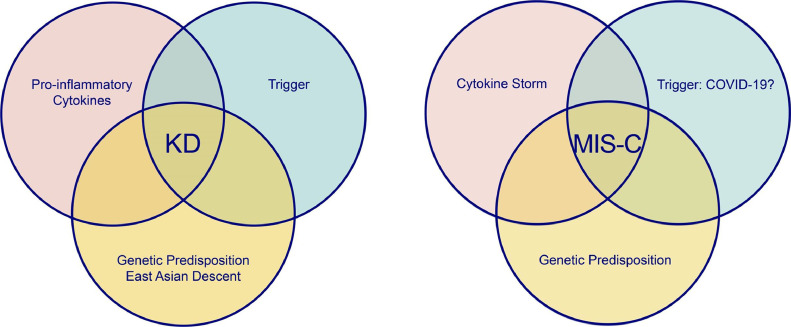
Ultimately, KD can be recognized as a complex inflammatory syndrome that occasionally affects the CAs of patients with genetic predisposition and a trigger. (Fig. 1 ) Children of East Asian and Pacific Islander descent are at highest risk – in Japan, the annual incidence is ~ 260 per 100,000. In the United States (US), the overall incidence is ~ 25 per 100,000, highest amongst Asian Americans (similar to Japan) [32]. Despite decades of research and hundreds of suggested pathogens, ranging from superantigens to rug shampoo [33], [34], no clear-cut antigen has been definitely linked to KD. The rise of COVID-19 and MIS-C has now further challenged our understanding of KD.
Fig. 1.
Pathogenetic Features of Kawasaki Disease and Multisystem Inflammatory Syndrome in Children (MIS-C).
KD: Kawasaki disease, MIS-C: Multisystem inflammatory syndrome in Children.
COVID-19 in pediatric population
Although children of all ages are susceptible to COVID-19, the impact and clinical manifestations have been less severe compared to the adult population. As of June 5th 2020, the US had 66,376 cases of COVID-19 in children <18 years old. The hospitalized rate is low with an estimated rate of 5.7%; the mortality rate is even lower in the US [35]. Proposed mechanisms include a stronger innate immune response compared to adults, and higher angiotensin converting enzyme (ACE)-2 expression which reduces the presence of angiotensin-2, known to correlate with COVID-19 viral load and clinical course [36], [37].
In a cross-sectional study of North American pediatric intensive care units (PICUs), between March and April 2020, 48 children were admitted to 14 PICUs in the US [38]. 73% presented with respiratory symptoms, and 38% required invasive ventilation. At our institution, which covers the District of Columbia (DC) metropolitan region (DC, Maryland, and Virginia), we have observed a steady increase of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) circulation in the 6 weeks since March 15, 2020 [39]. In our cohort, there was an association between other underlying comorbidities and hospitalization.
First report and case definitions for MIS-C
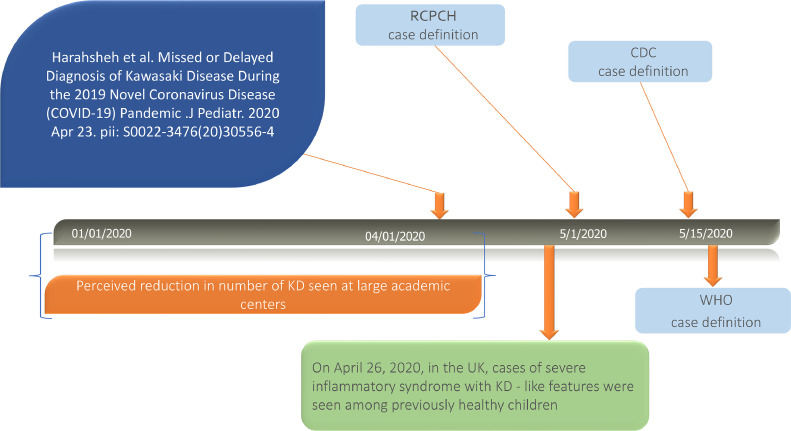
The “stay-at-home” self-quarantine orders, a necessity for controlling the COVID-19 pandemic, initially raised concern amongst pediatric cardiologists that the reduced contact with health care providers and over-emphasis of COVID-19 by Bayesian thinking would potentially lead to under recognition of KD [11]. A concerning surge in presence of CAAs was somewhat anticipated, although there was also speculation that social distancing would reduce exposure of plausible trigger agents for KD.
Thus, it was of great surprise to observe reports of pediatric patients treated for both COVID-19 and KD-like illness (Figure 2 ) [40]. The first case report was published in early April 2020 [41], but the first major call to attention was on April 26, 2020 when National Health Service in the United Kingdom (NHS UK) issued an alert to highlight a rise in cases of critically ill children with overlapping features of toxic shock syndrome, atypical KD and severe COVID-19 infection [42]. This was subsequently highlighted by Royal College of Paediatrics and Child Health (RCPCH), US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) [43], [44], [45]. Case definitions from all three institutions are listed in Table 1 , but none is neither specific nor evidence based. There have been multiple descriptions and names, including Pediatric Multisystem Inflammatory Syndrome (PMIS) and MIS-C. For consistency we will use the US CDC's definition “MIS-C”.
Fig. 2.
Timeline of Kawasaki Disease Through the COVID-19 Pandemic.
CDC: Centers for Disease Control and Prevention, KD: Kawasaki disease, RCPCH: Royal College of Paediatrics and Child Health, UK: United Kingdom, WHO: World health organization. Modified from The Journal of Pediatrics, Vol /edition number 224 , Harahsheh A, Dahdah N, Newburger J, Portman M, Tulloh R, McCrindle B, Cimaz R, Burns J, The COVID-19 Pandemic, From Worrying About Missed or Delayed Diagnosis of Kawasaki Disease to the Flood of Multisystem Inflammatory Syndrome in Children (MIS-C) (Reply), Pages No. pending, Copyright (2020), with permission from Elsevier
Table 1.
Case Definition of the New Multi-Systemic Inflammation Illness Temporarily Associated with the COVID-19 Pandemic.
| NHS- The Royal College of Paediatrics and Child Health | CDC | WHO | |
|---|---|---|---|
| Name of the condition | Paediatric multisystem inflammatory syndrome (PMIS) temporally associated with COVID-19 | Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) | Multisystem inflammatory syndrome in children and adolescents with COVID-19 |
| Release date | 05/01/2020 | 05/14/2020 | 05/15/2020 |
| Age (years) | Child | <21 | 0–19 |
| Fever | Persistent fever >38.5°C | Fever >38.0°C for ≥ 24 hours, or report of subjective fever lasting ≥24 hours | Fever ≥ 3 days |
| Clinical findings | Single or multi-organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal or neurological disorder) with additional features. This may include children fulfilling full or partial criteria for Kawasaki disease | Evidence of clinically severe illness requiring hospitalization, with multisystem (≥ 2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological) | Two of the following:1. Rash or bilateral non-purulent conjunctivitis or muco-cutaneous Inflammation signs (oral, hands or feet).2. Hypotension or shock.3. Cardiac (myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including echocardiographic findings or elevated troponin/NT-proBNP))4. coagulopathy (by PT, APTT, elevated D-dimers)5. Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain) |
| Evidence of COVID infection | SARS-CoV-2 PCR testing may be positive or negative | Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms | Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patients with COVID-19. |
| Evidence of inflammation | Neutrophilia, elevated CRP and lymphopaenia | Including, but not limited to, one or more of the following: an elevated CRP, ESR, fibrinogen, procalcitonin, d-dimer, ferritin, lactic acid dehydrogenase (LDH), or interleukin 6 (IL-6), elevated neutrophils, reduced lymphocytes and low albumin | Elevated markers of inflammation such as ESR, CRP, or procalcitonin. |
| Exclusion of other microbial cause | Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus (waiting for results of these investigations should not delay seeking expert advice) | No alternative plausible diagnoses | No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes. |
| Special note | May include children fulfilling full or partial criteria for KD. | Consider MIS-C in any pediatric death with evidence of SARS-CoV-2 infection Some individuals may fulfill full or partial criteria for Kawasaki disease but should be reported if they meet the case definition for MIS-C | |
| Reference | https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatricmultisystem-%20inflammatory%20syndrome-20200501.pd | https://emergency.cdc.gov/han/2020/han00432.asp | https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 |
APTT: Activated partial thromboplastin time, CDC: Centers for Disease Control and Prevention, CRP: C-reactive protein, COVID-19: 2019 Novel Coronavirus Disease, ESR: Erythrocyte sedimentation rate, KD: Kawasaki disease, NHS: National Health Service, NT-proBNP: N-terminal pro hormone B-type natriuretic peptide, PT: Prothrombin time, RT-PCR: Reverse transcription polymerase chain reaction, SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2, WHO: World health organization.
MIS-C clinical data
To date, the clinical data related to MIS-C are mainly collected from COVID-19 epicenters in Europe and the US. Multiple case series have been published in eastern US, Italy, UK and France [3], [4], [46], [47], [48], [49], [50], [51], [52]. The respective findings from several case series (with the largest representation for each region; combined for the US) are summarized in Table 2 and Table 3 . As of 6/16/2020, at least 130 patients have been reported in publications. The New York State Department of Health also identified 211 patients with similar presentations [53].
Table 2.
Clinical Features, Treatment, and Outcome of Multisystem Inflammatory Syndrome in Children (MIS-C). Clinical data extracted from Verdoni et al [47], Belhadjer et al [46], Whittaker et al [49], Cheung et al [50], Chiotos et al [48] and Waltuch et al [4] and grouped by country. For simplicity, Chiotos et al and Waltuch et al study information were combined.
| Italy | France and Switzerland | United Kingdom | United States | Summary | |
|---|---|---|---|---|---|
| Reference Source | Verdoni et al (Bergamo, Italy) | Belhadjer et al. (13 hospitals in France and 1 hospital in Switzerland) | Whittaker et al. (8 hospitals in the UK; includes 8 patients from Riphagen et al.) | § - Cheung et al (Morgan Stanley Children's Hospital, New York); ¶ - Chiotos et al (CHOP, Philadelphia) and Waltuch et al (Mount Sinai, New York) | |
| Number | 10 | 35 | 58 | § - 17 ¶ - 10 | 130 |
| Age (median + IQR) | 7.45 (6.5 – 8.45) | 10 | 9 (5.7 – 14) | § - 8 (1.8 – 16) ¶- 9.5 (5.25 – 12) | |
| Sex (%M) | 70% | 51% | 43% | 44% | |
| Reported Race/Ethnicity | N/A | N/A | 22 black, 18 Asian, 12 white, 6 other | 6 black, 4 Non-Hispanic white, 4 Hispanic, 6 Ashkenazi Jewish, 1 Asian, the rest unknown | |
| COVID Status | |||||
| COVID AB Assay positive | 8 | 30 | 40 | 18 | 74% |
| RT-PCR positive | 2 | 14 | 15 | 11 | 32% |
| Symptoms | |||||
| Fever | 6 Abdominal5 Complete KD5 Incomplete KD | 35 | 58 | 27 | 100% |
| Abdominal Symptoms | 29 | 31 | 24 | 70% | |
| Respiratory Symptoms | 23 | 12 | 13 | 40% | |
| Skin Rash | 20 | 30 | 16 | 55% | |
| Conjunctivitis | 31 | 26 | 16 | 61% | |
| Lymphadenopathy | 21 | 9 | 7 | 31% | |
| Extremity Edema | N/A | 9 | 2 | 26% | |
| Fissured lips/Strawberry Tongue | *&*) | 19 | 17 | 13 | 41% |
| Cardiac Features | |||||
| Chest pain | 0 | 6 | 0 | 0 | 5% |
| Shock (requiring fluids or vasoactives) | 5 | 28 | 27 | 23 | 64% |
| Arrhythmia | 0 | 1 | 4 | 3 | 6% |
| Coronary artery dilation (z-score >2; or >4 mm) | 2 | 6 | 8 (2 giant coronary artery aneurysms) | 4 (7 with prominent/echogenic coronaries) | 15% |
| Decreased ventricular function (EF < 55%) | 5 | 35 | 18 | 15 | 56% |
| Respiratory Features | |||||
| Positive CXR finding | 5 | N/A | N/A | 17 | |
| Non-invasive | N/A | 11 | N/A | 4 | |
| Mechanical Vent | N/A | 22 | 25 | 12 | |
| Treatment | |||||
| Vasoactive Support | 2 | 28 | 27 | 17 | 57% |
| IVIG | 10 | 25 | 41 | 22 | 75% |
| Steroids | 8 | 12 | 37 | 19 | 59% |
| IL-1 receptor antagonist (Anakinra) | 0 | 3 | 3 | 2 | 6% |
| IL-6 receptor antagonist (tocilizumab) | 0 | 0 | 0 | 4 | 3% |
| TNF-alpha antagonist (Infliximab) | 0 | 0 | 8 | 0 | 6% |
| Outcomes | |||||
| VA-ECMO | 0 | 10 | 3 | 0 | 10% |
| Death | 0 | 0 | 1 | 0 | 0.77% |
AB: Antibody, CXR: chest X-ray, EF: Ejection fraction, IVIG: Intravenous immunoglobulin, IL: Interleukin, KD: Kawasaki disease, N/A: not available, VA-ECMO: Veno-arterial extracorporeal membrane oxygenation.
Table 3.
Laboratory Finding of Multisystem Inflammatory Syndrome in Children (MIS-C). Clinical data extracted from Verdoni et al [47], Belhadjer et al [46], Whittaker et al [49], Cheung et al [50], Chiotos et al [48] and Waltuch et al [4] and grouped by country. For simplicity, Chiotos et al and Waltuch et al study information were combined. Verdoni et al lab findings are reported as mean (standard deviation), the rest are reported in median and interquartile ranges. *: NT-pro-BNP <300 is normal for adults (excluding the elderly); in children, the normal upper limit is much higher in infants (from newborn to 2 years of age) and progressively decreases in the first 6 years to adult range) [76]. **: The method of troponin testing was highly variable across case series. Belhadjer et al measured high-sensitivity troponin-I levels, whereas Cheung et al used high-sensitivity troponin-T levels. Verdoni et al reported troponin I levels, although the use of high-sensitivity lab technique was not specified. Whittaker et al, Cheung et al, Chiotos et al and Waltuch et al did not specify whether troponin-I or troponin-T was used, nor the use of high-sensitivity lab technique.
| Range | Italy | France and Switzerland | United Kingdom | United States | |
|---|---|---|---|---|---|
| Reference Source | Verdoni et al (Bergamo, Italy) | Belhadjer et al. (13 hospitals in Frances and 1 hospital in Switzerland) | Whittaker et al. (13 hospitals in the UK; includes the 8 patients from Riphagen et al.) | § - Cheung et al (Morgan Stanley Children's Hospital, New York); ¶ - Chiotos et al (CHOP, Philadelphia) and Waltuch et al (Mount Sinai, New York) | |
| Mean (SD) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| WBC (x103/L) | <12 | 10.8 (6.1) | 16 (12 – 23) | 17 (12 – 22) | § - 14.0 (4 – 35.9) ¶ - 20.6 (11.1 – 35.7) |
| Absolute lymphocyte count | >950 | 860 (40) | N/A | 800 (500 – 1500) | § - 1212.1 (115.5 – 6444.9) ¶ - 310 (262 – 412) |
| Platelets (103/uL) | <150 | 130 (32) | N/A | 151 (104 – 210) | § - 237 (69 – 892) ¶ - 120 (96.5 – 166.25) |
| Peak BNP (pg/mL) | <100 | N/A | 4256 (2340 – 6503) | N/A | § - N/A ¶ - 1411 (517 – 3068) |
| Peak NT-pro BNP (pg/mL) | * | 1255 (929) | 41484 (35811 – 52475) | 788 (172 – 10548) | § - 15833 (631 – 59291) ¶ - N/A |
| Peak Troponin (ng/mL) | ** | 1.0 (1.862) | 0.408 (0.258 – 0.679) | 0.045 (0.008 – 0.294) | § - 0.0568 (0.006 – 0.278) ¶ - 0.24 (0.0675 – 0.8775) |
| CRP (mg/dL) | <0.9 | 25 (15.3) | 24.1 (15.0 – 31.1) | 22.9 (15.6 – 33.8) | § - 20.0 (1.7 – 30.0) ¶ - 26.7 (17.6 – 30.2) |
| D-Dimer (ug/mL) | <0.5 | 3.798 (1.318) | 5.284 (4.069 – 9.095) | 3.578 (2.085 – 8.235) | § - 4 (0.9 – 11) ¶ - 4.46 (3.3 – 11.5) |
| Pro-Calcitonin (ng/mL) | <2 | N/A | 36 (8 – 99) | N/A | § - 21.7 (0.8 – 127) ¶ - 16.0 (15.08 – 58.85) |
| IL-6 (pg/mL) | <8.5 | 117.1 (137.4) | 135 (87 – 175) | N/A | § - 226.3 (3.1 – 315) ¶ - 330 (268 – 406.5) |
| Ferritin (ng/mL) | <150 | 1176 (1032) | N/A | 610 (359 – 1280) | § - 647.9 (83 – 1828) ¶ - 973 (762 – 1188) |
CRP: C-reactive protein, IL: Interleukin, NT-proBNP: N-terminal pro hormone B-type natriuretic peptide, WBC: White blood cells.
Symptoms included fever with variable degree of respiratory symptoms and chest X-ray findings. 70% of patients had abdominal pain/diarrhea as presenting symptom. Regarding COVID-19 exposure, 74% had positive antibody test and 32% had positive PCR test. Most of the affected children received IVIG (75%), with 57% requiring vasoactive support and 13 patients (10%) requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO) as part of mechanical circulatory assistance. In France, all patients were weaned off VA-ECMO. There was one death reported in the UK (secondary to cerebral ischemic infarction). None of the US case series reported any deaths, however 2 deaths were reported by the New York State Department of Health [49], [53].
The overall MIS-C presentation appears to overlap KD with noticeable differences. A significant portion of patients had findings consistent with KD including rash, conjunctivitis, lymphadenopathy, extremity edema and fissured lips. 56% had evidence of decreased LV function by echocardiography. Cardiac magnetic resonance imaging in several of these patients demonstrated increased signal intensity on T1 and T2-weighted imaging, consistent with diffuse myocardial edema, with no enhancement on late gadolinium imaging to suggest fibrosis [52]. Interestingly, only 15% had documented CA dilation, although the interpretation of CAA is affected by variations in image interpretation (i.e. absolute measurement vs. use of pediatric normative standards) and timing of echocardiography (i.e. whether echocardiography was performed before, during, or after immune modulation therapy). Laboratory findings include elevated troponin, NT-proBNP, inflammatory markers, as well as lymphopenia and thrombocytopenia (Table 3).
Is MIS-C a form of KD?
Proponents of a connection between MIS-C and KD would argue that both conditions have a common trigger that provokes a cascade reaction in genetically susceptible children leading to the phenotype described above (Fig. 1).
Opponents would argue that MIS-C children present with (1) older age of presentation, (2) more profound form of inflammation than that of KD or KD shock syndrome, (3) more gastrointestinal manifestation, (4) different laboratory findings including lymphopenia, thrombocytopenia, elevated troponin, elevated NT-proBNP, elevated D-Dimer, and elevated ferritin, and (5) have higher propensity towards LV dysfunction and shock compared to KD patients. (Table 4 ). They would also highlight how MIS-C seem to have less propensity towards children of east Asian and Pacific Islander descent [49], [54]. The clinical patterns resemble that of toxic shock syndrome, typically the result of massive cytokine production, leading to capillary leak and subsequent hypotension [55].
Table 4.
Comparison Between Multisystem Inflammatory Syndrome in Children, Kawasaki Disease, and Kawasaki Disease Shock Syndrome
| MIS-C | KD | KD-shock syndrome | |
|---|---|---|---|
| Median Age - Years (IQR) | 9 (5.7-14) | 2.7 (1.4-4.7) | 3.8 (0.2-18) |
| WBC | ↑↑ | ↑ | ↑ |
| Lymphocytes | ↓ | Normal | Normal |
| Neutrophils | ↑↑ | ↑ | ↑ |
| Hemoglobin | ↓↓ | Normal or ↓ | Normal or ↓ |
| Platelets | ↓ | ↑↑ | ↑↑ |
| CRP | ↑↑↑ | ↑ | ↑↑ |
| Ferritin | ↑ | Normal | Normal |
| Albumin | ↓↓ | Normal or ↓ | ↓ |
| NT-proBNP | ↑↑↑ | Normal or ↑ | ↑↑ |
| Troponin | ↑ | Normal | Normal |
| D-Dimer | ↑↑↑ | ↑ | ↑↑ |
Comparison extracted from Whittaker et al [49]. CRP: C-reactive protein, KD: Kawasaki disease, NT-proBNP: N-terminal pro hormone B-type natriuretic peptide, MIS-C: Multisystem Inflammatory Syndrome in Children.
Proponents of the MIS-C and KD connection would counter-argue that laboratory values difference noted in the Whittaker et al comparison might be in part explained by the different genetic compositions of the cohorts that were compared. The UK series showed that 37% of the patients were of African descent whereas the KD San Diego cohort contains a large Hispanic portion [49], [56]. They would also highlight that the geographic variation may be related to the differences in the sequences of SARS-CoV-2 [57]. The COVID-19 genomic data from GISAID [58] demonstrates that several variants (spike protein) have occurred more frequently in European countries and the eastern US, correlating with regions that produced the case series for MIS-C. Meanwhile, in regions where other variants of SARS-CoV-2 are more prevalent [59], [60], such as East Asian countries and the western US, there has been a paucity of reports despite being historically prevalent for KD. One recent computer model has suggested that the spike mutations create a structure motif similar to those of bacterial superantigens, directly binding to T cell receptors and potentially activating the cytokine storm observed in MIS-C [61]. The difference in race/ethnicity suggests that MIS-C is activated by a trigger (possibly a virus) in a population with different genetic susceptibility compared to KD.
The Cytokine storm
The massive activation of pro-inflammatory cytokines in MIS-C patients overlaps laboratory findings in both KD and the “cytokine storm” observed in adult patients with COVID-19 [62], [63]. Cytokine storm is observed in many viral infections and toxic shock syndrome [55], [63]; in the case of SARS-CoV-2, there is an associated increase in proinflammatory cytokines as well as macrophage activation [64]. Significant predictors of mortality in COVID-19 adult patients have included elevated serum ferritin and IL-6 [62]. The cytokine storm pattern in MIS-C has also implicated macrophage activating syndrome (MAS) [47], which is seen in KD and autoimmune diseases such as systemic lupus erythematosus [65]. This has informed the use of steroids to treat some MIS-C patients [47]. However, while these patients met ferritin criteria for MAS, the degree of elevation is considerably lower compared to historical cohorts (median 9,094 ng/mL, IQR of 2,000–19,767 ng/mL) [65].
Coronavirus as a trigger
The role of coronavirus as a potential trigger for KD has been previously proposed with mixed results. Coronavirus has been noted in KD patients but not in exclusive fashion (i.e. concurrent viral illness) [66]. Specific strains of coronavirus have also been associated with KD in small case series [67] that could not be reproduced in other locations [68]. The viral genomics of coronavirus challenges the identification of any triggers for KD, as coronavirus is a positive sense RNA virus [69] and its genome is prone to mutation and genetic drift [59]. Still, the absence of preceding symptoms, the 3 – 6 week lag between virus prevalence and MIS-C prevalence, and higher frequency of positive antibody results suggests that SARS-CoV-2 acts as a trigger [5].
“GOK”
It is poignant and bittersweet to note that the quick recognition of MIS-C is only possible because of meticulous research conducted by Dr. Tomisaku Kawasaki, who recently passed away on June 5th 2020. Dr. Kawasaki observed an unusual sickness not referenced in literature; as he saw more cases, he kept detailed observations in a folder labeled as “GOK” [70] - “God Only Knows.”
In the six weeks since the first NHS UK Alert, pediatricians and cardiologists now recognize the significance of MIS-C and a “call to action” [5], [11], [40], [71], [72] to prevent CAAs. Should we consider MIS-C as the same as KD? To conclude this review, the answer is complex and challenged by the unclear pathophysiology of both KD and MIS-C. If one were to consider KD as part of an inflammatory syndrome [22], it is reasonable to consider MIS-C as a unique and ubiquitous form [5] along its spectrum.
Nonetheless, the presentation of CAAs in a small but significant portion of MIS-C patients warrants caution and continued surveillance. Cardiologists will continue to have a role in providing accurate assessment of CAs and myocardial function to guide management [7]. Long-term studies are essential for MIS-C and COVID-19 patients, in the form of registry data and genomics [59], [73], [74], [75]. These studies will help define the clinical course, epidemiology, mechanism of MIS-C – and potentially even KD itself. The COVID-19 pandemic may serve as both challenge and opportunity for us to understand an inflammatory syndrome that “God Only Knows.”
Footnotes
Acknowledgment: The authors are grateful for Dr Angela J Doty and Lindsay Attaway for their editorial assistance.
Funding disclosures: None.
Author Contributions: Yue-Hin Loke was involved in paper design and drafting the manuscript. Charles I. Berul was involved in revising the manuscript. Ashraf S. Harahsheh was involved in paper design and revising the manuscript. This submission is with the full knowledge and approval of the listed co-authors.
Declaration of Competing Interest: None.
References
- 1.Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children] Arerugi Allergy. Mar 1967;16(3):178–222. [PubMed] [Google Scholar]
- 2.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;Apr 23;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. May 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. May 2020 doi: 10.1016/j.ajem.2020.05.058. S0735675720304034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrindle BW, Manlhiot C.Kawasaki disease and SARS-CoV-2–related multisystem inflammatory syndrome in children. 2020;3. [DOI] [PubMed]
- 6.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. Sep 1974;54(3):271–276. [PubMed] [Google Scholar]
- 7.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation [Internet] Apr 25, 2017;135(17) doi: 10.1161/CIR.0000000000000484. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000484 [cited 2020 Jun 7]Available from. [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. Aug 7, 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y. Kawasaki disease: epidemiology and the lessons from it. Int J Rheum Dis. Jan 2018;21(1):16–19. doi: 10.1111/1756-185X.13211. [DOI] [PubMed] [Google Scholar]
- 10.de Ferranti SD, Gauvreau K, Friedman KG, Tang A, Baker AL, Fulton DR, et al. Association of initially normal coronary arteries with normal findings on follow-up echocardiography in patients with Kawasaki disease. JAMA Pediatr. 2018;172(12) doi: 10.1001/jamapediatrics.2018.3310. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harahsheh AS, Dahdah N, Newburger JW, Portman MA, Piram M, Tulloh R, et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. Jul 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize kawasaki disease: a light and transmission electron microscopic study. PLoS One. Jun 18, 2012;7(6):e38998. doi: 10.1371/journal.pone.0038998. Moretti C, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada M, Yokouchi Y, Oharaseki T, Matsui K, Tobayama H, Tanaka N, et al. Histopathological characteristics of myocarditis in acute-phase Kawasaki disease: Myocarditis in Kawasaki disease. Histopathology. Dec 2012;61(6):1156–1167. doi: 10.1111/j.1365-2559.2012.04332.x. [DOI] [PubMed] [Google Scholar]
- 14.Yonesaka S, Takahashi T, Eto S, Sato T, Otani K, Ueda T, et al. Biopsy-proven myocardial sequels in Kawasaki disease with giant coronary aneurysms. Cardiol Young. Dec 2010;20(06):602–609. doi: 10.1017/S1047951109991132. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda E, Arakaki Y, Shimizu T, Sakaguchi H, Yoshimura S, Yazaki S, et al. Changes in causes of sudden deaths by decade in patients with coronary arterial lesions due to Kawasaki disease. Cardiol Young. Oct 2005;15(5):481–488. doi: 10.1017/S1047951105001344. [DOI] [PubMed] [Google Scholar]
- 16.Gatterre P, Oualha M, Dupic L, Iserin F, Bodemer C, Lesage F, et al. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. May 2012;38(5):872–878. doi: 10.1007/s00134-012-2473-8. [DOI] [PubMed] [Google Scholar]
- 17.Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with kawasaki disease. Pediatr Infect Dis J. Dec 2011;30(12):1022–1026. doi: 10.1097/INF.0b013e31822d4f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K-H, Chang S-S, Yu C-W, Lin S-C, Liu S-C, Chao H-y, et al. Usefulness of natriuretic peptide for the diagnosis of Kawasaki disease: a systematic review and meta-analysis. BMJ Open. Apr 14, 2015;5(4) doi: 10.1136/bmjopen-2014-006703. –e006703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionne A, Dahdah N. A decade of NT-proBNP in acute Kawasaki disease, from physiological response to clinical relevance. Children. Oct 12, 2018;5(10):141. doi: 10.3390/children5100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco A, Shimizu C, Tremoulet AH, Burns JC. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity. Jun 2010;43(4):317–324. doi: 10.3109/08916930903405891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco A, Touma R, Song Y, Shimizu C, Tremoulet AH, Kanegaye JT, et al. Specificity of regulatory T cells that modulate vascular inflammation. Autoimmunity. Mar 2014;47(2):95–104. doi: 10.3109/08916934.2013.860524. [DOI] [PubMed] [Google Scholar]
- 22.Marrani E, Burns JC, Cimaz R. How should we classify kawasaki disease? Front Immunol. Dec 14, 2018;9:2974. doi: 10.3389/fimmu.2018.02974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddox RA, Holman RC, Uehara R, Callinan LS, Guest JL, Schonberger LB, et al. Recurrent Kawasaki disease: USA and Japan: recurrent KD: USA and Japan. Pediatr Int. Dec 2015;57(6):1116–1120. doi: 10.1111/ped.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JC, Herzog L, Fabri O, Tremoulet AH, Rodó X, Uehara R, et al. Seasonality of Kawasaki disease: a global perspective. PLoS One. Sep 18, 2013;8(9):e74529. doi: 10.1371/journal.pone.0074529. Convertino M, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowley AH, Baker SC, Shulman ST, Garcia FL, Fox LM, Kos IM, et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS One. Feb 13, 2008;3(2):e1582. doi: 10.1371/journal.pone.0001582. Ratner A, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. Jan 15, 2001;166(2):1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 27.Hoang LT, Shimizu C, Ling L, Naim ANM, Khor CC, Tremoulet AH, et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. Nov 2014;6(11):541. doi: 10.1186/s13073-014-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunebaum E, Blank M, Cohen S, Afek A, Kopolovic J, Meroni PL, et al. The role of anti-endothelial cell antibodies in Kawasaki disease - in vitro and in vivo studies. Clin Exp Immunol. Nov 2002;130(2):233–240. doi: 10.1046/j.1365-2249.2002.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. Sep 2005;141(3):381–387. doi: 10.1111/j.1365-2249.2005.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillaume M-P, Reumaux H, Dubos F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol Young. May 2018;28(5):739–742. doi: 10.1017/S1047951117002864. [DOI] [PubMed] [Google Scholar]
- 31.Onouchi Y, Ozaki K, Burns JC, Shimizu C, Japan Kawasaki Disease Genome Consortium, US Kawasaki Disease Genetics Consortium A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. May 2012;44(5):517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]
- 32.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patriarca P, Morens D, Rogers M, Schonberger L, Kaminski R, Burns J, et al. Kawasaki syndrome: association with the application of rug shampoo. Lancet. Sep 1982;320(8298):578–580. doi: 10.1016/s0140-6736(82)90660-2. [DOI] [PubMed] [Google Scholar]
- 34.Al-Abbadi MA, Abuhammour W, Harahsheh A, Abdel-Haq NM, Hasan RA, Saleh HA. Conjunctival changes in children with Kawasaki disease: cytopathologic characterization. Acta Cytol. Jun 2007;51(3):370–374. doi: 10.1159/000325749. [DOI] [PubMed] [Google Scholar]
- 35.Bialek S, Gierke R, Hughes M, McNamara LA, CDC COVID-19 Response Team, CDC COVID-19 Response Team et al. Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. Apr 10, 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. May 8, 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr [Internet] May 11, 2020 doi: 10.1001/jamapediatrics.2020.1948. https://jamanetwork.com/journals/jamapediatrics/fullarticle/2766037 [cited 2020 Jun 7]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, et al. Severe COVID-19 in children and young adults in the Washington, DC Metropolitan Region. J Pediatr. May 2020 doi: 10.1016/j.jpeds.2020.05.007. S0022347620305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harahsheh AS, Dahdah N, Newburger JW, Portman MA, Tulloh R, McCrindle BW, et al. The COVID-19 pandemic, from worrying about missed or delayed diagnosis of Kawasaki disease to the flood of multisystem inflammatory syndrome in children (MIS-C) (Reply) J Pediatr. Jun 2020;S0022-3476(20)30826-X doi: 10.1016/j.jpeds.2020.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. Jun 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 42.Pediatric Intensive Care Society. PICS Statement: Increased number of reported cases of novel presentation of multisystem inflammatory disease. 2020 Apr p. 2.
- 43.Royal College of Paediatrics and Child Health. Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 [Internet]. 2020. Available from: http://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf.
- 44.US Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) [Internet]. 2020. Available from:)https://emergency.cdc.gov/han/2020/han00432.asp.
- 45.World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 46.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. May 17, 2020;120 doi: 10.1161/CIRCULATIONAHA.120.048360. CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- 47.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. Jun 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatr Infect Dis Soc. May 28, 2020 doi: 10.1093/jpids/piaa069. piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA [Internet] Jun 8, 2020 doi: 10.1001/jama.2020.10369. https://jamanetwork.com/journals/jama/fullarticle/2767209 [cited 2020 Jun 9]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA [Internet] Jun 8, 2020 doi: 10.1001/jama.2020.10374. https://jamanetwork.com/journals/jama/fullarticle/2767207 [cited 2020 Jun 9]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. Dec 2020;10(1):69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. Jun 9, 2020 doi: 10.1148/radiol.2020202288. 202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.New York State Department of Health. Childhood inflammatory disease related to COVID-19 [Internet]. Available from: https://coronavirus.health.ny.gov/childhood-inflammatory-disease-related-covid-19.
- 54.Shulman ST. Pediatric COVID-associated multi-system inflammatory syndrome (PMIS) J Pediatr Infect Dis Soc. May 22, 2020 [Google Scholar]
- 55.Low DE. Toxic shock syndrome. Crit Care Clin. Jul 2013;29(3):651–675. doi: 10.1016/j.ccc.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Portman MA. Cimaz R.Should Coronavirus Disease 2019-Associated Inflammatory Syndromes in Children Affect Social Reintegration? JAMA Pediatr. 2020;20 doi: 10.1001/jamapediatrics.2020.2810. [DOI] [PubMed] [Google Scholar]
- 57.Benvenuto D, Angeletti S, Giovanetti M, Bianchi M, Pascarella S, Cauda R, et al. Evolutionary analysis of SARS-CoV-2: how mutation of Non-Structural Protein 6 (NSP6) could affect viral autophagy. J Infect. Jul 2020;81(1):e24–e27. doi: 10.1016/j.jinf.2020.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall Hoboken NJ. Jan 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koyama T, Weeraratne D, Snowdon JL, Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens. Apr 26, 2020;9(5):324. doi: 10.3390/pathogens9050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Jackson CB, Mou H, Ojha A, Rangarajan ES, Izard T, et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv 2020 Jun 12;2020.06.12.148726. doi: 10.1101/2020.06.12.148726. [DOI]
- 61.Cheng MH, Zhang S, Porritt RA, Arditi M, Bahar I. An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations [Internet] Immunology. May 2020 http://biorxiv.org/lookup/doi/10.1101/2020.05.21.109272 [cited 2020 Jun 13]. Available from. [Google Scholar]
- 62.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. Mar 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang F-Y, Chen H-C, Chen P-J, Ho M-S, Hsieh S-L, Lin J-C, et al. Immunologic aspects of characteristics, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) J Biomed Sci. 2020;27(1):72. doi: 10.1186/s12929-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung CY, Poon LLM, Ng IHY, Luk W, Sia S-F, Wu MHS, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. Jun 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a european league against rheumatism/american college of rheumatology/paediatric rheumatology international trials organisation collaborat: eular/acr classification criteria for mas. Arthritis Rheumatol. Mar 2016;68(3):566–576. doi: 10.1002/art.39332. [DOI] [PubMed] [Google Scholar]
- 66.Turnier JL, Anderson MS, Heizer HR, Jone P-N, Glodé MP, Dominguez SR. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. Sep 2015;136(3):e609–e614. doi: 10.1542/peds.2015-0950. [DOI] [PubMed] [Google Scholar]
- 67.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. Feb 15, 2005;191(4):499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Lack of association between new haven coronavirus and Kawasaki disease. J Infect Dis. Jul 15, 2005;192(2):351–352. doi: 10.1086/430797. author reply 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. Apr 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schudel M. Tomisaku Kawasaki, doctor who identified inflammatory disease in children, dies at 95. The Washington Post [Internet]. 2020 Jun 13; Available from: https://www.washingtonpost.com/local/obituaries/tomisaku-kawasaki-doctor-who-identified-inflammatory-disease-in-children-dies-at-95/2020/06/13/35510b8e-ada0-11ea-9063-e69bd6520940_story.html.
- 71.Bassareo PP, Calcaterra G, Fanos V. Covid-19, Kawasaki disease, and multisystem inflammatory syndrome in children. J Pediatr. Jun 2020 doi: 10.1016/j.jpeds.2020.06.033. S0022347620307344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calabri GB, Formigari R. Covid-19 and Kawasaki disease: a glimpse at the past for a predictable future. Pediatr Cardiol [Internet] May 27, 2020 doi: 10.1007/s00246-020-02385-0. http://link.springer.com/10.1007/s00246-020-02385-0 [cited 2020 Jun 13]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park YR, Kim J-J, Yoon YJ, Yoon Y-K, Koo HY, Hong YM, et al. Establishment of Kawasaki disease database based on metadata standard. Database J Biol Databases Curation. 2016;2016 doi: 10.1093/database/baw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manlhiot C, Newburger JW, Low T, Dahdah N, Mackie AS, Raghuveer G, et al. Low molecular weight heparin vs. warfarin for thromboprophylaxis in children with coronary artery aneurysms after kawasaki disease: a pragmatic registry trial. Can J Cardiol. Jun 16, 2020 doi: 10.1016/j.cjca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 75.McCrindle B, Manlhiot C, Newburger J, Harahsheh A, Giglia T, Dallaire F et al. Medium-term complications associated with coronary artery aneurysms after Kawasaki disease: a study from the international Kawasaki disease registry. J Am Heart Assoc Accepted, DOI pending. [DOI] [PMC free article] [PubMed]
- 76.Nir A, Lindinger A, Rauh M, et al. NT-Pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009;30(1):3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]