Abstract
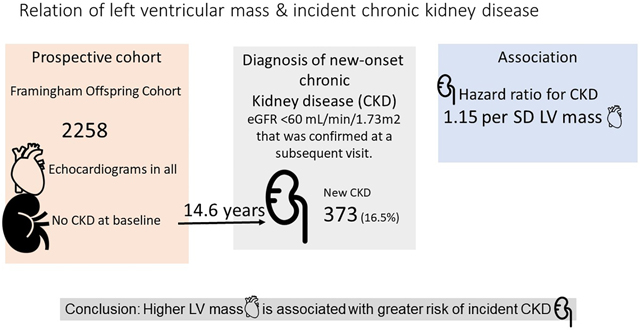
Chronic kidney disease (CKD) is associated with incident cardiovascular morbidity and mortality. Whether subclinical cardiovascular disease and target organ damage is associated with incident CKD is unknown. We investigated the relations of echocardiographic left ventricular (LV) mass with incident CKD. We evaluated 2258 Framingham Offspring cohort participants (mean age 57, 56% women) who underwent echocardiography at a routine examination and had an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73m2. We used Cox proportional-hazards regression with discrete time intervals to relate sex-standardized LV mass (independent variable) to the incidence of CKD, defined as eGFR <60 L/min/1.73m2, on follow-up. During a median follow-up of 14.6 years, 373 (16.5%) participants developed incident CKD. Higher LVM was associated with higher risk of CKD after adjusting for prevalent CVD, body mass index, systolic blood pressure, total and HDL cholesterol, anti-hypertensive medication, smoking and diabetes, (HR) and 95% confidence interval 1.15 (95% CI 1.03–1.29, p<=0.017) per 1 standard deviation (SD) increase in LV mass g/m2. Further adjustment for baseline eGFR (aHR 1.16 (95% CI 1.04 – 1.31, p=0.010) and baseline urine albumin/creatinine ratio (aHR 1.18 (95% CI 1.04 – 1.33, p=0.009) slightly attenuated the association. In our community-based sample, LV mass was associated with incident CKD prospectively, which suggests that the relations between CKD and subclinical cardiovascular disease may be bidirectional. Further studies are needed to confirm our findings.
Keywords: chronic kidney disease, target-organ damage, renal risk factor, cardiovascular disease, left ventricular hypertrophy, longitudinal study
Graphical Abstract
Introduction
Nearly four decades ago the Framingham Heart Study reported that proteinuria was associated with increased all-cause and cardiovascular mortality.(1) It is now well recognized that chronic kidney disease (CKD) is associated with an increased risk of cardiovascular disease (CVD) morbidity and mortality.(2–4) Data from multiple pooled cohorts also demonstrate the independent association of CKD with cardiovascular and all-cause mortality.(5) In those with pre-existing heart disease, such as heart failure, an elevated serum creatinine is a powerful indicator for future risk of CVD mortality.(6) Even more intriguing is the observation that healthy kidney donors develop an increase in left ventricular mass within a year after donating their kidneys, even though they may not experience any change in their 24-hour ambulatory blood pressure.(7) What remains less clear is whether the relation between CKD and subclinical CVD is bidirectional, i.e., does cardiovascular target organ damage portend an increased risk for incident CKD?(8)
We tested the hypothesis that higher left ventricular (LV) mass, as assessed by an echocardiogram, is associated with an increased risk of new-onset CKD.
Methods
Framingham Offspring data and materials in the current study will be made publicly available through the National Institute of Health Database Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) of the National Heart Lung and Blood Institute, Bethesda.
Participants
Framingham Offspring participants who attended examination cycle 6 (1995–1998) were eligible for the study (n=3532). Among the 3532 participants, 1274 participants were excluded for the following reasons: missing creatinine measurement at exam 6 (n=80), eGFR<60 at exam 6 (n=320), no eGFR measurement at any point during follow-up at examinations 7, 8, or 9 (n=196), or missing LVM values (n=678). There were 2258 participants in the final study sample (Figure 1).
Figure 1:

Study flow is shown. Among the 3532 participants at visit 6, 2258 were included in the study of which 1911 had complete data on all covariates.
Risk factor assessment
Demographic, anthropometric (height, weight, body mass index), and cardiovascular risk factors (current smoking, history of cardiovascular disease, total cholesterol, high density lipoprotein cholesterol, systolic blood pressure, use of anti-hypertensive medication, and diabetes status) were assessed in all participants at examination 6. In addition, serum creatinine, urine albumin to urine creatinine ratio on a morning urine specimen was measured in all participants at the time of examination 6. Seated clinic blood pressure measurements were made in duplicate by certified examiners at a single visit using a standardized methodology.
Exposure of interest
Left ventricular mass (LVM) was assessed using 2-D guided M-mode echocardiograms at baseline as previously described. Left ventricular mass was calculated using the Devereux formula, 0.8[1.04((LVEDD + IVSD + PWT)3 – LVEDD3)] + 0.6 where LVEDD is the left ventricular diameter in diastole, IVSD is the inter-ventricular septum thickness in diastole, and PWT is the left ventricular posterior wall thickness in diastole. LVM was standardized within each sex in all models.
Outcome ascertainment
Serum creatinine values was used in the CKD-EPI equation to obtain each participant’s eGFR. Incident CKD was defined as eGFR of <60 mL/min/1.73m2 at examinations 7, 8, or 9. Although the diagnosis of CKD requires that eGFR be below 60 mL/min/1.73m2 for three months or more, given the nature of the epidemiological cohort study, it was not feasible to evaluate serum creatinine on a second occasion. Participants who had an eGFR <60 but no measurements at subsequent examinations were considered as CKD cases. Participants who had an eGFR < 60 at one examination but had an eGFR≥60 at a subsequent examination, were not considered as having CKD to reduce misclassification of outcome based on use of single-occasion serum creatinine measurements.
Statistical methods
The outcome was incident CKD, defined as eGFR of <60 mL/min/1.73m2. We performed a Cox proportional-hazards regression model with discrete time intervals (given assessment of CKD status was at multiple FHS follow-up examinations) to relate baseline echocardiographic LV mass (independent variable) to time to CKD on follow-up (dependent variable). Model 1 adjusted for age and sex; model 2 additionally adjusted for prevalent CVD, body mass index, systolic blood pressure, blood total cholesterol, high density lipoprotein cholesterol, anti-hypertensive medications, smoking and diabetes mellitus; model 3 further adjusted for baseline eGFR; and model 4 also adjusted for baseline urine albumin/urine creatinine ratio. To support the findings of LV mass as a continuous variable, we also estimated the HRs of incident CKD for sex-specific tertiles of LV mass index.
Sensitivity analyses
We compared the baseline characteristics of participants who had missing LV mass assessment at baseline with those who had these measurements. We created a “missing LVM indicator” and added that in the outcome model to test the association of missingness with incident CKD for each of the Models 1–4. We assigned sex-specific median LV mass to those with missing LV mass and fitted each of the Models 1–4.
Results
Table 1 shows the baseline characteristics of the study sample. Participants had a mean BMI in the overweight range and approximately half of them had hypertension. Table S1 shows the characteristics of 678 participants who were missing the measurement of LV mass. Of note, CKD events in women with missing LV mass were nearly twice as much (26%) compared to those who had the measurements of LV mass (14%).
Table 1:
Clinical characteristics of the study sample
| Clinical characteristic* | Men (n=990) | Women (n=1268) |
|---|---|---|
| Age, years | 56 (9) | 57 (9) |
| Body mass index, kg/m2 | 28.0 (3.9) | 26.7 (5.1) |
| Systolic blood pressure, mmHg | 128 (17) | 125 (20) |
| Diastolic blood pressure, mmHg | 77 (9) | 74 (9) |
| Total cholesterol, mg/dL | 199 (35) | 212 (39) |
| HDL cholesterol, mg/dL | 44 (12) | 59 (16) |
| Hypertension, % | 56 | 41 |
| Hypertension medication use, % | 27 | 19 |
| Diabetes, % | 9.4 | 5.2 |
| Current smoking, % | 13 | 15 |
| History of CVD, % | 12 | 5.4 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 89 (15) | 90 (16) |
| UACR, mg/g (median, 25, and 75 percentiles) | 4.3 (1.8, 8.1) | 8.2 (3.5, 16.2) |
| LV mass, g (median, 25 and 75 percentiles) | 185 (161, 213) | 136 (116, 156) |
Values are mean(SD) unless otherwise noted
During a median follow-up of 14.6 years, 373 (16.5%) participants developed incident CKD. Adjusting for age and sex, (Model 1), higher LV mass was associated with a higher risk of CKD (Table 2). LV mass remained associated with incident CKD after multivariable adjustment (Table 2). Table S2 shows that HRs for the respective models did not change with the addition of the missing indicator. In Table S3 the missing LV mass was assigned the sex-specific median for the group. The HRs remained changed little in the sensitivity analysis. Table S4 shows the association of LV mass to CKD for each g/m2 of LV mass. To further dissect the contributions of LV muscular hypertrophy versus LV cavity diameter, we fitted three models to evaluate the effects of the each of these 2 components separately and jointly (Table S5). LV wall thickness and LV end-diastolic diameter were indexed to height in meters, and then standardized (mean=0, SD=1). In a fully adjusted model, height-indexed LV wall thickness was related to incident CKD whereas height-indexed LV end-diastolic diameter was not. In a joint model including both variables, wall thickness was related to incident CKD whereas the association with LV end-diastolic diameter was borderline statistically significant.
Table 2:
Association between Left Ventricular Mass and incidence of CKD
| Model | N of events/ No. at risk | Adjustment for covariates | Hazards ratio (95% CI)* |
p |
|---|---|---|---|---|
| 1 | 373/2258 | Age and sex | 1.28 (1.15–1.42) | <0.001 |
| 2 | 367/2239 | + CVD risk factors† | 1.15 (1.03–1.29) | 0.017 |
| 3 | 367/2239 | + baseline eGFR | 1.16 (1.04–1.31) | 0.010 |
| 4 | 312/1913 | + baseline UACR | 1.18 (1.04–1.33) | 0.009 |
CKD = chronic kidney disease, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, UACR = urinary albumin to creatinine ratio (mg/mg)
Hazards ratios are for a 1 standard deviation increase in LV mass.
CVD risk factors include prevalent CVD, body mass index, systolic blood pressure, total and HDL cholesterol, anti-hypertensive medication, smoking and diabetes
To further assess whether the relation of LV mass with incident CKD was linear, we fitted a restricted cubic spline model with knots placed at the 25th, 50th, and 75th percentiles. No evidence of non-linearity was noted (p for non-linearity 0.97) as shown in Figure 2. Next, we estimated HRs for incident CKD associated with sex-specific tertiles of indexed LV mass (lowest tertile serving as referent group). The incidence of CKD was as follows: in the lowest tertile, 93/750 (12.4%), middle tertile 108/752 (14.4%) and the highest tertile 172/750 (22.9%). Compared to the first tertile, the third tertile of LV mass index had HR 1.63 (95% CI 1.23 – 2.15, p<0.001) for model 1, HR 1.34 (1.01–1.79, p=0.04) for model 2, HR 1.43 (95% CI 1.07 to 1.91, p=0.02) for model 3, and HR 1.45 (95% CI 1.06 to 2.0, p=0.02) for model 4.
Figure 2:
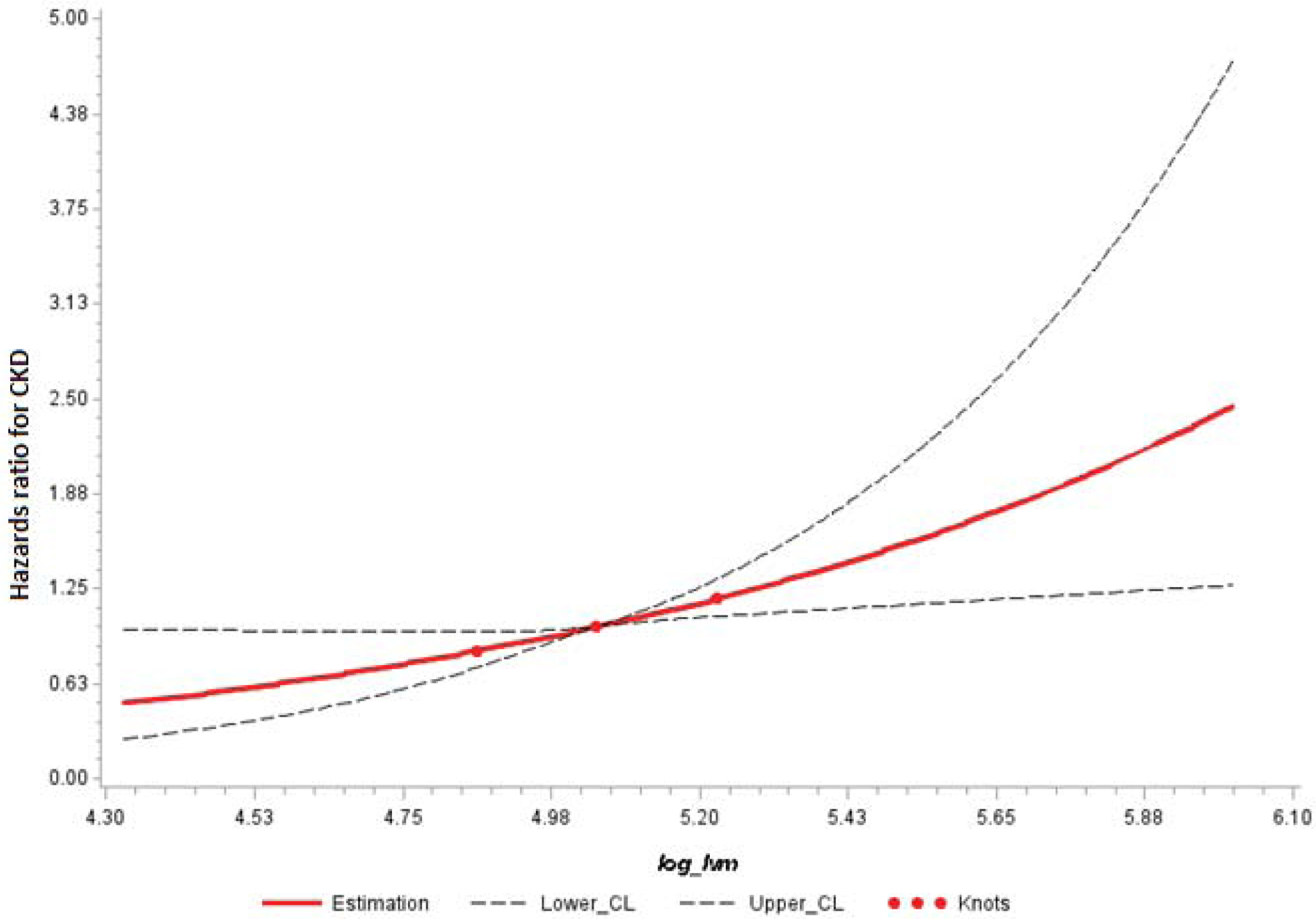
The log of LV mass is linearly related to the hazards of incident CKD. Reference value is the median LV mass. Knots for restricted cubic splines were placed at 25th, 50th, and 75th percentiles of LV mass.
Discussion
Richard Bright reported the results of autopsy of 8 cases on the disease that bears his name.(9) The first case described in February 1833 noted, “The heart very large in all its dimensions with all its cavities in a state of hypertrophy. The valves nearly healthy but a little thickened….”(9) This connection between nephritis at autopsy and left ventricular hypertrophy, about 200 years later has been extended to demonstrate the reverse relationship. We now show that those who have increased LV mass at baseline are at increased risk for developing incident CKD.
It is widely accepted that CKD is associated with incident cardiovascular morbidity and mortality. It is less clear whether subclinical CVD is associated with incident CKD. It is recognized also that in people with pre-existing CKD, the increase in left ventricular (LV) mass is rapid and related to carefully measured systolic BP. (10) It is not known, however, if greater LV mass in people with normal renal function is related to incident CKD. The present investigation demonstrates that target organ damage (as reflected by echocardiographic LV mass) is an important risk factor for the future development of CKD. The strength of this association was maintained upon adjustments for standard cardiovascular risk factors. More importantly, the association was maintained upon adjustment for baseline eGFR or UACR. Overall, our observations suggest that common pathways that damage the kidney and the heart (such as hypertension and diabetes) may not fully explain the bidirectional relationship of the heart and the kidney health/disease.
Our data extend cross-sectional, community-based studies, which demonstrate that both the systolic BP and the treatment of hypertension are associated with CKD.(11) Longitudinal studies have also observed an association between higher systolic BP and the occurrence of end-stage renal disease.(12) Similarly, examination of the placebo arm of a randomized trial has shown that higher levels of systolic BP are associated with a subsequent decline in kidney function.(13) However, lowering BP to levels has failed to abrogate this decline in eGFR.(14)
Despite both albuminuria and LV mass being markers of target organ damage, the association between LV mass and incident CKD remained statistically significant after adjustment for albuminuria. Although systolic blood pressure and its treatment are common upstream mediators of both albuminuria(1) and LV mass(15–17), we observed that the association between LV mass and incident CKD was maintained upon adjustment for these two key risk factors. Previous work has demonstrated that blood pressure measured in the clinic is a weaker correlate of albuminuria compared to 24-hour ambulatory blood pressure monitoring.(18) Thus, BP measured in the clinic—as compared to 24-hour ambulatory BP monitoring—may underestimate the risk of incident CKD. Albuminuria may, therefore, more accurately reflect the hemodynamic effects of blood pressure. On the other hand, unmeasured risk factors (such as fibrosis and inflammation in the heart and the kidney) may mediate the association of LV mass and incident CKD.(19)
Strengths and limitations
There are some strengths and limitations of our investigation. We evaluated a large prospective, community-based sample. However, our sample did not include black participants, a group that is known to have a heightened risk of CKD.(20) Accordingly, our results need to be confirmed in other racially diverse samples. Our observational data show a longitudinal association between target organ damage and incident CKD; a cause-and-effect relationship cannot be assumed especially because of unmeasured residual confounding.
We did not ascertain the mechanisms that may relate LV hypertrophy to incident CKD. However, if LV mass is related to the incident CKD, we can speculate on the mechanisms. LV mass is related to elevated 24-hour ambulatory BP and nocturnal hypertension often present in incipient CKD (21). In other words, LV mass may better reflect 24-hour ambulatory BP rather than clinic BP. An elevated 24-hour BP may provoke downstream CKD. Stimulation of the mineralocorticoid receptor provokes both LV hypertrophy and CKD by activating both hemodynamic and non-hemodynamic mechanisms such as inflammation and fibrosis (22). It is possible that we identified LV hypertrophy at an earlier time point that CKD as a manifestation of stimulation of the mineralocorticoid receptor. Perhaps kidney biopsies would have revealed vascular, glomerular, and tubular changes consistent with kidney disease. Future research should explore these possibilities.
Perspectives
In this community-based prospective study, we observed an association between higher LV mass at baseline with a greater risk of incident CKD on follow up. The relation between CKD and future cardiovascular disease is well recognized. This suggests that the relation between CKD and subclinical cardiovascular disease may be bidirectional. Further studies are needed to confirm these results.
Supplementary Material
Novelty and significance.
What is new?
Chronic kidney disease is a cardiovascular risk factor.
Whether subclinical cardiovascular disease is a harbinger of future chronic kidney disease
In this study we define the relations between left ventricular mass with subsequent incident chronic kidney disease.
What is relevant?
A community-based prospective study was conducted among 2258 Framingham Offspring cohort participants who were free of chronic kidney disease at baseline and were followed for median of 14.6 years.
Left ventricular mass was measured at baseline and the occurrence of incident chronic kidney disease evaluated at follow up.
A direct and linear association between higher left ventricular mass and a greater risk of incident chronic kidney disease on follow up was observed.
This association was robust despite adjustments for confounders and changed little after accounting for missing data.
Summary
Our study generates the novel observation that left ventricular mass is a kidney disease risk factor.
Acknowledgments and Funding
The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195, HHSN268201500001I and 75N92019D00031). Dr. Vasan is supported by an Evans Scholar award and the Jay and Louis Coffman Foundation from the Department of Medicine, Boston University School of Medicine.
Dr. Agarwal is supported by the National Heart, Lung, and Blood Institute (5 R01 HL126903) and the Veterans Administration (1 I01 CX001753).
Disclosures.
RA reports personal fees from Abbvie, Akebia, Amgen, AstraZeneca, Bayer, Birdrock Bio, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson & Johnson, Merck, Novartis, Opko, Otsuka, Reata, Relypsa, Sandoz, Sanofi, Takeda, ZS Pharma; has served as associate editor of the American Journal of Nephrology, Nephrology Dialysis and Transplantation; and an author on UpToDate; and received research grants from the US Veterans Administration and the National Institutes of Health.
References
- (1).Kannel WB, Stampfer MJ, Castelli WP, Verter J. The prognostic significance of proteinuria: the Framingham study. Am Heart J. 1984; 108:1347–1352. [DOI] [PubMed] [Google Scholar]
- (2).Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003; 108:2154–2169. [DOI] [PubMed] [Google Scholar]
- (3).Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- (4).Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van ZA, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015; 3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Matsushita K, van d V, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000; 102:203–210. [DOI] [PubMed] [Google Scholar]
- (7).Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin EL, Taylor RJ, Cockwell P, Steeds RP, Townend JN. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors. Hypertension. 2016; 67:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Matsushita K, Kwak L, Sang Y, Ballew SH, Skali H, Shah AM, Coresh J, Solomon S. Kidney Disease Measures and Left Ventricular Structure and Function: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Fine LG. Pathological specimens of the kidney examined by Richard Bright. Kidney Int. 1986; 29:779–783. [PubMed] [Google Scholar]
- (10).Agarwal R Longitudinal Study of Left Ventricular Mass Growth: Comparative Study of Clinic and Ambulatory Systolic Blood Pressure in Chronic Kidney Disease. Hypertension. 2016; 67:710–716. [DOI] [PubMed] [Google Scholar]
- (11).Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2001; 161:1207–1216. [DOI] [PubMed] [Google Scholar]
- (12).Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated Blood Pressure and Risk of End-stage Renal Disease in Subjects Without Baseline Kidney Disease. Arch Intern Med. 2005; 165:923–928. [DOI] [PubMed] [Google Scholar]
- (13).Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol. 2002; 13:2776–2782. [DOI] [PubMed] [Google Scholar]
- (14).Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011; 154:541–548. [DOI] [PubMed] [Google Scholar]
- (15).Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996; 275:1557–1562. [PubMed] [Google Scholar]
- (16).Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996; 275:1571–1576. [PubMed] [Google Scholar]
- (17).Kannel WB. Left ventricular hypertrophy as a risk factor: the Framingham experience. J Hypertens Suppl. 1991; 9:S3–S8. [DOI] [PubMed] [Google Scholar]
- (18).Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005; 46:514–520. [DOI] [PubMed] [Google Scholar]
- (19).Edwards NC, Moody WE, Yuan M, Hayer MK, Ferro CJ, Townend JN, Steeds RP. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol. 2015; 115:1311–1317. [DOI] [PubMed] [Google Scholar]
- (20).Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16- year MRFIT findings. JAMA. 1997; 277:1293–1298. [PubMed] [Google Scholar]
- (21).Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002; 347:797–805. [DOI] [PubMed] [Google Scholar]
- (22).Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 2019; 96:302–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


