Abstract
Introduction
The aim of this analysis was to characterize the safety and tolerability of empagliflozin in patients with type 2 diabetes mellitus (T2DM) who were randomized to empagliflozin (10/25 mg) or placebo in clinical trials.
Methods
Pooled data from 20 trials were analyzed for patients with T2DM treated with empagliflozin 10 mg (n = 4858), empagliflozin 25 mg (n = 5057), or placebo (n = 4904). The dataset comprised 15 randomized phase I–III trials, an extension trial and dose escalation studies. Adverse events (AEs) were assessed descriptively in participants who took ≥ 1 dose of study drug. AE incidence rates per 100 patient-years were calculated to adjust for differences in drug exposure between trials.
Results
Total exposure was 16,480 and 7857 patient-years in the pooled empagliflozin 10/25 mg and placebo groups, respectively. The incidence of any AEs, AEs leading to treatment discontinuation, severe AEs, and serious AEs was similar across groups. The frequency of serious AEs requiring hospitalization was 18.6% for the empagliflozin 10/25 mg group and 21.3% for the placebo group. The empagliflozin 10/25 mg group was not associated with a higher rate of confirmed hypoglycemia versus placebo, except in patients co-administered insulin and/or a sulfonylurea (31.5% vs. 30.2%, respectively). The incidence of events consistent with urinary tract infections (UTI) was also similar for the empagliflozin 10/25 mg group versus placebo (9.27 vs. 9.70/100 patient-years, respectively). History of UTI was identified as a risk factor for UTI during treatment. Events consistent with genital infections occurred more frequently with empagliflozin 10/25 mg than placebo (3.54 vs. 0.95/100 patient-years, respectively). The frequency of AEs consistent with volume depletion was similar across groups, but higher with empagliflozin 10/25 mg than placebo in patients aged 75 to < 85 years and those on loop diuretics at baseline.
Conclusion
This comprehensive analysis confirms that both empagliflozin 10 mg and 25 mg are well tolerated in patients with T2DM, reinforcing the established clinical safety profile of empagliflozin.
Keywords: Adverse drug event, Adverse drug reaction, Drug side effects, Hypoglycemia, Ketoacidosis, SGLT2 inhibitor
Plain Language Summary
Empagliflozin is approved to treat adults with type 2 diabetes mellitus (T2DM) insufficiently controlled by diet and exercise. It lowers blood glucose levels by inhibiting sodium-glucose co-transporter-2 (SGLT2), a protein involved in glucose reabsorption by the kidneys. By blocking SGLT2, glucose is removed in urine instead of being reabsorbed into the bloodstream. Numerous clinical studies have shown the effectiveness and safety of empagliflozin, but recent reports of two types of serious side effects [fractures and lower limb amputations (LLAs)] associated with another drug in the class, canagliflozin, has triggered a review of the risk associated with taking SGLT2 inhibitors. To examine the safety and tolerability of empagliflozin we pooled data from 20 clinical trials involving over 15,000 patients with T2DM who received either empagliflozin or placebo (control). We found that the risk of side effects was similar whether patients received empagliflozin or placebo. This included side effects that led to treatment being stopped as well as severe and serious side effects, including fractures and LLAs. Empagliflozin was not associated with a higher rate of hypoglycemia (low blood sugar) versus placebo, except in patients also treated with insulin and/or a sulfonylurea (31.5% vs. 30.2%, respectively). The risk of urinary tract infections was also similar for empagliflozin versus placebo (9.27 vs. 9.70/100 patient-years, respectively). However, genital infections, as anticipated, occurred more frequently in patients treated with empagliflozin than placebo (3.54 vs. 0.95/100 patient-years, respectively). Overall, this analysis confirms the results of previous studies showing that empagliflozin is well tolerated in patients with T2DM.
Key Summary Points
| Why carry out this study? |
| Empagliflozin is a potent sodium-glucose co-transporter-2 (SGLT2) inhibitor indicated for the treatment of type 2 diabetes mellitus (T2DM), including reduction of cardiovascular (CV) mortality in patients with T2DM and CV disease. |
| The clinical efficacy and safety profile of empagliflozin in T2DM has been well documented; however, new safety signals of increased lower limb amputations and fractures reported for another SGLT2 inhibitor have prompted a review of the risks associated with this drug class. |
| This study examined the safety and tolerability of empagliflozin in patients with T2DM using data pooled from 20 placebo-controlled clinical trials based on over 16,480 patient-years’ exposure to empagliflozin. |
| What was learned from the study? |
| This updated pooled analysis confirmed that both empagliflozin 10 mg and 25 mg are well tolerated in patients with T2DM. |
| These results reinforce the findings of a favorable benefit–risk profile for empagliflozin from previous clinical trials in patients with T2DM, including trials establishing the effects of empagliflozin on CV and all-cause mortality. |
Introduction
Empagliflozin, a potent and selective sodium-glucose co-transporter-2 (SGLT2) inhibitor, is indicated for the treatment of type 2 diabetes mellitus (T2DM) including reduction of cardiovascular (CV) mortality in patients with T2DM and CV disease. By blocking sodium-glucose co-transporters on proximal tubules, empagliflozin induces urinary glucose and sodium excretion which contribute to osmotic diuresis and reductions in plasma volume load [1–3]. The effects of SGLT2 inhibition on salt, water, and energy metabolism are thought to underlie the CV, renal, and metabolic benefits demonstrated by this drug class [4, 5]. Importantly, as this mechanism of action is independent of insulin modulation by β-cells, SGLT2 inhibitors are associated with a low risk of hypoglycemia [6].
The clinical efficacy and safety profile of empagliflozin in T2DM has been well documented. Treatment with empagliflozin at daily doses of 10 or 25 mg, either as monotherapy or add-on therapy, has been demonstrated to improve glycemic control and to result in reductions in body weight and blood pressure, and was well tolerated in placebo-controlled phase III trials in patients with T2DM [7–14]. Moreover, in the EMPA-REG OUTCOME® trial, empagliflozin, when given in addition to standard of care and compared with placebo, significantly reduced the risk of CV death by 38%, hospitalization for heart failure by 35%, improved clinically relevant kidney outcomes, and slowed the progression of kidney function decline in patients with T2DM and established CV disease [15, 16]. In the CANagliflozin cardioVascular Assessment Study (CANVAS), the SGLT2 inhibitor canagliflozin has also been shown to lower the risk of CV events in patients with T2DM and elevated risk of CV disease versus placebo [17]. However, a new and important safety signal was reported in the trial: there was a twofold increased risk of lower limb amputations (LLAs; primarily of the toe or metatarsal) in patients in the canagliflozin-treated group, versus placebo {hazard ratio (HR) 1.97 [95% confidence interval (CI) 1.41, 2.75]} [17]. The CANVAS program also reported an increased risk of all fractures with canagliflozin versus placebo [HR 1.26 (95% CI 1.04, 1.52)] [17]. Such new findings have prompted a review of the risks of LLAs and fractures associated with other SGLT2 inhibitors, including empagliflozin and dapagliflozin. For example, in the Dapagliflozin Effect on CardiovascuLAR Events (DECLARE–TIMI 58) and Dapagliflozin And Prevention of Adverse–outcomes in Heart Failure (DAPA-HF) trials, dapagliflozin showed no increased risk of either LLAs or fractures versus placebo [18, 19].
A comprehensive analysis of pooled safety profile data for empagliflozin, published in 2016 and derived from clinical trials of more than 9000 patient-years’ exposure to the drug, demonstrated that empagliflozin treatment was well tolerated and not associated with an increased risk of hypoglycemia compared with placebo, except in patients on background treatment with a sulfonylurea (SU) and/or insulin [20]. Furthermore, genital infection was reported to occur in a higher percentage of patients treated with empagliflozin versus placebo [20], which was consistent with findings from previous trials. An update of this pooled analysis, published in 2017, involved in excess of 15,000 patient-years’ exposure, and continued to support the favorable benefit–risk profile of empagliflozin in patients with T2DM [21]. However, a weakness of this larger analysis was that one of its component trials (the EMPA-REG OUTCOME® trial) contributed to over 55% (n = 7020) of the empagliflozin- or placebo-treated patients to the overall analysis population (n = 12,620) [21].
We report here the findings from an additional update on the pooled safety analysis of empagliflozin. This new analysis aims to further describe the safety and tolerability of empagliflozin based on 16,480 patient-years’ exposure to empagliflozin 10 mg or 25 mg in randomized, controlled phase I–III trials.
Methods
Patients
In this updated analysis, data were pooled from 20 trials (Table 1). This included the earlier dataset from 14 trials of 8 days’ to 78 weeks’ duration [7–14, 22–27], the 52-week extension trial to the phase III trials of empagliflozin as monotherapy, or as add-on to metformin, metformin plus an SU, and pioglitazone with or without metformin [28–31], and the CV outcomes trial EMPA-REG OUTCOME® [15]. It included all randomized, double-blind, placebo-controlled trials conducted in ambulatory patients with T2DM, including dose escalation trials and one extension trial of 52 weeks with patients enrolled from three main trials [32–36]). Data were only included for patients treated with empagliflozin (10/25 mg) or placebo who were randomized using either a 1:1 or 1:1:1 schedule.
Table 1.
Overview of the clinical trials included in the pooled safety analysis
| ClinicalTrials.gov identifier (BI study number) | Short title | Treatment duration | Dose escalationa |
|---|---|---|---|
| NCT00558571 (1245.4) | 4 weeks’ treatment in patients with T2DM | 28 days | No |
| NCT00789035 (1245.9) | Dose finder versus placebo as monotherapy | 12 weeks | No |
| NCT00749190 (1245.10) | Dose finder versus placebo as add-on therapy | 12 weeks | No |
| NCT00885118 (1245.15) | Treatment of patients with T2DM in Japan | 4 weeks | No |
| NCT01210001 (1245.19) | Efficacy on background TZD ± metformin | 24 weeks | No |
| NCT01177813 (1245.20) | Efficacy in drug-naïve patients | 24 weeks | No |
| NCT01159600 (metformin) (1245.23) | Efficacy on background metformin | 24 weeks | No |
| NCT01159600 (metformin + SU) (1245.23) | Efficacy on background metformin ± SU | 24 weeks | No |
| NCT01131676 (1245.25) | Safety cardiovascular outcome trial | Mean: 2.8 years | No |
| NCT02182830 (1245.29) | African American patients with T2DM and hypertension | 24 weeks | Yes |
| NCT01011868 (1245.33) | Efficacy on background basal insulin | 78 weeks | No |
| NCT01947855 (1245.35) | Japanese post-prandial glucose | 4 weeks | No |
| NCT01164501 (1245.36) | Renal safety study | 52 weeks | No |
| NCT01193218 (1245.38) | Japanese dose finder study plus extension | 52 weeks | No |
| NCT01370005 (1245.48) | Efficacy in patients with T2DM and hypertension | 12 weeks | No |
| NCT01306214 (1245.49) | Efficacy on background MDI insulin ± metformin | 52 weeks | No |
| NCT02589639 (1245.107) | Empagliflozin add-on to insulin (Japan) | 52 weeks | No |
| NCT01734785 (1275.9) | Empagliflozin add-on to linagliptin | 24 weeks | No |
| NCT02453555 (1275.19) | Empagliflozin add-on to linagliptin (Japan) | 52 weeks | Yes |
| NCT01649297 (1276.10) | Empagliflozin QD versus BID on background metformin | 16 weeks | No |
All trials were randomized, double-blind, and placebo-controlled in ambulatory patients with T2DM treated with empagliflozin 10 or 25 mg
BI Boehringer Ingelheim, BID twice daily, MDI multiple daily injections, QD once daily, SU sulfonylurea, T2DM type 2 diabetes mellitus, TZD thiazolidinedione
aIn some trials, the investigators could decide to increase the dose of empagliflozin from 10 to 25 mg in a blinded manner during the trial
The procedures followed in all studies were in accordance with the ethical standards of the responsible institutional and/or national committees on human experimentation, and with the Helsinki Declaration of 1964, as revised in 2013. An independent ethics committee or institutional review board approved the clinical protocol at each participating center. All patients provided their written informed consent prior to participation.
Assessments and Data Analyses
Safety and tolerability were assessed as for the earlier analysis [21], on the basis of investigator-reported adverse events (AEs) that were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1 preferred terms. The safety topics of interest were analyzed using MedDRA version 21.0 preferred terms. A severe AE was any AE adjudged by the investigator to be either incapacitating, causing inability to work, or to perform usual activities. A serious AE was any AE that resulted in death, was immediately life-threatening, resulted in persistent or marked disability/incapacity, required or prolonged patient hospitalization, was a congenital anomaly/birth defect, or was deemed serious for any other reason.
Similar to the previous analysis [21], safety topics of interest included events consistent with hypoglycemia (confirmed hypoglycemia was defined as a plasma glucose level of ≤ 3.9 mmol/l and/or requiring assistance), urinary tract infections, genital infections, volume depletion, diabetic ketoacidosis, urinary tract carcinogenicity, hepatic injury, bone fractures, acute pancreatitis, amputations, and decreased renal function. As LLAs were not systematically reported as separate AEs, the retrieval of these cases involved medical review of the narratives and concomitant therapy data. The present analysis also includes assessments of complicated urinary tract infections (UTIs) and complicated genital infections.
Analyses of AEs were descriptive and based on patients who received at least one dose of the study drug. Exposure-adjusted incidence rates were calculated per 100 patient-years as 100 × n/T where n was the number of patients with the event and T was the total number of patient-years at risk of the event. Patient-years at risk was defined for patients with an event as the time from first dose to the onset of a first event, or for patients without an event, as the time from first dose to the last dose plus 7 days. For LLAs, an intent-to-treat analysis was performed based on cases reported from the first intake of study drug up to trial termination in patients treated with at least one dose of the study drug. Additionally, a time-to-first-event analysis was performed.
The primary analysis was of placebo compared with the pooled empagliflozin 10/25 mg population, as the safety and tolerability of the two empagliflozin doses were shown to be similar in previous analyses [20, 21]. Data from the individual empagliflozin 10 mg and 25 mg groups are also presented in the tables and figure, but exclude the dose-escalation trials.
Results
Patient Disposition, Exposure and Baseline Characteristics
The analysis set included 10,177 patients treated with empagliflozin 10/25 mg and 4904 treated with placebo. Compared with the earlier pooled safety analysis of empagliflozin [21], the current dataset represents an approximate 20% increase in the number of patients analyzed overall (12,620 patients vs. 15,081 patients, respectively). In addition, the EMPA-REG OUTCOME® trial accounted for less than 50% of patients in the current analysis compared with earlier pooled safety analysis [21] (46.5% vs. 55.6%, respectively). However, patients aged ≥ 85 years old (0.2% of overall population) and those with an estimated glomerular filtration rate (eGFR) of < 30 ml/min/1.73 m2 (0.8% of the overall population), were under-represented in the present sample, similar to the earlier analysis [21]. The total study drug exposure was 16,480 and 7857 patient-years in the empagliflozin 10/25 mg and placebo groups, respectively. Patient baseline demographics and clinical characteristics were well balanced across the treatment groups. These are summarized in Table 2.
Table 2.
Demographics and baseline characteristics
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| Male, n (%) | 3119 (63.6) | 3094 (63.7) | 3249 (64.2) | 6529 (64.2) |
| Age, years | 60.5 (9.8) | 60.3 (9.7) | 60.4 (9.8) | 60.3 (9.7) |
| Age groups, years (%) | ||||
| < 65 | 3197 (65.2) | 3168 (65.2) | 3293 (65.1) | 6639 (65.2) |
| 65 to < 75 | 1377 (28.1) | 1390 (28.6) | 1426 (28.2) | 2887 (28.4) |
| 75 to < 85 | 318 (6.5) | 290 (6.0) | 327 (6.5) | 630 (6.2) |
| ≥ 85 | 12 (0.2) | 10 (0.2) | 11 (0.2) | 21 (0.2) |
| Race, n (%) | ||||
| White | 3044 (62.1) | 3256 (67.0) | 3346 (66.2) | 6602 (64.9) |
| Asian | 1347 (27.5) | 1252 (25.8) | 1349 (26.7) | 2601 (25.6) |
| Black/African–American | 279 (5.7) | 213 (4.4) | 219 (4.3) | 512 (5.0) |
| Othera | 51 (1.0) | 50 (1.0) | 51 (1.0) | 101 (1.0) |
| Missing | 183 (3.7) | 87 (1.8) | 92 (1.8) | 361 (3.5) |
| Region, n (%) | ||||
| Europe | 1821 (37.1) | 1939 (39.9) | 2013 (39.8) | 3952 (38.8) |
| North America | 1057 (21.6) | 1056 (21.7) | 1083 (21.4) | 2219 (21.8) |
| Latin America | 474 (9.7) | 503 (10.4) | 505 (10.0) | 1008 (9.9) |
| Africa/Middle East | 132 (2.7) | 129 (2.7) | 141 (2.8) | 270 (2.7) |
| Asia | 1420 (29.0) | 1231 (25.3) | 1315 (26.0) | 2728 (26.8) |
| Time since diabetes diagnosis, years, n (%) | ||||
| ≤ 1 | 259 (5.3) | 295 (6.1) | 308 (6.1) | 616 (6.1) |
| > 1 to ≤ 5 | 1077 (22.0) | 1048 (21.6) | 1100 (21.8) | 2216 (21.8) |
| > 5 | 3553 (72.5) | 3500 (72.0) | 3633 (71.8) | 7314 (71.9) |
| Missing | 15 (0.3) | 15 (0.3) | 16 (0.3) | 31 (0.3) |
| BMI, kg/m2b | 30.4 (5.5) | 30.5 (5.5) | 30.5 (5.5) | 30.5 (5.6) |
| eGFR, ml/min/1.73 m2, n (%)c | ||||
| ≥ 90 | 1933 (39.4) | 1998 (41.1) | 2041 (40.4) | 4177 (41.0) |
| ≥ 60 to < 90 | 2123 (43.3) | 2203 (45.3) | 2155 (42.6) | 4477 (44.0) |
| ≥ 45 to < 60 | 519 (10.6) | 464 (9.6) | 535 (10.6) | 1003 (9.9) |
| ≥ 30 to < 45 | 277 (5.6) | 182 (3.7) | 262 (5.2) | 445 (4.4) |
| < 30 | 52 (1.1) | 10 (0.2) | 61 (1.2) | 71 (0.7) |
| Missing | 0 | 1 (< 0.1) | 3 (0.1) | 4 (< 0.1) |
Data are mean (SD) unless otherwise indicated
BMI body mass index, eGFR estimated glomerular filtration rate, EMPA empagliflozin, SD standard deviation
aAmerican Indian/Alaska Native/Hawaiian/Pacific Islander
bPlacebo, n = 4883; EMPA 10 mg, n = 4838; EMPA 25 mg, n = 5038; EMPA 10/25 mg, n = 10,138
cPlacebo, n = 4904; EMPA 10 mg, n = 4857; EMPA 25 mg, n = 5054; EMPA 10/25 mg, n = 10,173
General Safety
The overall pattern of AEs observed in the earlier pooled safety analysis [21] was also seen in this updated analysis, with incidences of severe AEs, serious AEs, fatal AEs and AEs leading to treatment discontinuation being similar between the empagliflozin and placebo groups (Table 3). The percentage of patients with a serious AE requiring hospitalization was similar for the empagliflozin and placebo groups (empagliflozin 10/25 mg: 18.6%; placebo: 21.3%) (Table 4). The most common serious AEs requiring hospitalization (based on MedDRA terms) were cardiac disorders (empagliflozin 10/25 mg: 5.7%; placebo: 7.1%), infections and infestations (empagliflozin 10/25 mg: 3.8%; placebo: 4.6%), and nervous system disorders (empagliflozin 10/25 mg: 2.8%; placebo: 3.1%) (Table 5). In contrast, AEs that were deemed by the study investigator to be drug-related were more common in patients treated with empagliflozin compared with the placebo group (empagliflozin 10/25 mg: 25.3%; placebo: 21.0%) (Table 4).
Table 3.
Incidence of adverse events
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| ≥ 1 AE | 197.62 | 170.01 | 168.59 | 168.89 |
| ≥ 1 drug-related AEa | 15.45 | 19.57 | 19.38 | 19.54 |
| ≥ 1 AE leading to discontinuation | 7.40 | 6.57 | 6.38 | 6.43 |
| ≥ 1 severe AEb | 10.43 | 8.80 | 9.51 | 9.04 |
| ≥ 1 serious AEc | 18.61 | 15.29 | 16.07 | 15.52 |
| Fatal | 1.57 | 1.24 | 1.01 | 1.12 |
Data are the rate/100 patient-years. A patient may be counted in more than one seriousness criterion. MedDRA version used for reporting: 20.1
AE adverse event, EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities
aInvestigator-defined
bAn AE that is incapacitating or causing inability to work or perform usual activities
cAn AE that results in death, is immediately life-threatening, results in persistent or significant disability/incapacity, requires or prolongs patient hospitalization, is a congenital anomaly/birth defect, or is deemed serious for any other reason
Table 4.
Frequency of adverse events
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| ≥ 1 AE | 3942 (80.4) | 3740 (77.0) | 3896 (77.0) | 7805 (76.7) |
| ≥ 1 drug-related AEa | 1028 (21.0) | 1247 (25.7) | 1279 (25.3) | 2571 (25.3) |
| ≥ 1 AE leading to discontinuation | 565 (11.5) | 514 (10.6) | 512 (10.1) | 1033 (10.2) |
| ≥ 1 severe AEb | 747 (15.2) | 651 (13.4) | 718 (14.2) | 1371 (13.5) |
| ≥ 1 serious AEc | 1204 (24.6) | 1046 (21.5) | 1104 (21.8) | 2161 (21.2) |
| Fatal | 125 (2.5) | 101 (2.1) | 84 (1.7) | 186 (1.8) |
| Immediately life-threatening | 53 (1.1) | 54 (1.1) | 64 (1.3) | 118 (1.2) |
| Disability/incapacitation | 29 (0.6) | 20 (0.4) | 27 (0.5) | 47 (0.5) |
| Requiring hospitalization | 1044 (21.3) | 898 (18.5) | 986 (19.5) | 1891 (18.6) |
| Prolonged hospitalization | 79 (1.6) | 57 (1.2) | 76 (1.5) | 133 (1.3) |
| Congenital anomaly | 0 | 0 | 0 | 0 |
| Other | 193 (3.9) | 170 (3.5) | 175 (3.5) | 348 (3.4) |
Data are n (%). A patient may be counted in more than one seriousness criterion. MedDRA version used for reporting: 20.1
AE adverse event, EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities
aInvestigator-defined
bAn AE that is incapacitating or causing inability to work or perform usual activities
cAn AE that results in death, is immediately life-threatening, results in persistent or significant disability/incapacity, requires or prolongs patient hospitalization, is a congenital anomaly/birth defect, or is deemed serious for any other reason
Table 5.
Frequency of patients with serious adverse events requiring hospitalization
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| Number of patients | 1044 (21.3) | 898 (18.5) | 986 (19.5) | 1891 (18.6) |
| SOC | ||||
| Infections and infestations | 225 (4.6) | 187 (3.8) | 200 (4.0) | 387 (3.8) |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 45 (0.9) | 65 (1.3) | 75 (1.5) | 142 (1.4) |
| Blood and lymphatic system disorders | 18 (0.4) | 16 (0.3) | 14 (0.3) | 30 (0.3) |
| Immune system disorders | 4 (0.1) | 3 (0.1) | 4 (0.1) | 7 (0.1) |
| Endocrine disorders | 3 (0.1) | 3 (0.1) | 8 (0.2) | 11 (0.1) |
| Metabolism and nutrition disorders | 66 (1.3) | 42 (0.9) | 40 (0.8) | 82 (0.8) |
| Psychiatric disorders | 19 (0.4) | 11 (0.2) | 7 (0.1) | 18 (0.2) |
| Nervous system disorders | 152 (3.1) | 136 (2.8) | 153 (3.0) | 289 (2.8) |
| Eye disorders | 26 (0.5) | 29 (0.6) | 22 (0.4) | 51 (0.5) |
| Ear and labyrinth disorders | 17 (0.3) | 7 (0.1) | 16 (0.3) | 23 (0.2) |
| Cardiac disorders | 347 (7.1) | 282 (5.8) | 296 (5.9) | 580 (5.7) |
| Vascular disorders | 115 (2.3) | 74 (1.5) | 109 (2.2) | 183 (1.8) |
| Respiratory, thoracic and mediastinal disorders | 69 (1.4) | 43 (0.9) | 52 (1.0) | 95 (0.9) |
| Gastrointestinal disorders | 93 (1.9) | 94 (1.9) | 93 (1.8) | 187 (1.8) |
| Hepatobiliary disorders | 25 (0.5) | 28 (0.6) | 30 (0.6) | 59 (0.6) |
| Skin and subcutaneous tissue disorders | 28 (0.6) | 20 (0.4) | 34 (0.7) | 54 (0.5) |
| Musculoskeletal and connective tissue disorders | 98 (2.0) | 79 (1.6) | 81 (1.6) | 160 (1.6) |
| Renal and urinary disorders | 69 (1.4) | 43 (0.9) | 41 (0.8) | 84 (0.8) |
| Pregnancy, puerperium and perinatal conditions | 0 | 0 | 1 (< 0.1) | 1 (< 0.1) |
| Reproductive system and breast disorders | 10 (0.2) | 18 (0.4) | 19 (0.4) | 37 (0.4) |
| Congenital, familial and genetic disorders | 6 (0.1) | 4 (0.1) | 2 (< 0.1) | 6 (0.1) |
| General disorders and administration site conditions | 75 (1.5) | 58 (1.2) | 62 (1.2) | 121 (1.2) |
| Investigations | 18 (0.4) | 8 (0.2) | 15 (0.3) | 23 (0.2) |
| Injury, poisoning and procedural complications | 83 (1.7) | 69 (1.4) | 80 (1.6) | 151 (1.5) |
| Surgical and medical procedures | 15 (0.3) | 14 (0.3) | 16 (0.3) | 30 (0.3) |
| Social circumstances | 0 | 1 (< 0.1) | 0 | 1 (< 0.1) |
| Product issues | 4 (0.1) | 0 | 2 (< 0.1) | 2 (< 0.1) |
Data are n (%). A patient could have more than one event. MedDRA version used for reporting: 20.1
EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities, SOC System Organ Class
Safety Topics of Interest
Hypoglycemia
The frequency and incidence rate of hypoglycemic AEs were similar for empagliflozin and placebo in all-comers (empagliflozin 10/25 mg: 20.3%, 15.69 events per 100 patient-years; placebo: 21.3%, 16.32 events per 100 patient-years) (Table 6). However, a higher percentage of patients that used insulin and/or an SU at baseline in the empagliflozin 10/25 mg than placebo groups reported confirmed hypoglycemic AEs (empagliflozin 10/25 mg: 31.5%, 20.88 events per 100 patient-years; placebo: 30.2%, 20.36 per 100 patient-years) (Table 6).
Table 6.
Frequency and incidence rate of important identified risks
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) or n/N (%) | Rate/100 pt-yrs | n (%) or n/N (%) | Rate/100 pt-yrs | n (%) or n/N (%) | Rate/100 pt-yrs | n (%) or n/N (%) | Rate/100 pt-yrs | |
| UTIs (BIcMQ) | 691 (14.1) | 9.70 | 684 (14.1) | 9.45 | 675 (13.3) | 9.04 | 1382 (13.6) | 9.27 |
| Sex | ||||||||
| Male | 204/3119 (6.5) | 3.97 | 225/3094 (7.3) | 4.33 | 224/3249 (6.9) | 4.11 | 455/6529 (7.0) | 4.21 |
| Female | 487/1785 (27.3) | 24.50 | 459/1764 (26.0) | 22.43 | 451/1808 (24.9) | 22.35 | 927/3648 (25.4) | 22.55 |
| Age, years | ||||||||
| < 65 | 401/3197 (12.5) | 9.00 | 389/3168 (12.3) | 8.58 | 360/3293 (10.9) | 7.71 | 762/6639 (11.5) | 8.16 |
| 65 to < 75 | 220/1377 (16.0) | 10.35 | 216/1390 (15.5) | 9.64 | 243/1426 (17.0) | 10.59 | 466/2887 (16.1) | 10.14 |
| 75 to < 85 | 69/318 (21.7) | 13.39 | 79/290 (27.2) | 17.38 | 71/327 (21.7) | 14.72 | 153/630 (24.3) | 16.13 |
| ≥ 85 | 1/12 (8.3) | 4.61 | 0/10 | – | 1/11 (9.1) | 4.48 | 1/21 (4.8) | 2.98 |
| Complicated UTIs (BIcMQ) | 59 (1.2) | 0.75 | 39 (0.8) | 0.48 | 54 (1.1) | 0.65 | 93 (0.9) | 0.56 |
| Genital infections (BIcMQ) | 75 (1.5) | 0.95 | 278 (5.7) | 3.57 | 281 (5.6) | 3.52 | 565 (5.6) | 3.54 |
| Sex | ||||||||
| Male | 35/3119 (1.1) | 0.66 | 139/3094 (4.5) | 2.62 | 116/3249 (3.6) | 2.07 | 255/6529 (3.9) | 2.30 |
| Female | 40/1785 (2.2) | 1.58 | 139/1764 (7.9) | 5.61 | 165/1808 (9.1) | 6.98 | 310/3648 (8.5) | 6.33 |
| Age, years | ||||||||
| < 65 | 50/3197 (1.6) | 1.03 | 192/3168 (6.1) | 4.01 | 192/3293 (5.8) | 3.93 | 386/6639 (5.8) | 3.93 |
| 65 to < 75 | 21/1377 (1.5) | 0.88 | 69/1390 (5.0) | 2.81 | 71/1426 (5.0) | 2.82 | 144/2887 (5.0) | 2.86 |
| 75 to < 85 | 4/318 (1.3) | 0.68 | 17/290 (5.9) | 3.21 | 17/327 (5.2) | 3.13 | 34/630 (5.4) | 3.13 |
| ≥ 85 | 0/12 | – | 0/10 | – | 1/11 (9.1) | 4.35 | 1/21 (4.8) | 2.92 |
| Complicated genital infections (BIcMQ) | 24 (0.5) | 0.30 | 29 (0.6) | 0.36 | 26 (0.5) | 0.31 | 55 (0.5) | 0.33 |
| Hypoglycemia (narrow SMQ) | 1045 (21.3) | 16.32 | 1009 (20.8) | 15.79 | 1053 (20.8) | 16.02 | 2067 (20.3) | 15.69 |
| Confirmed hypoglycemic AEs | 987 (20.1) | 945 (19.5) | 1000 (19.8) | 1948 (19.1) | ||||
| Confirmed hypoglycemiaa with baseline use of insulin and/or SU | 915 (30.2) | 20.36 | 889 (31.9) | 20.92 | 942 (31.2) | 20.84 | 1,833 (31.5) | 20.88 |
| Confirmed hypoglycemia without baseline use of insulin and/or SU | 54 (2.9) | 2.66 | 38 (1.8) | 1.67 | 46 (2.3) | 2.13 | 85 (2.0) | 1.83 |
| Diabetic ketoacidosis (narrow BIcMQb) | 4 (0.1) | 0.05 | 4 (0.1) | 0.05 | 2 (< 0.1) | 0.02 | 6 (0.1) | 0.04 |
| Urinary tract carcinogenicityc (BIcMQ) | 9 (0.2) | 0.11 | 10 (0.2) | 0.12 | 13 (0.3) | 0.16 | 23 (0.2) | 0.14 |
| Onset after 6 months’ treatment | 7 (0.2) | 0.12 | 8 (0.2) | 0.13 | 10 (0.3) | 0.16 | 18 (0.3) | 0.15 |
| Liver injury (SMQ) | 157 (3.2) | 2.02 | 109 (2.2) | 1.36 | 135 (2.7) | 1.65 | 247 (2.4) | 1.51 |
| Bone fractures (BIcMQ) | 134 (2.7) | 1.72 | 121 (2.5) | 1.52 | 107 (2.1) | 1.30 | 233 (2.3) | 1.42 |
| Pancreatitis (SMQ) | 11 (0.2) | 0.14 | 8 (0.2) | 0.10 | 7 (0.1) | 0.08 | 15 (0.1) | 0.09 |
| Amputation risk (ITT population) | 46 (0.9) | 0.52 | 46 (0.9) | 0.51 | 49 (1.0) | 0.54 | 95 (0.9) | 0.52 |
| Minor | 27 (0.6) | 0.30 | 36 (0.7) | 0.40 | 40 (0.8) | 0.44 | 76 (0.8) | 0.41 |
| Major | 19 (0.4) | 0.21 | 10 (0.2) | 0.11 | 9 (0.2) | 0.10 | 19 (0.2) | 0.10 |
AE adverse event, BIcMQ Boehringer Ingelheim customized MedDRA query, EMPA empagliflozin, ITT intent-to-treat, MedDRA Medical Dictionary for Regulatory Activities, pt-yrs patient-years, SMQ standardized MedDRA queries, SU sulfonylurea, UTI urinary tract infection
aConfirmed hypoglycemia was defined as plasma glucose ≤ 3.9 mmol/l and/or requiring assistance
bThe lower number of events in the placebo group compared with the previously updated pooled safety analysis [21] is due to a change in definition resulting from a revision in MedDRA mapping between versions (i.e., the lowest level term ‘diabetic ketosis’ was mapped to the preferred term ‘diabetic ketoacidosis’ in version 18.0; however, this is no longer the case in version 21.0)
cBladder and renal malignancies
Urinary Tract Infections
The frequency and incidence of events consistent with UTI was higher among females compared with males in all treatment groups, but was similar when comparing empagliflozin with placebo in both males and females [empagliflozin 10/25 mg: (females) 25.4%, 22.55 events per 100 patient-years, (males) 7.0%, 4.21 events per 100 patient-years; placebo: (females) 27.3%, 24.50 events per 100 patient-years, (males) 6.5%, 3.97 events per 100 patient-years] (Table 6). Excluding patients aged ≥ 85 years, the frequency and incidence of UTI increased with age in all treatment groups, but, again, the frequency and incidence of UTI was similar when comparing empagliflozin with placebo in all age groups (Table 6). The majority of these events were non-serious, mild or moderate in intensity and led to treatment discontinuation in < 1% of treated patients in the empagliflozin 10/25 mg and placebo groups (Table 7). Similar proportions of patients (< 1%) with UTIs that either required or prolonged hospitalization were observed in the empagliflozin 10/25 mg and placebo groups (Table 7). Approximately one-third of patients in both the empagliflozin 10/25 mg and placebo groups with a history of chronic or recurrent UTIs had a UTI during treatment (Table 7). Similar proportions of patients in the empagliflozin 10/25 mg and placebo groups had complicated UTIs (0.9% vs. 1.2%, respectively; Table 7).
Table 7.
Frequency and incidence rate for UTIs and genital infections by seriousness, need for hospitalization, treatment discontinuation, history of chronic or recurrent infection, and complicated infection
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | |
| UTIs (BIcMQ)a | ||||||||
| Serious UTIs | 39 (0.8) | 0.49 | 27 (0.6) | 0.33 | 39 (0.8) | 0.47 | 66 (0.6) | 0.40 |
| Requires or prolongs hospitalization | 38 (0.8) | 0.5 | 26 (0.5) | 0.3 | 39 (0.8) | 0.5 | 65 (0.6) | 0.4 |
| Treatment discontinued | 15 (0.3) | 0.19 | 27 (0.6) | 0.33 | 25 (0.5) | 0.30 | 52 (0.5) | 0.31 |
| History of chronic or recurrent UTIb | ||||||||
| Yes | 100/277 (36.1) | 32.46 | 98/280 (35.0) | 28.58 | 102/313 (32.6) | 28.41 | 205/615 (33.5) | 28.82 |
| No | 573/4267 (13.4) | 8.71 | 559/4221 (13.2) | 8.39 | 550/4402 (12.5) | 7.98 | 1126/8863 (12.7) | 8.20 |
| Complicated UTIsc | 59 (1.2) | 0.75 | 39 (0.8) | 0.48 | 54 (1.1) | 0.65 | 93 (0.9) | 0.56 |
| Genital infections (BIcMQ)d | ||||||||
| Serious genital infections | 3 (0.1) | 0.04 | 8 (0.2) | 0.10 | 4 (0.1) | 0.05 | 12 (0.1) | 0.07 |
| Requires or prolongs hospitalization | 3 (0.1) | < 0.1 | 8 (0.2) | 0.1 | 4 (0.1) | < 0.1 | 12 (0.1) | 0.1 |
| Treatment discontinued | 2 (< 0.1) | 0.03 | 26 (0.5) | 0.32 | 21 (0.4) | 0.25 | 47 (0.5) | 0.28 |
| History of chronic or recurrent genital infectione | ||||||||
| Yes | 6/76 (7.9) | 5.25 | 17/76 (22.4) | 17.93 | 22/92 (23.9) | 23.47 | 39/172 (22.7) | 20.30 |
| No | 66/4468 (1.5) | 0.88 | 243/4425 (5.5) | 3.27 | 249/4623 (5.4) | 3.25 | 498/9306 (5.4) | 3.25 |
| Complicated genital infectionsf | 24 (0.5) | 0.30 | 29 (0.6) | 0.36 | 26 (0.5) | 0.31 | 55 (0.5) | 0.33 |
Data are n (%) except where indicated
AE adverse event, BIcMQ Boehringer Ingelheim customized MedDRA query, EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities, pt-yrs patient-years, UTI urinary tract infection
aBased on pre-defined MedDRA preferred terms, of which UTI, asymptomatic bacteriuria, and cystitis were the most frequent
bNumber of patients with UTIs/number of treated patients in subgroup (number of patients with UTIs as % of number of treated patients in subgroup)
cAll upper UTIs reported as serious or non-serious AEs and all lower UTIs reported as serious AEs
dBased on pre-defined MedDRA preferred terms, of which balanoposthitis, vulvovaginal mycotic infection, and vulvovaginal candidiasis were the most frequent
eNumber of patients with genital infections/number of treated patients in subgroup (number of patients with genital infections as % of number of treated patients in subgroup)
fEvents related to abscesses of external genital organs, endometritis, adnexitis, prostatitis, orchiepididymitis, pelvic infections, and serious AEs of vulvovaginitis and balanoposthitis
Genital Infections
The frequency and incidence of events consistent with genital infections was higher among females compared with males in all treatment groups, and was higher for empagliflozin than placebo in both males and females [empagliflozin 10/25 mg: (females) 8.5%, 6.33 events per 100 patient-years, (males) 3.9%, 2.30 events per 100 patient-years; placebo: (females) 2.2%, 1.58 events per 100 patient-years, (males) 1.1%, 0.66 events per 100 patient-years], and in all age groups (Table 6). As for UTIs, the majority of genital infections were non-serious, mild or moderate in intensity and led to treatment discontinuation in < 1% of patients in each of the treatment groups (Table 7). In addition, < 1% of patients across treatment groups had genital infections that required or prolonged hospitalization (Table 7). The frequency of events consistent with genital infections was higher in patients with a history of chronic or recurrent genital infections compared with patients without such a history for both empagliflozin 10/25 mg and placebo (empagliflozin 10/25 mg: 22.7% vs. 5.4%; placebo: 7.9% vs. 1.5%, respectively) (Table 7). Moreover, complicated genital infection rates were consistently low and similar across groups (0.5% for both empagliflozin 10/25 mg and placebo) (Table 7).
Volume Depletion
A higher rate of volume depletion was reported in patients with hypotension at baseline versus without, in patients treated with diuretics or loop diuretics at baseline than without, and in patients taking angiotensin-converting enzyme (ACE) inhibitors/angiotensin-receptor blockers (ARBs) or antihypertensive drugs at baseline than without (Table 8). However, the frequency of events consistent with volume depletion was similar between patients treated with empagliflozin 10/25 mg and placebo in these subgroups, except for patients aged 75 to < 85 years (5.9% vs. 5.0%, respectively), and patients treated with loop diuretics at baseline (9.8% vs. 7.4%, respectively), where the frequency was higher for empagliflozin 10/25 mg compared with placebo (Table 8).
Table 8.
Frequencies for volume depletion by age, hypotension at baseline, and concomitant drugs at baseline
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| Volume depletiona (BIcMQ) | 147 (3.0) | 150 (3.1) | 169 (3.3) | 320 (3.1) |
| Age (years) | ||||
| < 65 | 62/3197 (1.9) | 59/3168 (1.9) | 73/3293 (2.2) | 132/6639 (2.0) |
| 65 to < 75 | 67/1377 (4.9) | 73/1390 (5.3) | 75/1426 (5.3) | 149/2887 (5.2) |
| 75 to < 85 | 16/318 (5.0) | 18/290 (6.2) | 19/327 (5.8) | 37/630 (5.9) |
| ≥ 85 | 2/12 (16.7) | 0/10 | 2/11 (18.2) | 2/21 (9.5) |
| Hypotension at baseline | ||||
| Yes | 17/244 (7.0) | 15/254 (5.9) | 9/276 (3.3) | 24/542 (4.4) |
| No | 130/4602 (2.8) | 135/4548 (3.0) | 160/4727 (3.4) | 296/9525 (3.1) |
| Use of diuretics at baseline | ||||
| Yes | 83/1660 (5.0) | 79/1602 (4.9) | 96/1703 (5.6) | 175/3349 (5.2) |
| No | 64/3244 (2.0) | 71/3256 (2.2) | 73/3354 (2.2) | 145/6828 (2.1) |
| Use of loop diuretics at baseline | ||||
| Yes | 36/488 (7.4) | 42/415 (10.1) | 47/489 (9.6) | 89/909 (9.8) |
| No | 111/4416 (2.5) | 108/4443 (2.4) | 122/4568 (2.7) | 231/9268 (2.5) |
| Use of ACE inhibitor/ARB at baseline | ||||
| Yes | 121/3256 (3.7) | 126/3261 (3.9) | 142/3360 (4.2) | 269/6733 (4.0) |
| No | 26/1648 (1.6) | 24/1597 (1.5) | 27/1697 (1.6) | 51/3444 (1.5) |
| Use of antihypertensive drugs at baseline | ||||
| Yes | 140/3922 (3.6) | 142/3857 (3.7) | 158/3986 (4.0) | 301/7996 (3.8) |
| No | 7/982 (0.7) | 8/1001 (0.8) | 11/1071 (1.0) | 19/2181 (0.9) |
Data are n (%) except where indicated. Percentages are calculated using the total number of patients per treatment as the denominator
ACE angiotensin-converting enzyme, ARB angiotensin-receptor blocker, BIcMQ Boehringer Ingelheim customized MedDRA query, EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities
aBased on pre-defined MedDRA preferred terms, of which hypotension, syncope, and dehydration were the most frequent
Diabetic Ketoacidosis
The frequency and incidence of diabetic ketoacidosis were similar for patients treated with empagliflozin and placebo (empagliflozin 10/25 mg: 0.1%, 0.04 events per 100 patient-years; placebo: 0.1%, 0.05 events per 100 patient-years) (Table 6). Of four patients in the placebo group with diabetic ketoacidosis, none discontinued treatment and symptoms eventually resolved in all patients. Of six patients in the empagliflozin 10/25 mg group who experienced diabetic ketoacidosis, two discontinued treatment and symptoms resolved in five patients (the status of one patient was unknown).
Urinary Tract Carcinogenicity
The frequency and incidence of events consistent with urinary tract carcinogenicity (bladder and renal malignancies) with an onset of at least 6 months from the start of treatment were similar for the empagliflozin 10/25 mg and placebo groups (empagliflozin 10/25 mg: 0.3%, 0.15 events per 100 patient-years; placebo: 0.2%, 0.12 events per 100 patient-years) (Table 6).
Liver Injury
The frequency and incidence of events consistent with hepatic injury were similar for the empagliflozin and placebo groups (empagliflozin 10/25 mg: 2.4%, 1.51 events per 100 patient-years; placebo: 3.2%, 2.02 events per 100 patient-years) (Table 6). Elevations in alanine aminotransferase and/or aspartate aminotransferase ≥ 5 times the upper limit of normal were more frequent with empagliflozin 10/25 mg versus placebo (0.4% vs. 0.2%, respectively) (Table 9).
Table 9.
Elevations in liver enzymes and bilirubina
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |
|---|---|---|---|---|
| ALT and/or AST ≥ 3 × ULN | 65 (1.3) | 50 (1.0) | 45 (0.9) | 99 (1.0) |
| ALT and/or AST ≥ 5 × ULN | 11 (0.2) | 18 (0.4) | 21 (0.4) | 40 (0.4) |
| ALT and/or AST ≥ 3 × ULN with total bilirubin ≥ 2 × ULNb | 2 (< 0.1) | 5 (0.1) | 5 (0.1) | 10 (0.1) |
Data are n (%) in patients who received at least one dose of study drug
ALT alanine aminotransferase, AST aspartate aminotransferase, EMPA empagliflozin, ULN upper limit of normal
aPatients are presented regardless of baseline elevations
bPatients with ALT and/or AST ≥ 3 × ULN with concomitant or subsequent total bilirubin ≥ 2 × ULN in a 30-day period after ALT and/or AST elevation
Bone Fractures
The frequency and incidence of bone fractures were similar for the empagliflozin 10/25 mg and placebo groups (empagliflozin 10/25 mg: 2.3%, 1.42 events per 100 patient-years; placebo: 2.7%, 1.72 events per 100 patient-years) (Table 6).
Pancreatitis
The frequency and incidence of pancreatitis, including acute pancreatitis, were similar for the empagliflozin 10/25 mg and placebo groups (empagliflozin 10/25 mg: 0.1%, 0.09 events per 100 patient-years; placebo: 0.2%, 0.14 events per 100 patient-years) (Table 6).
Lower Limb Amputation Risk
In the current pooled safety analysis LLAs, including minor (at the ankle or below) and major (above the ankle) amputations, occurred in 95 and 46 patients in the intent-to-treat analysis who were treated with empagliflozin 10/25 mg and placebo, respectively. There was no difference in the overall incidence rate of LLAs between the empagliflozin 10/25 mg and placebo groups (0.52 vs. 0.52 cases per 100 patient-years, respectively) (Table 6). The degree of amputation was less often major in the empagliflozin 10/25 mg group than in the placebo group (0.10 vs. 0.21 cases per 100 patient-years, respectively), and more often minor in the empagliflozin 10/25 mg group than in the placebo group (0.41 vs. 0.30 cases per 100 patient-years, respectively) (Table 6). In addition, analysis of the EMPA-REG OUTCOME® trial, from which the majority of the cases of amputation were reported, showed that the proportion of patients with LLA was similar between the treatment groups, and that empagliflozin was not associated with an increased risk of LLA versus placebo in the trial [21, 37].
Renal Impairment
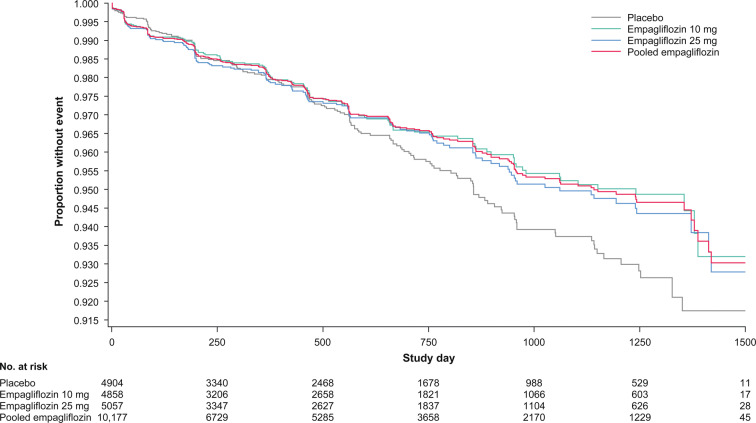
The frequency and incidence of renal impairment events, including acute kidney injury, were similar for the empagliflozin and placebo groups (empagliflozin 10/25 mg: 2.9%, 1.78 events per 100 patient-years; placebo: 2.4%, 2.18 events per 100 patient-years) (Table 10). In particular, the overall frequency of events across treatment groups, assessed by baseline eGFR range, was similar, including in patients with a reduced eGFR of ≥ 30 to < 45 ml/min/1.73 m2 (empagliflozin 10/25 mg: 12.8%; placebo: 13.4%) and < 30 ml/min/1.73 m2 (empagliflozin 10/25 mg: 12.7%; placebo: 11.5%). Kaplan–Meier estimates of time to first renal impairment event for patients treated with empagliflozin compared with placebo are shown in Fig. 1.
Table 10.
Frequency and incidence rate of user-defined renal impairment events
| Placebo (n = 4904) | EMPA 10 mg (n = 4858) | EMPA 25 mg (n = 5057) | EMPA 10/25 mg (n = 10,177) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | n (%) | Rate/100 pt-yrs | |
| Renal impairment | 169 (3.4) | 2.18 | 139 (2.9) | 1.75 | 152 (3.0) | 1.86 | 291 (2.9) | 1.78 |
| Acute kidney injurya | 44 (0.9) | 0.56 | 29 (0.6) | 0.36 | 28 (0.6) | 0.34 | 57 (0.6) | 0.34 |
| Renal impairment by baseline eGFR (CKD-EPI), ml/min/1.73 m2, n (%) | ||||||||
| ≥ 90 | 15/1933 (0.8) | 0.57 | 12/1998 (0.6) | 0.43 | 10/2041 (0.5) | 0.35 | 22/4177 (0.5) | 0.39 |
| 60 to < 90 | 63/2123 (3.0) | 1.73 | 57/2203 (2.6) | 1.48 | 60/2155 (2.8) | 1.56 | 117/4477 (2.6) | 1.50 |
| 45 to < 60 | 48/519 (9.2) | 4.92 | 46/464 (9.9) | 4.80 | 40/535 (7.5) | 4.09 | 86/1003 (8.6) | 4.44 |
| 30 to < 45 | 37/277 (13.4) | 7.85 | 21/182 (11.5) | 5.83 | 36/262 (13.7) | 8.38 | 57/445 (12.8) | 7.22 |
| < 30 | 6/52 (11.5) | 13.87 | 3/10 (30.0) | 20.63 | 6/61 (9.8) | 8.77 | 9/71 (12.7) | 10.85 |
CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, eGFR estimated glomerular filtration rate, EMPA empagliflozin, MedDRA Medical Dictionary for Regulatory Activities, pt-yrs patient-years
aBased on the MedDRA preferred term
Fig. 1.
Kaplan–Meier estimates of time to onset of first event suggestive of renal impairment
Discussion
In the EMPA-REG OUTCOME® trial in patients with T2DM and established CV disease, empagliflozin reduced the risk of CV death by 38% compared with placebo [15]. Patients treated with empagliflozin also experienced a 32% reduction in the risk of all-cause mortality, a 35% reduction in the risk of hospitalization for heart failure, and a 39% reduction in the risk of incident or worsening nephropathy [15, 16, 38]. In addition, empagliflozin has been estimated to increase life expectancy by an average of 1–4.5 years (depending on age), compared with placebo [39].
This analysis of pooled safety data for empagliflozin was of an expanded dataset previously used to investigate the safety and tolerability of empagliflozin in patients with T2DM [21]. Whereas the earlier analysis was based on more than 15,000 patient-years’ exposure to empagliflozin [21], the present expanded dataset was based on more than 16,480 patient-years’ exposure.
Following this earlier analysis [21], the present analysis showed a consistent AE profile and further continued to show that empagliflozin has a good safety profile and is well tolerated in patients with T2DM. In addition, the risk of hypoglycemia was similar for empagliflozin compared with placebo, except when co-administered with insulin and/or an SU.
Due to their mechanism of action, SGLT2 inhibitors cause transient increases in urine volume [1, 2], leading to acknowledgement of its potential for volume depletion and hypotension, particularly in the elderly population. The present analyses showed that the risk of volume depletion for empagliflozin compared with placebo was numerically increased in patients aged 75 to < 85 years, and in patients with concomitant use of loop diuretics, but not in patients with concomitant use of other diuretics, ACE inhibitors/ARBs, or other antihypertensive drugs.
Similarly, UTIs and genital infections are both identified risks associated with SGLT2 inhibitor use, with increases in urinary glucose concentration a potential exacerbating factor [40]. The present data continue to support previous findings that there is no empagliflozin-specific increased risk of UTIs when compared with placebo. Furthermore, the notion that increased urinary glucose concentration and excretion might predispose to UTIs may be counterbalanced with the hypothesis that increased urinary flow due to osmotic diuresis may reduce or balance the potential impact of increased urinary glucose concentration. The risk of genital infection was elevated for empagliflozin compared with placebo, but excess events associated with empagliflozin were predominantly mild or moderate in intensity and seldom led to treatment discontinuation. There was no increased risk of complicated UTIs or complicated genital infections associated with empagliflozin compared with placebo.
The risk of diabetic ketoacidosis, a serious complication associated with diabetes that arises when the body produces high levels of ketones, may be increased by the use of SGLT2 inhibitors [41]. No imbalance in diabetic ketoacidosis has been reported in clinical trials [15, 17, 18], and there was a similar frequency in the present data for patients treated with empagliflozin compared with placebo. Despite this, in 2015, the US Food and Drug Administration issued a safety announcement of the potential for an increased risk of diabetic ketoacidosis with SGLT2 inhibitor treatment [42]. This was the result of post-marketing reports, coupled with a potential mechanism of action of SGLT2 inhibitors involving the metabolic shift towards lipid utilization, leading to increased ketone body production, particularly during prolonged fasting [43]. As a result, the labels for all SGLT2 inhibitors were updated with a warning of this complication [44].
Recently, based on a number of post-marketing reports, the labels for SGLT2 inhibitors were also updated with an adverse reaction of Fournier’s gangrene, which is a rare but serious urological condition, characterized by a progressive necrotizing infection that affects the external genitalia or the perineum [45]. No cases of Fournier’s gangrene have been reported in clinical trials with empagliflozin. Six cases of Fournier’s gangrene were recorded in the DECLARE-TIMI 58 trial, one in the dapagliflozin group and five in the placebo group [18].
Previously, a concern of bladder cancer relating to SGLT2 inhibitor use was raised, particularly in relation to dapagliflozin [46, 47]. However, this risk was not confirmed in the DECLARE–TIMI 58 trial, a large placebo-controlled CV outcome trial [18]. There was also no increased risk of urinary tract cancer in the present analysis. In addition, an analysis of patients with at least 6 months’ drug exposure in EMPA-REG OUTCOME® was undertaken to assess the risk of bladder cancer [48]. The incidence, with an onset after 6 months’ cumulative exposure to the study drug, was reported in 10/4406 patients (0.2%) in the empagliflozin 10/25 mg group and 4/2187 patients (0.2%) in the placebo group. The authors concluded that, based on the totality of the data, no imbalance in bladder cancer cases between empagliflozin and placebo was observed in EMPA-REG OUTCOME® [48].
The CANVAS program reported an increased risk of fractures and LLAs with canagliflozin use, with LLAs consistent for minor and major amputations [17]. In 2017, regulators reviewed available data for possible associations between SGLT2 inhibitors and LLAs. They concluded that canagliflozin may increase the risk of LLA, and the US Food and Drug Administration issued a boxed warning to the canagliflozin label describing the increased risk of leg and foot amputations [49]. In the current analysis, there was no evidence of an association between empagliflozin use and LLAs. The overall frequency of amputations was the same for empagliflozin compared with placebo. In addition, the EMPA-REG OUTCOME® trial found that the proportion of patients with an LLA was similar between the empagliflozin and placebo groups [21]. Similarly, the DECLARE-TIMI 58 trial of over 17,000 patients found no increased risk of amputations between dapagliflozin and placebo [18]. A meta-analysis of the CANVAS program, EMPA-REG OUTCOME® and DECLARE-TIMI 58 trials showed that the increased risk of amputations and fractures in the CANVAS program contributed moderate to high percentages of the total variation across the three trials that was due to heterogeneity (I2 = 79.1% for amputations and I2 = 42.1% for fractures) [5]. In the recent CREDENCE study in 4401 patients with diabetes and albuminuric chronic kidney disease receiving canagliflozin or placebo on top of standard of care, there was no significant difference in the rates of amputation reported (HR 1.11 [95% CI 0.79, 1.56]) [50].
Other potential risks associated with SGLT2 inhibitor use, including liver injury, bone fracture, and pancreatitis, all occurred at a similar frequency for patients treated with empagliflozin and placebo. The events related to renal impairment for patients with reduced eGFR were similar for patients treated with empagliflozin compared with placebo.
Strengths of this analysis include the large sample size and patient exposure. Weaknesses include that the studies were of varying durations and that differences between groups were not compared using modeled analyses.
Conclusion
This pooled analysis, based on over 16,480 patient-years’ exposure to empagliflozin in placebo-controlled trials, confirms previous knowledge of the tolerability of empagliflozin 10 and 25 mg in patients with T2DM. Empagliflozin was not associated with a higher rate of confirmed hypoglycemic events compared with placebo, except when co-administered with insulin and/or an SU. In both the placebo and empagliflozin groups, there was a higher rate of volume depletion in patients with baseline hypotension versus normotension, and in patients taking diuretics, loop diuretics, ACE inhibitors/ARBs, or anti-hypertensive drugs, compared with patients not taking these medications. The frequency and incidence rates of events consistent with volume depletion were similar between patients treated with empagliflozin and placebo, except for patients aged 75 to < 85, and for patients treated with loop diuretics at baseline, where the frequency was higher for empagliflozin compared with placebo. Genital infections, but not UTIs, were more frequent in patients treated with empagliflozin than placebo. There was no difference in the incidence of LLA in the pooled empagliflozin group versus placebo. The incidences of bone fractures, urinary tract carcinogenicity, renal impairment, liver injury, pancreatitis, and diabetic ketoacidosis were not increased with empagliflozin compared with placebo in clinical trials. Further information on the safety and tolerability profile of empagliflozin will be provided by post-marketing surveillance and ongoing clinical trials. Overall, this analysis shows that empagliflozin is a well-tolerated SGLT2 inhibitor that reduces all-cause mortality in patients with T2DM and established CV disease while displaying a favorable benefit-risk profile.
Acknowledgements
The authors thank the participants of the studies that provided data for this analysis.
Funding
The studies were funded by Boehringer Ingelheim or the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. The article Rapid Service Fee and Open Access Fee were funded by Boehringer Ingelheim. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Medical Writing and/or Editorial Assistance
Medical writing assistance was supported financially by Boehringer Ingelheim and provided by Charlie Bellinger and Céline Tevlin of Elevate Scientific Solutions, Horsham, UK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Ona Kinduryte Schorling is employed by Boehringer Ingelheim. Douglas Clark is employed by Boehringer Ingelheim. Isabella Zwiener is employed by Boehringer Ingelheim. Stefan Kaspers is employed by Boehringer Ingelheim. Jisoo Lee is employed by Boehringer Ingelheim. Hristo Iliev is employed by Boehringer Ingelheim.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. An independent ethics committee or institutional review board approved the clinical protocol at each participating center. All participants gave signed and dated informed consent prior to inclusion.
Data Availability
The sponsor of the clinical trials (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient-level clinical study data. The datasets analyzed during the current study are available in the Boehringer Ingelheim repository (https://trials.boehringer-ingelheim.com/). Researchers are invited to submit inquiries via the Boehringer Ingelheim website.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12040413.
References
- 1.Heise T, Jordan J, Wanner C, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:2265–2276. doi: 10.1016/j.clinthera.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Heise T, Jordan J, Wanner C, et al. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin Ther. 2016;38:2248–2264. doi: 10.1016/j.clinthera.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Juni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet. 2019;393:3–5. doi: 10.1016/S0140-6736(18)32824-1. [DOI] [PubMed] [Google Scholar]
- 5.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 6.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diabetes Vasc Dis Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 8.Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–1659. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 11.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–948. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 15.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 16.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 17.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 18.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 20.Kohler S, Salsali A, Hantel S, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:1299–1313. doi: 10.1016/j.clinthera.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Kohler S, Zeller C, Iliev H, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I–III clinical trials. Adv Ther. 2017;34:1707–1726. doi: 10.1007/s12325-017-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrannini E, Seman L, Seewaldt-Becker E, et al. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–728. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 23.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–621. doi: 10.1111/dom.12073. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther. 2014;31:621–638. doi: 10.1007/s12325-014-0126-8. [DOI] [PubMed] [Google Scholar]
- 25.Kanada S, Koiwai K, Taniguchi A, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of 4 weeks' treatment with empagliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:613–617. doi: 10.1111/jdi.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-h glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–1160. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 28.Häring HU, Merker L, Christiansen AV, et al. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;110:82–90. doi: 10.1016/j.diabres.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773–88:e1. doi: 10.1016/j.clinthera.2015.05.511. [DOI] [PubMed] [Google Scholar]
- 30.Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555–1567. doi: 10.1111/dme.12814. [DOI] [PubMed] [Google Scholar]
- 31.Roden M, Merker L, Christiansen AV, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: a double-blind extension of a phase III randomized controlled trial. Cardiovasc Diabetol. 2015;14:154. doi: 10.1186/s12933-015-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross S, Thamer C, Cescutti J, et al. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17:699–702. doi: 10.1111/dom.12469. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinand KC, Seman L, Salsali A. Design of a 24-week trial of empagliflozin once daily in hypertensive black/African American patients with type 2 diabetes mellitus. Curr Med Res Opin. 2018;34:361–367. doi: 10.1080/03007995.2017.1405800. [DOI] [PubMed] [Google Scholar]
- 34.Søfteland E, Meier JJ, Vangen B, et al. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40:201–209. doi: 10.2337/dc16-1347. [DOI] [PubMed] [Google Scholar]
- 35.Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;20:2200–2209. doi: 10.1111/dom.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sone H, Kaneko T, Shiki K, et al. Efficacy and safety of empagliflozin as add-on to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2019. (in press). [DOI] [PMC free article] [PubMed]
- 37.Inzucchi SE, Iliev H, Pfarr E, et al. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:e4–e5. doi: 10.2337/dc17-1551. [DOI] [PubMed] [Google Scholar]
- 38.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claggett B, Lachin JM, Hantel S, et al. Long-term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease. Circulation. 2018;138:1599–1601. doi: 10.1161/CIRCULATIONAHA.118.033810. [DOI] [PubMed] [Google Scholar]
- 40.Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–381. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 41.Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33:e2886. doi: 10.1002/dmrr.2886. [DOI] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration. FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. https://www.fda.gov/media/92185/download. Accessed Sept 18, 2019.
- 43.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about. Accessed Sept 25, 2019.
- 45.Sorensen MD, Krieger JN, Rivara FP, et al. Fournier's gangrene: population based epidemiology and outcomes. J Urol. 2009;181:2120–2126. doi: 10.1016/j.juro.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AstraZeneca. Forxiga (dapagliflozin) US prescribing information. 2018. https://www.azpicentral.com/farxiga.pdf#page=1. Accessed Nov 18, 2018.
- 47.US Food and Drug Administration. Briefing document: NDA 202293 dapagliflozin oral tablets, 5 and 10 mg. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/202293Orig1s000RiskR.pdf. Accessed Nov 18, 2018.
- 48.Kohler S, Lee J, George JT, et al. Bladder cancer in the EMPA-REG OUTCOME trial. Diabetologia. 2017;60:2534–2535. doi: 10.1007/s00125-017-4430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US Food and Drug Administration. Canagliflozin (Invokana, Invokamet): Drug safety communication—increased risk of leg and foot amputations. 2017. https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm. Accessed Nov 18, 2018.
- 50.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sponsor of the clinical trials (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient-level clinical study data. The datasets analyzed during the current study are available in the Boehringer Ingelheim repository (https://trials.boehringer-ingelheim.com/). Researchers are invited to submit inquiries via the Boehringer Ingelheim website.