Abstract
Therapeutic approaches to decrease serum triglyceride (TG) concentrations are not successful mainly due to poor adherence or adverse effects of therapies. In consequence, the search for new low-cost and safer therapeutic alternatives is mandatory. Dark chocolate and cacao have shown promising results improving lipid profiles. Recently, using cacao by-products to reduce elevated cardiometabolic risk markers in an animal model of obesity induced by a high-fat diet and fructose, we showed that TGs, low-density lipoprotein cholesterol, and the TG/high-density lipoprotein (HDL) ratio decreased, suggesting that cacao by-products improved the metabolic function of obese animals. Based on these results, as a proof of concept, a blinded placebo-controlled study was implemented to explore the effects of cacao by-products on anthropometric and biochemical variables in a group of overweight subjects participating in a program composed of reduced-calorie-diet counseling plus a simple aerobic exercise plan. The results showed that counseling induced weight and abdominal circumference reductions in both groups. TGs did not change in the control group; however, TG decreased significantly by 54.9 mg/dL (27.9%) in the experimental group. The TG/HDL cholesterol ratio changed markedly (1.5) in the experimental group. The results reported suggest the use of cacao by-products as an alternative for the treatment of hypertriglyceridemia.
Keywords: cacao by-products, hypertriglyceridemia, obesity
Background
Treatment costs associated with obesity and diabetes threaten both national security and economic development worldwide.1–7 Among the cluster of pathological features that characterize dysmetabolic overweight/obesity (O/O), several abnormalities of lipid metabolism, such as hypertriglyceridemia, hypoalphalipoproteinemia, and low-density lipoprotein (LDL) hypercholesterolemia, stand out that when they occur together, they give rise to the so-called lipid triad or atherogenic dyslipidemia, which is highly predictive of myocardial infarction and/or stroke.8–12
Unfortunately, dyslipidemia treatment according to several international guidelines12 is focused mainly on LDL cholesterol (LDL-c) management, with just a marginal reference to hypertriglyceridemia, despite convincing evidence of its importance in many trials and meta-analyses.13
Given the rather limited success of therapeutic approaches to decrease serum triglyceride (TG) concentrations, mainly due to poor adherence to therapeutic lifestyle changes or to the several inconveniences associated with commonly used drugs, it is necessary to search for new low-cost and safer therapeutic alternatives.
In this regard, dark chocolate and cocoa have shown promising lipid profile results in several animal models and human studies.14,15 Our group, using the main flavonol of cacao, (−)-epicatechin (EPI), in both animal models and human clinical studies observed a substantial reduction in serum TGs.15,16
Recently, we reported the use of cacao by-products for the treatment of elevated cardiometabolic risk markers in an obesity animal model induced by high-fat diet and fructose ingestion. Our results showed that TGs were reduced by 55%, low-density lipoprotein cholesterol by 37%, and the TG/high-density lipoprotein (HDL) ratio by 54%, suggesting that cacao by-products improved the metabolic function of obese animals.17
Based on the results obtained in animals and as a proof of concept, this study explored the effects of cacao by-products on anthropometric and biochemical variables in a group of overweight human subjects participating in a program composed of reduced calorie diet counseling plus a simple aerobic exercise plan.
Methods
The study was approved by the Ethics and Research Institutional Committees of Hospital Juarez of Mexico and was conducted according to modified Declaration of Helsinki,18 Mexican Federal regulations and the Good Clinical Practice guidelines.
A group of overweight patients (body mass index [BMI] 25–29.9 kg/m2) of either gender who were 20–60 years old, who had serum TG concentrations of 150–350 mg/dL (1.69–3.95 mmol/L) and who signed the informed consent form were selected.
A convenience sample of 24 subjects were randomly assigned to one of two groups, (1) placebo or (2) experimental, on the basis of an initial screening of 50 subjects attending the hospital as a companion of the patients.
Patients with a previous diagnosis of diabetes mellitus (DM), hypercholesterolemia, systemic arterial hypertension (SAH), or established cardiovascular diseases; those who were already medicated with lipid-lowering drugs, metformin, or steroids; those who had suffered an acute physical stressful event, such as an acute infection, important loss of weight, or had undergone any surgical or instrumental procedures in the past 6 weeks; women who were pregnant or lactating; chronic smokers; persons with a history of alcohol or other drug abuse; and subjects with known allergies or intolerances to chocolate or its components were not included. Patients already admitted to the study were eliminated if they did not provide informed consent or developed any type of intolerance to the cacao by-products (cookies). Patients who developed DM or SAH during the trial, those who did not attend two consecutive visits, and those who failed to consume the prescribed cookies for 3 days were eliminated.
Subjects were randomized, using computational software, to Group A or B. Subjects in both groups received dietary instructions to reduce their habitual consumption by 250 kcal and were counseled to perform daily dynamic exercise to burn 250–300 kcal/day. Group A (placebo) received a placebo cookie (elaborated with wheat bran), whereas group B (experimental) received similar cookies made from cacao pericarp enriched with flavonoids extracted from the same cocoa husk containing 12.5 mg of EPI equivalents (the main flavonol of cacao). Procedures for obtaining cacao flour and extracting and quantifying flavonols have been previously reported.17
Experimental cookies were prepared using cocoa pericarp flour, wheat flour, water, butter, sugar, egg, baking powder, and salt; control cookies contain all the ingredients but cocoa pericarp flour.17
Both cookie types had the same characteristics and taste, weighed 4 g and contained 14 kcal. In addition, the treatment group cookies had an EPI content of 12.5 mg. In both groups, cookies were consumed twice a day, once before breakfast and once before dinner. The subjects in the experimental group received a total of 25 mg of EPI equivalents per day, EPI content was calculated in base of a standard curve developed with pure EPI and expressed as EPI equivalents.17
Dietary and exercise counseling
Instead of prescribing a rigorously restrictive diet of 20 calories per kilogram of “ideal weight,” which is known to be difficult for most patients to follow, we opted to counsel them to reduce their intake by 250 kcal per day, reducing, for example, the consumption of bread and sweetened drinks or other sources of simple and complex carbohydrates.
Furthermore, physical exercise counseling was given to patients in both groups, encouraging them to perform a type of moderate dynamic exercise (whose energy expenditure was between 3 and 6 metabolic equivalent of tasks [METs]) daily (or at least 5 days a week) for a length of time sufficient to expend 250 kcal.
The amount of energy expended depending on the type and duration of the exercise session was estimated with the following formula:
Energy spent per minute = (METs activity × 3.5 × body weight)/200, where METs activity is the energy expenditure per minute of each type of exercise (i.e., walking, swimming, or jogging).19
Patients were followed for 8 weeks. At each visit (0, 4, and 8 weeks), the following variables were assessed and recorded: dietary evaluation, which consisted of the application of a 24-h recall and the assessment of the frequency of food consumption, and a lifestyle assessment, which assessed the compliance with the prescription and counseling.
The anthropometric appraisal comprised the measure of body weight (kg) by means of a digital balance and height (m) with a stadiometer. These values were used to calculate BMI (kg/m2). Abdominal circumference was measured with a graduated fiberglass ribbon while the subject was standing erect, and the measuring tape was placed between the iliac crest and the last rib. In addition, at each visit, after 10 h of fasting, a venous blood sample was obtained to determine the serum glucose and lipid profile, employing commercial analysis kits (Randox).
Statistics
All data are presented as the mean ± standard error. Unifactorial analysis of variance (ANOVA) with Tukey's post-test was used to compare the differences between groups. Paired Student's t-tests were employed if necessary. A value of P < .05 was considered statistically significant. The data were processed using GraphPad Prism software, version 7.
Results
Table 1 shows the anthropometric baseline characteristics and metabolic profiles of the patients. A random distribution was confirmed since the anthropometric and basal metabolic profile data did not show significant differences, with the exception of age: patients in the experimental group were older than those in the control group.
Table 1.
Basal Characteristics of Both Groups
| Variable | Placebo | Experimental | P |
|---|---|---|---|
| N | 11 | 13 | |
| Age (years) | 42 ± 2.2 | 48.3 ± 1.9 | .03 |
| Weight (kg) | 73.5 ± 1.1 | 66.0 ± 1.2 | NS |
| BMI (kg/m2) | 28.4 ± 0.3 | 27.9 ± 0.3 | NS |
| Waist circumference (cm) | 91.4 ± 1.2 | 87.9 ± 1.3 | NS |
| Glucose (mg/dL) | 85.8 ± 3.2 | 91.0 ± 3.5 | NS |
| TC (mg/dL) | 188.5 ± 8.9 | 204.8 ± 3.4 | NS |
| LDL-c (mg/dL) | 102.8 ± 8.4 | 124.8 ± 11.1 | NS |
| HDL-c (mg/dL) | 38.9 ± 2.1 | 42.6 ± 2.0 | NS |
| TGs (mg/dL) | 195.9 ± 18.8 | 204.2 ± 17.4 | NS |
| TGs/HDL-c | 5.23 ± 0.7 | 4.9 ± 0.4 | NS |
| LDL-c/HDL-c | 2.9 ± 0.3 | 3.0 ± 0.3 | NS |
BMI, body mass index; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NS, no significant; TC, total cholesterol; TG, triglyceride
The sample was composed mostly of women. The BMI values indicated that all participants were overweight (BMI >25). Despite weight abnormalities, glycemia levels were normal in both groups. Because hypertriglyceridemia was a requisite for inclusion in the study, TG values were moderately high in both groups, without significant differences between them.
The basal values of total cholesterol (TC) and LDL-c were modestly higher in the experimental group than in the control group, whereas the HDL cholesterol (HDL-c) values were slightly lower in the control group (8.7%). These differences were not significant. The levels of HDL-c were low, taking into account that most of the participants were women, in whom the normal concentration is ≥50 mg/dL.
The ratio between TGs and HDL-c, an index with demonstrated cardiovascular predictive power and correlation with insulin resistance, was elevated in both groups, without a significant difference between them. The ratio between LDL-c/HDL-c was borderline in both groups (Table 1).
Table 2 shows changes in anthropometric and biochemical data after 8 weeks of treatment.
Table 2.
Anthropometric and Biochemical Changes
 |
Anthropometric
Weight loss occurred in both groups, 5.5 kg in the placebo group and 3.2 kg in the experimental group. BMI was reduced by 2 and 1.3 kg/m2 in the placebo and experimental groups, respectively, corresponding to a proportional change of 7.04% and 4.65%, respectively. Waist circumference was reduced to the same extent in both groups: 4.1 cm in the placebo group and 3.9 cm in the experimental group.
Biochemical
Glucose decreased slightly in both groups, by 4.3 mg/dL (5%, no significant) in the placebo group and 10 mg/dL (10.9%, P = .01) in the experimental group (Table 2).
TC and LDL-c did not exhibit significant changes in either group. HDL-c showed a moderate nonsignificant increase of 7% in the experimental group.
Interestingly, the TG concentration did not change significantly (4.08%) in the control group; notably, TGs decreased significantly by 54.9 mg/dL (27.9%) in the experimental group, and the comparison of the changes between the two groups was significant (P = .003) (Fig. 1).
FIG. 1.
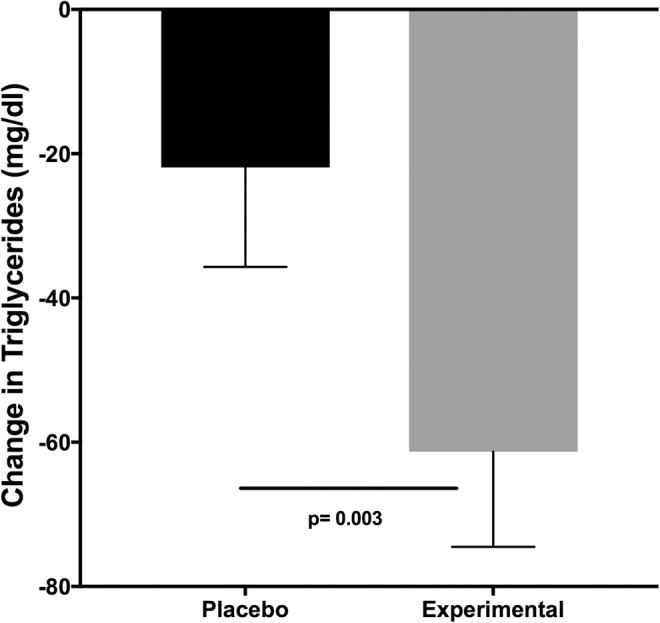
Change in TG concentration (week 8—basal) induced by the ingestion of cacao by-products. Subjects were blinded to the treatment. Treatment consisted of the ingestion of two cookies per day. Subjects in both groups received dietary instructions to reduce their habitual consumption by 250 kcal and received counseling to perform exercise to expend 250–300 kcal/day. Data are presented as the median ± SEM. SEM, standard error of the mean; TG, triglyceride.
The change in TG concentration was consistent throughout the evaluation since the decrease was also found during week 4 and continued until week 8 in the experimental group (Fig. 2).
FIG. 2.
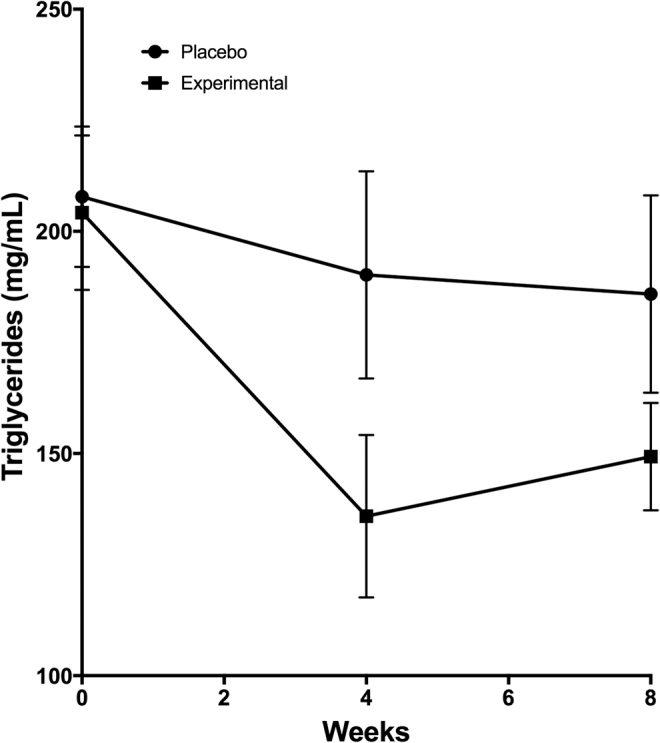
Changes in TG concentrations induced by the ingestion of cacao by-products. Treatment consisted of the ingestion of two cookies per day elaborated with cacao by-products or placebo. Subjects in both groups received dietary instructions to reduce their habitual consumption by 250 kcal and received counseling to perform exercise to expend 250–300 kcal/day. Data are presented as the median ± SEM.
The LDL-c/HDL-c ratio did not exhibit meaningful modifications in either group, whereas the TG/HDL-c ratio changed markedly (1.5) in the experimental group (30% from baseline, P < .008) but remained unchanged in the placebo group (Table 2).
Discussion
The main findings of this study were (1) counseling with the aim of limiting caloric ingestion and encouraging the performance of limited exercise (each resulting in a calorie deficit of ∼250 kcal) is enough to induce a decrease in body weight and waist circumference and (2) the combination of counseling and cookies made of cacao by-products induced a marked decrease in hypertriglyceridemia.
Apart from therapeutic lifestyle interventions, fibrates and omega-3 fatty acids are the classic drugs recommended for severe and moderate hypertriglyceridemia.
Dark chocolate, cacao-derived extracts, and EPI, the main flavonoid of cacao, have exhibited numerous notable metabolic actions in humans and animals, independent of their antioxidant effects. In particular, EPI, probably through its interaction with a surface receptor,20,21 exerts a set of metabolic actions, such as the activation of phosphorylating enzymes such as sirtuin 1 (NAD-dependent deacetylase), liver kinase B1 (LKB1), and 5′ adenosine monophosphate-activated protein kinase (AMPK), a key metabolic sensor that enhances both the transcription and translocation of glucose transporter type 4 (GLUT4), which increases glucose uptake. In addition, AMPK, through post-translational modification, controls the expression of the peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1α (PGC-1α), a transcription factor related to energy metabolism. Among many functions, this molecule intervenes in the process of lipid β-oxidation and can induce mitochondrial biogenesis.22 Through these mechanisms, EPI can reduce the levels of VLDL and reduce the concentrations of TGs and LDL-c.
In contrast, hypertriglyceridemia is frequently associated with low values of HDL-c (hypoalphalipoproteinemia), and the reduction in this protective lipoprotein is related to an increase in cardiovascular risk.
Traditional therapy for the treatment of hypertriglyceridemia using fibrates in dyslipidemic patients diminishes the relative risk by 41%.23 As cardiovascular risk increases with fasting TG concentrations even in a range as low as 150–200 mg/dL, it is recommendable to treat even moderate hypertriglyceridemia.
Data from this study are in agreement with previous results.23–25 In addition, in this rather small group of patients who were slightly overweight, our findings indicated that the strategy of “minimal” calorie reduction resulted in good clinical benefits by reducing weight, BMI, and abdominal circumference in both groups. The most notable finding, the decrease in TG concentrations in the treatment group (30% from baseline, P < .003), is similar to the results obtained from many fibrates. Furthermore, whereas HDL-c decreased by 3.85% among controls, it increased (nonsignificantly) by 7% in the active cookie group.
Interestingly, the TG/HDL-c index decreased in the active cookie group from 4.9 to 3.4, which was a 30% decrease (P < .008); however, this index remained unchanged in the control group. The TG/HDL-c index reflects the inverse relationship between TG and HDL concentrations, predicting the occurrence of coronary events, general mortality in women, and the extent of coronary lesions, and demonstrates a modest correlation with insulin resistance.
This proof-of-concept study showed that the combination of an “easy-to-maintain” diet (reducing intake by only 250 kcal/day) plus a very simple plan of dynamic mild exercise (30–60 min of brisk walking a day) and the ingestion of two cookies with cacao by-products that are rich in flavonoids decreases cardiovascular risk marker levels.
In summary, the administration of simple and relatively inexpensive cacao by-products can lower cardiovascular risk in overweight patients, demonstrating the possibility of using these by-products, which are essentially calorie free and rich in flavonols, in a systematic way to facilitate the attainment of preventive goals. The results reported here encourage the design of larger studies with longer durations to demonstrate that this strategy is beneficial in the long term while being easy for patients to accept and more useful from a cost-benefit point of view.
Author Disclosure Statement
Dr. Villarreal is a cofounder and stockholder of Cardero Therapeutics, Inc., and Dr. Ceballos is a stockholder. The rest of the authors declare no conflicts of interest.
Funding Information
Dr. Villarreal was supported by NIH DK98717 and Dr. Ceballos by a Conacyt #253769 grant.
References
- 1. Sánchez-Castillo CP, Velásquez-Monroy O, Lara-Esqueda A, Berber A, Sepulveda J, Tapia-Conyer R, et al. : Diabetes and hypertension increases in a society with abdominal obesity: Results of the Mexican National Health Survey 2000. Public Health Nutr 2005;8:53–60 [DOI] [PubMed] [Google Scholar]
- 2. Meaney A, Ceballos-Reyes G, Gutiérrez-Salmean G, Samaniego-Méndez V, Vela-Huerta A, Alcocer L, et al. : Cardiovascular risk factors in a Mexican middle-class urban population. The Lindavista Study. Baseline data. Arch Cardiol México 2013;83:249–256 [DOI] [PubMed] [Google Scholar]
- 3. Rtveladze K, Marsh T, Barquera S, Sanchez Romero LM ari., Levy D, Melendez G, et al. : Obesity prevalence in Mexico: Impact on health and economic burden. Public Health Nutr 2014;17:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fanghänel-Salmón G, Gutiérrez-Salmeán G, Samaniego V, Meaney A, Sánchez-Reyes L, Navarrete U, et al. : Obesity phenotypes in urban middle-class cohorts; the PRIT-Lindavista merging evidence in Mexico: The OPUS PRIME study. Nutr Hosp 2015;32:182–188 [DOI] [PubMed] [Google Scholar]
- 5. Barquera S, Campos-Nonato I, Aguilar-Salinas C, Lopez-Ridaura R, Arredondo A, Rivera-Dommarco J: Diabetes in Mexico: Cost and management of diabetes and its complications and challenges for health policy. Global Health 2013;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ginsberg HN, Zhang Y, Hernandez-ono A: Metabolic Syndrome: Focus on dyslipidemia metabolic syndrome: link to atherosclerosis. Focus (Madison). Obesity 2006;14(Suppl 1):41S–49S [DOI] [PubMed] [Google Scholar]
- 7. Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, Isenovic ER. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol 2017;15:30–39 [DOI] [PubMed] [Google Scholar]
- 8. Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol 1998;81:18B–25B [DOI] [PubMed] [Google Scholar]
- 9. Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS: Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation 2004;109:42–46 [DOI] [PubMed] [Google Scholar]
- 10. Wierzbicki AS. Diabetic dyslipidaemia: The triad. Eur Heart J Suppl 2006;8:30–33 [Google Scholar]
- 11. Estrada-Garcia T, Menaey A, López-Hernandez D, Meaney E, Sanchez-Hernandez O, Rodriguez-Arellano E, Solache G: Hypertension and lipid triad are the most important attributable risks for myocardial infarction in a middle class urban Mexican population. Ann Nutr Metab 2013;63(Suppl1):1343 [Google Scholar]
- 12. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. , AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209 [DOI] [PubMed] [Google Scholar]
- 13. Assmann G, Schulte H: Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Am J Cardiol 1992;70:733–737 [DOI] [PubMed] [Google Scholar]
- 14. Katz DL, Doughty K, Ali A: Cocoa and chocolate in human health and disease. Antioxid Redox Signal 2011;15:2779–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia L, Liu X, Bai YY, Li SH, Sun K, He C, et al. : Short-term effect of cocoa product consumption on lipid profile: A meta-analysis of randomized controlled trials. Am J Clin Nutr 2010;92:218–225 [DOI] [PubMed] [Google Scholar]
- 16. Gutiérrez-Salmeán G, Meaney E, Lanaspa MA, Cicerchi C, Johnson RJ, Dugar S, et al. : A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects. Int J Cardiol 2016;223:500–506 [DOI] [PubMed] [Google Scholar]
- 17. Hidalgo I, Ortiz A, Sanchez-Pardo M, Garduño-Siciliano L, Hernández-Ortega M, Villarreal F, et al. : Obesity and cardiovascular risk improvement using cacao by-products in a diet-induced obesity murine model. J Med Food 2019;22:567–577 [DOI] [PubMed] [Google Scholar]
- 18. Association WM: World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194 [DOI] [PubMed] [Google Scholar]
- 19. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. : 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581 [DOI] [PubMed] [Google Scholar]
- 20. Moreno-Ulloa A, Romero-Perez D, Villarreal F, Ceballos G, Ramirez-Sanchez I: Cell membrane mediated (-)-epicatechin effects on upstream endothelial cell signaling: Evidence for a surface receptor. Bioorg Med Chem Lett 2014;24:2749–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreno-Ulloa A, Mendez-Luna D, Beltran-Partida E, Castillo C, Guevara G, Ramirez-Sanchez I, et al. : The effects of (-)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol Res 2015;100:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramirez-Sanchez I, Moreno A, Ceballos G, Villarreal F: Epicatechin (EPI) induces mitochondrial biogenesis (MB) in human coronary artery endothelial cells (HCAEC) cultured under high glucose conditions. FASEB J 2011;25(Suppl):6866 [Google Scholar]
- 23. Gutierrez-Salmean G, Ciaraldi TP, Nogueira L, Barboza J, Taub PR, Hogan MC, et al. : Effects of (−)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J Nutr Biochem 2014;25:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutiérrez-Salmeán G, Ortiz-Vilchis P, Vacaseydel CM, Rubio-Gayosso I, Meaney E, Villarreal F, et al. : Acute effects of an oral supplement of (−)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct 2014;5:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutiérrez-Salmeán G, Ortiz-Vilchis P, Vacaseydel CM, Garduño-Siciliano L, Chamorro-Cevallos G, Meaney E, et al. : Effects of (−)-epicatechin on a diet-induced rat model of cardiometabolic risk factors. Eur J Pharmacol 2014;728:24–30 [DOI] [PubMed] [Google Scholar]


