Abstract
Significance: The primary function of NADPH oxidases (NOX1–5 and dual oxidases DUOX1/2) is to produce reactive oxygen species (ROS). If inadequately regulated, NOX-associated ROS can promote oxidative stress, aberrant signaling, and genomic instability. Correspondingly, NOX isoforms are known to be overexpressed in multiple malignancies, thus constituting potential therapeutic targets in cancer.
Recent Advances: Multiple genetic studies aimed at suppressing the expression of NOX proteins in cellular and animal models of cancer have provided support for the notion that NOXs play a pro-tumorigenic role. Further, large drug screens and rational design efforts have yielded inhibitor compounds, such as the diphenylene iodonium (DPI) analog series developed by our group, with increased selectivity and potency over “first generation” NOX inhibitors such as apocynin and DPI.
Critical Issues: The precise role of NOX enzymes in tumor biology remains poorly defined. The tumorigenic properties of NOXs vary with cancer type, and precise tools, such as selective inhibitors, are needed to deconvolute NOX contribution to cancer development. Most NOX inhibitors developed to date are unspecific, and/or their mechanistic and pharmacological characteristics are not well defined. A lack of high-resolution crystal structures for NOX functional domains has hindered the development of potent and selective inhibitors.
Future Directions: In-depth studies of NOX interactions with the tumor microenvironment (e.g., cytokines, cell-surface antigens) will help identify new approaches for NOX inhibition in cancer.
Keywords: NADPH oxidase, dual oxidase, cancer, inflammation, reactive oxygen species, small-molecule inhibitors
Introduction
Cellular reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide anion (O2•−) are generally produced in response to cytokine or growth factor stimuli, or, in the case of O2•−, as a by-product of mitochondrial oxidative phosphorylation (144). As such, ROS play the role of secondary messengers involved in the regulation of cell differentiation, growth, migration, and host defense (29, 66, 111). Under physiological conditions, ROS levels are tightly regulated through the action of various scavenging and antioxidant systems to mediate the normal activity of cellular signaling pathways (144). Enzymatic antioxidants such as glutathione peroxidases, catalase, and superoxide dismutase; and non-enzymatic antioxidants such as reduced glutathione, thioredoxins, peroxiredoxins, and glutaredoxins ensure that the redox balance of the cell is maintained (174).
Dysregulation of redox homeostasis due to increased ROS production or decreased cellular antioxidant capacity leads to pathological oxidative stress and oxidation of cellular components, such as DNA, lipids, and proteins (21). An aberrant oxidant environment has been linked to the development of a wide array of disease states, including hypertension, diabetes, neurogenerative disorders, chronic inflammation, and cancer (4, 71, 80, 130). Correspondingly, elevated ROS levels have been detected in cancer cells, resulting from increased metabolism, enhanced mitochondrial activity, and mitogenic signaling (89, 165, 170).
Outside of mitochondria, NADPH oxidases (NOXs) are the primary source of H2O2 and O2•− in non-phagocytic mammalian cells (22, 105, 134, 147). Although they share some structural and functional domain elements, the seven members of the NOX family (NOX1–5, dual oxidases [DUOX] 1, and DUOX2) vary in their architecture, tissue distribution, and activation mechanisms. NOX enzymes generate O2•− by catalyzing the NADPH-dependent transfer of an electron to oxygen across the cell membrane using their shared ferric reductase-like transmembrane element, which contains four highly conserved heme-binding histidine residues, along with cytosolic flavin adenine dinucleotide (FAD)- and nicotinamide adenine dinucleotide (NAD)-binding domains (22, 147). NOX5 and the DUOX also contain Ca2+-binding EF-hand motifs. Further, the highly homologous isoforms DUOX1 and DUOX2 have an additional extracellular peroxidase homology domain. DUOX1, DUOX2, and constitutively active NOX4 produce H2O2, either directly or via the spontaneous or enzymatic dismutation of O2•− (22, 60, 126, 127).
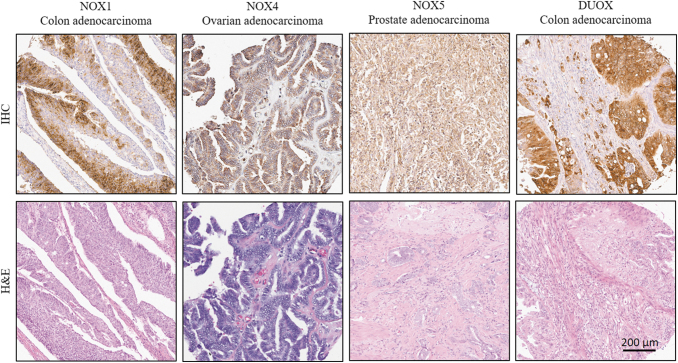
Study of the functional impact of NOX proteins and associated ROS on cancer signaling pathways has been hindered by the lack of specific, versatile, and high-quality monoclonal antibodies that are designed to recognize NOX proteins and confirm their expression in relevant model systems and tissues (4). Our research group has recently developed, validated, and made available for research use selective monoclonal antibodies targeting human DUOX1/2, NOX5, and NOX4 (8, 129, 189) (Fig. 1). The report of a validated human NOX1 monoclonal antibody also developed by our program will soon be published.
FIG. 1.
Immunohistochemical detection of NOXs in human tumors. Representative images showing positive IHC staining of NOX1 in colon adenocarcinoma, NOX4 in serous papillary ovarian adenocarcinoma, NOX5 in prostate adenocarcinoma, and DUOX in colon adenocarcinoma. The NOX1, NOX4, NOX5, and DUOX antibodies used for the IHC were developed by our group [(8, 129, 189) and unpublished results]. Tissues are from BioMax multi-tumor tissue arrays (BC05002 and BC051110a for the colon adenocarcinoma tissues, MC6163 for the ovarian adenocarcinoma, and PR2085b for the prostate adenocarcinoma). Images were taken at 10 × digital magnification. DUOX, dual oxidase; IHC, immunohistochemical; NOX, NADPH oxidase.
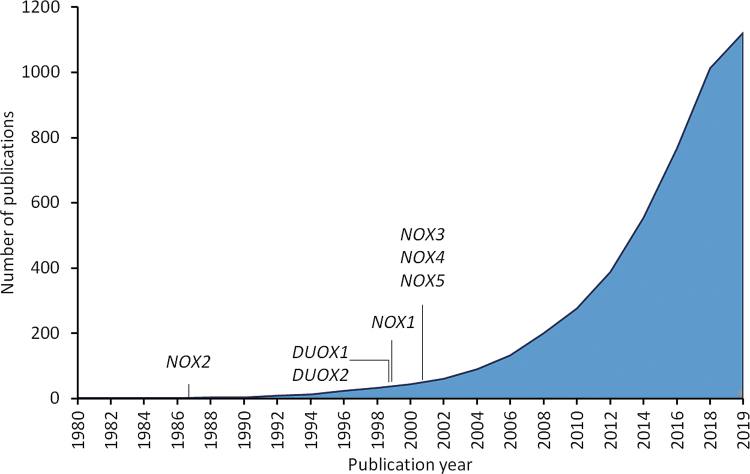
In addition to immunological means of detecting expressed NOX proteins, the ability to specifically and selectively inhibit NOX isoforms would allow for the unambiguous characterization of the role of NOX enzymes in cancer and could provide new therapeutic avenues for cancer treatment. At present, genetic depletion methods constitute a first-line, proof-of-concept method to help define the role of an NOX protein of interest in the etiology of malignancy. Such studies have revealed that the aberrant overexpression of NOX enzymes contributes to the etiology of many diseases (71, 127, 147, 190). Thus, NOX proteins are increasingly studied in the context of cancer. Effectively, the number of publications in PubMed referencing “NADPH oxidase” and “cancer” has been increasing exponentially since the discovery of gp91phox (NOX2) in 1986–1987 (55, 148, 171) (Fig. 2). In this work, we will review evidence supporting the NOX family as a therapeutic target in cancer, as well as past and ongoing efforts and shortcomings in inhibiting NOX activity and tumor growth using genetic and molecular approaches.
FIG. 2.
Cumulative number of publications referencing the terms “NADPH oxidase” and “cancer” in PubMed from 1980 to the present day. The discovery and first report of cloning is indicated for the various NOX isoforms (19, 20, 38, 50, 55, 60, 98, 148, 159, 166, 171).
NOXs as Therapeutic Targets in Cancer
Role of NOX isoforms in cancer
Over the past 20 years, a large number of studies involving genetic manipulation of NOX expression coupled with modulation of activity using inhibitors have implicated NOX-mediated ROS (particularly from NOX1 and NOX4) in cell division, cell survival, angiogenesis, and integrin signaling, suggestive of a causal role in cancer development (42). The tissue and subcellular compartment distribution of NOX isoforms varies such that they affect cell signaling in distinctive ways (28). Using small hairpin RNA (shRNA) technology to knock down NOX1, de Carvalho et al. demonstrated a clear role for NOX1 in O2•− production and proliferation of colon carcinoma cell lines (49). These results were further supported by a 2012 study by Kajla et al. (96a) wherein NOX1-mediated Wnt signaling induced cell growth and RAS transformation. Additional genetic knockdown studies detailing mechanisms through which NOX1-mediated ROS affect the proliferation of HT-29 and Caco-2 human colon cancer cells revealed that knockdown of NOX1 led to cell cycle arrest or apoptosis, depending on the cell line studied and extent of NOX1 knockdown (183). Wang et al. observed a significant decrease in the growth of HT-29-shNOX1 cells, which occurred, in part, by way of the ADAM17-EGFR-PI3K-Akt signaling pathway (181). Similarly, our laboratory found that silencing NOX1 inhibited the growth of HT-29 cells, and this effect was associated with the downregulation of several oncogenes, chemokines, and angiogenic factors (95). The significant decrease in tumor growth and angiogenesis on NOX1 attenuation was corroborated by in vivo studies from parental HT-29 cells versus NOX1 knockdown cell xenografts (95).
NOX2 expression has been associated with the growth of breast, colorectal, gastric, and prostate cancers, and with myelomonocytic leukemia (78, 81, 85, 100, 132). In breast cancer cells, silencing of the superoxide-generating NOX2 resulted in significant reduction of IKKɛ (inhibitor of NF-κB kinase) expression, a key player in cell transformation, invasiveness, and the development of chemoresistance (132). In gastric cancer, the small interfering RNA (siRNA)-mediated downregulation of NOX2 decreased cell viability and ROS content (100).
Several NOX4 genetic knockdown studies in various tumor types have been reported. In non-small cell lung cancer (NSCLC) cell lines, the depletion of NOX4 inhibited NSCLC cell aggressiveness and NOX4 overexpression-mediated metabolic effects. In gastric cancer, RNA interference (RNAi)-mediated silencing of NOX4 inhibited cell adhesion and invasive potential of gastric cancer cells through JAK2/STAT3 signaling (73). In human neuroblastoma cells, silencing of NOX4 suppressed glycolysis induced by hypoxia and cell growth through inhibition of the PI3K/Akt signaling pathway (198). One major weakness of many genetic knockdown studies, however, is the absence of a direct linkage between decreased gene expression and NOX-related production of ROS, limiting our understanding of the functional consequences of NOX inhibition.
Höll et al., using shRNA-mediated knockdown of NOX5 in NOX5-expressing (PC-3, LNCaP) but not NOX5-negative (DU145) prostate cancer cell lines, demonstrated that NOX5-derived ROS and the subsequent depletion of PKCζ and inaction of JNK played a critical role in the proliferation and survival of prostate cancer cells (86). The suppression of NOX5 expression produced a significant impact on the growth and proliferation of different tumor cell types in multiple studies, including in Barrett's esophageal adenocarcinoma cells involving cyclooxygenase 2, p16, silencer-of-death domain (SODD) and NF-κB regulation (113, 161), breast cancer cells via STAT5A-mediated NOX5-L expression (52), malignant melanoma involving pathways regulating HIF-1α and networks that signal through Akt/GSK3β/p27Kip1 (7), ALK-positive anaplastic large-cell lymphoma cell lines where NOX5-derived ROS contribute to apoptosis blockage (34), and adult T cell leukemia (ATL) where NOX5α was key to maintaining HTLV-1 transformation phenotype (157). In addition, depending on expression level and cellular context, NOX5 may play a dual role in cancer cells; NOX5 was shown to promote cell death via Ca2+ and c-ABL, while also being able to stimulate tumor growth in some cancers via STAT5A and CREB. Thus, NOX5 may determine the balance of cellular proliferation and death in skin, breast, and lung cancer cells (51).
The epigenetic silencing of DUOX1 has been reported in lung and liver cancers, suggesting a tumor suppressor role for DUOX1 in these diseases (117, 123). Stably knocking down the expression of DUOX1 in nontumor mammary cells (MCF12A) with shRNA led to higher cell proliferation rates and decreased migration and adhesion properties, suggesting that breast carcinogenesis may involve the silencing of DUOX1 (67). However, in contrast to its postulated tumor-suppressing ability in lung, liver, and breast cancer, DUOX1 has also been implicated in the promotion of genomic instability and radiation-induced thyroid tumorigenesis, as the downregulation of DUOX1 with a specific RNA abrogated the induction of DNA damage after ionizing radiation (6).
The expression of DUOX2 in human tumors has been associated with long-standing chronic inflammation. In pancreatic ductal adenocarcinoma cells, immunomodulatory and proinflammatory cytokines and bacterial cell wall component lipopolysaccharide (LPS) specifically upregulated DUOX2 and its maturation factor DUOXA2 (dual oxidase 2 maturation factor). Our group recently demonstrated the ability of inflammatory mediators such as interferon-gamma (IFN-γ), LPS, IL-4, and IL-17A to upregulate DUOX2 and increase the accumulation of H2O2 and DNA damage through STAT6 and NF-κB signaling, effects that could be suppressed with the NOX inhibitor diphenylene iodonium (DPI) (191, 193). In hepatocellular carcinoma (HCC), the depletion of DUOX2 abrogated PKCα-induced activation of Akt/MAPK signaling pathways as well as cell proliferation, migration, and invasion in HCC cells (182). These reports demonstrate that, depending on the cell context, DUOX-derived H2O2 might have tumor-promoting activities, but under some conditions the accumulation of DUOX-associated ROS may lead to growth arrest and senescence, thereby contributing to tumor suppression.
NOX and the malignant phenotype
NOX activity is induced by stimuli such as growth factors, cytokines, or calcium fluxes (127, 147). Excessive ROS produced by NOX proteins promote a pro-oxidative cellular environment that fosters genomic instability and cellular transformation due to the accumulation of macromolecular damage (24, 25, 39). Further, O2•− and H2O2 were found to contribute to multiple signal transduction pathways, in part through the activation of protein kinase and cytokine signaling (29, 106, 162). For instance, the increased glycolytic activity often observed in cancer cells, also known as the Warburg effect, that allows tumor cells to proliferate in spite of defective mitochondrial respiration caused by hypoxia or mitochondrial injury, can be supported by the NOX-dependent restoration of NAD+ stores, as reported by Lu et al.; and NOX-associated ROS may also promote the HIF-1α-dependent expression of glycolytic genes (46, 122, 195). A recent study by Bertram et al. demonstrating the involvement of NOX1 in the metabolic remodeling of hepatoblastoma cells toward a more proliferative state further supports this notion (23).
A plethora of studies have implicated NOX1, NOX2, NOX4, and DUOX2 as triggers of angiogenesis and promoters of invasiveness via the ERK-dependent upregulation of vascular endothelial growth factor (VEGF) and matrix metalloproteinases, and the activation of other redox-sensitive transcription factors such as HIF-1α, TP53, and NF-κB (11, 36, 74, 95, 128, 129, 175, 193, 194). A role for NOX1-generated ROS in cell invasion was reported in the context of metalloprotease production and cell motility, where the augmented invasiveness of KRAS-transformed normal rat kidney cells and epidermal growth factor-stimulated migration of Caco-2 cells was attenuated by transfection of NOX1 siRNAs (158). DUOX2 knockdown with siRNA significantly decreased IFN-γ-induced VEGF-A or HIF-1α upregulation in pancreatic cancer cells (193).
Increased tumor angiogenesis is associated with epithelial–mesenchymal transition (EMT), a process that facilitates tumor initiation, stemness, and resistance to anticancer therapy (65, 135). In fact, the activity of H2O2-producing enzymes NOX4 and DUOX2 in cancer cells was linked to EMT, a process that confers tumor cells with a phenotypic plasticity that allows for therapy evasion (84, 97, 110, 128). In breast cancer cells, NOX4 knockdown blunted TGF-β-induced wound healing and cell migration, key steps in TGF-β and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells (26). In human gastric cancer cells, the downregulation of DUOX2 increased radioresistance; whereas in colon cancer cells, the expression of DUOX2 promoted chemoresistance and aggressiveness via the TET1/DUOX2/ROS/EMT axis (97, 136). In addition, in prostate carcinoma (PCa), inhibition of stromal NOX4 abrogated the enhanced proliferation and migration of PCa cell lines induced by TGF-β1-activated prostate fibroblast conditioned media (149). In ovarian cancer cells, knockdown of either the low-affinity leukotriene B4 receptor BLT2 or NOX4 using specific siRNAs suppressed STAT3 stimulation and MMP2 expression, leading to attenuated invasiveness (155). Further, NOX1-, NOX2-, and NOX4-derived ROS increase resistance to apoptosis in cancers of the liver, bladder, and gastrointestinal tract (109, 112, 146, 152, 160, 177, 186). In contrast, RNAi-mediated DUOX1 silencing in NCI-H292 epithelial lung cancer cells promoted EMT, cancer stem cell characteristics, and invasive properties (118). Immunosuppression via inhibition of T cell responses, as reported for NOX2 in multiple studies, constitutes another avenue by which NOX proteins can mediate tumor growth (35, 37, 43, 186).
NOX protein expression in human malignancies
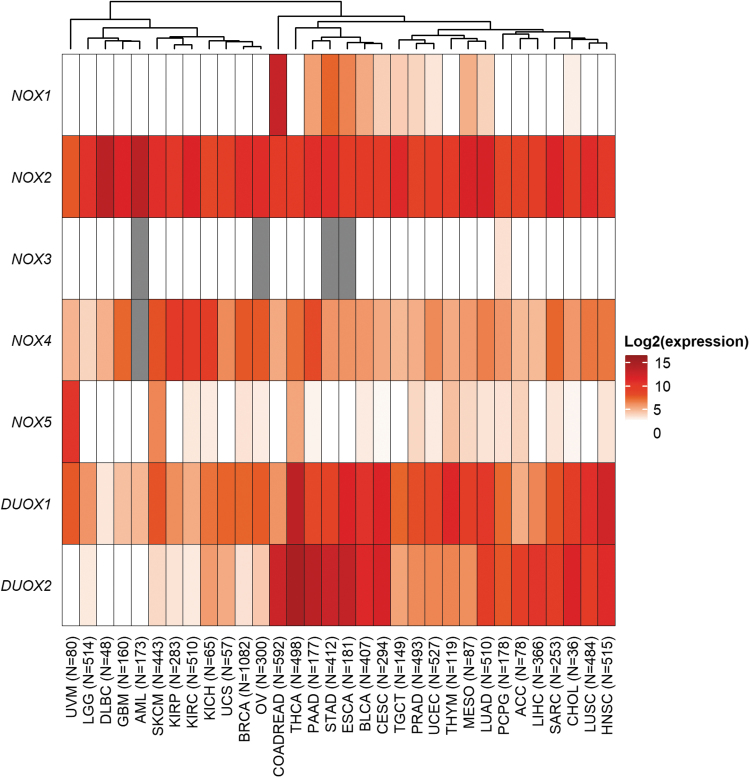
In line with the earlier findings linking NOX activity to tumor development and progression in preclinical models of cancer, overexpression of NOX1 has been observed in tissue specimens taken from patients with premalignant gastrointestinal inflammation or with colon adenocarcinoma (14, 33, 95, 107, 119, 169). In data from the PanCancer Atlas study of The Cancer Genome Atlas (TCGA), messenger RNA (mRNA) expression of NOX1 was notably higher in colorectal adenocarcinoma than in any other cancer types (Fig. 3). In addition, modest NOX1 mRNA levels are present in gastric and esophageal adenocarcinomas. Correspondingly, high levels of NOX1 expression may promote DNA breaks, base (guanine) oxidation, and general genomic instability; all of which are cellular events that are consistent with tumorigenesis (39). Juhasz et al. have provided a biological link between NOX1 expression and the pathogenesis of colon cancer by demonstrating the pro-proliferative effect of NOX1-associated ROS in colon cancer cells (95). Further, inhibition of NOX1, either genetically or chemically, was sufficient to prevent the onset of premalignant inflammatory states and to slow the growth of NOX1-containing murine tumors (74).
FIG. 3.
Landscape of NOX protein expression in human cancer specimens from TCGA. Hierarchical clustering on log-transformed mean gene expression values in 32 cohorts of TCGA (TCGA Research Network). Columns were clustered by using Euclidian distance and complex linkage. mRNA expression data (batch-normalized read counts from Illumina HiSeq_RNASeqV2) was downloaded from cBioPortal (72). Red: higher expression; white: lower expression; gray: missing value. The number of specimens per cohort is indicated in parentheses. ACC, adrenocortical carcinoma; AML, acute myeloid leukemia; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma; CHOL, cholangiocarcinoma; COADREAD, colorectal adenocarcinoma; DLBC, diffuse large B cell lymphoma; ESCA, esophageal adenocarcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, choromophobe renal cell carcinoma; KIRC, renal clear cell carcinoma; KIRP, renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; mRNA, messenger RNA; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TCGA, The Cancer Genome Atlas; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
In addition to its expression in phagocytes, NOX2 is highly expressed in hematopoietic malignancies (1, 13, 85, 94, 125). NOX2 can also be found in immune cells that infiltrate tumors, indicative of tissue inflammation. Correspondingly, in the TCGA-PanCancer Atlas study, NOX2 mRNA expression is high in the majority of cancer cohorts, including diffuse large B cell lymphoma (DLBC cohort), acute myeloid leukemia (AML), breast invasive carcinoma, and lung adenocarcinoma (Fig. 3). In marked contrast, to date, NOX3 has not been linked to any malignancy, in accordance with the lack of NOX3 mRNA expression in TCGA cohorts (Fig. 3).
NOX4, which is usually present in the kidney and in endothelial cells, has also been detected in multiple solid tumors, including malignancies of the brain, skin, kidney, and ovary (103, 129, 159, 160). NOX4 has also been implicated in TGF-β-induced EMT, a process that has been linked to the acquisition of chemoresistance (26, 84). In TCGA cohorts, the highest mRNA expression of NOX4 is observed in three kidney cancer cohorts: renal papillary cell carcinoma (KIRP), renal clear cell carcinoma (KIRC), and chromophobe renal cell carcinoma (KICH) (Fig. 3). Further, specimens from pancreatic adenocarcinoma, invasive breast carcinoma, ovarian serous cystadenocarcinoma, glioblastoma, and melanoma moderately express NOX4, in line with published reports (103, 129, 159, 160).
NOX5 has no orthologs in rodent genomes, thus rendering the study of NOX5 in preclinical cancer models difficult. NOX5 has been implicated in prostate cancer due to its reported expression in PC-3 prostate cancer cells (88). The recent development of a validated NOX5 antibody has allowed the immunodetection of the protein in UACC-257 malignant melanoma cells, as well as in multiple human tumors, including ovarian, prostate, and lung cancers, in tissue microarrays (8). In TCGA clinical specimens, NOX5 mRNA is highly expressed in uveal and skin melanoma, and to a lesser extent in thyroid carcinoma, as can be appreciated from the heatmap in Figure 3.
Both DUOX1 and DUOX2 are associated with normal thyroid function, as well as with host defense activities in airway epithelium and the gastrointestinal tract (30, 50, 60, 76). DUOX1 is frequently epigenetically silenced in lung and liver cancer, and it has generally low expression in other cancers (94, 117, 118, 123); however, DUOX1 appears to have moderate to high expression in a large fraction of TCGA cohorts, possibly related to its role in host defense (Fig. 3). DUOX2 has been shown to be overexpressed in inflammatory bowel disease, pancreatitis, and in breast, colorectal, gastric, lung, prostate, and pancreatic cancer compared with normal adjacent tissue (69, 141, 189–193). Bioinformatic analysis of TCGA cancer cohorts reveals high mRNA expression of DUOX2 in thyroid carcinoma, colorectal, pancreatic, stomach, and esophageal adenocarcinoma; moderate expression levels are observed in cholangiocarcinoma, head and neck squamous cell, and HCC (Fig. 3).
The tissue distribution of NOX proteins in normal tissue and in disease is summarized (22, 28, 94) and depicted in Table 1. Taken together, these results and observations confirm that NOX proteins constitute potential therapeutic targets for cancer treatment.
Table 1.
Tissue Distribution of NADPH Oxidase Enzymes in Normal Tissues and Malignant Diseases
| Enzyme | Normal tissue distribution | Associated malignancies |
|---|---|---|
| NOX1 | Colon epithelium (166) | Colorectal adenocarcinoma (14, 33, 39, 95, 119, 169) |
| NOX2 | Phagocytes (17) | Hematopoietic and lymphoid cancers (1, 13, 85, 125) |
| NOX3 | Inner ear (18) | N/A |
| NOX4 | Kidney, endothelial cells (75, 103, 159) | Kidney, ovary, brain, and bladder cancer; melanoma (103, 129, 159, 160) |
| NOX5 | Spleen, testis, and lymphoid tissue (20) | Prostate cancer, malignant melanoma (7, 86) |
| DUOX1 | Thyroid, airway epithelium, and prostate (30, 50, 60, 76, 180) | Thyroid and head and neck cancer; thymoma |
| DUOX2 | Thyroid, salivary gland, rectal mucosa, gastrointestinal tract, airway epithelium, and prostate (30, 50, 60, 63, 76, 180) | Thyroid, breast, colorectal, gastric, lung, prostate, and pancreatic cancer (69, 141, 189, 190, 192, 193) |
DUOX, dual oxidase; NOX, NADPH oxidase.
Prognostic value of NOX mRNA expression levels in cancer
Recent studies have leveraged the large collection of genomic data made available for research through TCGA to evaluate the prognostic potential of NOX mRNA expression in colorectal and gastric cancer, as well as in HCC. When comparing 458 colorectal cancer specimens with 41 normal samples, Cho et al. found the NOX family to be generally overexpressed at the mRNA level in tumor relative to normal tissue, and NOX4 and NOX5 to be associated with poor prognosis, whereas NOX1 and DUOX2 were associated with better prognosis (40). Eun et al. reported that NOX1, NOX2, and NOX5 were associated with poor prognosis in patients with HCC, in contrast to NOX4 and DUOX1 that were associated with better survival in these patients (64). You et al. reported the overexpression of NOX1, NOX2, and NOX4 in gastric cancer specimens, and they suggested that NOX4 and DUOX1 expression had a negative impact on the survival of these patients (197). Finally, we reported that DUOX2 mRNA expression was indicative of poor survival in the pancreatic adenocarcinoma patient cohort from TCGA (191). These results are by no means comprehensive, as TCGA comprises more than 30 cohorts spanning different malignancies. Further, the limits of using mRNA rather than protein expression for prognostication must be acknowledged. The variable and context-specific nature of these prognostic associations highlights potential challenges in applying NOX inhibition in the clinic for the treatment of cancer.
Genetic Inhibition of NOX in Preclinical Cancer Models
Physiological and pathophysiological functions of NOX members can be explored by using the genetically engineered mouse model (GEM) and/or the NOX-deficient mouse model. The NOX5 gene is absent from rodent genomes, and therefore cannot be studied by using knockout murine models.
Garrido-Urbani et al. discovered in 2011 that mice deficient in NOX1 have impaired angiogenesis. Silencing of NOX1 decreased endothelial cell migration and tube-like structure formation through the inhibition of PPARα, a regulator of the NF-κB signaling pathway (74). Further, Stalin et al. have recently reported the effect of the genetic deletion of NOX1 in preclinical immunocompetent mouse models of colorectal cancer and melanoma. In these experiments, NOX1 inhibition had potent antitumor effects: These effects were dependent on an intact immune system and required the inhibition of host NOX1 (163). Moreover, in GEM models of hepatocarcinogenesis, NOX1−/− mice developed 80% fewer tumors, which were 50% smaller than those of wild-type (WT) mice. Interestingly, the ablation of NOX1 in macrophages dramatically abolished the NOX1-mediated development of HCC by dampening the production of inflammatory cytokines in macrophages (115). NOX1-inhibitory effects have also been observed after the inhibition of NOX accessory proteins. In the K19-C2mE murine model of gastric cancer, disruption of NADPH oxidase organizer 1 (NOXO1), a component of the functional NOX1 enzymatic complex, significantly suppressed gastritis-associated metaplastic hyperplasia (61). In addition, a novel p47phox-dependent regulatory mechanism, shifting the immune balance toward colon cancer, was demonstrated when p47phox−/− mice defective in the IL-23/IL-17 axis were protected from colon cancer (145).
The influence of NOX2-derived O2•− and its oxidative metabolites on the acquisition of the metastatic phenotype using gp91phox−/− mice was initially reported by Okada et al. in 2006 (138). This effect of NOX2 on metastasis was further explored in genetically engineered NOX2-deficient mice using melanoma cells. The formation of lung metastases was reduced in B6.129S6-Cybbtm1DinK (NOX2-KO) versus NOX2-sufficient WT mice, in part due to a restored IFN-γ-dependent, NK cell-mediated clearance of melanoma cells (16). A similar decrease in pulmonary metastatic colonization was observed in mice deficient for all the individual subunits of NOX2 (encoded by CYBA, CYBB, NCF1, NCF2, and NCF4); however, the CYBA-deficient (Cybatm1a) mice showed the most significant decrease in metastatic colonization. NOX2 deficiency resulted in granulomas and in the accumulation of antitumoral immune cells in the lungs (176). In murine models of BCR-ABL1+ leukemia and a syngeneic, orthotopic model of prostate cancer, genetic ablation of NOX2 reduced the in vivo expansion of murine BCR-ABL1+ hematopoietic cells, and it caused a reduction in tumor growth and angiogenesis in the murine prostate cancer model NOX2−/y (78, 81). Recent reports have also demonstrated that NOX2-derived ROS contribute to the progression of KRAS-induced leukemia, in a series of experiments wherein the expansion of leukemia was markedly reduced in triple transgenic mice with genetic NOX2 deficiency (NOX2−/− × LSL-KrasG12D × Mx1-Cre) that expressed KRAS in their hematopoietic cells (15, 125).
The pro-angiogenic function of NOX4 was also investigated in vivo, notably with a model of chemically induced carcinogenesis in mice. Tumor angiogenesis induced by the carcinogen 3-methylcholanthrene (MCA) was significantly reduced, as indicated by the observed reduction in tumor vascularization and measured defects in hypoxia signaling in NOX4−/− mice compared with the WT, due, in part, to the decreased expression of HIF-1α and VEGF (82). Although additional in vivo studies are needed to unequivocally link NOX-mediated ROS to cancer, these results demonstrate that the inhibition of tumor growth through selective targeting of NOXs is achievable in vivo, due to the contribution of NOX-associated ROS in the regulation of cell division, survival, and angiogenesis in cancer. We will next consider the extent to which these antiproliferative effects can be recapitulated with the putative small-molecule NOX inhibitors that currently populate the field.
Small-Molecule Inhibitors of NOX Proteins
Unspecific NOX inhibitors
Small-molecule NOX inhibitors can be divided into three categories: ROS scavengers/antioxidants, inhibitors of NOX functional complex formation, and true enzyme antagonists (binding to one of the catalytic domains of the protein to suppress electron transfer). A short list of notable NOX inhibitory molecules is displayed in Table 2. Several anti-inflammatory molecules were discovered in the context of stroke prevention (99). Apocynin, or 4-hydroxy-3-methoxy-acetophenone, is a naturally occurring phenol derived from plants that inhibits inflammation via a dual mechanism, by preventing the assembly of an active NOX complex and in a myeloperoxidase-dependent manner, or by exerting nonspecific antioxidant effects (83, 164, 168). The IC50 (concentration with 50% inhibition of activity) of apocynin for the inhibition of the phagocytic respiratory burst was measured to be 10 μM. Suzuki et al. demonstrated that apocynin had antiproliferative effects in cellular and murine models of prostate cancer; apocynin suppressed the growth and metastasis of PLS10 prostate cancer xenografts in a dose-dependent manner (168). In this study, the antiproliferative effects of apocynin were attributed to the drug-induced dephosphorylation of NOX-complex protein RAC1 and the downregulation of cyclin D1, further confirming that NOXs are not direct targets of this agent (2, 168).
Table 2.
Summary of Notable NADPH Oxidase Inhibitors
| Inhibitor | Molecular target(s), mechanism of action | Activity in 3D or murine models of cancer | Potency (IC50) | Literature references |
|---|---|---|---|---|
| DPI | Flavoprotein inhibitor that inhibits all NOXs | Inhibited the growth of NOX1-expressing HT-29 and LS-174 colon cancer xenografts | Nanomolar range; see Figure 5B | Doroshow et al. (57); Lu et al. (121) |
| DPI analogs | Flavoprotein inhibitors with improved selectivity for NOX isoforms | Nanomolar range; see Figure 5B | Lu et al. (121) | |
| Apocynin | Antioxidant and inhibitor or NOX complex assembly | Dose-dependent suppression of tumor growth and metastasis in PLS10 mouse model of prostate cancer | ∼10 μM (NOX2) | Suzuki et al. (168) |
| AEBSF | Serine protease inhibitor hypothesized to inhibit NOX complex assembly | Inhibition of ROS production in DU145 prostate tumor spheroids | ∼10 mM (NOX2) | Wartenberg et al. (185); Diatchuk et al. (53) |
| Plumbagin | Antioxidant; anti-NOX4 activity | Inhibited the progression of gastric cancer in mice bearing anoikis-resistant MKN-45-AR xenografts | 2 μM (NOX4) | Du et al. (59); Ding et al. (56) |
| VAS2870, VAS3947 | Inhibitor of NOX complex assembly and activation; suppress PDGFR-dependent NOX activation | VAS2870: 2 μM (NOX2); VAS3947: 1–2 μM (NOX2) | Sancho and Fabregat (153); ten Freyhaus et al. (172); Wind et al. (187) | |
| GKT136901, GKT137831 | Specificity for NOX1 and NOX4; possible ROS scavengers | GKT136901 significantly attenuated the growth and vascularization of LLC1 Lewis lung carcinoma xenografts; GKT137831 slowed disease development in an FLT3ITD-transformed AML mouse model | GKT136901: 160–170 nM (NOX1/NOX4); 450 nM (NOX5); GKT137831: 110 nM (NOX1), 140 nM (NOX4), and 410 nM (NOX5) | Laleu et al. (104); Musset et al. (133); Garrido-Urbani et al. (74); Jayavelu et al. (92); Gaggini et al. (70) |
| ML171 | Selective NOX1 inhibitor; possibility of ROS-assay interference | 250 nM (NOX1) | Gianni et al. (77) | |
| NOS31 | Selective NOX1 inhibitor | 2 μM (NOX1) | Yamamoto et al. (196) | |
| GLX351322, GLX7013114, and GLX481372 | Selective NOX4, NOX5 inhibitors | GLX351322: 5 μM (NOX4); GLX7013114: 0.3 μM (NOX4); GLX481372: ∼0.6 μM (NOX4/NOX5) | Anvari et al. (9); Wang et al. (184) | |
| GKT771 | NOX1 | Inhibited the growth of MC38 colon carcinoma and B16F10 melanoma tumors in mouse models | Ki = 60 nM (NOX1) | Stalin et al. (163) |
| Nox2ds-tat (peptide) | NOX2 inhibitory peptide; binds p47phox-p22phox interface | Inhibited ROS production and vascular tissue damage | 740 nM (NOX2) | Jacobson et al. (90); Liu et al. (120); Csanyi et al. (44) |
| NoxA1ds (peptide) | Disrupts the interaction between NOX1 and NOXA1 | 20 nM (NOX1) | Ranayhossaini et al. (143) | |
| NF02 (peptide) | NOX1 inhibitory peptide | 63 μM (NOX1) | Mousslim et al. (131) |
AEBSF, 4-(2-aminoethyl)-benzenesulphonyl fluoride; AML, acute myeloid leukemia; DPI, diphenylene iodonium; IC50, concentration with 50% inhibition of activity; Ki, inhibition constant; ML171, 2-acetylphenothiazine; ROS, reactive oxygen species.
AEBSF, or 4-(2-aminoethyl)-benzenesulfonyl fluoride, is an irreversible serine protease inhibitor that was found to also inhibit NOX2 activation and O2•− production. Diatchuk et al. hypothesized that AEBSF may have a dual mode of action; the charged aminoethyl moiety mimics a basic amino acid, possibly binding to an acidic domain of the NOX complex, whereas the sulfonyl fluoride group may covalently and irreversibly bind to NOX and preclude binding of accessory proteins p47phox and/or p67phox (53). In a study by Wartenberg et al., AEBSF significantly inhibited ROS production in NOX1-overexpressing DU145 prostate tumor spheroids (185). Potency of AEBSF for NOX inhibition is lacking, however, with an IC50 ∼1 mM for the inhibition of O2•− production in intact macrophages (53).
Plumbagin, a plant-derived naphthoquinone, is another unspecific antioxidant molecule that exerts pleiotropic effects (56, 139). Although Ding et al. demonstrated the specificity of plumbagin for NOX4 using NOX4-expressing HEK293 and LN229 cells, and NOX4-transfected COS-7 cells, the exact mechanism of action of the molecule remains undefined. Several groups have reported on the antiproliferative effects of plumbagin, both in vitro and in vivo, mediated by the upregulation of p53 and p21, and the suppression of cell cycle regulators (87, 102, 200). A 2018 study by Du et al. described the plumbagin-induced growth suppression of NOX4-expressing, anoikis-resistant MKN45-AR xenografts (59).
Novel NOX inhibitors discovered through functional screens
Large-scale drug screening efforts have fostered the discovery of novel, more selective NOX inhibitors. The most notable inhibitors are listed in Table 2. Through a screen for the discovery of NOX2 inhibitors, the company Vasopharm GmbH discovered the triazolo pyrimidines VAS2870 and VAS3947. These molecules prevented the assembly and functional activation of NOX complexes (172, 188). VAS2870 was shown to inhibit the growth of human HCC cells, presumably through NOX inhibition (153). However, off-target effects have been reported, and these molecules have poor solubility and pharmacokinetic properties (5, 167).
The company GenKyoTex employed a structure–activity relationship screening approach to develop the pyrazolopyridine dione derivatives GKT136901 and GKT137831, two compounds with selective potency for the inhibition of NOX1 and NOX4, and to a lesser extent, NOX5 (10, 104, 133). GKT136901 significantly attenuated the growth and vascularization of LLC1 Lewis lung carcinoma xenografts, and GKT137831 slowed disease development in an FLT3ITD-transformed AML mouse model (74, 92). These compounds have favorable pharmacological properties, and GKT137831 is currently being evaluated in a clinical trial for patients with idiopathic pulmonary fibrosis (NCT03865927), a condition associated with NOX4-derived ROS (91, 93). Possible caveats to the use of these compounds as NOX inhibitors are the fact that their mechanism of action is unknown, and GKT136901 has been shown to function as an ROS scavenger (154). Moreover, Augsburger et al. have recently unequivocally established that the effect of both GKT136901 and GKT137831 was independent of NOX expression, and that the low Ki (inhibition constant) values for NOX inhibition are, in fact, attributable to nonspecific inhibition of the horseradish peroxidase enzyme used in the Amplex Red assay (12).
ML171 (2-acetylphenothiazine) is another putative NOX inhibitor with no known mechanism of action. Its discovery resulted from a large-throughput structure activity relationship screen of commercially available phenothiazine compounds for NOX1 inhibitors conducted by the Scripps Institute (77). ML171 effectively inhibited the ROS-dependent formation of extracellular matrix degrading invadopodia in DLD1 colon cancer cells (77). However, similar to other phenothiazine compounds, ML171 is also a weak inhibitor of serotonin and adrenergic receptors, thus opening the possibility of confounding off-target effects independent of NOX inhibition. Moreover, Seredenina et al. determined that ML171 actually interfered with peroxidase-dependent ROS-measuring assays rather than truly inhibiting NOXs (156).
Yamamoto et al. identified the microbial metabolite NOS31 produced by Streptomyces sp. as a low micromolar inhibitor of NOX1-associated PMA-stimulated production of ROS in colon cancer cells (196). NOS31 lacked both antioxidant and cytotoxic activities and appeared selective for NOX1 (IC50 ∼2 μM compared with ∼30 to >40 μM for other NOX isoforms). The proliferation of colon and stomach cancer cell lines was inhibited by this compound; however, the authors noted that the mechanism of NOX inhibition by NOS31 remains to be determined. To our knowledge, reports on NOS31 activity in vivo have not yet been published.
Wang et al. described the discovery and characterization, in collaboration with Glucox Biotech AB, of novel and selective inhibitors GLX351322, GLX7013114, and GLX481372 specific for NOX4 and NOX4/NOX5, respectively, with IC50 <1 μM (9, 184). The discovery of these compounds originated from a high-throughput screen of 40,000 chemicals by using an Amplex Red assay in a 384-well format. General antioxidant activity was ruled out with the 2,2-diphenyl-1-picrylhydrazyl-hydrate (DPPH) assay. GLX351322 prevented ROS production in pancreatic islet cells induced by high glucose in male C57BL/6 mice, and all three compounds protected islet cells from death induced by NOX4-associated ROS (184). To our knowledge, there are no published reports to date on the activity of these compounds in cancer models.
Finally, compound GKT771 developed by GenKyoTex shows promising antitumor activity in NOX1-expressing systems (163). GKT771 significantly attenuated the growth of xenografted MC38 colon carcinoma and B16F10 melanoma tumors in immunocompetent mice. Moreover, the authors noted a concurrent decrease in angiogenesis and lymphangiogenesis, and increased recruitment of tumor-associated immune cells. These effects were dependent on NOX1 expression, as NOX1-deficient mice did not respond to treatment with GKT771 (163). The authors concluded that GKT771 exerts NOX1-dependent immune-modulatory effects that, when used in combination with immune cell checkpoint inhibitor anti-PD1, further potentiate antitumor activity.
Iodonium class flavin dehydrogenase inhibitors
DPI, a flavoprotein inhibitor, inhibits electron-transporting enzymes, some of which are involved in normal cellular metabolism, including nitric oxide synthase, xanthine oxidase, mitochondrial complex I, and the microsomal cytochrome P450 reductase (2, 114). O'Donnell et al. empirically demonstrated that the mechanism by which DPI suppresses the flow of electrons from NADPH to FAD, heme redox centers, and, ultimately, to O2 may be by covalently binding to FAD, forming stable phenylated-FAD adducts (137). Despite its broad specificity for various flavoproteins, DPI is the most widely used NOX inhibitor (96). In a recent study aimed at evaluating the specificity and potency of NOX inhibitors using human cell lines expressing high levels of NOX isoforms, Augsburger et al. have shown that, in contrast to molecules such as apocynin, ebselen, GKT136901, and VAS2870, which lacked true NOX inhibition activity, DPI consistently elicited dose-dependent inhibition of all NOX isoforms in multiple assays (12). A study by our group revealed that DPI and a derivative di-2-thienyliodonium (DTI) inhibited the growth of multiple NOX1-expressing human colon cancer cell lines, suppressed ROS production at sub-micromolar concentrations, and decreased the growth of HT-29 and LS-174 colon cancer xenografts (57). We hypothesized that NOX inhibition may enhance the activity of protein tyrosine phosphatases (PTP) and inactivate key kinases involved in pro-survival signaling (Fig. 4) (57, 58).
FIG. 4.
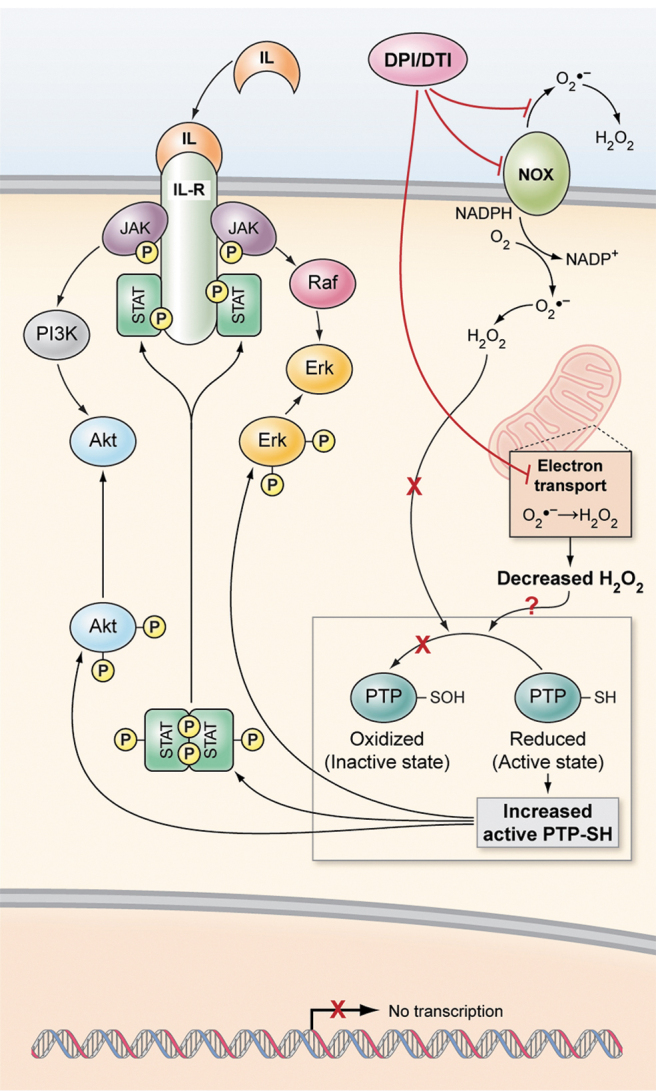
Hypothetical model of the effect of NOX inhibition on downstream cell signaling. Empirical evidence strongly suggests that NOX-associated ROS promote the activation of JAK/STAT and Akt signaling pathways, and that NOX inhibition with DPI or its analog DTI enhances the activity of intracellular PTP, thus dephosphorylating key signaling kinases. DPI, diphenylene iodonium; DTI, di-2-thienyliodonium; PTP, protein tyrosine phosphatases; ROS, reactive oxygen species. Adapted from Figure 8 in Doroshow et al. (58).
To circumvent off-target toxicities (178) and to allow for cancer cell targeting in spite of mitochondrial dysfunction—as is the case of many tumors that rely on glycolysis for energy production rather than the more efficient process of oxidative phosphorylation active in noncancerous cells (122)—we recently suggested that the specificity and selectivity of DPI could be optimized by modifying the position and identity of functional groups around the benzene rings of DPI, as well as the counter-ion to alter reactivity. We synthesized 36 DPI analogs and screened them for improved solubility, inhibition of cell proliferation, and inhibition of ROS production in different NOX-expressing systems (121). Because it has been demonstrated that mitochondrial metabolism can be inhibited by DPI (114), we further screened selected DPI derivatives for those compounds that did not disrupt normal mitochondrial energy metabolism, as assessed through measurements of oxygen consumption rate and extracellular acidification rate (121). This screen uncovered four DPI analogs (National Service Center [NSC] 740104, 751140, 734428, 737392) with potent inhibitory activity against HT-29 cell growth and ROS production (Fig. 5A). All four compounds were more potent than DPI with respect to the selective inhibition of NOX4 and DUOX2; 740104 and 734428 also demonstrated improved potency for NOX1 compared with the parent molecule DPI (Fig. 5B). Further, compounds 734428 and 737392, both of which feature nitro functional groups, had improved selective potency for DUOX2. To further investigate the observation that nitro substitution groups improve compound selectivity for DUOX2, a configurational isomer of NSC 737392 was prepared: NSC 780521 (121). In line with our hypothesis, compound 780521 exhibited a marked selectivity for inhibiting DUOX2-expressing cells, performing markedly better than DPI (Fig. 5B). Moreover, we have shown with a cell-free proof-of-concept experiment that the five novel DPI analogs were capable of covalently binding to reduced FAD to form stable adducts, consistent with O'Donnell et al.'s report of DPI binding to FAD as a mechanism of flavoprotein inhibition (137). Mechanistic experiments aimed at further characterizing the effects of these molecules at the cellular level are ongoing. It remains to be determined whether these compounds exert antiproliferative effects by blocking cell cycle progression, as was reported for DPI and DTI (58).
FIG. 5.
IC50 of DPI analogs relative to IC50 of DPI for inhibition of ROS production in NOX-expressing human cells. Adapted from Lu et al. (121). (A) Chemical structure of DPI analogs selected from a screen for O2•− inhibition and antiproliferative effects, and lack of potency for mitochondrial activity. The identity of substitution groups and counter ions for each compound are indicated in the table. (B) PMA-induced O2•− production was measured by luminescence assay in HEK293 NOX1, PMN (NOX2), and UACC-257 (NOX5) cells. H2O2 production was measured by Amplex Red assay of DPI or analog-treated NOX4 and DUOX2/DUOXA2 overexpression cell lines. The cells were incubated with DPI analogs for 30 min before assay measurement. IC50 (nM) for DPI in NOX-expressing cell lines is shown in the first row, and fold-ratio IC50 for DPI analogs compared with IC50 DPI is color coded in the following rows. Green shading denotes improved potency compared with DPI, and orange shading indicates decreased potency compared with DPI. DUOXA2, dual oxidase 2 maturation factor; H2O2, hydrogen peroxide; IC50, concentration with 50% inhibition of activity; O2•−, superoxide; PMN, polymorphonuclear leukocytes.
Inhibitory Peptides
To optimize selective targeting of NOX enzymes and decrease risks of off-target toxicity, the development of inhibitory monoclonal antibodies of NOX enzymes has also been explored, although with limited success, and further validation in model systems and against other NOXs to ascertain specificity is needed (4, 31, 199). Because the general domain architecture of NOX enzymes is well defined, and the protein–protein interaction partners that are necessary for functional activation have been extensively described for all NOXs, it follows that selective peptides (>20 residues in length) designed to mimic NOX complex components could be employed to specifically inhibit NOX activity. This approach holds promise for the development of isoform-specific NOX inhibitors (5). NOX2, the first identified NOX protein, has been the focus of most peptide development activities. An extensive review of peptide inhibitors of NOX2 activity corresponding to domains in NOX2, p47phox, p67phox, and RAC has been published by Dahan and Pick (48). Further, Pagano and colleagues have contributed two peptides with NOX isoform selectivity: Nox2ds-tat, a peptide that mimics the site of interaction between NOX2 and p47phox on the cytosolic B-loop of NOX2; NoxA1ds, which was designed to disrupt the interaction of NOXA1 with NOX1 (42, 44, 143).
The first and best characterized rationally designed NOX inhibitory peptide is Nox2ds-tat. The tat sequence, derived from the HIV virus, confers cell-membrane permeability. Isoform selectivity was confirmed in cell-free assays wherein the IC50 of NOX2ds-tat for ROS production was measured at 0.74 μM (44). In vivo results demonstrated that Nox2ds-tat given intravenously or delivered by adenoviral infection did inhibit ROS production and associated tissue damage (90, 120).
Peptide-based inhibition of NOX4 aimed at targeting the interaction between cytosolic NOX4 B-loop and p22phox N-terminus was unsuccessful, presumably due to the fact that NOX4 and functional activators exist in a constitutively active conformation, making disruption of complex formation difficult to achieve (45, 179). For the inhibition of NOX5-mediated O2•− production, peptides targeting the regulatory EF-hand Ca2+-binding domain disrupting its interaction with the C-terminal catalytic dehydrogenase domain were designed by Tirone et al. (173). No peptide-based inhibitors of NOX3, DUOX1, or DUOX2 have been described in the literature to date, although one could envision devising a peptide targeting the N-terminal peroxidase domain of the DUOX proteins to ensure selectivity for DUOX1/2.
Mousslim et al. recently presented NF02, a promising NOX1-specific inhibitory peptide identified through a screen with NOX1-expression HT-29 colon cancer cells (131). The authors demonstrated the lack of scavenger activity and cytotoxicity, as well as the selectivity for NOX1 over other NOX2 and other flavoenzymes. The IC50 for inhibition of HT-29 proliferation was ∼63 μM, and cell migration and invasion were significantly suppressed by NF02.
Although the peptide inhibitor approach is attractive due to the inherent specificity of such peptides for precise NOX isoform targeting, there are also limitations to translating these molecules into therapies for the clinic. First, peptides are readily degraded in animal systems and have limited bioavailability. Therefore, screening of peptide libraries for selective NOX inhibitors is mostly supported by cell-free assays (48). New delivery methods, such as nanoparticle formulations, and strategic design optimizing pharmacological properties for peptide-based inhibitors should be devised to optimize inhibitory activity in vivo (27, 48, 68, 143). Further, due to the strong conservation of functional domains between NOX proteins, isoform selectivity remains difficult to achieve. For instance, El-Benna et al. (62) and Dahan et al. (47) have created multiple NOX2-derived inhibitory peptides; however, the most potent peptides targeted the cytosolic FAD- and NADPH-binding domains conserved across all NOX proteins. To date, no peptide-based inhibitors of NOX proteins have entered clinical development.
Challenges and Perspectives
The unavailability of high-resolution full-length crystal structures for NOX isoforms has hindered efforts to develop specific and selective NOX inhibitors (124). Without such inhibitors and due to the lack of precision in assays commonly employed to measure ROS production (54, 79), the true impact of NOX enzymes in cancer remains to be fully elucidated. The use of unspecific NOX inhibitors in research can cause systemic ROS inhibition, having deleterious effects on important processes such as the innate immune system (142). For these reasons, the landscape of NOX inhibitors developed to date mostly comprises molecules having pleiotropic effects on cellular activities and metabolism, so that the reported antitumor effects cannot be unambiguously ascribed to NOX inhibition.
The fact that some studies have reported a pro-apoptotic or tumor-suppressing role for NOX enzymes in some cancers further complicates the matter (151). The role of NOX4 is complex and context specific. NOX4 is the main mediator of TGF-beta-induced apoptosis in many nontumoral and tumoral cells (150, 151). In addition to its oncogenic role in gastrointestinal cancers (197), NOX4 downregulation and deletion have been reported in HCC and liver cancer cells, and correspondingly, NOX4 expression was found to be associated with better prognosis for HCC patients (64). Similarly, DUOX1 is frequently epigenetically silenced in lung and liver cancers, suggesting a tumor suppressor role for DUOX1 in these diseases (117, 123). NOX5 also has both pro-apoptotic and pro-oncogenic roles in diseases such as skin, breast, and lung cancer (51).
Further, NOX-associated ROS is reported to potentiate cancer drug toxicity via ROS overproduction (140). Indeed, many chemotherapeutic agents exert antitumor effects by inducing ROS production and oxidative stress, and NOX1 activity was found to potentiate the activity of genotoxic agents such as paclitaxel and oxaliplatin (3, 41). In the latter case, Chocry et al. demonstrated that ROS could impact cell survival in dramatically different ways, depending on the level and duration of production; short-term NOX1 activation induced by oxaliplatin exposure favored cell death, whereas sustained NOX1 activation led to the activation of survival pathways through p38 MAPK signaling (41). In addition, per a recent study by Le Gal et al., antioxidant molecules N-acetylcysteine (NAC) and vitamin E analog Trolox significantly increased the invasiveness of a xenografted malignant melanoma tumor (108). Therefore, NOX inhibition, and more generally, antioxidant therapy, is only desirable in certain specific contexts.
Conclusions
There is ample empirical evidence that ROS promote the development and progression of human cancers. Therefore, it follows that NOX enzymes, which constitute a predominant source of ROS in mammalian cells, are likely to play an important role in tumorigenesis. This premise is supported by the fact that NOX overexpression was detected in specimens from various premalignant inflammatory diseases. Intracellular and extracellular ROS contribute to the biology of cancer by inducing DNA damage and oxidation of other cellular components, inducing the activation of oncogenes, such as RAS and c-Myc, and promoting mitogenic signaling by deactivating protein phosphatases (101, 116). ROS also induce the release of cytokines and chemokines, which, in turn, modify the tumor microenvironment. H2O2 has been shown to promote metastasis by inducing EMT—a process that is a feature of embryogenesis and wound healing whereby cell adhesion is disrupted and motility increased—but can be aberrantly activated in adult cells (32). Further, Chen et al. have recently described how NOX-associated ROS promote tumor-induced immunosuppression (37). Keeping in mind the caveats mentioned earlier regarding the context-specific properties and function of ROS, gaining a better understanding of the specific role of NOX in cancer and pleiotropic effects of associated ROS will require potent and selective inhibitors of NOX isoforms with favorable pharmacological properties. The rational design approach has already yielded some interesting candidates, although the mechanism of action has not been fully elucidated for some of these compounds. Continued efforts to resolve crystal structures for NOX proteins will allow for further optimization of lead NOX inhibitor compounds for in vivo evaluation, and ultimately, clinical development for the treatment of cancer.
Acknowledgments
The authors would like to thank Drs. Hala Makhlouf and Rodrigo Chuaqui for generating immunohistochemical and hematoxylin and eosin (H&E) staining images of NOX proteins in human tissues. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services.
Abbreviations Used
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- AML
acute myeloid leukemia
- DPI
diphenylene iodonium
- DTI
di-2-thienyliodonium
- DUOX
dual oxidase
- EMT
epithelial–mesenchymal transition
- FAD
flavin adenine dinucleotide
- GEM
genetically engineered mouse models
- H2O2
hydrogen peroxide
- HCC
hepatocellular carcinoma
- IC50
concentration with 50% inhibition of activity
- IFN-γ
interferon-gamma
- LPS
lipopolysaccharide
- ML171
2-acetylphenothiazine
- mRNA
messenger RNA
- NAD
nicotinamide adenine dinucleotide
- NOX
NADPH oxidase
- NSCLC
non-small cell lung cancer
- O2•−
superoxide
- PCa
prostate carcinoma
- RNAi
RNA interference
- ROS
reactive oxygen species
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
- TCGA
The Cancer Genome Atlas
- VEGF
vascular endothelial growth factor
- WT
wild-type
Funding Information
This work was supported by federal funds from the Center for Cancer Research and the Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health.
References
- 1. Adane B, Ye H, Khan N, Pei S, Minhajuddin M, Stevens BM, Jones CL, D'Alessandro A, Reisz JA, Zaberezhnyy V, Gasparetto M, Ho TC, Kelly KK, Myers JR, Ashton JM, Siegenthaler J, Kume T, Campbell EL, Pollyea DA, Becker MW, and Jordan CT. The hematopoietic oxidase NOX2 regulates self-renewal of leukemic stem cells. Cell Rep 27: 238–254, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, and Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Alexandre J, Hu Y, Lu W, Pelicano H, and Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res 67: 3512–3517, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, and Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23: 406–427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot F, Diallo I, de Vathaire F, Schlumberger M, and Dupuy C. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A 112: 5051–5056, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antony S, Jiang G, Wu Y, Meitzler JL, Makhlouf HR, Haines DC, Butcher D, Hoon DS, Ji J, Zhang Y, Juhasz A, Lu J, Liu H, Dahan I, Konate M, Roy KK, and Doroshow JH. NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1alpha and p27(Kip1) expression in malignant melanoma and other human tumors. Mol Carcinog 56: 2643–2662, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, and Doroshow JH. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 65: 497–508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anvari E, Wikstrom P, Walum E, and Welsh N. The novel NADPH oxidase 4 inhibitor GLX351322 counteracts glucose intolerance in high-fat diet-treated C57BL/6 mice. Free Radic Res 49: 1308–1318, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, and Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A 99: 715–720, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, Mahiout Z, Jansen-Durr P, Knaus UG, Doroshow J, Stocker R, Krause KH, and Jaquet V. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol 26: 101272, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aurelius J, Hallner A, Werlenius O, Riise R, Mollgard L, Brune M, Hansson M, Martner A, Thoren FB, and Hellstrand K. NOX2-dependent immunosuppression in chronic myelomonocytic leukemia. J Leukoc Biol 102: 459–466, 2017 [DOI] [PubMed] [Google Scholar]
- 14. Aviello G and Knaus UG. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol 11: 1011–1023, 2018 [DOI] [PubMed] [Google Scholar]
- 15. Aydin E, Hallner A, Grauers Wiktorin H, Staffas A, Hellstrand K, and Martner A. NOX2 inhibition reduces oxidative stress and prolongs survival in murine KRAS-induced myeloproliferative disease. Oncogene 38: 1534–1543, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aydin E, Johansson J, Nazir FH, Hellstrand K, and Martner A. Role of NOX2-derived reactive oxygen species in NK cell-mediated control of murine melanoma metastasis. Cancer Immunol Res 5: 804–811, 2017 [DOI] [PubMed] [Google Scholar]
- 17. Babior BM, Lambeth JD, and Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, and Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065–46072, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, and Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287: 138–142, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012: 137289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bedard K and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Bertram K, Valcu CM, Weitnauer M, Linne U, and Gorlach A. NOX1 supports the metabolic remodeling of HepG2 cells. PLoS One 10: e0122002, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhardwaj V, Gokulan RC, Horvat A, Yermalitskaya L, Korolkova O, Washington KM, El-Rifai W, Dikalov SI, and Zaika AI. Activation of NADPH oxidases leads to DNA damage in esophageal cells. Sci Rep 7: 9956, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Block K and Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12: 627–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boudreau HE, Casterline BW, Rada B, Korzeniowska A, and Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53: 1489–1499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouttefeux O, Beloqui A, and Preat V. Delivery of peptides via the oral route: diabetes treatment by peptide-loaded nanoparticles. Curr Pharm Des 22: 1161–1176, 2016 [DOI] [PubMed] [Google Scholar]
- 28. Brown DI and Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Caillou B, Dupuy C, Lacroix L, Nocera M, Talbot M, Ohayon R, Deme D, Bidart JM, Schlumberger M, and Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab 86: 3351–3358, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Campion Y, Paclet MH, Jesaitis AJ, Marques B, Grichine A, Berthier S, Lenormand JL, Lardy B, Stasia MJ, and Morel F. New insights into the membrane topology of the phagocyte NADPH oxidase: characterization of an anti-gp91-phox conformational monoclonal antibody. Biochimie 89: 1145–1158, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, and Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal 12: 1383–1430, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Cao WL, Xiang XH, Chen K, Xu W, and Xia SH. Potential role of NADPH oxidase in pathogenesis of pancreatitis. World J Gastrointest Pathophysiol 5: 169–177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carnesecchi S, Rougemont AL, Doroshow JH, Nagy M, Mouche S, Gumy-Pause F, and Szanto I. The NADPH oxidase NOX5 protects against apoptosis in ALK-positive anaplastic large-cell lymphoma cell lines. Free Radic Biol Med 84: 22–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cemerski S, Cantagrel A, Van Meerwijk JP, and Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem 277: 19585–19593, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Chen C, Li L, Zhou HJ, and Min W.. The role of NOX4 and TRX2 in angiogenesis and their potential cross-talk. Antioxidants (Basel) 6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen X, Song M, Zhang B, and Zhang Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid Med Cell Longev 2016: 1580967, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, Lambeth D, and Bignami M. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med 44: 332–342, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Cho SY, Kim JS, Eun HS, Kang SH, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, and Moon HS. Expression of NOX family genes and their clinical significance in colorectal cancer. Dig Dis Sci 63: 2332–2340, 2018 [DOI] [PubMed] [Google Scholar]
- 41. Chocry M, Leloup L, and Kovacic H. Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain/Nox1 pathway. Oncotarget 8: 103710–103730, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cifuentes-Pagano E and Pagano PJ. Rational design and delivery of NOX-inhibitory peptides. Methods Mol Biol 1982: 417–428, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, and Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182: 5693–5701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Csanyi G and Pagano PJ. Strategies aimed at Nox4 oxidase inhibition employing peptides from Nox4 B-loop and C-terminus and p22 (phox) N-terminus: an elusive target. Int J Hypertens 2013: 842827, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dabral S, Muecke C, Valasarajan C, Schmoranzer M, Wietelmann A, Semenza GL, Meister M, Muley T, Seeger-Nukpezah T, Samakovlis C, Weissmann N, Grimminger F, Seeger W, Savai R, and Pullamsetti SS. A RASSF1A-HIF1alpha loop drives Warburg effect in cancer and pulmonary hypertension. Nat Commun 10: 2130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dahan I, Molshanski-Mor S, and Pick E. Inhibition of NADPH oxidase activation by peptides mapping within the dehydrogenase region of Nox2-A “peptide walking” study. J Leukoc Biol 91: 501–515, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Dahan I and Pick E. Strategies for identifying synthetic peptides to act as inhibitors of NADPH oxidases, or “all that you did and did not want to know about Nox inhibitory peptides.” Cell Mol Life Sci 69: 2283–2305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Carvalho DD, Sadok A, Bourgarel-Rey V, Gattacceca F, Penel C, Lehmann M, and Kovacic H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int J Cancer 122: 1757–1764, 2008 [DOI] [PubMed] [Google Scholar]
- 50. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, and Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Dho SH, Kim JY, Kwon ES, Lim JC, Park SS, and Kwon KS. NOX5-L can stimulate proliferation and apoptosis depending on its levels and cellular context, determining cancer cell susceptibility to cisplatin. Oncotarget 6: 39235–39246, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dho SH, Kim JY, Lee KP, Kwon ES, Lim JC, Kim CJ, Jeong D, and Kwon KS. STAT5A-mediated NOX5-L expression promotes the proliferation and metastasis of breast cancer cells. Exp Cell Res 351: 51–58, 2017 [DOI] [PubMed] [Google Scholar]
- 53. Diatchuk V, Lotan O, Koshkin V, Wikstroem P, and Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Dikalov SI and Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 20: 372–382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, and Parkos CA. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327: 717–720, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Ding Y, Chen ZJ, Liu S, Che D, Vetter M, and Chang CH. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J Pharm Pharmacol 57: 111–116, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Doroshow JH, Gaur S, Markel S, Lu J, van Balgooy J, Synold TW, Xi B, Wu X, and Juhasz A. Effects of iodonium-class flavin dehydrogenase inhibitors on growth, reactive oxygen production, cell cycle progression, NADPH oxidase 1 levels, and gene expression in human colon cancer cells and xenografts. Free Radic Biol Med 57: 162–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu J, Antony S, Wu Y, Jiang G, and Roy K. Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem Pharmacol 83: 1195–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Du S, Miao J, Zhu Z, Xu E, Shi L, Ai S, Wang F, Kang X, Chen H, Lu X, Guan W, and Xia X. NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis 9: 948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, and Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem 274: 37265–37269, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Echizen K, Horiuchi K, Aoki Y, Yamada Y, Minamoto T, Oshima H, and Oshima M. NF-kappaB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene 38: 4250–4263, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. El-Benna J, Dang PM, and Perianin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem Pharmacol 80: 778–785, 2010 [DOI] [PubMed] [Google Scholar]
- 63. El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A, and Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288: G933–G942, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Eun HS, Cho SY, Joo JS, Kang SH, Moon HS, Lee ES, Kim SH, and Lee BS. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci Rep 7: 11060, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fantozzi A, Gruber DC, Pisarsky L, Heck C, Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U, Bentires-Alj M, and Christofori G. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res 74: 1566–1575, 2014 [DOI] [PubMed] [Google Scholar]
- 66. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fortunato RS, Gomes LR, Munford V, Pessoa CF, Quinet A, Hecht F, Kajitani GS, Milito CB, Carvalho DP, and Menck CFM. DUOX1 silencing in mammary cell alters the response to genotoxic stress. Oxid Med Cell Longev 2018: 3570526, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fosgerau K and Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today 20: 122–128, 2015 [DOI] [PubMed] [Google Scholar]
- 69. Fukushima N, Koopmann J, Sato N, Prasad N, Carvalho R, Leach SD, Hruban RH, and Goggins M. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod Pathol 18: 779–787, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19: 6989–6999, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Gao HM, Zhou H, and Hong JS. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 33: 295–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, and Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6: pl1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gao X, Sun J, Huang C, Hu X, Jiang N, and Lu C. RNAi-mediated silencing of NOX4 inhibited the invasion of gastric cancer cells through JAK2/STAT3 signaling. Am J Transl Res 9: 4440–4449, 2017 [PMC free article] [PubMed] [Google Scholar]
- 74. Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, and Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One 6: e14665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Geiszt M, Witta J, Baffi J, Lekstrom K, and Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17: 1502–1515, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, and Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grauers Wiktorin H, Nilsson T, Aydin E, Hellstrand K, Palmqvist L, and Martner A. Role of NOX2 for leukaemic expansion in a murine model of BCR-ABL1(+) leukaemia. Br J Haematol 182: 290–294, 2018 [DOI] [PubMed] [Google Scholar]
- 79. Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, and Bhatnagar A; American Heart Association Council on Basic and Cardiovascular Sciences. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res 119: e39–e75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harrison IP and Selemidis S. Understanding the biology of reactive oxygen species and their link to cancer: NADPH oxidases as novel pharmacological targets. Clin Exp Pharmacol Physiol 41: 533–542, 2014 [DOI] [PubMed] [Google Scholar]
- 81. Harrison IP, Vinh A, Johnson IRD, Luong R, Drummond GR, Sobey CG, Tiganis T, Williams ED, O’ Leary JJ, Brooks DA, and Selemidis S. NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development. Oncotarget 9: 35378–35393, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Helfinger V, Henke N, Harenkamp S, Walter M, Epah J, Penski C, Mittelbronn M, and Schroder K. The NADPH oxidase Nox4 mediates tumour angiogenesis. Acta Physiol (Oxf) 216: 435–446, 2016 [DOI] [PubMed] [Google Scholar]
- 83. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Hiraga R, Kato M, Miyagawa S, and Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res 33: 4431–4438, 2013 [PubMed] [Google Scholar]
- 85. Hole PS, Zabkiewicz J, Munje C, Newton Z, Pearn L, White P, Marquez N, Hills RK, Burnett AK, Tonks A, and Darley RL. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 122: 3322–3330, 2013 [DOI] [PubMed] [Google Scholar]
- 86. Höll M, Koziel R, Schafer G, Pircher H, Pauck A, Hermann M, Klocker H, Jansen-Durr P, and Sampson N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol Carcinog 55: 27–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hsu YL, Cho CY, Kuo PL, Huang YT, and Lin CC. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J Pharmacol Exp Ther 318: 484–494, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Huang WC, Li X, Liu J, Lin J, and Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res 10: 133–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, and Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, and Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92: 637–643, 2003 [DOI] [PubMed] [Google Scholar]
- 91. Jarman ER, Khambata VS, Cope C, Jones P, Roger J, Ye LY, Duggan N, Head D, Pearce A, Press NJ, Bellenie B, Sohal B, and Jarai G. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol 50: 158–169, 2014 [DOI] [PubMed] [Google Scholar]
- 92. Jayavelu AK, Muller JP, Bauer R, Bohmer SA, Lassig J, Cerny-Reiterer S, Sperr WR, Valent P, Maurer B, Moriggl R, Schroder K, Shah AM, Fischer M, Scholl S, Barth J, Oellerich T, Berg T, Serve H, Frey S, Fischer T, Heidel FH, and Bohmer FD. NOX4-driven ROS formation mediates PTP inactivation and cell transformation in FLT3ITD-positive AML cells. Leukemia 30: 473–483, 2016 [DOI] [PubMed] [Google Scholar]
- 93. Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, and Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 53: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, and Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res 43: 523–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Juhasz A, Markel S, Gaur S, Liu H, Lu J, Jiang G, Wu X, Antony S, Wu Y, Melillo G, Meitzler JL, Haines DC, Butcher D, Roy K, and Doroshow JH. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. J Biol Chem 292: 7866–7887, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, and Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38: 3000–3006, 2007 [DOI] [PubMed] [Google Scholar]
- 96a. Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, and Kamata T. A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. Faseb J 26: 2049-2059, 2012 [DOI] [PubMed] [Google Scholar]
- 97. Kang KA, Ryu YS, Piao MJ, Shilnikova K, Kang HK, Yi JM, Boulanger M, Paolillo R, Bossis G, Yoon SY, Kim SB, and Hyun JW. DUOX2-mediated production of reactive oxygen species induces epithelial mesenchymal transition in 5-fluorouracil resistant human colon cancer cells. Redox Biol 17: 224–235, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kikuchi H, Hikage M, Miyashita H, and Fukumoto M. NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene 254: 237–243, 2000 [DOI] [PubMed] [Google Scholar]
- 99. Kim JY, Park J, Lee JE, and Yenari MA. NOX inhibitors—a promising avenue for ischemic stroke. Exp Neurobiol 26: 195–205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim SM, Hur DY, Hong SW, and Kim JH. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem Biophys Res Commun 494: 550–555, 2017 [DOI] [PubMed] [Google Scholar]
- 101. Kumari S, Badana AK G MM, G S, and Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights 13: 1177271918755391, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kuo PL, Hsu YL, and Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther 5: 3209–3221, 2006 [DOI] [PubMed] [Google Scholar]
- 103. Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, and Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 104. Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 105. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Lambeth JD and Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014 [DOI] [PubMed] [Google Scholar]
- 107. Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, and Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer 123: 100–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyurek LM, Lindahl P, Nilsson J, and Bergo MO. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7: 308re8, 2015 [DOI] [PubMed] [Google Scholar]
- 109. Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, and Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133: 1637–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 110. Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, Han SI, and Kang HS. Reactive oxygen species induce epithelial-mesenchymal transition, glycolytic switch, and mitochondrial repression through the Dlx2/Snail signaling pathways in MCF7 cells. Mol Med Rep 20: 2339–2346, 2019 [DOI] [PubMed] [Google Scholar]
- 111. Leto TL and Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 8: 1549–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, Yuan J, and Zhang WJ. IL-4/Stat6 activities correlate with apoptosis and metastasis in colon cancer cells. Biochem Biophys Res Commun 369: 554–560, 2008 [DOI] [PubMed] [Google Scholar]
- 113. Li D, Hong J, and Cao W. Silencer-of-death domain mediates acid-induced decrease in cell apoptosis in Barrett's associated esophageal adenocarcinoma cells. J Pharmacol Exp Ther 360: 14–22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li Y and Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun 253: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 115. Liang S, Ma HY, Zhong Z, Dhar D, Liu X, Xu J, Koyama Y, Nishio T, Karin D, Karin G, McCubbin R, Zhang C, Hu R, Yang G, Chen L, Ganguly S, Lan T, Karin M, Kisseleva T, and Brenner DA. NADPH oxidase 1 in liver macrophages promotes inflammation and tumor development in mice. Gastroenterology 156: 1156–1172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Liao Z, Chua D, and Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 18: 65, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ling Q, Shi W, Huang C, Zheng J, Cheng Q, Yu K, Chen S, Zhang H, Li N, and Chen M. Epigenetic silencing of dual oxidase 1 by promoter hypermethylation in human hepatocellular carcinoma. Am J Cancer Res 4: 508–517, 2014 [PMC free article] [PubMed] [Google Scholar]
- 118. Little AC, Sham D, Hristova M, Danyal K, Heppner DE, Bauer RA, Sipsey LM, Habibovic A, and van der Vliet A. DUOX1 silencing in lung cancer promotes EMT, cancer stem cell characteristics and invasive properties. Oncogenesis 5: e261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu H, Antony S, Roy K, Juhasz A, Wu Y, Lu J, Meitzler JL, Jiang G, Polley E, and Doroshow JH. Interleukin-4 and interleukin-13 increase NADPH oxidase 1-related proliferation of human colon cancer cells. Oncotarget 8: 38113–38135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu J, Yang F, Yang XP, Jankowski M, and Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 121. Lu J, Risbood P, Kane CT Jr., Hossain MT, Anderson L, Hill K, Monks A, Wu Y, Antony S, Juhasz A, Liu H, Jiang G, Harris E, Roy K, Meitzler JL, Konate M, and Doroshow JH.. Characterization of potent and selective iodonium-class inhibitors of NADPH oxidases. Biochem Pharmacol 143: 25–38, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lu W, Hu Y, Chen G, Chen Z, Zhang H, Wang F, Feng L, Pelicano H, Wang H, Keating MJ, Liu J, McKeehan W, Wang H, Luo Y, and Huang P. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol 10: e1001326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Luxen S, Belinsky SA, and Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68: 1037–1045, 2008 [DOI] [PubMed] [Google Scholar]
- 124. Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, and Mattevi A. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci U S A 114: 6764–6769, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Martner A, Aydin E, and Hellstrand K. NOX2 in autoimmunity, tumor growth and metastasis. J Pathol 247: 151–154, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 127. Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, Lu J, Roy K, and Doroshow JH. NADPH oxidases: a perspective on reactive oxygen species production in tumor biology. Antioxid Redox Signal 20: 2873–2889, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Meitzler JL, Konate MM, and Doroshow JH. Hydrogen peroxide-producing NADPH oxidases and the promotion of migratory phenotypes in cancer. Arch Biochem Biophys 675: 108076, 2019 [DOI] [PubMed] [Google Scholar]
- 129. Meitzler JL, Makhlouf HR, Antony S, Wu Y, Butcher D, Jiang G, Juhasz A, Lu J, Dahan I, Jansen-Durr P, Pircher H, Shah AM, Roy K, and Doroshow JH. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol 13: 182–195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Montezano AC and Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 20: 164–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mousslim M, Pagano A, Andreotti N, Garrouste F, Thuault S, Peyrot V, Parat F, Luis J, Culcasi M, Thetiot-Laurent S, Pietri S, Sabatier JM, and Kovacic H. Peptide screen identifies a new NADPH oxidase inhibitor: impact on cell migration and invasion. Eur J Pharmacol 794: 162–172, 2017 [DOI] [PubMed] [Google Scholar]
- 132. Mukawera E, Chartier S, Williams V, Pagano PJ, Lapointe R, and Grandvaux N. Redox-modulating agents target NOX2-dependent IKKepsilon oncogenic kinase expression and proliferation in human breast cancer cell lines. Redox Biol 6: 9–18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, and El Jamali A. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem 287: 9376–9388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem 283: 16961–16965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Navas T, Kinders RJ, Lawrence SM, Ferry-Galow KV, Borgel S, Hollingshead MG, Srivastava AK, Alcoser SY, Makhlouf HR, Chuaqui R, Wilsker DF, Konate MM, Miller SB, Voth AR, Chen L, Vilimas T, Subramanian J, Rubinstein L, Kummar S, Chen AP, Bottaro DP, Doroshow JH, and Parchment RE. Clinical evolution of epithelial-mesenchymal transition in human carcinomas. Cancer Res 80: 304–318, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nguyen DM, Parekh PR, Chang ET, Sharma NK, and Carrier F. Contribution of dual oxidase 2 (DUOX2) to hyper-radiosensitivity in human gastric cancer cells. Radiat Res 184: 151–160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. O'Donnell BV, Tew DG, Jones OT, and England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290(Pt 1): 41–49, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Okada F, Kobayashi M, Tanaka H, Kobayashi T, Tazawa H, Iuchi Y, Onuma K, Hosokawa M, Dinauer MC, and Hunt NH. The role of nicotinamide adenine dinucleotide phosphate oxidase-derived reactive oxygen species in the acquisition of metastatic ability of tumor cells. Am J Pathol 169: 294–302, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Padhye S, Dandawate P, Yusufi M, Ahmad A, and Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev 32: 1131–1158, 2012 [DOI] [PubMed] [Google Scholar]
- 140. Paletta-Silva R, Rocco-Machado N, and Meyer-Fernandes JR. NADPH oxidase biology and the regulation of tyrosine kinase receptor signaling and cancer drug cytotoxicity. Int J Mol Sci 14: 3683–3704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Qi R, Zhou Y, Li X, Guo H, Gao L, Wu L, Wang Y, and Gao Q. DUOX2 expression is increased in Barrett esophagus and cancerous tissues of stomach and colon. Gastroenterol Res Pract 2016: 1835684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rada B and Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15: 164–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, and Pagano PJ. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem 288: 36437–36450, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ray PD, Huang BW, and Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Richter C, Herrero San Juan M, Weigmann B, Bergis D, Dauber K, Muders MH, Baretton GB, Pfeilschifter JM, Bonig H, Brenner S, and Radeke HH. Defective IL-23/IL-17 axis protects p47phox−/− mice from colon cancer. Front Immunol 8: 44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Sekiyama A, and Teshima-Kondo S. NADPH oxidases in the gastrointestinal tract: a potential role of Nox1 in innate immune response and carcinogenesis. Antioxid Redox Signal 8: 1573–1582, 2006 [DOI] [PubMed] [Google Scholar]
- 147. Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, and Doroshow JH. NADPH oxidases and cancer. Clin Sci 128: 863–875, 2015 [DOI] [PubMed] [Google Scholar]
- 148. Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, and Orkin SH. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature 322: 32–38, 1986 [DOI] [PubMed] [Google Scholar]
- 149. Sampson N, Brunner E, Weber A, Puhr M, Schafer G, Szyndralewiez C, and Klocker H. Inhibition of Nox4-dependent ROS signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int J Cancer 143: 383–395, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sanchez A, Alvarez AM, Benito M, and Fabregat I. Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J Biol Chem 271: 7416–7422, 1996 [DOI] [PubMed] [Google Scholar]
- 151. Sancho P, Bertran E, Caja L, Carmona-Cuenca I, Murillo MM, and Fabregat I. The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim Biophys Acta 1793: 253–263, 2009 [DOI] [PubMed] [Google Scholar]
- 152. Sancho P and Fabregat I. NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J Biol Chem 285: 24815–24824, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sancho P and Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-beta-induced apoptosis of liver tumor cells. Biochem Pharmacol 81: 917–924, 2011 [DOI] [PubMed] [Google Scholar]
- 154. Schildknecht S, Weber A, Gerding HR, Pape R, Robotta M, Drescher M, Marquardt A, Daiber A, Ferger B, and Leist M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr Med Chem 21: 365–376, 2014 [DOI] [PubMed] [Google Scholar]
- 155. Seo JM, Park S, and Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem 287: 13840–13849, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Seredenina T, Chiriano G, Filippova A, Nayernia Z, Mahiout Z, Fioraso-Cartier L, Plastre O, Scapozza L, Krause KH, and Jaquet V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic Biol Med 86: 239–249, 2015 [DOI] [PubMed] [Google Scholar]
- 157. Shigemura T, Shiohara M, Kato M, Furuta S, Kaneda K, Morishita K, Hasegawa H, Fujii M, Gorlach A, Koike K, and Kamata T. Superoxide-generating Nox5alpha is functionally required for the human T-cell leukemia virus type 1-induced cell transformation phenotype. J Virol 89: 9080–9089, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Shinohara M, Adachi Y, Mitsushita J, Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K, Miyazaki H, and Kamata T. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem 285: 4481–4488, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, and Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 160. Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, and Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer 123: 787–792, 2008 [DOI] [PubMed] [Google Scholar]
- 161. Si J, Fu X, Behar J, Wands J, Beer DG, Souza RF, Spechler SJ, Lambeth D, and Cao W. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett's esophageal adenocarcinoma cells. J Biol Chem 282: 16244–16255, 2007 [DOI] [PubMed] [Google Scholar]
- 162. Simon AR, Rai U, Fanburg BL, and Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol 275: C1640–C1652, 1998 [DOI] [PubMed] [Google Scholar]
- 163. Stalin J, Garrido-Urbani S, Heitz F, Szyndralewiez C, Jemelin S, Coquoz O, Ruegg C, and Imhof BA. Inhibition of host NOX1 blocks tumor growth and enhances checkpoint inhibitor-based immunotherapy. Life Sci Alliance 2: e201800265, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Stolk J, Hiltermann TJ, Dijkman JH, and Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994 [DOI] [PubMed] [Google Scholar]
- 165. Storz P. Reactive oxygen species in tumor progression. Front Biosci 10: 1881–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 166. Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 167. Sun QA, Hess DT, Wang B, Miyagi M, and Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Suzuki S, Pitchakarn P, Sato S, Shirai T, and Takahashi S. Apocynin, an NADPH oxidase inhibitor, suppresses progression of prostate cancer via Rac1 dephosphorylation. Exp Toxicol Pathol 65: 1035–1041, 2013 [DOI] [PubMed] [Google Scholar]
- 169. Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, and Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 170. Szatrowski TP and Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798, 1991 [PubMed] [Google Scholar]
- 171. Teahan C, Rowe P, Parker P, Totty N, and Segal AW. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. Nature 327: 720–721, 1987 [DOI] [PubMed] [Google Scholar]
- 172. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 173. Tirone F, Radu L, Craescu CT, and Cox JA. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry 49: 761–771, 2010 [DOI] [PubMed] [Google Scholar]
- 174. Trachootham D, Lu W, Ogasawara MA, Nilsa RD, and Huang P. Redox regulation of cell survival. Antioxid Redox Signal 10: 1343–1374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ushio-Fukai M and Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 266: 37–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. van der Weyden L, Speak AO, Swiatkowska A, Clare S, Schejtman A, Santilli G, Arends MJ, and Adams DJ. Pulmonary metastatic colonisation and granulomas in NOX2-deficient mice. J Pathol 246: 300–310, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, and Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279: 34643–34654, 2004 [DOI] [PubMed] [Google Scholar]
- 178. Varga ZV, Ferdinandy P, Liaudet L, and Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol 309: H1453–H1467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. von Lohneysen K, Noack D, Hayes P, Friedman JS, and Knaus UG. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J Biol Chem 287: 8737–8745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang D, De Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, and Miot F. Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem 280: 3096–3103, 2005 [DOI] [PubMed] [Google Scholar]
- 181. Wang HP, Wang X, Gong LF, Chen WJ, Hao Z, Feng SW, Wu YB, Ye T, and Cai YK. Nox1 promotes colon cancer cell metastasis via activation of the ADAM17 pathway. Eur Rev Med Pharmacol Sci 20: 4474–4481, 2016 [PubMed] [Google Scholar]
- 182. Wang J, Shao M, Liu M, Peng P, Li L, Wu W, Wang L, Duan F, Zhang M, Song S, Jia D, Ruan Y, and Gu J. PKCalpha promotes generation of reactive oxygen species via DUOX2 in hepatocellular carcinoma. Biochem Biophys Res Commun 463: 839–845, 2015 [DOI] [PubMed] [Google Scholar]
- 183. Wang R, Dashwood WM, Nian H, Lohr CV, Fischer KA, Tsuchiya N, Nakagama H, Ashktorab H, and Dashwood RH. NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Int J Cancer 128: 2581–2590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wang X, Elksnis A, Wikstrom P, Walum E, Welsh N, and Carlsson PO. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS One 13: e0204271, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wartenberg M, Hoffmann E, Schwindt H, Grunheck F, Petros J, Arnold JR, Hescheler J, and Sauer H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett 579: 4541–4549, 2005 [DOI] [PubMed] [Google Scholar]
- 186. Weyemi U, Redon CE, Parekh PR, Dupuy C, and Bonner WM. NADPH oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem 13: 502–514, 2013 [PMC free article] [PubMed] [Google Scholar]
- 187. Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wingler K, Altenhoefer SA, Kleikers PW, Radermacher KA, Kleinschnitz C, and Schmidt HH. VAS2870 is a pan-NADPH oxidase inhibitor. Cell Mol Life Sci 69: 3159–3160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wu Y, Antony S, Hewitt SM, Jiang G, Yang SX, Meitzler JL, Juhasz A, Lu J, Liu H, Doroshow JH, and Roy K. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol 42: 1229–1238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wu Y, Antony S, Meitzler JL, and Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 345: 164–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wu Y, Konate MM, Lu J, Makhlouf H, Chuaqui R, Antony S, Meitzler JL, Difilippantonio MJ, Liu H, Juhasz A, Jiang G, Dahan I, Roy K, and Doroshow JH. IL-4 and IL-17A cooperatively promote hydrogen peroxide production, oxidative DNA damage, and upregulation of dual oxidase 2 in human colon and pancreatic cancer cells. J Immunol 203: 2532–2544, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, Meitzler JL, Hollingshead M, Haines DC, Butcher D, Roy K, and Doroshow JH. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-gamma and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol 190: 1859–1872, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wu Y, Meitzler JL, Antony S, Juhasz A, Lu J, Jiang G, Liu H, Hollingshead M, Haines DC, Butcher D, Panter MS, Roy K, and Doroshow JH. Dual oxidase 2 and pancreatic adenocarcinoma: IFN-gamma-mediated dual oxidase 2 overexpression results in H2O2-induced, ERK-associated up-regulation of HIF-1alpha and VEGF-A. Oncotarget 7: 68412–68433, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, and Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67: 10823–10830, 2007 [DOI] [PubMed] [Google Scholar]
- 195. Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, and Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65: 613–621, 2005 [PubMed] [Google Scholar]
- 196. Yamamoto T, Nakano H, Shiomi K, Wanibuchi K, Masui H, Takahashi T, Urano Y, and Kamata T. Identification and characterization of a novel NADPH oxidase 1 (Nox1) inhibitor that suppresses proliferation of colon and stomach cancer cells. Biol Pharm Bull 41: 419–426, 2018 [DOI] [PubMed] [Google Scholar]
- 197. You X, Ma M, Hou G, Hu Y, and Shi X. Gene expression and prognosis of NOX family members in gastric cancer. Onco Targets Ther 11: 3065–3074, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yu T, Li L, Liu W, Ya B, Cheng H, and Xin Q. Silencing of NADPH oxidase 4 attenuates hypoxia resistance in neuroblastoma cells SH-SY5Y by inhibiting PI3K/Akt-dependent glycolysis. Oncol Res 27: 525–532, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang XQ, Yang CY, Rao XF, and Xiong JP. Plumbagin shows anti-cancer activity in human breast cancer cells by the upregulation of p53 and p21 and suppression of G1 cell cycle regulators. Eur J Gynaecol Oncol 37: 30–35, 2016 [PubMed] [Google Scholar]