Abstract
Significance: Fibrosis is a stereotypic, multicellular tissue response to diverse types of injuries that fundamentally result from a failure of cell/tissue regeneration. This complex tissue remodeling response disrupts cellular/matrix composition and homeostatic cell–cell interactions, leading to loss of normal tissue architecture and progressive loss of organ structure/function. Fibrosis is a common feature of chronic diseases that may affect the lung, kidney, liver, and heart.
Recent Advances: There is emerging evidence to support a combination of genetic, environmental, and age-related risk factors contributing to susceptibility and/or progression of fibrosis in different organ systems. A core pathway in fibrogenesis involving these organs is the induction and activation of nicotinamide adenine dinucleotide phosphate oxidase (NOX) family enzymes.
Critical Issues: We explore current pharmaceutical approaches to targeting NOX enzymes, including repurposing of currently U.S. Food and Drug Administration (FDA)-approved drugs. Specific inhibitors of various NOX homologs will aid establishing roles of NOXs in the various organ fibroses and potential efficacy to impede/halt disease progression.
Future Directions: The discovery of novel and highly specific NOX inhibitors will provide opportunities to develop NOX inhibitors for treatment of fibrotic pathologies.
Keywords: NADPH oxidases, pharmacology, tissue repair and remodeling, myofibroblasts, aging, clinical
Introduction
Tissue fibrosis is invariably associated with a failure to maintain a normal epithelial/endothelial barrier in affected organs. This failure in efficient/effective cellular regeneration at the vascular interface (endothelium) or the luminal surfaces of the lung (airway epithelium), kidney (tubular epithelium), or liver (hepatic/biliary epithelium) can result in the activation of the subtending mesenchyme. This localized mesenchymal activation typically involves the expansion of tissue-resident mesenchymal progenitor cells, which differentiate into contractile cells known as myofibroblasts. Although both fibroblasts and myofibroblasts secrete/remodel the extracellular matrix (ECM), it is the myofibroblasts that contribute to tissue contracture and architectural distortion. The sustained and relentless activation of the mesenchyme may be further fueled by immune dysregulation, including the profibrotic polarization of tissue-resident/recruited macrophages. Thus, damage to epithelium/endothelium, fibroblast/myofibroblast activation, ECM remodeling, and immune dysregulation are salient and essential features of fibrosis involving all organs.
Over the past decade, accumulating evidence supports a role of nicotinamide adenine dinucleotide phosphate oxidases (NOXs) in tissue inflammation and fibrosis. The NOX family of enzymes comprises seven family members: NOX1, NOX2, NOX3, NOX4, NOX5; and dual oxidase (DUOX) homologs, Duox1 and Duox2. All NOX enzymes generate reactive oxygen species (ROS), either as superoxide anion (O2·−) or as hydrogen peroxide (H2O2), as the primary product of enzymatic catalysis and reduction of molecular oxygen (O2). While the preponderance of data supports a critical role for NOX4 in fibrosis across all organ systems, in this review, we will explore reported functions of other NOX enzymes in the tissue injury, inflammation, and/or repair process. The homeostatic roles of NOX enzymes in some tissues/organs are also discussed. Finally, we highlight emerging strategies to target NOX enzymes in fibrotic pathologies involving different organ systems.
Lung Fibrosis
Causes and pathogenic mechanisms
Fibrosis involving the lung may be due to known causes such as occupational exposures, drugs, or an associated systemic connective tissue disease, or represent an “idiopathic” form referred to as idiopathic pulmonary fibrosis (IPF), the most common and lethal of all the fibrotic lung diseases. Although a specific “cause” of IPF has not been identified, there are known risk factors that include genetic and environmental factors, as well as aging. Based on this increased understanding of IPF pathogenesis, there is a growing impetus to eliminate the term “idiopathic” and introduce alternative classification schemes (246, 256–258, 264).
Genetic variants have been identified in both sporadic and familial cases of IPF. Familial cases have been estimated to account for between 2% and 20% (81, 124, 200); this variability is likely related to the populations studied as well as disease definitions. In general, rare variants are highly penetrant and have a large effect size. These variants include “mutations” in genes related to alveolar stability, including the surfactant proteins (SFTPs): SFTPC, SFTPA1, and SFTPA2; and another protein involved in surfactant metabolism, ATP-binding cassette-type 3 (ABCA3); and genes associated with telomere biology: TERT, TERC, DKC1, TINF2, RTEL1, PARN, and nuclear assembly factor 1 ribonucleoprotein (NAF1) (124). Sporadic cases of IPF have also been linked to a number of gene variants, which are more common, but typically with smaller effect sizes; these include genes linked to telomere maintenance (TERT, TERC), host defense functions (MUC5B, TOLLIP), and epithelial integrity (DSP, DPP9) (124). Among these, a MUC5B risk variant has a relatively high effect size and, due to its higher prevalence in the general population, may account for up to 30% of the genetic risk of developing IPF (66).
Environmental factors almost certainly contribute to risk of developing IPF. Although it is difficult to conduct and interpret such studies, most evidence supports the concept that IPF is a heterogeneous disorder caused by a number of environmental and occupational exposures; such exposures may include smoking, agriculture/farming, livestock, wood dust, metal dust, and stone/sand (241).
The pathogenesis of IPF involves the interactions between different cell types and the emergence of profibrotic phenotypes (Fig. 1). Alveolar epithelial cell apoptosis (50, 193, 251), senescence (215, 247), and epithelial-to-mesenchymal transition (EMT; 225, 277, 285) are thought to contribute to the early development of lung fibrosis. Impaired re-epithelialization, due to exhaustion of epithelial cell progenitors in response to chronic exposure to injury and/or aging, is likely to be a key driver of the pathogenesis of the disease (44). A pathological hallmark of lung fibrosis is the differentiation of lung fibroblasts into myofibroblasts and their accumulation within fibroblastic foci. The profibrotic cytokine transforming growth factor beta-1 (TGF-β1) stimulates myofibroblast differentiation (245). Myofibroblast differentiation is accompanied by enhanced secretion of ECM components and resistance to apoptosis (102–104). Pulmonary fibrosis has also been associated with the recruitment of macrophages and neutrophils at site of tissue remodeling (78, 89, 94, 172). In addition to profibrotic mediators, recruited macrophages and neutrophils release ROS that may perpetrate the fibrogenesis via the modulation of ECM synthesis and the polarization of macrophages toward a profibrotic phenotype (8, 84, 95, 96). In addition to the pathogenic mechanisms described above, cellular senescence has been the focus of numerous studies following the observation that senescent cells accumulate in the fibrotic lung (98, 215). Epithelial cells and fibroblasts have been shown to undergo senescence in IPF lungs (98, 215). One of the mechanisms by which senescent cells may influence phenotypes of neighboring cells is through secreted mediators referred to as the senescence-associated secretory phenotype (SASP) (242). Among SASP components are inflammatory cytokines (interleukin [IL]-6, IL-8, IL-1α), plasminogen activator inhibitor-1 (PAI-1), matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), IGF-binding proteins, monocyte chemoattractant protein 1 (MCP-1), prostaglandin E2 (PGE2), and nitric oxide (NO) (52). These SASP-associated factors promote inflammation, tissue remodeling, and cell growth (188). The targeting of senescent cells with senolytics has been shown to ameliorate the severity of lung fibrosis in a murine experimental model of the disease (215).
FIG. 1.
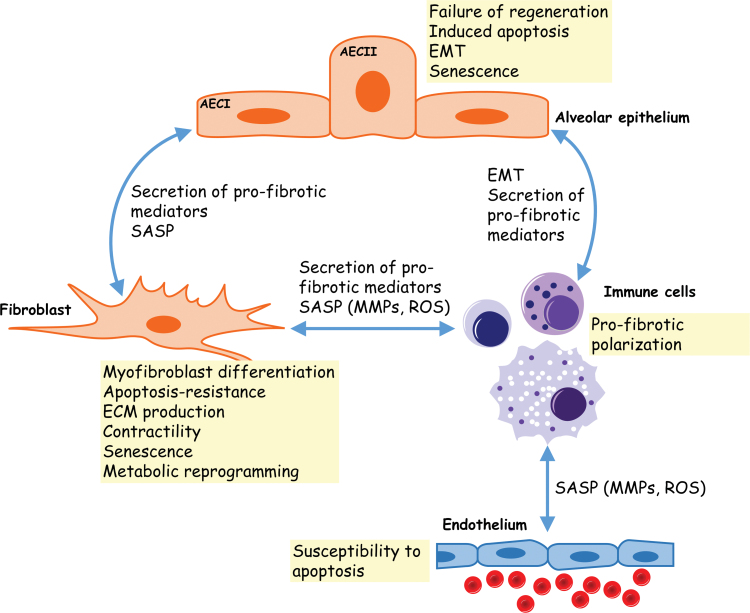
Cellular interactions in pulmonary fibrosis. Communication between the different cellular effectors of pulmonary fibrosis involves secreted/paracrine mediators. Injured alveolar epithelial cells secrete proinflammatory factors that may sustain the recruitment/activation of immune cells to the site of injury. Immune cells amplify the inflammatory response and may polarize to a more profibrotic response that promotes myofibroblast differentiation and ECM remodeling. The SASP of epithelial cells and myofibroblasts has also been proposed to alter the phenotype of neighboring cells. AECI, alveolar epithelial cell type I; AECII, alveolar epithelial cell type II; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; MMPs, matrix metalloproteinases; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype. Color images are available online.
Role of NOX enzymes in lung fibrosis
The rationale for targeting NOX4 as a therapeutic target to ameliorate lung fibrosis is based on the finding of high expression of this homolog in human IPF and from in vivo genetic/pharmacological targeting in murine experimental models of the disease (98, 99) (Fig. 2). NOX4 expression and activity increase after the stimulation of lung fibroblasts, in vitro, with TGF-β1 (99). NOX4 expression and activity are constitutively increased in lung fibroblasts isolated from IPF patients. NOX4, through the production of H2O2, mediates tissue-repair functions of myofibroblasts, which are effectors of ECM production and contraction (99). In addition to its role in sustaining myofibroblast activation, NOX4 supports lung fibroblast migration via a ROS-mediated RhoA/Rho kinase pathway (168).
FIG. 2.
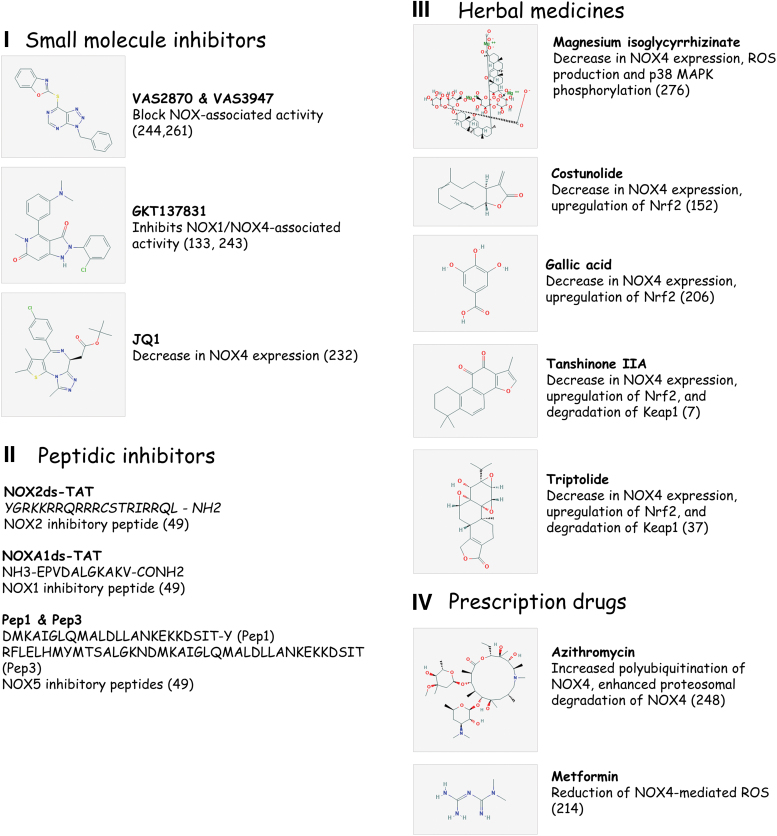
NOX enzyme inhibition and antifibrotic therapies. Compounds that have proven beneficial in ameliorating fibrosis in animal models of organ fibrosis and/or in cellular models of myofibroblast differentiation. The discovery of these compounds resulted from three different drug discovery strategies: the development of small molecule inhibitors through high-throughput screening, application of herbal medicines, and the repurposing of prescription drugs. H2O2, hydrogen peroxide; NF-κB, nuclear factor-kappa B; NOX, nicotinamide adenine dinucleotide phosphate oxidase; VSMCs, vascular smooth muscle cells. Color images are available online.
More recently, we reported that metabolic reprogramming is required for myofibroblast activation (20, 267). This reprogramming relies on enhanced mitochondrial bioenergetics and biogenesis (20). These studies demonstrated that NOX4 regulates mitochondrial bioenergetics and biogenesis in lung fibroblasts by modulating levels of nuclear factor erythroid-derived 2-like 2 (Nrf2) (19). The balance between NOX4 and Nrf2 also influences the fate of myofibroblasts to a senescent and apoptotic-resistant phenotype (98, 212).
Epithelial cell death and/or epithelial-to-mesenchymal cell transition are two mechanisms that have been proposed to contribute to the establishment of lung fibrosis along with myofibroblast differentiation (126, 127, 138, 165, 259). NOX4-deficient mice show decrease in epithelial cell apoptosis in response to airway injury with bleomycin (33). Differential expression analysis of pro-oxidant and antioxidant transcripts after EMT of lung epithelial cells also showed that downregulation of antioxidant transcripts correlated with an upregulation of NOX4 expression (196).
Macrophage recruitment and activation respiratory burst have been associated with the early inflammatory phase of lung fibrosis (72). Activation of the inflammasome and production of IL-1β in response to NOX-dependent release of ROS have been suggested to link macrophage respiratory burst and inflammation in lung fibrosis (234).
The progression of lung fibrosis depends on the development of comorbidities such as pulmonary hypertension (PAH) (70). Hypoxia, which may lead to PAH, stimulates the release of superoxide by endothelial cells via a NOX2-dependent pathway. NOX2-mediated ROS serve to mobilize endothelial cell progenitors that are necessary for vascular repair (219). Enhanced NOX activity also contributes to endothelial apoptosis and the remodeling of pulmonary arteries. In particular, NOX4 promotes the proliferation of smooth muscle cells involved in vascular remodeling (58, 63).
Mesenchymal stem cells (MSCs) participate in the repair process after tissue injury by “homing” to site of injury. MSCs can differentiate into another cell type to regenerate the epithelial barrier and/or mediate paracrine actions on resident cells to promote repair responses. Some evidence suggests that the self-renewal and recruitment of MSCs depend on a crosstalk between gp91phox and matrix metalloproteinase-12 (MMP-12) (16). The crosstalk between vascular factors and perivascular NOX4 has also been implicated in establishing a favorable environment for engraftment of MSCs in fibrotic tissues (31). In particular, ectopic upregulation of hepatocyte growth factor (HGF) in endothelial cells inhibits perivascular NOX4 and facilitates engraftment of MSCs in fibrotic tissues of the lung and liver (31). On the contrary, targeted deletion of HGF in mice endothelial cells induces perivascular NOX4, and recapitulates the lung and liver fibrotic phenotypes (31).
Therapeutic strategies
Two U.S. Food and Drug Administration (FDA)-approved drugs, pirfenidone and nintedanib, are currently available to treat IPF patients (128, 204). These two drugs, however, have a limited efficacy and are not curative. They merely slow down the progression of IPF without increasing survival and ameliorating symptoms of the disease (202). Therefore, the development of alternative treatments remains a priority. Numerous studies have been undertaken to identify novel inhibitors of NOXs through drug library screening of small molecule inhibitors, traditional herbal medicines, and repurposing of prescription drugs (Fig. 3).
FIG. 3.
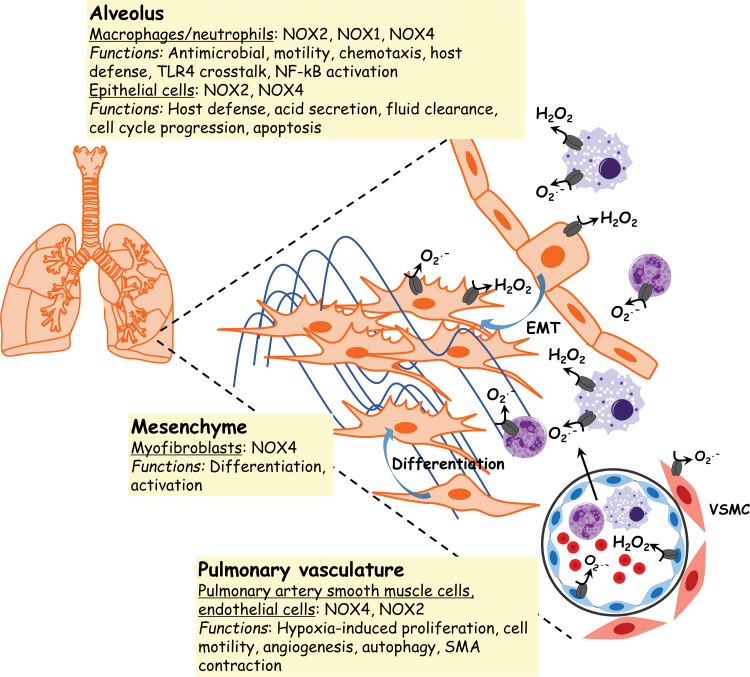
NOX expression and function in lung fibrosis. NOXs are a source of ROS in alveolar epithelial cells, macrophages, neutrophils, myofibroblasts, endothelial cells, and VSMCs. H2O2 and O2· − participate in determination of cell phenotype and fate by regulating immune cell recruitment/activation, EMT, myofibroblast differentiation, and ECM remodeling, which may all contribute to the pathogenesis of lung fibrosis. H2O2, hydrogen peroxide; Keap1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; Nrf2, nuclear factor erythroid-derived 2-like 2. Color images are available online.
Small molecule inhibitors of NOX enzymes
The development of specific inhibitors of NOX enzymes has proved challenging due to the structure of NOX enzymes, their regulatory interactions, and the lack of a crystal structure, although partial structural information is obtained from modeling in cyanobacteria (163). The following gives an overview of the progresses made in the development of small molecule inhibitors of NOX enzymes.
VAS2870 and VAS3947 are triazolopyrimidine derivatives that have been reported to completely block NOX-associated activity in a variety of cellular models without specificity toward any NOX homologs (244, 261).
GKT137831 is the first small molecule inhibitor targeting NOX1/NOX4 (133). GKT137831 belongs to the pyrazolopyridine chemical series (133, 243). Preclinical studies using GKT137831 have demonstrated promising effects of this compound in ameliorating fibrosis in a murine experimental model of the disease (98). Notably, GKT137831 reverses age-associated persistent fibrosis in a murine experimental model of lung fibrosis in aged mice. In these studies, in vivo targeting of NOX4 decreased accumulation of myofibroblasts and collagen deposition at sites of remodeling. It also downregulated the expression of the senescent markers p16 and p21 (98). These data suggest that GKT137831 modulates the senescent and apoptosis-resistant phenotype of myofibroblasts.
Peptidic inhibitors of NOX enzymes have been designed to block the assembly of the functional NOX enzyme, promote autoinhibition of the NOX enzyme, or disrupt regulatory interaction of the NOXs with p22phox, p47phox, or p67phox (49). Among peptides inhibitory of NOX2, NOX2ds-tat has been described as isoform specific (49). NOXA1ds targets the interaction of NOX1 and the activation domain of p67phox (49). Pep1 and Pep3 target the interaction between NOX5 N-terminal tail containing the EF domain, responsible for NOX5 stimulation by Ca2+, and its C-terminal catalytic dehydrogenase domain (49).
JQ1 is an inhibitor of the epigenetic readers, bromodomain-containing protein 3 and 4 (Brd3 and Brd4) (3). Pretreatment with JQ1 prevents TGF-β1-induced activation and differentiation of lung fibroblasts (232). These studies show that JQ1 treatment reduces NOX4 and SOD2 messenger RNA (mRNA) expression, thus shifting the imbalance between NOX4 and SOD2 that results from TGF-β1 stimulation of fibroblasts.
Herbal medicines
Magnesium isoglycyrrhizinate (MgIG) is a magnesium salt of 18α-glycyrrhizic acid stereoisomer. Glycyrrhizic acid is the main active ingredient in licorice, and has antioxidant and anti-inflammatory properties (145). MgIG treatment improves radiation-induced pulmonary fibrosis by attenuating lung collagen deposition and reducing levels of TGF-β1. MgIG mediates its effect on radiation-induced pulmonary fibrosis through a decrease in NOX4 expression, ROS production, and p38 mitogen-activated protein kinase (MAPK) phosphorylation (276).
Three compounds derived from herbal Chinese medicine have been reported to attenuate bleomycin or TGF-β1-induced lung fibrosis in vivo. These compounds restore the redox balance in myofibroblasts by reducing NOX4 and upregulating Nrf2 (98). These compounds are as follows: (i) costunolide, a natural occurring sesquiterpene lactone isolated from Saussurea lappa root (152); (ii) a novel gallic acid derivative derived from Quercus infectoria (206); and (iii) tanshinone IIA (Tan-IIA), isolated from the root of Salvia miltiorrhiza Bunge (7). Tan-IIa augments Nrf2 through promoting the degradation of Kelch-like ECH-associated protein 1 (Keap1). Of note, Tan-IIA-induced Nrf2 has been reported to increase intracellular glutathione (GSH) by shifting glutamate utilization by the tricarboxylic acid cycle toward GSH production (7).
Triptolide (TPL) is a diterpenoid epoxide isolated from Tripterygium wilfordii. It has been used in Eastern Asia to treat inflammatory and autoimmune disorders such as systemic lupus erythematosus, psoriasis, rheumatoid arthritis, and asthma (39). TPL has been reported to reduce the recruitment of alveolar macrophages in a model of irradiation-induced lung fibrosis in mice. TPL has also been shown to inhibit NOX2- and NOX4-mediated ROS in alveolar macrophages, thus attenuating effects of paracrine actions of ROS on myofibroblast activation induced by irradiation (37).
Prescription drugs
Azithromycin is a macrolide-type antibiotic prescribed for respiratory, dermal, and urogenital bacterial infections. Because of its immunomodulatory property, it is also used to treat chronic inflammatory disorders (190). Azithromycin ameliorates bleomycin-induced lung fibrosis in mice and prevents TGF-β1-induced myofibroblast differentiation in vitro. These effects of azithromycin have been associated with the stimulation of NOX4 degradation by the proteasome. In these studies, enhanced proteosomal degradation of NOX4 was shown to result from increased polyubiquitination of NOX4 after reduction of autophagy and activation of the unfolded protein response by azithromycin (248).
Metformin is a FDA-approved drug for treatment of type 2 diabetes. Metformin or 3-(diaminomethylidene)-1,1-dimethylguanidine is an AMP-activated kinase (AMPK) activator. AMPK is a bioenergetics sensor and metabolic regulator of cell adaptation to low energy conditions. In the bleomycin-injury model of lung fibrosis, AMPK activity is lower in fibrotic regions associated with metabolically active and apoptosis-resistant myofibroblasts (201). Treatment with metformin attenuates the fibrotic response to injury (201, 214). Sato et al. demonstrated that metformin prevents myofibroblasts differentiation by reducing NOX4-mediated ROS, which support TGF-β1-induced Smad2/3 phosphorylation. These studies show that inhibition of NOX4 expression by metformin leads to reduction in NOX4-mediated ROS (214).
Kidney Fibrosis
Causes and pathogenic mechanisms
Diabetes and hypertension are the most prominent causes of progressive kidney diseases characterized by renal fibrosis. Pathological renal fibrosis can affect the glomeruli (glomerulosclerosis), the tubulointerstitium (interstitial fibrosis), and the vasculature (vascular sclerosis). As described for the lung, myofibroblasts are responsible for the accumulation and the contractility of the scar tissue observed in renal fibrosis. EMT has been reported to give rise to myofibroblasts along with the differentiation of mesenchymal fibroblasts, pericytes, and perivascular fibroblasts into myofibroblasts upon exposure to profibrotic factors (151, 156, 169). EMT of podocytes is involved in glomerulosclerosis (253), while EMT of tubular epithelial cells has been reported in conjunction with interstitial renal fibrosis (286). Many profibrotic factors have been described as mediators of renal fibrosis in response to injury. However, angiotensin II (Ang II) drives the fibrogenic process via stimulating the release of other profibrotic factors such as TGF-β1 (22, 235), platelet-derived growth factor (PDGF) (107), connective-tissue-derived growth factor (CTGF) (274), NOX (199), PAI-1 (170), tumor necrosis factor-alpha (TNF-α) (198), osteopontin (263), and vascular cell adhesion molecule-1 (VCAM-1) (130). Mesangial cell-, macrophage-, and ECM-derived TGF-β1 sustains ECM deposition through stimulation of ECM synthesis and inhibition of its degradation (24, 91). TGF-β1 also promotes EMT of tubular epithelial cells (156). CTGF contributes to the pathogenesis of renal fibrosis through the modulation of matrix-degrading metalloproteinases (275). PAI-1 has been suggested to promote fibrogenesis via upregulation of fibronectin and type III collagen in the kidney (183). In addition to the release of profibrotic mediators, Ang II induces nuclear translocation of the transcription factor nuclear factor-kappa B (NF-κB), which induces gene expression of a number of proinflammatory and profibrotic mediators (129, 158, 268, 272). Ang II-mediated activation of NF-κB has been described to support the expression of tissue transglutaminase (153). Ang II-mediated expression of tissue transglutaminase in the fibrotic kidney may participate in the activation of ECM-bound latent TGF-β1 (217). TGF-β1-induced oxidative stress responses in mesangial and endothelial cells have been implicated in the pathogenesis of renal fibrosis (34). One proposed mechanism may be oxidative-stress-mediated neutralization of the protective role of mesangial NO against renal fibrosis (155). In the unilateral ureteral obstruction (UUO) model of renal fibrosis, alteration of renal hemodynamics is an early event triggered by increased activity of vasoconstrictor systems such as the renin–angiotensin system (73). Mesangial cells have been implicated in the regulation of the kidney tubuloglomerular feedback (203). Notably, mesangial cells have been suggested as a source of ROS that enhance tubuloglomerular feedback by quenching NO, thus counteracting NO-mediated vasodilation of glomerular afferent arterioles (155). TGF-β1 treatment of primary mouse mesangial cells has been shown to upregulate the production of ROS via augmenting the activity of NOX2, NOX4, and Duox2 (34). Of note, as opposed to the antifibrotic role of NO suggested above, some studies have reported an association between high levels of inducible nitric oxide synthase (iNOS) and the progression of kidney remodeling in UUO mice (230).
Role of NOX enzymes in kidney fibrosis
NOX enzymes have been implicated in the regulation of the kidney function through their role in gluconeogenesis (262), glucose transport (93), tubuloglomerular feedback (260), renal hemodynamics (218, 222), and electrolyte transport (112, 226) (Fig. 4). NOX4 is the NOX isoform that is most abundantly expressed in the renal cortex. NOX4 localizes to mesangial cells, podocytes, tubular epithelial cells, and endothelial cells. p47phox and p22phox are expressed in tubular epithelial cells along with NOX4 (85). NOX2, p67phox, p47phox, and p22phox mRNA are also expressed in podocytes (90). NOX1 is localized with NOX2 and NOX4 in different regions of the nephron (thick ascending limb, macula densa, apical area of distal convoluted tubules) as well as in the vasculature and glomeruli (35, 36). NOX5 has been localized to the tubular and glomerular regions in biopsies of human kidney tissue (113), and NOX3 has been reported to be expressed in the fetal kidney (42).
FIG. 4.
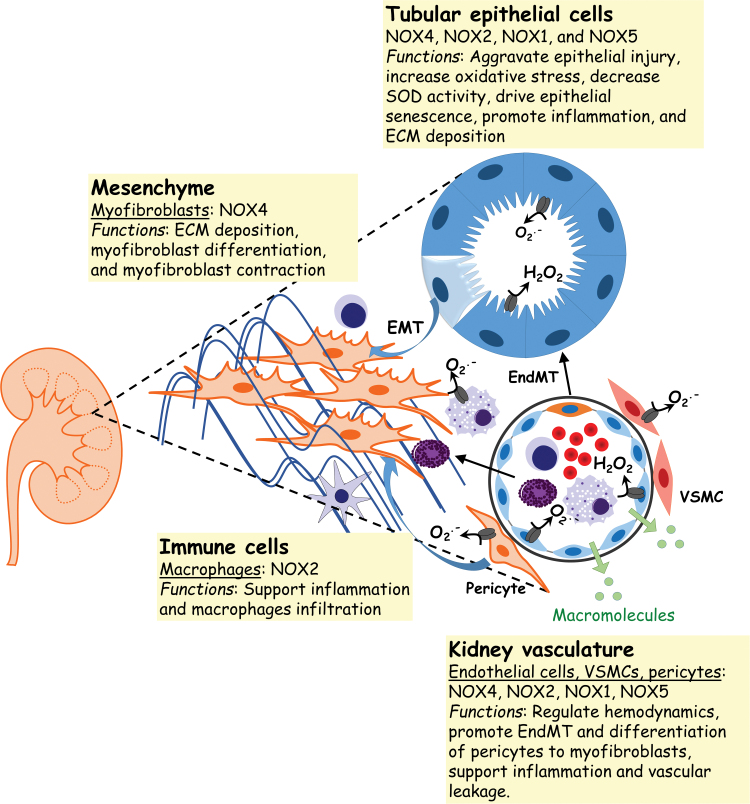
NOX expression and function in kidney fibrosis. NOXs constitute a major source of ROS in the kidney. Renal NOXs modulate a variety of cell functions that are involved in kidney fibrosis such as inflammation, ECM deposition, myofibroblast differentiation/contraction, and EndMT. Renal NOXs have also been shown to regulate vascular tone, hemodynamics, inflammation, and remodeling. EndMT, endothelial to mesenchymal; SOD, superoxide dismutase. Color images are available online.
NOX4 has been shown to play a role in diabetes-induced renal fibrosis in mice and rats. In these experimental models, hyperglycemia increases the expression and activity of NOX4. This increase in NOX4 expression has been linked to the upregulation of TGF-β1 levels, phosphorylation of p38 MAPK, and fibronectin deposition in the kidney (220). Transient receptor potential cation channel 6 (TRPC6), a member of the family of transmembrane Ca2+ channels, is an important modulator of glomerular dynamics, alterations of which have been associated with the development of renal fibrosis (106). NOX4-generated ROS have been implicated in the downregulation of TRPC6 expression in mesangial cells (88). Cannabinoid receptor type 1 (CB1) is Gi/G0 protein-coupled receptor that is activated upon binding of endocannabinoids such as anandamide and 2-arachidonyl glycerol (160). While identified as one of the most abundant G-protein-coupled receptors in the brain (101), CB1 is also expressed in adipocytes (207), liver (55), skeletal muscle (105), and kidney (137). In the kidney, CB1 has been localized in podocytes (118), mesangial cells (149), proximal tubule cells (136, 250), distal tubule cells (136), and intercalated cells (136). In the murine model of cisplatin-induced renal fibrosis, upregulation of NOX4 gene expression has been linked to the activation of the CB1 (175). In this experimental model, CB1 antagonists or genetic deletion of CB1 receptors have been shown to reduce the expression of NOX2 and NOX4, thus reducing oxidative stress induced by cisplatin (175). Evidence of NOX2 involvement in renal fibrosis comes from the use of experimental models of diabetes-induced renal fibrosis. NOX2 expression is upregulated in the renal cortex of diabetic mice, and its inhibition has been linked to lower oxidative stress, enhanced superoxide dismutase (SOD) activity, and amelioration of renal fibrosis (77). However, contrary to these studies, NOX2 expression has been reported to remain unchanged in the renal cortex of db/db mice, a widely used experimental model of type 2 diabetes (220). NOX1 upregulation in the glomeruli and cortical tubules of diabetic mice has been associated with larger glomerular volume, increased mesangial matrix, and upregulated levels of the DNA double-strand break markers p27 and γH2AX; these effects on glomerular structure, ECM deposition, and senescence were attenuated in NOX1 KO mice (288). Data related to the role of NOX5 in renal fibrosis in vivo come from studies using a humanized murine model of NOX5 overexpression (113). In these studies, overexpression of NOX5 in mesangial cells and vascular smooth muscle cells (VSMCs) was associated with enhanced ROS production, deposition of collagen IV and fibronectin, immune infiltration, mesangial cells expansion, and glomerulosclerosis (113).
Therapeutic strategies
In the following sections, we discuss pharmacological approaches that have proven beneficial in the amelioration of experimental renal fibrosis through the inhibition of NOX-mediated oxidative stress and/or expression.
Small molecule inhibitors
Lysophosphatidic acid (LPA) is a proinflammatory mediator that is elevated in the serum of diabetes patients and in the kidney of a murine model of type 2 diabetes mellitus (281). LPA acts through binding/activation of its G protein-coupled receptors (LPAR1–6) (233). AM095 is a specific inhibitor of LPAR1 (237). Studies using the streptozotocin (STZ)-induced murine model of diabetic nephropathy showed that treatment with AM095, a specific antagonist of LPAR1, reduces inflammation through the modulation of the TLR4/NF-κB pathway; this is associated with a reduction in ROS levels, in part by decreasing NOX2 expression (139).
NOX1, NOX2, and NOX4 expression is augmented in diabetic end-stage kidney disease (132). Oral administration of APX-115, a pan-inhibitor of NOX enzymes, attenuates fibrosis associated with diabetic end-stage kidney disease in a murine model of STZ-induced kidney disease. Treatment with APX-115 decreases the expression of profibrotic markers such as TGF-β1, α-smooth muscle actin (SMA), fibronectin, and collagen IV (132). Restoration of peroxisomal function and mitochondrial biogenesis are among the mechanisms suggested to mediate effects of APX-115 on kidney fibrosis downstream of NADPH inhibition (132). APX-115 also reduces kidney remodeling in a rat model of diabetic kidney disease by reducing inflammation, evidenced by decreases in the expression of the proinflammatory marker CD68, and reduced levels of IL-6 and MCP-1 (61).
Fluorofenidone or [1-(3-fluorophenyl)-5-methyl-2-(1H)-pyridone] (FD) is a derivative of the antifibrotic drug, pirfenidone (157). FD treatment reduces NOX2 and NOX4-mediated oxidative stress as well as p47phox expression in rat proximal tubular epithelial (NRK-52E) cells in vitro and in the kidney of a rat model of UUO-induced renal fibrosis in vivo (199). In this model, hampered oxidative stress upon FD treatment is associated with a reduction in the expression of collagen Ia1 and fibronectin as well as lower levels of the oxidative stress marker, malondialdehyde, and MAPK signaling. FD has also been shown to decrease the expression of the profibrotic markers, collagen I and TGF-β1, via inhibition of NOX4 in NRK-52E cells (192). Of note, most studies used high concentrations of FD (2 mM for in vitro studies and 500 mg/kg/day for in vivo studies), thus raising concerns related to the specificity of this compound toward NOXs.
Tissue expression of NOX4, p22phox, and p47phox is upregulated in the renal cortex of db/db mice. Treatment of db/db mice with GKT136901, a NOX1/NOX4 inhibitor, blocks NOX4-dependent fibrotic signaling in the kidney after exposure to high glucose. Notably, GKT136901 inhibits high-glucose-mediated ROS, prevents phosphorylation of p38 MAPK, and decreases the expression of TGF-β1/2 and fibronectin (220).
Herbal medicines
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a phytoestrogen that has been identified in >70 species of plants and their products such as grapes, wine, and berries (209). Resveratrol is an activator of sirtuins (23) that mediates antisenescence (144), antioxidant (141), and anti-inflammatory properties (51, 143). Studies using dietary resveratrol showed that resveratrol ameliorates renal function and tubulointerstitial fibrosis in the kidney of aged mice (111). These beneficial effects of resveratrol appear to be through the modulation of the renin–angiotensin system. Resveratrol downregulates the angiotensin-converting enzyme/Ang II/angiotensin II type 1 receptor (ACE/Ang II/AT1R) signaling axis and upregulates the ACE2/Ang 1–7/Mas axis that is protective against kidney remodeling. Resveratrol also reduces the expression of the oxidative stress markers NOX4, 8-hydroxy 2′-deoxyguanosine (8-OHdG), 3-nitrotyrosine in the aged murine kidney. Of note, in these studies, resveratrol had no significant effect on the expression of NOX2 (111). In diabetic nephropathy, interstitial fibrosis results, in part, from the proliferation of interstitial fibroblasts and their activation to myofibroblasts. The proliferation of interstitial fibroblasts depends on the activation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) by NOX4-mediated ROS. Studies using the rat kidney fibroblast cell line (NRK-49F) and db/db mice demonstrate that resveratrol attenuates interstitial fibrosis by inhibiting fibroblast proliferation through activation of AMPK and subsequent downregulation of NOX4 expression (97).
Rosmarinic acid is an ester of caffeic acid and 3, 4-dihydroxyphenyllactic acid. Rosmarinic acid is a component of plants of the Lamiaceae family, including Rosmarinus officinalis (rosemary), Perilla frutescens (perilla), Salvia officinialis (sage), Mentha arvense (mint), and Ocimum basilicum (basil) (142). It has antioxidant and anti-inflammatory properties, and is used to treat reactive airway diseases, in particular asthma (210). Rosmarinic acid attenuates CdCl2-induced renal inflammation and fibrosis through reduction of the TGF-β1/SMAD3 signaling pathway and associated NOX activity. Overall decreases in H2O2 and NO production have been observed in response to rosmarinic acid treatment in vitro (studies using renal mesangial cells) and in vivo (studies in a murine experimental model of cadmium-induced nephrotoxicity) (116). P. frutescens has also been reported to reduce high-glucose-induced kidney remodeling via the suppression of NOX4 and NOX2 activity in association with the activation of the metabolic regulator AMPK (125).
Icariin is a bioactive constituent isolated from the Chinese medicine, Ying Yang Huo. It is a prenylated flavonol glycoside with reported potent cardiovascular protective functions (265). Icariin treatment reduces inflammation and fibrosis in the UUO model of renal fibrosis in mice. Icariin mediates its effect by decreasing the proinflammatory mediators, NF-κB, cyclooxygenase-2, and IL-1-β. Icariin also restores the renal oxidant/antioxidant balance through inhibition of TGF-β1-induced NOX4 activity and increases in the expression of the antioxidant enzymes, catalase and SOD (38).
Punica granatum (pomegranate) is rich in phenolic compounds, such as tannins (gallotannins and ellagitannins) and anthocyanins (rutinosides, pentosides, and glucosides of cyanidin, pelargonidin, and delphinidin) (6, 74). Pomegranate peels contain higher amounts of bioactive compounds than those found in the juice (135, 185); these peels are recognized as dietary antioxidants with antimicrobial, anticancer, antiobesity, antidiabetic, antiulcerogenic and antihypertensive, and antimutagenic properties (15, 17, 62, 65, 92, 185, 231). In vivo studies using pomegranate peel extract-stabilized gold nanoparticles (PPE-AuNP) have been shown to attenuate nephropathy and associated kidney fibrosis in the murine model of STZ-induced diabetic nephropathy. In this model, STZ-induced hyperglycemia sustains the production of NOX4-mediated ROS via the formation of advanced glycation end products (AGE). The binding of AGE to the receptor for advanced glycation end products (RAGE) increases NOX4 expression and leads to phosphorylation of p47phox. Beneficial effects of PPE-AuNP on diabetic nephropathy are mediated, in part, by a decrease in AGE-RAGE stimulation of NOX4 expression and p47phox phosphorylation. In addition, PPE-AuNP reduces inflammation through modulation of the MAPK/NF-κB/STAT3 axis and promotes antioxidant defenses via activation of the PI3K/AKT/Nrf2 signaling (164).
Peptides/amino acids
Chronic activation of the renin–angiotensin system via stimulation of the ACE/Ang II/type 1 angiotensin receptor (AT1) signaling pathway leads to hypertensive kidney disease (HKD), characterized by glomerular sclerosis and interstitial fibrosis (289). The heptapeptide Ang1–7 is derived from the hydrolysis of Ang II by the angiotensin-converting enzyme ACE2 (29). Ang1–7 mediates vasodilation through its binding to the G protein-coupled receptor, Mas, and this ACE2/Ang1–7/Mas axis counteracts profibrotic effects of ACE/Ang II/AT1 activation (290). Aldosterone-induced HKD in rats recapitulates the glomerular damage/fibrosis associated with the disease; in this HKD model, infusion of Ang1–7 decreases levels of the profibrotic factors, TGF-β1, TIMP-1/TIMP-2, FGF-1 and downregulates NOX2 mRNA expression in the kidney (40). The protective effect of Ang1–7 against kidney remodeling has also been demonstrated in Akita mice, a murine model of type 1 diabetes. Ang1–7 administered to Akita mice reduces kidney oxidative stress and NOX4 expression, in association with decreased expression of the profibrotic markers, TGF-β1 and collagen IV (223).
Glycine is a nonessential amino acid that is a precursor of reduced GSH, the most abundant intracellular antioxidant (167). Glycine is also used for the synthesis of creatine, purines, and heme (26, 68, 82). Glycine treatment has been shown to attenuate tubular interstitial fibrosis in the murine model of STZ-induced diabetes. In this model, amelioration of kidney remodeling in response to glycine treatment was associated with a decrease in renal oxidative stress and NOX4 mRNA expression (255).
Prescription drugs
Sitagliptin is a dipeptidyl peptidase 4 inhibitor that is approved for the treatment of type 2 diabetes mellitus (79). Sitagliptin ameliorates tubulointerstitial fibrosis associated with doxorubicin (DOX)-induced nephropathy in rats. This effect of sitagliptin results from a decrease in the expression of the NLRP3 inflammasome, and a decrease in the mRNA expression of NOX2, p47phox, and p67phox (115).
Aliskiren is a renin inhibitor that causes vasodilatation and is prescribed to treat hypertension (221). Paricalcitol is a synthetic vitamin D analog that is prescribed to treat hyperparathyroidism (179). Administration of aliskiren (via a miniosmotic pump) or paricalcitol (intraperitoneal injection) decreases the expression of the profibrotic markers, α-SMA and collagen IV, in a murine experimental model of UUO-induced kidney fibrosis (47); antifibrotic effects of these drugs are associated with a decrease in NOX1 and NOX2 expression and a decrease in levels of p-Erk, p-p38 MAPK, and NF-κB activation. Administration of aliskiren alone decreases the expression of NOX1 and NOX2 in the kidneys of UUO mice without having a significant effect on NOX4 expression; in contrast, paricalcitol alone significantly reduces NOX4 expression in the obstructed kidney. Monotherapy with either drug reduces the number of apoptotic cells detected by TUNEL in the fibrotic kidney (47).
Candesartan, approved for the treatment of hypertension, blocks Ang II receptors (87). Pioglitazone is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonist (56) that restores insulin sensitivity and is a prescribed drug for treatment of type 2 diabetes (59). The administration of candesartan and pioglitazone to db/db mice decreases glomerulosclerosis and glomerular oxidative stress. This effect of candesartan and pioglitazone on renal fibrosis is associated with a decrease in the expression of NOX2 and p22phox and enhanced expression of Cu/Zn-SOD and extracellular SOD (77).
Liver Fibrosis
Causes and pathogenic mechanisms
Liver fibrosis results from chronic exposure to injuries of diverse etiology (249). Sources of such injury may include viral infections, alcoholism, xenobiotic intoxication, and systemic metabolic diseases such as diabetes, obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis (NASH) (25). As described for other organs, liver fibrosis is thought to result from common fibrogenic pathways linked to a dysregulated tissue-repair response (191). Epithelial injury may initiate this dysregulated repair process (53), often associated with release of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) (5, 171). The recognition of DAMPs and PAMPs by Kupffer cells (hepatic-resident macrophages), hepatocytes, and immune cells triggers hepatic inflammation (12, 57). Hepatic inflammation involves the mobilization of macrophages, lymphocytes, eosinophils, and dendritic cells. Inflammatory mediators responsible for the mobilization and activation of these immune cells in the liver include TNF-α, IL-1, IL-6, C-X-C motif chemokines, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage-CSF, and the NLRP3 inflammasome (57). Injured epithelial cells, endothelial cells, and activated immune cells secrete profibrotic mediators that set the stage for the fibrogenic phase of liver injury. These profibrotic mediators include PDGF, Ang II, CTGF, and TGF-β1 (67, 187, 254, 278). Fibrogenesis leads to progressive accumulation of ECM, destruction of the liver tissue architecture, decline in liver function, and portal hypertension (194). The putative cell-of-origin of liver myofibroblasts that deposit and remodel the ECM in the liver have been intensely studied. EMT and activation of resident mesenchymal progenitors, bone-marrow-derived fibrocytes, or hepatic stellate cells (HSCs) have all been implicated in contributing to the myofibroblast population in the fibrotic liver (1, 60, 122, 131, 182, 205). However, in vivo lineage tracing studies carried out in a murine model of liver fibrosis have shown that >95% of liver myofibroblasts come from the transdifferentiation of HSCs and the activation of portal fibroblasts (108).
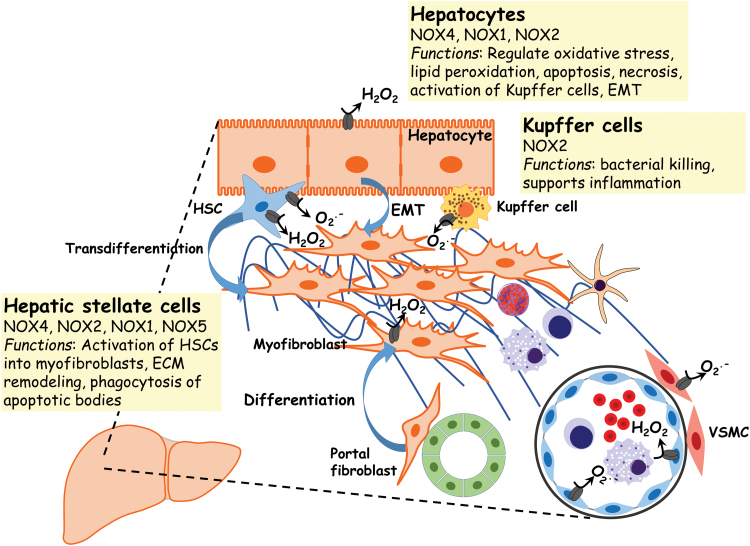
Role of NOX enzymes in liver fibrosis
The role of increased oxidative stress in the pathogenesis of liver fibrosis is based on studies using clinical samples and experimental animal models of liver fibrosis (11, 211, 273) (Fig. 5). NOX4 expression has been shown to be elevated in a murine model of steatosis-induced fibrosis (21). In this model, hepatocyte-specific deletion of NOX4 results in lower oxidative stress, reduced lipid peroxidation, and attenuation of liver fibrosis (21). NOX4-derived ROS have also been implicated in the transdifferentiation and proliferation of HSCs (211). NOX2−/− mice are protected against CCl4-induced liver fibrosis (11). These studies support a role of NOX2 in the regulation of both the expression of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) and the expression of the MMPs tissue inhibitors, TIMP-1 and TIMP-2. NOX2 has also been shown, in vitro, to be the source of ROS in HSCs and Kupffer cells (186). NOX2 has been shown to mediate the activation of HSCs into myofibroblasts after phagocytosis of dying hepatocytes (114). NOX1 KO mice are also protected against CCl4-induced liver fibrosis (134). NOX1 is also expressed and activated in HSCs. NOX1, similar to NOX2 and NOX4, has also been implicated in the transdifferentiation and proliferation of HSCs (134). In vitro studies using a genetic approach to delete NOX5 in HSCs have also suggested that NOX5 may be involved in the activation of HSCs through the modulation of p38 MAPK (9).
FIG. 5.
NOX expression and function in liver fibrosis. The expression/activity of NOXs supports the increase in oxidative stress and altered redox signaling in the pathogenesis of liver fibrosis. NOX-generated ROS contribute to apoptosis and/or necrosis of hepatocytes after injury. Hepatocyte NOX-dependent ROS may activate hepatic Kupffer cells, resident macrophages of the liver. H2O2 and O2· − from activated NOXs mediate the activation and transdifferentiation of HSCs into myofibroblasts, as well as in the phagocytosis of apoptotic bodies by HSCs. HSCs, hepatic stellate cells. Color images are available online.
Therapeutic strategies
Small molecule inhibitors
The NOX1/NOX4 inhibitor, GKT137831, ameliorates liver inflammation and fibrosis in a murine model of CCl4-induced liver fibrosis (10). These studies applied the CCl4-induced liver fibrosis model to WT mice and to mice harboring the SOD1 G37R mutation (SOD1mu), which increases the catalytic activity of NOX1; however, the drug protected both WT and SOD1mu mice. GKT137831 impedes hepatic lipid peroxidation, as evidenced by lower levels of 4-HNE, in liver tissue, along with a downregulation in the gene expression of profibrotic markers and NOX4 in HSCs isolated from WT or SOD1mu mice (10).
Herbal medicines
2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside (TSG) is a bioactive polyphenolic component of Polygonum multiflorum (266). TSG has been reported to protect against end-stage hepatic fibrosis associated with NASH (269). TSG treatment of low-density lipoprotein receptor knockout mice fed a high fat diet to induce NASH resulted in lowering of collagen accumulation in the liver with reduced expression of TGF-β1 and α-SMA. In addition, TSG decreased NOX2 and NOX4 expression, and enhanced the hepatic antioxidant activity via the upregulation of SOD, catalase, and GSH (269). Of note, TSG administration has been reported to aggravate acetaminophen-induced hepatotoxicity in mice due to enhanced expression of cytochrome P450 (271).
Ferulic acid is a dietary antioxidant found in many plants and vegetables. It is a phenolic compound particularly abundant in bamboo sprouts, beetroot, cabbage, broccoli, and spinach (284). In vitro treatment of human HSCs (LX-2 cells) with methyl ferulic acid (MFA) inhibited TGF-β1-induced expression of α-SMA, collagen I, TGF-β receptor I, NOX4, and p22phox (43). This reduction in the expression of profibrotic markers was associated with a decrease in the phosphorylation levels of Smad2/3 (43). Furthermore, MFA reduced fibrosis markers such as hydroxyproline, hyaluronic acid, procollagen III, and collagen IV in the serum of a rat model of hepatic fibrosis (43). Modulation of the TGF-β1/NOX4-mediated ROS signaling pathway has been suggested as the mechanism of MFA's protective effect on liver fibrosis (43).
Ursolic acid is a lipophilic pentacyclic triterpenoid found in the peel of fruits and herbs such as apples, bearberry, lavender, and coffee leaves (110). Pretreatment of HSCs with ursolic acid prevents TGF-β1-induced differentiation of HSCs into myofibroblasts (279). This effect of ursolic acid in vitro on HSC activation has been linked to both a reduction in the expression of the NOX2 and its subunits, p67phox, p22phox, and Rac1 and inhibition of the hedgehog signaling pathway (279). More recent studies have shown that ursolic acid also protects against CCl4-induced liver fibrosis in rats (80). These studies showed that administration of ursolic acid ameliorates fibrosis via downregulation of TGF-β1, collagen I, and TIMP-1 gene expression (80); this decrease in profibrotic marker expression is accompanied by a reduction in CCl4-induced expression of NOX4, p67phox, NOX2, p47phox, NOX1, p22phox, and Rac1 proteins in the liver tissue (80).
Polydatin is a glucoside of resveratrol that has been shown to attenuate CCl4-induced liver fibrosis in mice (283). Polydatin treatment reduces CCl4-induced liver fibrosis in mice via the attenuation of oxidative stress and inflammation. Lower hepatic levels of the oxidative stress markers, 4-hydroxy-2-nonenal (4-HNE) and NOX4, have been shown in response to polydatin treatment (283). The reduction of the inflammatory response upon polydatin administration is characterized by decreased macrophage recruitment and reduced gene expression of TNF-α and MCP-1 (283).
Quercetin or 3, 3′, 4′, 5, 7-pentahydroxyflavone is a plant pigment that is abundant in many fruits, vegetables, and grains (147). Quercetin has been reported to improve bile duct ligation (BDL)-induced liver fibrosis in rats through downregulation of NOX1 expression (120). Quercetin also decreases the expression of Rac1, a small GTPase protein that enhances NOX1 activity, and upregulates the activity of SOD and catalase in the liver tissue of rats in the BDL liver fibrosis model (120).
Antioxidants
Apocynin is a natural organic compound that has been described as a NOX inhibitor (14). α-Lipoic acid (ALA) is an antioxidant produced by plants, animals, and humans. In cells, ALA serves as a cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in mitochondria (208). Administration of both apocynin and ALA attenuated concanavalin A-induced liver fibrosis in rats (71). These studies showed that apocynin and ALA treatment reduces hepatic levels of hydroxyproline, α-SMA, and TGF-β1 as well as diminish levels of the proinflammatory markers, IL-6 and TNF-α. This treatment also restores the antioxidant capacity of the liver through augmentation of SOD and GSH while decreasing mRNA expression of NOX4 and NOX1 (71).
Prescription drugs
Fluvastatin (Flu) is a prescription drug that limits the production of cholesterol and prevents its accumulation in blood vessel walls (189). Flu treatment ameliorates steatohepatitis-induced liver fibrosis in rats via lowering collagen accumulation and decreasing the expression of α-SMA (45). Flu treatment also downregulates mRNA expression of collagen 1, TIMP-1, IL-6, iNOS, and intercellular adhesion molecule 1 (ICAM-1). In vitro treatment with Flu prevents palmitate-induced injury of hepatocytes by reducing intracellular ROS and the secretion of proinflammatory factors such as TNF-α and IL-6 (45). These studies also show that Flu treatment downregulates NOX2 mRNA.
Peptides
Similarly to kidney fibrosis, delivery of Ang1–7 attenuates liver fibrosis induced by BDL in rats (30). In these studies, the authors demonstrate that fibrogenesis depends on the activation of the NLRP3 inflammasome/IL-1β signaling axis by NOX4. Protective effects of Ang1–7 were proposed to be mediated by restoration of the altered redox balance in HSCs by lowering NOX4-driven oxidative stress and augmenting the Nrf2/ARE antioxidant defenses (30).
Cardiac Fibrosis
Causes and pathogenic mechanisms
The term cardiac fibrosis encompasses two subtypes of fibrosis affecting the heart, namely replacement fibrosis and reactive fibrosis. Replacement fibrosis is a reparative process initiated upon cardiomyocyte injury. Replacement fibrosis deposits scar tissue to replace the loss of cardiomyocytes due to necrosis, and is associated with systolic ventricular dysfunction. Reactive fibrosis is triggered by stimuli such as mechanical stress, myocardial inflammation, and metabolic dysregulation. These stimuli activate fibrogenic responses in absence of cardiomyocyte death. Reactive fibrosis leads to the accumulation of fibrous tissue around cardiomyocytes and the heart vasculature; in contrast to replacement fibrosis, reactive fibrosis is associated with diastolic dysfunction (216).
Activated myofibroblasts deposit fibrils of collagen I and III that constitute scar tissue in cardiac fibrosis (216). Lineage tracing studies have shown that resident cardiac fibroblasts are the main source of activated myofibroblasts in the heart (4, 174). Profibrotic mediators support the activation and differentiation of cardiac myofibroblasts. Macrophage-derived IL-10, TGF-β1, and PDGF are prominent profibrotic factors that mediate myofibroblast differentiation and activation (69, 195). Degranulation of mast cells after cardiomyocyte injury also releases proinflammatory and profibrotic mediators such as TNF-α, TGF-β1, IL-4, PDGF, chymase, and tryptase (76, 121, 162, 178, 224, 228). Recruited T cells have been suggested to participate in the reprogramming of macrophage toward a profibrotic phenotype, and to support the activation and differentiation of myofibroblasts. Direct interactions between Th1 lymphocytes and cardiac fibroblasts have been suggested to enhance the production of fibroblast-derived TGF-β1 (180). Th2 lymphocyte-derived IL-4 and IL-13 (48) have been implicated in the stimulation of collagen synthesis by myofibroblasts (83). Neutrophils have been proposed to play a role in the development of fibrosis in the aging heart through the establishment of a peptidylarginine deiminase 4-dependent neutrophil extracellular trap formation (166). In addition to the release of profibrotic mediators by recruited immune cells, the renin–angiotensin–aldosterone system promotes the differentiation of cardiac fibroblasts into myofibroblasts through the engagement of AT1 and mineralocorticoid receptor-driven signaling (119, 252). Furthermore, crosstalk between injured cardiomyocytes, endothelial cells, and resident cardiac fibroblasts has been reported to stimulate and sustain the differentiation of fibroblasts into myofibroblasts (213).
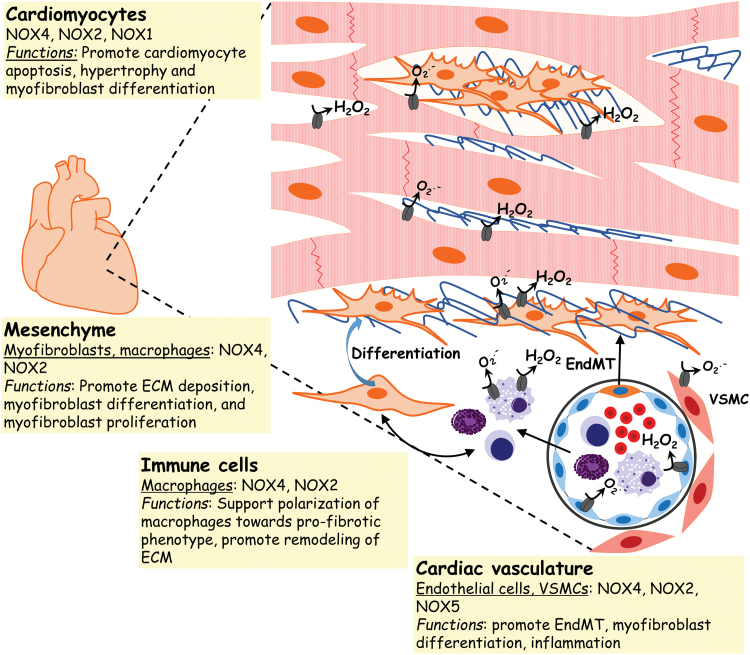
Role of NOX enzymes in cardiac fibrosis
Many of the profibrotic mediators described above, in particular Ang II and TGF-β1, mediate their effects on cardiac myofibroblast activation and differentiation via stimulating ROS production (159, 197). Cardiac fibroblast-derived ROS participates in matrix accumulation and remodeling (148). The ROS-generating enzymes, NOX4 and NOX2, are the predominant NOX isoforms expressed in the heart (2, 18). Both NOX4 and NOX2 are expressed in cardiomyocytes, endothelial cells, and fibroblasts (280). NOX4 is also found in smooth muscle cells (270). In vivo studies using NOX4- or NOX2-deficient mice have confirmed a role for both these homologs in the pathogenesis of cardiac fibrosis (2, 54, 117). NOX4-produced H2O2 mediates the differentiation of cardiac fibroblasts (54), and may contribute to cardiomyocyte apoptosis (2). NOX2 has been reported to mediate Ang II-induced cardiac fibrosis (117). More recently, studies have shown increased cardiac NOX1 mRNA expression in a murine model of DOX-induced cardiac fibrosis; in addition, conditioned medium from NOX1-depleted H9c2 cardiomyocytes loses its ability to upregulate collagen3a1 mRNA expression in cardiac fibroblasts (109). NOX2 has been suggested to mediate endothelial-to-mesenchymal transition in cardiac fibrosis (176). Selective expression of NOX5 in VSMCs of humanized transgenic mice increases cardiac oxidative stress and fibrosis (173) (Fig. 6).
FIG. 6.
NOX expression and function in cardiac fibrosis. In the fibrotic heart, NOXs have been implicated in cardiomyocyte apoptosis and hypertrophy. NOXs are also involved in the differentiation of mesenchymal fibroblasts into myofibroblasts that deposit exuberant ECM. NOXs also contribute to the polarization of macrophages to a profibrotic phenotype. Color images are available online.
Therapeutic strategies
Small molecule inhibitors
Mitoquinone (MitoQ) is a mitochondria-targeted antioxidant that accumulates within the mitochondrial matrix due to the positive charge of its triphenylphosphonium moiety (227). MitoQ treatment attenuates left ventricular pressure overload-induced cardiac remodeling (86). These studies showed that MitoQ treatment, in vivo, restores the cardiac oxidant/antioxidant balance. The protective effect of MitoQ appears to result from both a reduction in NOX4-mediated oxidative stress and an increase in Nrf2-driven antioxidant defenses (86). Furthermore, MitoQ administration lowers levels of the profibrotic cytokine, TGF-β1 (86).
As with fibrosis involving the lung, kidney, and liver, the NOX1/4 inhibitor GKT137831 has been shown to attenuate cardiac fibrosis in a murine model overexpressing human NOX4 in cardiac myocytes (282). These studies showed that GKT137831 treatment reduces oxidative-stress-mediated activation of the AKT/mTOR and NFκB signaling pathways.
Herbal medicines
Protocatechuic acid or 3,4-dihydroxy-benzoic acid is a metabolite that is derived from poylphenols found in green tea (140). Protocatechuic acid has antioxidant properties and has been shown to prevent differentiation of cardiac fibroblasts into myofibroblasts (229). Protocatechuic acid blocks myofibroblast differentiation by inhibiting NOX4-mediated ROS production and phosphorylation of p38 MAPK. Inhibition of the NOX4/ROS/p38 signaling pathway leads to reduced expression of the profibrotic markers, α-SMA, collagen I, and CTGF (229).
Astragaloside IV is a traditional Chinese medicinal herb derived from Astragalus membranaceus that is proposed to mediate antioxidant, anti-inflammatory, immunoregulatory, anticancer, hypolipidemic, and antihyperglycemic properties (154). A. membranaceus extracts contain polysaccharides, flavonoids, and saponins (154). Astragaloside IV ameliorates cardiac fibrosis and cardiac function in a murine model of DOX-induced cardiomyopathy (150). Astragaloside IV mediates its protective effects against DOX-induced cardiac fibrosis via the inhibition of NOX2- and NOX4-mediated oxidative stress (150). This reduction in NOX-mediated ROS is associated with a decrease in cardiomyocyte apoptosis triggered by DOX injury (150). Astragaloside IV treatment has also been reported to ameliorate cardiac fibrosis in ApoE−/− mice, a murine experimental model of hyperlipidemia that develops cardiac hypertrophy and fibrosis (146). In this model, astragaloside IV restored the expression of the proliferative marker Ki67 and decreased that of NOX4 and p16INK4a, a biomarker of cellular senescence (146).
Carthamus tinctorius L. or safflower belongs to the family of Asteraceae. Safflower extracts contain bioactive components such as flavonoids, quinochalcones, alkaloids, and polyacetylenes (287). Treatment with safflower extracts reduces NOX2-mediated oxidative stress in the heart of NO-deficient hypertensive rats (28). In this experimental model, lower levels of the profibrotic mediators TGF-β1 and MMP-9 were linked to a decrease in NOX2 expression (27).
Luteolin-7-diglucuronide (L7DG) is a bioactive compound extracted from the leaves of Verbena officinalis (32). L7DG pretreatment prevents the development of cardiac fibrosis in response to isoproterenol-induced myocardial injury in mice (181). These studies showed that L7DG pretreatment decreases the accumulation of interstitial collagen and α-SMA levels in heart tissues of isoproterenol-injured mice. Moreover, L7DG treatment reduced the expression of genes encoding NOX subunits (181), namely CYBA (p22phox), CYBB (gp91phox), NCF1 (p47phox), NCF2 (p67phox), NCF4 (p40phox), and RAC2 (240). L7DG also downregulated mRNA expression of the profibrotic markers, Col1a1, Col1a2, Col3a1, Col2a1, fibronectin, elastin, CTGF, and collagen triple helix repeat containing-1 (181).
Prescription drugs
Trimetazinide (TMZ) is a piperazine compound that prevents ischemia and is a prescribed drug for treatment of angina (46). TMZ was first described as a modulator of metabolism that shifts mitochondrial substrate utilization (75). TMZ ameliorates cardiac fibrosis associated with diabetes-mellitus-induced cardiomyopathy in rats (239). In this experimental model of cardiomyopathy, TMZ reduced the occurrence of oxidative DNA damage, and decreased the expression of NOX2 and p47phox mRNA. Of note, these studies also showed that TMZ does not reverse the increase in p22phox mRNA expression observed in the myocardium of diabetic rats. Furthermore, reduction of myocardial oxidative stress in diabetic rats upon TMZ treatment attenuates the cardiac inflammatory response (239).
Febuxostat is a xanthine oxidase inhibitor (XO) that is approved for the treatment of gout (64). High salt intake leads to increased cardiac mass in rats due to cardiomyocyte hypertrophy and interstitial fibrosis (123). Febuxostat administration reversed the increase in XO and NOX activity associated with high-salt-induced hypertension in rats (177). Febuxostat treatment also reduced the expression of collagen I, TGF-β1, phospho-ERK, ACE, and ATR1 (177).
Similar to protective effects of metformin on lung fibrosis, administration of metformin after myocardial infarction ameliorated cardiac fibrosis and decreased levels of cardiac galectin-3 (13). Galectin-3 is a β-galactoside binding lectin that mediates myofibroblast proliferation and activation (100, 161, 238). A reduction in galectin-3 release by myofibroblasts, through the modulation of the mitochondrial NOX4/PKCα signaling pathway by AMPK, has been proposed as a mechanism for the salutary effects of metformin on cardiac fibrosis after myocardial infarction (13).
Peptide
As described for kidney fibrosis, Ang1–7 has been reported to ameliorate cardiac fibrosis, although by apparently different mechanisms (41). Activation of cardiac fibroblasts observed in cardiac fibrosis is mediated by enhanced extracellular calcium influx via TRPC3 channels (184), as well as by augmented NOX4 expression (54). Administration of Ang1–7 reduces collagen accumulation and mitigates the activation of cardiac fibroblasts by AngII (41). These studies suggest that Ang1–7 exerts its effect on cardiac remodeling through the reduction of either (or both) extracellular calcium influx and oxidative stress. The mechanism proposed to support the protective effects of Ang1-7 is by a reduction in NOX4-mediated oxidation of CaMKIIδ and GSSG, and thus lower levels of CTGF and ERK1/2 MAPK activation (41).
Conclusion
Fibrosis involving diverse organ systems such as the lung, kidney, liver, and heart may involve common pathophysiologic mechanisms. Accumulating evidence indicates that an upregulation in the expression and activation of ROS-generating NOX enzymes represents a conserved fibrogenic mechanism across these organs. As such, NOX enzymes constitute a promising therapeutic target to ameliorate fibrosis, either through a preventative strategy or by arresting progression of established fibrosis. A large number of preclinical studies in experimental animal models have reinforced the legitimacy of NOXs as attractive targets for organ fibrosis. Notably, these studies have demonstrated in vivo attenuation of the severity of fibrosis, acceleration of its resolution, and/or arrest of its progression. Some of the NOX-targeted therapies appear to have redox-modulatory effects rather than a specific effect on NOX enzymes (14). The currently available preclinical models of organ fibrosis do not recapitulate the progressive nature of the disease in humans; using aging models may be more representative of the pathology (98), and the emerging use of human organoid models may be more predictive of therapeutic responses (236). Finally, a more thorough understanding of signaling targets/intermediates of the NOXs, their cell-specific expression and targeting, and the development of more specific, potent, and safe strategies to inhibit specific NOX homologs will advance drug discovery/development for fibrotic pathologies that currently carry high morbidity and mortality.
Funding Information
This work was supported by NIH grants P01 HL114470, R01 AG046210 (to V.J.T.), R01HL139617 (K.B. and V.J.T.), and U.S. Department of Veterans Affairs Merit Award I01BX003056 (to V.J.T.).
Abbreviations Used
- 4-HNE
4-hydroxy-2-nonenal
- 8-OHdG
8-hydroxy 2′-deoxyguanosine
- ABCA3
ATP-binding cassette-type 3
- ACE
angiotensin-converting enzyme
- AECI
alveolar epithelial cell type I
- AECII
alveolar epithelial cell type II
- AGE
advanced glycation end products
- ALA
α-lipoic acid
- AMPK
AMP-activated kinase
- Ang II
angiotensin II
- AT1
type 1 angiotensin receptor
- AT1R
angiotensin II type 1 receptor
- BDL
bile duct ligation
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CB1
cannabinoid receptor type 1
- CTGF
connective tissue-derived growth factor
- DAMPs
danger-associated molecular patterns
- DOX
doxorubicin
- Duox
dual oxidase
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- EndMT
endothelial-to-mesenchymal
- ERK1/2
extracellular signal-regulated protein kinase 1 and 2
- FD
fluorofenidone or [1-(3-fluorophenyl)-5-methyl-2-(1H)-pyridone]
- FDA
U.S. Food and Drug Administration
- Flu
fluvastatin
- G-CSF
granulocyte-colony stimulating factor
- GSH
glutathione
- H2O2
hydrogen peroxide
- HGF
hepatocyte growth factor
- HKD
hypertensive kidney disease
- HSCs
hepatic stellate cells
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IPF
idiopathic pulmonary fibrosis
- Keap1
Kelch-like ECH-associated protein 1
- L7DG
luteolin-7-diglucuronide
- LPA
lysophosphatidic acid
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MFA
methyl ferulic acid
- MgIG
magnesium isoglycyrrhizinate
- MitoQ
Mitoquinone
- MMPs
matrix metalloproteinases
- mRNA
messenger RNA
- MSCs
mesenchymal stem cells
- NAF1
nuclear assembly factor 1 ribonucleoprotein
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- Nrf2
nuclear factor erythroid-derived 2-like 2
- PAH
pulmonary hypertension
- PAI-1
plasminogen activator inhibitor-1
- PAMPs
pathogen-associated molecular patterns
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PPAR-γ
peroxisome proliferator-activated receptor-gamma
- PPE-AuNP
pomegranate peel extract-stabilized gold nanoparticles
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SFTPs
surfactant proteins
- SMA
smooth muscle actin
- SMAD
mothers against decapentaplegic homolog
- SOD
superoxide dismutase
- STZ
streptozotocin
- Tan-IIA
tanshinone IIA
- TGF-β1
transforming growth factor-beta 1
- TIMPs
tissue inhibitors of matrix metalloproteinases
- TMZ
trimetazinide
- TNF-α
tumor necrosis factor-alpha
- TPL
triptolide
- TRPC6
transient receptor potential cation channel 6
- TSG
2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside
- UUO
unilateral ureteral obstruction
- VCAM-1
vascular cell adhesion molecule-1
- VSMCs
vascular smooth muscle cells
- XO
xanthine oxidase inhibitor
References
- 1. Abe R, Donnelly SC, Peng T, Bucala R, and Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alghamdi S, Khan I, Beeravolu N, McKee C, Thibodeau B, Wilson G, and Chaudhry GR. BET protein inhibitor JQ1 inhibits growth and modulates WNT signaling in mesenchymal stem cells. Stem Cell Res Ther 7: 22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, and Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635, 2014 [DOI] [PubMed] [Google Scholar]
- 5. Alisi A, Carsetti R, and Nobili V. Pathogen- or damage-associated molecular patterns during nonalcoholic fatty liver disease development. Hepatology 54: 1500–1502, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Amri Z, Zaouay F, Lazreg-Aref H, Soltana H, Mneri A, Mars M, and Hammami M. Phytochemical content, Fatty acids composition and antioxidant potential of different pomegranate parts: comparison between edible and non edible varieties grown in Tunisia. Int J Biol Macromol 104: 274–280, 2017 [DOI] [PubMed] [Google Scholar]
- 7. An L, Peng LY, Sun NY, Yang YL, Zhang XW, Li B, Liu BL, Li P, and Chen J. Tanshinone IIA activates nuclear factor-erythroid 2-related factor 2 to restrain pulmonary fibrosis via regulation of redox homeostasis and glutaminolysis. Antioxid Redox Signal 30: 1831–1848, 2019 [DOI] [PubMed] [Google Scholar]
- 8. Anderson MM, Hazen SL, Hsu FF, and Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest 99: 424–432, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andueza A, Garde N, Garcia-Garzon A, Ansorena E, Lopez-Zabalza MJ, Iraburu MJ, Zalba G, and Martinez-Irujo JJ. NADPH oxidase 5 promotes proliferation and fibrosis in human hepatic stellate cells. Free Radic Biol Med 126: 15–26, 2018 [DOI] [PubMed] [Google Scholar]
- 10. Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, and Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology 49: 911–919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armengol C, Bartoli R, Sanjurjo L, Serra I, Amezaga N, Sala M, and Sarrias MR. Role of scavenger receptors in the pathophysiology of chronic liver diseases. Crit Rev Immunol 33: 57–96, 2013 [PubMed] [Google Scholar]
- 13. Asensio-Lopez MDC, Lax A, Fernandez Del Palacio MJ, Sassi Y, Hajjar RJ, and Pascual-Figal DA. Pharmacological inhibition of the mitochondrial NADPH oxidase 4/PKCalpha/Gal-3 pathway reduces left ventricular fibrosis following myocardial infarction. Transl Res 199: 4–23, 2018 [DOI] [PubMed] [Google Scholar]
- 14. Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, Mahiout Z, Jansen-Durr P, Knaus UG, Doroshow J, Stocker R, Krause KH, and Jaquet V. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol 26: 101272, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bagheri M, Fazli M, Saeednia S, Kor A, and Ahmadiankia N. Pomegranate peel extract inhibits expression of beta-catenin, epithelial mesenchymal transition, and metastasis in triple negative breast cancer cells. Cell Mol Biol (Noisy-le-grand) 64: 86–91, 2018 [PubMed] [Google Scholar]
- 16. Banerjee ER and Henderson WR Jr. Characterization of lung stem cell niches in a mouse model of bleomycin-induced fibrosis. Stem Cell Res Ther 3: 21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banihani S, Swedan S, and Alguraan Z. Pomegranate and type 2 diabetes. Nutr Res 33: 341–348, 2013 [DOI] [PubMed] [Google Scholar]
- 18. Bendall JK, Cave AC, Heymes C, Gall N, and Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM, and Thannickal VJ. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J Biol Chem 292: 3029–3038, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernard K, Logsdon NJ, Ravi S, Xie N, Persons BP, Rangarajan S, Zmijewski JW, Mitra K, Liu G, Darley-Usmar VM, and Thannickal VJ. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J Biol Chem 290: 25427–25438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, Tian J, Katsuyama M, Yabe-Nishimura C, Xi Y, Szyndralewiez C, Schroder K, Shah A, Brandes RP, Haj FG, and Torok NJ. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 149: 468–480.e10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Border WA and Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension 31: 181–188, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Borra MT, Smith BC, and Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280: 17187–17195, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Branton MH and Kopp JB. TGF-beta and fibrosis. Microbes Infect 1: 1349–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Brenner C, Galluzzi L, Kepp O, and Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol 59: 583–594, 2013 [DOI] [PubMed] [Google Scholar]
- 26. Brosnan JT, da Silva RP, and Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids 40: 1325–1331, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Bunbupha S, Pakdeechote P, Maneesai P, Prachaney P, and Boonprom P. Carthamus tinctorius L. extract attenuates cardiac remodeling in L-NAME-induced hypertensive rats by inhibiting the NADPH oxidase-mediated TGF-beta1 and MMP-9 pathway. Ann Anat 222: 120–128, 2019 [DOI] [PubMed] [Google Scholar]
- 28. Bunbupha S, Prachaney P, Kukongviriyapan U, Kukongviriyapan V, Welbat JU, and Pakdeechote P. Asiatic acid alleviates cardiovascular remodelling in rats with L-NAME-induced hypertension. Clin Exp Pharmacol Physiol 42: 1189–1197, 2015 [DOI] [PubMed] [Google Scholar]
- 29. Burrell LM, Johnston CI, Tikellis C, and Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 15: 166–169, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai SM, Yang RQ, Li Y, Ning ZW, Zhang LL, Zhou GS, Luo W, Li DH, Chen Y, Pan MX, and Li X. Angiotensin-(1–7) improves liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxid Redox Signal 24: 795–812, 2016 [DOI] [PubMed] [Google Scholar]
- 31. Cao Z, Ye T, Sun Y, Ji G, Shido K, Chen Y, Luo L, Na F, Li X, Huang Z, Ko JL, Mittal V, Qiao L, Chen C, Martinez FJ, Rafii S, and Ding BS. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci Transl Med 9: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carnat A, Carnat AP, Chavignon O, Heitz A, Wylde R, and Lamaison JL. Luteolin 7-diglucuronide, the major flavonoid compound from Aloysia triphylla and Verbena officinalis. Planta Med 61: 490, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, and Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cha JJ, Min HS, Kim KT, Kim JE, Ghee JY, Kim HW, Lee JE, Han JY, Lee G, Ha HJ, Bae YS, Lee SR, Moon SH, Lee SC, Kim G, Kang YS, and Cha DR. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab Invest 97: 419–431, 2017 [DOI] [PubMed] [Google Scholar]
- 35. Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, and Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, and Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Chen C, Yang S, Zhang M, Zhang Z, Hong J, Han D, Ma J, Zhang SB, Okunieff P, and Zhang L. Triptolide mitigates radiation-induced pulmonary fibrosis via inhibition of axis of alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biol Ther 17: 381–389, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen HA, Chen CM, Guan SS, Chiang CK, Wu CT, and Liu SH. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine 59: 152917, 2019 [DOI] [PubMed] [Google Scholar]
- 39. Chen SR, Dai Y, Zhao J, Lin L, Wang Y, and Wang Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol 9: 104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y, Zhao W, Liu C, Meng W, Zhao T, Bhattacharya SK, and Sun Y. Molecular and cellular effect of angiotensin 1–7 on hypertensive kidney disease. Am J Hypertens 32: 460–467, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen YL, Fan J, Cao L, Han TL, Zeng M, Xu Y, Ling Z, and Yin Y. Unique mechanistic insights into the beneficial effects of angiotensin-(1–7) on the prevention of cardiac fibrosis: a metabolomic analysis of primary cardiac fibroblasts. Exp Cell Res 378: 158–170, 2019 [DOI] [PubMed] [Google Scholar]
- 42. Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Cheng Q, Li C, Yang CF, Zhong YJ, Wu D, Shi L, Chen L, Li YW, and Li L. Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-beta1/Smad and NOX4/ROS pathways. Chem Biol Interact 299: 131–139, 2019 [DOI] [PubMed] [Google Scholar]
- 44. Chilosi M, Doglioni C, Murer B, and Poletti V. Epithelial stem cell exhaustion in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 27: 7–18, 2010 [PubMed] [Google Scholar]
- 45. Chong LW, Hsu YC, Lee TF, Lin Y, Chiu YT, Yang KC, Wu JC, and Huang YT. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol 15: 22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chrusciel P, Rysz J, and Banach M. Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs 74: 971–980, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung S, Kim S, Kim M, Koh ES, Shin SJ, Park CW, Chang YS, and Kim HS. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS One 12: e0181757, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, and Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol 50: 248–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cifuentes-Pagano ME, Meijles DN, and Pagano PJ. Nox inhibitors & therapies: rational design of peptidic and small molecule inhibitors. Curr Pharm Des 21: 6023–6035, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coalson JJ. The ultrastructure of human fibrosing alveolitis. Virchows Arch A Pathol Anat Histol 395: 181–199, 1982 [DOI] [PubMed] [Google Scholar]
- 51. Cong X, Li Y, Lu N, Dai Y, Zhang H, Zhao X, and Liu Y. Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Mol Med Rep 9: 2528–2532, 2014 [DOI] [PubMed] [Google Scholar]
- 52. Coppe JP, Desprez PY, Krtolica A, and Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cordero-Espinoza L and Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest 128: 85–96, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, and Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Dai E, Zhang L, Ye L, Wan S, Feng L, Qi Q, Yao F, and Li Z. Hepatic expression of cannabinoid receptors CB1 and CB2 correlate with fibrogenesis in patients with chronic hepatitis B. Int J Infect Dis 59: 124–130, 2017 [DOI] [PubMed] [Google Scholar]
- 56. Dana N, Vaseghi G, and Haghjooy Javanmard S. PPAR gamma agonist, pioglitazone, suppresses melanoma cancer in mice by inhibiting TLR4 signaling. J Pharm Pharm Sci 22: 418–423, 2019 [DOI] [PubMed] [Google Scholar]
- 57. Del Campo JA, Gallego P, and Grande L. Role of inflammatory response in liver diseases: therapeutic strategies. World J Hepatol 10: 1–7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Diebold I, Petry A, Hess J, and Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Domecq JP, Prutsky G, Elraiyah T, Wang Z, Mauck KF, Brito JP, Undavalli C, Sundaresh V, Prokop LJ, Montori VM, and Murad MH.. Medications affecting the biochemical conversion to type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2019. [Epub ahead of print]; DOI: 10.1210/jc.2019-01269 [DOI] [PubMed] [Google Scholar]
- 60. Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, and Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 135: 642–659, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Dorotea D, Kwon G, Lee JH, Saunders E, Bae YS, Moon SH, Lee SJ, Cha DR, and Ha H. A pan-NADPH oxidase inhibitor ameliorates kidney injury in type 1 diabetic rats. Pharmacology 102: 180–189, 2018 [DOI] [PubMed] [Google Scholar]
- 62. Dos Santos RL, Dellacqua LO, Delgado NT, Rouver WN, Podratz PL, Lima LC, Piccin MP, Meyrelles SS, Mauad H, Graceli JB, and Moyses MR. Pomegranate peel extract attenuates oxidative stress by decreasing coronary angiotensin-converting enzyme (ACE) activity in hypertensive female rats. J Toxicol Environ Health A 79: 998–1007, 2016 [DOI] [PubMed] [Google Scholar]
- 63. Drummond GR and Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25: 452–463, 2014 [DOI] [PubMed] [Google Scholar]
- 64. Edwards NL. Febuxostat: a new treatment for hyperuricaemia in gout. Rheumatology (Oxford) 48 Suppl 2: ii15–ii19, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Elwej A, Ben Salah G, Kallel C, Fakhfakh F, Zeghal N, and Ben Amara I. Protective effects of pomegranate peel against hematotoxicity, chromosomal aberrations, and genotoxicity induced by barium chloride in adult rats. Pharm Biol 54: 964–974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, and Schwartz DA. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 96: 1567–1591, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ezquerro IJ, Lasarte JJ, Dotor J, Castilla-Cortazar I, Bustos M, Penuelas I, Blanco G, Rodriguez C, Lechuga Mdel C, Greenwel P, Rojkind M, Prieto J, and Borras-Cuesta F. A synthetic peptide from transforming growth factor beta type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine 22: 12–20, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Fan TWM, Bruntz RC, Yang Y, Song H, Chernyavskaya Y, Deng P, Zhang Y, Shah PP, Beverly LJ, Qi Z, Mahan AL, Higashi RM, Dang CV, and Lane AN. De novo synthesis of serine and glycine fuels purine nucleotide biosynthesis in human lung cancer tissues. J Biol Chem 294: 13464–13477, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fang L, Ellims AH, Beale AL, Taylor AJ, Murphy A, and Dart AM. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res 9: 5063–5073, 2017 [PMC free article] [PubMed] [Google Scholar]
- 70. Farkas L, Gauldie J, Voelkel NF, and Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1–15, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Fayed MR, El-Naga RN, Akool ES, and El-Demerdash E. The potential antifibrotic impact of apocynin and alpha-lipoic acid in concanavalin A-induced liver fibrosis in rats: role of NADPH oxidases 1 and 4. Drug Discov Ther 12: 58–67, 2018 [DOI] [PubMed] [Google Scholar]
- 72. Fazzi F, Njah J, Di Giuseppe M, Winnica DE, Go K, Sala E, St Croix CM, Watkins SC, Tyurin VA, Phinney DG, Fattman CL, Leikauf GD, Kagan VE, and Ortiz LA. TNFR1/phox interaction and TNFR1 mitochondrial translocation Thwart silica-induced pulmonary fibrosis. J Immunol 192: 3837–3846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fern RJ, Yesko CM, Thornhill BA, Kim HS, Smithies O, and Chevalier RL. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest 103: 39–46, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fischer UA, Jaksch AV, Carle R, and Kammerer DR. Determination of lignans in edible and nonedible parts of pomegranate (Punica granatum L.) and products derived therefrom, particularly focusing on the quantitation of isolariciresinol using HPLC-DAD-ESI/MSn. J Agric Food Chem 60: 283–292, 2012 [DOI] [PubMed] [Google Scholar]
- 75. Folmes CD, Clanachan AS, and Lopaschuk GD. Fatty acid oxidation inhibitors in the management of chronic complications of atherosclerosis. Curr Atheroscler Rep 7: 63–70, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, and Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98: 699–710, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, Ogawa H, and Kim-Mitsuyama S. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens 28: 340–352, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Fulmer JD, Roberts WC, von Gal ER, and Crystal RG. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest 63: 665–676, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gallwitz B. Review of sitagliptin phosphate: a novel treatment for type 2 diabetes. Vasc Health Risk Manag 3: 203–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gan D, Zhang W, Huang C, Chen J, He W, Wang A, Li B, and Zhu X. Ursolic acid ameliorates CCl4-induced liver fibrosis through the NOXs/ROS pathway. J Cell Physiol 233: 6799–6813, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garcia CK. Insights from human genetic studies of lung and organ fibrosis. J Clin Invest 128: 36–44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garcia-Santos D, Schranzhofer M, Bergeron R, Sheftel AD, and Ponka P. Extracellular glycine is necessary for optimal hemoglobinization of erythroid cells. Haematologica 102: 1314–1323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gasparini G, Cozzani E, and Parodi A. Interleukin-4 and interleukin-13 as possible therapeutic targets in systemic sclerosis. Cytokine 125: 154799, 2019 [DOI] [PubMed] [Google Scholar]
- 84. Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, and Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A 98: 11961–11966, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gill PS and Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Goh KY, He L, Song J, Jinno M, Rogers AJ, Sethu P, Halade GV, Rajasekaran NS, Liu X, Prabhu SD, Darley-Usmar V, Wende AR, and Zhou L. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol 21: 101100, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gohlke P, Jurgensen T, von Kugelgen S, and Unger T. Candesartan cilexetil: development and preclinical studies. Drugs Today (Barc) 35: 105–115, 1999 [DOI] [PubMed] [Google Scholar]
- 88. Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, Ding Y, and Ma R. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol 301: C304–C315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, Agostini BA, Crum LT, Oczypok EA, Oury TA, and Houghton AM. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol 98: 143–152, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Greiber S, Munzel T, Kastner S, Muller B, Schollmeyer P, and Pavenstadt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int 53: 654–663, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Gupta S, Clarkson MR, Duggan J, and Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int 58: 1389–1399, 2000 [DOI] [PubMed] [Google Scholar]
- 92. Hajimahmoodi M, Shams-Ardakani M, Saniee P, Siavoshi F, Mehrabani M, Hosseinzadeh H, Foroumadi P, Safavi M, Khanavi M, Akbarzadeh T, Shafiee A, and Foroumadi A. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat Prod Res 25: 1059–1066, 2011 [DOI] [PubMed] [Google Scholar]
- 93. Han HJ, Lee YJ, Park SH, Lee JH, and Taub M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol 288: F988–F996, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Hasan SA, Eksteen B, Reid D, Paine HV, Alansary A, Johannson K, Gwozd C, Goring KA, Vo T, Proud D, and Kelly MM. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J Allergy Clin Immunol 131: 1663–1673, 2013 [DOI] [PubMed] [Google Scholar]
- 95. He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, and Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem 286: 15597–15607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He C, Ryan AJ, Murthy S, and Carter AB. Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem 288: 20745–20757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. He T, Xiong J, Nie L, Yu Y, Guan X, Xu X, Xiao T, Yang K, Liu L, Zhang D, Huang Y, Zhang J, Wang J, Sharma K, and Zhao J. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J Mol Med (Berl) 94: 1359–1371, 2016 [DOI] [PubMed] [Google Scholar]
- 98. Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, and Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, and Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A 103: 5060–5065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, and Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, and Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem 279: 1359–1367, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, and Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19: 761–771, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Horowitz JC, Rogers DS, Simon RH, Sisson TH, and Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 38: 78–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Iannotti FA, Silvestri C, Mazzarella E, Martella A, Calvigioni D, Piscitelli F, Ambrosino P, Petrosino S, Czifra G, Biro T, Harkany T, Taglialatela M, and Di Marzo V. The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc Natl Acad Sci U S A 111: E2472–E2481, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, and Staruschenko A. The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci Rep 7: 299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ishizawa K, Izawa-Ishizawa Y, Dorjsuren N, Miki E, Kihira Y, Ikeda Y, Hamano S, Kawazoe K, Minakuchi K, Tomita S, Tsuchiya K, and Tamaki T. Angiotensin II receptor blocker attenuates PDGF-induced mesangial cell migration in a receptor-independent manner. Nephrol Dial Transplant 25: 364–372, 2010 [DOI] [PubMed] [Google Scholar]
- 108. Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, and Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 111: E3297–E3305, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Iwata K, Matsuno K, Murata A, Zhu K, Fukui H, Ikuta K, Katsuyama M, Ibi M, Matsumoto M, Ohigashi M, Wen X, Zhang J, Cui W, and Yabe-Nishimura C. Up-regulation of NOX1/NADPH oxidase following drug-induced myocardial injury promotes cardiac dysfunction and fibrosis. Free Radic Biol Med 120: 277–288, 2018 [DOI] [PubMed] [Google Scholar]
- 110. Jager S, Trojan H, Kopp T, Laszczyk MN, and Scheffler A. Pentacyclic triterpene distribution in various plants—rich sources for a new group of multi-potent plant extracts. Molecules 14: 2016–2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jang IA, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, Park CW, Chang YS, and Choi BS. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 10: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Javkhedkar AA, Lokhandwala MF, and Banday AA. Defective nitric oxide production impairs angiotensin II-induced Na-K-ATPase regulation in spontaneously hypertensive rats. Am J Physiol Renal Physiol 302: F47–F51, 2012 [DOI] [PubMed] [Google Scholar]
- 113. Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, Thallas-Bonke V, De Vos L, Holterman CE, Coughlan MT, Power DA, Skene A, Ekinci EI, Cooper ME, Touyz RM, Kennedy CR, and Jandeleit-Dahm K. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes 66: 2691–2703, 2017 [DOI] [PubMed] [Google Scholar]
- 114. Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, Devaraj S, Shah V, Gershwin ME, Friedman SL, and Torok NJ. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology 139: 1375–1384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jo CH, Kim S, Park JS, and Kim GH. Anti-inflammatory action of sitagliptin and linagliptin in doxorubicin nephropathy. Kidney Blood Press Res 43: 987–999, 2018 [DOI] [PubMed] [Google Scholar]
- 116. Joardar S, Dewanjee S, Bhowmick S, Dua TK, Das S, Saha A, and De Feo V. Rosmarinic acid attenuates cadmium-induced nephrotoxicity via inhibition of oxidative stress, apoptosis, inflammation and fibrosis. Int J Mol Sci 20: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Johar S, Cave AC, Narayanapanicker A, Grieve DJ, and Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006 [DOI] [PubMed] [Google Scholar]
- 118. Jourdan T, Park JK, Varga ZV, Paloczi J, Coffey NJ, Rosenberg AZ, Godlewski G, Cinar R, Mackie K, Pacher P, and Kunos G. Cannabinoid-1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes Metab 20: 698–708, 2018 [DOI] [PubMed] [Google Scholar]
- 119. Ju H, Zhao S, Jassal DS, and Dixon IM. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc Res 35: 223–232, 1997 [DOI] [PubMed] [Google Scholar]
- 120. Kabirifar R, Ghoreshi ZA, Safari F, Karimollah A, Moradi A, and Eskandari-Nasab E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. Hepatobiliary Pancreat Dis Int 16: 88–95, 2017 [DOI] [PubMed] [Google Scholar]
- 121. Kanellakis P, Ditiatkovski M, Kostolias G, and Bobik A. A pro-fibrotic role for interleukin-4 in cardiac pressure overload. Cardiovasc Res 95: 77–85, 2012 [DOI] [PubMed] [Google Scholar]
- 122. Karin D, Koyama Y, Brenner D, and Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 92: 84–92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Katayama IA, Pereira RC, Dopona EP, Shimizu MH, Furukawa LN, Oliveira IB, and Heimann JC. High-salt intake induces cardiomyocyte hypertrophy in rats in response to local angiotensin II type 1 receptor activation. J Nutr 144: 1571–1578, 2014 [DOI] [PubMed] [Google Scholar]
- 124. Kaur A, Mathai SK, and Schwartz DA. Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front Med (Lausanne) 4: 154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kim HR and Kim SY. Perilla frutescens sprout extract protect renal mesangial cell dysfunction against high glucose by modulating AMPK and NADPH oxidase signaling. Nutrients 11: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, and Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, and Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, and Noble PW; ASCEND Study Group.. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014 [DOI] [PubMed] [Google Scholar]
- 129. Klahr S and Morrissey J. Angiotensin II and gene expression in the kidney. Am J Kidney Dis 31: 171–176, 1998 [DOI] [PubMed] [Google Scholar]
- 130. Klahr S and Morrissey JJ. The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int Suppl 75: S7–S14, 2000 [PubMed] [Google Scholar]
- 131. Kong M, Chen X, Lv F, Ren H, Fan Z, Qin H, Yu L, Shi X, and Xu Y. Serum response factor (SRF) promotes ROS generation and hepatic stellate cell activation by epigenetically stimulating NCF1/2 transcription. Redox Biol 26: 101302, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kwon G, Uddin MJ, Lee G, Jiang S, Cho A, Lee JH, Lee SR, Bae YS, Moon SH, Lee SJ, Cha DR, and Ha H. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 8: 74217–74232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 134. Lan T, Kisseleva T, and Brenner DA. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS One 10: e0129743, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lansky EP and Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 109: 177–206, 2007 [DOI] [PubMed] [Google Scholar]
- 136. Larrinaga G, Varona A, Perez I, Sanz B, Ugalde A, Candenas ML, Pinto FM, Gil J, and Lopez JI. Expression of cannabinoid receptors in human kidney. Histol Histopathol 25: 1133–1138, 2010 [DOI] [PubMed] [Google Scholar]
- 137. Lecru L, Desterke C, Grassin-Delyle S, Chatziantoniou C, Vandermeersch S, Devocelle A, Vernochet A, Ivanovski N, Ledent C, Ferlicot S, Dalia M, Said M, Beaudreuil S, Charpentier B, Vazquez A, Giron-Michel J, Azzarone B, Durrbach A, and Francois H. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 88: 72–84, 2015 [DOI] [PubMed] [Google Scholar]
- 138. Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, and Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 200: 377–389, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lee JH, Sarker MK, Choi H, Shin D, Kim D, and Jun HS. Lysophosphatidic acid receptor 1 inhibitor, AM095, attenuates diabetic nephropathy in mice by downregulation of TLR4/NF-kappaB signaling and NADPH oxidase. Biochim Biophys Acta Mol Basis Dis 1865: 1332–1340, 2019 [DOI] [PubMed] [Google Scholar]
- 140. Lee SH, Choi BY, Kho AR, Jeong JH, Hong DK, Lee SH, Lee SY, Lee MW, Song HK, Choi HC, and Suh SW. Protective effects of protocatechuic acid on seizure-induced neuronal death. Int J Mol Sci 19: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lejri I, Agapouda A, Grimm A, and Eckert A. Mitochondria- and oxidative stress-targeting substances in cognitive decline-related disorders: from molecular mechanisms to clinical evidence. Oxid Med Cell Longev 2019: 9695412, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Levsh O, Pluskal T, Carballo V, Mitchell AJ, and Weng JK. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J Biol Chem 294: 15193–15205, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C, and Wang H. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-kappaB signaling pathway. Mol Med Rep 11: 1120–1126, 2015 [DOI] [PubMed] [Google Scholar]
- 144. Li J, Zhang CX, Liu YM, Chen KL, and Chen G. A comparative study of anti-aging properties and mechanism: resveratrol and caloric restriction. Oncotarget 8: 65717–65729, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Li JY, Cao HY, Liu P, Cheng GH, and Sun MY. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res Int 2014: 872139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Li XZ, Ding YZ, Wu HF, Bian ZP, Xu JD, Gu CR, Chen XJ, and Yang D. Astragaloside IV prevents cardiac remodeling in the apolipoprotein E-deficient mice by regulating cardiac homeostasis and oxidative stress. Cell Physiol Biochem 44: 2422–2438, 2017 [DOI] [PubMed] [Google Scholar]
- 147. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, and Yin Y. Quercetin, inflammation and immunity. Nutrients 8: 167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lijnen P, Papparella I, Petrov V, Semplicini A, and Fagard R. Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. J Hypertens 24: 757–766, 2006 [DOI] [PubMed] [Google Scholar]
- 149. Lim JC, Lim SK, Park MJ, Kim GY, Han HJ, and Park SH. Cannabinoid receptor 1 mediates high glucose-induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. Am J Physiol Renal Physiol 301: F179–F188, 2011 [DOI] [PubMed] [Google Scholar]
- 150. Lin J, Fang L, Li H, Li Z, Lyu L, Wang H, and Xiao J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur J Pharmacol 859: 172490, 2019 [DOI] [PubMed] [Google Scholar]
- 151. Lin SL, Kisseleva T, Brenner DA, and Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, and Wang T. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-beta1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun 510: 329–333, 2019 [DOI] [PubMed] [Google Scholar]
- 153. Liu C, Luo R, Wang W, Peng Z, Johnson GVW, Kellems RE, and Xia Y. Tissue transglutaminase-mediated AT1 receptor sensitization underlies pro-inflammatory cytokine LIGHT-induced hypertension. Am J Hypertens 32: 476–485, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Liu P, Zhao H, and Luo Y. Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 8: 868–886, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liu R, Ren Y, Garvin JL, and Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 156. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lou Q, Meng X, Lao Z, Xuan L, Bai J, Hou Q, Hu G, Luo R, Tao L, and Li Z. Design, synthesis and antifibrotic activities of carbohydrate-modified 1-(substituted aryl)-5-trifluoromethyl-2(1H) pyridones. Molecules 17: 884–896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lu Q, Ma Z, Ding Y, Bedarida T, Chen L, Xie Z, Song P, and Zou MH. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-kappaB/p65 regulatory axis. Nat Commun 10: 2145, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, and Liu DP. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J 38: 1389–1398, 2017 [DOI] [PubMed] [Google Scholar]
- 160. Mackie K. Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. Int J Obes (Lond) 30 Suppl 1: S19–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 161. Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K, and Yoshizato K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem 278: 18938–18944, 2003 [DOI] [PubMed] [Google Scholar]
- 162. Maeda Y, Inoguchi T, Takei R, Hendarto H, Ide M, Inoue T, Kobayashi K, Urata H, Nishiyama A, and Takayanagi R. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig 3: 354–361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, and Mattevi A. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci U S A 114: 6764–6769, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Manna K, Mishra S, Saha M, Mahapatra S, Saha C, Yenge G, Gaikwad N, Pal R, Oulkar D, Banerjee K, and Das Saha K. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-kappaB and Nrf2 signaling system. Int J Nanomedicine 14: 1753–1777, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, Chapman HA, and Wolters PJ. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 301: L71–L78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, and Wagner DD. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med 214: 439–458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. McCarty MF, O'Keefe JH, and DiNicolantonio JJ. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection. Ochsner J 18: 81–87, 2018 [PMC free article] [PubMed] [Google Scholar]
- 168. Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX, Li W, Li Y, Zhang WY, and Li X. The angiotensin-converting enzyme 2/angiotensin (1–7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox Signal 22: 241–258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Meran S and Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92: 158–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mia S, Castor T, Musculus K, Voelkl J, Alesutan I, and Lang F. Role of AMP-activated protein kinase alpha1 in angiotensin-II-induced renal Tgfss-activated kinase 1 activation. Biochem Biophys Res Commun 476: 267–272, 2016 [DOI] [PubMed] [Google Scholar]
- 171. Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci 19: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia H, Yacoub TJ, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, and Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214: 2387–2404, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Montezano AC, De Lucca Camargo L, Persson P, Rios FJ, Harvey AP, Anagnostopoulou A, Palacios R, Gandara ACP, Alves-Lopes R, Neves KB, Dulak-Lis M, Holterman CE, de Oliveira PL, Graham D, Kennedy C, and Touyz RM. NADPH Oxidase 5 Is a Pro-contractile Nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J Am Heart Assoc 7: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, and Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 124: 2921–2934, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mukhopadhyay P, Pan H, Rajesh M, Batkai S, Patel V, Harvey-White J, Mukhopadhyay B, Hasko G, Gao B, Mackie K, and Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 160: 657–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, and Shah AM. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J Am Coll Cardiol 63: 2734–2741, 2014 [DOI] [PubMed] [Google Scholar]
- 177. Namai-Takahashi A, Sakuyama A, Nakamura T, Miura T, Takahashi J, Kurosawa R, Kohzuki M, and Ito O. Xanthine oxidase inhibitor, febuxostat ameliorates the high salt intake-induced cardiac hypertrophy and fibrosis in Dahl salt-sensitive rats. Am J Hypertens 32: 26–33, 2019 [DOI] [PubMed] [Google Scholar]
- 178. Nazari M, Ni NC, Ludke A, Li SH, Guo J, Weisel RD, and Li RK. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J Mol Cell Cardiol 94: 32–42, 2016 [DOI] [PubMed] [Google Scholar]
- 179. Negrea L. Active Vitamin D in chronic kidney disease: getting right back where we started from? Kidney Dis (Basel) 5: 59–68, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, and Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 214: 3311–3329, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ning BB, Zhang Y, Wu DD, Cui JG, Liu L, Wang PW, Wang WJ, Zhu WL, Chen Y, and Zhang T. Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacol Sin 38: 331–341, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nitta T, Kim JS, Mohuczy D, and Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology 48: 909–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Nogueira A, Pires MJ, and Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo 31: 1–22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Numaga-Tomita T, Oda S, Shimauchi T, Nishimura A, Mangmool S, and Nishida M. TRPC3 channels in cardiac fibrosis. Front Cardiovasc Med 4: 56, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pagliarulo C, De Vito V, Picariello G, Colicchio R, Pastore G, Salvatore P, and Volpe MG. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem 190: 824–831, 2016 [DOI] [PubMed] [Google Scholar]
- 186. Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, and Brenner DA. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 53: 1730–1741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V, and Bedossa P. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology 30: 968–976, 1999 [DOI] [PubMed] [Google Scholar]
- 188. Parikh P, Wicher S, Khandalavala K, Pabelick CM, Britt RD Jr., and Prakash YS. Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol 316: L826–L842, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Park HS, Jang JE, Ko MS, Woo SH, Kim BJ, Kim HS, Park HS, Park IS, Koh EH, and Lee KU. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J 40: 376–385, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, and Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 143: 225–245, 2014 [DOI] [PubMed] [Google Scholar]
- 191. Pellicoro A, Ramachandran P, Iredale JP, and Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14: 181–194, 2014 [DOI] [PubMed] [Google Scholar]
- 192. Peng ZZ, Hu GY, Shen H, Wang L, Ning WB, Xie YY, Wang NS, Li BX, Tang YT, and Tao LJ. Fluorofenidone attenuates collagen I and transforming growth factor-beta1 expression through a nicotinamide adenine dinucleotide phosphate oxidase-dependent way in NRK-52E cells. Nephrology (Carlton) 14: 565–572, 2009 [DOI] [PubMed] [Google Scholar]
- 193. Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, and Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 127: 266–274, 2005 [DOI] [PubMed] [Google Scholar]
- 194. Povero D, Busletta C, Novo E, di Bonzo LV, Cannito S, Paternostro C, and Parola M. Liver fibrosis: a dynamic and potentially reversible process. Histol Histopathol 25: 1075–1091, 2010 [DOI] [PubMed] [Google Scholar]
- 195. Prabhu SD and Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Prasanphanich AF, Arencibia CA, and Kemp ML. Redox processes inform multivariate transdifferentiation trajectories associated with TGFbeta-induced epithelial-mesenchymal transition. Free Radic Biol Med 76: 1–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Purnomo Y, Piccart Y, Coenen T, Prihadi JS, and Lijnen PJ. Oxidative stress and transforming growth factor-beta1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug Targets 13: 165–172, 2013 [DOI] [PubMed] [Google Scholar]
- 198. Pushpakumar S, Ren L, Kundu S, Gamon A, Tyagi SC, and Sen U. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci Rep 7: 6349, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Qin J, Mei WJ, Xie YY, Huang L, Yuan QJ, Hu GY, Tao LJ, and Peng ZZ. Fluorofenidone attenuates oxidative stress and renal fibrosis in obstructive nephropathy via blocking NOX2 (gp91phox) expression and inhibiting ERK/MAPK signaling pathway. Kidney Blood Press Res 40: 89–99, 2015 [DOI] [PubMed] [Google Scholar]
- 200. Raghu G, Weycker D, Edelsberg J, Bradford WZ, and Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816, 2006 [DOI] [PubMed] [Google Scholar]
- 201. Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, and Zmijewski JW. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 24: 1121–1127, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rangarajan S, Locy ML, Luckhardt TR, and Thannickal VJ. Targeted therapy for idiopathic pulmonary fibrosis: where to now? Drugs 76: 291–300, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ren Y, Carretero OA, and Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney Int 62: 525–531, 2002 [DOI] [PubMed] [Google Scholar]
- 204. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, and Collard HR; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014 [DOI] [PubMed] [Google Scholar]
- 205. Rockey DC, Du Q, and Shi Z. Smooth muscle alpha-actin deficiency leads to decreased liver fibrosis via impaired cytoskeletal signaling in hepatic stellate cells. Am J Pathol 189: 2209–2220, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Rong Y, Cao B, Liu B, Li W, Chen Y, Chen H, Liu Y, and Liu T. A novel Gallic acid derivative attenuates BLM-induced pulmonary fibrosis in mice. Int Immunopharmacol 64: 183–191, 2018 [DOI] [PubMed] [Google Scholar]
- 207. Ruiz de Azua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, Zingaretti CM, Sassmann A, Quarta C, Schwitter C, Conrad A, Wettschureck N, Vemuri VK, Makriyannis A, Hartwig J, Mendez-Lago M, Bindila L, Monory K, Giordano A, Cinti S, Marsicano G, Offermanns S, Nisoli E, Pagotto U, Cota D, and Lutz B. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest 127: 4148–4162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Salehi B, Berkay Yilmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, Akram M, Riaz M, Capanoglu E, Sharopov F, Martins N, Cho WC, and Sharifi-Rad J. Insights on the use of alpha-lipoic acid for therapeutic purposes. Biomolecules 9: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, and Sharifi-Rad J. Resveratrol: a double-edged sword in health benefits. Biomedicines 6: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, Inoue KI, Sadakane K, Ichinose T, and Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin Exp Allergy 34: 971–977, 2004 [DOI] [PubMed] [Google Scholar]
- 211. Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, and Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 7: e45285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sanders YY, Liu H, Liu G, and Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med 79: 197–205, 2015 [DOI] [PubMed] [Google Scholar]
- 213. Sassi Y, Ahles A, Truong DJ, Baqi Y, Lee SY, Husse B, Hulot JS, Foinquinos A, Thum T, Muller CE, Dendorfer A, Laggerbauer B, and Engelhardt S. Cardiac myocyte-secreted cAMP exerts paracrine action via adenosine receptor activation. J Clin Invest 124: 5385–5397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, Ito S, Hara H, Kadota T, Yanagisawa H, Hashimoto M, Utsumi H, Wakui H, Kojima J, Numata T, Kaneko Y, Odaka M, Morikawa T, Nakayama K, Kohrogi H, and Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 17: 107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, and LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Schelbert EB. Myocardial scar and fibrosis: the ultimate mediator of outcomes? Heart Fail Clin 15: 179–189, 2019 [DOI] [PubMed] [Google Scholar]
- 217. Schelling JR. Tissue transglutaminase inhibition as treatment for diabetic glomerular scarring: it's good to be glueless. Kidney Int 76: 363–365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Schluter T, Zimmermann U, Protzel C, Miehe B, Klebingat KJ, Rettig R, and Grisk O. Intrarenal artery superoxide is mainly NADPH oxidase-derived and modulates endothelium-dependent dilation in elderly patients. Cardiovasc Res 85: 814–824, 2010 [DOI] [PubMed] [Google Scholar]
- 219. Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, and Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res 105: 537–544, 2009 [DOI] [PubMed] [Google Scholar]
- 220. Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 221. Shafiq MM, Menon DV, and Victor RG. Oral direct renin inhibition: premise, promise, and potential limitations of a new antihypertensive drug. Am J Med 121: 265–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sharma K, Cook A, Smith M, Valancius C, and Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 223. Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, and Chan JS. Angiotensin-(1–7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond) 128: 649–663, 2015 [DOI] [PubMed] [Google Scholar]
- 224. Shiota N, Rysa J, Kovanen PT, Ruskoaho H, Kokkonen JO, and Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens 21: 1935–1944, 2003 [DOI] [PubMed] [Google Scholar]
- 225. Sides MD, Klingsberg RC, Shan B, Gordon KA, Nguyen HT, Lin Z, Takahashi T, Flemington EK, and Lasky JA. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor—beta1 synergistically induce epithelial—mesenchymal transition in lung epithelial cells. Am J Respir Cell Mol Biol 44: 852–862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Silva E and Soares-da-Silva P. Reactive oxygen species and the regulation of renal Na+-K+-ATPase in opossum kidney cells. Am J Physiol Regul Integr Comp Physiol 293: R1764–R1770, 2007 [DOI] [PubMed] [Google Scholar]
- 227. Smith RA, Porteous CM, Gane AM, and Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A 100: 5407–5412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, Caughey GH, Entman ML, and Frangogiannis NG. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol 205: 102–111, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Song H and Ren J. Protocatechuic acid attenuates angiotensin II-induced cardiac fibrosis in cardiac fibroblasts through inhibiting the NOX4/ROS/p38 signaling pathway. Phytother Res 33: 2440–2447, 2019 [DOI] [PubMed] [Google Scholar]
- 230. Song L, Shi S, Jiang W, Liu X, and He Y. Protective role of propofol on the kidney during early unilateral ureteral obstruction through inhibition of epithelial-mesenchymal transition. Am J Transl Res 8: 460–472, 2016 [PMC free article] [PubMed] [Google Scholar]
- 231. Sorrenti V, Randazzo CL, Caggia C, Ballistreri G, Romeo FV, Fabroni S, Timpanaro N, Raffaele M, and Vanella L. Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front Microbiol 10: 660, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Stock CJW, Michaeloudes C, Leoni P, Durham AL, Mumby S, Wells AU, Chung KF, Adcock IM, Renzoni EA, and Lindahl GE. Bromodomain and extraterminal (BET) protein inhibition restores redox balance and inhibits myofibroblast activation. Biomed Res Int 2019: 1484736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Suckau O, Gross I, Schrotter S, Yang F, Luo J, Wree A, Chun J, Baska D, Baumgart J, Kano K, Aoki J, and Brauer AU. LPA1, LPA2, LPA4, and LPA6 receptor expression during mouse brain development. Dev Dyn 248: 375–395, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sun B, Wang X, Ji Z, Wang M, Liao YP, Chang CH, Li R, Zhang H, Nel AE, and Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small 11: 2087–2097, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Sun Y, Zhang J, Zhang JQ, and Ramires FJ. Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension 35: 1078–1084, 2000 [DOI] [PubMed] [Google Scholar]
- 236. Surolia R, Li FJ, Wang Z, Li H, Dsouza K, Thomas V, Mirov S, Perez-Sala D, Athar M, Thannickal VJ, and Antony VB. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 4: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, Lee C, Parr TA, Roppe JR, Seiders TJ, Ziff J, Prasit P, Hutchinson JH, Evans JF, and Lorrain DS. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther 336: 693–700, 2011 [DOI] [PubMed] [Google Scholar]
- 238. Takemoto Y, Ramirez RJ, Yokokawa M, Kaur K, Ponce-Balbuena D, Sinno MC, Willis BC, Ghanbari H, Ennis SR, Guerrero-Serna G, Henzi BC, Latchamsetty R, Ramos-Mondragon R, Musa H, Martins RP, Pandit SV, Noujaim SF, Crawford T, Jongnarangsin K, Pelosi F, Bogun F, Chugh A, Berenfeld O, Morady F, Oral H, and Jalife J. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl Sci 1: 143–154, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Tang SG, Liu XY, Wang SP, Wang HH, Jovanovic A, and Tan W. Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress. J Pharmacol Sci 139: 311–318, 2019 [DOI] [PubMed] [Google Scholar]
- 240. Tarazona-Santos E, Machado M, Magalhaes WC, Chen R, Lyon F, Burdett L, Crenshaw A, Fabbri C, Pereira L, Pinto L, Redondo RA, Sestanovich B, Yeager M, and Chanock SJ. Evolutionary dynamics of the human NADPH oxidase genes CYBB, CYBA, NCF2, and NCF4: functional implications. Mol Biol Evol 30: 2157–2167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Taskar VS and Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc 3: 293–298, 2006 [DOI] [PubMed] [Google Scholar]
- 242. Tchkonia T, Zhu Y, van Deursen J, Campisi J, and Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123: 966–972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, and Wood JM. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br J Pharmacol 174: 1647–1669, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 245. Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, and Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003 [DOI] [PubMed] [Google Scholar]
- 246. Thannickal VJ, Wells A, and Kolb M. Idiopathic pulmonary fibrosis: idiopathic no more? Lancet Respir Med 6: 84–85, 2018 [DOI] [PubMed] [Google Scholar]
- 247. Tian Y, Li H, Qiu T, Dai J, Zhang Y, Chen J, and Cai H. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-kappaB activation. Aging Cell 18: e12858, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Tsubouchi K, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N, Kadota T, Sato N, Yoshida M, Kurita Y, Kobayashi K, Ito S, Fujita Y, Utsumi H, Yanagisawa H, Hashimoto M, Wakui H, Yoshii Y, Ishikawa T, Numata T, Kaneko Y, Asano H, Yamashita M, Odaka M, Morikawa T, Nakayama K, Nakanishi Y, and Kuwano K. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy 13: 1420–1434, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Tsuchida T and Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14: 397–411, 2017 [DOI] [PubMed] [Google Scholar]
- 250. Udi S, Hinden L, Earley B, Drori A, Reuveni N, Hadar R, Cinar R, Nemirovski A, and Tam J. Proximal tubular cannabinoid-1 receptor regulates obesity-induced CKD. J Am Soc Nephrol 28: 3518–3532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, and Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 275: L1192–L1199, 1998 [DOI] [PubMed] [Google Scholar]
- 252. van den Borne SW, Isobe S, Zandbergen HR, Li P, Petrov A, Wong ND, Fujimoto S, Fujimoto A, Lovhaug D, Smits JF, Daemen MJ, Blankesteijn WM, Reutelingsperger C, Zannad F, Narula N, Vannan MA, Pitt B, Hofstra L, and Narula J. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC Cardiovasc Imaging 2: 187–198, 2009 [DOI] [PubMed] [Google Scholar]
- 253. Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J, and Wu B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-kappaB p65 axis. Sci Rep 9: 323, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Wang X, Wu X, Zhang A, Wang S, Hu C, Chen W, Shen Y, Tan R, Sun Y, and Xu Q. Targeting the PDGF-B/PDGFR-beta Interface with Destruxin A5 to Selectively Block PDGF-BB/PDGFR-betabeta signaling and attenuate liver fibrosis. EBioMedicine 7: 146–156, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Wang Z, Zhang J, Wang L, Li W, Chen L, Li J, Zhao D, Zhang H, and Guo X. Glycine mitigates renal oxidative stress by suppressing Nox4 expression in rats with streptozotocin-induced diabetes. J Pharmacol Sci 137: 387–394, 2018 [DOI] [PubMed] [Google Scholar]
- 256. Wells AU, Brown KK, Flaherty KR, Kolb M, and Thannickal VJ; IPF Consensus Working Group. What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J 51: pii: , 2018 [DOI] [PubMed] [Google Scholar]
- 257. Wells AU, Brown KK, Flaherty KR, Kolb M, and Thannickal VJ; IPF Consensus Working Group; IPF Consensus Working Group. Idiopathic interstitial pneumonia or idiopathic interstitial pneumonitis: what's in a name? Eur Respir J 53: pii: , 2019 [DOI] [PubMed] [Google Scholar]
- 258. Wells AU, Brown KK, Flaherty KR, Kolb M, and Thannickal VJ; IPF Consensus Working Group; IPF Consensus Working Group. Pulmonary fibrosis: “idiopathic” is not “cryptogenic”. Eur Respir J 53: pii: , 2019 [DOI] [PubMed] [Google Scholar]
- 259. Wheaton AK, Velikoff M, Agarwal M, Loo TT, Horowitz JC, Sisson TH, and Kim KK. The vitronectin RGD motif regulates TGF-beta-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol 310: L1206–L1217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand 179: 217–223, 2003 [DOI] [PubMed] [Google Scholar]
- 261. Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Winiarska K, Grabowski M, and Rogacki MK. Inhibition of renal gluconeogenesis contributes to hypoglycaemic action of NADPH oxidase inhibitor, apocynin. Chem Biol Interact 189: 119–126, 2011 [DOI] [PubMed] [Google Scholar]
- 263. Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, and Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int 76: 32–43, 2009 [DOI] [PubMed] [Google Scholar]
- 264. Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, Maher TM, Molina-Molina M, Noble PW, Raghu G, Richeldi L, Schwarz MI, Selman M, Wuyts WA, and Schwartz DA. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med 6: 154–160, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Wu B, Feng JY, Yu LM, Wang YC, Chen YQ, Wei Y, Han JS, Feng X, Zhang Y, Di SY, Ma ZQ, Fan CX, and Ha XQ. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br J Pharmacol 175: 4137–4153, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Xiang K, Liu G, Zhou YJ, Hao HZ, Yin Z, He AD, Da XW, Xiang JZ, Wang JL, and Ming ZY. 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) attenuates human platelet aggregation, secretion and spreading in vitro. Thromb Res 133: 211–217, 2014 [DOI] [PubMed] [Google Scholar]
- 267. Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, and Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Xiong D, Hu W, Ye ST, and Tan YS. Isoliquiritigenin alleviated the Ang II-induced hypertensive renal injury through suppressing inflammation cytokines and oxidative stress-induced apoptosis via Nrf2 and NF-kappaB pathways. Biochem Biophys Res Commun 506: 161–168, 2018 [DOI] [PubMed] [Google Scholar]
- 269. Xu J, Peng Y, Zeng Y, Hua YQ, and Xu XL. 2, 3, 4′, 5-tetrahydroxystilbene-2-0-beta-d glycoside attenuates age- and diet-associated non-alcoholic steatohepatitis and atherosclerosis in LDL receptor knockout mice and its possible mechanisms. Int J Mol Sci 20: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Xu S, Chamseddine AH, Carrell S, and Miller FJ Jr. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol 2: 642–650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Xu S, Liu J, Shi J, Wang Z, and Ji L. 2,3,4′,5-tetrahydroxystilbene-2-O-beta-D-glucoside exacerbates acetaminophen-induced hepatotoxicity by inducing hepatic expression of CYP2E1, CYP3A4 and CYP1A2. Sci Rep 7: 16511, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Xu Z, Li W, Han J, Zou C, Huang W, Yu W, Shan X, Lum H, Li X, and Liang G. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2). Sci Rep 7: 44911, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Yadav D, Hertan HI, Schweitzer P, Norkus EP, and Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol 97: 2634–2639, 2002 [DOI] [PubMed] [Google Scholar]
- 274. Yang F, Chung AC, Huang XR, and Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension 54: 877–884, 2009 [DOI] [PubMed] [Google Scholar]
- 275. Yang M, Huang H, Li J, Huang W, and Wang H. Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen 15: 817–824, 2007 [DOI] [PubMed] [Google Scholar]
- 276. Yang Q, Zhang P, Liu T, Zhang X, Pan X, Cen Y, Liu Y, Zhang H, and Chen X. Magnesium isoglycyrrhizinate ameliorates radiation-induced pulmonary fibrosis by inhibiting fibroblast differentiation via the p38MAPK/Akt/Nox4 pathway. Biomed Pharmacother 115: 108955, 2019 [DOI] [PubMed] [Google Scholar]
- 277. Yao L, Conforti F, Hill C, Bell J, Drawater L, Li J, Liu D, Xiong H, Alzetani A, Chee SJ, Marshall BG, Fletcher SV, Hancock D, Coldwell M, Yuan X, Ottensmeier CH, Downward J, Collins JE, Ewing RM, Richeldi L, Skipp P, Jones MG, Davies DE, and Wang Y. Paracrine signalling during ZEB1-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ 26: 943–957, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, and Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745–750, 2001 [DOI] [PubMed] [Google Scholar]
- 279. Yu SS, Chen B, Huang CK, Zhou JJ, Huang X, Wang AJ, Li BM, He WH, and Zhu X. Ursolic acid suppresses TGF-beta1-induced quiescent HSC activation and transformation by inhibiting NADPH oxidase expression and Hedgehog signaling. Exp Ther Med 14: 3577–3582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Zhang M, Perino A, Ghigo A, Hirsch E, and Shah AM. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18: 1024–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Zhang MZ, Wang X, Yang H, Fogo AB, Murphy BJ, Kaltenbach R, Cheng P, Zinker B, and Harris RC. Lysophosphatidic acid receptor antagonism protects against diabetic nephropathy in a type 2 diabetic model. J Am Soc Nephrol 28: 3300–3311, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, and Abboud HE. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 131: 643–655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Zhao X, Li R, Liu Y, Zhang X, Zhang M, Zeng Z, Wu L, Gao X, Lan T, and Wang Y. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Arch Biochem Biophys 629: 1–7, 2017 [DOI] [PubMed] [Google Scholar]
- 284. Zhao Z and Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem 109: 691–702, 2008 [DOI] [PubMed] [Google Scholar]
- 285. Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, Kim KJ, duBois RM, Crandall ED, Beers MF, and Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 45: 498–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhou J, Cheng H, Wang Z, Chen H, Suo C, Zhang H, Zhang J, Yang Y, Geng L, Gu M, and Tan R. Bortezomib attenuates renal interstitial fibrosis in kidney transplantation via regulating the EMT induced by TNF-alpha-Smurf1-Akt-mTOR-P70S6K pathway. J Cell Mol Med 23: 5390–5402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhou X, Tang L, Xu Y, Zhou G, and Wang Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol 151: 27–43, 2014 [DOI] [PubMed] [Google Scholar]
- 288. Zhu K, Kakehi T, Matsumoto M, Iwata K, Ibi M, Ohshima Y, Zhang J, Liu J, Wen X, Taye A, Fan C, Katsuyama M, Sharma K, and Yabe-Nishimura C. NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Radic Biol Med 83: 21–30, 2015 [DOI] [PubMed] [Google Scholar]
- 289. Zhuo JL and Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides 32: 1551–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Zimmerman D and Burns KD. Angiotensin-(1–7) in kidney disease: a review of the controversies. Clin Sci (Lond) 123: 333–346, 2012 [DOI] [PubMed] [Google Scholar]