Abstract
Purpose
We report the novel use of 3-charge coupled device camera technology to infer tissue oxygenation. The technique can aid surgeons to reliably differentiate vascular structures and noninvasively assess laparoscopic intraoperative changes in renal tissue perfusion during and after warm ischemia.
Materials and Methods
We analyzed select digital video images from 10 laparoscopic partial nephrectomies for their individual 3-charge coupled device response. We enhanced surgical images by subtracting the red charge coupled device response from the blue response and overlaying the calculated image on the original image. Mean intensity values for regions of interest were compared and used to differentiate arterial and venous vasculature, and ischemic and nonischemic renal parenchyma.
Results
The 3-charge coupled device enhanced images clearly delineated the vessels in all cases. Arteries were indicated by an intense red color while veins were shown in blue. Differences in mean region of interest intensity values for arteries and veins were statistically significant (p >0.0001). Three-charge coupled device analysis of pre-clamp and post-clamp renal images revealed visible, dramatic color enhancement for ischemic vs nonischemic kidneys. Differences in the mean region of interest intensity values were also significant (p <0.05).
Conclusions
We present a simple use of conventional 3-charge coupled device camera technology in a way that may provide urological surgeons with the ability to reliably distinguish vascular structures during hilar dissection, and detect and monitor changes in renal tissue perfusion during and after warm ischemia.
Keywords: kidney, nephrectomy, video-assisted surgery, laparoscopy, ischemia
Since widespread introduction of laparoscopic surgery in the early 1990s and robotics in early 2000, urological surgeons have offered their patients many safe, minimally invasive choices to treat complex urological diseases. Today minimally invasive nephron sparing surgery is generating much attention due to postoperative morbidity,1 renal function preservation and 5-year oncologic outcomes2–4 comparable to those of the standard open approach at several high volume academic centers.
Contemporary accomplishments in laparoscopic urology are closely coupled to steady upgrading in video endoscopic equipment, instrumentation and hemostatic agents as well as refinement in surgical technique.5,6 Innovations in optical imaging, video cameras and digital technology have decreased the steep learning curve associated with laparoscopic surgery. A high resolution, magnified image of the operative field is a substantial advantage to the laparoscopic surgeon.5
Despite numerous advantages of laparoscopy, limitations exist due to its 2-dimensional view, lack of tactile feedback and poor surgeon ability to detect tissue perfusion and ischemia. In renal surgery surgeons frequently identify arterial and venous anomalies at the level of the renal hilum, and preoperative imaging can miss early branching and/or aberrant vessels to and from the kidney. Reliable identification and control of all vessels are paramount for successful operative results. Also, in patients with familial renal tumors significant perihilar scarring and fibrosis can make repeat LPN difficult and time-consuming.7 Thus, we propose that the 3-CCD camera may help further define anatomy and provide another tool to assist the laparoscopic surgeon.
As mentioned, intraoperative monitoring of renal perfusion is a significant limitation of LPN. Monitoring during LPN is currently limited to visual clues, such as increased pallor and/or mottling. Currently to our knowledge no immediate, tissue specific method exists to monitor warm ischemia during and after vascular clamping. While some biomarkers such as myoglobin, creatinine kinase-MB, and troponin I and T8 are used as indicators of ischemia, the results of these tests are neither immediate nor easily accessible during a surgical procedure. These technical limitations led us to examine 3-CCD enhanced imaging technology to monitor renal perfusion during and after warm ischemia.
We previously used 3-CCD technology during laparoscopic donor nephrectomy to assist with blood vessel identification and measure tissue oxygenation.9,10 Three-CCD contrast enhancement serves as a tool to extrapolate tissue oxygenation from the hemoglobin absorbance detected by the red and blue channels of the 3-CCD camera. Figure 1 shows how this innovative camera design provides a more sensitive color palate and perception of detail.11,12 A digital converter captures each voltage signal as an image and translates voltage values in numbers or pixels, including information on color, light intensity and contrast. These variables are then modified using image processing software in the camera or a computer and may potentially indicate organ perfusion. Thus, this technology provides a possible means to differentiate vessels and objectively monitor renal ischemia during LPN.
Figure 1.
Reflected light in abdominal cavity is collected by laparoscope and separated into 3 individual responses by prisms, which diffract light into red, green and blue visible light regions, respectively. Wavelength filtered images are detected by 3 individual monochrome CCDs. Surgeon observes composite of 3 CCD responses on high definition monitor in operating room.
MATERIALS AND METHODS
Laparoscopic Partial Nephrectomy
Informed consent was obtained from the 10 patients undergoing LPN. All patients were participants in an institutional review board approved protocol in compliance with all applicable federal regulations governing the protection of human subjects. The 10 LPNs were done by a single surgeon between January 2005 and February 2007. Nine cases were performed to resect renal cell carcinoma and 1 was done to remove an oncocytic neoplasm. Tumors in each case were 1.9 to 3 cm. Cases were recorded on DVD-R media using a laparoscopic tower equipped with a 3-CCD camera attached to a 10 mm 30-degree laparoscope.
Analysis
Three-CCD camera
We used images from the 10 videotaped LPN cases to calculate mean intensity values from the 3-CCD camera, as described previously.9,10 Briefly, we extracted select video images before and after renal arterial clamping as uncompressed TIFF files using Adobe® Premier® 6.0. Using MATLAB® software and in-house written scripts we subtracted the blue CCD response from the red CCD response, where SRed is the absorbance signal over the wavelength range of approximately 545 to 635 nm and SBlue is the absorbance signal over the wavelength range of approximately 415 to 505 nm. The oxyhemoglobin spectrum is red shifted relative to the spectrum of deoxyhemoglobin (fig. 2). By subtracting the red CCD response from the blue CCD response, images were enhanced to compare renal tissue before and after arterial clamping as well as arterial and venous vessels.13
Figure 2.
Regions representing individual CCD responses (blue, green and red curves, respectively). Solid lines represent oxygenated (HbO2) and deoxygenated (Hb) hemoglobin with absorbance maxima of 416, 541 and 577, and 430 and 556 nm, respectively.
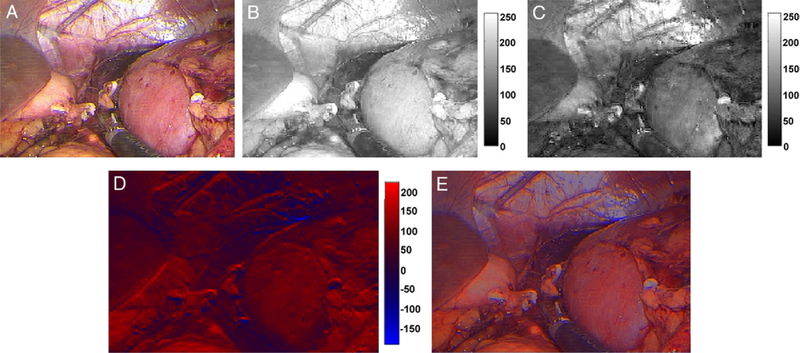
The resulting image was plotted in a modified color scale and the calculated image was overlaid on the original extracted laparoscopic image, allowing complete visual registry of the organs along with enhancement (fig. 3). A pre-clamp image of the kidney was split into the individual red, green and blue CCD responses (fig. 3, A). The blue CCD response was subtracted from the red CCD response, yielding an image with primarily red tones (fig. 3, B to D). A somewhat transparent calculated image was then overlaid on the original image for the final enhanced image (fig. 3, E).
Figure 3.
Original laparoscopic image with kidney clearly visible at right (A), and response detected by red (B) and blue (C) CCDs in grayscale. Calculated image created by subtracting blue from red CCD response (D) is shown as modified color scale. Red areas indicate regions with smallest CCD response difference, that is oxygenated, while blue areas indicate regions with greatest difference, that is deoxygenated. Calculated image was overlaid on original image to obtain enhanced image (E).
In each case mean values for the ROI in the images were derived from the calculated images using rectangular ROIs containing about 100 to 2,000 pixels for vessels and about 1,000 to 6,000 pixels for kidneys. Due to almost constant renal reorientation the size of the rectangle was not consistent among images. Vessel ROIs were intensity normalized to the entire image while renal ROIs were not. For vessel differentiation vessels must be distinguished from other tissue and, thus, values are intensity normalized. To monitor the kidney the calculated values only need to be relative to the previous image (before vs after clamping) so that normalization is not required. To further evaluate renal ischemia we then converted mean renal ROI intensity values to SaO2 using the formula, SaO2 (x) = mean ROI − 28.231555/0.866275. This formula was derived from pig calibration data,10 in which mean ROI values calculated for kidneys were directly correlated with SaO2 values derived from blood gas measurements of renal artery blood draws.
Cases were not considered for analysis if images indicated that glare saturated the camera response, the angle of the laparoscope provided inadequate illumination, the camera was not properly white balanced, or the kidney or vessels were not sufficiently defatted and exposed.
Statistics
We used Student’s t test to determine significant differences between mean ROI intensity values. Means were considered significantly different at p <0.05. Normally for a dependent sample such as a kidney before and after vessel clamping the paired t test would be used. However, in these cases we used the unpaired t test to compare the mean ROI intensity values of ischemic and nonischemic kidneys due to inconsistent feature orientation and illumination. Since all conditions for vessel images were equal for artery and vein, vessel mean ROI intensity values were evaluated using the paired t test.
RESULTS
Vessel Differentiation
We calculated and compared mean ROI intensity values for the artery and vein in each case. Figure 4, C shows mean ± SD ROI intensity values for 10 cases. We noted a clear difference between values calculated for the renal vein and the renal artery(s). Figure 4, A shows a case with multiple arteries. All vessels were dissected with the vein on the left side and the 2 arteries on the right side. The 3-CCD enhanced image clearly showed the vein as blue and each artery as red with a mean ROI intensity of 48.3 ± 1.3, 58.6 ± 6.1 and 66.9 ± 6.5 arbitrary units, respectively (fig. 4, B). Figure 4, C shows mean ROI intensity values with a clear difference between values calculated for the renal vein and the renal artery(s).
Figure 4.
Original image obtained and shown by laparoscopic tower reveals renal vein and 2 renal arteries (A). On enhanced image colored boxes indicating each ROI for which we calculated mean ROI intensity values, including 48.3 ± 1.3 (white boxes), 58.6 ± 6.1 (yellow boxes) and 66.9 ± 6.5 (green boxes) (B). Mean ROI intensity values in all 10 cases revealed clear distinction between venous and arterial vessels (p <0.0001) (C). Dotted line indicates arbitrary threshold value. a.u., arbitrary units.
Renal Ischemia
We also performed 3-CCD analysis of renal parenchyma before and after vessel clamping as well as after the clamps were released and the kidney was allowed to reperfuse. Calculated mean ROI intensity was significantly different before vs after clamping (83.30 ± 6.57 vs 27.11 ± 5.49 arbitrary units). Mean ROI after reperfusion was 76.42 ± 10.60 arbitrary units, which did not statistically differ from the pre-clamp value. Figure 5, A and B show the dramatic spectral response of the 3-CCD camera to renal ischemia after vascular clamping. Figure 5, C shows results in all 10 cases. There was a clear difference between mean ROI intensity values in the kidneys before and after vessel clamping. Mean ROI intensity values converted to SaO2 were 63.47% before clamping, 0% after clamping and 55.63% for reperfusion.
Figure 5.
Boxes indicate ROIs (A and B). Mean ROI intensity values of baseline renal image before vessel clamping and ischemic kidney image after clamping was 83.30 ± 6.57 (A) and 27.42 ± 5.49 (B). Mean ROI intensity value measured after clamp was released was 76.42 ± 10.60 (C). a.u., arbitrary units.
DISCUSSION
LPN is rapidly becoming established as the standard of care for renal tumors less than 4 cm.14 However, this procedure is technically challenging and its widespread use has been limited to surgeons with advanced laparoscopic skills at high volume institutions. Original reports of LPN were limited to small, superficial, exophytic, noninfiltrating tumors at anatomically favorable, peripheral, nonhilar sites. Mastery and adaptation of surgical technique, and appropriate experience as well as improved instrumentation, hemostatic agents and video endoscopic imaging technology have given some urologists confidence to continually expand LPN indications. In 2006 Gill et al reported the feasibility of LPN for centrally located tumors in 154 patients15 and in 25 patients with hilar tumors16 in whom perioperative outcomes were comparable to those of peripheral tumors. In a retrospective study Richstone et al also reported the safe performance of LPN for hilar tumors in 17 patients.17 Repeat partial nephrectomy for ipsilateral tumor has also been done successfully at experienced centers.7,18
As more complex laparoscopic procedures are being performed, it is often necessary to interrupt renal blood flow via pedicle clamping to safely repair collecting system and parenchymal defects. Unfortunately renal hypothermia and its proven benefit to protect the kidney from ischemic damage during open partial nephrectomy have not been widely used in LPN.19 Thus, the longer warm ischemia time that is typical during LPN is one of the greatest issues and risks of the case.20
Warm ischemia time was recently recognized as the strongest modifiable predictor of renal function after open and laparoscopic nephron sparing surgery.24 The strongest predictors of the postoperative glomerular filtration rate are the preoperative glomerular filtration rate, age, gender, tumor size and warm ischemia time. The accepted safe duration of warm ischemia time is 30 minutes but some studies suggest that it can be longer without significant clinical sequelae.21,22 However, those studies were limited by small size and the fact that serum creatinine was used to measure postoperative renal function outcome, which was criticized by some groups as an inadequate measurement.23 Three-CCD technology may provide a means to continuously monitor the ischemia duration and extent, and assess renal reperfusion during these cases.
The current noninvasive techniques to monitor organ function during surgery are NCLBF, pulse oximetry, fluorescein,24 laser autofluorescence imaging,13,25 erythrocyte velocity measurement12 and visible reflectance imaging.26 Ando et al performed a comparative analysis of NCBLF, pulse oximetry and fluorescein for the ability to assess tissue ischemia.27 NCBLF outperformed pulse oximetry and fluorescein in its accuracy and sensitivity to predict the viability of ischemic bowel. The disadvantage of NCLBF is that the measurement is made by a pencil probe, which is appropriate for open surgery only in its current form.
Another modality to detect tissue ischemia is laser autofluorescence imaging. This operates on the assumption that autofluorescence changes with 335 nm excitation are attributable to nicotinamide adenine dinucleotide, which accumulates in ischemic tissue.13,25 This technique is promising since it allows real-time in vivo imaging. Several important limitations are special instrumentation, including 335 nm laser illumination and optics, a liquid nitrogen cooled CCD and a control kidney to normalize image intensity.13,25 This approach cannot be easily converted to a format compatible in a laparoscopic tower.
Measuring erythrocyte velocity with a magnifying endoscope12,28 has potential application for laparoscopic surgery. However, the pencil lens probe of the endoscope can only sample and evaluate a small portion of the tissue at a time. Whole renal inspection would require a large number of sampling points, proving inefficient in a time limited scenario. While Tracy et al were able to measure global renal tissue oxygenation using visible reflectance imaging, the instrumentation was used in an open porcine model and has not yet shown laparoscopic capability.26 Thus, currently to our knowledge no noninvasive techniques are well suited to monitor the kidney during laparoscopic surgery.
We report a technique that has real-time capability with straightforward incorporation of additional software and computer interfacing. Real-time function does not require additional hardware or equipment that is not readily available in the operating suite. This technique provides a potential way to monitor oxygen saturation throughout the case. In our calculations of oxygen saturation, which was lower than one would expect, the open porcine cases on which these calculations were based may not reflect LPN cases due to the different ways in which light reflects in open and laparoscopic cases. However, there is obviously a correlation and it may currently be used to qualitatively evaluate the progression of ischemia. Also, when evaluating images, while glare proves troublesome in some images, including polarizing optics directly into the laparoscope would obviate this problem. Although the technique probes the surface of tissue, since shortwave visible light only penetrates 0.5 to 2.5 mm into tissue29 and cannot detect tissue oxygenation covered by fatty regions or other organs, this obstacle is typically negated by the need for surgical dissection.
We studied the usefulness of conventional 3-CCD camera technology to enhance blood vessel visualization during hilar dissection and measure tissue ischemia in 10 LPN cases. This study shows a noninvasive use of conventional camera technology available in most laparoscopic camera systems. A noted limitation of our study is digital image processing after LPN, which can be overcome by designing cameras with built-in software analysis and 3-CCD response on-off capability. This would allow real-time enhancement capacity and renal perfusion monitoring. Work is currently in progress to provide this real-time application of 3-CCD technology. After real-time capability is implemented, the ability to dynamically assess the renal parenchyma and identify renal vessels should decrease the learning curve associated with LPN, improve operative time, and possibly decrease intraoperative and postoperative outcomes such as bleeding or renal ischemia. An example of the usefulness of this approach concerns hilar dissection. By taking advantage of the ability to differentiate arteries from other structures, extended dissection surrounding secondary or tertiary renal arteries may be avoided, thus improving achievement of the mentioned goal of an improved operative outcome.
CONCLUSIONS
Practical application of modern camera and video technologies has revolutionized laparoscopic surgery. Our novel technique of using readily available 3-CCD cameras for reliable vascular differentiation and tissue oxygenation may aid experienced laparoscopic urologists to treat difficult, complex renal tumors with more confidence and speed. Real-time on-off switch application of this modality may result in less operative time, decreased bleeding and a briefer learning curve for LPN. Applying the 3-CCD spectral response to renal oxygenation may potentially enable noninvasive monitoring of renal tissue perfusion during and after warm ischemia.
Acknowledgments
Supported by the United States Navy Bureau of Medicine and Surgery under the Medical Development Program (PE 0604771N), Office of Naval Research work unit No. 604771N.0933. 001.A0812, Combat Wound Initiative Program and intramural program of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Abbreviations and Acronyms
- CCD
charge coupled device
- LPN
laparoscopic partial nephrectomy
- NCLBF
noncontact laser tissue blood flowmeter
- ROI
region of interest
- SaO2
oxygen saturation
Contributor Information
Nicole J. Crane, Department of Regenerative Medicine, Naval Medical Research Center, Bethesda, Maryland.
Suzanne M. Gillern, Department of Regenerative Medicine, Naval Medical Research Center, Bethesda, Maryland; Department of Surgery, Walter Reed Army Medical Center, Washington, D.C..
Kambiz Tajkarimi, Silver Spring and Urologic Oncology, National Cancer Institute, Bethesda, Maryland; Department of Urology, George Washington University, Washington, D.C..
Ira W. Levin, Laboratory of Chemical Physics, National Institutes of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland
Peter A. Pinto, Silver Spring and Urologic Oncology, National Cancer Institute, Bethesda, Maryland
Eric A. Elster, Department of Regenerative Medicine, Naval Medical Research Center, Bethesda, Maryland; National Institutes of Health and Department of Surgery, Uniformed Service University, Bethesda, Maryland.
REFERENCES
- 1.Link RE, Bhayani SB, Allaf ME et al. : Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy performed for renal mass. J Urol 2005; 173: 1690. [DOI] [PubMed] [Google Scholar]
- 2.Lane BR and Gill IS: 5-Year outcomes of laparoscopic partial nephrectomy. J Urol 2007; 177: 70. [DOI] [PubMed] [Google Scholar]
- 3.Allaf ME, Bhayani SB, Rogers C et al. : Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol 2004; 172: 871. [DOI] [PubMed] [Google Scholar]
- 4.Permpongkosol S, Bagga HS, Romero FR et al. : Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol 2006; 176: 1984. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy TJ: Impact of video on endourology. J Endourol 1987; 1: 75. [Google Scholar]
- 6.Litwiller DA and Preminger GM: Advances in electronic imagery for laparoscopy. J Endourol 1993; 7: 5195. [Google Scholar]
- 7.Johnson A, Sudarshan S, Liu J et al. : Feasibility and outcomes of repeat partial nephrectomy. J Urol 2008; 180: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozbas H, Yildirir A and Muderrisoglu H: Cardiac enzymes, renal failure and renal transplantation. Clin Med Res 2006; 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane NJ, McHone B, Hawksworth J et al. : Enhanced surgical imaging: laparoscopic vessel identification and assessment of tissue oxygenation. J Am Coll Surg 2008; 206: 1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane NJ, Pinto PA, Hale D et al. : Non-invasive monitoring of tissue oxygenation during laparoscopic donor nephrectomy. BMC Surg 2008; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna G and Cuschieri A: Image display technology and image processing. World J Surg 2001; 25: 1419. [DOI] [PubMed] [Google Scholar]
- 12.Knyrim K, Seidlitz H, Vakil N et al. : Perspectives in “electronic endoscopy.” Past, present and future of fibers and CCDs in medical endoscopes. Endoscopy, suppl., 1990; 221: 2. [DOI] [PubMed] [Google Scholar]
- 13.Crane NJ, Kansal NS, Dhanani N et al. : Visual enhancement of laparoscopic nephrectomies using the 3-CCD camera. Proc SPIE 2006; 6081: 60810G1. [Google Scholar]
- 14.Eng MK, Bernstein AJ, Katz MH et al. : Impact of renal lesion size on perioperative and pathologic outcomes in patients undergoing laparoscopic partial nephrectomy. J Endourol 2009; 23: 439. [DOI] [PubMed] [Google Scholar]
- 15.Frank I, Colombo JR Jr, Rubinstein M et al. : Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol 2006; 175: 849. [DOI] [PubMed] [Google Scholar]
- 16.Gill IS, Colombo JR Jr, Frank I et al. : Laparoscopic partial nephrectomy for hilar tumors. J Urol 2005; 174: 850. [DOI] [PubMed] [Google Scholar]
- 17.Richstone L, Montag S, Ost M et al. : Laparoscopic partial nephrectomy for hilar tumors: evaluation of short-term oncologic outcome. Urology 2008; 71: 36. [DOI] [PubMed] [Google Scholar]
- 18.Turna B, Aron M, Frota R et al. : Feasibility of laparoscopic partial nephrectomy after previous ipsilateral renal procedures. Urology 2008; 72: 584. [DOI] [PubMed] [Google Scholar]
- 19.Gill IS, Kavoussi LR, Lane BR et al. : Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007; 178: 41. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Leibovich BC, Lohse CM et al. : Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol 2005; 174: 855. [DOI] [PubMed] [Google Scholar]
- 21.Bhayani SB, Rha KH, Pinto PA et al. : Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol 2004; 172: 1264. [DOI] [PubMed] [Google Scholar]
- 22.Desai MM, Gill IS, Ramani AP et al. : The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int 2005; 95: 377. [DOI] [PubMed] [Google Scholar]
- 23.Yossepowitch O, Eggener SE, Serio A et al. : Temporary renal ischemia during nephron sparing surgery is associated with short-term but not long-term impairment in renal function. J Urol 2006; 176: 1339. [DOI] [PubMed] [Google Scholar]
- 24.Kontos MC, Garg R, Anderson FP et al. : Outcomes in patients admitted for chest pain with renal failure and troponin I elevations. Am Heart J 2005; 150: 674. [DOI] [PubMed] [Google Scholar]
- 25.Boppart SA, Deutsch TF and Rattner DW: Optical imaging technology in minimally invasive surgery. Current status and future directions. Surg Endosc 1999; 13: 718. [DOI] [PubMed] [Google Scholar]
- 26.Tracy CR, Terrell JD, Francis RP et al. : Characterization of renal ischemia using DLP® hyperspectral imaging: a pilot study comparing artery-only occlusion versus artery and vein occlusion. J Endourol 2010; 24: 321. [DOI] [PubMed] [Google Scholar]
- 27.Ando M, Ito M, Nihei Z et al. : Assessment of intestinal viability using a non-contact laser tissue blood flowmeter. Am J Surg 2000; 180: 176. [DOI] [PubMed] [Google Scholar]
- 28.Hattori R, Ono Y, Kato M et al. : Direct visualization of cortical peritubular capillary of transplanted human kidney with reperfusion injury using a magnifying endoscopy. Transplantation 2005; 79: 1190. [DOI] [PubMed] [Google Scholar]
- 29.Tuchin V: Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 2nd ed. Bellingham: SPIE Press; 2007; p 841. [Google Scholar]