Abstract
The formation of the central nervous system (CNS) involves multiple cellular and molecular interactions between neural progenitor cells (NPCs) and blood vessels to establish extensive and complex neural networks and attract a vascular supply that support their function. In this review, we discuss studies that have performed genetic manipulations of chick, fish and mouse embryos to define the spatiotemporal roles of molecules that mediate the reciprocal regulation of NPCs and blood vessels. These experiments have highlighted core functions of NPC-expressed ligands in initiating vascular growth into and within the neural tube as well as establishing the blood–brain barrier. More recent findings have also revealed indispensable roles of blood vessels in regulating NPC expansion and eventual differentiation, and specific regional differences in the effect of angiocrine signals. Accordingly, NPCs initially stimulate blood vessel growth and maturation to nourish the brain, but blood vessels subsequently also regulate NPC behaviour to promote the formation of a sufficient number and diversity of neural cells. A greater understanding of the molecular cross-talk between NPCs and blood vessels will improve our knowledge of how the vertebrate nervous system forms and likely help in the design of novel therapies aimed at regenerating neurons and neural vasculature following CNS disease or injury.
Keywords: angiogenesis, blood brain barrier, central nervous system, embryogenesis, neurogenesis, neural stem cells
Introduction
The formation of the central nervous system (CNS) begins early in development following the specification of the ectodermal germ layer [1]. The neural ectoderm develops into the neuroepithelium, which is initially comprised of a small pool of highly proliferative neural progenitor cells (NPCs) that will ultimately give rise to all glia and neurons in the adult nervous system via several NPC-derived lineages [2]. These NPC lineages generate functionally specialised neurons in a process termed neurogenesis, which is tightly regulated by both cell-intrinsic mechanisms and extrinsic signals from surrounding neural and non-neural cells. The neuroepithelium must also attract blood vessels to sustain the high metabolic demands of NPCs and their neuronal progeny. Like elsewhere in the body, vessel formation involves the proliferation and migration of endothelial cells (ECs) that form the inner layer of all blood vessels to contain the vascular lumen and the recruitment of supporting mural cells that are termed pericytes.
Here, we will review signals produced by the neuroepithelium that direct the growth and maturation of blood vessels in the CNS, as well as reciprocal roles of CNS vasculature in regulating NPC behaviour. We will particularly focus on studies that have explored relevant molecular mechanisms through the analysis of conditional mouse mutants, because this allows us to distinguish the relative contribution of specific factors in endothelial versus neural cells during neurogenesis. We will further highlight gaps in our current knowledge about the molecular interplay between both developing systems and provide an outlook on potential directions for future research in this field.
Role of NPCs in brain vascularisation and vascular maturation
To ensure an adequate supply of oxygen and nutrients, the neuroepithelium has to produce chemoattractive guidance cues that ensure the formation of patent blood vessels in positions where neurons form and function.
Key steps in brain vascularisation
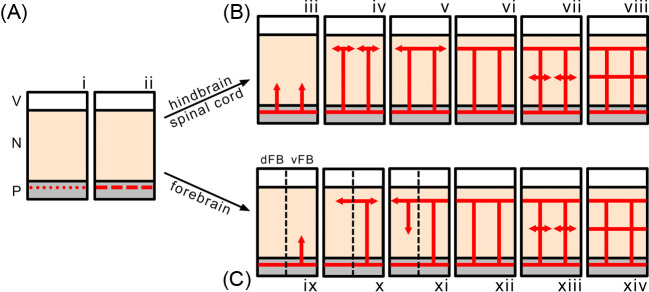
The main steps in neural tube vascularisation are by now well characterised, with many similarities and some differences along the rostrocaudal axis [3]. To vascularise the neuroepithelium, vessel sprouts ingress from a perineural vascular plexus (PNP) directly outside the pial surface of the neural tissue [4–6]. The PNP forms via vasculogenesis, a process in which angioblasts differentiate from the presomitic mesoderm to form blood vessels de novo [7,8] (Figure 1). Angioblasts are initially attracted to the basal surface of the neural tube by neural VEGF-A [8] (see below). The next steps of neural tube vascularisation are particularly well characterised for the hindbrain [9] and the spinal cord [10,11]. In both parts of the neural tube, vessels sprout from the PNP into the neural parenchyma, grow radially towards the ventricular zone and turn laterally before reaching the ventricular surface; the laterally sprouting vessels then fuse with one another into a periventricular vessel network known as the subventricular vascular plexus (SVP) [4–6] (Figure 1B). Subsequently, lateral sprouts emerge from radial vessels in deeper brain layers and then fuse into additional plexi. This process appears to be conserved in the zebrafish and avian hindbrain, possibly reflecting the physiological need for evolutionary conservation of hindbrain anatomy across the vertebrate phylum [12]. Studies of spinal cord vascularisation have further shown that radial vessels initially invade the neural parenchyma at several stereotypical entry points around the circumference of the spinal cord, except at the level of the ventral motor neuron (MN) columns, which remain avascular until SVP formation is complete in the remainder of the CNS [13].
Figure 1. Spatial progression of mammalian CNS vascularisation.
(A) Angioblasts (red dots, i) converge and align outside the neural tube to form the PNP (red dashes, ii). (B) Radial vessels sprout from the PNP into hindbrain and spinal cord and grow radially towards the ventricular zone (iii). Before reaching the ventricular surface, the sprouts turn laterally (iv) and fuse into the SVP (v). After SVP formation is complete (vi), a deeper plexus sprouts from the radial vessels (vii, viii). (C) Vascularisation proceeds in a similar fashion in the ventral portion of the forebrain to more caudal regions of the neural tube (as shown in iii–viii), but the dorsal forebrain is vascularised by lateral SVP sprouts (x) that extend radial vessels back to the underlying PNP (xi). Abbreviations: dFB, dorsal forebrain; N, neuroepithelium; P, pial tissue; V, ventricle; vFB, ventral forebrain.
Vascularisation of the forebrain differs from that of the hindbrain and spinal cord with respect to the spatiotemporal progression of vascular outgrowth [14] (Figure 1C). Radial vessels ingress initially in the ventral compartment of the telencephalic vesicle similar to the process of hindbrain vascularisation; however, even though an SVP forms in the ventrolateral forebrain, dorsomedial regions remain temporarily avascular [14]. Indeed, vascularisation of the dorsal forebrain is not initiated by vessels originating from the PNP, but via vascular spouting from the SVP in the ventral forebrain, whereby sprouts extend tangentially around the telencephalon to vascularise the dorsal regions [14].
During neural tube vascularisation at all rostrocaudal levels, ECs recruit mural cells called pericytes, which ensheath the blood vessel endothelium, but are separated from it by a basement membrane. Loss of pericytes causes excessive endothelial proliferation, but also increases endothelial apoptosis or transcytosis [26]. Accordingly, several forms of paediatric brain haemorrhage are thought to result from poor pericyte recruitment to the vascular network of the germinal matrix in human foetuses [27].
Subsequent to its formation as pericyte-ensheathed endothelial networks, the CNS vasculature undergoes specialisation. Thus, ECs extend their interaction with pericytes to astrocytes, whose endfeet make direct contact with the endothelium [28,29]. The reciprocal interaction of these three cell types results in the formation of the blood–brain barrier (BBB), which maintains essential tissue homoeostasis by establishing selective permeability to fluid, molecules and cells between the blood stream and neural parenchyma. The level of extracellular fluid in neural tissue is tightly regulated by the BBB to maintain physiological levels of water and prevent fluid build-up in the brain [30]. Glucose is actively pumped into the CNS by BBB ECs through the glucose transporter GLUT1, whose expression is regulated in part by NPC signals [18] in a mechanism that likely supports the metabolic demand of neural activity (see below). Additional transmembrane pumps are responsible for the movement of metabolites, toxins and drugs bidirectionally across the barrier [31]. The BBB also acts as a physical barrier to harmful pathogens and ensures that immune cells can only access the brain in certain physiological states to reduce the opportunity for auto-immunity [32].
Molecules regulating brain vascular development
The processes by which new blood vessels sprout from pre-existing ones, migrate into the neural parenchyma, branch and fuse into a network and then undergo maturation is collectively termed ‘angiogenesis’ and involves regulation by specific gene families. We will highlight several of these genes below, with specific consideration given to pro-vascular molecules expressed by NPC populations (Table 1).
Table 1. NPC regulation of CNS vascularisation.
| Process | NPC signal/molecule | Species/CNS region | References |
|---|---|---|---|
| (1) Vascular ingression and intraneural branching | VEGF-A | Fish spinal cord | [15] |
| Mouse forebrain | [16,17] | ||
| Mouse mid-/hindbrain | [17] | ||
| WNT7A/7B | Mouse spinal cord | [18,19] | |
| (2) Branching/angiogenesis | VEGF-A | Fish spinal cord | [15] |
| Mouse forebrain | [16,17] | ||
| Mouse mid-/hindbrain | [17] | ||
| (3) Vessel maturation/BBB formation | WNT7A/7B | Mouse spinal cord | [18,19] |
| (4) Vascular stability (via TGFβ activation) | Integrins αvβ6 and αvβ8 | Mouse forebrain | [20,21] |
Vascular endothelial growth factor (VEGF)
The VEGF family of secreted glycoproteins is the best-studied group of angiogenic molecules to date. The importance of VEGF-A for mammalian development is demonstrated by the early lethality of mouse embryos that lack even one copy of the Vegfa gene that encodes VEGF-A [33]. In these mice, ECs do not differentiate, and blood vessels accordingly cannot assemble. This phenotype is also observed in mice lacking both copies of the Kdr gene, which encodes the VEGF-A receptor tyrosine kinase VEGFR2, also known as FLK1 or KDR [34]. VEGF-A binding to VEGFR2 also activates intracellular signalling cascades in ECs that drive their proliferation for vascular expansion [35,36]. Furthermore, VEGF-A acts as a guidance cue for sprouting vessels by directing the filopodia that extend from the endothelial ‘tip cells’ to lead the vessel sprout into avascular areas [37,38].
The Vegfa mRNA transcripts include several differentially spliced forms that are translated into VEGF-A isoforms of varying lengths, which possess varying affinities for the extracellular matrix (ECM) and different receptor binding properties [39]. In the mouse, the main isoforms are termed VEGF120, VEGF164 and VEGF188. The corresponding human isoforms are all one amino acid longer in length and accordingly termed VEGF121, VEGF165 and VEGF189, respectively. VEGF120 is a diffusible isoform with low ECM affinity that binds VEGFR2, whilst the VEGF164 and VEGF188 isoforms have a high ECM affinity and bind both VEGFR2 and the non-catalytic receptor neuropilin 1 (NRP1) [40] (see below). All VEGF isoforms promote EC proliferation similarly, and therefore each type is sufficient for vessel formation during embryonic development [38]. However, the differential affinity of the isoforms for the ECM is essential for proper vascular patterning in the developing brain. Accordingly, mouse embryos expressing only VEGF120 exhibit defective branching of vessels in the hindbrain because VEGF-A fails to form proper growth factor gradients to guide the tip cell filopodia [38]. As the ECs in the growing vessels continue to proliferate, the vessels in mice expressing VEGF120 only increase their luminal diameter excessively. Vice versa, the vessels of mice expressing only VEGF188 as the isoform that is most tightly bound to ECM show vascular hypersprouting, leading to excessively thin vessels [38]. Together, these findings show that the different VEGF-A isoforms are cooperatively required for proper vascular morphogenesis in the brain [38].
VEGF164 and VEGF188 can also bind to a co-receptor complex composed of VEGFR2 and NRP1, with the VEGF isoforms believed to form a bridge between both receptors [41]. However, VEGF-A does not require NRP1 to direct angiogenesis in the developing brain; this is illustrated by the finding that both forebrain and hindbrain vascularisation are unaffected in mouse embryos that express NRP1 with a defective binding pocket for VEGF-A [42,43]. Nevertheless, embryo-wide or endothelial Nrp1 deletion impairs CNS vascularisation [5,6,44], suggesting that NRP1 acts in ECs to promote brain vascularisation through a VEGF-independent signalling mechanism. This mechanism may involve the modulation of TGF-β signalling [45,46] and the promotion of tip cell filopodia extension and actin cytoskeletal reorganisation via integrin-associated signalling pathways [47].
Experiments in avian embryos support the idea that NPCs modulate the ingression of PNP vessels into the neural tube by releasing VEGF-A [8]. Vegfa is expressed abundantly in the spinal cord at a stage when it is composed mainly of NPCs [8]. Furthermore, ectopic expression of VEGF165 and VEGF189 in the neural tube, achieved through targeted in ovo electroporation of expression vectors, induces supernumerary radial vessel entry points into the neural parenchyma [11].
NPC expression of VEGF-A is also required for neural tube vascularisation in the zebrafish [15], with fish VEGF-A encoded by not one, but two genes that are termed vegfaa and vegfab [48]. Vegfaa and vegfab are collectively expressed across the neuroepithelium, including in NPCs and the neuron-populated mantle zone [48]. The depletion of vegfab from radial glia, the predominant NPC subtype in the fish spinal cord, prevented the formation of the bilateral vertebral arteries that flank the neural tube and represent the fish equivalent of the PNP [15]. Preventing their formation subsequently blocks the vascularisation of the zebrafish neural tube from these vessels [15]. Inhibiting endothelial responsiveness to both VEGF-A ligands by mutating kdrl, the fish orthologue of mammalian VEGFR2, also blocks the formation of the basilar artery and sprouting of the central arteries in the brain [48].
NPCs have been shown to be the main cellular source of VEGF-A during mammalian CNS vascularisation. Thus, loss of Vegfa expression from NPCs perturbs cortical vascular development in mice [16,17]. Specifically, both the number of endothelial filopodia and filopodial length, and consequently vessel branching and vascular coverage, are decreased in the cortex of mouse embryos with reduced, but not absent Vegfa expression, demonstrating that brain angiogenesis is regulated by VEGF-A in a dose-dependent manner [16] (Figure 2A). These experiments were performed by Cre-LoxP-mediated recombination of one conditional Vegfa null allele in cells expressing the NPC gene nestin, together with a hypomorphic mutation of the second Vegfa allele [16]. This strategy was required to overcome the effect of nestin-driven, undesired germline expression of Cre, which effectively rendered the Vegfa deletion embryo-wide in the F1 generation [33,16]. Thus, a male carrying the Nestin-Cre transgene and a hypomorphic Vegfa allele was mated to females carrying conditional Vegfa null alleles to obtain embryos with an approximately 75% reduction in brain VEGF-A levels. The consequence of complete loss of Vegfa expression from NPCs on brain vascularisation has therefore not yet been demonstrated.
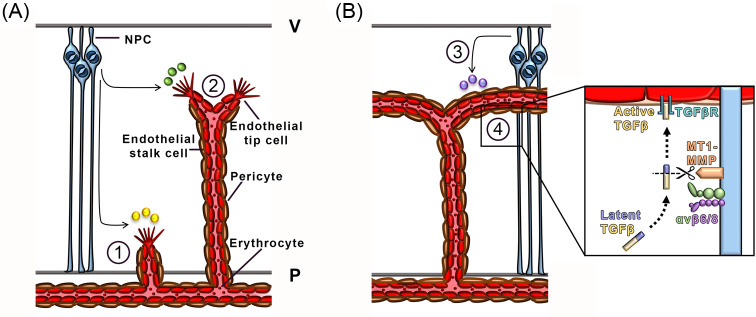
Figure 2. NPC regulation of CNS vascularisation in the developing brain.
Schematic representation of molecular mechanisms by which NPCs promote CNS vascularisation. (A) NPC-derived secreted cues such as VEGF and WNT ligands promote vascular ingression and radial outgrowth, indicated with (1), and lateral branching, indicated with (2), respectively. (B) NPC-secreted WNT ligands and NPC-activated TGFβ regulate vascular maturation and stability, respectively, indicated with (3) and (4). Inset, NPCs (light blue) express integrins αvβ6 or αvβ8 (green/purple) to promote the cleavage of latent TGFβ (blue/yellow) by the matrix metalloprotease MT1-MMP (orange) to release active TGFβ (yellow). Active TGFβ then binds TGFβ receptors (cyan) on ECs (red). Abbreviations: P, pial surface; V, ventricular surface.
VEGF-A signalling in the CNS is modulated positively by the hypoxia-inducible factor 1α (HIF1A). HIF1A is up-regulated and stabilised in cells under low oxygen tension to increase VEGF-A expression, which then helps to attract new blood vessels into the hypoxic areas to restore normoxia e.g. in the tumour microenvironment [49]. HIF1A is expressed by NPCs prior to the onset of brain vascularisation [50,51] and is thought to regulate VEGF-A expression to drive angiogenesis into avascular regions of the expanding neuroepithelium [51]. Thus, mouse embryos lacking HIF1A specifically in NPCs following Cre-LoxP mediated recombination of conditional null Hif1a alleles, under the control of the nestin promoter, have reduced tissue-wide VEGF-A levels and reduced blood vessel extension into the cortical plate [51]. Furthermore, cortical vasculature regresses at later stages of gestation in these mutants, indicating that HIF1A-driven VEGF expression supports vascular maintenance [51].
VEGF-A signalling in the CNS is modulated negatively by a VEGF-A binding decoy receptor termed soluble (s) fms-related tyrosine kinase 1 (FLT1). Thus, sFLT1 expression in the zebrafish neural tube, regulated non-cell autonomously by resident radial glia, restricts VEGF-induced radial vessel ingression into the spinal cord to specific sites and limits oversprouting within the parenchyma, thus demonstrating that endogenous flt1 expression is essential for normal CNS vascularisation [11,52]. It is not yet known whether vertebrate NPCs modulate vessel ingression into the neural tube through secreting sFLT1. Nevertheless, post-mitotic MNs have been shown to express sFLT1 in the chick to delay VEGF-induced vascularisation of their own domain, the nascent bilateral motor columns in the ventral spinal cord [13]. Specifically, it was shown that blocking Flt1 expression in the MNs of the avian spinal cord via forced expression of Flt1-targeting microRNAs under a MN-specific promoter caused ectopic vessel ingression sites and precocious motor column vascularisation [13]. Studies in fish demonstrate also that neuronal flt1 prevents ectopic vessel sprouting in the neural tube, and its function is therefore conserved across different vertebrate species [53].
WNTs
The WNT family of secreted ligands is crucial to many developmental mechanisms, including brain vascular development [54]. WNT proteins bind to a co-receptor complex comprised of a lipoprotein receptor-related protein (LRP) and a frizzled (Fz) receptor to inhibit the proteolytic degradation of β-catenin and therefore the translocation of β-catenin to the nucleus, where it would otherwise act as a transcriptional coactivator. Thus, β-catenin forms part of the TCF/LEF transcription factor complex that drives the expression of genes responsible for processes such as cell cycle progression and fate determination.
WNT proteins can influence EC behaviour in vitro; for example, WNT7A promotes the migration of cultured ECs across a fibronectin-coated filter, suggesting that it acts as a guidance cue [19]. WNT7A and WNT7B are expressed in the murine CNS, and the analysis of genetic mouse mutants lacking these WNT ligands has highlighted their important roles in establishing and maintaining CNS vascularisation [18]. Specifically, the combinatorial deletion of conditional null alleles of the Wnt7a and Wnt7b genes in NPCs with Cre expressed under the control of either the Sox2 or nestin promoters shows that WNT signalling is required for vascularisation of the mouse CNS [18] (Figure 2A). Vascular outgrowth is impaired in the spinal cord of Wnt7a/7b compound mutants; however, ablation of either ligand alone has no effect on neural tube vascularisation, demonstrating that they can compensate for each other during this process [18]. A large number of other WNTs are also expressed across the forebrain and spinal cord [19], but it is not yet known if they too are required for CNS vascularisation, or instead drive other aspects of CNS development, such as neuronal specification [55].
WNT signalling is also necessary for establishing patent and selectively permeable CNS vasculature (Figure 2B). Firstly, NPC-specific Wnt7a/b mutant embryos have haemorrhagic brain vasculature, in addition to the angiogenesis defects described above [18]. Secondly, Wnt3a, expressed in mouse forebrain and spinal cord [19], promotes the expression of the tight junction protein claudin 3 in cultured brain microvascular ECs [56]. Thirdly, the conditional inactivation of WNT signalling in brain ECs through endothelial deletion of β-catenin increases the extravasation of injectable vascular permeability tracers into the brain [56,57]. Finally, NPC-derived WNT signals promote GLUT1 expression in ECs and therefore enable glucose transport into the brain, further demonstrating the importance of WNT proteins in establishing the BBB [18].
Despite being important for blood vessel formation, another study proposed that prolonged WNT signalling induces vascular regression and prevents vessel stabilisation during embryogenesis and thereby causes vascular leakage in the cortex after birth [58]. In particular, radial glia loss in the developing forebrain by conditional Orc3 deletion results in ectopic activation of WNT signalling in the SVP at later stages of gestation; this consequently impairs vessel maturation through increased expression of matrix metalloproteases that mediate vascular remodelling [58]. In agreement, vessel regression is not as severe in mouse mutants with radial glia ablation on a Wnt7b-null background, or after pharmacological blockade of WNT signalling or inhibition of metalloprotease function [58]. It seems possible that NPCs regulate WNT signalling in ECs through other molecules, such as through NOTCH-based regulation of β-catenin [59].
Recent research suggests that the orphan G-protein coupled receptor, GPR124, plays a role in WNT-regulated angiogenesis and BBB formation. Thus, Gpr124−/− mutant mice possess cerebrovascular malformations in different areas of the developing CNS that are characteristic of defective WNT signalling, including poor endothelial barrier maturation and haemorrhaging [60–64]. However, it is not yet known which ligand (if any) stimulates GPR124 and whether this signalling pathway operates in NPCs.
Integrins
Integrins are heterodimeric membrane-bound receptors that are composed of an α and a β subunit and are vital for establishing tissue architecture and maintaining structural integrity during development. Integrins regulate cell behaviour by transducing signals after binding cell contact-dependent cues in the ECM, such as fibronectin and laminin. NPCs express integrins abundantly during neural development to transduce signals from the basal lamina into NPCs, and also between NPCs, to dictate the onset of neurogenesis (e.g. [65,66]).
In the developing CNS, integrins also promote normal vascular development by modulating transforming growth factor beta (TGF-β) signalling (Figure 2B). In particular, both αvβ6 and αvβ8 complexes promote cleavage of pro-TGF-β by the matrix metalloprotease MT1-MMP to convert TGF-β into an active form that is able to signal to ECs [67–69]. As TGF-β promotes vessel stabilisation and BBB establishment [70,71], mice lacking the integrin subunits αv or β8 have brain vascular defects reminiscent of TGF-β mutants [20,21]. Specifically, the Cre-LoxP-mediated ablation of the Itgb8 gene that encodes β8 in nestin-expressing NPCs disrupts vessel morphology, in addition to compromising the adhesion of NPCs to each other [20]. In contrast, ablating β8 integrin later on in cortical neurons does not impair vascularisation [20]. The NPC-specific deletion of the αv integrin subunit also results in abnormal cerebral vascularisation and brain haemorrhage [21]. These findings, coupled with the knowledge of integrin-based activation of latent TGF-β, are consistent with a role for NPCs in helping to stabilise nascent vessels by modulating endothelial TGF-β signalling [20,21]. Interestingly, mice lacking either αv or β8 expression in NPCs reach birth and do not exhibit vascular leakage postnatally, suggesting that unidentified mechanisms can compensate for their function during postnatal CNS vascularisation [20,21].
Netrins
Netrins are laminin-related secreted molecules that bind to UNC5 and DCC receptors to exert repulsive or attractive actions in axons, respectively. Netrin signalling can also have pro- or anti-angiogenic effects during developmental angiogenesis, but it is not known whether this is due to the use of alternative receptors [72]. It has also been discussed that differing netrin roles may be explained by different netrin concentrations or the initiation of alternate cellular behaviours depending on the nature of the recipient cell [72]. For example, one study reported that netrin promotes EC survival by binding to and subsequently preventing pro-apoptotic signalling through UNC5B in ECs [73], whilst another found that netrins promote post-ischaemic neovascularisation [74]. Yet another study found that UNC5B is required to restrict vessel branching and ectopic vessel growth in the CNS [75]. In particular, blood vessels in the Unc5b-null mouse cortex and spinal cord and the morpholino-treated zebrafish neural tube have significantly more tip cell filopodia [75]. However, mice lacking netrin 1 do not possess any obvious defects in CNS vascularisation, suggesting that other netrins can compensate for netrin 1 in this process [75]. In support of this idea, both netrins 1 and 4 are expressed abundantly in the mouse forebrain and spinal cord [76,77]. As they are expressed highly in the ventricular zone, they may be secreted by NPCs to restrict vessel growth in the germinal region during neurogenesis. However, NPC-specific compound netrin mutants have not been analysed to date to investigate these possibilities, and additional work is therefore required to understand the complexity of mechanisms by which netrin signalling affects ECs.
Role of vascular signals in NPC regulation and neurogenesis
During neurogenesis in vertebrate embryos, NPCs expand in number and specialise to give rise to partially committed progenitors that then differentiate into diverse subtypes of neurons and glia. Initially, the neuroepithelium contains primitive NPCs [2], which differentiate into a self-renewing NPC subtype called apical radial glia. Both subtypes make up the broader class of NPCs known as ‘apical progenitors’ (APs) that are defined primarily by their position in the neuroepithelium during mitosis and reside in the apical portion of the neurogenic zone [78,79]. At the beginning of CNS development, APs divide rapidly to expand in number and establish a large progenitor pool. Following expansion, APs differentiate into ‘basal progenitors’ (BPs), a subclass of NPCs that contains a varying proportion of basal radial glia and resides in the basal portion of the neurogenic zone [80]. Basal radial glia are sparse in the forebrain of lower mammals with smooth (lissencephalic) brains such as rodents, but are more prevalent in higher mammals with larger and gyrified (gyrencephalic) brains, including humans [81]. APs and BPs are the most abundant types of progenitors in the early mammalian brain, but other progenitor subtypes with intermediate properties are also present in smaller numbers [82].
The symmetry of NPC cell division controls whether progenitors self-renew or differentiate into more committed NPCs and post-mitotic neurons [83]. The division mode is linked closely to cell cycle progression and the polarised distribution of intracellular fate determinants (e.g. [84,85]). These two processes are regulated by molecular cues originating from the surrounding tissue, which therefore acts as a ‘regulatory niche’. Many of these extrinsic signals are generated by other neural cells [86] and also by vasculature, as discussed below (Table 2).
Table 2. Vascular regulation of Neurogenesis.
| Vessel-derived signals | NPC target | Murine CNS region | Effect | References |
|---|---|---|---|---|
| Oxygen | HIF1A | Forebrain | AP differentiation into BPs | [22] |
| Secreted signals, unidentified | Unidentified | Forebrain-derived neurospheres hindbrain | NPC self-renewal | [23,24] |
| Extracellular matrix, unidentified component(s) | ITGB1 | Ventral forebrain | Vessel anchorage/proliferation of APs for interneuron generation | [25] |
Blood vessels regulate NPC commitment along specific lineages through tissue oxygenation
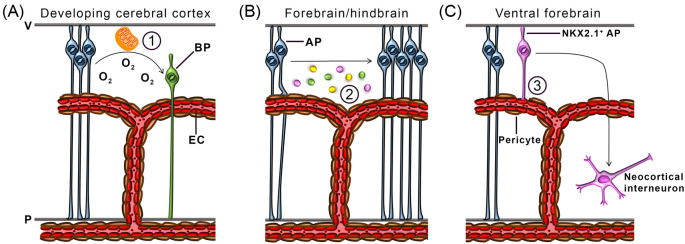
Two studies have shown that blood vessels promote NPC differentiation into more committed subtypes. Firstly, ectopic Vegfa expression in the mouse cortex, achieved through in utero electroporation, promotes angiogenesis in the targeted areas and is accompanied by the supernumerary generation of TBR2+ BPs [87]. Newly-formed BPs are generated in regions far from the ventricle, but close to ectopically sprouting blood vessels, suggesting that vasculature facilitates the conversion to, or the expansion of, this NPC subclass [87]. Even though it was not tested whether the excessive generation of BPs occurred at the expense of less differentiated progenitors or if it represents a selective amplification of BPs, this study suggested that VEGF-induced blood vessels alter NPC commitment along specific lineages. In agreement with this idea, a recent study showed that blood vessels regulate NPC commitment by alleviating tissue hypoxia and altering NPC metabolism [22] (Figure 3A). Firstly, the onset of cortical vascularisation relieves local tissue hypoxia, which in turn destabilises HIF1A [22]. Secondly, it was shown that these processes correlate temporally with a decline of PAX6+ APs and an increase of TBR2+ BPs, suggesting increased progenitor commitment [22]. Thirdly, preventing cortical vascularisation through endothelial GPR124 deletion (discussed above) increased hypoxia and therefore HIF1A levels, which resulted in NPC expansion at the expense of commitment to a BP fate [22]. Finally, HIF1A was shown to control NPC fate by maintaining NPC metabolism in a state of glycolysis, favoured by APs, and concomitantly preserving expression of the stemness gene Myc [22].
Figure 3. Vascular regulation of embryonic NPCs.
Schematic representation of molecular mechanisms by which blood vessels regulate NPC behaviour in the embryonic brain. (A) APs (blue) in the developing cerebral cortex differentiate into BPs (green) following a switch in metabolism, which is enabled by blood vessel-derived oxygen. (B) ECs secrete unidentified diffusible factors (indicated by pink, green and yellow spheres), which promote AP self-renewal and expansion in the forebrain and hindbrain. (C) NKX2.1+ APs in the ventral forebrain attach to unidentified matrix components on periventricular vasculature via an integrin containing the β1 subunit. Vessel anchorage maintains NKX2.1+ AP proliferation and therefore enhances their generation of neocortical interneurons. Abbreviations: P, pial surface; V, ventricular surface.
Endothelial cells secrete factors that prevent NPC differentiation
The first indication of angiocrine signalling to NPCs was demonstrated when NPCs were propagated as ‘neurospheres’ derived from e10 mouse cortical tissue and co-cultured with either bovine pulmonary artery or adult brain microvascular EC lines [23]. By using a transwell system that physically separated both cell types, it was shown that ECs released secreted factors that stimulated neurosphere expansion significantly more than co-culture with non-ECs [23]. The EC-conditioned media promoted a stem-like character in the cultured NPCs, because they formed neurospheres comprised of many more NPCs than neurons [23]. In fact, single NPCs propagated in co-culture with ECs did not produce any neurons during a 4-day time-lapse video recording, indicating that soluble factors produced by ECs promote NPC self-renewal and delay neurogenesis, at least in vitro [23]. More recently, studies of the mouse embryonic hindbrain have suggested that neural vasculature regulates NPC self-renewal by releasing diffusible regulatory signals also in vivo (Figure 3B). Hindbrain progenitors divide most actively following the main phase of vascularisation, and the temporal profile of progenitor mitosis is disturbed by impairing vessel outgrowth in the NPC-populated germinal zone [24]. Thus, constitutive and endothelial NRP1 mutant hindbrains are poorly vascularised at early phases of hindbrain neurogenesis [5,24], which results in failed cell cycle re-entry of early-formed NPCs and their increased differentiation into neural cell types [24]. Taken together, these findings illustrate that the embryonic endothelium supports NPC expansion, for example by secreting regulatory molecules.
Molecular regulation of neurogenesis by vascular signals
VEGF regulation of NPCs
As discussed above, several lines of evidence suggest that NPCs secrete VEGF-A to promote CNS vascularisation. Vice versa, blood vessels have been suggested to also release VEGF-A to regulate neurogenesis. In one study, conditional mouse mutants lacking Vegfa expression in Tie2-expressing ECs were reported to have profound cortical malformations and heterotopias [88]. Yet, the precise mechanisms involved have not yet been elucidated. On the one hand, it was not examined whether a specific neural VEGF-A receptor might be required for this process; on the other hand, it was reported that endothelial Vegfa deletion disrupts forebrain vascularisation [88] and vascular homoeostasis [89], and an endothelial Vegfa mutation may therefore affect cortical development indirectly by compromising the vasculature, rather than by impairing direct VEGF-A signalling to NPCs or their progeny.
Another study sought to identify whether VEGF-A signals directly to embryonic NPCs. Recombinant VEGF165 was found to promote the formation of neurospheres in vitro after serial passaging, but this effect was lost in VEGFR2-deficient neurospheres [90]. In contrast, other observations, made in the developing mammalian CNS, argue against direct effects of VEGF-A on NPCs. Firstly, two independent studies show that murine NPCs lack VEGFR2 expression in vivo, with one study having examined the embryonic mouse forebrain mid-way through cortical neurogenesis [87], and the other having studied the embryonic mouse hindbrain through the main phases of hindbrain neurogenesis [24]. Secondly, the alternative VEGF-A receptor NRP1 is expressed in hindbrain NPCs, but their proliferation is unaffected by progenitor-specific NRP1 ablation [24]. The divergent results obtained for VEGFR2 roles in NPCs through culture models versus in vivo studies may be that NPCs up-regulate VEGFR2 after they are cultured. It is not known why this might occur, but one possible explanation could be that dissociation of NPCs from other cells within the neuroepithelium alters NPC gene expression patterns.
Blood vessels regulate neurogenesis via cell contact with NPCs
Many stem cell populations share physical contacts with neighbouring cells, from which they receive paracrine niche signals. For example, adult neural stem cells (NSCs) project endfeet onto periventricular blood vessels and receive endothelial-derived notch and ephrin signals to remain in quiescence [91,92]. In analogy, a fate-restricted subset of forebrain NPCs contacts the SVP in the ventral telencephalon [25]. Accordingly, it was shown that NKX2.1+ radial glial cells in the medial ganglionic eminence, which are destined to generate neocortical interneurons, project endfeet onto periventricular ECs in pericyte-free vessel areas [25]. This adhesion is, at least in part, integrin-mediated, because genetic targeting of the Itgb1 gene causes process retraction. This detachment subsequently impairs the mitotic capacity of the progenitors, as well as their ability to generate interneurons [25] (Figure 3C). In agreement, the disruption of vessel networks by injecting function blocking antibodies against VEGFR2 into the forebrain ventricle also disrupts anchorage of these NPCs to blood vessels and reduces their mitotic capacity [25]. However, the specific integrin ligands involved in radial glia anchorage to blood vessels for neurogenic regulation remain unidentified. Notably, very few radial glia in the dorsal telencephalon terminate endfeet on cortical blood vessels [25]. Vessel association therefore appears to be important for specific NPC subsets.
Conclusions
The studies described above demonstrate the importance of extensive molecular crosstalk between NPCs and ECs in the developing CNS of several vertebrate species. We have discussed how NPCs direct vascularisation of the neuraxis through a number of complementary signalling mechanisms that regulate the initial invasion of the parenchyma by blood vessels, as well as their branching and eventual maturation within the neural tube. Yet, many outstanding questions remain to be answered before we will fully understand the process of CNS vascularisation. For example, we know now that VEGF-A has a key role in promoting mammalian CNS vascularisation, but we still need to define how it cooperates with other signalling pathways discussed here or described elsewhere to fine-tune the formation and maturation of neural vasculature. Moreover, it remains to be investigated which of these pro-angiogenic factors are of NPC or neuronal origin. Answering these questions may help devise effective therapies aimed at regenerating defective or regressed cerebrovasculature in ischaemic or degenerative CNS diseases.
We have also discussed recent studies that highlight the importance of blood vessels in regulating neurogenesis. However, the specific mechanisms involved appear to differ, at least in part, across different regions of the developing CNS. For example, we have described above that a subset of forebrain NPC processes terminate with endfeet on periventricular vessels [22], whereas hindbrain NPC processes are more loosely associated with germinal zone vasculature [24]. Moreover, we have discussed that restoring normoxia in poorly vascularised forebrains rescues hypoxia-induced NPC commitment defects in the forebrain [22], even though restoring normoxia does not prevent precocious NPC commitment in the hindbrain of mouse mutants possessing an avascular hindbrain germinal zone [24]. These regional differences in NPC behaviour may stem from differences in the NPC subtypes present in each brain region; for example, hindbrains lack basal TBR2+ NPC subtypes [93], whose generation in the forebrain depends on vascularisation-driven relief from hypoxia [22]. Indeed, prior studies having focused predominantly on neurogenesis regulation within the context of only one defined subregion of the developing brain. Future work should therefore compare different parts of the embryonic CNS in a systematic manner to distinguish the cellular and molecular mechanisms that are shared between different brain regions from those that are unique to specific compartments.
Extending the idea that blood vessels regulate embryonic neurogenesis via oxygen provision, both forebrain and hindbrain studies have shown that blood vessels additionally provide angiocrine signals that regulate NPC behaviour, independently of oxygenation [23,24]. This observation is reminiscent of findings in the adult neurogenic niche, where ECs in the lateral subventricular zones regulate resident NSC behaviour [94]. However, whether the angiocrine signals in the adult and embryonic niches are similar or distinct remains to be established. In particular, it will be interesting to examine whether embryonic NPCs respond to contact-dependent vascular regulation via notch-delta signalling [91] or endothelial cytokines such as PEDF, PLGF, BDNF or NTF3 [94–97], which are all key for stem and progenitor cell regulation in the adult neurogenic niche. This is an important consideration, because findings made in the adult brain are not necessarily predictive of angiocrine regulation of NPCs in utero, as demonstrated, for example, by the divergent roles of NTF3 during adult and embryonic neurogenesis [97,98]. It will also be intriguing to define whether ECs in extra-neural vessel networks contribute to the neurogenic niche, in particular in the case of the PNP, which is close to NPC endfeet contacting the basal surface of the neuroepithelium [24] and is therefore ideally placed to provide diffusible regulatory cues.
Ultimately, the knowledge gained on the vascular regulation of embryonic neurogenesis will increase our understanding of CNS formation and hopefully lay the foundation for innovative repair strategies in adult brain disease by exploiting developmental principles. For example, activating vasculature in the adult brain may be an ideal means to stimulate existing NSC niches and also create new niches that can support the survival and function of transplanted neural stem and progenitor cells for adult brain repair [99–102].
Acknowledgements
The authors thank Dr. Camille Charoy for helpful comments on the manuscript.
Abbreviations
- AP
apical progenitor
- BBB
blood–brain barrier
- BP
basal progenitor
- CNS
central nervous system
- EC
endothelial cell
- ECM
extracellular matrix
- FLT1
fms-related tyrosine kinase 1
- HIF1A
hypoxia-inducible factor 1α
- MN
motor neuron
- MT1-MMP
membrane type 1 metalloprotease
- NKX2.1
NK2 homeobox 1
- NPC
neural progenitor cell
- NRP1
neuropilin 1
- NSC
neural stem cell
- PAX6
paired box protein Pax-6
- PNP
perineural vascular plexus
- SVP
subventricular vascular plexus
- TBR2
T-box brain protein 2
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
Competing interests
The authors declare that there are no competing interests associated with this article.
Funding
This work was supported by a Wellcome Trust Investigator Award to Christiana Ruhrberg [grant number 095623/Z/11/Z].
References
- 1.Spemann H. and Mangold H. (1924) Über Induktion von Embryonalanlagen durch implantation artfremder Organisatoren. Roux’s Arch. Entw. Mech. Org. 100, 599–638 [Google Scholar]
- 2.Rakic P. (1995) A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388 10.1016/0166-2236(95)93934-P [DOI] [PubMed] [Google Scholar]
- 3.Tata M., Ruhrberg C. and Fantin A. (2015) Vascularisation of the central nervous system. Mech. Dev. 138, 26–36 10.1016/j.mod.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S. et al. (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fantin A., Vieira J.M., Plein A., Denti L., Fruttiger M., Pollard J.W. et al. (2013) NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 121, 2352–2362 10.1182/blood-2012-05-424713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhardt H., Ruhrberg C., Abramsson A., Fujisawa H., Shima D. and Betsholtz C. (2004) Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev. Dyn. 231, 503–509 10.1002/dvdy.20148 [DOI] [PubMed] [Google Scholar]
- 7.Wilting J., Brand-Saberi B., Huang R., Zhi Q., Kontges G., Ordahl C.P. et al. (1995) Angiogenic potential of the avian somite. Dev. Dyn. 202, 165–171 10.1002/aja.1002020208 [DOI] [PubMed] [Google Scholar]
- 8.Hogan K.A., Ambler C.A., Chapman D.L. and Bautch V.L. (2004) The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 131, 1503–1513 10.1242/dev.01039 [DOI] [PubMed] [Google Scholar]
- 9.Ruhrberg C. and Bautch V.L. (2013) Neurovascular development and links to disease. Cell. Mol. Life Sci. 70, 1675–1684 10.1007/s00018-013-1277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao T., Ishizawa A. and Ogawa R. (1988) Observations of vascularization in the spinal cord of mouse embryos, with special reference to development of boundary membranes and perivascular spaces. Anat. Rec. 221, 663–677 10.1002/ar.1092210212 [DOI] [PubMed] [Google Scholar]
- 11.James J.M., Gewolb C. and Bautch V.L. (2009) Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 136, 833–841 10.1242/dev.028845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland L.Z. (2009) Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nat. Rev. Neurosci. 10, 736–746 10.1038/nrn2703 [DOI] [PubMed] [Google Scholar]
- 13.Himmels P., Paredes I., Adler H., Karakatsani A., Luck R., Marti H.H. et al. (2017) Motor neurons control blood vessel patterning in the developing spinal cord. Nat. Commun. 8, 14583 10.1038/ncomms14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan A., Long J.E., Crandall J.E., Rubenstein J.L. and Bhide P.G. (2008) Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 11, 429–439 10.1038/nn2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka R.L., Rossi A., Stone O.A. and Stainier D.Y.R. (2017) CNS-resident progenitors direct the vascularization of neighboring tissues. Proc. Natl. Acad. Sci. USA 114, 10137–10142 10.1073/pnas.1619300114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haigh J.J., Morelli P.I., Gerhardt H., Haigh K., Tsien J., Damert A. et al. (2003) Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225–241 10.1016/S0012-1606(03)00356-7 [DOI] [PubMed] [Google Scholar]
- 17.Raab S., Beck H., Gaumann A., Yuce A., Gerber H.P., Plate K. et al. (2004) Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595–605 [DOI] [PubMed] [Google Scholar]
- 18.Stenman J.M., Rajagopal J., Carroll T.J., Ishibashi M., McMahon J. and McMahon A.P. (2008) Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- 19.Daneman R., Agalliu D., Zhou L., Kuhnert F., Kuo C.J. and Barres B.A. (2009) Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641–646 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proctor J.M., Zang K., Wang D., Wang R. and Reichardt L.F. (2005) Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J. Neurosci. 25, 9940–9948 10.1523/JNEUROSCI.3467-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty J.H., Lacy-Hulbert A., Charest A., Bronson R.T., Crowley D., Housman D. et al. (2005) Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132, 165–176 10.1242/dev.01551 [DOI] [PubMed] [Google Scholar]
- 22.Lange C., Turrero Garcia M., Decimo I., Bifari F., Eelen G., Quaegebeur A. et al. (2016) Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 35, 924–941 10.15252/embj.201592372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Q., Goderie S.K., Jin L., Karanth N., Sun Y., Abramova N. et al. (2004) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- 24.Tata M., Wall I., Joyce A., Vieira J.M., Kessaris N. and Ruhrberg C. (2016) Regulation of embryonic neurogenesis by germinal zone vasculature. Proc. Natl. Acad. Sci. USA 113, 13414–13419 10.1073/pnas.1613113113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan X., Liu W.A., Zhang X.J., Shi W., Ren S.Q., Li Z. et al. (2016) Vascular influence on ventral telencephalic progenitors and neocortical interneuron production. Dev. Cell 36, 624–638 10.1016/j.devcel.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daneman R., Zhou L., Kebede A.A. and Barres B.A. (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballabh P. (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr. Res. 67, 1–8 10.1203/PDR.0b013e3181c1b176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott N.J., Ronnback L. and Hansson E. (2006) Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 29.Janzer R.C. and Raff M.C. (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325, 253–257 10.1038/325253a0 [DOI] [PubMed] [Google Scholar]
- 30.Guerra M., Blazquez J.L. and Rodriguez E.M. (2017) Blood–brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS 14, 19 10.1186/s12987-017-0067-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Z., Nelson A.R., Betsholtz C. and Zlokovic B.V. (2015) Establishment and dysfunction of the. Cell 163, 1064–1078 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S. et al. (2011) The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M. et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- 34.Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L. et al. (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- 35.Sakurai Y., Ohgimoto K., Kataoka Y., Yoshida N. and Shibuya M. (2005) Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 102, 1076–1081 10.1073/pnas.0404984102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong C. and Jin Z.G. (2005) Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J. Biol. Chem. 280, 33262–3326 9 10.1074/jbc.M503198200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A. et al. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H. et al. (2002) Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16, 2684–2698 10.1101/gad.242002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.E., Keller G.A. and Ferrara N. (1993) The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 10.1091/mbc.4.12.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker M.W., Xu P., Li X. and Vander Kooi C.W. (2012) Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 287, 11082–11089 10.1074/jbc.M111.331140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soker S., Miao H.Q., Nomi M., Takashima S. and Klagsbrun M. (2002) VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J. Cell. Biochem. 85, 357–368 10.1002/jcb.10140 [DOI] [PubMed] [Google Scholar]
- 42.Gelfand M.V., Hagan N., Tata A., Oh W.J., Lacoste B., Kang K.T. et al. (2014) Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. Elife 3, e03720 10.7554/eLife.03720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fantin A., Herzog B., Mahmoud M., Yamaji M., Plein A., Denti L. et al. (2014) Neuropilin 1 (NRP1) hypomorphism combined with defective VEGF-A binding reveals novel roles for NRP1 in developmental and pathological angiogenesis. Development 141, 556–562 10.1242/dev.103028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T. et al. (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126, 4895–4902 [DOI] [PubMed] [Google Scholar]
- 45.Aspalter I.M., Gordon E., Dubrac A., Ragab A., Narloch J., Vizan P. et al. (2015) Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat. Commun. 6, 7264 10.1038/ncomms8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirota S., Clements T.P., Tang L.K., Morales J.E., Lee H.S., Oh S.P. et al. (2015) Neuropilin 1 balances beta8 integrin-activated TGFbeta signaling to control sprouting angiogenesis in the brain. Development 142, 4363–4373 10.1242/dev.113746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantin A., Lampropoulou A., Gestri G., Raimondi C., Senatore V., Zachary I. et al. (2015) NRP1 regulates CDC42 activation to promote filopodia formation in endothelial tip cells. Cell Rep. 11, 1577–1590 10.1016/j.celrep.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bussmann J., Wolfe S.A. and Siekmann A.F. (2011) Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development 138, 1717–1726 10.1242/dev.059881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blouw B., Song H., Tihan T., Bosze J., Ferrara N., Gerber H.P. et al. (2003) The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4, 133–146 10.1016/S1535-6108(03)00194-6 [DOI] [PubMed] [Google Scholar]
- 50.Jain S., Maltepe E., Lu M.M., Simon C. and Bradfield C.A. (1998) Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech. Dev. 73, 117–123 10.1016/S0925-4773(98)00038-0 [DOI] [PubMed] [Google Scholar]
- 51.Tomita S., Ueno M., Sakamoto M., Kitahama Y., Ueki M., Maekawa N. et al. (2003) Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol. Cell. Biol. 23, 6739–6749 10.1128/MCB.23.19.6739-6749.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuoka R.L., Marass M., Avdesh A., Helker C.S., Maischein H.M., Grosse A.S. et al. (2016) Radial glia regulate vascular patterning around the developing spinal cord. Elife 5, 10.7554/eLife.20253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wild R., Klems A., Takamiya M., Hayashi Y., Strahle U., Ando K. et al. (2017) Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization. Nat. Commun. 8, 13991 10.1038/ncomms13991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis M. and Liebner S. (2013) Wnt signaling in the vasculature. Exp. Cell Res. 319, 1317–1323 10.1016/j.yexcr.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 55.Lee K.J. and Jessell T.M. (1999) The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 22, 261–294 10.1146/annurev.neuro.22.1.261 [DOI] [PubMed] [Google Scholar]
- 56.Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C.J. et al. (2008) Wnt/beta-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 183, 409–417 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y., Wang Y., Tischfield M., Williams J., Smallwood P.M., Rattner A. et al. (2014) Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 124, 3825–3846 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma S., Kwon H.J., Johng H., Zang K. and Huang Z. (2013) Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 11, e1001469 10.1371/journal.pbio.1001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collu G.M., Hidalgo-Sastre A. and Brennan K. (2014) Wnt-Notch signalling crosstalk in development and disease. Cell. Mol. Life Sci. 71, 3553–3567 10.1007/s00018-014-1644-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuhnert F., Mancuso M.R., Shamloo A., Wang H.T., Choksi V., Florek M. et al. (2010) Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330, 985–989 10.1126/science.1196554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanhollebeke B., Stone O.A., Bostaille N., Cho C., Zhou Y., Maquet E. et al. (2015) Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. Elife 4, 10.7554/eLife.06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y. and Nathans J. (2014) Gpr124 controls CNS angiogenesis and blood–brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 31, 248–256 10.1016/j.devcel.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Posokhova E., Shukla A., Seaman S., Volate S., Hilton M.B., Wu B. et al. (2015) GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 10, 123–130 10.1016/j.celrep.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson K.D., Pan L., Yang X.M., Hughes V.C., Walls J.R., Dominguez M.G. et al. (2011) Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 108, 2807–2812 10.1073/pnas.1019761108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loulier K., Lathia J.D., Marthiens V., Relucio J., Mughal M.R., Tang S.C. et al. (2009) beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 7, e1000176 10.1371/journal.pbio.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stenzel D., Wilsch-Brauninger M., Wong F.K., Heuer H. and Huttner W.B. (2014) Integrin alphavbeta3 and thyroid hormones promote expansion of progenitors in embryonic neocortex. Development 141, 795–806 10.1242/dev.101907 [DOI] [PubMed] [Google Scholar]
- 67.Mu D., Cambier S., Fjellbirkeland L., Baron J.L., Munger J.S., Kawakatsu H. et al. (2002) The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 157, 493–507 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cambier S., Gline S., Mu D., Collins R., Araya J., Dolganov G. et al. (2005) Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am. J. Pathol. 166, 1883–1894 10.1016/S0002-9440(10)62497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Z., Mu Z., Dabovic B., Jurukovski V., Yu D., Sung J. et al. (2007) Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 176, 787–793 10.1083/jcb.200611044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mu Z., Yang Z., Yu D., Zhao Z. and Munger J.S. (2008) TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech. Dev. 125, 508–516 10.1016/j.mod.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 71.Sridurongrit S., Larsson J., Schwartz R., Ruiz-Lozano P. and Kaartinen V. (2008) Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev. Biol. 322, 208–218 10.1016/j.ydbio.2008.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castets M. and Mehlen P. (2010) Netrin-1 role in angiogenesis: to be or not to be a pro-angiogenic factor? Cell Cycle 9, 1466–1471 10.4161/cc.9.8.11197 [DOI] [PubMed] [Google Scholar]
- 73.Castets M., Coissieux M.M., Delloye-Bourgeois C., Bernard L., Delcros J.G., Bernet A. et al. (2009) Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev. Cell 16, 614–620 10.1016/j.devcel.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 74.Wilson B.D., Ii M., Park K.W., Suli A., Sorensen L.K., Larrieu-Lahargue F. et al. (2006) Netrins promote developmental and therapeutic angiogenesis. Science 313, 640–644 10.1126/science.1124704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu X., Le Noble F., Yuan L., Jiang Q., De Lafarge B., Sugiyama D. et al. (2004) The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432, 179–186 10.1038/nature03080 [DOI] [PubMed] [Google Scholar]
- 76.Kennedy T.E., Serafini T., de la Torre J.R. and Tessier-Lavigne M. (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 10.1016/0092-8674(94)90421-9 [DOI] [PubMed] [Google Scholar]
- 77.Yin Y., Sanes J.R. and Miner J.H. (2000) Identification and expression of mouse netrin-4. Mech. Dev. 96, 115–119 10.1016/S0925-4773(00)00369-5 [DOI] [PubMed] [Google Scholar]
- 78.Misson J.P., Edwards M.A., Yamamoto M. and Caviness V.S. Jr (1988) Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Brain Res. Dev. Brain Res. 44, 95–108 10.1016/0165-3806(88)90121-6 [DOI] [PubMed] [Google Scholar]
- 79.Gotz M., Stoykova A. and Gruss P. (1998) Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031–1044 10.1016/S0896-6273(00)80621-2 [DOI] [PubMed] [Google Scholar]
- 80.Betizeau M., Cortay V., Patti D., Pfister S., Gautier E., Bellemin-Menard A. et al. (2013) Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80, 442–457 10.1016/j.neuron.2013.09.032 [DOI] [PubMed] [Google Scholar]
- 81.Lui J.H., Hansen D.V. and Kriegstein A.R. (2011) Development and evolution of the human neocortex. Cell 146, 18–36 10.1016/j.cell.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pilz G.A., Shitamukai A., Reillo I., Pacary E., Schwausch J., Stahl R. et al. (2013) Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 4, 2125 10.1038/ncomms3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noctor S.C., Martinez-Cerdeno V., Ivic L. and Kriegstein A.R. (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- 84.Calegari F., Haubensak W., Haffner C. and Huttner W.B. (2005) Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J. Neurosci. 25, 6533–6538 10.1523/JNEUROSCI.0778-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kosodo Y., Roper K., Haubensak W., Marzesco A.M., Corbeil D. and Huttner W.B. (2004) Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 23, 2314–2324 10.1038/sj.emboj.7600223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paridaen J.T. and Huttner W.B. (2014) Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 15, 351–364 10.1002/embr.201438447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Javaherian A. and Kriegstein A. (2009) A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb. Cortex 19, i70–i77 10.1093/cercor/bhp029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S., Haigh K., Haigh J.J. and Vasudevan A. (2013) Endothelial VEGF sculpts cortical cytoarchitecture. J. Neurosci. 33, 14809–14815 10.1523/JNEUROSCI.1368-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee S., Chen T.T., Barber C.L., Jordan M.C., Murdock J., Desai S. et al. (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 10.1016/j.cell.2007.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wada T., Haigh J.J., Ema M., Hitoshi S., Chaddah R., Rossant J. et al. (2006) Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J. Neurosci. 26, 6803–6812 10.1523/JNEUROSCI.0526-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ottone C., Krusche B., Whitby A., Clements M., Quadrato G., Pitulescu M.E. et al. (2014) Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 16, 1045–1056 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirzadeh Z., Merkle F.T., Soriano-Navarro M., Garcia-Verdugo J.M. and Alvarez-Buylla A. (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278 10.1016/j.stem.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon G.S. and Hadjantonakis A.K. (2007) Eomes::GFP – a tool for live imaging cells of the trophoblast, primitive streak, and telencephalon in the mouse embryo. Genesis 45, 208–217 10.1002/dvg.20293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crouch E.E., Liu C., Silva-Vargas V. and Doetsch F. (2015) Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J. Neurosci. 35, 4528–4539 10.1523/JNEUROSCI.1188-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andreu-Agullo C., Morante-Redolat J.M., Delgado A.C. and Farinas I. (2009) Vascular niche factor PEDF modulates notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 12, 1514–1523 10.1038/nn.2437 [DOI] [PubMed] [Google Scholar]
- 96.Snapyan M., Lemasson M., Brill M.S., Blais M., Massouh M., Ninkovic J. et al. (2009) Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 29, 4172–4188 10.1523/JNEUROSCI.4956-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delgado A.C., Ferron S.R., Vicente D., Porlan E., Perez-Villalba A., Trujillo C.M. et al. (2014) Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83, 572–585 10.1016/j.neuron.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 98.Parthasarathy S., Srivatsa S., Nityanandam A. and Tarabykin V. (2014) Ntf3 acts downstream of Sip1 in cortical postmitotic neurons to control progenitor cell fate through feedback signaling. Development 141, 3324–3330 10.1242/dev.114173 [DOI] [PubMed] [Google Scholar]
- 99.Fox I.J., Daley G.Q., Goldman S.A., Huard J., Kamp T.J. and Trucco M. (2014) Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 10.1126/science.1247391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldman S.A. and Chen Z. (2011) Perivascular instruction of cell genesis and fate in the adult brain. Nat. Neurosci. 14, 1382–1389 10.1038/nn.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okano H. and Sawamoto K. (2008) Neural stem cells: involvement in adult neurogenesis and CNS repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2111–2122 10.1098/rstb.2008.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obernier K., Tong C.K. and Alvarez-Buylla A. (2014) Restricted nature of adult neural stem cells: re-evaluation of their potential for brain repair. Front. Neurosci. 8, 162 10.3389/fnins.2014.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]