Abstract
Community-acquired pneumonia (CAP) is the prominent cause of mortality and morbidity with important clinical impact across the globe. India accounts for 23 per cent of global pneumonia burden with case fatality rates between 14 and 30 per cent, and Streptococcus pneumoniae is considered a major bacterial aetiology. Emerging pathogens like Burkholderia pseudomallei is increasingly recognized as an important cause of CAP in Southeast Asian countries. Initial management in the primary care depends on clinical assessment while the hospitalized patients require combinations of clinical scores, chest radiography and various microbiological and biomarker assays. This comprehensive diagnostic approach together with additional sampling and molecular tests in selected high-risk patients should be practiced. Inappropriate therapy in CAP in hospitalized patients lengthens hospital stay and increases cost and mortality. In addition, emergence of multidrug-resistant organisms poses tough challenges in deciding empirical as well as definitive therapy. Developing local evidence on the cause and management should be a priority to improve health outcomes in CAP.
Keywords: Antimicrobial resistance, bacteria, CABP, community acquired pneumonia, diagnosis, management, Streptococcus pneumoniae
Introduction
Community-acquired pneumonia (CAP) is the leading cause of mortality and morbidity with substantial clinical and economic impact. Although several organisms are implicated with the disease, data on the pathogen distribution are not uniformly represented across the countries. Several factors such as geographical region, age and study period influence the incidence of CAP in adults. However, reliable and consistent data over a prolonged period are available from only a few countries. Reports suggest nearly 2.4 million deaths occur among all ages due to lower respiratory tract infections (LRTIs)1. Among these, sub-Saharan Africa, Southeast Asia and South Asia have documented higher fatality. In 2016, 197.05 million episodes (112.83-287.64) of pneumococcal pneumonia were reported worldwide and thus represented the leading cause of LRTI morbidity and mortality. Globally, mortality due to LRTI remained unchanged from 2005 to 2015 although age standardized death rates fell by 19.5 per cent1. In recent years, there has been a steady increase in the hospitalization rates including intensive care units (ICU) due to CAP, especially in the older population2. The case fatality rate ranges from 2 to 20 per cent reaching up to 50 per cent in patients admitted to ICUs and varies between healthcare settings, geographical region, patient categories and age3. This narrative review focuses on the bacterial CAP in immunocompetent adults with special emphasis on existing modalities and gaps in diagnostics, optimum utilization of testing strategies, and individualized therapy decisions with a focus on Indian scenarios.
Disease burden of community-acquired pneumonia in India and Southeast Asia
India contributes about 23 per cent of global pneumonia burden and 36 per cent WHO regional burden in patients under five years4. Reliable estimates of disease burden are not available particularly for the adult population. The sparse data for adults come from tertiary care teaching hospitals using cross-sectional studies5. A study from Mumbai reported that severe CAP (SCAP) reached 19 per cent of all patients and Streptococcus pneumoniae and Gram-negative bacteria (Pseudomonas aeruginosa and Klebsiella pneumoniae) had increased occurrence in severe pneumonia6. A recent review underscores the importance of pneumococci in the invasive pneumococcal diseases in India7. The reported case fatalities are between 14 and 30 per cent in all CAP patients and 47 per cent in SCAP. An overview of studies representing CAP in India is presented in Table I.
Table I.
Indian studies on community-acquired pneumonia highlighting the geographical distribution, aetiology and diagnostic tests
| Author | Site | Period | Number | Age | Methods | Pathogens (%) | Overall diagnostic yield (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Para et al8 | Kashmir | 2013-2015 | 225 | All adults | Blood culture Sputum culture Antigen detection Viral PCR |
Streptococcus pneumoniae (30.5) Legionella (17.5) Mycoplasma (7.2) Chlamydia pneumophila (5.5) Staphylococcus aureus (5.2) Klebsiella pneumoniae (4.8) Mycobacterium tuberculosis (4.8) Pseudomonas aeruginosa (3.1) Influenza viruses (15.4) |
72 | 8 |
| Nagesh Kumar et al9 | Bengaluru | 2012-2014 | 122 | All adults | Sputum culture Blood culture Immunofluorescence for IgM antibody against atypical bacterial and viruses (Pneumoslide-M assay) |
S. pneumoniae (15.6) K. pneumoniae (8.2) Mycoplasma pneumoniae (7.4) Legionella (5.7) Haemophilus influenzae (6.6) S. aureus (3.3) P. aeruginosa (3.3) |
60.7 | 8.2 |
| Bin et al10 | Bijapur | 2008-2010 | 50 | Adults ≥65 yr |
Sputum culture |
S. pneumoniae (16) K. pneumoniae (6) H. influenzae (4) P. aeruginosa (4) S. aureus (2) |
32 | 16 |
| Shah et al11 | Kashmir | 1998-2000 | 100 | All adults | Sputum culture Blood culture Transthoracic needle aspiration |
P. aeruginosa (9) S. aureus (6) Escherichia coli (5) K. pneumoniae (3) S. pneumoniae (1) |
29 | 14 |
| Dagaonkar et al6 | Mumbai | NR | 100 | All adults | Sputum culture Blood culture Urinary antigen Serology for atypical bacteria |
S. pneumoniae (23) Chlamydia (11) H. influenzae (9) Moraxella (6) Mycoplasma (5) Legionella (3) Klebsiella (3) P. aeruginosa (2) |
58 | 9 |
| Chaudhry et al12* | Delhi | 2011-2014 | 453 | Adults and children | Any respiratory specimen Legionella and Mycoplasma culture Urinary antigen Serology PCR |
M. pneumoniae (25.6) Legionella (27.2) |
NR | NR |
| Prasad and Bhat13 | Mangalore | NR | 165 | All adults | Sputum, BAL, other respiratory culture |
K. pneumoniae (29) P. aeruginosa (18.1) S. pneumoniae (13.1) H. influenzae (4.8) |
48 | 2.4 |
| Sharma et al14 | Pune | 2010-2012 | 85 | All adults | Sputum cultures |
K. pneumoniae (21.7) S. aureus (15.2) S. pneumoniae (12.9) |
NR | |
| Acharya et al15 | Mangalore | NR | 100 | All adults | Sputum cultures |
S. pneumoniae (31) P. aeruginosa (15) K. pneumoniae (13) S. aureus (8) Moraxella (8) E. coli (8) H. influenzae (5) |
39 | NR |
| Menon et al16 | Cochin | 2009 | 145 | All adults | Sputum cultures |
S. pneumoniae (32.4) K. pneumoniae (20) P. aeruginosa (8.9) E. coli (6.2) |
76 | NR |
*Tests done only for atypical bacterial pathogens. NR, not reported; BAL, bronchoalveolar lavage
The WHO global health estimates for 2016 shows 783,000 deaths due to LRTIs in Southeast Asia17. Comprehensive data on aetiology, clinical outcome and risk factors were reported by the Asian Network for Surveillance of Resistant Pathogens between 2002 and 2004 from eight Asian countries18. Pneumonia Severity Index (PSI) categories 4 and 5 comprised 28.4 per cent of patients. Among hospitalized (62.1%) patients, 9.4 per cent were admitted to ICUs. The overall mortality was 7.3 and 50.6 per cent among patients in PSI class 4 and 518. S. pneumoniae was the commonest pathogen implicated (29.2%), followed by atypical pathogens in 25 per cent and Gram-negative bacteria (K. pneumoniae, P. aeruginosa) in 22 per cent. Acute respiratory infections were the major contributors to sepsis in a Southeast Asian multicentre study19.
Bacterial pathogens in community-acquired pneumonia
Bacterial pathogens implicated in CAP [community-acquired bacterial pneumonia (CABP)] vary with geographic distribution and host characteristics. The laboratory test utilization practices, access to healthcare, guideline recommendations for testing and extent of laboratory facilities might further influence the reported pathogen frequency. Despite the geographical disparities, S. pneumoniae remains a predominant pathogen globally in all ages. Staphylococcus aureus, Haemophilus influenzae, K. pneumoniae, P. aeruginosa, and atypical pathogens, Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumophila are other pathogens contributing to the majority of CABP aetiology. A subset of bacterial pathogens that are resistant to multiple antimicrobial agents, sometimes referred as PES pathogens (Pseudomonas, Enterobacteriaceae, methicillin-resistant S. aureus), are of major concern due to challenging antimicrobial therapy20,21,22.
In a systematic review from India, S. pneumoniae was the predominant pathogen in CAP with the pooled proportion of 19 per cent [95% confidence interval (CI): 12-26%; I2=94.5%; P<0.01]. Other pathogens were M. pneumoniae [15.5% (1.1-35.5%)], K. pneumoniae [10.5% (1.6-24.0%)] and L. pneumophila [7.3% (2.5-23.8%)]23. Putting together data from all Asian studies, Peto et al24 reported Gram-negative bacilli (GNB) in 13 per cent of hospitalized CAP, more in Southeast Asia and India, increasing to 21.5 per cent in SCAP.
CABP pathogens with special relevance to India and other tropical countries
Aetiology of CABP shows variations in several tropical Asian countries posing challenges in diagnosis and management. Although S. pneumoniae was recognized as the most common aetiology of CAP in two systematic reviews, national and regional variations exist23,24. In a systematic review, >10 per cent of cases of CAP in Asia were attributed to Mycobacterium tuberculosis25. Due to overlapping features of acute respiratory distress syndrome (ARDS) and pneumonia, several fever syndromes in tropics are initially assessed as CAP. Scrub typhus, leptospirosis, malaria and dengue among others are important distractors in early recognition of CAP25. B. pseudomallei, a soil bacterium and causative agent of melioidosis is an important cause of CAP and sepsis in Thailand, India, Vietnam, Malaysia and other Southeast Asian countries26,27,28. B. pseudomallei was the second commonest pathogen in hospitalized CAP as reported from Thailand27. In India, several reports of melioidosis presenting as CAP exist, however, denote only a tip of the iceberg28. The lack of widely available standard tests and awareness has led to clinical and laboratory oversight in correctly diagnosing this condition.
Clinical diagnosis and assessment of the severity of CAP
CAP is suspected by acute symptoms such as dyspnoea, cough and fever and presence of new focal chest signs without other obvious cause, whereas new pulmonary infiltrate on a chest radiograph is required for a definite diagnosis29,30,31. Subgroups of patients as in elderly people, the clinical presentation can have less evident classical symptoms (may present with an altered state of consciousness, gastrointestinal discomfort and fever may be absent) delaying the diagnosis frequently.
RTIs are the most common reasons for unnecessary and inappropriate antimicrobial prescriptions in both primary and hospital settings, contributing significantly to the development of antimicrobial resistance (AMR)32. Since a vast majority of CAP are managed in primary care, it is essential for the primary care physicians to correctly identify and manage patients with CAP. Management of CAP focuses particularly on early identification of risk for severe disease and early administration of the appropriate antimicrobial agent, not ignoring the risk of development of AMR. Individual components of the history or physical examination are not reliable in accurately diagnosing pneumonia while the presence of several findings assists in the clinical decision. Only a few clinical scores have been developed to increase the likelihood of CAP diagnosis in primary care. These scores help ruling out bronchitis or upper respiratory infections. The Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) and European guidelines differ in their viewpoints on chest radiography (CR) in all cases of suspected pneumonia29,31, although studies have found CR as a useful tool in primary care33.
In the settings where CR is not routinely available, several clinical decision support system based on combinational symptoms and C-reactive protein (CRP) may be considered. The diagnosis of CAP was strongly associated with elevated CRP and positive CR (P<0.001)33. Negative CR may not, however, rule out pneumonia as it may not be present if the patient presents early. Risk for severity may be assessed by CRB 65 in locations where urea testing is not available. The measurement of oxygen saturation on room air using pulse oximetry is a simple non-invasive tool endorsed in numerous guidelines to aid the assessment of CAP30. A study involving 2,923 patients of CAP managed in outpatient departments in Canada analyzed the oxygen saturation and its association with patient outcome and reported oxygen saturation <90 per cent was significantly associated with 30 days mortality34. However, the physicians should adhere to the right application of this test and use only the approved instruments for measurements.
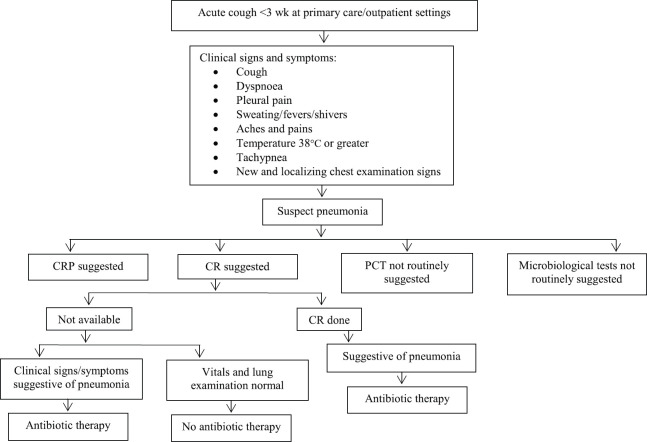
In adult outpatient settings, the American College of Chest Physicians has provided recommendations to rationalize the antibiotic use, reduce hospitalizations and improve outcome in patients presenting with acute cough for less than three weeks (Fig. 1)35 along with diagnostic indicators (Table II)36.
Fig. 1.
Summary of guidelines on the management of acute cough at primary care. CRP, C-reactive protein; CR, chest radiograph; PCT, procalcitonin. Source : Refs 35,36.
Table II.
Diagnostic performance measures of indicator tests at primary care in the diagnosis of community acquired pneumonia
| Specificity >80%* | Positive LR >2.0* | High diagnostic odds ratio* |
|---|---|---|
| Temperature >38°C | Temperature >38°C | Cough |
| Pulse rate >100/min | Pulse rate >100/min | Crackles |
| Crackles | Respiratory rate ≥20/min | Respiratory rate ≥20/min |
| Reduced breath sound | Crackles | Temperature >38°C |
| PCT >0.25 ng/ml and CRP >20 mg/l | Pulse rate >100/min | |
| Reduced breath sound | ||
| PCT >0.25 ng/ml and CRP >20 mg/l |
*Diagnostic performances for individual factors. PCT, procalcitonin; CRP, C-reactive protein; LR, likelihood ratio
Source: Ref. 36
SCAP is a progressive disease evolving from a local to systemic infection with the spectrum of sepsis-related complications requiring ICU admission. In the management of CAP patients, assessment of severity is fundamental not only to assign the appropriate site of care but also to select empirical antibiotic and adjuvant therapy. During the assessment of pneumonia, it is crucial to identify organ dysfunctions and disease severity as even a mild dysfunction is associated with 10 per cent excess mortality37.
Predisposing factors contributing to SCAP have been identified as increasing age, alcoholism, chronic obstructive pulmonary disease (COPD), renal disease, chronic heart disease and immunosuppression38. The mortality in SCAP may go up to 50 per cent39, however, a few studies have shown a decline in mortality presumably due to advancement in intensive care management, adherence to treatment guidelines and early administration of appropriate therapy40. In a review article, Pereira et al41 narrated the comparative benefits of several pneumonia specific severity scores in the management of CAP. The PSI and CURB 65 scores are good at predicting 30 days mortality but do not assess for CAP complications which is an important step in the early stabilization of SCAP patients. The latter has been better addressed by IDSA/ATS 2007 and SMART-COP scores29,42. Studies have identified delayed ICU admission as a short-term risk factor for mortality in CAP patients29,42. Besides, differing epidemiology and aetiology of CAP in different geographical regions highlight the need for research with alternate clinical endpoints other than mortality alone37,43.
Microbiological diagnostics in CABP-bridging ideal and real with a quest to future diagnostics
Respiratory infections are the most common precipitating conditions leading to sepsis. The aetiological diagnosis of bacterial pneumonia supports early appropriate antimicrobial therapy and reduce mortality and morbidity. In the absence of a gold standard diagnostic test for CAP, establishing aetiological diagnosis fails in >50 per cent of patients due to the challenges in identifying the implicating pathogen in the laboratory44. According to REACH multinational study, 35-67 per cent patients with CAP did not have microbiological diagnosis resulting in high empirical antibacterial therapy45. Diagnostic testing in patients with suspected pneumonia is driven mostly by the type of care facility (inpatient, outpatient, ICU), disease severity, access to healthcare and availability of clinically useful tests46. Several guidelines recommend microbiological testing based on clinical severity29,30,31. Comprehensive microbiological assessment in CAP has shown to be a useful approach in antimicrobial stewardship.
Diagnostic utility of sputum Gram's stain and culture in CABP
The yield of sputum cultures for bacteria in patients with suspected pneumonia has a variable outcome and influenced by the quality of the specimen, subsequent analytical process and prior antibiotic therapy29. In elderly patients, the inability to expectorate good-quality sputum limits its usefulness. Studies show varying reports on the culture requests, quality and yield of sputum specimen. A study from Brazil reported 78.8 per cent of patients of CAP presented with expectoration while only 33.6 per cent of them were subjected to bacteriological tests. Despite productive cough, 45 per cent were unable to provide a sample for testing or physician failed to order the tests. Only 13.5 per cent samples were satisfactory for analysis and aetiological agents were detected only in 28.2 per cent of these samples47. Antibiotic exposure before LR sampling occurred in 84.8 per cent patients in another study, significantly reducing the culture yield (P<0.0001; odds ratio: 9.1; 95% CI: 4.1-22.4)48. Further, culture return is influenced by the time to transport and processing of specimen and relative abundance of oral flora. Interpreting positive cultures would be problematic in situations where pathogens are also known to be the colonizers of airway or in mixed infections.
In Asian countries, detection of B. pseudomallei, a common cause of severe pneumonia and sepsis, from expectorated sputum is a challenge due to overgrowth by other commensals and the delayed growth of B. pseudomallei which may take three or more days to grow in cultures. The prior exposure to amoxicillin-clavulanic acid and fluoroquinolones to which this bacterium is susceptible, further reduces the culture yield in respiratory samples. Inexperienced laboratory personnel might disregard the culture growth as non-fermenting GNB leading to underreporting. In a study from south India, the routine culture of expectorated sputum could detect only one in six cases of polymerase chain reaction (PCR) confirmed melioidosis49. In the endemic regions, high index of suspicion, special culture methods (enrichment culture) and prolonged incubation of culture plates are essential in patients with risk factors for melioidosis.
Analyzing cellular response in the expectorated specimen by Gram's stain is a good tool to screen sample quality in CAP, however, the interpreter must be aware of the other conditions displaying similar results as in acute exacerbation of COPD or acute/chronic bronchitis. Culture from these conditions shows similar pathogens as CAP such as S. pneumoniae, H. influenzae or Moraxella50. Gram's stain is variable in its sensitivity for early identification of aetiology. From a good-quality specimen, the sensitivity for the detection of S. pneumoniae and H. influenzae was 35.4 and 42.8 per cent, and specificity 96.7 and 99.4 per cent, respectively, when there was a single or predominant morphotype (90%)51,52. Another study on good-quality sputum samples which was possible in 63 per cent of patients showed a diagnostic sensitivity of 76 per cent for S. aureus, 79 per cent H. influenzae, 82 per cent S. pneumoniae and 78 per cent Gram-negative bacteria53. A recent meta-analysis considering the diagnostic threshold of >50 per cent for predominant morphotype reported a pooled sensitivity of 64 per cent for GNB, 72 per cent for S. aureus and 78 per cent for H. influenzae. The positive likelihood ratio was highest for GNB (37.49), followed by H. influenzae (21.08), S. aureus (16.27) and least for S. pneumoniae (4.60)54. Besides poor to moderate sensitivity, Grams stain is affected by lack of quality control tool and high interobserver variations when observed by different technologists55. Gram's stain is still a useful tool in the early recognition of S. pneumoniae and H. influenzae pneumonia in antibiotic-naïve patients in both outpatient and inpatient settings. In primary care, non-availability of the skilled microbiologist is another limitation for Gram's stain utility.
Invasive techniques to collect respiratory samples
Several invasive techniques such as thoracocentesis, transthoracic needle aspiration (TNA) of infected lung site, bronchoscopic protected specimen brush and bronchoalveolar lavage (BAL) are practiced in different clinical situations and severity states. Diagnostic thoracocentesis should be attempted in patients with pneumonia and an associated pleural effusion although effusion occurs in 40 per cent of patients. Despite poor sensitivity, the bacterial organism when detected reflects an accurate aetiology. However, published reports represent poor clinical relevance of the pleural fluid culture with regard to therapy modifications and patient outcome56. To improve the disease ascertainment, pleural fluid should be additionally cultured into the commercial automated blood culture bottles wherein 21 per cent incremental increase in the yield is demonstrated57. TNA allows specimen collection directly from the infected focus in the lung without contamination by the upper airway flora. Culture of TAN has shown varied sensitivities between 33 and 80 per cent58 and overall yield increased by combinational testing procedures. Culture of BAL demonstrated good sensitivity (80%) in the detection of bacterial pathogens in patients who did not show improvement in the initial three days of therapy59. In contrast, the low sensitivity is reported in studies involving subgroup of patients who received antibiotics60. Early stratification of patients with a risk for severe pneumonia is valuable in determining who would benefit from the invasive procedures for microbiological sampling. Although invasive sampling increases the yield slightly as the disease progresses in severity, the laboratory result might not be clinically relevant at the stage of sepsis.
Antigen tests
Urinary antigen testing (UAT) is a useful rapid point-of-care (POC) test in diagnosing respiratory infection caused by S. pneumoniae and L. pneumophila. Guidelines from developed nations recommend UAT in moderate and severe grade CAP29,30. In a large European multicentre study, S. pneumoniae emerged as a predominant pathogen in CAP and 71 per cent of 916 patients with pneumococcal CAP was exclusively diagnosed by UAT with a sensitivity and specificity of 60 and 99.7 per cent, respectively61. Incremental diagnosis of pneumococcal pneumonia in 43.8 per cent was demonstrated in another study using UAT. Authors demonstrated targeted antimicrobial therapy in 8.6 per cent of all CAP with favourable outcome62. The sensitivity of pneumococcal urinary antigen (UA) did not decline despite prior antibiotic therapy63. Disease severity positively correlated with UA detection. A meta-analysis reported a pooled sensitivity and specificity of 74 and 97.2 per cent in diagnosing pneumococcal CAP64. Apart from urine, empyema fluid was also found to be a useful sample for pneumococcal antigen test with a sensitivity and specificity of 71 and 93 per cent65.
Legionella UAT has gained prominence due to the lack of alternate diagnostic strategies. UA is detected in 2-3 days after clinical symptoms appear66. Concentrating urine specimen increases the sensitivity of Legionella UAT. Despite early initiation of specific treatment and direct impact on clinical management, routine testing of Legionella may not influence outcome and cost benefit is debatable in low prevalence settings or in patients without some clinical features suggestive of legionellosis67. An introduction of rapid POC test for the detection of B. pseudomallei antigen in any clinical specimen (whole blood has poor yield) has shown to be a useful tool in early recognition of melioidosis. This test has a comparable performance with PCR and special enrichment culture. The high negative predictive value of this test [98.57% (CI: 94.65 to 99.63%) 0.846; P<0.001] is an added advantage to rule out this disease during the early evaluation of SCAP as the antimicrobial therapy of this disease differs from the conventional CAP therapy68.
The universal UAT recommendations by CAP guidelines have prompted the evaluation of the usefulness of these tests and the results are presented recently69. The results indicate that the CAP guidelines show poor sensitivity in identifying patients with positive results. No clinical characteristics were strongly associated with positive pneumococcal UATs, while features associated with positive Legionella UATs were hyponatremia, fever, diarrhoea and recent travel.
Blood cultures in CABP
Fever is a common symptom in pneumonia, and reflex blood culture orders have been a common practice. IDSA/ATS guidelines29 recommend blood cultures in SCAP and those with risk for SCAP while European Respiratory Society and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommend in all patients hospitalized with CAP31. In uncomplicated CAP, blood cultures have a relatively low yield of 6-9 per cent. A recent study on 517 consecutive hospitalizations with CAP, 95 per cent had blood cultures drawn resulting in overall positivity of 8.5 per cent. SCAP showed 13.8 per cent positivity while non-SCAP had only 7.9 per cent yield of blood cultures. Only 65 per cent of bacteraemic CAP had organisms that were likely pneumonia related while 35 per cent had bacteria implicated from non-pulmonary source70.
The limitations of sputum cultures such as low yield, difficulty in differentiating colonizers and pathogens and loss of viability of pathogens particularly S. pneumoniae and H. influenzae if specimen transport is delayed, might be partially overcome by blood cultures in SCAP. The blood cultures techniques need special attention in developing nations such as to collect appropriate volume and number of sets, entering the blood culture system with minimum delay (<2 h) and immediate processing of positive signalled bottles, all contributing to better yield in pneumococcal bacteremia71. With the high background resistance and increasing occurrence of CAP by Gram-negative bacteria, it is appropriate to perform blood cultures in severe disease. Despite low yield, it is still beneficial to collect blood cultures in all hospitalized patients with CAP.
The microbial aetiology and bacteraemia are independently associated with severity of illness and sepsis in CAP38. Blood culture yield increases in severe pneumonia needing ICU admissions and in those with risk factors such as asplenia, chronic liver diseases, leukopenia and alcoholism. In these situations, blood culture positivity goes up to 33 per cent72. Bacteria associated with SCAP such as S. aureus, S. pneumoniae, Enterobacteriaceae and Pseudomonas are likewise responsible for bacteraemia. Blood culture positivity rose from 9.5 (0-1 organ failure) to 15.6 per cent (≥2 organ failure) with increasing numbers of organ failure in a study72.
Development of bacteraemia has been studied by several investigators in pneumococcal CAP. Varying blood culture positivity has been shown reaching up to 45 per cent in CAP patients. Most studies demonstrated an increase in the in-hospital mortality in bacteraemic pneumococcal pneumonia73. Analysis of Etiology of Pneumonia in the Community (EPIC) study data from the USA showed the presence of bacteraemia in 56.7 per cent of cases of CAP by S. aureus74. Another study demonstrated bacteraemia in 20 per cent of S. aureus pneumonia, and presence of bacteraemia independently contributed to a six-fold risk of mortality75. An Indian study on S. aureus bacteraemia revealed respiratory source in 24 per cent of patients76.
In Asian and African countries, K. pneumoniae has been found to be increasingly associated with bacteraemia CAP77,78. Increased mortality due to SCAP by this bacteria and detection of hypervirulence strains have been demonstrated. Pneumonia by B. pseudomallei in the Asian region is associated with a high rate of bacteraemia and mortality. Reports from India showed lungs as a common source of bacteraemic melioidosis while 54 per cent of pulmonary melioidosis had bacteraemia79,80. In a Thai study, 56 per cent of bacteraemic melioidosis had pneumonia81.
Molecular tests
The recent outbreaks such as pandemic influenza and Middle East respiratory syndrome coronavirus (MERS CoV) have largely contributed to the wider availability and renewed interests on the molecular assays in CAP diagnosis. The nucleic acid tests (NAT) have several advantages as these detect low levels of pneumonia pathogens, not affected by prior antibiotic therapy and provide results within a clinically relevant time frame. Further, atypical bacterial pathogens not routinely detected by conventional culture are increasingly diagnosed by NATs82. In recent years, molecular techniques based on multiplex PCR are developed to simultaneously detect and quantify multiple respiratory pathogens along with resistance genes83. The NATs are generally customized to individual healthcare settings providing an enhanced aetiological diagnosis over routine culture and antigen tests48,84.
Several commercial multiplex platforms are available for comprehensive molecular testing of CAP pathogens including atypical bacteria and viruses. Curetis Unyvero, a cartridge-based PCR to detect 18 bacterial and one fungal pathogen, has shown enhanced yield over conventional culture (55 vs. 8.2%) in the diagnosis of severe nosocomial pneumonia. Short turnaround time (TAT) of 6.5 h favours its usefulness in ICU settings85. This system has been tested on BAL samples in ICU patients and shown to increase pathogen detection and positive predictive value in diagnosing Gram-negative bacteria with a sensitivity and specificity of 68.4 and 86 per cent86. Another multiplex PCR, Fast Track Diagnostics FTD-29.19, tests nine bacterial pathogens and has demonstrated more pathogen detection in 37 per cent over 11 per cent by conventional methods in elderly patients87. Multiplexing several gene targets for detection of atypical pathogens has shown comparable performance with in-house individual PCRs in a study demonstrating overall 52 per cent PCR positivity in patients suspected with atypical pneumonia88.
The BioFire FilmArray Pneumonia panel plus is a recent Food and Drug Administration (FDA) cleared rapid POC NAT to detect 18 bacteria (11 Gram-negative, 4 Gram-positive, 3 atypical, 14 reported semiquantitatively), seven antibiotic resistance markers and nine viruses causing pneumonia and other LRTIs with a total TAT of 60 min. A study comparing the investigational use only version of this test with standard of care (SOC) methods such as culture and PCR on BAL samples showed a positive and negative correlation of 96.2 and 97.6 per cent. Depicted false-positive and false-negative results by FilmArray was found in patients receiving antibiotics within 72 h and in those specimen containing significant normal flora obscuring the pathogen in SOC cultures. Among the evaluable patients, antibiotic modifications were achievable in 68 per cent patients89.
Even after demonstrating satisfactory performance and good analytical sensitivity, interpretations of molecular tests face the challenges of discriminating pathogen from colonization for those organisms forming a part of normal flora. Certain platforms providing semiquantitative results might be a useful solution in this regard. The wide pathogen spectra in the commercial molecular tests are alluring for POC tests but at the same time suffer setbacks due to high cost involved and the inability to customize the test panel to individual patient settings, risk categories and geographical locations. The absence of antiviral treatment for most of the identified viruses further impedes the test utilization.
Biomarkers in CABP
Fundamental problems in establishing pneumonia aetiology using conventional methods have prompted the search for a biomarker in the bloodstream as a result of the infection process in the lung. Two approaches, to differentiate bacterial or viral aetiology of CAP and predict the severity of the disease are of special interest to researchers and care providers. In differentiating viral or bacterial aetiologies, proteins of acute phase inflammation and signalling molecules are potential indicators90,91. Cytokine regulatory network as a result of alveolar macrophage recruitment as first line defence marks the basis of the immune response in lung pathologies. Only procalcitonin and C-reactive protein are the most used biomarkers in clinical practice while several others are fully investigated for their clinical utility.
A systematic review assessed the diagnostic value of CRP in primary care and emergency department to rule in or rule out CAP92. At the cut-off value of CRP ≤20 mg/l, the pooled positive likelihood ratio (LR) was 2.1 (95% CI: 1.8-2.4) and pooled negative LR 0.33 (95% CI: 0.25-0.43). The results did not produce homogenous LR at the cut-off values of ≤50 and >100 mg/l. Based on several randomized controlled trials and other studies, the National Institute for Health and Care Excellence guidelines suggest not to use antibiotics routinely if CRP is <20 mg/l in patients with symptoms of LRTI in primary care93. In patients admitted to ICUs, the diagnostic ability of CRP to identify bacterial pneumonia is only 0.64 by area under the curve (AUC)94.
PCT levels showed good sensitivity (84%) to differentiate mixed bacterial and viral pneumonia in a meta-analysis95. The specificity was 64 per cent when viral pneumonia had a secondary bacterial infection. The discriminatory power of PCT to differentiate viral and bacterial pneumonia was better than CRP (AUC 0.76)94. There was no association of PCT levels with individual bacterial pathogens; however, the value was higher in pneumonia by typical bacteria and not atypical bacteria96,97. The science and practicality of other less important biomarkers are reviewed by a few others but none have shown a wide clinical application98.
Comprehensive diagnostic approach and future diagnostics
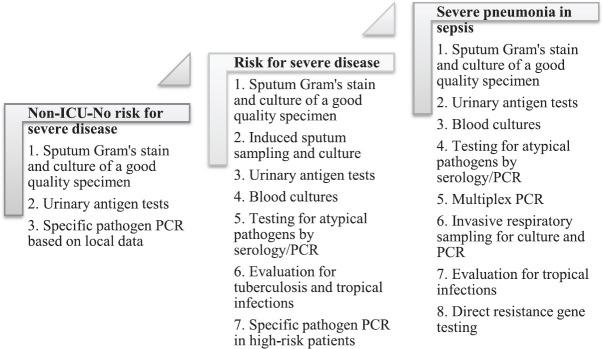
A variety of diagnostic tools targeting pathogens and biomarkers have gained prominence in the aetiological diagnosis of CABP while very few tests have been considered in well-designed clinical outcome studies. Not any one test is likely to replace another in the time to come but only to complement each other to offer an overall betterment in the clinically relevant yield. Pulmonary infections, being one of the greatest challenges to diagnostics, suffer an inherent drawback of best sampling time and methods. Globally, CAP diagnostics face contrasting situations in developed and developing nations. On one hand, there is an advancement in syndromic and high sensitivity detection methods while on the other hand, there is a lack of healthcare access, non-availability of diagnostic tests including RDTs in the developing nations where highest disease burden and AMR are prevalent. Most of the management guidelines are based on data from developed nations and are mostly country/region specific while there is a paucity of high-quality data from developing nations. As no single test detects all pathogens in a given setting, the approach of customized sampling and testing should be the priority. Moreover, any approach should be based on the reliable baseline data, local epidemiology, and type of healthcare settings and appropriate risk stratification of the patients with CAP. Management protocols should target alternate outcome priorities such as length of stay, time to clinical resolution and antibiotic days/de-escalation time. A comprehensive and schematic guide to diagnostics based on large studies on hospitalized patients is provided in Figure 2.
Fig. 2.
Microbiological tests that may be adapted for comprehensive sampling strategy in community acquired pneumonia-based on disease severity and underlying risk in hospitalized patients. ICU, intensive care unit; PCR, polymerase chain reaction. Source : Refs 29,38,54,58,59,82,85,86,88.
Recent advancement in sepsis research has added a new dimension to the management of severe infections including CAP by precision medicine. Besides pathogen-specific diagnostics including rapid tests, a new approach is to understand the molecular endotypes, gene expressions and transcriptomic analysis. Blood microarray analysis has identified a few molecular biomarkers that are expected to improve knowledge on host response to infection and customize the therapy99.
Outcome of CABP and associated factors
CAP is identified as the commonest cause of sepsis and septic shock in adults. GenOSept study from Europe showed ICU mortality of 19 per cent and independent factors associated with outcome were APACHE II score, haematocrit, mechanical ventilation and blood pH. S. aureus was related with SCAP fatality100. Another study on non-streptococcal SCAP demonstrated shock at admission and acute kidney injury as significant risk factors for mortality while combined antibiotic therapy and early antibiotic therapy within three hours were associated with a favourable outcome. Legionella and P. aeruginosa were less likely to be covered empirically and hence showed worse outcome101. The association of microbial aetiology with mortality is less understood. Gram-negative bacterial pneumonia and bacteraemia were found to be independent risk factors for mortality, and S. aureus pneumonia was shown to be associated with more organ failure72.
Optimum therapy and individualization
Inappropriate initial antimicrobial therapy (IIAT) in patients with CAP is associated with longer hospital stays, increased hospital costs and mortality102. Prediction of likely pathogen and knowledge of local susceptibility patterns is the key to initiate appropriate therapy (IAT). Adherence to guidelines has shown better outcomes in American and European studies. Guidelines tailored to national and regional contexts are essential considering the differences in socio-economic factors, healthcare systems, local healthcare access, variations in pathogen occurrence and susceptibility. Data on the common CAP pathogens and susceptibilities are lacking from the developing world. In India, due to the high overall prevalence of Gram negative bacterial infections including pneumonia, the CAP national guidelines suggest empirical therapy with β-lactam-β-lactamase inhibitor combinations combinations along with macrolide in hospitalized CAP. The empirical use of fluoroquinolones is generally avoided due to the high tuberculosis incidence103.
In India, high prevalence of tuberculosis and noteworthy proportion presenting as CAP points towards the urgent need of locally relevant management guidelines. Decisions to cover atypical pathogens empirically are controversial among international guidelines. A systematic review from China showed Mycoplasma as a predominant CAP pathogen in adults104. Following the global reduction in drug-resistant S. pneumoniae (DRSP), it is prudent to use β-lactam antibiotics as empirical therapy in hospitalized patients. Analysis of global data identified asthma, liver disease and non-cystic fibrosis bronchiectasis as independent risk factors for DRSP. Another determinant of IIAT is the local prevalence of multidrug-resistant organisms (MDROs). However, the health benefit of IIAT using broad spectrum and last resort antibiotics is a double edged sword. The risk of MDROs increases with prior hospitalization, antibiotic exposure and the presence of MDRO in the local environment. The mere isolation of easily cultivable MDROs such as methicillin-resistant S. aureus, K. pneumoniae and P. aeruginosa may not always indicate disease but might mask the isolation of S. pneumoniae or prevent further workup on other atypical/viral pathogens in resource constrained settings. Combined clinical scores and risk for MDROs should be used before the selection of empirical therapy in selected situation105. For that, a careful assessment of risk before initiation of empirical therapy could improve outcome.
The duration of treatment of CAP has gained attention in the era of AMR. International guidelines recommend a minimum five days of therapy and early discontinuation based on clinical stability criteria29,31, but the information regarding the real clinical practice is minimal in the literature. A RCT endorsed the short duration therapy based on clinical stability criteria in non-ICU hospitalized patients without any adverse outcome106. A longer duration therapy is considered for extrapulmonary involvement, delay in establishing aetiological diagnosis and pulmonary complications29,31.
Immunization in CABP
Given the increased mortality and morbidity of CAP, particularly in older adults, Advisory Committee on Immunization Practices (ACIP) recommends107 routine use of pneumococcal vaccines for all adults ≥65 yr and adults >18 yr with risk factors. Two vaccines, PCV 13 and PPSV23, have been used with varied coverage between the countries. In line with ACIP, Association of Physicians of India (API) recommends pneumococcal and influenza vaccines for all adults >18 yr in India108. However, data on vaccine coverage and health benefits are lacking. The clinical benefit of adult pneumococcal vaccination is conflicting109,110. The lack of clinical benefit might be due to the shift in pathogen occurrence and thus showing a null effect on all cause pneumonia outcomes. Despite controversies, it is prudent to administer the vaccine to high-risk group adults given the benefits and safety of the vaccine.
Conclusions
CABP contributes to significant healthcare burden with higher impact on the developing countries. Lack of appropriate and rapid diagnostics delay the care adding to the adverse outcomes. Aetiological variations are driven by the geographical regions, climate, environmental factors, AMR, quality of healthcare and test availability. The transition towards resistant GNB infections challenges the therapy choices. Clinical and diagnostic decision support systems should be developed to assist the risk stratification of patients and utilize the laboratory tests optimally. Knowledge of endemic pathogens of CAP will further clarify the management pathways.
Acknowledgment
Authors thank Dr Joao Coimbra, Internal Medicine Physician, Sao Joao Hospital, Porto, Portugal, for his helpful comments on the manuscript.
Footnotes
Financial support & sponsorship: The study was funded in part by Centro de Investigacion Biomedica en Red de Enfermedades Respiratorias (CIBERES), Institute of Health Carlos III, Madrid, Spain
Conflicts of Interest: Dr Rello served in the speaker's bureau or consultant for Pfizer, Anchoagen, and ROCHE. The remaining authors have no conflicts of interest to declare.
References
- 1.GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806–12. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 3.Heo JY, Song JY. Disease burden and etiologic distribution of community-acquired pneumonia in adults: Evolving epidemiology in the era of pneumococcal conjugate vaccines. Infect Chemother. 2018;50:287–300. doi: 10.3947/ic.2018.50.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooqui H, Jit M, Heymann DL, Zodpey S. Burden of severe pneumonia, pneumococcal pneumonia and pneumonia deaths in Indian states: Modelling based estimates. PLoS One. 2015;10:E0129191. doi: 10.1371/journal.pone.0129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghimire M, Bhattacharya SK, Narain JP. Pneumonia in South-East Asia Region: Public health perspective. Indian J Med Res. 2012;135:459–68. [PMC free article] [PubMed] [Google Scholar]
- 6.Dagaonkar RS, Udwadia ZF, Sen T, Nene A, Joshi J, Rastogi SA, et al. Severe community acquired pneumonia Mumbai, India: Etiology and Predictive Value of the Modified British Thoracic Society Rule, American Thoracic Society. 2012:A6060. [Google Scholar]
- 7.Koul PA, Chaudhari S, Chokhani R, Christopher D, Dhar R, Doshi K, et al. Pneumococcal disease burden from an Indian perspective: Need for its prevention in pulmonology practice. Lung India. 2019;36:216–25. doi: 10.4103/lungindia.lungindia_497_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Para RA, Fomda BA, Jan RA, Shah S, Koul PA. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India. 2018;35:108–15. doi: 10.4103/lungindia.lungindia_288_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagesh Kumar TC, Rafiudeen R, Rashmi K. A study of clinical and etiological profile of community-acquired pneumonia with special reference to atypical pneumonia. Ann Niger Med. 2017;11:11. [Google Scholar]
- 10.Bin AB, Zoheb M, Ashraf SM, Ali S, Nausheen N. A study of community-acquired pneumonias in elderly individuals in Bijapur, India. ISRN Pulmonol. 2012;2012:936790. [Google Scholar]
- 11.Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Lung India. 2010;27:54–7. doi: 10.4103/0970-2113.63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry R, Valavane A, Sreenath K, Choudhary M, Sagar T, Shende T, et al. Detection of Mycoplasma pneumoniae and Legionella pneumophila in patients having community-acquired pneumonia: A multicentric study from New Delhi, India. Am J Trop Med Hyg. 2017;97:1710–6. doi: 10.4269/ajtmh.17-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad P, Bhat S. Clinicomicrobiological study of community-acquired pneumonia. Lung India. 2017;34:491–2. doi: 10.4103/lungindia.lungindia_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R, Deoskar R, Bargaje M, Kumar P, Agarwal Y. A study of etiological and clinical profile of community acquired pneumonia in a tertiary care hospital in Western India. Eur Respir J. 2013;42:P2766. [Google Scholar]
- 15.Acharya VK, Padyana M, Unnikrishnan B, Anand R, Acharya PR, Juneja DJ. Microbiological profile and drug sensitivity pattern among community acquired pneumonia patients in tertiary care centre in Mangalore, coastal Karnataka, India. J Clin Diagn Res. 2014;8:MC04–6. doi: 10.7860/JCDR/2014/7426.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon RU, George AP, Menon UK. Etiology and anti-microbial sensitivity of organisms causing community acquired pneumonia: A single hospital study. J Family Med Prim Care. 2013;2:244–9. doi: 10.4103/2249-4863.120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Disease burden and mortality estimates. Geneva: WHO; 2019. [Google Scholar]
- 18.Song JH, Oh WS, Kang CI, Chung DR, Peck KR, Ko KS, et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: A prospective study by the Asian Network for Surveillance of Resistant Pathogens. Int J Antimicrob Agents. 2008;31:107–14. doi: 10.1016/j.ijantimicag.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southeast Asia Infectious Disease Clinical Research Network. Causes and outcomes of sepsis in Southeast Asia: A multinational multicentre cross-sectional study. Lancet Glob Health. 2017;5:E157–67. doi: 10.1016/S2214-109X(17)30007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prina E, Ranzani OT, Polverino E, Cillóniz C, Ferrer M, Fernandez L, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12:153–60. doi: 10.1513/AnnalsATS.201407-305OC. [DOI] [PubMed] [Google Scholar]
- 21.Restrepo MI, Babu BL, Reyes LF, Chalmers JD, Soni NJ, Sibila O, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur Respir J. 2018:52. doi: 10.1183/13993003.01190-2017. pii: 1701190. [DOI] [PubMed] [Google Scholar]
- 22.Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis. 2013;13:268. doi: 10.1186/1471-2334-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghia CJ, Dhar R, Koul PA, Rambhad G, Fletcher MA. Streptococcus pneumoniae as a cause of community-acquired pneumonia in Indian adolescents and adults: A systematic review and meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2019;13:1179548419862790. doi: 10.1177/1179548419862790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peto L, Nadjm B, Horby P, Ngan TT, van Doorn R, Van Kinh N, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: A systematic review. Trans R Soc Trop Med Hyg. 2014;108:326–37. doi: 10.1093/trstmh/tru058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim TK, Siow WT. Pneumonia in the tropics. Respirology. 2018;23:28–35. doi: 10.1111/resp.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rammaert B, Beauté J, Borand L, Hem S, Buchy P, Goyet S, et al. Pulmonary melioidosis in Cambodia: A prospective study. BMC Infect Dis. 2011;11:126. doi: 10.1186/1471-2334-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reechaipichitkul W, Lulitanond V, Tantiwong P, Saelee R, Pisprasert V. Etiologies and treatment outcomes in patients hospitalized with community-acquired pneumonia (CAP) at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. 2005;36:156–61. [PubMed] [Google Scholar]
- 28.Mukhopadhyay C, Shaw T, Varghese GM, Dance DAB. Melioidosis in South Asia (India, Nepal, Pakistan, Bhutan and Afghanistan) Trop Med Infect Dis. 2018:3. doi: 10.3390/tropicalmed3020051. pii: E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.British Thoracic Society. BTS Guidelines for the management of community acquired pneumonia in adults. Br Thorac Soc. 2009;64:1–22. [Google Scholar]
- 31.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections - Summary. Clin MicrobiolInfect. 2011;17(Suppl 6):1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 32.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moberg AB, Taléus U, Garvin P, Fransson SG, Falk M. Community-acquired pneumonia in primary care: Clinical assessment and the usability of chest radiography. Scand J Prim Health Care. 2016;34:21–7. doi: 10.3109/02813432.2015.1132889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: A population-based cohort study. Clin Infect Dis. 2011;52:325–31. doi: 10.1093/cid/ciq076. [DOI] [PubMed] [Google Scholar]
- 35.Hill AT, Gold PM, El Solh AA, Metlay JP, Ireland B, Irwin RS, et al. Adult outpatients with acute cough due to suspected pneumonia or influenza: CHEST guideline and expert panel report. Chest. 2019;155:155–67. doi: 10.1016/j.chest.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Htun TP, Sun Y, Chua HL, Pang J. Clinical features for diagnosis of pneumonia among adults in primary care setting: A systematic and meta-review. Sci Rep. 2019;9:7600. doi: 10.1038/s41598-019-44145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira JM, Paiva JA, Rello J. Assessing severity of patients with community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33:272–83. doi: 10.1055/s-0032-1315639. [DOI] [PubMed] [Google Scholar]
- 38.Montull B, Menéndez R, Torres A, Reyes S, Méndez R, Zalacaín R, et al. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS One. 2016;11:E0145929. doi: 10.1371/journal.pone.0145929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Solé-Violán J, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–8. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 40.Rello J, Diaz E, Mañez R, Sole-Violan J, Valles J, Vidaur L, et al. Improved survival among ICU-hospitalized patients with community-acquired pneumonia by unidentified organisms: A multicenter case-control study. Eur J Clin Microbiol InfectDis. 2017;36:123–30. doi: 10.1007/s10096-016-2779-5. [DOI] [PubMed] [Google Scholar]
- 41.Pereira JM, Paiva JA, Rello J. Severe sepsis in community-acquired pneumonia – Early recognition and treatment. Eur J Intern Med. 2012;23:412–9. doi: 10.1016/j.ejim.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137:552–7. doi: 10.1378/chest.09-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupisan S, Suzuki A, Macalalad N, Egos R, Sombrero L, Okamoto M, et al. Etiology and epidemiology of community-acquired pneumonia in adults requiring hospital admission: A prospective study in rural Central Philippines. Int J Infect Dis. 2019;80:46–53. doi: 10.1016/j.ijid.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Bjarnason A, Westin J, Lindh M, Andersson LM, Kristinsson KG, Löve A, et al. Incidence, etiology, and outcomes of community-acquired pneumonia: A population-based study. Open Forum Infect Dis. 2018;5:ofy010. doi: 10.1093/ofid/ofy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blasi F, Ostermann H, Racketa J, Medina J, McBride K, Garau J, et al. Early versus later response to treatment in patients with community-acquired pneumonia: Analysis of the REACH study. Respir Res. 2014;15:6. doi: 10.1186/1465-9921-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carugati M, Aliberti S, Reyes LF, Franco Sadud R, Irfan M, Prat C, et al. Microbiological testing of adults hospitalised with community-acquired pneumonia: An international study. ERJ Open Res. 2018:4. doi: 10.1183/23120541.00096-2018. pii: 00096-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Signori LGH, Ferreira MW, Vieira LCH, Müller KR, De Mattos WLL. Sputum examination in the clinical management of community-acquired pneumonia. J Bras Pneumol. 2008;34:152–8. doi: 10.1590/s1806-37132008000300005. [DOI] [PubMed] [Google Scholar]
- 48.Gadsby NJ, Russell CD, McHugh MP, Mark H, Conway Morris A, Laurenson IF, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62:817–23. doi: 10.1093/cid/civ1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tellapragada C, Shaw T, D'Souza A, Eshwara VK, Mukhopadhyay C. Improved detection of Burkholderia pseudomallei from non-blood clinical specimens using enrichment culture and PCR: Narrowing diagnostic gap in resource-constrained settings. Trop Med Int Health. 2017;22:866–70. doi: 10.1111/tmi.12894. [DOI] [PubMed] [Google Scholar]
- 50.Barnes P, Drazen J, Rennard S, Thomson N, editors. Asthma and COPD: Basic mechanisms and clinical management. 2nd ed. Cambridge (MA): Academic Press; 2008. [Google Scholar]
- 51.Miyashita N, Shimizu H, Ouchi K, Kawasaki K, Kawai Y, Obase Y, et al. Assessment of the usefulness of sputum Gram stain and culture for diagnosis of community-acquired pneumonia requiring hospitalization. Med Sci Monit. 2008;14:CR171–6. [PubMed] [Google Scholar]
- 52.Butler JC, Bosshardt SC, Phelan M, Moroney SM, Tondella ML, Farley MM, et al. Classical and latent class analysis evaluation of sputum polymerase chain reaction and urine antigen testing for diagnosis of pneumococcal pneumonia in adults. J Infect Dis. 2003;187:1416–23. doi: 10.1086/374623. [DOI] [PubMed] [Google Scholar]
- 53.Anevlavis S, Petroglou N, Tzavaras A, Maltezos E, Pneumatikos I, Froudarakis M, et al. A prospective study of the diagnostic utility of sputum Gram stain in pneumonia. J Infect. 2009;59:83–9. doi: 10.1016/j.jinf.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Del Rio-Pertuz G, Gutiérrez JF, Triana AJ, Molinares JL, Robledo-Solano AB, Meza JL, et al. Usefulness of sputum gram stain for etiologic diagnosis in community-acquired pneumonia: A systematic review and meta-analysis. BMC Infect Dis. 2019;19:403. doi: 10.1186/s12879-019-4048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper GM, Jones JJ, Arbique JC, Flowerdew GJ, Forward KR. Intra and inter technologist variability in the quality assessment of respiratory tract specimens. Diagn Microbiol Infect Dis. 2000;37:231–5. doi: 10.1016/s0732-8893(00)00156-5. [DOI] [PubMed] [Google Scholar]
- 56.Jiménez D, Díaz G, García-Rull S, Vidal R, Sueiro A, Light RW. Routine use of pleural fluid cultures Are they indicated Limited yield, minimal impact on treatment decisions. Respir Med. 2006;100:2048–52. doi: 10.1016/j.rmed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Menzies SM, Rahman NM, Wrightson JM, Davies HE, Shorten R, Gillespie SH, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax. 2011;66:658–62. doi: 10.1136/thx.2010.157842. [DOI] [PubMed] [Google Scholar]
- 58.Skerrett SJ. Diagnostic testing for community-acquired pneumonia. Clin Chest Med. 1999;20:531–48. doi: 10.1016/s0272-5231(05)70234-3. [DOI] [PubMed] [Google Scholar]
- 59.El-Shabrawy M, EL-Sokkary RH. Role of fiberoptic bronchoscopy and BAL in assessment of the patients with non-responding pneumonia. Egypt J Chest Dis Tuberc. 2016;65:613–20. [Google Scholar]
- 60.Kim ES, Kim EC, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Bacterial yield from quantitative cultures of bronchoalveolar lavage fluid in patients with pneumonia on antimicrobial therapy. Korean J Intern Med. 2012;27:156–62. doi: 10.3904/kjim.2012.27.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molinos L, Zalacain R, Menéndez R, Reyes S, Capelastegui A, Cillóniz C, et al. Sensitivity, specificity, and positivity predictors of the pneumococcal urinary Antigen test in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12:1482–9. doi: 10.1513/AnnalsATS.201505-304OC. [DOI] [PubMed] [Google Scholar]
- 62.Sordé R, Falcó V, Lowak M, Domingo E, Ferrer A, Burgos J, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med. 2011;171:166–72. doi: 10.1001/archinternmed.2010.347. [DOI] [PubMed] [Google Scholar]
- 63.Chen M, Zhou M, Xiao W, Ai B, Liu X, Li Y. The urinary antigen tests have high sensitivity in diagnosis of Pneumococcus caused community-acquired pneumonia posterior to antimicrobial therapy. Cell Biochem Biophys. 2014;70:1029–34. doi: 10.1007/s12013-014-0015-4. [DOI] [PubMed] [Google Scholar]
- 64.Sinclair A, Xie X, Teltscher M, Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 2013;51:2303–10. doi: 10.1128/JCM.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porcel JM, Ruiz-González A, Falguera M, Nogués A, Galindo C, Carratalá J, et al. Contribution of a pleural antigen assay (Binax NOW) to the diagnosis of pneumococcal pneumonia. Chest. 2007;131:1442–7. doi: 10.1378/chest.06-1884. [DOI] [PubMed] [Google Scholar]
- 66.Sopena N, Sabrià M, Pedro-Botet ML, Reynaga E, García-Núñez M, Domínguez J, et al. Factors related to persistence of Legionella urinary antigen excretion in patients with legionnaires' disease. Eur J Clin Microbiol Infect Dis. 2002;21:845–8. doi: 10.1007/s10096-002-0839-5. [DOI] [PubMed] [Google Scholar]
- 67.Esnault P, Nguyen C, Bordes J, D'Aranda E, Montcriol A, Contargyris C, et al. Early-onset ventilator-associated pneumonia in patients with severe traumatic brain Injury: Incidence, risk factors, and consequences in cerebral oxygenation and outcome. Neurocrit Care. 2017;27:187–98. doi: 10.1007/s12028-017-0397-4. [DOI] [PubMed] [Google Scholar]
- 68.Shaw T, Tellapragada C, Ke V, AuCoin DP, Mukhopadhyay C. Performance evaluation of active melioidosis detect-lateral flow assay (AMD-LFA) for diagnosis of melioidosis in endemic settings with limited resources. PLoS One. 2018;13:E0194595. doi: 10.1371/journal.pone.0194595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellew S, Grijalva CG, Williams DJ, Anderson EJ, Wunderink RG, Zhu Y, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026–33. doi: 10.1093/cid/ciy826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang D, Yang D, Makam AN. Utility of blood cultures in pneumonia. Am J Med. 2019;132:1233–8. doi: 10.1016/j.amjmed.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eshwara VK, Shaw T, Mukim Y, Kumar G, Kamath A, Mukhopadhyay C. Perform or perish: Laboratory optimization to avoid false negative blood cultures in pneumococcal bacteremia. J Lab Med. 2019;43:217–20. [Google Scholar]
- 72.Menéndez R, Montull B, Reyes S, Amara-Elori I, Zalacain R, Capelastegui A, et al. Pneumonia presenting with organ dysfunctions: Causative microorganisms, host factors and outcome. J Infect. 2016;73:419–26. doi: 10.1016/j.jinf.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Amaro R, Liapikou A, Cilloniz C, Gabarrus A, Marco F, Sellares J, et al. Predictive and prognostic factors in patients with blood-culture-positive community-acquired pneumococcal pneumonia. Eur Respir J. 2016;48:797–807. doi: 10.1183/13993003.00039-2016. [DOI] [PubMed] [Google Scholar]
- 74.Self WH, Wunderink RG, Williams DJ, Zhu Y, Anderson EJ, Balk RA, et al. Staphylococcus aureus Community-acquired pneumonia: Prevalence, clinical characteristics, and outcomes. Clin Infect Dis. 2016;63:300–9. doi: 10.1093/cid/ciw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: Outcomes and epidemiology. J Crit Care. 2011;26:395–401. doi: 10.1016/j.jcrc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Eshwara VK, Munim F, Tellapragada C, Kamath A, Varma M, Lewis LE, et al. Staphylococcus aureus bacteremia in an Indian tertiary care hospital: Observational study on clinical epidemiology, resistance characteristics, and carriage of the Panton-Valentine leukocidin gene. Int J Infect Dis. 2013;17:E1051–5. doi: 10.1016/j.ijid.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: Clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect Dis. 2010;10:307. doi: 10.1186/1471-2334-10-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, et al. Community-acquired Klebsiella pneumoniae bacteremia: Global differences in clinical patterns. Emerg Infect Dis. 2002;8:160–6. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaw T, Tellapragada C, Kamath A, Kalwaje Eshwara V, Mukhopadhyay C. Implications of environmental and pathogen-specific determinants on clinical presentations and disease outcome in melioidosis patients. PLoS Negl Trop Dis. 2019;13:E0007312. doi: 10.1371/journal.pntd.0007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patra S, Shaw T, Eshwara VK, Saravu K, Hande M, Mukhopadhyay C. Pulmonary melioidosis: An experience over years from a tertiary care hospital from southwest India. Indian J Med Sci. 2017;69:21–6. [Google Scholar]
- 81.Jatapai A, Gregory CJ, Thamthitiwat S, Tanwisaid K, Bhengsri S, Baggett HC, et al. Hospitalized bacteremic melioidosis in rural Thailand: 2009-2013. Am J Trop MedHyg. 2018;98:1585–91. doi: 10.4269/ajtmh.17-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torres A, Lee N, Cilloniz C, Vila J, Van der Eerden M. Laboratory diagnosis of pneumonia in the molecular age. Eur RespirJ. 2016;48:1764–78. doi: 10.1183/13993003.01144-2016. [DOI] [PubMed] [Google Scholar]
- 83.Babady NE. The FilmArray® respiratory panel: An automated, broadly multiplexed molecular test for the rapid and accurate detection of respiratory pathogens. Expert Rev Mol Diagn. 2013;13:779–88. doi: 10.1586/14737159.2013.848794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Çaǧlayan Serin D, Pullukçu H, Ciçek C, Sipahi OR, Taşbakan S, Atalay S, et al. Bacterial and viral etiology in hospitalized community acquired pneumonia with molecular methods and clinical evaluation. J Infect Dev Ctries. 2014;8:510–8. doi: 10.3855/jidc.3560. [DOI] [PubMed] [Google Scholar]
- 85.Kunze N, Moerer O, Steinmetz N, Schulze MH, Quintel M, Perl T. Point-of-care multiplex PCR promises short turnaround times for microbial testing in hospital-acquired pneumonia - An observational pilot study in critical ill patients. Ann Clin Microbiol Antimicrob. 2015;14:33. doi: 10.1186/s12941-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Affolter K, Schumann DM, Tamm M, Jahn K, Siebeneichler A, Junker L, et al. Multiplex PCR on the bronchoalveolar lavage fluid of immunocompromised patients. Chest. 2018;154:722–5. doi: 10.1016/j.chest.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Prendki V, Huttner B, Marti C, Mamin A, Fubini PE, Meynet MP, et al. Accuracy of comprehensive PCR analysis of nasopharyngeal and oropharyngeal swabs for CT-scan-confirmed pneumonia in elderly patients: A prospective cohort study. Clin Microbiol Infect. 2019;25:1114–9. doi: 10.1016/j.cmi.2018.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner K, Springer B, Imkamp F, Opota O, Greub G, Keller PM. Detection of respiratory bacterial pathogens causing atypical pneumonia by multiplex LightmixRT-PCR. Int J Med Microbiol. 2018;308:317–23. doi: 10.1016/j.ijmm.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 89.Buchan B, Windham S, Faron M, Balada-Llasat J, Relich R, Humphries R, et al. Clinical evaluation and potential impact of a semi-quantitative multiplex molecular assay for the identification of pathogenic bacteria and viruses in lower respiratory specimens. Am J Respir Crit Care Med. 2018;197:A2617. [Google Scholar]
- 90.Principi N, Esposito S. Biomarkers in pediatric community-acquired pneumonia. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020447. pii: E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blasi F, Bocchino M, Di Marco F, Richeldi L, Aliberti S. The role of biomarkers in low respiratory tract infections. Eur J Intern Med. 2012;23:429–35. doi: 10.1016/j.ejim.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: Systematic review of diagnostic accuracy studies. Fam Pract. 2009;26:10–21. doi: 10.1093/fampra/cmn095. [DOI] [PubMed] [Google Scholar]
- 93.National Institute for Health and Care Excellence. Pneumonia: diagnosis and management of community- and hospital-acquired pneumonia in adults. [accessed on September 2, 2019]. Available from: https://wwwniceorguk/guidance/cg191/documents/pneumonia-guideline-consultation-nice-guideline2 . [PubMed]
- 94.Pfister R, Kochanek M, Leygeber T, Brun-Buisson C, Cuquemelle E, Machado MB, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: A prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18:R44. doi: 10.1186/cc13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu MH, Lin CC, Huang SL, Shih HM, Wang CC, Lee CC, et al. Can procalcitonin tests aid in identifying bacterial infections associated with influenza pneumonia? A systematic review and meta-analysis. Influenza Other Respir Viruses. 2013;7:349–55. doi: 10.1111/j.1750-2659.2012.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krüger S, Ewig S, Papassotiriou J, Kunde J, Marre R, von Baum H, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: Results from the German competence network CAPNETZ. Respir Res. 2009;10:65. doi: 10.1186/1465-9921-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Self WH, Balk RA, Grijalva CG, Williams DJ, Zhu Y, Anderson EJ, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65:183–90. doi: 10.1093/cid/cix317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Müller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scicluna BP, Klein Klouwenberg PM, van Vught LA, Wiewel MA, Ong DS, Zwinderman AH, et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am J Respir Crit Care Med. 2015;192:826–35. doi: 10.1164/rccm.201502-0355OC. [DOI] [PubMed] [Google Scholar]
- 100.Walden AP, Clarke GM, McKechnie S, Hutton P, Gordon AC, Rello J, et al. Patients with community acquired pneumonia admitted to European intensive care units: An epidemiological survey of the GenOSept cohort. Crit Care. 2014;18:R58. doi: 10.1186/cc13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gattarello S, Lagunes L, Vidaur L, Solé-Violán J, Zaragoza R, Vallés J, et al. Improvement of antibiotic therapy and ICU survival in severe non-pneumococcal community-acquired pneumonia: A matched case-control study. Crit Care. 2015;19:335. doi: 10.1186/s13054-015-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oster G, Berger A, Edelsberg J, Weber DJ. Initial treatment failure in non-ICU community-acquired pneumonia: Risk factors and association with length of stay, total hospital charges, and mortality. J Med Econ. 2013;16:809–19. doi: 10.3111/13696998.2013.794805. [DOI] [PubMed] [Google Scholar]
- 103.Indian Council of Medical Research. Treatment guidelines for antimicrobial use in common syndromes. New Delhi: ICMR; 2017. pp. 1–106. [Google Scholar]
- 104.Zhu YG, Tang XD, Lu YT, Zhang J, Qu JM. Contemporary situation of community-acquired pneumonia in China: A systematic review. J Transl Int Med. 2018;6:26–31. doi: 10.2478/jtim-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gross AE, Van Schooneveld TC, Olsen KM, Rupp ME, Bui TH, Forsung E, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58:5262–8. doi: 10.1128/AAC.02582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uranga A, España PP, Bilbao A, Quintana JM, Arriaga I, Intxausti M, et al. Duration of antibiotic treatment in community-acquired pneumonia: A multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257–65. doi: 10.1001/jamainternmed.2016.3633. [DOI] [PubMed] [Google Scholar]
- 107.Centers for Disease Control and Prevention. ACIP Recommendations. [accessed on September 2, 2019]. Available from: https://wwwcdcgov/vaccines/acip/recommendationshtml .
- 108.Dhar R. Review of guidelines for the use of vaccines to prevent community-acquired pneumonia in Indian adults. J Assoc Physicians India. 2016;64(Suppl):45–51. [Google Scholar]
- 109.Vila-Corcoles A, Ochoa-Gondar O, de Diego C, Satue E, Aragón M, Vila-Rovira A, et al. Evaluating clinical effectiveness of 13-valent pneumococcal conjugate vaccination against pneumonia among middle-aged and older adults in Catalonia: Results from the EPIVAC cohort study. BMC Infect Dis. 2018;18:196. doi: 10.1186/s12879-018-3096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–43. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]