Abstract
Background
Inflammation and mechanical demands play a role in the development of tendon conditions and the dysregulation of tendon healing. In patients with obesity, high levels of pro-inflammatory cytokines and a high mechanical demand promote chronic low-grade inflammation. Although controversial results have been reported, we aimed to summarize current evidence while highlighting the role of obesity in tendinopathy.
Questions/purposes
(1) Do patients with obesity have a greater risk of tendinopathy, stratified by upper and lower extremity sites, than patients who do not have obesity? (2) Is obesity associated with a higher risk of upper and lower extremity tendon tear and ruptures? (3) Is obesity associated with an increased risk of complications after upper and lower extremity tendon surgery?
Methods
We performed a systematic review by searching the PubMed, Embase, and Cochrane Library databases, combining the term “tendon” with common terms for tendinopathy and rupture such as “tendon injury OR tendinopathy OR tendon rupture” and “obese” OR “obesity.” We included studies with any level of evidence published from January 2000 to July 10, 2019 in peer-reviewed journals reporting clinical results. After we removed the duplicates, there were 365 records. Two independent authors screened these records and excluded 320 based on abstract and title screening. Of the remaining 45 studies, 23 were excluded because the topic did not address the research questions (n = 19), the article was outdated (n = 3), or because there was a serious risk of bias (n = 1). Finally, we included 22 studies with 49,914 participants (5984 with obesity), 31,100 (1884 with obesity) of whom had upper-extremity tendinopathy, while 18,814 (4010 with obesity) had lower-extremity tendinopathy. Obesity was defined as a BMI ≥ 30 kg/m2 according to the WHO’s criteria. Data were extracted and analyzed critically. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were applied, and the risk of bias (ROBINS tool) of the studies was assessed, as was the methodological quality (Coleman score). The assessment was performed independently by two authors. Inter-rater agreement for the assessments of the risk of bias and methodological quality were 89% and 94%, respectively. All studies were observational, and most were retrospective case-control studies. Any discrepancy was discussed and solved by consensus. The articles had a moderate risk of bias (eight articles) or a low risk of bias (fourteen articles). We excluded one article because of a serious risk of bias. The mean (range) Coleman score was 53.5 (42-74).
Results
Obesity was associated with a greater risk of upper extremity tendinopathy (rotator cuff: odds ratio 1.25 [95% confidence interval 1.12 to 1.40]; p < 0.001; medial epicondylitis: OR 1.9 [95% CI 1.0 to 3.7]; p < 0.05) and lower-extremity tendinopathy (Achilles tendon: OR 3.81 [95% CI 2.57 to 5.63]; OR 3.77 [95% CI 2.24 to 6.34]; OR 6.56 [95% CI 3.18 to 13.55], for obesity Classes I, II and III, respectively; patellar tendon: OR 1.10 [95% CI 1.05 to 1.90]; p = 0.001; plantar fascia: OR 2.97 [95% CI 1.64 to 5.37]; p = 0.004). Obesity was associated with a greater risk of upper extremity tendon tear (rotator cuff: OR 2.35 [95% CI 1.62 to 3.40]; p < 0.001) and rupture leading to tendon surgery (rotator cuff in men: OR 3.13 [95% CI 1.29 to 7.61]; p < 0.001 and women: OR 3.51 [95% CI 1.80 to 6.85]; p < 0.001). However, no association was found between BMI and lower extremity rupture (Achilles mean BMI: 27.77 kg/m2 [95% CI 26.94 to 28.49] versus control: 26.66 kg/m2 [95% CI 26.06 to 27.27]; p = 0.047). Upper extremity complications (n = 359) after tendon repair surgery had a weighted incidence of 13.27% and 8.13% for rotator cuff surgery in patients with and without obesity, respectively. In the lower extremity (n = 21,152), the weighted incidence for Achilles tendon surgery was 11.28% and 8.6% in patients with and without obesity, respectively.
Conclusions
Obesity is associated with a higher risk of tendinopathy, tendon tear and rupture, and complications after tendon surgery than non-obesity. However, the high heterogeneity and observational nature of the studies highlight the need to be cautious about the results of our study. We encourage researchers to perform clinical and preclinical studies to explore pathways related to the metabolic state of this population.
Level of Evidence
Level IV, prognostic study.
Introduction
Obesity has been investigated as a possible risk factor for impaired healing in patients with tendinopathy because it could increase mechanical stress and support an inflammatory environment [58]. Low-grade, chronic inflammation [2], which affects this population, seems to be caused by adipocytes [57]. Because of hypertrophy, adipose cells suffer from hypoxic, mechanical, and oxidative stress, ultimately leading to cellular apoptosis [38] with the release of intracellular pro-inflammatory molecules [30]. Additionally, continuous macrophage recall increases the production of pro-inflammatory cytokines [35]. Strong evidence confirmed that this inflammatory condition predisposes patients with obesity to have chronic diseases such as Type II diabetes mellitus, cardiovascular diseases, degenerative diseases, and autoimmune conditions, which are well-known risk factors for musculoskeletal diseases [7, 27, 42, 43].
Although these diseases may partly explain the higher burden of musculoskeletal issues among these patients, chronic low-grade inflammation was recently associated with a failed healing response in tendons [54]. However, this evidence is still inconclusive. Although obesity is a known risk factor for complications after tendon surgery and increases failure rates, there is still a lack of evidence on how obesity clinically affects the outcome of tendinopathy treatment.
Our main aims were to critically describe the influence of obesity on the occurrence, severity, and progression of tendinopathy; identify how obesity is associated with surgical and non-surgical treatment; and evaluate the difference in the postoperative risk of rupture in this population.
Therefore, we asked: (1) Do patients with obesity have a greater risk of tendinopathy, stratified by upper and lower extremity sites, than patients who do not have obesity? (2) Is obesity associated with a higher risk of upper and lower extremity tendon tear and ruptures? (3) Is obesity associated with an increased risk of complications after upper and lower extremity tendon surgery?
Materials and Methods
Study Search Strategy
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [37]. A comprehensive search was performed by two independent authors (MM, MS) using three medical electronic databases (PubMed, Embase, and Cochrane Library) from January 2000 to July 10, 2019 (Fig. 1). To achieve the maximum sensitivity of the search strategy, we combined the terms “tendon” with some common terms of tendon conditions such as “tendon injury OR (tendon damage) OR tendonitis OR tendinopathy OR (chronic tendonitis) OR tendinosis OR (chronic tendinopathy) OR enthesitis)” AND “obese” OR “obesity” OR “overweight” OR “BMI” as either keywords or Medical Subject Heading terms. The reference lists of all articles, previous reviews on the topic, and top hits from Google Scholar were reviewed to further identify potentially relevant studies, which were assessed using the inclusion and exclusion criteria. To avoid overlap with other ongoing review studies, we searched PROSPERO for any similar reviews.
Fig. 1.
The distribution of the published articles split by year is shown here.
Selection Criteria
Eligible studies included those investigating the influence of obesity on patients with tendinopathy and their outcome after treatment. The titles and abstracts were screened by including only clinical studies with any level of evidence published in peer-reviewed journals reporting clinical results in English. We excluded studies investigating the tendon’s response to regenerative treatments (such as platelet-rich plasma and mesenchymal stem cells) or new drugs related to tissue healing, which may act as a confounding factor for the effect of obesity. Additionally, we excluded studies in which data were not accessible or missing, those without an available full-text article, or those that were not well-reported. We also excluded duplicates and studies with poor scientific methodology, assessed as described below. Abstracts, case reports, conference presentations, reviews, editorials, and expert opinions were excluded. Two authors (MM, MS) performed the search and evaluated the articles independently. A researcher experienced in systematic reviews (EC) resolved cases of doubt. First, each investigator read the abstracts of all articles, selected relevant articles according to the inclusion and exclusion criteria, and compared the results with those of the other investigators. After 4 weeks, the same studies were read again to ensure the investigators agreed about article selection. There was no disagreement among the investigators. One investigator (MM) entered data from the full-text articles into an Excel spreadsheet with structured tables to analyze each study descriptively. Another investigator (MS) independently double-checked the primary data from all articles. Doubts and inconsistencies were grouped and resolved.
Data Extraction and Criteria Appraisal
Data were extracted from article texts, tables, and figures using the Population, Intervention, Comparison, Outcome framework [47] and included the title, year of publication, study design, sample size, study population, patient characteristics, intervention and comparator groups (where applicable), outcomes, funding, and conclusions. Two investigators (SE, GS) independently reviewed each article. Discrepancies between the two reviewers were resolved by discussion and consensus.
Risk of Bias Assessment
A risk of bias assessment of all included clinical studies was performed according to the ROBINS risk of bias tool [51] (see Supplemental Digital Content 1, http://links.lww.com/CORR/A335). This assessment used low, moderate, and high as judgement keys. Low indicated a low risk of bias, moderate indicated a moderate risk of bias, and high indicated a high risk of bias. The assessment was performed by two authors (SE, GS) independently. The inter-rater agreement was 89%. Any discrepancy was solved by consensus. The articles were considered to have a moderate (eight articles) or low risk of bias (14 articles). We excluded one article because of a serious risk of bias.
Study Quality Assessment
The Coleman score was used to assess the studies [17] (see Supplemental Digital Content 1, http://links.lww.com/CORR/A335). It is based on two parts (A and B) with eight and three items, respectively. Part A comprises study size, the mean follow-up duration, percentage of patients with follow-up, the number of interventions per group, study type, diagnostic certainty, description of the surgical procedure, and postoperative rehabilitation. Part B comprises outcome criteria, procedure for assessing outcomes, and description of the participant selection process. A score of 100 indicates that the study largely avoids confounding factors and biases. The subsections that comprise the Coleman score are based on the subsections of the CONSORT statement for randomized controlled trials but are modified to allow for other trial designs. The assessment was performed by two authors (SE, GS) independently. The inter-rater agreement was 94%. Any discrepancy was solved by consensus among the authors. The mean (range) score was 53.5 (42-74).
Study Selection
The initial study search resulted in 365 studies. After reading the title and abstract and removing duplicates, we selected 45 for full-text reading. Ultimately, we selected 22 articles for analysis, excluding 23 articles because the topic did not address our study questions (n = 19) or the article was outdated (n = 3) and because of a serious risk of bias (n = 1) (Fig. 2) [37].
Fig. 2.
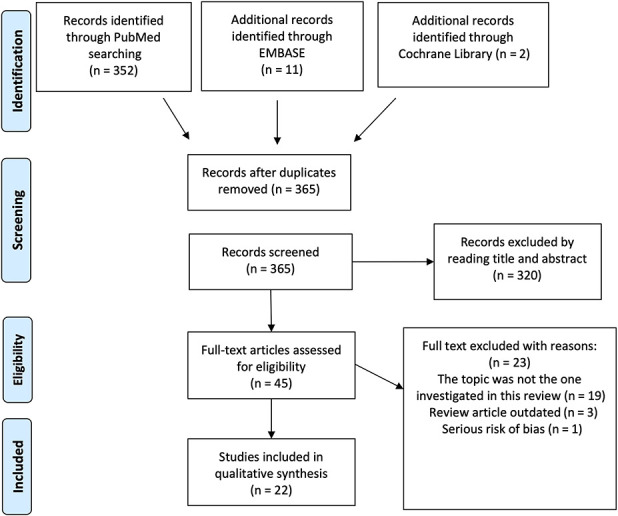
A PRISMA flowchart showing the selection process is shown here.
Study Characteristics
Of the 22 studies included in our systematic review [3, 5, 9, 11, 15, 19, 21, 24, 28, 29, 31–34, 36, 40, 44–46, 50, 55, 60, ], eight were case-control studies [3, 5, 19, 29, 34, 40, 55, 60], five were retrospective cohort studies [9, 11, 15, 31, 33], and one was a prospective study [46]. Additionally, six were cross-sectional studies [21, 24, 32, 36, 45, 50] and two were case series [28, 44].
In the 22 included studies, we analyzed the data of 49,914 participants (5984 with obesity), 31,100 (1884 with obesity) who had upper extremity tendinopathy while 18,814 (4010 with obesity) had lower extremity tendinopathy. We only examined patients with obesity (BMI ≥ 30 kg/m2), defined according to the WHO’s criteria [41]. The total number of patients with obesity was 5912. The weighted mean age was 50.5 ± 4.6 years, and the male-to-female ratio was 8:10.
The tissues involved were the Achilles tendon [3, 5, 9, 15, 31, 32, 34, 40], rotator cuff [11, 19, 29, 33, 45, 55, 60], patellar tendon [21], tibialis posterior tendon [44], plantaris tendon [36], ankle and foot tendons [24, 28], upper-extremity soft tissues [46], and insertion of the elbow epicondyle tendon [50].
The evaluated outcomes were the effect of obesity on the etiopathogenesis of tendinopathy. The most investigated outcomes were the association between obesity and tendinopathy or tendon rupture [5, 19, 45, 50, 55, 60, 21, 24, 28, 32, 34, 36, 40, 44], the outcome and complications after tendon surgery in patients with obesity [9, 11, 15, 31, 33], and the effect of obesity on the treatment of tendinopathy [34, 60]. Other outcomes were the effect of exercise on tendinopathy in patients with obesity [3, 5, 40] and on recovery from upper extremity soft-tissue disorders [46].
The main characteristics of all included studies were extracted and described (see Supplemental Digital Content 2, http://links.lww.com/CORR/A336)
Statistical Analysis
The statistical analysis were performed using SPSS software version 22.0 (IBM Corp, Armonk, NY, USA) and MedCalc program for Windows. Continuous variables were presented as the mean value and categorical data were reported following the study authors preference. When possible, we used the data reported in the text and tables to perform a univariate analysis (t test and chi-square test for continuous and categorical data, respectively). However, after reviewing the data, the heterogeneity of outcomes, follow-up, and assessments, in addition to unreported data, prevented us from performing a meta-analysis. Thus, a descriptive analysis was performed for this systematic review.
Results
Risk of Tendinopathy
Obesity was associated with a greater risk of upper extremity tendinopathy (rotator cuff: odds ratio 1.26 [95% confidence interval 1.13 to 1.40]; p < 0.001; medial epicondylitis: OR 1.9 [95% CI 1.0 to 3.7]; p < 0.05) and lower extremity tendinopathy (Achilles tendon: OR 3.81 [95% CI 2.57 to 5.63]; OR 3.77 [95% CI 2.24 to 6.34]; OR 6.56 [95% CI 3.18 to 13.55] for obesity Classes I, II, and III, respectively; patellar tendon: OR 1.10 [95% CI 1.05 to 1.90]; p = 0.001; plantar fascia: OR 2.98 [95% CI 1.65 to 5.37]; p = 0.004). A matched case-control study [55] and a cross-sectional study [45] found that being overweight or obese is associated with rotator cuff tendinopathy. In particular, although the quality of the design affected the matched case-control study’s [55] reporting, both studies reported similar trends, with a reported higher risk of tendon-related pain (men: OR 1.4 [95% CI 1.0 to 2.0]; p < 0.05; women: OR 1.6 [95% CI 1.0 to 2.4]; p < 0.05) and overall risk of tendinopathy (OR 1.26 [95% CI 1.13 to 1.40]). Similar results were confirmed in another study [28]. One study investigated lateral and medial epicondylitis with evidence supporting a role in only medial epicondylitis (OR 1.9 [95% CI 1.0 to 3.7]; p < 0.05). When restricted to people with obesity only, data in multivariate analyses were inconsistent, which may have been because of unreported data [50]. In 2014, a cross-sectional study [21] including 213 patents with patellar tendinopathy and 84 healthy participants found that BMI was associated with the prevalence of patellar tendinopathy (OR 1.10 [95% CI 1.05 to 1.90]; p = 0.001). Four studies [4, 24, 32, 34] evaluated the effect of obesity on the Achilles tendon. Two matched case-control studies [4, 34] investigated the role of BMI in the occurrence of Achilles tendinopathy. Patients with obesity had a higher prevalence of tendinopathy than patients without obesity [4, 32]. A cross-sectional study [24] including 1411 patients, 738 of whom had a BMI > 25 kg/m2, found that being overweight or obese increases the chances that tendinitis (OR 1.92; p < 0.001) and plantar fasciitis (OR 1.40; p < 0.040) will occur. Another study [36] reported similar results on the risk of plantar fasciitis tendinopathy (OR 2.98 [95% CI 1.65 to 5.37]; p = 0.004). However, a large study [44] on 6879 patients with tibialis posterior tendon tendinitis, plantar fasciitis, or both concluded that were no differences in the mean BMI between any two groups.
Risk of Tendon Tear and Rupture
Obesity was associated with a greater risk of upper extremity tendon tear than non-obesity (rotator cuff: OR 2.35 [95% CI 1.62 to 3.40]; p < 0.001) and rupture treated with tendon surgery (rotator cuff in men: OR 3.13 [95% CI 1.29 to 7.61]; p < 0.001 and women: OR 3.51 [95% CI 1.80 to 6.85]; p < 0.001); however, no association was found between BMI and lower-extremity rupture (Achilles mean BMI: 27.77 kg/m2 [95% CI 26.94 to 28.49] versus control 26.66 kg/m2 [95% CI 26.06 to 27.27]; p = 0.047). Patients with obesity also had a greater risk of tendon rupture than patients without [19, 29, 60]. One study [29] investigated the risk of a rotator cuff tear and two [19, 60] evaluated rupture that eventually led to surgery. A case-control study [29], in which patients were divided into two groups based on BMI, found that patients with obesity had a higher incidence of rotator cuff tears than did those without (OR 2.35 [95% CI 1.62 to 3.40]; p < 0.001). On the other hand, a matched case-control study [60] using a multiple linear regression analysis showed an association between BMI and shoulder surgery in men (OR 3.13 [95% CI 1.29 to 7.61]) and women (OR 3.51 [95% CI 1.80 to 6.85]). Moreover, similar results were reported in another study [19], in which patients who underwent arthroscopic rotator cuff repair had a higher BMI than healthy control participants (OR 2.11; p < 0.001). However, this was not the same for lower extremity tendons. A matched case-control study [40] comparing 93 patients who had Achilles tendon ruptures with 186 controls found no difference in the mean BMI (rupture: 27.77 kg/m2 [95% CI 26.94 to 28.49] versus control: 26.66 kg/m2 [95% CI 26.06 to 27.27]; p = 0.047).
Complications
After tendon surgery, obesity was associated with a higher proportion of complications that ranged from 2.78% to 28.6% (weighted mean: 3.07%; median: 3.1%). By contrast, in patients without obesity, this incidence ranged from 0.56% to 15.8% (weighted mean: 1.77%; median: 6.2%) [9, 11, 15, 31, 33]. When divided by tendon location, the upper extremity (n = 359) weighted incidence was 13.27% versus 8.13% for rotator cuff surgery in patients with and without obesity, respectively. In the lower extremity (n = 21,152 patients), the weighted incidence of rotator cuff surgery was 11.28% versus 8.6% in patients with and without obesity, respectively [9, 11, 15, 31, 33]. The analyzed complications were wound complications [9, 15, 31]; infections [15, 31]; venous thromboembolism [15]; joint stiffness [15]; re-tear, meaning that tendon rupture occurred after surgery to treat a primary tendon tear [11, 33]; and other medical complications [15]. The incidences of wound complications were 3.8% [15], 3.1% [9], and 1.8% [31]. Conflicting evidence was reported about the influence of obesity on complications after tendon surgery [1, 4, 9, 11, 15, 24, 31–34]. One retrospective study [15] reported that after Achilles tendon surgery, patients with obesity had a greater risk of complications, including wound complications (OR 2.1 [95% CI 1.7 to 2.6]; p < 0.001), infections (OR 1.8 [95% CI 1.5 to 2.3]; p < 0.001), venous thromboembolism (OR 1.7 [95% CI 1.3 to 2.4]; p < 0.001), medical complications (OR 3.9 [95% CI 2.9 to 5.1]; p < 0.001), and ankle stiffness (OR 0.4 [95% CI 0.3 to 0.6]; p < 0.001) than patients without obesity. However, two studies did not find any difference in the outcome of tendon surgery between patients with obesity and those without [9, 31]. Similar findings were reported for rotator cuff repair after tendon surgery [11]. In addition, the incidence of re-tear was higher in patients with obesity than in patients without (OR 4.3 [95% CI 1.6 to 11.4]; p = 0.008), and these patients’ postoperative quality of life was impaired. ROM was limited in patients with obesity (p < 0.001) [11]. Controversially, Kessler et al. [33], who used a similar sample, did not report a higher proportion of complications in patients with obesity. However, the follow-up duration was shorter and the authors were not able to get a good estimate of the re-tear risk after surgery. Although the overall number of participants with postoperative complications was high, we caution the reader about the studies’ heterogenous designs and observational value.
Discussion
Studies have demonstrated an association between tendinopathy and BMI [23, 26, 49], dyslipidemia [25], and waist circumference (often used as a measure of body composition and obesity) [25, 26]. However, clinical observational studies were inconclusive on the topic, leaving clinicians without clear guidance on whether or not to treat these patients differently. To our knowledge, this is the first study that systematically reviewed clinical studies on the relationship between obesity and tendinopathy and its main consequences. The collected evidence showed that obesity had a clinically significant role in the risk that tendinopathy will develop and that these patients will have a tear or rupture and suffer complications after tendon repair.
Limitations
The most important limitation of our systematic review was the heterogeneity of the studies, not only because of the type of study and outcomes, but also because of the quality and risk of bias. Thus, because it was impossible to perform a meta-analysis, this might affect the ability to translate our findings into clinical practice. Most of the included studies were retrospective, cross-sectional, or case-control studies, and we found no randomized controlled clinical trials. However, because randomizing patients as being obese or not is an impossible study design, we decided to perform a review and try to describe the studies’ trends.
Because of the studies’ designs, none identified obesity as playing a major role in the etiopathogenesis of tendinopathy, and these studies considered it only a risk factor. Furthermore, they often did not refer specifically to obesity but to metabolic syndrome or diabetes mellitus. In these cases, it was crucial for us to extract data from the entire text and tables. In addition, data about sex, the mean age, and mean BMI were not available in some studies, so we were unable to discuss some of the findings in depth. However, all authors agreed to conduct some minor descriptive analyses to provide more data about the risk and burden of obesity and tendinopathy. The evidence we provide should be interpreted with caution, but it is based on the best evidence we can currently collect and should be considered to provide better care to patients.
Because it was impossible to conduct a randomized controlled trial, we encourage researchers to perform preclinical animal studies and prospective cohort studies in the future. Although research studies with models of tendinopathy using animals that are randomized to a high-fat diet or not may help us highlight the molecular pathogenesis of tendinopathy, well-designed, multicenter, prospective, cohort human studies examining patients with obesity matched by age and sex to people without obesity might shed light on the topic from a clinical point of view. In addition, a study evaluating patients with obesity matched by age and sex with patients who were obese in the past may help to understand how to prevent this condition.
Obesity and the Risk of Tendinopathy
Our findings suggest that obesity is associated with a higher risk of tendinopathy. Higher BMI was associated with macroscopic tendon changes such as greater thickness and lower stiffness [2, 6, 10, 22, 53, 59]. However, although mechanical changes and systematic comorbidities could explain the higher risk of tendinopathy in individuals with obesity than in individuals without, the findings we showed in upper extremity tendons weaken this conclusion. The molecular aspects of the disease, particularly adipokines, should be studied further for these reasons. Only one study [46] considered this aspect and found that higher levels of leptin were associated with a lower incidence of recovery from upper extremity soft-tissue disorders. In contrast, higher levels of resistin and visfatin were associated with a higher recovery rate. Thus, the role of leptin resistance, which is commonly found in people with obesity, may elucidate this further [20, 39]. Mechanical changes cannot be considered the cause of the increased risk of tendinopathy and should be considered a contributing factor only. We should aim to evaluate the metabolic state of patients with obesity.
Obesity and the Risk of Tendon Rupture
Obesity is associated with a higher risk of tear and rupture in upper-extremity tendons. This was not true for lower-extremity tendons. As emerging studies are confirming, although moderate exercise plays an essential role in tendon homeostasis, a lack or excess of it and overuse are associated with tendinopathy and tendon tears [3, 5, 40]. Patients with a sedentary lifestyle are more likely to have a higher BMI than those with a more active lifestyle, which is ultimately associated with enthesopathy and tendon abnormalities [1]. Our results seem to agree with previous reviews examining a sedentary state as an explanation for the increased risk associated with obesity [4, 12, 13, 16, 23, 25].
However, the controversial absence of greater tendon tear and rupture might be difficult to interpret. We concluded that mechanical stress that affects the feet of patients with obesity during daily activities such as walking is unavoidable in this population and might somehow protect against degenerative changes that lead to rupture but not tendon inflammation and pain.
Obesity and the Risk of Complications After Tendon Surgery
The risk of complications after tendon surgery was higher in patients with obesity than in patients without obesity, both for upper and lower extremity tendons [9, 11, 15, 31, 33]. However, three studies did not find a difference between patients with obesity and patients without [46, 49, 51]. These studies had a lower risk of bias and a higher quality, according to our assessment; thus, we recommend further investigation into this topic. The greater risk of tendinopathy and complications after tendon surgery in patients with obesity than in individuals without could be explained by the role of obesity in impaired tendon healing. In some preclinical studies [8, 12–14, 18, 52] in which mice were placed on either a high-fat or low-fat diet, the tendons of rats with obesity had impaired biomechanical and healing properties, with increased M2 polarization [8, 14, 18, 52] and microscopic collagen fibril alterations with a lower amount of glycosaminoglycans [12, 13]. Another explanation is the role of adipokines; leptin has been shown to promote osteogenic differentiation of tendon-derived stem cells and formation of heterotopic bone via mTORC1 signalling. Adiponectin stimulated the proliferation and differentiation of the tenocyte progenitor, and tenomodulin played a role in the pathogenesis of obesity [48, 56, 61]
Conclusion
Obesity is associated with tendinopathy and its main features. The risk that this condition will develop, together with the risk of complications after surgery, was higher when either upper and lower extremity tendons were investigated. When we evaluated tendon tear and rupture, only upper extremity tendons seemed to be affected. This review is the first, to our knowledge, to systematically investigate this association, and it is based only on observational evidence that did not show causation but only association. Thus, the results should be interpreted with caution. However, our findings highlight that obesity has a clear role in tendinopathy, and patients should be guided in weight loss and proper progressive physical activity. In addition, surgery might not be the best option to treat tendon tear and rupture because of the higher risk of complications and impaired healing response in clinical and preclinical studies, respectively. Future preclinical studies are needed to confirm our results and investigate the inflammatory and molecular pathways of this relationship, while prospective, multicenter, matched-cohort studies may elucidate its role clinically. In particular, they will need to identify whether obesity is just an associated factor or whether it has a central role in the pathogenesis of tendinopathy.
Acknowledgments
We thank the Medical Student Research Academy for supporting this project with lessons about research methodology and manuscript preparation; without its network and support this project would not have been completed.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Catania, Catania, Italy.
References
- 1.Abate M, Di Carlo L, Salini V, Schiavone C. Metabolic syndrome associated to non-inflammatory Achilles enthesopathy. Clin Rheumatol . 2014;33:1517–1522. [DOI] [PubMed] [Google Scholar]
- 2.Abate M, Oliva F, Schiavone C, Salini V. Achilles tendinopathy in amateur runners: Role of adiposity (Tendinopathies and obesity). Muscles Ligaments Tendons J . 2012;2:44–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Abate M, Salini V. Mid-portion Achilles tendinopathy in runners with metabolic disorders. Eur J Orthop Surg Traumatol . 2019;29:697–703. [DOI] [PubMed] [Google Scholar]
- 4.Abate M, Salini V, Andia I. How Obesity Affects Tendons? Adv Exp Med Biol. 920; 2016:167–177. [DOI] [PubMed] [Google Scholar]
- 5.Abate M, Salini V, Schiavone C. Achilles tendinopathy in elderly subjects with type II diabetes: the role of sport activities. Aging Clin Exp Res . 2016;28:355–358. [DOI] [PubMed] [Google Scholar]
- 6.Abate M, Schiavone C, Di Carlo L, Salini V. Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index. Clin Rheumatol . 2012;31:1109–1113. [DOI] [PubMed] [Google Scholar]
- 7.Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology. 2013;52:599–608. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman JE, Geary MB, Orner CA, Bawany F, Loiselle AE. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing respons. PLoS One. 2017;12:e0181127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad J, Jones K. The effect of obesity on surgical treatment of Achilles tendon ruptures. J Am Acad Orthop Surg . 2017;25:773–779. [DOI] [PubMed] [Google Scholar]
- 10.Al-Qahtani M, Al-Tayyar S, Mirza EH, Al-Musallam A, Al-Suwayyid A, Javed R. Body Mass Index and Segmental Mass Correlation With Elastographic Strain Ratios of the Quadriceps Tendon. J Ultrasound Med . 2018;34:754-761 [DOI] [PubMed] [Google Scholar]
- 11.Ateschrang A, Eggensperger F, Ahrend MD, Schröter S, Stöckle U, Kraus TM. Obesity causes poorer clinical results and higher re-tear rates in rotator cuff repair. Arch Orthop Trauma Surg . 2018;138:835–842. [DOI] [PubMed] [Google Scholar]
- 12.Biancalana A, Velloso LA, Taboga SR, Gomes L. Implications of obesity for tendon structure, ultrastructure and biochemistry: A study on Zucker rats. Micron. 2012;43:463–469. [DOI] [PubMed] [Google Scholar]
- 13.Biancalana A, Veloso L, Gomes L. Obesity Affects Collagen Fibril Diameter and Mechanical Properties of Tendons in Zucker Rats. Connect Tissue Res . 2010;51:171–178. [DOI] [PubMed] [Google Scholar]
- 14.Boivin GP, Platt KM, Corbett J, Reeves J, Hardy AL, Elenes EY, Charnigo RJ, Hunter SA, Pearson KJ. The effects of high-fat diet, branched-chain amino acids and exercise on female C57BL/6 mouse Achilles tendon biomechanical properties. Bone Joint Res . 2013;2:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrus MT, Werner BC, Park JS, Perumal V, Cooper MT. Achilles Tendon Repair in Obese Patients Is Associated With Increased Complication Rates. Foot Ankle Spec . 2016;9:208–214. [DOI] [PubMed] [Google Scholar]
- 16.Castro AA, Skare TL, PAN Nassif, Sakuma AK, Barros WH. Tendinopathy and obesity. Arq Bras Cir Dig (São Paulo). 2016;29:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2–11. [DOI] [PubMed] [Google Scholar]
- 18.David MA, Jones KH, Inzana JA, Zuscik MJ, Awad HA, Mooney RA. Tendon Repair Is Compromised in a High Fat Diet-Induced Mouse Model of Obesity and Type 2 Diabetes. PLoS One. 2014;9:e91234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djerbi I, Chammas M, Mirous M-P, Lazerges C, Coulet B. Impact of cardiovascular risk factor on the prevalence and severity of symptomatic full-thickness rotator cuff tears. Orthop Traumatol Surg Res . 2015;101:S269–S273. [DOI] [PubMed] [Google Scholar]
- 20.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin Resistance and Obesity. Obesity. 2006;14:254S-258S. [DOI] [PubMed] [Google Scholar]
- 21.Fairley J, Toppi J, Cicuttini FM, Wluka AE, Giles GG, Cook J, O’Sullivan R, Wang Y. Association between obesity and magnetic resonance imaging defined patellar tendinopathy in community-based adults: a cross-sectional study. BMC Musculoskelet Disord . 2014;15:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faria A, Gabriel R, Abrantes J, Brás R, Moreira H. Triceps-surae musculotendinous stiffness: Relative differences between obese and non-obese postmenopausal women. Clin Biomech . 2009;24:866–871. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi F, Papalia R, Paciotti M, Franceschetti E, Di Martino A, Maffulli N, Denaro V. Obesity as a Risk Factor for Tendinopathy: A Systematic Review. Int J Endocrinol . 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey C, Zamora J. The Effects of Obesity on Orthopaedic Foot and Ankle Pathology. Foot Ankle Int . 2007;28:996–999. [DOI] [PubMed] [Google Scholar]
- 25.Gaida JE, Alfredson L, Kiss ZS, Wilson AM, Alfredson H, Cook JL. Dyslipidemia in Achilles Tendinopathy Is Characteristic of Insulin Resistance. Med Sci Sport Exerc . 2009;41:1194–1197. [DOI] [PubMed] [Google Scholar]
- 26.Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum . 2009;61:840–849. [DOI] [PubMed] [Google Scholar]
- 27.Gaida JE, Cook JL, Bass SL. Adiposity and tendinopathy. Disabil Rehabil . 2008;30:1555–1562. [DOI] [PubMed] [Google Scholar]
- 28.Galli MM, Protzman NM, Mandelker EM, Malhotra A, Schwartz E, Brigido SA. Comparing Tendinous and Ligamentous Ankle Pathology in Atraumatic Overweight and Nonoverweight Patients. Foot Ankle Spec . 2014;7:449–456. [DOI] [PubMed] [Google Scholar]
- 29.Gumina S, Candela V, Passaretti D, Latino G, Venditto T, Mariani L, Santilli V. The association between body fat and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg . 2014;23:1669–1674. [DOI] [PubMed] [Google Scholar]
- 30.Haanen C, Vermes I. Apoptosis and inflammation. Mediators Inflamm . 1995;4:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillam JS, Mohile N, Smyth N, Kaplan J, Aiyer A. The Effect of Obesity on Achilles Rupture Repair. Foot Ankle Spec . 2018:193864001881637. [DOI] [PubMed] [Google Scholar]
- 32.Holmes GB, Lin J. Etiologic Factors Associated with Symptomatic Achilles Tendinopathy. Foot Ankle Int . 2006;27:952–959. [DOI] [PubMed] [Google Scholar]
- 33.Kessler KE, Robbins CB, Bedi A, Carpenter JE, Gagnier JJ, Miller BS. Does Increased Body Mass Index Influence Outcomes After Rotator Cuff Repair? Arthrosc J Arthrosc Relat Surg . 2018;34:754–761. [DOI] [PubMed] [Google Scholar]
- 34.Klein EE, Weil L, Weil LS, Fleischer AE. Body Mass Index and Achilles Tendonitis: A 10-Year Retrospective Analysis. Foot Ankle Spec . 2013;6:276–282. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda M, Sakaue H. Adipocyte Death and Chronic Inflammation in Obesity. J Med Investig . 2017;64:193–196. [DOI] [PubMed] [Google Scholar]
- 36.Labovitz JM, Yu J, Kim C. The Role of Hamstring Tightness in Plantar Fasciitis. Foot Ankle Spec . 2011;4:141–144. [DOI] [PubMed] [Google Scholar]
- 37.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med . 2010;16:247–256. [DOI] [PubMed] [Google Scholar]
- 39.Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab . 2010;21:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noback PC, Jang ES, Cuellar DO, Seetharaman M, Malagoli E, Greisberg JK, Vosseller JT. Risk factors for achilles tendon rupture: A matched case control study. Injury. 2017;48:2342–2347. [DOI] [PubMed] [Google Scholar]
- 41.Nuttall FQ. Body mass index: Obesity, BMI, and health: A critical review. Nutr Today. 2015;50:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta . 2003;331:25–28. [DOI] [PubMed] [Google Scholar]
- 43.Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, Hamilton J, Heycock C, Saravanan V, Kelly C. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med . 2009;20:718–721. [DOI] [PubMed] [Google Scholar]
- 44.Reb CW, Schick FA, Karanjia HN, Daniel JN. High Prevalence of Obesity and Female Gender Among Patients With Concomitant Tibialis Posterior Tendonitis and Plantar Fasciitis. Foot Ankle Spec . 2015;8:364–368. [DOI] [PubMed] [Google Scholar]
- 45.Rechardt M, Shiri R, Karppinen J, Jula A, Heliövaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: A population-based study. BMC Musculoskelet Disord . 2010;11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rechardt M, Viikari-Juntura E, Shiri R. Adipokines as predictors of recovery from upper extremity soft tissue disorders. Rheumatology (Oxford) . 2014;53:2238–2242. [DOI] [PubMed] [Google Scholar]
- 47.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12. [PubMed] [Google Scholar]
- 48.Rothan HA, Suhaeb AM, Kamarul T. Recombinant human adiponectin as a potential protein for treating diabetic tendinopathy promotes tenocyte progenitor cells proliferation and tenogenic differentiation in vitro. Int J Med Sci . 2013;10:1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott RT, Hyer CF, Granata A. The Correlation of Achilles Tendinopathy and Body Mass Index. Foot Ankle Spec . 2013;6:283–285. [DOI] [PubMed] [Google Scholar]
- 50.Shiri R, Viikari-Juntura E, Varonen H, Heliovaara M. Prevalence and Determinants of Lateral and Medial Epicondylitis: A Population Study. Am J Epidemiol . 2006;164:1065–1074. [DOI] [PubMed] [Google Scholar]
- 51.Sterne JA Hernán MA Reeves BC Savović J Berkman ND Viswanathan M Henry D Altman DG Ansari MT Boutron I Carpenter JR Chan AW Churchill R Deeks JJ Hróbjartsson A Kirkham J Jüni P Loke YK Pigott TD Ramsay CR Regidor D Rothstein HR Sandhu L Santaguida PL Schünemann HJ Shea B Shrier I Tugwell P Turner L Valentine JC Waddington H Waters E Wells GA Whiting PF Higgins JP. ROBINS -I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Studentsova V, Mora KM, Glasner MF, Buckley MR, Loiselle AE. Obesity/Type II Diabetes Promotes Function-limiting Changes in Murine Tendons that are not reversed by Restoring Normal Metabolic Function. Sci Rep . 2018;8:9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taş S, Yilmaz S, Onur MR, Soylu AR, Altuntaş O, Korkusuz F. Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop Traumatol Turc . 2017;51:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res . 2015;33:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Titchener AG, White JJE, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg . 2014;23:1282–1288. [DOI] [PubMed] [Google Scholar]
- 56.Tolppanen A-M, Pulkkinen L, Kolehmainen M, Schwab U, Lindström J, Tuomilehto J, Uusitupa M, The Finnish Diabetes Prevention Study. Tenomodulin is Associated with Obesity and Diabetes Risk: The Finnish Diabetes Prevention Study. Obesity. 2007;15:1082–1088. [DOI] [PubMed] [Google Scholar]
- 57.Wasim M, Awan FR, Najam SS, Khan AR, Khan HN. Role of Leptin Deficiency, Inefficiency, and Leptin Receptors in Obesity. Biochem Genet . 2016;54:565–572. [DOI] [PubMed] [Google Scholar]
- 58.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev . 2006;7:239–250. [DOI] [PubMed] [Google Scholar]
- 59.Wearing SC, Hooper SL, Grigg NL, Nolan G, Smeathers JE. Overweight and obesity alters the cumulative transverse strain in the Achilles tendon immediately following exercise. J Bodyw Mov Ther . 2013;17:316–321. [DOI] [PubMed] [Google Scholar]
- 60.Wendelboe AM, Hegmann KT, Gren LH, Alder SC, White GL, Lyon JL. Associations between Body-Mass Index and Surgery for Rotator Cuff Tendinitis. J Bone Joint Surg Am. 2004;86:743–747. [DOI] [PubMed] [Google Scholar]
- 61.Xu J-C, Wu T, Wu G-H, Zhong Z-M, Tang Y-Z, Chen J-T. Leptin expression by heterotopic ossification-isolated tissue in rats with Achilles’ tenotomy. Saudi Med J 2009;30:605–610. [PubMed] [Google Scholar]