History
Total hip arthroplasty remains one of the most commonly performed and clinically successful procedures available to patients with severe degenerative arthritis and other painful conditions of the hip, such as osteonecrosis [17]. Implant design and philosophy have evolved since the use of the first THA, which was described by Professor Themistocles Glick in 1891; his ivory implant was used to replace the femoral heads of patients with tuberculosis [7]. Sir John Charnley [2] is widely considered the father of modern THA. He advocated for the use of acrylic bone cement to allow for fixation of the acetabular and femoral components, in conjunction with a small-diameter femoral head to minimize wear (“the low-friction arthroplasty”).
Before Dorr’s classification [6], Noble et al. [24] measured the canal flare index to divide the proximal femoral geometry among the stovepipe, normal, and champagne flute shapes. The canal flare index was measured as the ratio of the intracortical width of the proximal femur 20 mm proximal to the lesser trochanter to the intracortical width of the canal isthmus. Femurs with ratios of less than 3 were categorized as “stovepipe,” those with ratios of 3 to 4.7 as “normal,” and those with ratios of 4.7 to 6.5 as “champagne-fluted.” Dorr et al. [6] expanded on these findings to investigate the biochemical and histologic properties associated with differences in the morphology of the proximal femur.
Long-term follow-up of early cemented femoral designs suggested that the most-common cause of revision was aseptic loosening [14, 26]. Furthermore, revision arthroplasty with cemented femoral fixation demonstrated higher rates of failure than did primary THA [15]. “Cement disease” as a potential cause of late failure spurred investigation in alternative forms of fixation [12]. In North America in particular, the use of uncemented implants began to see wider use in the 1980s as advances in mechanical engineering facilitated osteointegration after initial press-fit fixation. In this context, a classification system was needed to help determine which patients were good candidates for uncemented fixation, the success of which was felt to depend to some degree on the quality of femoral cortical bone.
Purpose
Early cementless implants were challenging to implant, and proponents of those first-generation devices recommended limiting their use to patients with robust femoral cortical bone [13, 19]. In this context, Dorr et al. [6] described a classification system to evaluate the quality of the femur on plain radiographs in patients who undergo THA. There is debate even today about whether cementless devices should be used in patients whose femoral bone quality is poor [1, 4], and for that reason, the criteria initially developed by Dorr et al. [6] to some extent remain in use today.
As with most orthopaedic classification systems, the Dorr classification system of femoral bone quality can be used for four main purposes in patients undergoing THA: clinical decision-making, communication among providers, determining to what degree the prognosis of reconstructions may depend on bone quality, and research. It is also critical that surgeons understand the calcar-canal ratio, described in Dorr et al. [6], which can be used in templating to decrease risk of intraoperative fracture in THA.
Description
The Dorr classification evaluates the quality of the proximal femur according to radiographic, biochemical, and histologic data (Fig. 1). The study described three types of proximal femoral geometry based on 52 consecutive patients undergoing primary THA [6]. The differences in bone type can especially be appreciated on a lateral radiograph. Type A indicates thick and distinct cortices seen on AP and lateral radiographs, creating a narrow diaphyseal canal and “funnel shape” of the proximal femur. The lateral radiograph shows a thick curved posterior cortex (fin). In the original study, this type was more frequently found in younger, heavier, and male patients. Dorr Type A femurs (“champagne flute”) typically accommodate a flat, tapered, proximally porous coated stem (that is, a “single wedge” or “blade”), although the length of the stem as well as the specific implant’s geometry must be considered. Flat, tapered, proximally fitting stems typically achieve three points of fixation: two points near the metaphysis as the proximal aspect of the implant engages the lateral shoulder and medial calcar, and a third point near the distal aspect of the stem in the diaphysis [16]. In some Dorr Type A femurs, the meta-diaphyseal diameter can be excessively narrow, causing the implant to engage or get “caught up” distally before maximizing their fit in the metaphysis [3]. The may result in some degree of axial stability, but often results in a femoral implant that is undersized and fails to achieve rotational stability within the metaphysis. Stems that are caught up distally also may be excessively proud regardless of mechanical stability, resulting in the potential for leg length discrepancy or the need for reduced head or neck lengths to achieve equality. This may result in a leg-length discrepancy or the need for reduced head or neck lengths to achieve equality. Although patients with Dorr Type A femoral bone often have thick cortical densities, excessive impaction of tapered broaches may result in fracture in the metadiaphyseal region [27].
Fig. 1.
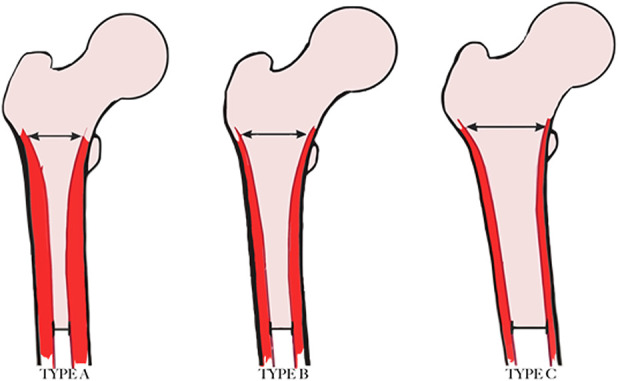
This figure shows the Dorr classification.
Type B indicates bone loss from the medial and posterior cortices resulting in a wider diaphyseal canal, which was found to be more prevalent in men than in women. Type B femurs on a lateral radiograph have erosion of the posterior fin with flattening of the cortex and proximal “rat bites” from active osteoclast activity. The distal end of the posterior fin may be absent. Dorr Type B femurs will typically accommodate most stem designs, both cemented and uncemented. However, flat, tapered stems largely depend on the quality of the proximal cortical bone and three-dimensional anatomy, and even if axial stability is achieved and the mediolateral cortical width is filled, rotational stability may be inadequate if the quality of cancellous bone is poor or becomes deficient because of inaccurate broaching. As such, a metadiaphyseal or fully diaphyseal engaging stem (or cemented stem) might be considered [25].
Type C indicates substantial loss of the medial and posterior cortices (loss of the posterior fin) with decreased bony definition of the cortices (a “fuzzy” appearance). Type C proximal femurs were described as having a “stovepipe” appearance. They had a wide canal diameter and were more often found in thinner, elderly, and female patients (Fig. 1). Dorr C Type femurs (“stovepipe”) have a geometry that often favors the use of a cemented stem. Although the use of flat, tapered metadiaphyseal and diaphyseal stems has been described with clinical success in Dorr Type C femurs in several studies [5, 22, 28], reliance on these styles of implant may be associated with an increased risk of complications [8, 30]. Dorr Type C femoral anatomy is typically associated with underlying osteopenia, as confirmed histologically in Dorr et al.’s original study [6]. The force necessary to achieve mechanical stability with a tapered broach thus may result in femoral fracture, typically at the level of the calcar. A diaphyseal-engaging stem may result in fracture at the diaphysis, either with reaming or final implant positioning. Second, the integrity of bone necessary to achieve mechanical support with an uncemented stem may result in axial or rotational instability at the time of surgery or early postoperative subsidence. The use of cement to allow for immediate mechanical interdigitation typically negates this consideration [10].
Each type was analyzed using quantitative indices: the cortical index and the canal-to-calcar isthmus ratio. The cortical index was defined as the ratio of the difference between the femoral diaphyseal diameter and intramedullary canal diameter over the femoral diaphyseal diameter at a point 10 cm distal to the mid-lesser trochanter as a reflection of the cortical thickness (Fig. 2). The canal-to-calcar isthmus ratio was calculated on an AP radiograph as a fraction of the intramedullary canal’s isthmus over the diameter of the intramedullary canal at the calcar. Widened distal intramedullary canals will have higher ratios for this measurement (Fig. 3). Cortical indices were higher in Type A bone (0.58 ± 0.01) than in Type B bone (0.50 ± 0.0) and higher in Type B bone than in Type C bone (0.42 ± 1 0.01), with all measures being statistically significant. Type C bone also had higher canal-to-calcar ratios (0.64 ± 0.02) than Type A bone did (0.57 ± 0.02), but no difference was identified between Type B (0.59 ± 0.02) and Types A or C. Calcar-canal ratios are an important aspect of preoperative templating to avoid intraoperative fracture. In Type A bone, the canal is small compared with the metaphysis, so templating to fill the metaphysis may result in fracture of the medial calcar or lesser trochanter. Additionally, the thick posterior fin in Type A bone may displace the stem anteriorly in the diaphysis, forcing the femoral head into a posterior position. This can retrovert the stem and impact stability. In Type C bone, the metaphysis might be smaller than the diaphysis, so templating to fill the diaphysis may result in fracture of the medial calcar.
Fig. 2.
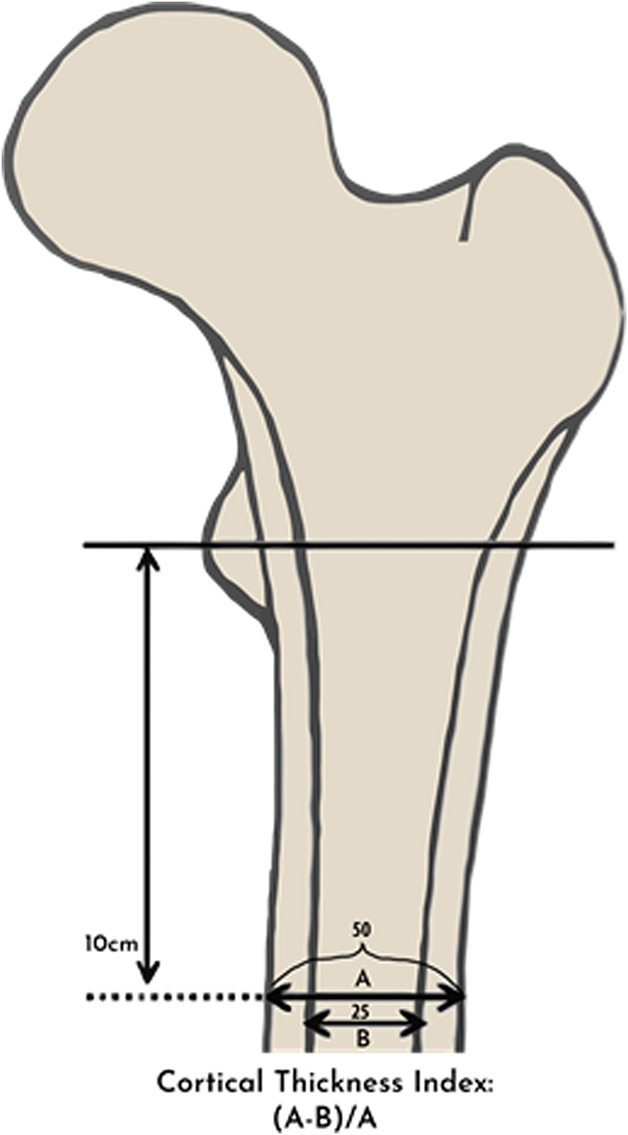
This image shows the cortical thickness index. In this example, the cortical index is 0.5 ([A-B/A] = 50-25/50).
Fig. 3.
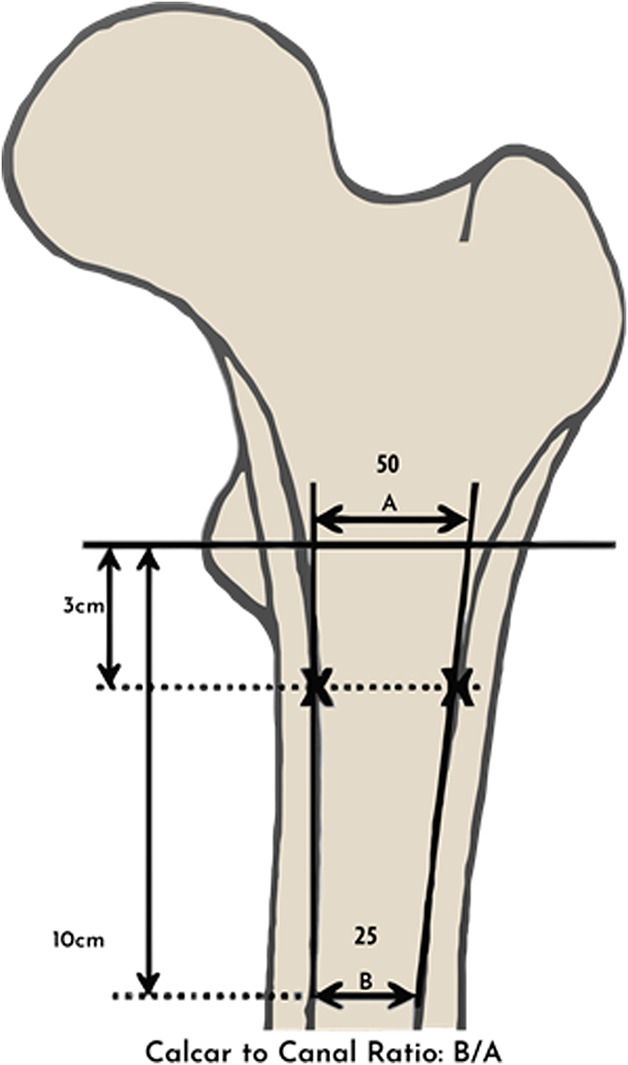
This image shows the calcar-to-canal ratio. In this example, the calcar-to-canal ratio would be 50% (B/A= 25/50).
Dorr et al. [6] measured serum calcium (measured by atomic absorption spectrophotometry), parathyroid hormone (by radioimmunoassay), and vitamin D levels (by liquid chromatography) in each patient and found these values to be within normal limits and not statistically different among the three Dorr types.
A histologic assessment of intraoperative bone biopsy results from each type of bone determined that Type C proximal femurs had deficiencies at both the structural and cellular level. Structurally, Type A bone had thicker cortical bone than Types B and C bone, and Type B bone was thicker than Type C bone. Osteoid volume, osteoid surface, the mean formation period of osteons, osteoblast surface, osteoclast surface, and numbers of osteoblasts and osteoclasts were all greater in Type C bone than in Types A and B bone. There were no differences in these parameters between Types A and B bone. Type B bone had greater cortical porosity than Types A and C bone, but no differences were detected between Types A and C for this parameter.
Validation
The Dorr classification is used in both orthopaedic and non-orthopaedic specialties to assess the morphology of the proximal femur. In the original study, AP and lateral radiographs from 52 patients were examined by two observers twice for classification. The interobserver variation was less than 20% at the first reading and less than 5% at the second reading [6]. Multiple studies have assessed the validity of the Dorr classification since then, with varied results [20, 23, 29].
Mazhar et al. [20] evaluated the interobserver and intraobserver reliability of the Dorr classification among two senior orthopaedic residents, two orthopaedic surgeons, and two arthroplasty fellowship-trained surgeons. The intraobserver reliability was calculated after individuals performed first and second reviews of 50 AP hip radiographs 6 weeks apart, and interobserver reliability was assessed within and between experience level groups. The authors used Cohen’s kappa value to calculate reliability; Cohen’s kappa ranged from -1 to 1, with 0 reflecting a random chance of agreement and 1 reflecting perfect agreement [21]. In Mazhar et al.’s study [20], the mean total kappa value (intraobserver and intraobserver) for residents was 0.576, 0.553 for orthopaedic surgeons, and 0.484 for fellowship-trained surgeons; these kappa values are too low to support adoption of the Dorr classification, and in that study, the authors found that experience did not improve reliability by very much. However, Mazhar et al. [20] only reviewed AP radiographs.
However, other studies have found greater reliability, particularly among more-experienced users. In 2018, Nakaya et al. [23] assessed the reproducibility of the Dorr classification between experience levels (three junior and three senior arthroplasty surgeons) and the effect of quantitative indices on observer reliability. The intraexaminer reproducibility was 0.36, 0.62, and 0.65 for junior hip surgeons and 0.7, 0.86, and 0.87 for senior hip surgeons. Meanwhile, the interexaminer reproducibility was 0.32 for junior hip surgeons and 0.52 for expert hip surgeons. Their finding suggested that interobserver and intraobserver reliability positively correlated with levels of clinical experience, although interobserver reliability remained a serious concern, even among more-experienced users. When observers also used cortical indices as an assistive measure (the ratio of the difference between the femoral diaphyseal diameter and intramedullary canal diameter over the femoral diaphyseal diameter at a point 10 cm distal to the mid-lesser trochanter) (Fig. 2), the reliability of the Dorr classification improved substantially, with an intraexaminer reproducibility of 0.89 and 0.86 for junior and senior hip surgeons, respectively [23]. Based on this, we strongly recommend that surgeons using the Dorr classification employ it in conjunction with carefully calculated cortical indices (Fig. 2).
Outside orthopaedic surgery, Sah et al. [29] found that patients with Type C bone had lower T scores than those with Type A bone did, in contrast to the Singh index and canal-to-calcar ratio, which were not associated with T scores. This demonstrated that the Dorr classification may indicate a patient’s bone quality. However, a prior study showed that plain radiographs are not the most-reliable was to assess patient bone quality [11]. In the study by Sah et al. [29], intraobserver reliability was 92% and is somewhat reassuring in light of an earlier study that reported much lower intraobserver reliability metrics [20]. We believe the higher intraobserver reliability reported by Sah et al. [29] may be because they used AP and lateral radiographs for evaluation, as opposed to the study by Mazhar et al. [20].
Limitations
The most-important limitation of the Dorr classification has been its inconsistent reliability across the studies that have evaluated it [20]. Kappa values of approximately 0.5 or less do not support the wide use of a classification system; however, two studies have found much better reliability [23, 29], with kappa values and agreement percentages easily high enough to recommend the classification’s use, as we noted earlier. In general, the studies that showed higher reliability had more-experienced reviewers and used quantitative indices such as the cortical index as an assistive measure. For readers classifying proximal femoral morphology according to Dorr et al. [6], we recommend that cortical indices be used in addition to qualitative bone assessments, and that classifications are discussed with experienced surgeons to improve reliability.
Although very early uncemented components were difficult to implant and risked causing femoral fractures in patients with poorer cortical bone quality [13, 19], making the Dorr classification essential for decision-making vis-à-vis implant selection, most recent studies have found that modern uncemented components are generally reliable, even in patients with poor (Dorr Type C) femoral cortical bone quality (Table 1) [5, 9, 22, 28]. This may call into question whether the Dorr classification offers as valuable a contribution to clinical decision-making in the era of contemporary components as it once did. Regardless, reliable mechanical fixation with uncemented implants cannot always be assured, and a critical evaluation of femoral geometry using Dorr’s method may help identify patients who may benefit from a cemented techique.
Table 1.
Studies in support of uncemented fixation in Dorr C Type bone
Conclusions
Although the Dorr classification has been found to have inconsistent interexaminer and intraexaminer reliability, use of the cortical index appears to improve its reproducibility. We recommend that the Dorr classification be used with attention to lateral radiographs for classification into type and calculation of cortical indices. Although Dorr Type C bone once was thought to preclude the use of cementless fixation, studies of modern femoral stem designs have demonstrated durable fixation with this technique [5, 9, 22, 28]. Surgeons must also consider, however, that patients with Type C bone are often elderly and inactive, and the consequences of intraoperative fracture using cementless THA may result in a second surgery. Furthermore, several of these studies [5, 9, 22] demonstrating efficacy of cementless implants in poor femoral bone have had relatively few patients and the procedures were performed by experienced surgeons. As stated by Leopold [18], some of the long-term dangers of newer procedures or techniques may not be discovered until years later and would require very large sample sizes to accurately detect. We believe the Dorr classification remains useful in patient selection, particularly for lower-volume surgeons who may consider cemented techniques to decrease early mechanical loosening or femoral fractures during hip arthroplasty. We strongly emphasize the importance of preoperative templating to ensure a stem will fit the geometry of a patient’s femoral canal to decrease the risk of iatrogenic fractures in patients who undergo THA. Further investigations should be performed to identify reliable and useful assessments of a patient’s bone morphology to decrease the risk of complications such as intraoperative fracture during THA.
Acknowledgments
We thank Arien Cherones BS, Director of Technology and Systems Management for UW Orthopaedics and Sports Medicine for his assistance with creating and formatting the table.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Both authors certify that they has no commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aro HT, Alm JJ, Moritz N, Makinen TJ, Lankinen P. Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: a 2-year RSA study of 39 patients. Acta Orthop. 2012;83:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charnley J. Arthroplasty of the hip. A new operation. Lancet. 1961;1:1129-1132. [DOI] [PubMed] [Google Scholar]
- 3.Cooper HJ, Jacob AP, Rodriguez JA. Distal fixation of proximally coated tapered stems may predispose to a failure of osteointegration. J Arthroplasty. 2011;26:78-83. [DOI] [PubMed] [Google Scholar]
- 4.Cooper HJ, Rodriguez JA. Early postoperative periprosthetic femur fracture in the presence of a non-cemented tapered wedge femoral stem. HSS J. 2010;6:150-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalury DF, Kelley TC, Adams MJ. Modern proximally tapered uncemented stems can be safely used in Dorr type C femoral bone. J Arthroplasty. 2012;27:1014-1018. [DOI] [PubMed] [Google Scholar]
- 6.Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231-242. [DOI] [PubMed] [Google Scholar]
- 7.Gomez PF, Morcuende JA. Early attempts at hip arthroplasty--1700s to 1950s. Iowa Orthop J . 2005;25:25-29. [PMC free article] [PubMed] [Google Scholar]
- 8.Gromov K, Bersang A, Nielsen CS, Kallemose T, Husted H, Troelsen A. Risk factors for post-operative periprosthetic fractures following primary total hip arthroplasty with a proximally coated double-tapered cementless femoral component. Bone Joint J . 2017;99:451-457. [DOI] [PubMed] [Google Scholar]
- 9.Hatem MA, Ferreira da Luz B, Nishi RN, Cimbalista de Alencar PG. Evaluation of the results from proximal fixation of uncemented conical femoral components in Dorr type C femurs. Rev Bras Ortop . 2014;49:260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy WL. Hip implant selection for total hip arthroplasty in elderly patients. Clin Orthop Relat Res. 2002:54-64. [DOI] [PubMed] [Google Scholar]
- 11.Jensen T, Hansen M, Jensen KE, Podenphant J, Hansen TM, Hyldstrup L. Comparison of dual X-ray absorptiometry (DXA), digital X-ray radiogrammetry (DXR), and conventional radiographs in the evaluation of osteoporosis and bone erosions in patients with rheumatoid arthritis. Scand J Rheumatol . 2005;34:27-33. [DOI] [PubMed] [Google Scholar]
- 12.Jones LC, Hungerford DS. Cement disease. Clin Orthop Relat Res. 1987;225:192-206. [PubMed] [Google Scholar]
- 13.Judet R, Siguier M, Brumpt B, Judet T. A noncemented total hip prosthesis. Clin Orthop Relat Res. 1978;137:76-84. [PubMed] [Google Scholar]
- 14.Kavanagh BF, Dewitz MA, Ilstrup DM, Stauffer RN, Coventry MB. Charnley total hip arthroplasty with cement. Fifteen-year results. J Bone Joint Surg Am. 1989;71:1496-1503. [PubMed] [Google Scholar]
- 15.Kavanagh BF, Ilstrup DM, Fitzgerald RH., Jr Revision total hip arthroplasty. J Bone Joint Surg Am. 1985;67:517-526. [PubMed] [Google Scholar]
- 16.Kim JT, Yoo JJ. Implant design in cementless hip arthroplasty. Hip Pelvis. 2016;28:65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet . 2007;370:1508-1519. [DOI] [PubMed] [Google Scholar]
- 18.Leopold SS. Editorial: When “safe and effective” becomes dangerous. Clin Ortho Relat Res . 2014;472:1999-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord GA, Hardy JR, Kummer FJ. An uncemented total hip replacement: Experimental study and review of 300 madreporique arthroplasties. Clin Orthop Relat Res. 1979; 141:2-16. [PubMed] [Google Scholar]
- 20.Mazhar FN, Jafari D, Nojoomi M, Mirzaei A, Tayebi H. Inter and intra-observer reliability of Dorr classification in proximal femur morphology. Shafa Ortho J. 2018;5:e64801. [Google Scholar]
- 21.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) . 2012;22:276-282. [PMC free article] [PubMed] [Google Scholar]
- 22.Meding JB, Galley MR, Ritter MA. High survival of uncemented proximally porous-coated titanium alloy femoral stems in osteoporotic bone. Clin Orthop Relat Res. 2010;468:441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakaya R, Takao M, Hamada H, Sakai T, Sugano N. Reproducibility of the Dorr classification and its quantitative indices on plain radiographs. Orthop Traumatol Surg Res. 2019;105:17-21. [DOI] [PubMed] [Google Scholar]
- 24.Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS. The anatomic basis of femoral component design. Clin Orthop Relat Res. 1988:148-165. [PubMed] [Google Scholar]
- 25.Nourbash PS, Paprosky WG. Cementless femoral design concerns. Rationale for extensive porous coating. Clin Orthop Relat Res. 1998:189-199. [PubMed] [Google Scholar]
- 26.Olsson SS, Jernberger A, Tryggo D. Clinical and radiological long-term results after Charnley-Müller total hip replacement: a 5-10 year follow-up study with special reference to aseptic loosening. Acta Orthop Scand 1981;52:531-542. [DOI] [PubMed] [Google Scholar]
- 27.Park CW, Eun HJ, Oh SH, Kim HJ, Lim SJ, Park YS. Femoral stem survivorship in Dorr type A femurs after total hip arthroplasty using a cementless tapered wedge stem: a matched comparative study with type B femurs. J Arthroplasty. 2019;34:527-533. [DOI] [PubMed] [Google Scholar]
- 28.Reitman RD, Emerson R, Higgins L, Head W. Thirteen year results of total hip arthroplasty using a tapered titanium femoral component inserted without cement in patients with type C bone. J Arthroplasty. 2003;18:116-121. [DOI] [PubMed] [Google Scholar]
- 29.Sah AP, Thornhill TS, LeBoff MS, Glowacki J. Correlation of plain radiographic indices of the hip with quantitative bone mineral density. Osteoporos Int . 2007;18:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taunt CJ, Jr, Finn H, Baumann P. Immediate weight bearing after cementless total hip arthroplasty. Orthopedics. 2008;31:223. [DOI] [PubMed] [Google Scholar]