Where Are We Now?
In the current study, Rohrer and colleagues [15] performed a single-blinded randomized trial to determine whether preoperative decolonization reduces surgical site infections (SSIs) in a European orthopaedic surgery tertiary care center. Two weeks before elective orthopaedic procedures, patients were screened for methicillin-sensitive Staphylococcus aurea carriers (MSSA) carrier status. Two groups were formed, based on whether patients carried MSSA or did not carry that bacterium. Decolonization was multimodal. The researchers found no difference in the risk of SSI between the decolonization and control groups, both in carriers and noncarriers. Since decolonization did not mitigate SSIs, the medical community must seek other protocols to reduce SSIs.
Generally, there are two categories contributing to surgical complications like SSI. The first is the surgeon, who must take reasonable precautions against infection and operate efficiently. The second is the host, which sometimes is out of the surgeon’s control; some patients carry nonmodifiable factors that increase their risk for SSI, but in other respects, are good candidates for elective orthopaedic surgery.
The current study [15] brings to light that bacterial decolonization (of MSSA, at least) is not effective against reducing SSIs. We know that perioperative intravenous antibiotics can decrease SSIs. So too, does patient selection and preoperative medical optimization. These are preventive measures. A better understanding of effective treatment protocols may provide insight into more-effective preventive pathways. For example, judicious use of intravenous antibiotics is paramount to prevent emergence of resistant bacteria. We also know that many infections are polymicrobial and that treatment requires removing all implants that may have a biofilm. The biofilm is a glycocalyx formed by bacteria, which is often referred to as the extracellular polymeric substance (EPS). The EPS prevents antibiotics from reaching the deeply hidden bacteria. Moreover, the EPS can shed bacteria at any time point postoperatively resulting in a late-onset SSI. Studies have shown [1, 8, 12] that in fracture-related infections, antibiotics may be used to suppress the infection until union occurs, and then the hardware can be removed.
Where Do We Need To Go?
Going forward, we need several things to diagnose, prevent, and treat SSIs more effectively. First, we need to develop some point-of-care diagnostics; the 5 to 10 or more days currently necessary for culture results to finalize is just too long in many situations. For example, during the second stage revision for an infected total joint arthroplasty, the surgeon and patient would greatly benefit from reliable intra-operative knowledge about whether the infection is still present.
We also need a better understanding of biofilm formation. Surgeons who treat infections cannot typically ascertain the margins of infected and non-infected tissue by visual inspection. Surgeons who treat musculoskeletal infections have adopted the principle of excising a margin of healthy tissue along with infected tissue from our orthopaedic oncology colleagues. Currently, this tissue margin is a subjective boundary based upon experience, and it may not be the most effective method, as evidenced by the high risk of recurrence following revision procedures for infection [10, 13]. We need an objective method of identifying infection margins, especially when the medullary canal may be involved. This process begins with understanding that S. aureus species have been identified in osteoblasts and dermatocytes [10]. Therefore, incomplete infection débridements are probably commonplace, and this may contribute to the frequent recurrences of these infections. Tumor surgeons assess for adequate tumor resection margins based upon intraoperative biopsies, and surgeons who treat infections may have to develop similar approaches. This would depend upon establishing a method of confirming bacteria-free margins, which may come from new special stains for bacteria, or PCR/deoxyribonucleic acid (DNA) detection methods on bone and soft-tissue samples.
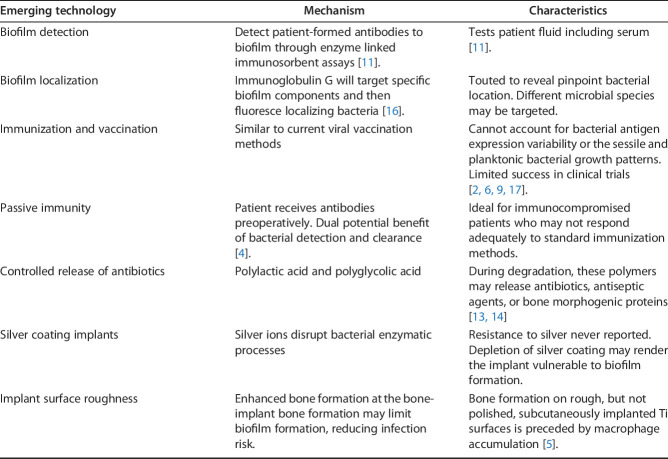
While many strategies are currently under development for improved SSI management, simply using more antibiotics probably is not the answer. Topical application before incision or wound closure has shown mixed results [1, 8, 12]. In fact, the CDC has a position statement out against topical application [7]. Generally, two arguments are made against topicals. First, they may induce resistant strains. The second objection is rooted in the fact that the reports of topical efficacy in the spine often lacked controls. Therefore, from an evidence-based perspective, the conclusions from these studies [1, 8, 12] do not apply to other orthopaedic infections. Immunization, vaccination, and immune modulation, however, are emerging approaches to infection prevention and treatment (Table 1).
Table 1.
Emerging technologies in infection management [3].
How Do We Get There?
Several promising nonantibiotic therapeutics are nearing clinical readiness for infection management. One intriguing and promising area is Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), which are the hallmark of a bacterial defense system that forms the basis for CRISPR-Cas9 genome editing technology. CRISPR has been proposed as a treatment for human diseases with a genetic cause. Its ability to modify specific DNA sequences makes CRISPR a tool with the potential to fix disease-causing mutations and infections by drug-resistant bacteria.
Alpha defensin is a molecule currently used as a marker for periprosthetic joint infections. White blood cells produce alpha defensin. If alpha defensin is detected in a joint aspiration, then the implication is that an infection must be present because white blood cells are detected indirectly via alpha defensin levels. Some patients with infections may lack the ability to synthesize infection fighting molecules such as alpha defensin. Hypothetically, CRISPR could be used to correct a genetic defect in alpha defensin production and improve one’s infection fighting capability. Furthermore, patients could be screened for infecting fighting protein deficiencies and treated accordingly preoperatively.
One final treatment frontier currently only available for diagnostic purposes are exosomes. Exosomes are microvesicles shed from the membranes of cells. They contain snippets of ribonucleic acid, (RNA), which regulates protein synthesis in target cells. Exosomes function in a paracrine like fashion. Since exosomes bud from cell membranes, their surface markers are identical to their cellular origin. This specificity has enabled early detection of cancers, cardiac, and neuro diseases because cells in these disease states shed numerous exosomes enhancing early detection. Due to their protein synthesis, regulatory properties, and cellular specificity, exosomes may treat certain diseases. For example, in infection scenarios, a patient could receive exosomes that would target their white blood cells. The exosomes would bind to white blood cells, and then the exomeRNA would enter the white blood cell, triggering increased production of phagocytosis proteins or even alpha defensin proteins. This process of altering protein synthesis is under investigation for other disease treatments, including myocardial infarctions and cerebrovascular events. Application in infection prevention and treatment would require establishing baseline quantitative and qualitative exosome expressions in patients with infections. This information would provide insight into the regulatory pathways for all of the human defense factors involved in infection. We would have to identify which exosomes assist our bodies in fighting an infection
Finally, we should devise a way to either actively or passively increase serum levels of select exosomes. Many of the currently known exosomes are numbered such as R-124 This exosome is associated with neural recovery after strokes. Researchers are currently looking at ways to increase R-124 exosome levels in animal stroke models to enhance recovery. Something similar would be done for infections.
Footnotes
This CORR Insights® is a commentary on the article “Does Preoperative Decolonization Reduce Surgical Site Infections in Elective Orthopaedic Surgery? A Prospective Randomized Controlled Trial” by Rohrer and colleagues available at: DOI: 10.1097/CORR.0000000000001152.
The author certifies that neither he, nor any members of his immediate family, have any commercial associations (such as consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The opinions expressed are those of the writer, and do not reflect the opinion or policy of CORR® or The Association of Bone and Joint Surgeons®.
References
- 1.Berkes M, Obremskey WT, Scannell B, Ellington JK, Hymes RA, Bosse M; Southeast Fracture Consortium. Maintenance of Hardware After Early Postoperative Infection Following Fracture Internal Fixation. J Bone Joint Surg Am. 2010;92:823-828. [DOI] [PubMed] [Google Scholar]
- 2.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey A, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000–2007. J Perinatol. 2010;30:135-139. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborti M, Jackson JK, Plackett D, Gilchrist SE, Burt HM. The application of layered double hydroxide clay (LDH)-poly(lactide-co-glycolic acid) (PLGA) film composites for the controlled release of antibiotics. J Mater Sci Mater Med. 2012;23:1705–1713. [DOI] [PubMed] [Google Scholar]
- 5.Chehroudi B, Ghrebi S, Murakami H, Waterfield JD, Owen G, Brunette DM. Bone formation on rough, but not polished, subcutaneously implanted Ti surfaces is preceded by macrophage accumulation. J Biomed Mater Res A. 2010;93:724-737. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: A randomized trial. JAMA. 2013;309:1368–1378. [DOI] [PubMed] [Google Scholar]
- 7.Garner J. CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in November 1985). Revised. Infect Control. 1986;7:193-200. [DOI] [PubMed] [Google Scholar]
- 8.Geronemus G, Mertz P, Eaglestein WH. Wound Healing The Effects of Topical Antimicrobial Agents. Arch Dermatol. 1979;115:1311-1314. [DOI] [PubMed] [Google Scholar]
- 9.Holt J, Hertzberg B, Weinhold P, Storm W, Schoenfisch M, Dahners L. Decreasing bacterial colonization of external fixation pins through nitric oxide release coatings. J Orthop Trauma. 2011;25:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT: Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. [DOI] [PubMed] [Google Scholar]
- 11.Leid JG, Vail TL, Kofonow JM, Shirtliff ME, Brady RA. Methods and devices for the detection of biofilm. Available at: https://patents.google.com/patent/US8541006B2/en. Accessed February 7, 2020.
- 12.Lineaweaver W, Howard R, Soucy D. Topical antimicrobial toxicity. Arch Surg. 1985;120:267-270. [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J, Alijanipour P, Barberi EF, Hickok NJ, Phillips KS, Shapiro IM, Schwarz EM, Stevens MH, Wang Y, Shirtliff ME. Novel Developments in the Prevention, Diagnosis, and Treatment of Periprosthetic Joint Infections. J Am Acad Orthop Surg. 2015;23: S32-S43. [DOI] [PubMed] [Google Scholar]
- 14.Reef SE, Lasker BA, Butcher DS, McNeil MM, Pruitt R, Keyserling H, Jarvis WR. Nonperinatal Nosocomial Transmission of Candida albicans in a Neonatal Intensive Care Unit: Prospective Study. J Clin Microbiol. 1998;36:1255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrer F, Nötzli H, Risch L, Bodmer T, Cottagnoud P, Hermann T, Limacher A, Fankhauser N, Wagner K, Brügger J. Does preoperative decolonization reduce surgical site infections in elective orthopaedic surgery? Clin Orthop Relat Res. A prospective randomized controlled trial. [Published online ahead of print]. DOI: 10.1097/CORR.0000000000001152. [DOI] [PMC free article] [PubMed]
- 16.Schaffer AC, Lee JC: Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents. 2008;32:S71–S78. [DOI] [PubMed] [Google Scholar]
- 17.Weems JJ, Jr, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R, Moore T, Patti JM, Hetherington S, Texter M, Wenzel E, Kelley VA, Fowler VG., Jr Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:2751–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]