Abstract
Background
Surgical site infections (SSIs) after elective orthopaedic surgery are very stressful for patients due to frequent rehospitalizations with reoperations and poorer functional outcomes. Prevention of such events is therefore crucial. Although an evidence-based consensus is still lacking, preoperative decolonization could decrease SSI. Specifically, more information is needed about the effect of a preoperative decolonization procedure on SSI proportions in both Staphylococcus aureus carriers and non-S. aureus carriers after general orthopaedic surgery.
Questions/purposes
Our study addressed the following questions: (1) Does preoperative decolonization reduce the risk of SSI after general elective orthopaedic surgery in patients colonized with S. aureus? (2) Does preoperative decolonization reduce the risk of SSI among patients who are not colonized with S. aureus?
Methods
In this prospective, randomized, single-blinded trial, we recruited patients undergoing general elective orthopaedic surgery in one tertiary care center in Switzerland. Between November 2014 and September 2017, 1318 of 1897 screened patients were enrolled. Patients were allocated into either the S. aureus carrier group (35%, 465 of 1318 patients) or the noncarrier group (65%, 853 of 1318 patients) according to screening culture results. In the S. aureus group, 232 patients were allocated to the intervention arm and 233 were allocated to the control arm. Intervention was 5 days of daily chlorhexidine showers and mupirocin nasal ointment twice a day. Of the 853 noncarriers, 426 were allocated to the intervention arm and 427 were allocated to the control arm. All patients in both groups were analyzed in an intention-to-treat manner. The primary endpoint was SSI occurrence at 90 days postoperative and the secondary endpoint was SSI occurrence at 30 days postoperative.
The initial sample size calculation was made for the S. aureus carrier group. Based on the literature review, a 4% proportion of SSI was expected in the control group. Thus, 726 carriers would have been needed to detect a relative risk reduction of 80% with a power of 80% at a two-sided α-error of 0.048 (adjusted for interim analysis). Assuming carrier prevalence of 27%, 2690 patients would have been needed in total. An interim analysis was performed after including half of the targeted S. aureus carriers (363 of 726). Based on the low infection rate in the control group (one of 179), a new sample size of 15,000 patients would have been needed. This was deemed not feasible and the trial was stopped prematurely.
Results
Among carriers, there was no difference in the risk of SSI between the intervention and control arms (decolonized SSI risk: 0.4% [one of 232], control SSI risk: 0.4% [one of 233], risk difference: 0.0% [95% CI -1.2% to 1.2%], stratified for randomization stratification factors; p > 0.999). For noncarriers, there was no difference in risk between the intervention and control arms (decolonized SSI risk: 0.2% [one of 426], control SSI risk: 0.2% [one of 247], stratified risk difference: -0.0% [95% CI -0.7 to 0.6]; p = 0.973).
Conclusions
We found no difference in the risk of SSI between the decolonization and control groups, both in S. aureus carriers and noncarriers. Because of the low event numbers, no definite conclusion about efficacy of routine preoperative decolonization can be drawn. The results, however, may be helpful in future meta-analyses.
Level of Evidence
Level II, therapeutic study
Introduction
Surgical site infections (SSIs) are a major postoperative complication caused mainly by the patient's endogenous organisms such as Staphylococcus aureus [21]. Given that approximately 25% of the general population is permanently colonized with S. aureus [36], prevention is crucial as treatment options for SSI are very stressful for patients (rehospitalizations, reoperations, reduction in quality of life) [9, 38]. One longitudinal study used whole genome sequencing to evaluate the routes of S. aureus transmission in an intensive care unit [29]. They found that colonization occurred with genetically diverse S. aureus strains. Transmission from healthcare workers to patients was infrequent and mostly originated from the patient’s own flora [8]. Consequently, concentrating on vertical transmission control could be more effective than adding horizontal infection control measures. Decolonization can be effective in S. aureus carriers by following protocols stipulating the use of intranasal mupirocin and showering with chlorhexidine [4]. One groundbreaking randomized controlled trial by Bode et al. [1] analyzed the effect of preoperative decolonization of S. aureus carriers on the incidence of hospital-associated infections. The procedure reduced infection rates from 7.7% in the control group to 3.4% in the intervention group.
In general orthopaedic surgery, however, the possibility of reducing SSI through decolonization remains controversial. In the trial by Bode et al. [1] , most patients underwent cardiothoracic surgery, which presents a higher risk for SSI than orthopaedic surgery. As a result, no conclusion from this trial can be transferred to orthopaedic surgery. To date, only retrospective studies favor preoperative decolonization procedures in orthopaedic surgery [7, 12, 31, 32] and other studies failed to find any substantial effect of a “screen and treat” strategy [13, 20, 22, 28]. A randomized controlled trial did not demonstrate any reduction in SSI, but patients underwent decolonization independent of their carrier status [15]. “Non-carriers only” were never addressed separately in these trials and the possible effects of decolonization in this population is unknown. A further difficulty of implementing decolonization is ensuring adherence to the protocol; which can be as low as one third [3]. Studies investigating decolonization protocols should, therefore, also focus on methods to improve how patients follow instructions. As a whole, systematic reviews recommended further prospective randomized trials on the effect of decolonization before orthopaedic surgery and the subsequent creation of widely applicable evidence-based guidelines [9, 33].
Therefore, we asked: (1) Does preoperative decolonization reduce the risk of SSI after general elective orthopaedic surgery in patients colonized with S. aureus? (2) Does preoperative decolonization reduce the risk of SSI among patients who are not colonized with S. aureus?
Patients and Methods
Study Design
The present DECO-SSI (DECOlonization and SSI) study is a prospective, randomized, controlled, interventional, single-blinded trial (the outcome assessors were blinded) performed at one tertiary care center in Bern, Switzerland. It was designed with carrier and noncarrier groups, which included two parallel arms each. Patients with a positive nose swab culture for S. aureus were allocated to the carrier group and those with a negative nose swab culture were allocated to the noncarrier group. Patients in each group were then randomized to either a control or an intervention arm. Intervention consisted of a decolonization procedure 5 days before surgery.
The primary outcome was the overall 90-day postoperative incidence of SSI. Secondary outcomes were defined as early (30-day postoperative) and late (31-day to 90-day postoperative) SSI, death related to infection, SSI caused by documented bacteria and time from surgery to SSI or death. S. aureus strains identified in SSI were compared with colonizing strains from the same patient using next-generation sequencing (see Appendix, Supplemental Digital Content, http://links.lww.com/CORR/A295).
The study protocol was approved by the local ethics committee (PB_2016_00256). Written informed consent was obtained from each patient during a screening visit. The trial was monitored by an external expert of the clinical trial unit, University of Bern, Switzerland.
Participants
All patients scheduled for elective orthopaedic surgery were evaluated for eligibility. Inclusion criteria were a minimum age of 16 years, written and signed informed consent, and a period of at least 14 days before surgery. This timeframe was needed to perform nasal swabbing and decolonization. Exclusion criteria were allergy to mupirocin or chlorhexidine, the presence of any foreign nasal body, pregnancy, ongoing intervention for a documented infection or prior enrollment in the study. Withdrawal criteria were the withdrawal of patient consent, death, and loss to follow-up. All available data were evaluated in an intention-to-treat manner.
Randomization and Masking
Patients in the carrier and noncarrier groups were allocated either to an intervention or a control arm on a 1:1 basis in the electronic data entry system (REDCap v 8.5.19, Vanderbilt University, Nashville, TN, USA). Randomization was stratified by procedure type (upper extremities and pelvic/hip versus spine versus knee and foot) and American Society of Anesthesiologists criteria (I versus II versus III-V). A randomization list was generated by an independent statistician not otherwise involved in the trial. Allocation was concealed using central randomization, which was implemented in REDCap.
Enrollment, Intervention and Follow-up
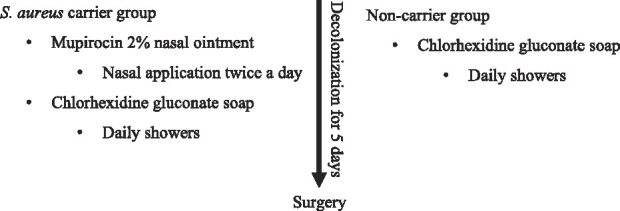
Patients scheduled for surgery after an orthopaedic consultation were enrolled, and informed consent was obtained by a member of the trial team. To screen for S. aureus, a nasal swab was obtained 2 to 4 weeks before surgery; either by a trained clinical research coordinator or by the patients themselves according to verbal and written instructions. The intervention arms of both groups received a decolonization kit per mail one week before surgery. For S. aureus carriers, the kit contained mupirocin 2% nasal ointment (BACTROBAN® Nasal ong, 3 g, GSK, Muenchenbuchsee, Switzerland) with instructions to apply the ointment to each nostril twice a day for 5 days before admission and to shower daily using ample amounts of chlorhexidine gluconate soap (Lifo-Scrub sol 4%®, 500 mL, B. Braun, Sempach, Switzerland). For non-carriers, the decolonization procedure was performed using only chlorhexidine gluconate soap (Lifo-Scrub sol 4% 500 mL, B. Braun) (Table 1). For practical reasons, we did not consider the use of a placebo kit in the control group. To improve and measure compliance, mobile phone text message reminders were sent at the start of decolonization and patients had to complete a daily checklist during the decolonization period. Participants in the control arm were instructed to shower before the intervention with conventional soap according to our standard infection prevention protocol. Patients in both arms received cefuroxime antibiotic prophylaxis (1.5 g, IV) 30 minutes to 60 minutes before incision and at 8 hours and 16 hours postoperatively. The operative field was disinfected three times with povidone-iodine alcoholic solution (Betaseptic®, Mundipharma Medical Company, Basel, Switzerland), in line with national hygiene and infection control guidelines [27]. No specific antiseptic wound dressings were applied postoperatively.
Table 1.
Study intervention according to groups
At 30 days and 90 days after surgery, a clinical research coordinator interviewed patients by telephone using a standardized form to assess the possibility of SSI (local wound symptoms, fever, and antibiotic treatment prescribed). The questionnaire was designed by the Swiss National Nosocomial Infection Monitoring Institution and is used routinely in this context. When SSI was suspected, the responsible orthopaedic surgeon was contacted and asked to confirm the diagnosis. SSIs were defined by Centers for Disease Control criteria [14]. Surgeons were blinded to the carrier status and the study arm of their patient. Patients were instructed, both verbally and in writing, not to inform their surgeon about the possible decolonization treatment.
Accounting for Patients
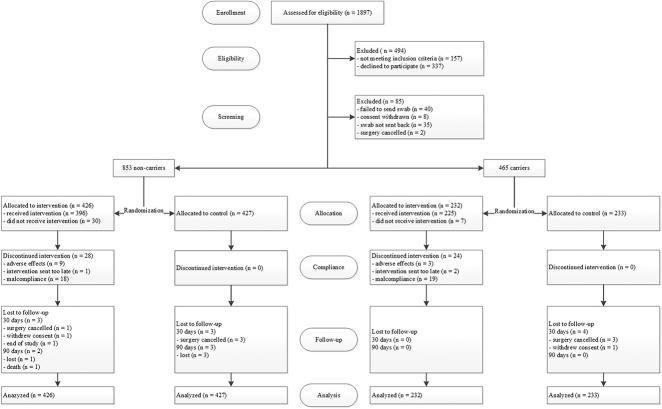
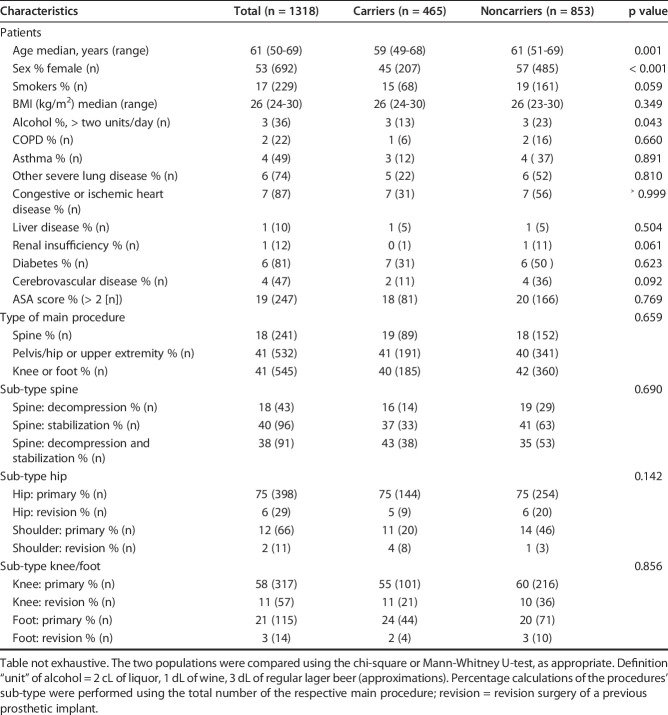
Between November 1, 2014 and September 30, 2017, 1897 patients were screened for study eligibility; 1403 met the inclusion criteria and were enrolled (Fig. 1). The main reason patients declined to participate was the additional time and effort required before surgery. Data was not collected for non-participants; thus, no comparison could be made with the active participants. A further 85 patients were excluded before randomization because of missing nose swabbing results. Of 1318 returned nose swabs, 465 tested positive for S. aureus (35%) and 853 negative (65%). S. aureus carriers were slightly younger than noncarriers (median 59 years versus 61 years; p = 0.001) and predominantly male (56% versus 43%, p < 0.001) (Table 2). Other baseline characteristics were not markedly different between carriers and noncarriers. The most common operations were on the hip, knee, and spine. Overall, 43% (571 of 1318) of the patients underwent prosthetic surgery. Baseline characteristics of the two arms of the carrier and noncarrier groups were well-balanced. Adherence to the treatment regimen was defined as the use of the intervention drugs for at least 4 days, and was 87% and 86% in the carrier and noncarrier groups, respectively.
Fig. 1.
This flow chart shows the patients included in the study.
Table 2.
Participant baseline characteristics and comparison between carriers and noncarriers
Of the 465 carriers, 232 were allocated to the intervention arm and 233 were allocated to the control arm. In the intervention arm, 225 patients received the decolonization kit. No patient was lost to follow-up. Of the 853 noncarriers, 426 were allocated to the intervention arm and 427 were allocated to the control arm. In the intervention arm, 396 patients received the decolonization kit. In total, four patients were lost to follow-up.
Procedures
Nasal swabs were taken using nylon fiber flocked swabs treated with Amies liquid transport medium (SwabAX, Axon Lab AG, Baden, Switzerland) and processed on a BD Kiestra Total Laboratory Automation system (Becton-Dickinson, Sparks, MD, USA). Specimens were vortexed, flocked swabs were removed, and 100 µL of transport medium was transferred into tubes containing 3 mL of selective enrichment broth (Staphylokokken Anreicherungsbouillon, Axon Lab AG, Baden, Switzerland). After incubation at 35° C for 16 to 24 hours, broths were sub-cultured onto chromID S. aureus Elite agar media (bioMérieux, Marcy l’Etoile, France). Agar media were incubated at 35° C in an aerobic atmosphere and observed after 16 hours to 24 hours and after 32 hours to 48 hours. Suspicious colonies were identified at the species level using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany). Comprehensive antibiograms of S. aureus isolates were obtained using VITEK-2 AST-P636 (bioMérieux) and interpreted according to current European Committee on Antimicrobial Susceptibility Testing guidelines [10]. Isolates from patients with S. aureus were stored at -80° C (Cryobank, Mast Diagnostica, Germany).
Sample Size
The sample size calculation was based on the S. aureus carrier group. Previous studies reported an approximate 80% relative risk reduction of SSI in decolonized S. aureus carriers and an SSI proportion of 4% in general elective orthopaedic surgery in S. aureus carriers [5, 12, 28, 31, 32]. As internal SSI proportions at our hospital were unknown, estimations were based on a review of previous studies. Assuming a 4% proportion of SSI in the control group, 726 carriers would have been needed to detect a relative risk reduction of 80% with a power of 80% at a two-sided α-error of 0.048 (adjusted for interim analysis). Based on 27% carrier prevalence in the general population, 2690 patients would have been needed overall [5, 6, 11-13, 15, 28]. We also performed a power analysis for the noncarrier group. An assumed prevalence of noncarriers of 73%, results in 1964 patients for this study group. Assuming a 2% risk of SSI in noncarriers, we would have detected a relative risk reduction of 71% with a power of 80% at a two-sided α-level of 0.05 [6, 11, 12, 15, 28].
Outcomes: Interim Analysis
An interim analysis was pre-specified in the study protocol and statistical analysis plan to assess efficacy, futility, and recalculate the sample size. Sample size recalculations were based on the carrier group only; as the most pronounced effect was expected in this group. This was conducted after evaluating 363 of the targeted 726 carriers. The sample size re-estimation was based on the originally predicted risk reduction of 80%, the actually observed proportion of SSI in the S. aureus carrier control group, and the prevalence of S. aureus carriers at the time of the interim analysis. To assess futility, we calculated the conditional power to obtain a statistically significant risk difference in the carrier group at the end of the trial based on the original sample size assumptions. We pre-specified that if the conditional power was less than 10%, the trial would be stopped for futility. To assess efficacy, we adopted the O’Brien-Fleming group sequential design: testing the primary hypothesis of the carrier group at a significance level of 0.0052 for the interim analysis and 0.048 for the final analysis. An independent statistician performed the work, which was evaluated by a data safety monitoring board. In the end, the data safety monitoring board halted the trial because of futility and non-feasibility. The conditional power for the original sample size was only 4.2%. The sample size recalculation stipulated that 14,752 patients instead of the original 2690 would be needed, and this was deemed unfeasible. Patient recruitment continued during the interim analysis. The final analysis was then conducted with all patients included. No SSIs were observed in the carrier group between the interim analysis and termination of the trial.
Statistical Analysis
We applied a hierarchical test procedure to account for double hypothesis testing in the carrier and noncarrier groups. Expecting a more pronounced effect in the carrier group, we first tested the superiority of the decolonization procedure to control infection in the carrier group at the two-sided α-level of 0.048. Secondary hypothesis testing in the noncarrier group would have been performed only if the primary null hypothesis in the carrier group was rejected. The primary analysis was performed according to an intention-to-treat principle. A per-protocol analysis was performed for sensitivity, excluding patients who did not fulfill the minimal adherence criteria or had missing outcome data. We compared the characteristics of the two study populations using the chi-square or Mann-Whitney U test, as appropriate. For the primary binary outcome, we calculated the proportion and Mantel-Haenszel risk difference with a corresponding 95% confidence interval stratified for randomization stratification factors (surgical procedure type and American Society of Anesthesiologists category) according to Klingenberg et al.’s method [19]. We compared groups using the stratified Cochran-Mantel-Haenszel test. “R” software (R core team, version 3.5.1, Vienna, Austria,) for the statistical analysis.
Results
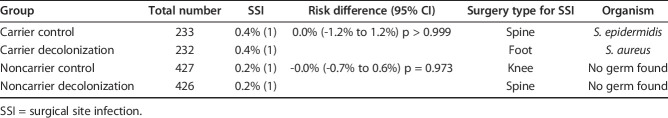
Among carriers, there was no difference in the risk of SSI between the intervention and control arms (risk difference: 0.0% [95% CI -1.2 to 1.2], stratified for randomization stratification factors; p ≥ 0.999) (Table 3). The overall SSI risk was 0.4% (one of 232 patients) in decolonized carriers and 0.4% (one of 233 patients) in control S. aureus carriers. Both SSIs were early-onset, and no deaths occurred. A subgroup analysis was not conducted because of low SSI numbers. In the per-protocol analysis, 32 patients in the intervention arm and five in the control arm were excluded, mainly because the intervention was discontinued. There was no difference in risk between the arms (stratified risk difference: -0.1% [95% CI -1.4 to 1.2]; p = 0.941).
Table 3.
Proportions of patients with SSI and types of SSI
S. aureus carrier proportions were 35% (465 of 1318) and 0.08% of patients (one of 1318) was a carrier of methicillin-resistant S. aureus (MRSA). All S. aureus isolates responded to mupirocin.
For noncarriers, there was no risk difference between the intervention and control arms (stratified risk difference: -0.0% [95% CI -0.7 to 0.6]; p = 0.973) (Table 3). Overall, the SSI incidence was 0.2% (one of 426 patients) in decolonized carriers and 0.2% (one of 427 patients) in control noncarriers. Both SSIs were early-onset. One death unrelated to SSI occurred in the intervention arm. A subgroup analysis was not conducted because of low SSI numbers. In the per-protocol analysis, 64 patients in the intervention arm and seven in the control arm were excluded, mainly because the intervention was discontinued. There was no risk difference between the arms (stratified risk difference: -0.1% [95% CI -0.8 to 0.6]; p = 0.896).
Overall, one of four SSIs occurred due to documented S. aureus. The patient had been identified as a nasal carrier before undergoing elective orthopaedic surgery and had completed our decolonization protocol. Vertical transmission was suspected and confirmed by whole genome sequence strain typing (Fig. 2). One SSI was caused by Staphylococcus epidermidis and in two SSIs, no pathogen was identified.
Fig. 2.
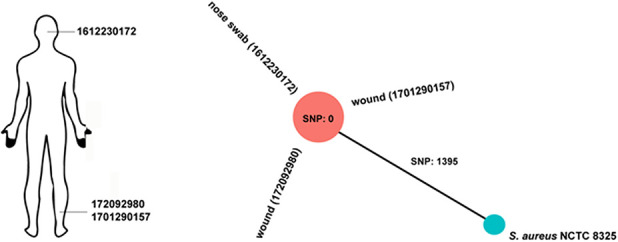
A minimum spanning tree was calculated for the allelic profiles of the 1865 cgMLST targets of Staphylococcus aureus, including an S. aureus reference strain and three S. aureus strains isolated from different sites in the same patient.
Discussion
SSI is a major complication and patients are severely affected by the subsequent long and stressful treatments. One independent risk factor for SSI is colonization with S aureus [21]. Preoperative decolonization has therefore been proposed as a preventive measure to reduce SSI. It has been shown to be effective in cardiac and vascular surgery but its benefits remain controversial in connection with orthopaedic surgery [1, 37]. There is insufficient data at this time to implement a routine preoperative decolonization procedure as it requires more effort from the patient and there are concerns regarding the development of microbial resistance [2]. Specifically, more information is needed about the effect of preoperative decolonization on SSI proportions after elective orthopaedic surgery in S. aureus carriers and non-S. aureus carriers. In this prospective, randomized trial, a preoperative decolonization procedure did not decrease SSI risk in patients undergoing elective orthopaedic surgery, but these results should be interpreted with caution because event numbers were small. The procedure was not effective in either the S. aureus carrier group or the noncarrier group. We encountered an unexpectedly low level of SSI at our center compared with that reported in previous studies [5, 12, 28, 31, 32], even though the S. aureus carrier proportion was above average and represented a population of patients at high risk of SSI.
Limitations
The key limitation in this study is the theoretical statistical uncertainty. The study was prematurely halted after interim analysis because of the low risk of SSI. Interim analysis calculations were based on the carrier group only, as a more pronounced effect was expected in this high-risk group for SSI. Sample size recalculation based on the S. aureus colonization proportions and SSI proportions in the carrier control group found that around 15,000 patients would be necessary to establish definitive proof. Such a mega trial is not justified in our view as, even if a benefit was shown, clinical relevance would be questionable.
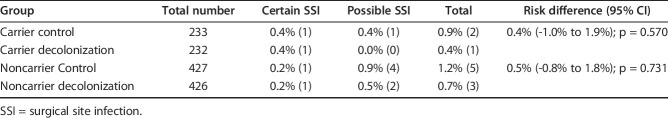
The low risk of SSI in our trial was surprising because earlier studies had reported baseline SSI of 2% to 4% [5, 12, 28, 31, 32]. As there was no internal monitoring of SSI at our institution before this trial, SSI risks were based on reported statistics. Possible methodological factors contributing to the low infection proportions at our institution were the follow-up time of 90 days, possible selection bias, and population baseline characteristics and the single-blinded design. We chose the 90-day follow-up according to CDC criteria for SSI. Periprosthetic joint infections (PJIs), however, can occur even up to 2 years after implantation. In our cohort, 43% of interventions involved prosthetic surgery. According to previous reports involving knee and hip arthroplasty, up to 80% of SSIs are diagnosed within 90 days [23]. The analysis showed further that seven of 11 SSI occurring after 90 days were due to S. aureus, but the diagnosis in five of these patients was potentially delayed by the antibiotic treatment administered for poor wound healing. To account for this issue and to substitute for an external outcome assessor—which could have evaluated all study participants for SSI—we performed an ad-hoc analysis adding “possible SSI” to the confirmed cases of SSI (Table 4). We defined possible SSIs as antibiotic treatment prescribed postoperatively by the orthopaedic surgeon despite criteria for SSI having not been met. We identified seven such patients in our study population. Reasons for prescribing antibiotic therapy were delayed wound healing (four patients) or suspected SSI (three patients). Antibiotic therapy was stopped after the wound healed or after negative intraoperative bacterial culture results. The statistical ad-hoc analysis did not show any difference between the intervention and control arms in carriers (stratified risk difference: -0.4% [95% CI -1.0 to 1.9]; p = 0.570) or in noncarriers (stratified risk difference: -0.5% [95% CI -0.8 to 1.8]; p = 0.731).
Table 4.
Patients with certain and possible SSI
Another concern were the baseline characteristics of our study population. As patients appear rather healthy (Table 2), the question arises whether this could be the result of a selection bias. No data was collected on patients who were not screened or on patients who were screened but did not consent or were not eligible. Comparison with other studies is difficult due to the lack of data, but in relation to Sousa et al. [34] and Ramos et al. [30], who reported higher SSI proportions of 2% to 4% for TKA and THA, our population is slightly younger (mean age 57 versus 65 reported in Sousa et al. [34] and 61 in Ramos et al. [30]), lower diabetes proportions (6.7% versus 21.9% in Sousa et al. [34]), lower mean BMI (26 versus 29 in Ramos et al. [30]) and lower ASA ≥ 3 proportions (28.5 versus 17.5% in Sousa et al. [34]). Furthermore, our trial included arthroscopies, diverse foot surgeries, and removal of osteosynthesis material that could have contributed to the low proportion of SSI. According to our study protocol all patients with at least 2 weeks’ time to surgery were eligible for participation and were approached systematically; meaning our study population represents an average preoperative orthopaedic outpatient population. The protocol was designed to answer the question of routine decolonization in elective surgery, not in a specifically selected high-risk population. Other possible factors contributing to the low SSI proportions in our clinic are our highly standardized procedures with strong aseptic conditions and timely antibiotic prophylaxis, which received a rating of 100% in Swiss infection control report that is, unfortunately, not publicly available. Other “soft” factors thought to contribute to low SSI proportions include well-trained surgeons with high workloads and short intervention times [25, 26].
Another limitation is the lack of a placebo in the control group. Opting for a single-blind design was advantageous because no placebo product was available and a double-blinded approach would have increased costs greatly. We do not believe it would have measurably improved our study’s robustness; any crossover from the control to the intervention arm seems improbable because patients were not informed about colonization status and the control group did not receive instructions for a full decolonization course. Furthermore, surgeons were blinded to the colonization status and treatment arm, making an influence on SSI rates unlikely.
We only screened for S. aureus in the nose; not the throat, axilla, groin or perianal region. With this procedure, we may have missed approximately 10% of positive swab results, but the nose remains the most significant reservoir for S. aureus [24, 39].
SSI Reduction in Patients Colonized with S. aureus
We found no benefit of our decolonization protocol in S. aureus carriers in terms of reducing the SSI risk in those individuals. Another prospective randomized trial with much higher proportions of 3% to 4% SSI also failed to find any effect with the same decolonization regimen in TKA and THA, but the sample size was too small to reach explanatory power [34]. Studies reporting benefits of decolonization should be interpreted with caution because of their “before and after implementation” or observational study design [12, 31, 35]. SSI risk depends on many factors and prevention bundles often consist of a multimodal approach, which may possibly affect results. For example, the additional use of vancomycin in MRSA carriers could bias effects attributed to decolonization [35]. Our study specifically adds information on decolonization only, as no other measures were added during the study period and we included a broad orthopaedic population. In addition, the very low proportion of MRSA carriers in our study population removes a further bias. Nevertheless, in a population with a high proportion of MRSA carriers, selected decolonization for these patients could be beneficial, as this population is at even greater risk for SSI compared with methicillin-sensitive Staphylococcus aureus (MSSA) carriers [18]. This topic requires further studies.
The S. aureus carrier proportion was higher in this study than described elsewhere. Only one other screening study by Mertz et al. [24] in Switzerland found more (Fig. 3). Two methodological features of our study may partly explain this phenomenon. First, both we and Mertz et al. [24] used a selective enrichment broth instead of the conventional agar culture media in other studies [1, 12, 13, 15, 28, 31, 35]. This technique may have identified more S. aureus in the two studies. Indeed, one Scandinavian screening study tested the difference and found 5% more S. aureus carriers with enrichment broth than with agar culture media [17]. However, we still observed higher proportions than described elsewhere, even after subtracting 5% from our carrier proportion. Secondly, our novel and simplified home-based screening approach differs from ambulant or hospital-based methods used in other studies. Our participants received detailed verbal and written instruction on how to swab their noses themselves. A recent study screening of 102 participants found no substantial difference in detection proportions between swabbing performed by a nurse and swabbing by the patients [39]. A home-based approach is also easier for patients, ensures higher recruitment numbers, and has the shortest possible interval between nose swabbing and the operation date. This in turn minimizes the bias of intermittent carriers.
Fig. 3.
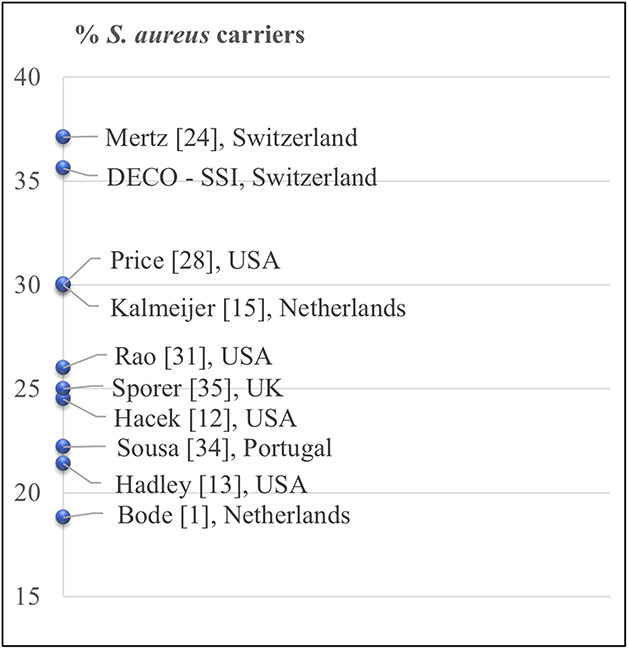
This figure shows the percentage of methicillin-sensitive Staphylococcus aureus carriers in other studies [1, 12, 13, 15, 24, 28, 31, 34, 35] (name of first author and the country of study, are shown).
SSI Reduction in Patients not Colonized with S. aureus
We also found no benefit of decolonization in noncarrier patients with respect to reducing SSI risk. Ours is the first trial we know of to study the efficacy of decolonization in noncarriers. This is relevant as universal decolonization protocols without prior screening of S. aureus carriers are promoted as more cost-effective than the screen-and treat strategy [16]. Our results suggest that decolonization in this group may be of no benefit. Even worse, such a practice may promote the development of bacterial resistance [2] and expose patients to possible adverse events. In fact, we recorded nine adverse reactions possibly connected to chlorhexidine in the noncarrier group.
In a large retrospective cohort, 2519 decolonized S. aureus carriers were identified among 13,828 patients undergoing spine surgery or arthroplasty [30]. Despite decolonization, carriers remained at a substantially higher risk of SSI than noncarriers. Our study adds novel information because results show that decolonized noncarriers did not experience less SSI than noncarriers, in the control arm or in both carriers’ arms. This indicates no added benefit of decolonization in practice in this respect as well.
We found no difference in the SSI risk between the decolonization and the control groups; both in S. aureus carriers and noncarriers. Due to the low event numbers, no definite conclusion about the efficacy of preoperative decolonization can be drawn, but these results could be helpful for future meta-analyses. A mega trial to establish definitive proof on routine preoperative decolonization in a population with a low prevalence of SSI is probably not justified. Future studies may focus on high-risk patients for SSI or on bundle approaches, as these show the best results.
Acknowledgments
We thank medical editor Erica Holt B Sc, of Bern, who edited the final version of the manuscript. We thank Peter Keller MD, Institute for Infectious Diseases, University of Bern, Switzerland for his fruitful input and whole genome sequencing of the S. aureus strain. We also thank Beat Jordi MD, Lindenhofgruppe, Bern, Switzerland for IT support throughout the study.
Footnotes
The institution of the authors (FR, HN, LR, TB, PC, TH, AL, NF, JB) has received, during the study period, funding from Fonds fuer Lehre und Forschung Lindenhof (FLF), (The Lindenhof Fund for Teaching and Research, Bern, Switzerland) Grant no. 14-11-F and 16-04-F. The FLF had no influence on study design, enrolment or data evaluation. The manufacturer of Lifo-Scrub® (B. Braun) provided medication free of charge without any influence on study design, enrolment and data evaluation.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and may be viewed on request.
This trial is registered at Clinicaltrials.gov: NCT02560155, kofam.ch SNCTP000001557.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Sonnenhofspital, Lindenhofgruppe, Bern, Switzerland.
References
- 1.Bode LG Kluytmans JA Wertheim HF Bogaers D Vandenbroucke-Grauls CM Roosendaal R Troelstra A Box AT Voss A van der Tweel I van Belkum A Verbrugh HA, and Vos MC. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9-17. [DOI] [PubMed] [Google Scholar]
- 2.Caffrey AR Quilliam BJ, and LaPlante KL. Risk factors associated with mupirocin resistance in meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76:206-210. [DOI] [PubMed] [Google Scholar]
- 3.Caffrey AR Woodmansee SB Crandall N Tibert C Fielding C Mikolich DJ Vezeridis MP, and LaPlante KL. Low adherence to outpatient preoperative methicillin-resistant Staphylococcus aureus decolonization therapy. Infect Control Hosp Epidemiol. 2011;32:930-932. [DOI] [PubMed] [Google Scholar]
- 4.Chen AF Heyl AE Xu PZ Rao N, and Klatt BA. Preoperative decolonization effective at reducing staphylococcal colonization in total joint arthroplasty patients. J Arthroplasty. 2013;28:18-20. [DOI] [PubMed] [Google Scholar]
- 5.Chen AF Wessel CB, and Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res. 2013;471:2383-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun D and Aytac J. Decrease in Staphylococcus aureus surgical-site infection rates after orthopaedic surgery after intranasal mupirocin ointment. J Hosp Infect. 2004;58:90-91. [DOI] [PubMed] [Google Scholar]
- 7.Courville XF Tomek IM Kirkland KB Birhle M Kantor SR, and Finlayson SR. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2012;33:152-159. [DOI] [PubMed] [Google Scholar]
- 8.Denis O. Route of transmission of Staphylococcus aureus. Lancet Infect Dis. 2017;17:124-125. [DOI] [PubMed] [Google Scholar]
- 9.Fry DE. The continued challenge of Staphylococcus aureus in the surgical patient. Am Surg. 2013;79:1-10. [PubMed] [Google Scholar]
- 10.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. 2018; version 8.0: Available at: http://www.eucast.org/clinical_breakpoints/. Date Accessed March 25, 2018. [Google Scholar]
- 11.Gernaat-van der Sluis AJ Hoogenboom-Verdegaal AM Edixhoven PJ, and Spies-van Rooijen NH. Prophylactic mupirocin could reduce orthopedic wound infections. 1,044 patients treated with mupirocin compared with 1,260 historical controls. Acta Orthop Scand. 1998;69:412-414. [DOI] [PubMed] [Google Scholar]
- 12.Hacek DM Robb WJ Paule SM Kudrna JC Stamos VP, and Peterson LR. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadley S Immerman I Hutzler L Slover J, and Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horan TC Gaynes RP Martone WJ Jarvis WR, and Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606-608. [PubMed] [Google Scholar]
- 15.Kalmeijer MD Coertjens H van Nieuwland-Bollen PM Bogaers-Hofman D de Baere GA Stuurman A van Belkum A, and Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353-358. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel YE Sunkerneni AR Kirchner GJ Prodromo JP, and Moretti VM. The cost-effectiveness of preoperative Staphylococcus aureus screening and decolonization in total joint arthroplasty. J Arthroplasty. 2018;33:S191-S195. [DOI] [PubMed] [Google Scholar]
- 17.Kerttula AM Lyytikainen O Virolainen A Finne-Soveri H Agthe N, and Vuopio-Varkila J. Staphylococcus aureus colonization among nursing home residents in a large Finnish nursing home. Scan J Infect Dis. 2007;39:996-1001. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH Spencer M Davidson SM Li L Shaw JD Gulczynski D Hunter DJ Martha JF Miley GB Parazin SJ Dejoie P, and Richmond JC. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am. 2010;92:1820-1826. [DOI] [PubMed] [Google Scholar]
- 19.Klingenberg B. A new and improved confidence interval for the Mantel-Haenszel risk difference. Stat Med. 2013:2968-2983. [DOI] [PubMed] [Google Scholar]
- 20.Konvalinka A Errett L, and Fong IW. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect. 2006;64:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leaper DJ van Goor H Reilly J Petrosillo N Geiss HK Torres AJ, and Berger A. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J. 2004;1:247-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy PY Ollivier M Drancourt M Raoult D, and Argenson JN. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthop Traumatol Surg Res. 2013;99:645-651. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SS Dicks KV Chen LF Bolognesi MP Anderson DJ Sexton DJ, and Moehring RW. Delay in diagnosis of invasive surgical site infections following knee arthroplasty versus hip arthroplasty. Clin Infect Dis. 2015;60:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertz D Frei R Jaussi B Tietz A Stebler C Fluckiger U, and Widmer AF. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007;45:475-477. [DOI] [PubMed] [Google Scholar]
- 25.Meyer E Weitzel-Kage D Sohr D, and Gastmeier P. Impact of department volume on surgical site infections following arthroscopy, knee replacement or hip replacement. BMJ Qual Saf. 2011;20:1069-1074. [DOI] [PubMed] [Google Scholar]
- 26.Muilwijk J van den Hof S, and Wille JC. Associations between surgical site infection risk and hospital operation volume and surgeon operation volume among hospitals in the Dutch nosocomial infection surveillance network. Infect Control Hosp Epidemiol. 2007;28:557-563. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner PE Borens O Bodler P Broger I Eich G Hefti F Marurer T Nötzli H Seiler S Suvà D Trampuz A Uçkay I Vogt M, and Zimmerli W. Infections of the Musculoskeletal System. Heraeus; 2014:28. [Google Scholar]
- 28.Price CS Williams A Philips G Dayton M Smith W, and Morgan S. Staphylococcus aureus nasal colonization in preoperative orthopaedic outpatients. Clin Orthop Relat Res. 2008;466:2842-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JR Cole K Bexley A Kostiou V Eyre DW Golubchik T Wilson DJ Crook DW Walker AS Peto TEA Llewelyn MJ, and Paul J. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis. 2017;17:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos N Stachel A Phillips M Vigdorchik J Slover J, and Bosco JA. Prior Staphylococcus aureus nasal colonization: a risk factor for surgical site infections following decolonization. J Am Acad Orthop Surg. 2016;24:880-885. [DOI] [PubMed] [Google Scholar]
- 31.Rao N Cannella B Crossett LS Yates AJ Jr., and McGough R 3rd.. A preoperative decolonization protocol for staphylococcus aureus prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466:1343-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao N Cannella B Crossett LS Yates AJ Jr. McGough RL 3rd, and Hamilton CW. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection: prospective cohort study with 2-year follow-up. J Arthroplasty. 2011;26:1501-1507. [DOI] [PubMed] [Google Scholar]
- 33.Savage JW and Anderson PA. An update on modifiable factors to reduce the risk of surgical site infections. Spine J. 2013;13:1017-1029. [DOI] [PubMed] [Google Scholar]
- 34.Sousa RJ Barreira PM Leite PT Santos AC Ramos MH, and Oliveira AF. Preoperative Staphylococcus aureus screening/decolonization protocol before total joint arthroplasty-results of a small prospective randomized trial. J Arthroplasty. 2016;31:234-239. [DOI] [PubMed] [Google Scholar]
- 35.Sporer SM Rogers T, and Abella L. Methicillin-resistant and methicillin-sensitive Staphylococcus aureus screening and decolonization to reduce surgical site infection in elective total joint arthroplasty. J Arthroplasty. 2016;31:144-147. [DOI] [PubMed] [Google Scholar]
- 36.van Belkum A Verkaik NJ de Vogel CP Boelens HA Verveer J Nouwen JL Verbrugh HA, and Wertheim HF. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009;199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 37.Verhoeven PO Berthelot P Chapelle C Gagnaire J Grattard F Pozzetto B Farizon F Lucht F, and Botelho-Nevers E. Letter to the editor: Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res. 2013;471:3709-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse JD Friedman ND Kirkland KB Richardson WJ, and Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183-189. [DOI] [PubMed] [Google Scholar]
- 39.Young BC Votintseva AA Foster D Godwin H Miller RR Anson LW Walker AS Peto TEA Crook DW, and Knox K. Multi-site and nasal swabbing for carriage of Staphylococcus aureus: what does a single nose swab predict? J Hosp Infect. 2017;96:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]