Abstract
The three common intestinal Cryptosporidium species in cattle differ significantly in host range, pathogenicity and public health significance. While Cryptosporidium parvum is pathogenic in pre-weaned calves and has a broad host range, C. bovis and C. ryanae are largely non-pathogenic and bovine-specific species in post-weaned calves. Thus far, only the genome of C. parvum has been sequenced. To improve our understanding of the genetic determinants of biological differences among Cryptosporidium spcies, we sequenced the genomes of C. bovis and C. ryanae and conducted a comparative genomics analysis. The genome of C. bovis has a gene content and organization more similar to C. ryanae than to other Cryptosporidium species sequenced to date; the level of similarity in amino acid and nucleotide sequences between the two species is 75.2 and 69.4 %, respectively. A total of 3723 and 3711 putative protein-encoding genes were identified in the genomes of C. bovis and C. ryanae, respectively, which are fewer than the 3981 in C. parvum. Metabolism is similar among the three species, although energy production pathways are further reduced in C. bovis and C. ryanae. Compared with C. parvum, C. bovis and C. ryanae have lost 14 genes encoding mucin-type glycoproteins and three for insulinase-like proteases. Other gene gains and losses in the two bovine-specific and non-pathogenic species also involve the secretory pathogenesis determinants (SPDs); they have lost all genes encoding MEDLE, FLGN and SKSR proteins, and two of the three genes for NFDQ proteins, but have more genes encoding secreted WYLE proteins, secreted leucine-rich proteins and GPI-anchored adhesin PGA18. The only major difference between C. bovis and C. ryanae is in nucleotide metabolism. In addition, half of the highly divergent genes between C. bovis and C. ryanae encode secreted or membrane-bound proteins. Therefore, C. bovis and C. ryanae have gene organization and metabolic pathways similar to C. parvum, but have lost some invasion-associated mucin glycoproteins, insulinase-like proteases, MEDLE secretory proteins and other SPDs. The multiple gene families under positive selection, such as helicase-associated domains, AMP-binding domains, protein kinases, mucins, insulinases and TRAPs could contribute to differences in host specificity and pathogenicity between C. parvum and C. bovis. Biological studies should be conducted to assess the contribution of these copy number variations to the narrow host range and reduced pathogenicity of C. bovis and C. ryanae.
Keywords: Cryptosporidium bovis, Cryptosporidium ryanae, comparative genomics, host specificity, pathogenicity
Data Summary
1. All sequencing reads of Cryptosporidium bovis and Cryptosporidium ryanae have been submitted to the NCBI Sequence Read Archive (SRA) under accessions SRR9329505 and SRR9329807, respectively. The assemblies of C. bovis and C. ryanae are deposited in GenBank under accession VHIT00000000 and VHLK00000000, respectively.
Impact Statement.
Cryptosporidium species are important apicomplexan parasites, causing diarrhoea and enteric diseases in humans and domestic animals. Cryptosporidium parvum, Cryptosporidium bovis and Cryptosporidium ryanae are three common intestinal Cryptosporidium species in cattle. As a zoonotic pathogen, C. parvum is the only major species responsible for diarrhoea in pre-weaned calves. As bovine-specific species, C. bovis and C. ryanae often infect post-weaned calves and yearlings mostly without any clinical signs of disease. We sequenced the genomes of C. bovis and C. ryanae for the first time and conducted a comparative genomic analysis. We found that C. bovis and C. ryanae have lost many secretory pathogenesis determinants, such as mucin-type glycoproteins, insulinase-like proteases, secreted MEDLE proteins, FLGN, SKSR and NFDQ proteins, which could potentially contribute to the reduced host range and pathogenicity of C. bovis and C. ryanae. The results of our study are useful in understanding differences in pathogenicity of various Cryptosporidium species within the same host.
Introduction
Cryptosporidiosis is well recognized as an important cause of diarrhoea and enteric diseases in humans and domestic animals [1]. In addition to moderate-to-severe diarrhoea, it can cause weight loss and death in neonatal animals, children and immunocompromised persons [2, 3]. As Cryptosporidium infections are common in cattle, calves are considered major reservoir hosts [4].
Cryptosporidium species vary in host range and public health significance. Thus far, over 40 Cryptosporidium species have been recognized [5]. Among them, Cryptosporidium parvum and Cryptosporidium hominis are two dominant species in humans. The former is also commonly found in cattle. In addition, cattle are frequently infected with Cryptosporidium bovis, Cryptosporidium ryanae and Cryptosporidium andersoni [5]. Among the four bovine Cryptosporidium species, C. parvum, which infects the small intestine of pre-weaned calves, is the only major species responsible for diarrhoea [3, 6]. C. bovis and C. ryanae often infect the small intestine of post-weaned calves and yearlings mostly without any clinical signs of disease [7, 8]. In contrast, C. andersoni infects the abomasum of mature cattle, leading to poor weight gain and reduced milk production [9]. Among the three intestinal species, C. parvum has a broad host range, while C. bovis and C. ryanae infect exclusively bovine animals [4].
Comparative genomics analysis of human-pathogenic Cryptosporidium species has revealed significant diversification in secretory pathogenesis determinants (SPDs), which include MEDLE proteins, insulinase-like proteases and mucin-type glycoproteins. Therefore, SPDs are suggested to be involved in differences in host range, tissue tropism and pathogenicity among Cryptosporidium species [10, 11]. Among them, MEDLE proteins were named after a conserved sequence motif at the C terminus and are expressed in the invasion stages of C. parvum [12, 13]. Insulinase-like proteases are widespread in apicomplexans, and are known to be involved in processing invasion-related proteins or modifying host cell activities [14]. Mucin-type glycoproteins are a large family of secreted proteins in micronemes and could be involved in the initial attachment and invasion of Cryptosporidium spcies [15].
Genes encoding SPDs are often arranged in the genome as clusters in the subtelomeric regions, which facilitates gene duplication, deletion and genetic recombination [11]. For example, compared with C. parvum, one gene encoding insulinase-like protease was lost in the 3′ subtelomeric region of chromosome 6 of C. hominis. In contrast, the gastric species C. andersoni has lost the subtelomeric regions encoding MEDLE proteins and insulinases entirely [11]. Similarly, a major difference between C. parvum and Cryptosporidium chipmunk genotype I is the loss of four subtelomeric genes encoding MEDLE proteins and one subtelomeric gene encoding an insulinase-like protease in the latter [16]. Copy number variations in the genes encoding MEDLE and insulinase-like proteases have also been seen among subtype families of C. parvum, which have different host preferences [17, 18]. In addition, an enrichment of positively selected genes encoding SPDs was observed in subtelomeric regions between zoonotic and anthroponotic C. parvum subtypes [19]. Differences in the number and sequences of genes encoding mucin-type glycoproteins could also be partially responsible for the tissue tropism between the intestinal and gastric Cryptosporidium species [11].
In this study, to improve our understanding of potential genetic determinants of the host range and pathogenicity in Cryptosporidium species, we sequenced the genomes of C. bovis and C. ryanae and performed a comparative genomics analysis of the three intestinal species infecting cattle and available whole genome sequence data from other Cryptosporidium species [10, 11, 20–22].
Methods
Specimen collection and whole-genome sequencing
C. bovis isolate 42482 and C. ryanae isolate 45019 were collected from dairy calves in Shanghai and Guangdong, China, respectively. They were diagnosed by sequence analysis of the small subunit rRNA gene [23]. Sucrose and caesium chloride density gradient centrifugations and immunomagnetic separation were used to purify the oocysts from the specimens [24]. The purified oocysts were subjected to five freeze–thaw cycles and digested with proteinase K overnight. The QIAamp DNA Mini Kit (Qiagen Sciences) was used in extracting genomic DNA from the oocysts. The REPLI-g Midi Kit (Qiagen) was used to amplify the DNA harvested. The genomes were sequenced using Illumina HiSeq 2500 analysis of 250 bp paired-end libraries constructed using the Illumina TruSeq (v3) library preparation kit (Illumina). The sequence reads were trimmed to remove adapter sequences and regions of poor sequence quality (Phred score <25) and assembled de novo using the CLC Genomics Workbench Version 9.0 with word size of 63 and bulb size of 500.
Genome structure analysis and gene prediction
The C. bovis and C. ryanae genomes obtained were aligned with the published genomes of the C. parvum IOWA isolate [20], C. ubiquitum [11] and C. andersoni [11] using Mauve 2.3.1 [25] with default parameters. The syntenic relationship (regions with orthologous genes) among the C. bovis genome and the other four genomes was illustrated using Circos 0.69 [26]. We used Bowtie2 to map the reads on the C. bovis genome, and the Integrative Genomics Viewer was used to check the coverage of the regions which connect large rearrangements between C. bovis and C. parvum.
After training the software with the gene model of the C. parvum IOWA genome, protein-encoding genes in C. bovis and C. ryanae were predicted using GeneMark-ES [27], AUGUSTUS 3.2.1 [28] and Geneid 1.4 [29] with default settings, as described previously [16]. The final gene set was generated by consensus predictor EVidence Modeler [30] based on the prediction outcomes using the three software packages.
Functional annotation
blast p [31] and Hidden Markov Model (HMMER) analysis (http://hmmer.org) were used to annotate the predicted genes of C. bovis and C. ryanae by searching in the GenBank NR and Pfam databases. SignalP 4.1 [32], TMHMM 2.0 [33] and the GPI-SOM webserver [34] were used to identify signal peptides, transmembrane domains and GPI anchor sites, respectively. The KAAS web server [35] was used to analyse the metabolism with the BBH (Bi-directional Best Hit) method and eukaryote gene model. The annotations of functional proteins, catalytic enzymes and metabolic pathways within the genomes were conducted using Pfam (http://pfam.xfam.org/) [36], the online database KEGG (Kyoto Encyclopedia of Genes and Genomes) (http://www.genome.jp/kegg/) and LAMP (Library of Apicomplexan Metabolic Pathways, release-2) [37], respectively.
Comparative genomics analysis
Sequence similarities among C. bovis, C. ryanae, C. parvum and other Cryptosporidium genomes in CryptoDB (http://cryptodb.org/cryptodb/) were assessed by using blast p and HMMER with e-value thresholds of 1e-3. OrthoMCL [38] was used to identify homologous gene families among Cryptosporidium spcies with e-value thresholds of 1e-5. VennPainter (https://github.com/linguoliang/VennPainter) was used to draw the Venn diagram of shared orthologues and species-specific genes in C. parvum, C. ubiquitum, C. andersoni, C. bovis and C. ryanae. Based on results of blast p homology analysis (threshold of protein pairs sharing 30 % identity over 100 amino acids), the relationship among proteins in C. bovis, C. parvum and C. ryanae was visualized using Gephi (https://gephi.org/) with the Fruchterman–Reingold layout. The data of KAAS and LAMP were used in comparative analyses of metabolism in Cryptosporidium species. Comparisons of transporter proteins and invasion-related proteins among Cryptosporidium species were based on results of Pfam searches.
Phylogenetic analysis
Amino acid sequences encoded by single-copy orthologous genes shared among Cryptosporidium species and Gregarina niphandrodes were concatenated and aligned with each other using muscle [39]. Poorly aligned positions were eliminated from the sequence alignments using Gblocks [40]. RAxML was used to reconstruct maximum-likelihood (ML) trees with 1000 bootstrap replications [41]. The concatenated sequence from G. niphandrodes was used as the outgroup in the phylogenetic analysis.
Results
Genome features
A total of 7.08 million and 5.13 million of 250 bp paired-end reads were obtained from C. bovis isolate 42 482 (from a dairy calf in Shanghai) and C. ryanae isolate 45 019 (from a dairy calf in Guangdong) using Illumina sequencing, respectively. The reads were assembled into a 9.11 Mb C. bovis genome of 55 contigs and a 9.06 Mb C. ryanae genome of 93 contigs after removing contigs from contaminants. We identified 3723 protein-encoding genes in C. bovis and 3711 in C. ryanae by combining the gene prediction results from GeneMark, Augustus and Geneid. The gene content of C. bovis and C. ryanae is similar to that of C. baileyi but smaller than the genomes of C. parvum and C. hominis (Table 1). Compared with eight other Cryptosporidium species, C. bovis has a relatively high similarity in amino acid and nucleotide sequences to C. ryanae (75.2 and 69.4 %, respectively). The GC content of C. ryanae is slightly higher than that of C. bovis in the overall genome and coding regions (32.9 and 33.9% versus 30.7 and 31.8 %, respectively) (Table 1).
Table 1.
Genomic features of Cryptosporidium bovis and Cryptosporidium ryanae in comparison with Cryptosporidium parvum and other Cryptosporidium species
|
C. muris |
C. andersoni |
C. parvum |
C. hominis UdeA01 |
C. meleagridis |
Cryptosporidium chipmunk genotype I |
C. ubiquitum |
C. bovis |
C. ryanae |
C. baileyi |
|
|---|---|---|---|---|---|---|---|---|---|---|
|
Total length (Mb) |
9.21 |
9.09 |
9.1 |
9.06 |
8.97 |
9.05 |
8.97 |
9.11 |
9.06 |
8.5 |
|
No. of super contigs |
45 |
135 |
8 |
97 |
57 |
50 |
27 |
55 |
93 |
153 |
|
GC content (%) |
28.4 |
28.5 |
30.3 |
30.1 |
31 |
32 |
30.8 |
30.7 |
32.9 |
24.3 |
|
Nucleotide sequence similarity (%) |
24.8 |
25.6 |
38.6 |
38.7 |
38.4 |
38.4 |
38.5 |
– |
69.4 |
40.9 |
|
No. of genes |
3937 |
3905 |
3981 |
3819 |
3782 |
3783 |
3767 |
3723 |
3711 |
3728 |
|
Total length of CDS (Mb)* |
6.93 |
6.86 |
6.83 |
6.81 |
6.91 |
6.94 |
6.94 |
6.8 |
6.74 |
6.69 |
|
GC content in CDS (%) |
30 |
30.1 |
31.9 |
31.8 |
32.4 |
33.6 |
33 |
31.8 |
33.9 |
25.6 |
|
Amino acid sequence similarity (%) |
46.9 |
46.7 |
55.1 |
54.8 |
54.5 |
54.6 |
54.8 |
– |
75.2 |
57.1 |
|
GC content at third position in codons (%) |
17.8 |
18.1 |
22.5 |
23.5 |
24.1 |
26.9 |
24.5 |
25.4 |
30.2 |
12.6 |
|
Gene density (genes/Mb) |
427.5 |
429.6 |
418.1 |
421.5 |
421.6 |
418 |
420 |
408.7 |
409.6 |
438.6 |
|
Percentage coding (%) |
75.2 |
75.5 |
75 |
75.2 |
77 |
76.7 |
77.4 |
74.6 |
74.4 |
78.7 |
|
No. of genes with intron |
798 |
832 |
163 |
417 |
506 |
515 |
758 |
571 |
602 |
763 |
|
Genes with intron (%) |
20.3 |
21.3 |
4.2 |
10.9 |
13.4 |
13.6 |
20.1 |
15.3 |
16.2 |
20.5 |
|
No. of tRNAs |
45 |
44 |
45 |
45 |
45 |
45 |
45 |
45 |
45 |
46 |
|
No. of tRNAmet |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Proteins with signal peptide |
323 |
309 |
397 |
391 |
397 |
396 |
399 |
366 |
329 |
344 |
|
Proteins with transmembrane domain |
836 |
839 |
832 |
817 |
805 |
793 |
772 |
781 |
774 |
813 |
|
Proteins with GPI anchor |
52 |
47 |
63 |
54 |
55 |
57 |
50 |
62 |
57 |
57 |
*CDS, coding sequences.
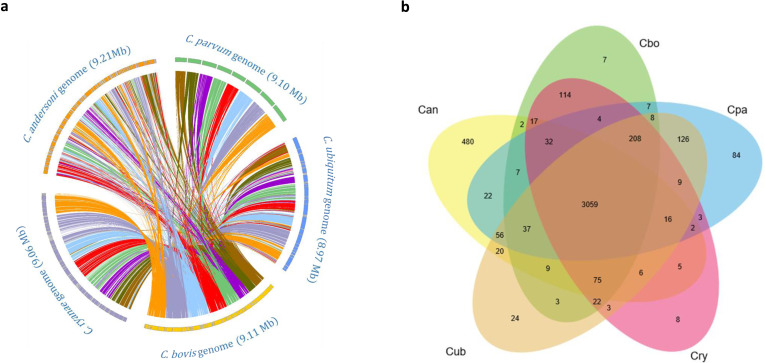
A complete synteny in gene organization was observed between the C. bovis and C. ryanae genomes, but some large rearrangements were observed between the C. bovis and C. parvum genomes (Fig. 1a). For example, in a rearrangement of ~150 kb in chromosome 1 of C. parvum that contains 52 genes (cgd1_500 to 11_1010), the homologous region in C. bovis is fragmented into different contigs, including contig_1 (chromosome 5), contig_16 (chromosome 7) and contig_32 (chromosome 6). Similarly, an ~480 kb fragment containing 175 genes at the 5′ region of chromosome 3 of C. parvum is translocated to chromosomes 1, 5 and 6 in C. bovis. In addition, an ~303 kb fragment containing 134 genes in chromosome 2 of C. parvum is translocated to the 5′ subtelomeric region (contig 6) of chromosome 8 in C. bovis. Several other rearrangements were seen in C. bovis, involving the 5′ region of chromosomes 5, 6 and 8 of C. parvum. We found that all junction regions of the large rearrangements in C. bovis were mapped to reads, and most of them had high coverage (50–794-fold). Two had lower coverage, including the regions in contig_23 (coverage: 6–70-fold) and contig_6 (coverage: 20–40-fold).
Fig. 1.
Syntenic relationship and shared orthologous genes among Cryptosporidium species. (a) The syntenic relationship in gene organization among genomes of Cryptosporidium parvum, C. ubiquitum, C. bovis, C. ryanae and C. andersoni. Syntenic blocks (regions with orthologous genes) are connected with lines, with the different colours representing eight chromosomes of C. parvum. (b) Venn diagram of orthologous genes shared by five Cryptosporidium species. Abbreviations of taxa: Cryptosporidium parvum IOWA (Cpa); C. ubiquitum (Cub); C. bovis (Cbo); C. ryanae (Cry); C. andersoni (Can).
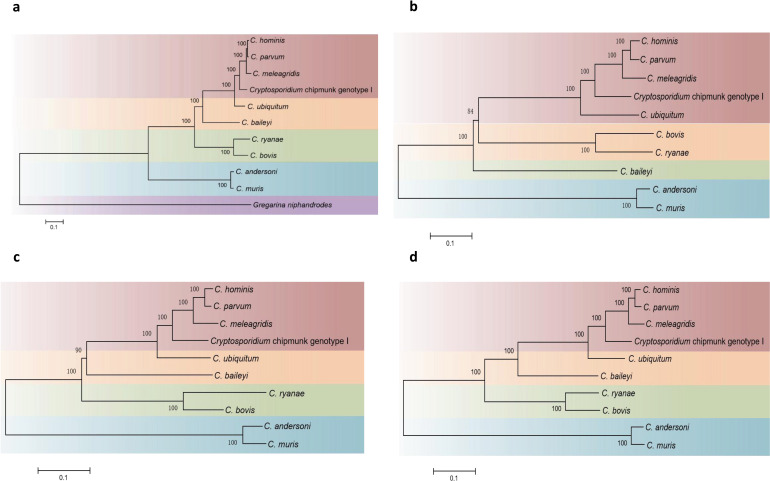
Based on orthology delineation, 3059 genes are shared by C. parvum, C. bovis, C. ryanae, C. ubiquitum and C. andersoni. Among the remaining genes, the genes shared between C. bovis and C. ryanae are different from those shared between C. parvum and C. ubiquitum. Thus, there are 126 genes shared only by C. parvum and C. ubiquitum, two human-pathogenic species with broad host ranges, while 114 other genes are shared only by C. bovis and C. ryanae, two bovine-specific species (Tables S1 and S2, available in the online version of this article). Among these five Cryptosporidium species, C. parvum has 84 species-specific genes, compared with only a few species-specific genes in C. bovis and C. ryanae. The latter was largely due to the fact that C. bovis and C. ryanae share a virtually identical set of genes (Fig. 1b). Phylogenetic analysis of amino acid sequences of 100 orthologous genes supported the close relationship of C. bovis to C. ryanae (Fig. 2a). This was confirmed by phylogenetic analysis of amino acid sequences of invasion-related protein families, including mucin-type glycoproteins, insulinase-like proteases and thrombospondin-related adhesive proteins (TRAPs) (Fig. 2b–d).
Fig. 2.
Phylogenetic relationships among Cryptosporidium species. (a) Phylogenetic relationship of Cryptosporidium species based on ML analysis of sequences of 100 single-copy orthologous proteins. (b) Phylogenetic relationship of Cryptosporidium species based on ML analysis of insulinase-like proteases. (c) Phylogenetic relationship of Cryptosporidium species based on ML analysis of TRAP sequences. (d) Phylogenetic relationship of Cryptosporidium species based on ML analysis of mucin-type glycoproteins.
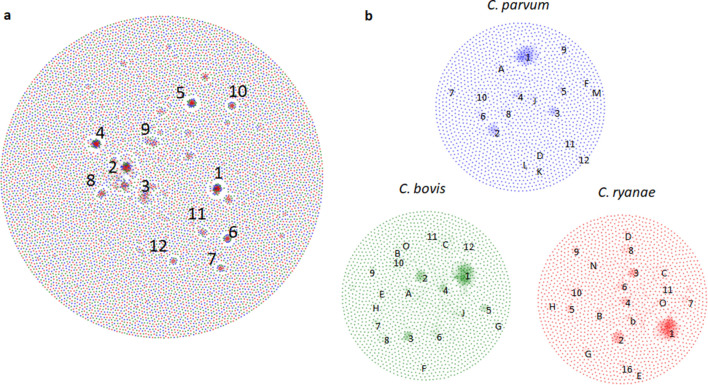
Network analysis of the C. parvum, C. bovis and C. ryanae proteomes based on sequence similarity identified multiple gene families in clusters (Fig. 3a). Members of AAA proteins formed cluster 1. Cryptosporidium species possess a large number of protein kinases, which were included in cluster 2. Clusters 3, 4 and 7 in the network consisted of helicases with the DEAD, HA2 and SNF2 domains, respectively. Ras proteins involved in signalling pathways formed cluster 5. The metallophos domain was found in a diverse range of phosphoesterases, which formed cluster 6. Ubiquitin-conjugating enzymes involved in the second step of ubiquitination formed cluster 8. There are seven members of peptidyl-prolyl cis-trans isomerases in each of the three Cryptosporidium species, forming cluster 10 in the network. Protein network analysis indicated conservation in the members of these major protein families among C. parvum, C. bovis and C. ryanae (Fig. 3b). We found three unique clusters in C. parvum, namely cluster K (FGLN), cluster L (insulinase-like proteases) and cluster M (MEDLE proteins). Proteins containing the RNA recognition motif (cluster C), IMCp domain (cluster E) and ketoacyl synthase domain (cluster O) were only found in C. bovis and C. ryanae.
Fig. 3.
Protein architecture network of Cryptosporidium bovis, Cryptosporidium ryanae and Cryptosporidium parvum. (a) Protein architecture network based on sequence similarity of all proteins in proteomes of C. bovis (green), C. ryanae (red) and C. parvum (blue). 1, AAA proteins; 2, protein kinase; 3, DEAD; 4, SNF2 family; 5, Ras protein; 6, metallophos; 7, HA2 helicase; 8, ubiquitin-conjugating enzyme; 9, ABC transporter; 10, cyclophilin type peptidyl-prolyl cis-trans isomerase; 11, TRAP; 12, zinc finger C3H1-type domain containing protein. (b) Protein architecture network based on sequence similarity of proteins in proteomes of C. parvum (blue), C. bovis (green) and C. ryanae (red). 1, protein kinase; 2, DEAD; 3, AAA proteins; 4, SNF2 family; 5, Ras protein; 6, ABC transporter; 7, TRAP; 8, DnaJ domain; 9, ubiquitin-conjugating enzyme; 10, WD40; 11, cyclophilin type peptidyl-prolyl cis-trans isomerase; 12 proteasome; A, metallophos; B, HA2 helicase; C, RNA recognition motif; D, minichromosome maintenance; E, inner membrane complex protein; F, DHHC domain; G, HSP60; H, CCCH type domain; J, ankyrin repeat; K, FGLN; L, IDE; M, MEDLE; N, Sec7 domain; O, beta-ketoacyl synthase.
Divergent metabolic pathways among intestinal bovine Cryptosporidium species
Terpenoid metabolism
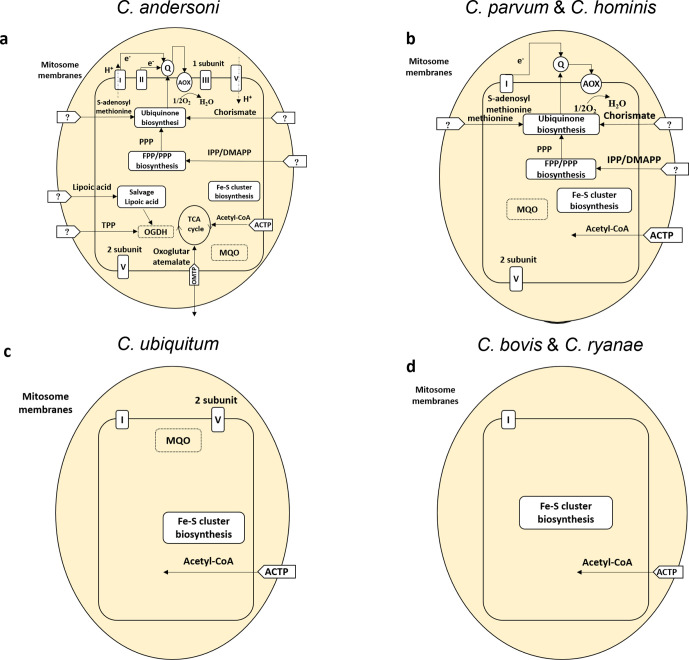
In C. parvum, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMPP) are two important five-carbon isoprene substrates in terpenoid metabolism. They are synthesized by farnesyl diphosphate (FPP) synthase and polyprenyl synthase (encoded by cgd4_2550 and cgd7_3730, respectively). The genes encoding these two enzymes were shown to have high expression in C. parvum according to data in CryptoDB (https://cryptodb.org/), but they are lost in the predicted proteomes of C. bovis and C. ryanae (Fig. 4b and d) as well as C. ubiquitum [11]. In other apicomplexans, IPP biosynthesis is one of the major metabolic pathways in the apicoplast. However, the apicoplast is lost in Cryptosporidium species, and the remaining IPP biosynthesis apparently has been further reduced in some species within the genus. The progressive loss of IPP biosynthesis pathways in Cryptosporidium species further confirms that the lipid metabolism in the parasites is not dependent on the apicoplast. Instead, they could salvage the nutrients from the host.
Fig. 4.
Mitochondrial metabolism in several Cryptosporidium species. Abbreviations of enzymes: AOX, alternative oxidase; MAT, methionine adenosyl transferase; OGDH, oxoglutarate dehydrogenase complex; MQO, malate quinone oxidoreductase. Abbreviations of metabolites: Q, ubiquinone (coenzyme Q); CoA, coenzyme A; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; FPP, farnesyl diphosphate; PPP, polyprenyl diphosphate. Abbreviations of transporter proteins: OMTP, oxoglutarate/malate transporter protein; AATP, amino acids transporter protein; ACTP, acetyl-CoA transporter protein.
Electron transport chain
A further reduction in the electron transport chain was detected in C. bovis and C. ryanae. C. bovis and C. ryanae have lost all genes encoding ATP synthase and the alternative oxidase (AOX) (Table 2). In particular, the gene encoding malate quinone oxidoreductase (MQO) is lost in C. bovis and C. ryanae, whereas the orthologous genes are present in other Cryptosporidium species (Fig. 4). Similarly, the gene encoding the oxoglutarate/malate translocator protein (cgd1_600 in C. parvum) is absent in C. bovis and C. ryanae.
Table 2.
Comparison of essential metabolic pathways among Cryptosporidium species and other common apicomplexan parasites
|
Category |
Metabolic pathway |
Cpar |
Chom |
Cmel |
Cchi |
Cubi |
Cbov |
Crya |
Cbai |
Cand |
Pfal |
Tgon |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Carbohydrate and energy metabolism |
Glycolysis |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Methylcitrate cycle |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
TCA cycle |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
+ |
|
|
Pentose phosphate pathway |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Shikimate biosynthesis |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Folate biosynthesis |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of pterin |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Galactose metabolism |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Synthesis of starch |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Synthesis of trehalose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Synthesis of 1,3-beta-glucan |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Conversion between UDP-Glc and UDP-Gal |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Conversion between GDP-Man and GDP-Fuc |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Conversion from UDP-Glc to UDP-GlcA to UDP-Xyl |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
− |
|
|
Synthesis of mannitol from fructose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
− |
|
|
Fatty acid biosynthesis in cytosol (FAS I) |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Fatty acid biosynthesis in apicoplast (FAS II) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Fatty acid degradation |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Oxidative phosphorylation (NADH dehydrogenase) |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Oxidative phosphorylation (Complex II) |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
+ |
|
|
Oxidative phosphorylation (Complex III) |
− |
− |
− |
− |
− |
− |
− |
− |
one sub |
+ |
+ |
|
|
Oxidative phosphorylation (Complex IV) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
F-ATPase |
two sub |
two sub |
two sub |
two sub |
two sub |
− |
− |
two sub |
+ |
+ |
+ |
|
|
Alternative oxidase (AOX) |
+ |
+ |
+ |
+ |
− |
− |
− |
− |
+ |
− |
− |
|
|
Glyoxalase metabolism producing d-lactate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of isoprene (MEP/DOXP) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of farnesyl/polyprenyl diphosphate |
+ |
+ |
+ |
+ |
− |
− |
− |
− |
+ |
+ |
+ |
|
|
Nucleotide metabolism |
Synthesis of purine rings de novo |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
|
Conversion from IMP to XMP |
+ |
+ |
+ |
+ |
+ |
+ |
− |
− |
− |
+ |
+ |
|
|
Conversion from XMP to GMP |
+ |
+ |
− |
− |
− |
+ |
− |
− |
− |
+ |
+ |
|
|
Synthesis of pyrimidine de novo |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Conversion from uracil to UMP |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
+ |
+ |
+ |
|
|
Conversion from dCMP to dUMP |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
+ |
+ |
+ |
|
|
Amino acid metabolism |
Synthesis of alanine from pyruvate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
Synthesis of glutamate from nitrite/nitrate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Conversion from glutamate to glutamine |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Synthesis of aspartate from oxaloacetate and glutamate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Conversion from aspartate to asparagine |
+ |
+ |
+ |
+ |
+ |
− |
− |
− |
− |
+ |
+ |
|
|
Conversion from glutamate to proline |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Synthesis of serine from glycerate/glycerol phosphate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Conversion from serine to cysteine |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Conversion from serine to glycine |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Recycle homocysteine into methionine |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of lysine from aspartate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Synthesis of threonine from aspartate |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Synthesis of ornithine from arginine |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
− |
|
|
Synthesis of ornithine from proline |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of polyamine from ornithine |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
− |
|
|
Polyamine pathway backward |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
− |
+ |
|
|
Degradation of branched-chain amino acids |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Synthesis of tryptophan |
+ |
+ |
− |
+ |
+ |
− |
− |
− |
− |
− |
− |
|
|
Aromatic amino acid hydroxylases (AAAH) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Vitamins and others |
Synthesis of ubiquinone (coenzyme Q) |
+ |
+ |
+ |
+ |
− |
− |
− |
− |
+ |
+ |
+ |
|
Synthesis of Fe-S cluster |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Synthesis of haem |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of thiamine (vitamin B1) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
− |
|
|
Conversion from thiamine to thiamine pyrophosphate (TPP) |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of FMN/FAD from riboflavin |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of pyridoxal phosphate (vitamin B6) de novo |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of NAD(P)+ de novo from nicotinate/nicotinamide |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Synthesis of pantothenate from valine |
− |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
|
|
Synthesis of CoA from pantothenate |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
Synthesis of lipoic acid de novo in apicoplast |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
|
|
Salvage of lipoic acid in mitochondria |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
+ |
|
|
Synthesis of porphyrin/cytochrome proteins |
− |
− |
− |
− |
− |
− |
− |
− |
− |
+ |
+ |
Plus symbols denote that these metabolic pathways were identified in this apicomplexan parasite, whereas minus symbols denote that these metabolic pathways were absent. Abbreviations: Cryptosporidium parvum (Cpar); C. hominis (Chom); C. meleagridis (Cmel); Cryptosporidium chipmunk genotype I (Cchi); C. ubiquitum (Cubi); C. bovis (Cbov); C. ryanae (Crya); C. baileyi (Cbai); C. andersoni (Cand); Plasmodium falciparum (Pfal); Toxoplasma gondii (Tgon).
Sub, abbreviation of subunit. One sub means only one subunit of the protein was detected in the species.
Coenzyme Q (CoQ), also known as ubiquinone, is involved in transferring electrons from nicotinamide adenine dinucleotide (NADH) dehydrogenase (complex I), MQO and complex II to the cytochrome bc1 complex (complex III). In comparison with C. parvum, C. ubiquitum has lost four of the eight genes encoding enzymes in CoQ metabolism, while C. bovis and C. ryanae have lost one additional such gene.
The number of mitochondrial carrier proteins is reduced in C. bovis and C. ryanae due to simplification of the electron transport system. There are only three genes encoding mitochondrial carrier proteins in C. bovis and four in C. ryanae (Table 3). In comparison, C. parvum has nine such genes while C. ubiquitum has six (Table 3). Moreover, the number of triose phosphate transporters (six in C. bovis and seven in C. ryanae) and ABC transporters (22 in C. bovis and 20 in C. ryanae) is also different between C. bovis and C. ryanae.
Table 3.
Putative transporters in Cryptosporidium species and other common apicomplexan parasites*
|
Substrate |
Cellular location |
Tgon |
Pfal |
Cand |
Cmur |
Cpar |
ChomUde |
Cmel |
Cchi |
Cubi |
Cbov |
Crya |
Cbai |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hexose |
5 |
2 |
2 |
3 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
|
Triose phosphate |
Plasma/apicoplast membrane |
4 |
4 |
8 |
8 |
8 |
8 |
8 |
7 |
8 |
6 |
7 |
7 |
|
Amino acids |
Plasma membrane |
6 |
1 |
12 |
12 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Nucleobase/nucleoside |
Plasma membrane |
4 |
4 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Nucleotide-sugar |
Plasma membrane |
4 |
1 |
2 |
2 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
2 |
|
Folate/pterine |
Plasma membrane |
7 |
2 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Formate/nitrite |
3 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
GABA (aminobutanoate) |
Plasma/mitochondrial membrane |
5 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Acetyl-CoA |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Chloride |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Inorganic phosphate |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Sulfate |
4 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Sodium/potassium/calcium |
9 |
0 |
3 |
3 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
|
Zinc |
4 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
|
Copper |
3 |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Choline |
Plasma membrane |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Cadmium/zinc/cobalt (efflux) |
Plasma membrane |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Glycerol/water |
Plasma membrane |
2 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
ABC transporter |
Plasma membrane |
24 |
16 |
21 |
21 |
21 |
21 |
21 |
21 |
21 |
22 |
20 |
22 |
|
Mitochondrial carrier |
Mitochondrial membrane |
21 |
14 |
13 |
12 |
9 |
9 |
8 |
8 |
6 |
3 |
4 |
6 |
*The detection of these transporter proteins was based on the Pfam search.
Tgon: Toxoplasma gondii; Pfal: Plasmodium falciparum; Cand: Cryptosporidium andersoni; Cmur: C. muris; Cpar: C. parvum; ChomUde: C. hominis UdeA01; Cmel: C. meleagridis; Cchi: Cryptosporidium chipmunk genotype I; Cubi: C. ubiquitum; Cbov: C. bovis; Crya: C. ryanae; Cbai: C. baileyi.
Nucleotide metabolism
Compared with C. parvum, C. bovis possesses all 42 orthologous genes encoding enzymes involved in the interconversion of purines and pyrimidines, whereas five such genes are absent in C. ryanae. In purine metabolism, the genes encoding inosine monophosphate (IMP) dehydrogenase (cgd6_20), guanosine monophosphate (GMP) synthase (cgd5_4520) and nucleoside-triphosphate pyrophosphatase (cgd4_4150) are absent in C. ryanae. The three enzymes are involved in the conversion of IMP to xanthosine 5′-phosphate (XMP), XMP to GMP, and deoxyguanosine triphosphate (dGTP) to deoxyguanosine monophosphate (dGMP), respectively (Table 2). In pyrimidine metabolism, uracil phosphoribosyltransferase (cgd4_4460) and deoxycytidine monophosphate (dCMP) deaminase (cgd2_2780) are absent in C. ryanae. In other Cryptosporidium spcies, uracil is transported into the parasites by nucleobase transporter and catalysed to uridine monophosphate (UMP) by uracil phosphoribosyltransferase (Table 2). The loss of dCMP deaminase indicates that C. ryanae does not have the ability to convert dCMP to deoxyuridine monophosphate (dUMP).
Other losses in metabolic pathways
Compared with C. bovis and C. ryanae, C. parvum has 462 species-specific genes, 276 of which encode putative proteins with unknown functions. The genes lost in C. bovis and C. ryanae encode proteins involved in various metabolic pathways. In amino acid metabolism, the gene encoding tryptophan synthase is present in C. parvum (cgd5_4560), but absent in C. bovis and C. ryanae. A gene encoding asparagine synthase A, which could convert aspartate into asparagine, is also absent in C. bovis and C. ryanae. The orthologue of E3 ubiquitin ligase (cgd6_2490), which catalyses the transfer of ubiquitin from the E2 ubiquitin-conjugating enzyme to the protein substrate, was not detected in C. bovis or C. ryanae, indicating that the protein degradation ability is decreased in these two species. Dynamin is a GTPase involved in endocytosis, division of organelles, cytokinesis and microbial pathogen resistance in eukaryotic cells. The gene encoding dynamin (cgd8_1990) in C. parvum is absent in C. bovis and C. ryanae. In addition, three genes encoding ribosomal proteins (cgd1_300, cgd3_2250 and cgd7_4050) are lost in C. bovis and C. ryanae. The gene (cgd3_2840) encoding a protein that has two C2H2 zinc fingers and is involved in RNA metabolism is also absent in C. bovis and C. ryanae. Furthermore, C. bovis and C. ryanae have lost one member of the polypeptide N-acetylgalactosaminyltransferase family and histidine phosphatase superfamily, which each possess two adjacent genes in other intestinal Cryptosporidium species.
Gains and losses in subtelomeric genes encoding invasion-related proteins
Compared with other Cryptosporidium species, the genes encoding mucin-type glycoproteins have high divergence in C. bovis and C. ryanae (Table S3). Among them, the gene encoding CP2 (cgd6_5410), which is involved in the invasion process and the integrity of the parasitophorous vacuole membrane (PVM), is absent in C. bovis, C. ryanae and C. andersoni. Similarly, the cluster of seven mucin genes (encoding Muc1–Muc7) in the 5′ subtelomeric regions of chromosome 2 in C. parvum were not detected in C. bovis or C. ryanae. In addition, the genes encoding Muc12, Muc14, Muc17, Muc20 and Muc24 are lost in C. bovis and C. ryanae. In contrast, C. bovis and C. ryanae have several genes (C_bov_6.3221, C_bov_8.3556, C_bov_4.2822, C_bov_4.2823, C_bov_42.2912, C_bov_6.3080, C_bov_8.3622, C_bov_8.3594, C_bov_1.182, C_bov_10.262, C_bov_20.1093, C_bov_3.2223, C_bov_8.3592, C_bov_8.3638, C_bov_1.152, C_rya_29.1908, C_rya_26.1661, C_rya_6.2899, C_rya_45.2592, C_rya_23.1311, C_rya_23.1284, C_rya_11.174, C_rya_19.991, C_rya_25.1585, C_rya_23.1281, C_rya_96.3702) encoding novel mucin-type glycoproteins. Among them, C_bov_6.3080 and C_rya_45.2592 are subtelomeric, while C_bov_4.2822 and C_bov_4.2823 are adjacent to each other.
Compared with C. parvum, three of the 23 genes encoding insulinase-like proteases are lost in C. bovis and C. ryanae (Table S3). Two of them are C. parvum-specific genes located in 3′ subtelomeric regions of chromosomes 5 and 6. Furthermore, the gene (cgd3_4270) encoding INS16, which is a paralogue of cgd3_4260 with 83 % amino acid sequence similarity, is absent in C. ubiquitum, C. bovis and C. ryanae, but present in other Cryptosporidium species. As in C. ubiquitum, C. baileyi and C. andersoni, C. bovis and C. ryanae have lost all genes encoding MEDLE family proteins (Table S3).
Gene gains and losses in other multigene protein families
C. bovis and C. ryanae have gained members of several multigene families compared with C. parvum (Table S4). The WYLE protein family contains secreted proteins with the WYLE sequence in the middle of the proteins. In C. parvum, C. hominis and C. meleagridis, there are six genes encoding WYLE proteins, five of which form a cluster in chromosome 8. Interestingly, three and two additional genes encoding WYLE proteins were detected in the gene cluster in C. bovis and C. ryanae, respectively. In contrast, only four genes of the WYLE protein family were detected in the gastric species C. andersoni and C. muris. Furthermore, two genes (C_bov_31.2447 and C_bov_31.2452) encoding secreted leucine-rich proteins form a new gene family in C. bovis. One orthologue of the gene, C_bov_31.2447, was found in C. ryanae. Similarly, two genes (C_bov_11.434 and C_bov_18.914) encoding a new protein family annotated as GPI-anchored adhensin were detected in C. bovis with only one orthologue in C. ryanae.
More often, members of multigene families are lost in C. bovis and C. ryanae. The FLGN and SKSR families of secreted proteins are present in all major human-infecting Cryptosporidium species. Between them, the FLGN protein family has six, six, six, six and four members in C. parvum, C. hominis, C. meleagridis, Cryptosporidium chipmunk genotype I and C. ubiquitum, respectively. Similarly, the SKSR protein family has nine, 11, 10, nine and seven members in C. parvum, C. hominis, C. meleagridis, Cryptosporidium chipmunk genotype I and C. ubiquitum, respectively. None of these FLGN and SKSR genes were detected in C. bovis or C. ryanae. The NFDQ protein family has three subtelomeric genes (cgd6_5500, cgd5/6_5500 and cgd8_10) in C. parvum, six in C. hominis, four in Cryptosporidium chipmunk genotype I, two in C. meleagridis and one in C. ubiquitum. Among them, only the orthologue of cgd6_5500 was detected in C. bovis (C_bov_16.739) and C. ryanae (C_rya_14.480). Similar to other Cryptosporidium species, C. bovis and C. ryanae have only one orthologue of cgd8_680_90, which encodes a large low-complexity protein; a paralogue (cgd8_660_70) of cgd8_680_90 is present in C. parvum.
Highly divergent genes between C. bovis and C. ryanae
We compared the genomes of C. bovis and C. ryanae and found 46 highly divergent genes encoding proteins with an amino acid identity below 65 % (Table S5). Among them, 22 (47.8 %) genes encode secreted proteins, 18 (39.8 %) encode membrane-bound proteins, 17 (37.0 %) are located in the subtelomeric regions and 21 (45.7 %) have paralogous genes in C. bovis. Notably, C_bov_10.237 encodes a secreted mucin-like glycoprotein that has only 51.3 % sequence identity to the protein encoded by C_rya_24.1464; C_bov_21.1320 encodes a secreted insulinase-like peptidase, which has only 47.5 % sequence identity to the homologue in C. ryanae; and C_bov_5.3046 encodes a membrane-associated aspartyl protease with three paralogous genes, and has 59.8 % sequence similarity to the homologe in C. ryanae. The same is also true for genes encoding oocyst wall protein (C_bov_26.1848), ubiquitin-activating enzyme E1 (C_bov_6.3147) and secreted low-complexity containing protein (C_bov_8.3456). The functions of other proteins involved are unknown.
Genes under selection pressure
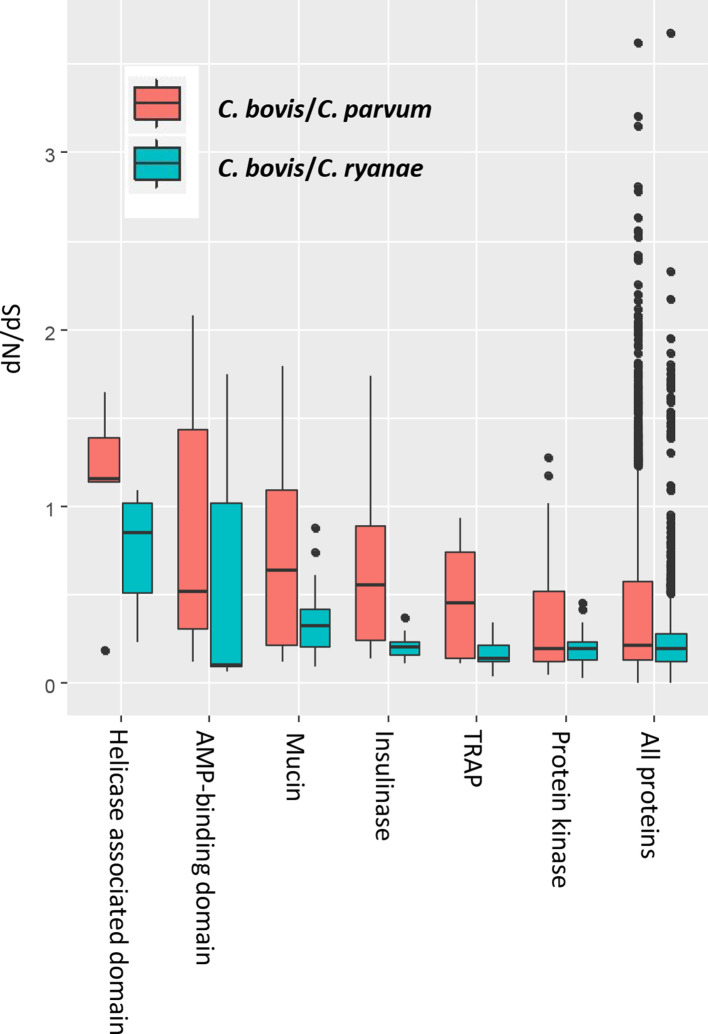
The orthologous genes between C. bovis and C. parvum exhibited elevated dN/dS ratios compared with those between C. bovis and C. ryanae, especially in the gene families that encode proteins involved in host–pathogen interactions. We found that the gene families encoding helicase-associated domains, AMP-binding domains, protein kinases, mucins, insulinases and TRAPs have higher dN/dS ratios between C. bovis and C. parvum than between C. bovis and C. ryanae (Fig. 5). The genes under positive selection between C. bovis and C. parvum include six helicases, four RNA polymerases, four protein kinases, three insulinase-like peptidases and two ABC transporters (Table 4). The three insulinase-like peptidases under positive selection are in a gene cluster within chromosome 3 in C. parvum. The gene cgd3_4270 also is among them but is lost in C. bovis and C. ryanae.
Fig. 5.
Selective pressure in multigene families between Cryptosporidium parvum and Cryptosporidium bovis and between C. bovis and Cryptosporidium ryanae as indicated by the dN/dS ratio.
Table 4.
Multigene protein families under positive selective pressure between Cryptosporidium bovis and Cryptosporidium ryanae
|
Gene family |
Gene in C. parvum |
Gene in C. bovis |
dN/dS ratio |
Annotation |
|---|---|---|---|---|
|
Helicase |
cgd1_2650 |
C_bov_13.593 |
1.64629 |
SNF2 helicase |
|
cgd6_1410 |
C_bov_13.593 |
1.64629 |
Pre-mRNA splicing factor ATP-dependent RNA helicase |
|
|
cgd6_3860 |
C_bov_25.1726 |
1.12968 |
SNF2 helicase |
|
|
cgd7_640 |
C_bov_4.2704 |
1.38836 |
Prp16p pre-mRNA splicing factor. HrpA family SFII helicase |
|
|
cgd8_2770 |
C_bov_42.2905 |
1.08081 |
SNF2L orthologue with an SWI/SNF2 like ATPase and an Myb domain |
|
|
cgd8_4100 |
C_bov_13.593 |
1.64629 |
PRP43 involved in spliceosome disassembly mRNA splicing |
|
|
Insulinase-like peptidase |
cgd3_4250 |
C_bov_21.1320 |
1.73579 |
Secreted insulinase-like peptidase |
|
cgd3_4260 |
C_bov_21.1321 |
1.28419 |
Secreted insulinase-like peptidase |
|
|
cgd3_4280 |
C_bov_21.1322 |
1.11455 |
Secreted insulinase-like peptidase |
|
|
Protein kinase |
cgd5_250 |
C_bov_24.1656 |
1.01196 |
Ser/Thr protein kinase |
|
cgd5_3180 |
C_bov_17.879 |
1.27347 |
Ser/Thr protein kinase |
|
|
cgd6_4960 |
C_bov_30.2379 |
1.01594 |
Ser/Thr protein kinase |
|
|
cgd6_540 |
C_bov_23.1582 |
1.17354 |
Ser/Thr protein kinase |
|
|
ABC transporter |
cgd2_90 |
C_bov_6.3084 |
1.80151 |
ABC transporter with 9× transmembrande domains and 2× AAA |
|
cgd4_4440 |
C_bov_27.1928 |
1.19132 |
ABC transporter with 9× transmembrande domains and 2× AAA |
|
|
RNA polymerase |
cgd7_3720 |
C_bov_6.3158 |
1.75622 |
RNA polymerase beta subunit |
|
cgd8_170 |
C_bov_10.307 |
1.28553 |
DNA-directed RNA polymerase beta subunit |
|
|
cgd3_2620 |
C_bov_20.1075 |
1.60633 |
DNA-directed RNA polymerase, possible RNA polymerase |
|
|
cgd6_3290 |
C_bov_36.2567 |
1.60829 |
DNA-directed RNA polymerase III C1 subunit |
|
|
Acyl transferase domain |
cgd3_2180 |
C_bov_14.664 |
2.0781 |
Type I fatty acid synthase |
|
cgd4_2900 |
C_bov_36.2532 |
2.03806 |
Polyketide synthase |
Discussion
The results of this study have shown significant differences among the genomes of the three common intestinal Cryptosporidium species in bovine animals. The nucleotide and amino acid sequence identities between C. bovis and C. parvum are 38.6 and 55.1 %, respectively, while those between C. bovis and C. ryanae are 69.4 and 75.2 %, respectively. In contrast, the nucleotide and amino acid sequence identities between Cryptosporidium chipmunk genotype I and other major human-pathogenic species such as C. hominis, C. parvum, C. meleagridis and C. ubiquitum are 78.7–82.5 and 79.0–84.0 %, respectively [16]. These genomic differences among Cryptosporidium species are in agreement with their phylogenetic relationship (Fig. 2). They could contribute to the differences in human infectivity and pathogenicity among intestinal Cryptosporidium species.
Accompanying the significant sequence differences is a reduction in synteny in gene organization between the C. bovis/C. ryanae and C. parvum genomes. Compared with the large syntenic regions among C. hominis, C. parvum and Cryptosporidium chipmunk genotype I, the syntenic regions between C. bovis/C. ryanae and C. parvum are more fragmented. Blocks of rearrangements and deletions were observed in some chromosomes between C. bovis and C. parvum, especially in the subtelomeric regions, leading to losses in the former of some subtelomeric genes encoding secreted proteins. Breaks in genome synteny are common in other apicomplexans, leading to the losses of multigene families and species-specific genes [42].
Compared with C. parvum and other human-pathogenic intestinal Cryptosporidium species, C. bovis and C. ryanae appear to have more streamlined metabolism. The gene content of the C. bovis and C. ryanae genomes is smaller than that of the C. hominis and C. parvum genomes. There are nearly 3300 genes shared by all intestinal Cryptosporidium species. Compared with C. parvum, the genes lost in C. bovis mostly encode metabolism-related enzymes and secreted proteins. The loss of enzymes involved in the metabolic pathways leads to further reduced biosynthesis capacity and energy production in C. bovis and C. ryanae. As a result, these two parasites could be more dependent on specific hosts to salvage nutrients. Previous studies have shown a progressive reduction in the electron transport chain in Cryptosporidium species [11]. The loss of the genes encoding ATP synthase and MQO in C. bovis and C. ryanae has provided new evidence for progressive reduction in energy production within the genus Cryptosporidium. Variations in metabolism are thought to contribute to lineage-specific adaptation to the host environment and virulence of apicomplexan parasites. In Toxoplasma gondii, altered capacity for energy production is associated with strain-specific differences in growth rates and virulence across different hosts, organs and cell types [43]. Because of the importance of some metabolic pathways in pathogen growth and survival, they could be potential targets for drug development, such as isoprenoid biosynthesis [44] and the shikimate pathway [45]. MQO could be such a potential target against C. parvum, but not against C. bovis or C. ryanae.
A major difference among the three bovine intestinal Cryptosporidium species is in the number of mucin-type glycoproteins, which are important SPDs involved in the attachment of sporozoites to the host cells [46]. C. bovis and C. ryanae have lost a series of mucin-type glycoproteins, including CP2, Muc1–Muc7, Muc12, Muc14, Muc17 and Muc20. In addition to the loss of mucin-type glycoproteins, several novel mucin-type glycoproteins were observed in C. bovis and C. ryanae. Thus, Muc25–Muc39 have no orthologues in C. parvum and most of them are present in both C. bovis and C. ryanae. These copy number variations in mucin-type glycoproteins could potentially contribute to the phenotypic differences among intestinal Cryptosporidium species, such as variations in growth rate of the pathogens and duration and intensity of infections [46].
Similarly, subtelomeric genes encoding other invasion-associated proteins, such as secreted MEDLE proteins and insulinase-like proteases, are also divergent among C. parvum, C. bovis and C. ryanae. Three insulinase-like proteases are lost in C. bovis and C. ryanae, two of which are in the subtelomeric region and one is in the multigene cluster. Similarly, genes encoding MEDLE proteins located in the subtelomeric region are completely absent in C. bovis and C. ryanae. The number of invasion-related proteins is known to be different among apicomplexans. For example, Neospora caninum and Sarcocystis neurona have 227 and 23 SAG1-related sequences (SRS), respectively, which are involved in modulation of host immune responses [47]. Similarly, Toxoplasma gondii strains Me49 (less virulent) and GT1 (more virulent) have 109 and 90 such genes, respectively [48]. Theileria parva and Theileria annulata are known to have different numbers (85 and 51 members, respectively) of genes encoding subtelomeric variable secreted proteins (SVSPs) [47], which could contribute to differences in host range and pathogenicity between the two species. Cryptosporidium species do not have homologous proteins of these families, but subtelmoeric genes encoding secreted proteins account for the majority of multigene families in their genomes. They were previously suggested to be SPDs in Cryptosporidium species [16].
Our comparative genomics analysis has revealed some gains and losses of other potential SPDs among the three bovine Cryptosporidium species. They include genes encoding secreted WYLE, FLGN, SKSR and NFDQ proteins. Previous studies have suggested that differences in pathogenicity, transmission modes and host range among Toxoplasma gondii strains could have been caused by differences in copy numbers of genes encoding SRS proteins and secretory proteins from micronemes (MICs), dense granules (GRAs) and rhoptries (ROPs), which appear to be SPDs in Toxoplasma gondii [49]. In Cryptosporidium species, differences in copy numbers of genes encoding SKSR proteins have been observed between C. parvum IIa and IId subtype families [17]. The subtelomeric genes encoding these SPDs, except for those encoding WYLE proteins, are mostly lost in C. bovis and C. ryanae. In contrast, the latter two species have additional members of WYLE proteins, which could contribute to the biological uniqueness of these two bovine Cryptosporidium species.
Compared with other Cryptosporidium species, C. bovis and C. ryanae have similar gene contents and the closest genetic relationship. Minor differences in gene content between the two species include genes encoding enzymes in nucleotide metabolism, ABC transporters, mitochondrial carriers, mucin-type glycoproteins and several hypothetical proteins. However, 46 genes with highly divergent sequences are present between C. bovis and C. ryanae. Half of the highly divergent genes between C. bovis and C. ryanae encode secreted proteins or membrane-bound proteins and one-third of the highly divergent genes are located in the subtelomeric regions. While most of the genes encode proteins with unknown functions, some are specific to C. bovis and C. ryanae, including members of invasion-related protein families, ubiquitin-activating enzymes and oocyst wall proteins. More functional studies on these proteins are needed to understand the importance of the sequence divergence between these two species.
The elevated dN/dS ratios in the orthologous genes between C. bovis and C. parvum reveal a divergence in the evolution between these two species. The positive selection identified in some multigene families could be a reflection of the proteins encoded by the genes in host specificity and pathogenicity between the two species. In addition to the gains and losses of invasion-related protein families between C. parvum and C. bovis, some members of these families are also under positive selection, including three insulinase-like peptidases located in a gene cluster within chromosome 3. Previous studies have shown that only a few orthologous genes are under positive selection among closely related Cryptosporidium species [50, 51], and most of them are located in the subtelomeric regions. Between C. parvum and C. bovis, however, some of the positively selected genes are distributed in various parts of the chromosomes. Furthermore, multiple gene families encoding helicases and polymerases are also among those with high dN/dS ratios. These genes are relatively conservative between C. parvum and other intestinal Cryptosporidium species sequenced thus far. Sequence polymorphisms in these genes could affect the efficiency of transcription and translation, leading to the divergence in biological characteristics between C. parvum and C. bovis. More transcriptomic and proteomic studies of Cryptosporidium species are needed to understand the significance of this finding. As expected, two genes encoding ABC transporters are also under positive selection, which could be involved in endobiotic and xenobiotic detoxification [52]. They could be potential targets for drug development.
In conclusion, C. bovis and C. ryanae apparently have high similarities in gene organization, metabolic pathways and SPDs. They have reduced metabolic capacity compared with C. parvum and other Cryptosporidium species. The loss of some mucin-type glycoproteins and insulinase-like proteases and all six secreted MEDLE proteins could potentially be responsible for the narrowed host range of C. bovis and C. ryanae. The loss of some other SPDs such as FLGN, SKSR and NFDQ proteins might contribute to the reduced pathogenicity of C. bovis and C. ryanae. Highly divergent genes encoding secreted and surface-associated proteins could contribute to the biological differences between C. bovis and C. ryanae. These hypotheses, however, should be examined in future studies using the functional genomics approach to confirm the findings from comparative genomics. Multiple isolates of C. bovis and C. ryanae should be sequenced and analysed to support some of the conclusions. This will probably lead to improved understanding of determinants of the host specificity and pathogenicity of different Cryptosporidium species.
Data Bibliography
1. Lihua Xiao. NCBI BioProject PRJNA545588 and PRJNA545579 (2019).
2. Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304, 441-445 NCBI BioProject PRJNA15586 (2004).
3. Isaza JP, Galvan AL, Polanco V, Huang B, Matveyev AV, Serrano MG, Manque P, Buck GA, Alzate JF. Revisiting the reference genomes of human pathogenic Cryptosporidium species: reannotation of C. parvum Iowa and a new C. hominis reference. Sci Rep 5, 16324 European Nucleotide Archive PRJEB10000 (2015).
4. Ifeonu OO, Chibucos MC, Orvis J, Su Q, Elwin K, Guo F, Zhang H, Xiao L, Sun M, Chalmers RM et al. Annotated draft genome sequences of three species of Cryptosporidium: Cryptosporidium meleagridis isolate UKMEL1, C. baileyi isolate TAMU-09Q1 and C. hominis isolates TU502_2012 and UKH1. Pathog Dis 74. NCBI BioProject PRJNA222838 and PRJNA22283574 (2016).
5. Liu S, Roellig DM, Guo Y, Li N, Frace MA, Tang K, Zhang L, Feng Y, Xiao L. Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium. BMC Genomics 17, 1006 NCBI BioProject PRJNA246478 and PRJNA308889 (2016).
6. Xu, Z., Guo, Y., Roellig, D.M., Feng, Y., Xiao, L. Comparative analysis reveals conservation in genome organization among intestinal Cryptosporidium species and sequence divergence in potential secreted pathogenesis determinants among major human-infecting species. BMC genomics 20, 406. NCBI BioProject PRJNA511361 2019.
Supplementary Data
Funding information
This work was supported in part by the National Natural Science Foundation of China (31 630 078 and 31602042), National Key R and D Program of China (2017YFD0500404), and the 111 Project (D20008).
Author contributions
Conceptualization: Y.F. and L.X.; methodology: Z.X. and L.X.; formal analysis: Z.X. and L.X.; investigation and resources: N.L. and Y.G.; writing – original draft preparation: Z.X.; writing – review and editing: L.X. and Y.F.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
This study was approved by the Ethics Committee of the East China University of Science and Technology. Faecal specimens from dairy cattle were collected with the permission of the farm manager. During specimen collection, cattle were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China.
Footnotes
Abbreviations: ML, maximum-likelihood; MQO, malate quinone oxidoreductase; SPD, secreted pathogenesis determinant; TRAP, thrombospondin-related adhesive protein.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Five supplementary tables are available with the online version of this article.
References
- 1.Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium . Lancet Infect Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Santín M. Clinical and subclinical infections with Cryptosporidium in animals. N Z Vet J. 2013;61:1–10. doi: 10.1080/00480169.2012.731681. [DOI] [PubMed] [Google Scholar]
- 4.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Ryan UM, Xiao L. Genetic diversity and population structure of Cryptosporidium . Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Rieux A, Paraud C, Pors I, Chartier C. Molecular characterization of Cryptosporidium isolates from beef calves under one month of age over three successive years in one herd in western France. Vet Parasitol. 2014;202:171–179. doi: 10.1016/j.vetpar.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayer R, Santin M, Trout JM. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet Parasitol. 2007;145:260–266. doi: 10.1016/j.vetpar.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Santín M, Trout JM, Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet Parasitol. 2008;155:15–23. doi: 10.1016/j.vetpar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Ralston B, Thompson RCA, Pethick D, McAllister TA, Olson ME. Cryptosporidium andersoni in Western Australian feedlot cattle. Aust Vet J. 2010;88:458–460. doi: 10.1111/j.1751-0813.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Tang K, Rowe LA, Li N, Roellig DM, et al. Comparative genomic analysis reveals occurrence of genetic recombination in virulent Cryptosporidium hominis subtypes and telomeric gene duplications in Cryptosporidium parvum . BMC Genomics. 2015;16:320. doi: 10.1186/s12864-015-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Roellig DM, Guo Y, Li N, Frace MA, et al. Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium . BMC Genomics. 2016;17:1006. doi: 10.1186/s12864-016-3343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei J, Wu H, Su J, Jin C, Li N, et al. Characterization of MEDLE-1, a protein in early development of Cryptosporidium parvum . Parasit Vectors. 2018;11:312. doi: 10.1186/s13071-018-2889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J, Jin C, Wu H, Fei J, Li N, et al. Differential expression of three Cryptosporidium species-specific MEDLE proteins. Front Microbiol. 2019;10:1177. doi: 10.3389/fmicb.2019.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Wang Y, Wu H, Li N, Jiang J, et al. Characterization of a species-specific insulinase-like protease in Cryptosporidium parvum . Front Microbiol. 2019;10:354. doi: 10.3389/fmicb.2019.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouzid M, Hunter PR, Chalmers RM, Tyler KM. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013;26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Guo Y, Roellig DM, Feng Y, Xiao L. Comparative analysis reveals conservation in genome organization among intestinal Cryptosporidium species and sequence divergence in potential secreted pathogenesis determinants among major human-infecting species. BMC Genomics. 2019;20:406. doi: 10.1186/s12864-019-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Li N, Roellig DM, Kelley A, Liu G, et al. Comparative genomic analysis of the IId subtype family of Cryptosporidium parvum . Int J Parasitol. 2017;47:281–290. doi: 10.1016/j.ijpara.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Chen L, Li F, Li N, Feng Y, et al. Divergent Copies of a Cryptosporidium parvum-Specific Subtelomeric Gene. Microorganisms. 2019;7:366.:E366. doi: 10.3390/microorganisms7090366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nader JL, Mathers TC, Ward BJ, Pachebat JA, Swain MT, et al. Evolutionary genomics of anthroponosis in Cryptosporidium . Nat Microbiol. 2019;4:826–836. doi: 10.1038/s41564-019-0377-x. [DOI] [PubMed] [Google Scholar]
- 20.Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum . Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 21.Ifeonu OO, Chibucos MC, Orvis J, Su Q, Elwin K, et al. Annotated draft genome sequences of three species of Cryptosporidium: Cryptosporidium meleagridis isolate UKMEL1, C. baileyi isolate TAMU-09Q1 and C. hominis isolates TU502_2012 and UKH1. Pathog Dis. 2016;74:ftw080. doi: 10.1093/femspd/ftw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, et al. The genome of Cryptosporidium hominis . Nature. 2004;431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/AEM.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Cebelinski E, Matusevich C, Alderisio KA, Lebbad M, et al. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J Clin Microbiol. 2015;53:1648–1654. doi: 10.1128/JCM.03436-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005;33:6494–6506. doi: 10.1093/nar/gki937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanke M, Steinkamp R, Waack S, Morgenstern B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 2004;32:W309–W312. doi: 10.1093/nar/gkh379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parra G, Blanco E, Guigó R. Geneid in Drosophila. Genome Res. 2000;10:511–515. doi: 10.1101/gr.10.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 32.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 33.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 34.Fankhauser N, Mäser P. Identification of GPI anchor attachment signals by a Kohonen self-organizing map. Bioinformatics. 2005;21:1846–1852. doi: 10.1093/bioinformatics/bti299. [DOI] [PubMed] [Google Scholar]
- 35.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanmugasundram A, Gonzalez-Galarza FF, Wastling JM, Vasieva O, Jones AR. Library of apicomplexan metabolic pathways: a manually curated database for metabolic pathways of apicomplexan parasites. Nucleic Acids Res. 2013;41:D706–D713. doi: 10.1093/nar/gks1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 41.Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- 42.DeBarry JD, Kissinger JC. Jumbled genomes: missing apicomplexan synteny. Mol Biol Evol. 2011;28:2855–2871. doi: 10.1093/molbev/msr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song C, Chiasson MA, Nursimulu N, Hung SS, Wasmuth J, et al. Metabolic reconstruction identifies strain-specific regulation of virulence in Toxoplasma gondii . Mol Syst Biol. 2013;9:708. doi: 10.1038/msb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno SNJ, Li Z-H. Anti-infectives targeting the isoprenoid pathway of Toxoplasma gondii . Expert Opin Ther Targets. 2008;12:253–263. doi: 10.1517/14728222.12.3.253. [DOI] [PubMed] [Google Scholar]
- 45.McConkey GA, Pinney JW, Westhead DR, Plueckhahn K, Fitzpatrick TB, et al. Annotating the Plasmodium genome and the enigma of the shikimate pathway. Trends Parasitol. 2004;20:60–65. doi: 10.1016/j.pt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Lendner M, Daugschies A. Cryptosporidium infections: molecular advances. Parasitology. 2014;141:1511–1532. doi: 10.1017/S0031182014000237. [DOI] [PubMed] [Google Scholar]
- 47.Reid AJ. Large, rapidly evolving gene families are at the forefront of host-parasite interactions in Apicomplexa. Parasitology. 2015;142 Suppl 1:S57–S70. doi: 10.1017/S0031182014001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasmuth JD, Pszenny V, Haile S, Jansen EM, Gast AT, et al. Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence. mBio. 2012;3:e00321-12. doi: 10.1128/mBio.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun. 2016;7:10147. doi: 10.1038/ncomms10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazurie AJ, Alves JM, Ozaki LS, Zhou S, Schwartz DC, et al. Comparative genomics of Cryptosporidium . Int J Genomics. 2013;2013:832756. doi: 10.1155/2013/832756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaza JP, Galván AL, Polanco V, Huang B, Matveyev AV, et al. Revisiting the reference genomes of human pathogenic Cryptosporidium species: reannotation of C. parvum Iowa and a new C. hominis reference. Sci Rep. 2015;5:16324. doi: 10.1038/srep16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zapata F, Perkins ME, Riojas YA, Wu TW, Le Blancq SM. The Cryptosporidium parvum ABC protein family. Mol Biochem Parasitol. 2002;120:157–161. doi: 10.1016/S0166-6851(01)00445-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.