Abstract
Introduction
Epidemiological studies indicate an inverse association between nut consumption and body mass index (BMI). However, clinical trials evaluating the effects of nut consumption compared with a nut-free diet on adiposity have reported mixed findings with some studies reporting greater weight loss and others reporting no weight change. This paper describes the rationale and detailed protocol for a randomised controlled trial assessing whether the inclusion of almonds or carbohydrate-rich snacks in an otherwise nut-free energy-restricted diet will promote weight loss during 3 months of energy restriction and limit weight regain during 6 months of weight maintenance.
Methods and analysis
One hundred and thirty-four adults aged 25–65 years with a BMI of 27.5–34.9 kg/m2 will be recruited and randomly allocated to either the almond-enriched diet (AED) (15% energy from almonds) or a nut-free control diet (NFD) (15% energy from carbohydrate-rich snack foods). Study snack foods will be provided. Weight loss will be achieved through a 30% energy restriction over 3 months, and weight maintenance will be encouraged for 6 months by increasing overall energy intake by ~120–180 kcal/day (~500-750kJ/day) as required. Food will be self-selected, based on recommendations from the study dietitian. Body composition, resting energy expenditure, total daily energy expenditure (via doubly labelled water), physical activity, appetite regulation, cardiometabolic health, gut microbiome, liver health, inflammatory factors, eating behaviours, mood and personality, functional mobility and pain, quality of life and sleep patterns will be measured throughout the 9-month trial. The effects of intervention on the outcome measures over time will be analysed using random effects mixed models, with treatment (AED or NFD) and time (baseline, 3 months and 9 months) being the between and within factors, respectively in the analysis.
Ethics and dissemination
Ethics approval was obtained from the University of South Australia Human Research Ethics Committee (201436). Results from this trial will be disseminated through publication in peer-reviewed journals, national and international presentations.
Trial registration number
Australian New Zealand Clinical Trials Registry (ACTRN12618001861246).
Keywords: nutrition & dietetics, clinical physiology, public health
Strengths and limitations of this study.
To our knowledge, this will be the first randomised controlled trial to assess whether the inclusion of almonds versus carbohydrate-rich snack foods in an otherwise nut-free diet will improve weight loss and limit weight regain.
A wide range of outcomes will be assessed including but not limited to: body composition, resting and total daily energy expenditure, appetite regulation, cardiometabolic health, liver health, inflammatory markers and effects on the gut microbiome.
Both objective and subjective appetite regulation will be evaluated, adding to our current limited knowledge of the effects of almonds on appetite control.
The metagenomic analysis that will be performed will be a substantial advance on our current understanding of the impact of almonds on the gut microbiome (previously limited to amplicon sequencing approaches).
A potential limitation of this study is that it will only be feasible to follow participants for 6 months after initial weight loss.
Introduction
Epidemiological studies report associations between increased frequency of nut consumption and lower body weight.1–4 This is supported by clinical data which suggest that regular nut intake has a beneficial impact on adiposity, insulin resistance and related metabolic abnormalities.5–11 Despite this, many people still avoid eating nuts due to the perception that they lead to weight gain based on their high energy and fat content. Nut consumption in many countries including Australia and USA is low (6 g/day and 3.3 g/day per capita, respectively),12–14 with the prevalence of adult nut consumers being ~16%–20% in Australia15 and USA.16 Such low consumption suggests there is scope to increase consumption in both countries.
Data from the National Health and Nutrition Examination Survey indicated that nut consumption was associated with a lower body mass index (BMI) (27.7±0.2 kg/m2 vs 28.1±0.1 kg/m2, p<0.05) and waist circumference (95.6±0.4 cm vs 96.4±0.3 cm, p<0.05), and tree nut consumers had lower body weight than non-consumers (78.8±0.7 kg vs 80.7±0.3 kg, p<0.05).16 When considering almonds specifically, randomised controlled trials have reported greater weight loss17 18 or improved body composition (reduced total fat and truncal fat)19 20 on hypocaloric diets with the inclusion of almonds compared with a nut-free diet. However, recent meta-analyses of clinical trials evaluating the effects of nut consumption on adiposity have reported no difference in body weight, BMI or waist circumference when comparing diets including nuts against control diets.5 9 Nevertheless, changes in body fat distribution and reductions in fat stored in the liver can improve metabolic outcomes independent of weight changes. Inclusion of nuts may help prevent and manage non-alcoholic fatty liver disease21 22 and almond consumption has been associated with reductions in circulating liver enzyme concentrations.23 Additionally, data from large cohort studies suggest that frequent nut consumption may lower the risk of weight gain,4 with consumption on 5 or more days per week showing the greatest effect.24 25 Prospective analysis of cohorts of healthy adults show that the average weight gain over 4 years was 3.3 lb (~1.5 kg). This weight gain was inversely associated with nut consumption.26
Weight regain following initial weight loss is common and contributes, in part, to the obesity epidemic.27 Randomised controlled trials assessing weight loss maintenance report a 30%–35% weight regain in the first year following a weight loss intervention with a 76% weight regain at 4 years post-treatment.28 This suggests there is a need for nutritional strategies that prevent weight regain. Therefore, assessing the effects of nutritional strategies, such as the inclusion of nuts for the prevention of weight regain, is paramount. Diets high in protein appear to limit weight regain29 with higher intakes of non-cereal plant proteins, such as in nuts, associated with a protective effect.30 Nuts are rich in protein, fibre and monounsaturated fat, which have been suggested to contribute to their positive effect on appetite regulation31 32 and almonds, specifically, have been shown to have positive effects on subjective ratings of appetite.33 34 However, the majority of assessments of appetite regulation with nuts have been conducted acutely and subjectively, and few have assessed appetite hormones.35 Reduced food cravings have been associated with long-term weight loss success36; thus, assessing both of these outcomes following regular almond consumption is important.
Previous studies have reported significant differences in gut microbiota between lean and obese individuals37 and modulation at the phylum and genus levels following weight loss.38 Changes in gut microbiota have also been observed following consumption of diets containing almonds39 and almond skins,40 although not consistently in all populations.41 Significant increases in the relative abundance of bacterial taxa comprising Ruminiclostridium, and members of the Ruminococcaceae and Lachnospiraceae families, under trial conditions are implicated in the degradation of complex dietary plant-derived polysaccharides, and the production of beneficial short-chain fatty acids.42 It has been suggested that bacterial products may impact on short-term intestinal satiety pathways and long-term appetite control.43 However, it is unclear whether the changes in gut microbiota associated with almond consumption may help limit weight regain and the relationship with other biomarkers of cardiometabolic risk.
Objectives
Primary objective
The primary aim of this project is to evaluate whether inclusion of 15% of energy from almonds (almond-enriched diet (AED)) compared with carbohydrate-rich snacks in an otherwise nut-free energy-restricted diet (nut-free control diet (NFD)), will improve weight loss during 3 months of dietary energy restriction and limit weight regain during 6 months of weight maintenance. We hypothesise that the AED will lead to greater weight loss during the energy restriction phase of 3 months and limit weight regain during the weight maintenance period of 6 months compared with the NFD.
Secondary objectives
The secondary aim is to evaluate whether an AED compared with a NFD improves body composition and body fat distribution (reduced waist circumference and abdominal fat depots), impacts resting and total daily energy expenditure and improves subjective and objective measures of satiety.
Tertiary objectives
The tertiary aim is to evaluate whether an AED compared with a NFD reduces fat accumulation in the liver and improves liver enzyme profiles, results in beneficial changes in the composition of the gut microbiome, improves inflammatory biomarkers and cardiometabolic health outcomes including blood lipid profiles, glucose and insulin, and improves self-reported eating behaviours, mood, personality, pain, functional mobility, quality of life, sleep and physical activity patterns.
Methods and analysis
Study design
The study is designed as a 9-month randomised controlled parallel-arm dietary intervention. The study will be conducted in the research facilities of the Alliance for Research in Exercise, Nutrition and Activity at the University of South Australia, Adelaide. The Standard Protocol Items: Recommendations for Interventional Trials guidelines were used in the development of this protocol.44
Patient and public involvement
Development of this research protocol was done without patient involvement. The final study results will be disseminated to all participants.
Participants
Eligibility criteria
Participants will be male and female volunteers, aged 25–65 years, with a BMI of 27.5–34.9 kg/m2. This age range ensures physical maturity has been achieved and limits the possibility of chronic health conditions that would exclude the volunteer from participation. This BMI range ensures sufficient weight available to lose and reduces the risk of chronic health conditions. Participants will be non-smokers (minimum 6 months) and weight stable (within 5 kg) for 3 months prior to enrolment. Detailed inclusion and exclusion and withdrawal criteria are listed in box 1.
Box 1. Eligibility criteria.
Inclusion criteria
Men and women aged 25–65 years.
Body mass index 27.5–34.9 kg/m2.
Weight stable (within 5 kg) in the past 3 months.
Non-smoker (minimum 6 months).
Exclusion criteria
Cardiovascular disease.
Type 1 or type 2 diabetes.
Thyroid disorders.
Kidney or liver disease.
Gastrointestinal disorders requiring medical nutrition therapy (eg, Crohn’s disease, irritable bowel, coeliac disease).
Are pregnant or breast feeding.
Allergies to nuts, gluten or other components of the test foods.
Unable to chew hard foods such as nuts.
Consumed more than 30 g of nuts per day in the month prior to beginning the trial.
Alcohol (>14 standard drinks/week) (1 standard drink=100 mL wine, 285 mL beer, 30 mL spirit) or drug dependency.
Have changed medications or supplements in the last 3 months.
Take vitamin, mineral, herbal supplementation or medications that may have an impact on study outcomes.
Unwilling to stop dietary supplements that influence weight.
Suffer claustrophobia or a fear of enclosed spaces.
Show unwillingness to be randomised to either experimental group (based on liking and palatability questionnaires).
Withdrawal criteria
Adverse reaction to test foods.
The need to take a medication or treatment, which in the opinion of the investigator, may interfere with study measurements.
Consistent non-compliance (<80% compliance) with consuming study foods.
Failure to satisfy the investigator regarding suitability to participate for any other reason.
Recruitment/screening
Participants will be recruited from the public through radio, TV, printed media, internet-based advertisements and flyer distribution. Procedures will occur in accordance with ethical standards, including obtaining written informed consent.
Interested participants will be sent the participant information and a Diet and Lifestyle Questionnaire (DLQ) to determine eligibility. Participants who appear eligible from DLQ responses will be assigned a screening number and will undergo an initial screening interview over the telephone to review medical history, concomitant medication and supplementation. Prospective participants will attend the clinical research facility approximately 3 weeks before baseline where eligibility will be confirmed, and the likelihood to consume test foods will be established via liking and palatability questionnaires (see table 1). Participants who meet the required criteria and are deemed eligible, will be asked to provide written informed consent in the presence of the investigator and will proceed with a pre-baseline clinic appointment, 2 weeks prior to baseline.
Table 1.
Outcome measures at each time point
| Time (weeks) from start of dietary intervention | Screening | Pre-baseline | Baseline | Study period | Close-out | |||||
| -3 | -2 | −1(D1) | 0 (D2) | 10 | 12 (D1) | 13 (D2) | 35 | 36 (D1) | 37 (D2) | |
| Study food liking and palatability scores | ||||||||||
| Labelled Affect Magnitude Scale | X | X | ||||||||
| Food Action Rating Scale | X | X | ||||||||
| Body composition | ||||||||||
| Height | X | X | ||||||||
| Weight (and body mass index) | X | X | X* | X | X | X* | X | X | X* | X |
| Total and truncal fat mass (DXA) | X | X | X | |||||||
| Total and truncal fat-free mass (DXA) | X | X | X | |||||||
| Visceral adipose tissue (DXA) | X | X | X | |||||||
| Waist circumference | X | X | X | |||||||
| Energy expenditure | ||||||||||
| Accelerometry | X | X | X | |||||||
| Resting energy expenditure (indirect calorimetry) | X | X | X | |||||||
| Total daily energy expenditure (doubly labelled water)† | X | X | X | |||||||
| International Physical Activity Questionnaire | X | X | X | |||||||
| Appetite regulation and eating behaviour | ||||||||||
| Energy intake | ||||||||||
| 4-day food diary | X | X | X | |||||||
| 24 hours diet recalls‡ | X | X | ||||||||
| Fasting and postprandial gut hormones and glucose | ||||||||||
| Glucagon-like peptide-1 | X | X | X | |||||||
| Ghrelin | X | X | X | |||||||
| Leptin | X | X | X | |||||||
| Pancreatic polypeptide | X | X | X | |||||||
| Glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide) | X | X | X | |||||||
| Peptide tyrosine tyrosine | X | X | X | |||||||
| C-peptide | X | X | X | |||||||
| Cholecystokinin | X | X | X | |||||||
| Glucagon | X | X | X | |||||||
| Glucose | X | X | X | |||||||
| Drive to eat and eating behaviour | ||||||||||
| Subjective drive to eat (Visual Analogue Scales)—fasting and postprandial | X | X | X | |||||||
| Energy consumed at a buffet meal | X | X | X | |||||||
| Power of Food Survey | X | X | X | |||||||
| Food Craving Scale | X | X | X | |||||||
| Pickiness/Finickiness Questionnaire | X | X | ||||||||
| Eating Attitude Test | X | X | X | |||||||
| Control of Eating Questionnaire | X | X | X | |||||||
| Psychology and health | ||||||||||
| General health, pain, mobility, mood and personality | ||||||||||
| Short-Form 36 Questionnaire | X | X | X | |||||||
| Profile of Mood States | X | X | X | |||||||
| Perceived Stress Scale | X | X | X | |||||||
| Zung Self-Rating Scale | X | X | X | |||||||
| McGill Pain Scale and Chronic and Acute Pain Scales (Visual Analogue Scales) | X | X | X | |||||||
| Timed up and go (functional mobility) | X | X | X | |||||||
| Eysenck Personality Questionnaire | X | |||||||||
| Brief Sensation Seeking Scale | X | |||||||||
| Gut health | ||||||||||
| Faecal microbiome composition | X | X | X | |||||||
| Liver health | ||||||||||
| Liver ultrasound | X | X | X | |||||||
| Alanine aminotransferase | X | X | X | |||||||
| Aspartate aminotransferase | X | X | X | |||||||
| Alkaline phosphatase | X | X | X | |||||||
| γ-glutamyltransferase | X | X | X | |||||||
| Cardiometabolic health | ||||||||||
| Blood pressure | X | X | X | X | X | |||||
| Flash glucose monitoring | X | X | X | |||||||
| Insulin | X | X | X | |||||||
| HOMA | X | X | X | |||||||
| Triglycerides | X | X | X | X | X | X | ||||
| Cholesterols (TC, HDL-C, LDL-C, VLDL-C, IDL-C and subclasses, lipoprotein (a), oxidised LDL, LDL-C particle size) | X | X | X | |||||||
| Apolipoprotein B | X | X | X | |||||||
| Apolipoprotein A1 | X | X | X | |||||||
| Inflammatory markers | ||||||||||
| F2-isoprostane levels (plasma+urine) | X | X | X | |||||||
| C-reactive protein | X | X | X | |||||||
| Adiponectin | X | X | X | |||||||
| Sleep patterns | ||||||||||
| Pittsburg Sleep Quality Index | X | X | X | |||||||
| 14-day sleep diary | X | X | X | |||||||
| Biomarkers of compliance | ||||||||||
| Alpha-tocopherol | X | X | X | |||||||
*Indicates primary outcome timepoints (fasted weight).
†Subsample only.
‡3×24-hour recalls were completed at random intervals between 0–13 weeks and 13–37 weeks.
D1, day 1; D2, day 2; DXA, dual-energy X-ray absorptiometry; HDL-C, high-density lipoprotein-cholesterol; IDL-C, intermediate-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; TC, total cholesterol; VLDL-C, very low-density lipoprotein-cholesterol.
Randomisation, allocation concealment and sequence generation
Data collected at the screening visit will be used to assign participants to groups based on age, sex and BMI in the process of randomisation by minimisation.45 Minimisation will ensure baseline characteristics are balanced between the treatment groups and has been proposed to be the most suitable randomisation method for small clinical trials, such as the proposed study, to reduce bias.45 46 A staff member independent of the study outcome assessments and statistical analysis will perform the treatment allocation and maintain the randomisation list in a secure location with access limited to authorised personnel. As the participants are consuming whole foods, which are easily identified, the participants and staff involved in diet management cannot be blinded. Staff conducting clinical assessments at baseline, 3 months and 9 months will remain blinded to treatment group allocation. Participants will be asked not to disclose the foods they are consuming to the researchers. Researchers conducting assessments and analysing data will remain blinded until the completion of statistical analysis.
Sample size calculation
The study is powered on the primary outcomes of weight loss and weight regain. One hundred participants will provide 80% power to detect a 2.4 kg difference in weight loss18 and a 1.7 kg difference in weight regain (based on variance in our pilot data) (Wilson AL et al. Nudging Weight Loss Maintenance in Adults with Type-2 Diabetes: A Pilot Intervention) between the two groups (α-level of 0.05). One hundred and thirty-four participants will be recruited (n=67 in each group) to allow for a 25% dropout.
Preintervention
Two weeks prior to baseline visits participants will be asked to attend a pre-baseline session. During this visit, a flash blood glucose monitoring sensor (FreeStyle Libre, Abbott, Australia) will be inserted for collection of blood glucose measures for 14 days, a wrist-worn accelerometer (GENEActiv, Activinsights, UK) will be provided for measuring physical activity and sleep patterns for 14 days and a test kit will be provided for a single stool sample collection. Participants will be asked to keep a 4-day weighed food diary (non-consecutive days with 1 weekend day) and a 14-day sleep diary. Several questionnaires will also be administered to assess eating behaviour, mood, stress and personality (see table 1).
Study intervention
The intervention will be a 9-month protocol, consisting of 3 months weight loss and 6 months weight maintenance. During the 9-month study period, participants in the AED will incorporate 15% of their energy as unsalted, whole, natural almonds with skins while participants in the NFD will include 15% of their energy consumed as carbohydrate-rich snack foods (oven-baked fruit cereal bar and rice crackers) as part of a 30% energy restricted weight loss diet. It is expected that the minimum quantity of almonds required to contribute 15% of energy will be 30 g, which is consistent with dietary guidelines.47 The control foods have been selected as they are commonly consumed snacks, and do not contain the beneficial micro and macro-nutrients available in almonds but can provide equal energy density (see table 2). Participants will be provided with test foods to consume 6 days per week so that they have 1 day per week free from consuming test food. This has previously been found to support compliance.48 Checklists will be used to record daily consumption of study food and participants will be asked to return leftover food to calculate compliance scores. The threshold of compliance with test food consumption is >80%. All participants will be asked to avoid all other nuts and nut products during the entire study.
Table 2.
Macronutrient composition of test foods
| Per 100 g | Unsalted, whole, natural almonds with skin | Weight watchers apple crumble bar | Rice crackers, white rice, other |
| Energy (kJ) | 2385 | 1270 | 1646 |
| Protein (g) (%) | 19.7 (14.0) | 4.4 (5.9) | 9.4 (9.8) |
| Total fat (g) (%) | 50.5 (78.3) | 1.0 (2.9) | 5.6 (12.5) |
| Saturated fat (g) | 3.8 | 0.3 | 1.4 |
| Polyunsaturated fat (g) | 12.8 | 0.3 | 1.2 |
| Monounsaturated fat (g) | 30.7 | 0.3 | 2.6 |
| Carbohydrate (g) (%) | 5.4 (3.6) | 55.7 (72.4) | 74.6 (76.8) |
| Sugars (g) | 5.2 | 27.5 | 1.7 |
| Starch (g) | 0.2 | 28.2 | 72.8 |
| Fibre (g) (%) | 10.9 (3.7) | 14.7 (9.3) | 1.8 (0.9) |
Foods analysed in Foodworks Nutritional Analysis Software V.9 (Xyris Software, Brisbane, Queensland, Australia).
Energy requirements will be determined by using the Schofield equation, based on age, sex and baseline body weight, as well as self-reported physical activity captured via the International Physical Activity Questionnaire (IPAQ).49 Energy recommendation for weight loss will be 30% less than requirements to achieve 0.5–1 kg weight loss per week. Participants will be guided to consume a variety of foods within all five food groups to ensure they are still meeting nutrient reference guidelines consistent with Australian Dietary Guidelines.47 Participants will be provided with food group serve advice consistent with an energy restriction plan closest to their weight loss energy requirement of either 1200, 1500 or 1800 kcal/day (5000, 6300 or 7600 kJ/day). Weight loss plans will be adapted if more energy is required. We will lend participants a set of kitchen food scales to weigh food to ensure serve size accuracy. Three sample meal plans will be provided, as well as a recommended discretionary serve allowance of two per week, consistent with their energy requirement. Diet checklists will be used to assist with dietary compliance. During the weight maintenance phase, participants will be encouraged to stabilise their weight by increasing their overall energy intake by ~120–180 kcal/day (~500–750 kJ/day), with additional adjustments as required through dietetic consultation.
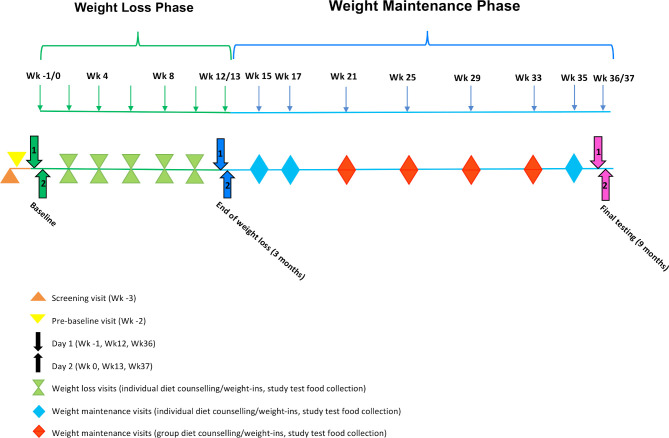
Participants will attend two baseline appointments (day 1, –1 week and day 2, 0 week before intervention) and will be asked to refrain from alcohol for 24 hours and fast for a minimum of 10 hours prior to assessments on both days. Day 1 and day 2 testing will occur again at 3 months and at 9 months. Details of tests, assessments and outcome measures completed on days 1 and 2 (baseline, 3 months and 9 months) are available in table 1. Participants will meet with the study dietitian at the end of the baseline day two appointment to receive initial dietary counselling and instructions on test food consumption requirements. They will then meet individually with a dietitian every 2 weeks during the weight loss phase to have their weight monitored and test food compliance checked. During weight maintenance, participants will meet individually with the dietitian every 2 weeks for the first month and then monthly in small groups. Figure 1 outlines the study timeline. Adherence to energy-restricted diets will be assessed using 3×24-hour dietary recalls (via phone, at random times) during each phase, with weighed food diaries at baseline and at the end of the weight loss and weight maintenance phases. Participants will also be encouraged to meet national physical activity guidelines for Australian adults: 150–300 min (2½–5 hours) of moderate intensity physical activity or equivalent, per week.47 Accelerometer data, as well as self-reported activity via the IPAQ, will monitor whether ‘unexpected’ weight change might be explained by physical activity levels, and will assist in understanding the weight loss effects attributable to consuming almonds.
Figure 1.
Study Timeline.
Data collection
The following section outlines the data and biochemical samples being collected during the test periods (see table 1 for a summary).
Anthropometry
Anthropometric assessments will be taken at day 1 baseline, 3-month and 9-month appointments. All anthropometric assessments will be conducted with participants barefoot and wearing light clothing. Height will be measured twice to the nearest 1 mm with the average value calculated and recorded using a stadiometer at baseline (SECA 216 Height Measuring Rod, SECA). Body weight will be recorded to the nearest 100 g following an overnight fast and will be measured twice on each occasion using calibrated electronic scales (TANITA Ultimate Scale 2000, Tanita Corporation, Tokyo, Japan) and the average value calculated. The same scales will be used throughout the intervention. BMI will be calculated as weight/height squared (kg/m2). Waist circumference will be measured, according to the protocol of the International Society for the Advancement of Kinanthropometry,50 using a metal measuring tape at the midpoint between the lower costal (10th rib) border and the iliac crest. Three measurements will be taken unless they differ by more than 2% whereby a fourth measurement will be obtained. The mean of the measurements will be used for analysis. Body composition will be determined from a whole-body dual-energy X-ray absorptiometry (DXA) scan (Lunar ProdigyModel, GE Healthcare, Madison, Wisconsin, USA). Participants will wear a light disposable gown, and all external metal objects will be removed. Total body fat mass (%, kg), total body lean mass (%, kg), regional fat mass (arms, legs, trunk and abdominal fat mass, android, gynoid (kg)) and visceral adipose tissue amount and volume (kg and cm3) will be obtained using enCORE 2015 software (GE Healthcare enCORE V.13.31). visceral adipose tissue (VAT) mass is converted to volume (cm3) at 1 g=1.06 cm3 (GE, Europe). Precision estimates for iDXA measurements on the DXA scanner are 0.8% coefficient of variation (CV) for total fat mass and 0.5% CV for VAT mass.51
In addition to the measurements taken at baseline, at the end of weight loss and weight maintenance phases, non-fasting weight will also be measured during dietetic counselling appointments to provide feedback to participants. We will also lend participants scales (Withings/Nokia WBS06, Nokia) with Bluetooth capacity to enable them to monitor their weight at least two times per week at home and for these data to be sent to research staff to assist with weight monitoring. Regular weight monitoring has been shown to enhance success in weight loss52 and weight maintenance trials.53
Biochemical measures
At day 1 baseline, 3-month and 9-month appointments, fasting (>10 hours) venous blood samples will be taken by a trained phlebotomist. Collected blood samples will be centrifuged (4°C, 4000 rpm, 10 min) to separate plasma or serum and stored at −80°C for later analysis (see table 3).
Table 3.
Blood, urine and faecal analysis
| Parameter | Analysis method | Sample collected (additives) |
| TC | Vertical auto profile (VAP II)54 | Serum |
| HDL-C+subclass | Vertical auto profile (VAP II)54 | Serum |
| LDL-C+subclass | Vertical auto profile (VAP II)54 | Serum |
| IDL-C+subclass | Vertical auto profile (VAP II)54 | Serum |
| VLDL-C+subclass | Vertical auto profile (VAP II)54 | Serum |
| Lipoprotein(a) | Vertical auto profile (VAP II)54 | Serum |
| Oxidised LDL-C | Solid phase 2-site ELISA | Serum |
| LDL particle number and size | Nuclear magnetic resonance spectroscopy55 | Serum |
| Triglyceride | Konelab Auto Analyser | Plasma |
| APOB | Vertical auto profile (VAP II)54 and patented equations20 | Serum |
| APOA1 | Vertical auto profile (VAP II)54 and patented equations20 | Serum |
| hs-CRP | Konelab Auto Analyser | Serum |
| Adiponectin | ELISA | Serum |
| F2-isoprostanes | Electron-capture negative-ion gas chromatography–mass spectrometry56 | Plasma+urine (butylated hydroxytoluene) |
| Alpha-tocopherol | High-performance liquid chromatography using the photo-diode array method60 | Plasma |
| ALT | Abbott Alinity C | Serum |
| AST | Abbott Alinity C | Serum |
| ALP | Abbott Alinity C | Serum |
| GGT | Abbott Alinity C | Serum |
| Glucose | Konelab Auto Analyser | Plasma |
| Insulin | Mercodia ELISA | Plasma (protease inhibitor and DPP-IV) |
| HOMA | Calculated using the Homeostasis Model Assessment Calculator V.2.3.3104 | – |
| Glucagon | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| GLP-1 | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| Ghrelin | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| Leptin | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| Pancreatic polypeptide | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| GIP | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| PYY | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| C-peptide | Multiplex analysis system | Plasma (protease inhibitor and DPP-IV) |
| CCK | ELISA (Ray Biotech) | Plasma (protease inhibitor and DPP-IV) |
| Total daily energy expenditure | Doubly labelled water | Urine |
| Faecal microbiota | MoBio Powerlyzer Powersoil DNA Isolation Kit | OMNIgene GUT DNA Stabilization Kit |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; APOA1, apolipoprotein A1; APOB, apolipoprotein B; AST, aspartate aminotransferase; CCK, cholecystokinin; DPP-IV, dipeptidyl peptidase-4; GGT, γ-glutamyltransferase; GIP, glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide); GLP-1, glucagon-like peptide-1; HDL-C, high-density lipoprotein-cholesterol; hs-CRP, high-sensitivity c-reactive protein; IDL-C, intermediate-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; PYY, peptide tyrosine tyrosine (peptide YY); TC, total cholesterol; VLDL-C, very low-density lipoprotein-cholesterol.
Lipids
Serum lipids, cholesterol lipoprotein subclasses and apolipoproteins will be assayed using vertical auto profile (VAP II) (Atherotech Diagnostics Lab, Birmingham, Alabama, USA), which directly measures cholesterol in all lipoprotein classes.54 Serum lipoprotein particle number and size will be assessed by a proton magnetic resonance spectroscopy assay (NMR LipoProfile III; LipoScience, Raleigh, North Carolina, USA), which measures the particle concentrations of lipoprotein subclasses and average particle size of lipoproteins.55 VAP II quantifies cholesterol concentrations of total lipoprotein, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), lipoprotein(a) (Lp(a)), intermediate-density lipoprotein (IDL), and HDL, LDL, VLDL and IDL subclasses. Apolipoprotein B and apolipoprotein A1 will be calculated using results from the VAP test and patented equations.20 Serum oxidised LDL will be measured in duplicate by a solid phase 2-site ELISA (Mercodia, Uppsala, Sweden). Plasma triglyceride (%CV <5%) will be measured using a Konelab Auto Analyser.
Inflammatory markers
Fasting plasma and a spot urine sample will be collected for analysis of F2-isoprostanes as biomarkers of oxidative stress. Samples will be stabilised with butylated hydroxytoluene. Plasma and urine F2-isoprostanes will be measured as total (free plus esterified) F2-isoprostanes using electron-capture negative-ion gas chromatography–mass spectrometry as described previously.56 Serum will be collected for assessment of adiponectin by ELISA (Linco Research, St. Charles, Missouri, USA)57 and high-sensitivity C-reactive protein (hs-CRP) (%CV intra-assay 2.9%, interassay 1.9%) will be measured using a Konelab Auto Analyser.
Liver enzymes
Fasting serum levels of alanine aminotransferase (ALT) (%CV intra-assay 0.5%, interassay 1.0%), aspartate aminotransferase (AST) (%CV intra-assay 0.6%, interassay 0.8%), alkaline phosphatase (ALP) (%CV intra-assay 0.3%, interassay 1.7%) and γ-glutamyltransferase (GGT) (%CV intra-assay 0.4%, interassay 1.1%) and will be measured using a local pathology service.23 To eliminate the effect of freeze-thawing of samples that may lower enzyme activity values, ALT, AST, ALP and GGT testing will be conducted on samples immediately transferred to the pathology laboratory.
Biomarkers of compliance
Compliance with long-term almond consumption will be confirmed by measuring alpha-tocopherol levels.58 59 Plasma alpha-tocopherol levels will be analysed using high-performance liquid chromatography with photo-diode array method according to Liu et al.60
Appetite regulation
At day 2 baseline, 3-month and 9-month appointments, objective and subjective measures of appetite and satiety will be assessed. A test snack will be consumed following an overnight fast (>10 hours). The test snack for the AED group will comprise 15% of daily energy intake from almonds, and the NFD group will have 15% of daily energy intake from a high carbohydrate snack (oven-baked fruit filled bar). Blood samples will be obtained at time 0 (before the test snack) and every 30 min for 2 hours post the test snack, with participants asked to consume the test snack within 10 min. Blood samples will be collected via a BD Nexiva cannula blood collection system by a trained phlebotomist. Collected blood samples will be centrifuged (4°C, 4000 rpm, 10 min) to separate plasma or serum and stored at −80°C for later analysis of gut hormones and glucose at all time points and triglycerides at time 0 min only (see table 3). Both groups will be given 200 mL of water to consume with their snack food. A further 300 mL of water will be provided over the 2-hour testing period. Participants will be required to drink all water provided. After 2 hours, a buffet meal will be provided, and participants will be advised to eat as much or as little as they like within 30 min. The buffet meal will be free of nuts and will provide a selection of core and non-core foods and beverages as defined by the Australian Dietary Guidelines for participants to select.47 The foods chosen from the buffet meal will be assessed for total energy consumed, macronutrient and micronutrient composition using Foodworks Nutritional Analysis Software V.9 (Xyris Software, Brisbane, Queensland, Australia).
A protease inhibitor cocktail (protease inhibitor (Sigma P2714) and DPP-IV (Millipore DPP4-010)) will be added immediately to the blood sample intended for testing; glucagon-like peptide-1, ghrelin, leptin, pancreatic polypeptide, glucose-dependent insulinotropic polypeptide, peptide tyrosine tyrosine, cholecystokinin (CCK), C-peptide, insulin and glucagon. The samples will be analysed using a multiplex analysis system (LUMINEX MAGPIX, ThermoScientific). CCK will be assessed using ELISA (Ray Biotech) (%CV intra-assay <10%, interassay <15%) and insulin will be assessed at baseline only using Mercodia ELISA (%CV <5%) and insulin resistance will be calculated using the Homeostasis Model Assessment (HOMA2) Calculator V.2.2.3 82. Glucose and triglycerides will be analysed via Konelab Auto Analyser (%CV <5%).
Subjective ratings of hunger, appetite and fullness will be measured by Visual Analogue Scale (VASs) at time 0 (before the test snack) and every 30 min for 2 hours post the test snack, as well as post buffet meal. VASs will be presented on separate sheets and recorded to the nearest mm. VAS responses will be recorded on a 100 mm line and measured to the nearest mm as the distance from the left hand anchor, with ‘not/none at all/no desire’ on the left and ‘extremely/great desire’ on the right. Questions will include ‘How hungry do you feel?’, ‘How thirsty do you feel?’, ‘How satisfied do you feel?’, ‘How full do you feel?’ and ‘How much do you think you can eat?’. The validity and reliability of this approach have previously been established.61 62 Completed VAS score sheets will be removed, so participants are not able to see their previous scores. While participants will not be blinded to the food consumed, the researchers evaluating VAS data will remain blinded. Area under the curve (AUC) for VAS (mm) responses will be plotted over time and calculated for each satiety/hunger measure using the trapezoidal estimation method.63
Accelerometry
Physical activity will be measured using triaxial accelerometers (GENEActiv Original, Activinsights), which will be worn on the non-dominant wrist. Participants will be asked to wear the monitor 24 hours/day for 14 consecutive days at pre-baseline, and 14 consecutive days before the end of the weight loss and weight maintenance phases (see table 1), removing it for showering/bathing or any other water-based activities. Devices will be configured through the manufacturer’s software (GENEActiv PC Software, Activinsights) to record at 50 Hz for 14 days, starting at midnight of the first day of the monitoring period.
Participants will be provided with a paper-based record sheet to document: (1) the time they went to bed (‘bedtime’) (2) the time they woke up (‘get up time’) and (3) the time the device was removed (‘non-wear’) and put back on again as well as the reason for removal (eg, showing).
After the device is returned, the research team will download the raw acceleration data through the manufacturer’s software. The signal vector magnitude of the acceleration, minus gravity, will be computed and summed over 60 s epochs:
, where ax, ay, az are the three components of the acceleration signal and g the acceleration of gravity (9.81 m/s2). The 60 s epoch data will then be imported into custom Matlab software for further processing. This software (Cobra, developed at the University of South Australia) provides a user-friendly graphical user interface for processing accelerometer data. Each 60 s epoch of waking wear time will be classified into one of four physical activity levels: sedentary, light, moderate or vigorous physical activity (PA). Cutpoints for PA levels are defined according to Esliger et al for adults64 and adjusted proportionally to account for the 50 Hz sampling frequency.65 The resulting cutpoints between sedentary and light, light and moderate, and moderate and vigorous PA are 188, 403 and 1131 gravity units per minute (g/min), respectively. Device removals (non-wear) will be identified using the self-reported records and excluded from analysis. Where the reason given for removal is ‘sport’, the removal period will be replaced with a period of moderate to vigorous physical activity.
Sleep will be identified using the self-reported records. Sleep times will be corrected by visual inspection when necessary, that is, in case sleep times were not reported or when obvious discrepancies were observed between reported sleep and accelerometer trace. Sleep quality will be assessed through total sleep time and sleep fragmentation.66 Each minute between ‘bed time’ and ‘get up time’ will be classified as sleep or wake using the algorithm developed by van Hees et al to detect wake periods during the night.67 Total sleep time is the sum of all sleep minutes between ‘bedtime’ and ‘get up time’. Sleep fragmentation is the ratio of total sleep time over time in bed.
All sleep and physical activity variables will be averaged over monitoring days for each participant. Averages over weekdays (Monday to Friday) and weekend days (Saturday, Sunday) will also be computed to assess any potential differences in physical activity between the two. A day will be considered invalid and excluded from analysis if it included ≤10 hours wear during waking hours.68 A participant will be considered invalid and excluded from analysis if they provide <4 valid days of accelerometry data over the 14-day testing period on any testing occation.68 69
Resting energy expenditure
At day 1 baseline, 3-month and 9-month appointments, resting energy expenditure (REE) will be measured using a ventilated hood system (TrueOne 2400 Metabolic System, ParvoMedics, Sandy, Utah, USA), which will be calibrated before each measurement using standardised gases. All testing will be conducted in the morning after a minimum 10 hours overnight fast. Testing will be performed in a thermo-neutral environment with participants lying supine in a comfortable position, head on a pillow and a transparent ventilated hood placed over their head. During the measurement period, participants will be asked to remain as relaxed as possible without falling asleep and instructed not to talk or fidget. Oxygen consumption (VO2) and carbon dioxide production (VCO2) will be measured continuously for 30 min. After discarding the first 10 min of data, REE will be calculated as the lowest consecutive 10 min average value, provided that the coefficient of variation within that 10 min interval is <5%. REE will be calculated using the Weir equation (metabolic rate (kcal per day)=1.44 (3.94 VO2+1.11 VCO2)).70–72
Doubly labelled water
Total daily energy expenditure during free-living conditions over 14 days (see table 1) will be quantified using the criterion doubly labelled water (DLW) technique at pre-baseline, and at day 1 3-month and 9-month appointments in a subsample of participants (n=24 total, 12 per group). Each participant will be asked if they would like to participate in DLW testing until the required number of participants is achieved. On each occasion, participants will be provided with a dose of isotope labelled water (10 atom% oxygen 18 (18O) and 99.9 atom% deuterium (2H)) with the dose based on body mass (1.35 g of DLW×body mass in kg). Participants will be asked to collect urine specimens daily over a 2-week period. Samples will be analysed by isotope ratio mass spectrometry. Total daily energy expenditure (kJ) over the 2-week period will be divided by 14 to estimate mean total daily energy expenditure.73 74
Blood pressure
At day 1 baseline, 3-month and 9-month appointments, seated blood pressure will be recorded in a controlled environment using an automated sphygmomanometer and appropriately sized cuffs after a 5 min quiet rest according to JNC (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) 7 guidelines.75 The same arm will be used for all assessment visits with the appropriately sized cuff. Four consecutive readings will be recorded at ~2 min intervals with the mean of the last three measurements used for analysis.
Flash glucose monitoring
Flash glucose monitoring will be used to assess dynamic changes in glucose over a 14-day period at pre-baseline and 14 days before the end of both the weight loss and weight maintenance periods (FreeStyle Libre Flash Glucose Monitoring System).76 Participants will wear a sensor on the back of their upper arm for up to 14 days and have a reader to scan the sensor every 6–8 hours. This system measures interstitial glucose concentrations and continuously stores measurement values every 15 min, which will provide information about postprandial glucose responses as well as changes in glucose regulation during weight loss. Average interstitial glucose and AUC77 will be calculated and evaluated at each time point (see table 1).
Dietary analysis
Adherence to energy-restricted diets will be assessed using 3×24 hours dietary recalls (via phone, at random times) during the weight loss and weight maintenance phases. Participants will also be asked to complete a 4-day weighed food record at pre-baseline, and 2 weeks before the end of both the weight loss and weight maintenance periods. Participants will be asked to record all foods and drinks consumed during this time and to record weights or estimate volumes using standard measures where possible and provide as much detail as possible about branded products. If required, we will lend participants a set of kitchen food scales.
Data will be collected on non-consecutive days and entered into Foodworks Nutritional Analysis Software V.8 (Xyris Software) for analysis of macronutrient and micronutrient intake as well as total energy intake.
Study food liking and palpability scores
A liking score for almonds and the carbohydrate-rich snack foods will be assessed using a Labelled Affect Magnitude Scale78 and a Food Action Rating Scale79 which will rate foods for overall liking, liking of textures and liking of flavours. These tests will occur at screening and at day 1 9 months to determine any change following long-term consumption of the test foods.
Eating behaviour, mood and personality
Eating behaviour, mood and personality will be assessed at day 1 and day 2 appointments at baseline, 3 months and 9 months (see table 1). Change over time will be assessed using a series of validated questionnaires. Eating behaviour via: Power of Food,80 Food Craving Scale,81 Eating Attitude Test,82 Pickiness/Finickiness Questionnaire,83 EAT-26,82 Control of Eating Questionnaire.84 Mood and personality via: Profile of Mood States,85 Perceived Stress Scale,86 Zung Self-Rating Scale,87 Eysenck Personality Questionnaire88 and Brief Sensation Seeking Scale.89
Quality of life, functional mobility and pain
The timed up and go (TUG) test is a test of functional mobility. This test requires participants to be timed while getting up, walking 3 m, turning, returning to the chair and sitting down again.90 Previous studies in adults have reported the CV error was 6% for the TUG test.91 The Short-Form 36 (SF36) questionnaire will be used for assessing overall quality of life, and pain will be assessed with the SF36 bodily pain subscale as well as a VAS to rate the intensity of pain at each major chronic and/or acute pain site. The nature of pain (at each site) will be rated using a short-form of the McGill Pain Questionnaire.92 All pain measures have been shown to be reliable and valid in adults,93 with the psychometrics of the SF36 specific to the Australian population.94 95 Assessments will occur at day 1 and day 2 appointments at baseline, 3 months and 9 months (see table 1).
Faecal microbiota
Stool samples will be collected at pre-baseline and 2 weeks before the end of the weight loss and weight maintenance phases using OMNIgene GUT DNA Stabilization Kits (DNA Genotek). DNA extraction will be performed using MoBio Powerlyzer Powersoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, California, USA) as described previously.96 DNA concentration will be quantified fluorometrically with a Qubit dsDNA HS Assay kit (Life Technologies). Faecal microbial composition will be assessed by 16S rRNA amplicon sequencing.97 This analysis will include determination of Firmicutes:Bacteroidetes ratios and quantification of Akkermansia muciniphila abundance. A subsample of highest responders (n=10) and lowest responders (n=10) (based on weight regain) will also undergo more detailed analysis of samples from pre-baseline, end of weight loss and end of weight maintenance. Samples will be assessed for identification of responder/non-responder phylogenetic and functional traits using shotgun metagenomic sequencing.98
Liver health
The amount of fat stored within the liver will be assessed using ultrasound at day 2 baseline, 3-month and 9-month appointments. Participants will have their livers imaged using a clinical ultrasound scanner (Philips iU22 Ultrasound Imaging System, Bothell, Washington, DC, USA) equipped with a 5–1 MHz high-resolution curved array transducer (Model c5-1, Philips). The amount of fat stored within the liver will be assessed using three different methods. First, the size of the liver will be determined using the technique by Childs et al.99 Three linear measurements of the liver will be taken and then volume calculated by using the following equation: liver volume (cm3) = 343.71+[0.84×ABC], where ABC is the product of the three linear measurements.99 Second, a visual assessment of fat accumulation will be evaluated according to the technique by Ballestri et al, adapted to liver images.100 A fatty liver indicator score ranging from 0 to 9 will be calculated using three indicators: (1) presence or absence of liver-kidney contrast; (2) assessment of beam penetration and (3) level of vessel wall blurring. Each measure will be graded as normal (score of 0), mild (score of 1), moderate (score of 2) or severe (score of 3).100 Finally, quantification of liver fibrosis, a consequence of fatty liver, will be performed using shear wave elastography. The ultrasound machine emits a low-frequency pulse and software inbuilt into the ultrasound machine indicates the density of the liver tissue. The more fibrotic the tissue, the denser the tissue and the higher the reading.101
Data management
All participants will be given a unique code, which will be used to identify their electronic and paper-based data and biological samples. A password-protected computer database, accessible by the researchers only, will store participant identifiers (eg, name, email address, phone number) and their associated code. Paper-based data will be stored securely at the University of South Australia for 15 years, after which it may be destroyed. Paper case report forms will be used to collect data at all clinic visits. Data will be entered twice into two separate password-protected Excel data files. Before analysis, data will be compared between files to ensure it has been correctly recorded. Biological samples will be stored in a swipe card secured −80°C freezer, with an alarm system that alerts a staff member if the temperature rises above a predetermined temperature. Samples will be stored for up to 15 years from collection date and disposed of accordingly after that time.
Protocol deviations
Deviations from the proposed protocol will be communicated via an update of the Australian and New Zealand Clinical Trial Registry and also through a letter to the editor of this journal.
Adverse events
Adverse events will be recorded in the case report form and will be reported to the University of South Australia Human Research Ethics Committee. Adverse events that lead to withdrawals will be reported in future publications. We do not intend to formally analyse adverse events.
Statistical analysis plan
Statistical analysis will be performed using SPSS for Windows V.24.0. Data will be tested for normality, and where possible non-normally distributed data will be log transformed prior to analysis. The effects of diet treatment on the dependent measures over time will be analysed using random effects mixed models, with treatment (AED or NFD) and time (baseline, end of weight loss and end of weight maintenance) being the between and within factors respectively in the analysis. Both intention-to-treat (ITT) and per-protocol analyses (for those who achieve a minimum of 80% compliance with test food consumption) will be completed. Where main effects are identified, Bonferroni post hoc tests will be performed to identify significant differences between means (p set at <0.05). While the ITT analysis will be the main analysis, the per-protocol analysis will allow us to decipher that the effects are due to participants being compliant with consuming test foods. We will also run sub-analyses for both the ITT and per-protocol analyses which will stratify participants according to weight loss during the weight loss phase to determine whether there is an interaction between dietary treatment and weight loss in terms of effects on the various outcomes. This will allow for determination of whether almonds, compared with control, provide greater improvements in outcomes for any given level of weight loss.
Data access
There are no contractual agreements that require the data from this trial to be shared.
Ethics and dissemination
Ethics approval was obtained from the University of South Australia Human Research Ethics Committee (201436).
Participants will receive a copy of their individual results (with the exception of liver scans, faecal sample testing, REE, DLW and some blood analysis results) as well as a summary of the study findings. Participants who complete all aspects of the study will receive an honorarium of $400 to compensate for their time and travel expenses. The subsample of participants who also participate in DLW assessments will be provided with an additional $150 honorarium. Findings of the study will be disseminated at scientific conferences and in published papers. Further, where appropriate outcomes will be made available to the public via media releases.
Discussion
Nut consumption in many countries is low, and it has been suggested that people may avoid eating nuts as they may perceive that the high-fat content will lead to weight gain.12 13 15 16 However, various mechanisms have been hypothesised regarding the association between nut consumption and weight loss success. Nuts are rich in protein, fibre and monounsaturated fat, macronutrients known to have a positive effect on appetite control.25 The high unsaturated fat content and protein in nuts may lead to an increase in REE and diet-induced thermogenesis.31 Additionally, the structure of the lipid-storing granules and high fibre content of nuts, as well as incomplete mastication may cause reduced fat absorption resulting in a loss of available energy.31 Despite this, clinical trials evaluating the effects of nut consumption compared with a nut-free diet on adiposity have reported mixed findings, with some studies reporting greater weight loss17 18 or improved body composition,19 20 and others reporting no weight change,1 5 9 suggesting further investigation is required.
Strengths
There are a number of novel features in this study design. To our knowledge, this will be the first trial to assess whether the inclusion of 15% of energy from almonds versus 15% of energy from carbohydrate-rich snack foods in an otherwise nut-free diet will improve weight loss and limit weight regain. As such, this work has the potential to expand the current understanding of how regular consumption of almonds can aid weight loss and weight maintenance, while also providing beneficial cardiometabolic, liver and gut health effects. Furthermore, the metagenomic analysis that will be performed will be a substantial advance on our current understanding of the impact of almonds on the gut microbiome (previously limited to amplicon sequencing approaches).102
Limitations
One of the main challenges with any dietary intervention study is recruiting participants and keeping them motivated. We have previously successfully recruited large numbers of participants for similar intervention trials and plan to support participants with regular appointments with the dietitian, as well as the provision of test foods and simple food checklists to assist with compliance. A potential limitation of this study is that it will only be feasible to follow participants for 6 months after initial weight loss, although data from our recent pilot study suggests that this will be sufficient to observe differential changes in weight regain due to the rapidity of weight regain once intensive dietary support is removed (Wilson AL et al. Nudging Weight Loss Maintenance in Adults with Type-2 Diabetes: A Pilot Intervention).
This 9-month randomised controlled trial will add to the evidence based strategies to facilitate weight loss and prevent regain. This may lead to recommendations to frequently substitute energy‐dense snacks that lack nutritional value with nuts, which, in turn, could lead to improved weight loss outcomes and facilitate beneficial dietary habits.13 103
Supplementary Material
Acknowledgments
Professor Kevin Croft, at The University of Western Australia, will analyse F2-isoprostanes as biomarkers of oxidative stress.
Footnotes
Contributors: AMC, JDB, AMH, S-YT, GBR were coapplicants on the grant application and as such were involved with the original design. AMC was the lead applicant and is the principal investigator for the study. AMC, JDB, AMH, SC, CY are involved with study coordination and responsible for the day to day running of the trial, recruitment and sample collection. All authors (SC, AMH, CY, JDB, S-YT, GBR, JC, MM, KL, SW, TRS, FF, AH, AMC) contributed to method development and the writing and development of the protocol paper and all authors (as above) will have responsibility for analysis, statistical interpretation of outcomes and preparation of manuscripts for publication post-study completion.
Funding: This work was funded by the Almond Board of California.
Disclaimer: This funding source had no role in the design of this study and will have no role in the analysis or interpretation of the data.
Competing interests: AMC has consulted for Nuts for Life (an initiative of the Australian Tree Nut Industry).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Casas-Agustench P, Bulló M, Ros E, et al. Cross-sectional association of nut intake with adiposity in a Mediterranean population. Nutr Metab Cardiovasc Dis 2011;21:518–25. 10.1016/j.numecd.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 2.Martínez-González MA, Bes-Rastrollo M. Nut consumption, weight gain and obesity: epidemiological evidence. Nutr Metab Cardiovasc Dis 2011;21(Suppl 1):S40–5. 10.1016/j.numecd.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Vadivel V, Kunyanga CN, Biesalski HK. Health benefits of nut consumption with special reference to body weight control. Nutrition 2012;28:1089–97. 10.1016/j.nut.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Freisling H, Noh H, Slimani N, et al. Nut intake and 5-year changes in body weight and obesity risk in adults: results from the EPIC-PANACEA study. Eur J Nutr 2018;57:2399-2408. 10.1007/s00394-017-1513-0 [DOI] [PubMed] [Google Scholar]
- 5.Flores-Mateo G, Rojas-Rueda D, Basora J, et al. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr 2013;97:1346–55. 10.3945/ajcn.111.031484 [DOI] [PubMed] [Google Scholar]
- 6.Bitok E, Rajaram S, Jaceldo-Siegl K, et al. Effects of long-term walnut supplementation on body weight in free-living elderly: results of a randomized controlled trial. Nutrients 2018;10. 10.3390/nu10091317. [Epub ahead of print: 18 Sep 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantino M, Bichard C, Mistretta F, et al. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: a randomized controlled intervention. Appetite 2020;144:104483. 10.1016/j.appet.2019.104483 [DOI] [PubMed] [Google Scholar]
- 8.Gulati S, Misra A, Pandey RM, et al. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition 2014;30:192–7. 10.1016/j.nut.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Blanco Mejia S, Kendall CWC, Viguiliouk E, et al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2014;4:e004660. 10.1136/bmjopen-2013-004660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajaram S, Sabaté J. Nuts, body weight and insulin resistance. Br J Nutr 2006;96 Suppl 2:S79–86. 10.1017/BJN20061867 [DOI] [PubMed] [Google Scholar]
- 11.Dhillon J, Thorwald M, De La Cruz N, et al. Glucoregulatory and cardiometabolic profiles of almond vs. cracker snacking for 8 weeks in young adults: a randomized controlled trial. Nutrients 2018;10. 10.3390/nu10080960. [Epub ahead of print: 25 Jul 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carughi A, Feeney MJ, Kris-Etherton P, et al. Pairing nuts and dried fruit for cardiometabolic health. Nutr J 2016;15:23. 10.1186/s12937-016-0142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neil CE, Nicklas TA, Fulgoni VL. Tree nut consumption is associated with better nutrient adequacy and diet quality in adults: National health and nutrition examination survey 2005-2010. Nutrients 2015;7:595–607. 10.3390/nu7010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Bureau of Statistics Australian health survey: nutrition first results – foods and nutrients: 2011–2. [Google Scholar]
- 15.Nuts for Life Australian nut consumption patterns from the National nutrition and physical activity survey released 2015. Personal communication with Lisa Yates 2016.
- 16.O'Neil CE, Keast DR, Nicklas TA, et al. Nut consumption is associated with decreased health risk factors for cardiovascular disease and metabolic syndrome in U.S. adults: NHANES 1999-2004. J Am Coll Nutr 2011;30:502–10. 10.1080/07315724.2011.10719996 [DOI] [PubMed] [Google Scholar]
- 17.Wien MA, Sabaté JM, Iklé DN, et al. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord 2003;27:1365–72. 10.1038/sj.ijo.0802411 [DOI] [PubMed] [Google Scholar]
- 18.Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J Res Med Sci 2014;19:457–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Dhillon J, Tan S-Y, Mattes RD. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J Nutr 2016;146:2513–9. 10.3945/jn.116.238444 [DOI] [PubMed] [Google Scholar]
- 20.Berryman CE, West SG, Fleming JA, et al. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J Am Heart Assoc 2015;4:e000993. 10.1161/JAHA.114.000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han JM, Jo AN, Lee SM, et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 2014;29:1265–72. 10.1111/jgh.12520 [DOI] [PubMed] [Google Scholar]
- 22.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int 2017;37:936–49. 10.1111/liv.13435 [DOI] [PubMed] [Google Scholar]
- 23.Abazarfard Z, Eslamian G, Salehi M, et al. A randomized controlled trial of the effects of an Almond-enriched, hypocaloric diet on liver function tests in overweight/obese women. Iran Red Crescent Med J 2016;18:e23628. 10.5812/ircmj.23628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bes-Rastrollo M, Sabaté J, Gómez-Gracia E, et al. Nut consumption and weight gain in a Mediterranean cohort: the sun study. Obesity 2007;15:107–16. 10.1038/oby.2007.507 [DOI] [PubMed] [Google Scholar]
- 25.Jackson CL, Hu FB. Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutr 2014;100(Suppl 1):408S–11. 10.3945/ajcn.113.071332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell NS, Catenacci VA, Wyatt HR, et al. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32. 10.1016/j.psc.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turk MW, Yang K, Hravnak M, et al. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 2009;24:58–80. 10.1097/01.JCN.0000317471.58048.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aller EEJG, Larsen TM, Claus H, et al. Weight loss maintenance in overweight subjects on AD libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes 2014;38:1511–7. 10.1038/ijo.2014.52 [DOI] [PubMed] [Google Scholar]
- 30.van Baak MA, Larsen TM, Jebb SA, et al. Dietary intake of protein from different sources and weight regain, changes in body composition and cardiometabolic risk factors after weight loss: the DIOGenes study. Nutrients 2017;9. 10.3390/nu9121326. [Epub ahead of print: 06 Dec 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan SY, Dhillon J, Mattes RD. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am J Clin Nutr 2014;100(Suppl 1):412S–22. 10.3945/ajcn.113.071456 [DOI] [PubMed] [Google Scholar]
- 32.Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr 2013;67:1205–14. 10.1038/ejcn.2013.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori AM, Considine RV, Mattes RD. Acute and second-meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutr Metab 2011;8:6. 10.1186/1743-7075-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hull S, Re R, Chambers L, et al. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur J Nutr 2015;54:803–10. 10.1007/s00394-014-0759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rock CL, Flatt SW, Barkai H-S, et al. A walnut-containing meal had similar effects on early satiety, CCK, and PYY, but attenuated the postprandial GLP-1 and insulin response compared to a nut-free control meal. Appetite 2017;117:51–7. 10.1016/j.appet.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalton M, Finlayson G, Walsh B, et al. Early improvement in food cravings are associated with long-term weight loss success in a large clinical sample. Int J Obes 2017;41:1232–6. 10.1038/ijo.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Million M, Angelakis E, Maraninchi M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes 2013;37:1460–6. 10.1038/ijo.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Ott B, Skurk T, Hastreiter L, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep 2017;7:11955. 10.1038/s41598-017-12109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns AM, Zitt MA, Rowe CC, et al. Diet quality improves for parents and children when almonds are incorporated into their daily diet: a randomized, crossover study. Nutr Res 2016;36:80–9. 10.1016/j.nutres.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Lin X, Huang G, et al. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014;26:1–6. 10.1016/j.anaerobe.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Ukhanova M, Wang X, Baer DJ, et al. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br J Nutr 2014;111:2146–52. 10.1017/S0007114514000385 [DOI] [PubMed] [Google Scholar]
- 42.Holscher HD, Taylor AM, Swanson KS, et al. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: a randomized controlled trial. Nutrients 2018;10:126. 10.3390/nu10020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 2017;13:11–25. 10.1038/nrendo.2016.150 [DOI] [PubMed] [Google Scholar]
- 44.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330:843. 10.1136/bmj.330.7495.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taves DR. The use of minimization in clinical trials. Contemp Clin Trials 2010;31:180–4. 10.1016/j.cct.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 47.National Health and Medical Research Council Australian dietary guidelines. Canberra: National Health and Medical Research Council, 2013. [Google Scholar]
- 48.Barbour JA, Howe PRC, Buckley JD, et al. Lower energy intake following consumption of Hi-oleic and regular peanuts compared with iso-energetic consumption of potato crisps. Appetite 2014;82:124–30. 10.1016/j.appet.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 49.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 50.Norton K, Olds T, Anthropometrica : a textbook of body measurement for sports and health courses. Sydney, Australia: UNSW Press, Australian Sports, 1996. [Google Scholar]
- 51.Swainson MG, Batterham AM, Hind K. Age- and sex-specific reference intervals for visceral fat mass in adults. Int J Obes 2020;44:289–96. 10.1038/s41366-019-0393-1 [DOI] [PubMed] [Google Scholar]
- 52.Burke LE, Wang J, Sevick MA. Self-Monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011;111:92–102. 10.1016/j.jada.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crain AL, Sherwood NE, Martinson BC, et al. Mediators of weight loss maintenance in the keep it off trial. Ann Behav Med 2018;52:9–18. 10.1007/s12160-017-9917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med 2006;26:787–802. 10.1016/j.cll.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 55.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 2002;48:171–80. [PubMed] [Google Scholar]
- 56.Barden AE, Mas E, Croft KD, et al. Minimizing artifactual elevation of lipid peroxidation products (F2-isoprostanes) in plasma during collection and storage. Anal Biochem 2014;449:129–31. 10.1016/j.ab.2013.12.030 [DOI] [PubMed] [Google Scholar]
- 57.Hill AM, Coates AM, Buckley JD, et al. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr 2007;26:396S–402. 10.1080/07315724.2007.10719628 [DOI] [PubMed] [Google Scholar]
- 58.Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr 2007;98:651–6. 10.1017/S0007114507734608 [DOI] [PubMed] [Google Scholar]
- 59.Li S-C, Liu Y-H, Liu J-F, Chang W-H, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9. 10.1016/j.metabol.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Lee H-J, Garofalo F, Jenkins DJ, et al. Simultaneous measurement of three tocopherols, all-trans-retinol, and eight carotenoids in human plasma by isocratic liquid chromatography. J Chromatogr Sci 2011;49:221–7. 10.1093/chrsci/49.3.221 [DOI] [Google Scholar]
- 61.Jamison RN, Gracely RH, Raymond SA, et al. Comparative study of electronic vs. paper vas ratings: a randomized, crossover trial using healthy volunteers. Pain 2002;99:341–7. 10.1016/S0304-3959(02)00178-1 [DOI] [PubMed] [Google Scholar]
- 62.Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]
- 63.Doucet E, St-Pierre S, Alméras N, et al. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite 2003;40:137–43. 10.1016/S0195-6663(02)00143-5 [DOI] [PubMed] [Google Scholar]
- 64.Esliger DW, Rowlands AV, Hurst TL, et al. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085–93. 10.1249/MSS.0b013e31820513be [DOI] [PubMed] [Google Scholar]
- 65.Fraysse F, Grobler AC, Muller J, et al. Physical activity and sedentary activity: population epidemiology and concordance in Australian children aged 11-12 years and their parents. BMJ Open 2019;9:136–46. 10.1136/bmjopen-2018-023194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matricciani L, Fraysse F, Grobler AC, et al. Sleep: population epidemiology and concordance in Australian children aged 11-12 years and their parents. BMJ Open 2019;9:127–35. 10.1136/bmjopen-2017-020895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a Wrist-Worn Accelerometer. PLoS One 2015;10:e0142533. 10.1371/journal.pone.0142533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–8. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 69.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017;47:1821–45. 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weir JBDEB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davison K, Coates AM, Buckley JD, et al. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes 2008;32:1289–96. 10.1038/ijo.2008.66 [DOI] [PubMed] [Google Scholar]
- 72.Tan S-Y, Peh E, Lau E, et al. Physical form of dietary fat alters postprandial substrate utilization and glycemic response in healthy Chinese men. J Nutr 2017;147:1138–44. 10.3945/jn.116.246728 [DOI] [PubMed] [Google Scholar]
- 73.Bluck LD FE, Hills A, Kurpad A. Assessment of body composition and total energy expenditure in humans using stable isotope technique. Vienna: International Atomic Energy Agency, 2009. [Google Scholar]
- 74.Tanhoffer RA, Tanhoffer AIP, Raymond J, et al. Comparison of methods to assess energy expenditure and physical activity in people with spinal cord injury. J Spinal Cord Med 2012;35:35–45. 10.1179/2045772311Y.0000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 76.Bailey T, Bode BW, Christiansen MP, et al. The performance and usability of a Factory-Calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787–94. 10.1089/dia.2014.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective flash glucose monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol 2016;10:1294–302. 10.1177/1932296816648165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawless HT, Sinopoli D, Chapman KW. A comparison of the labeled affective magnitude scale and the 9‐point hedonic scale and examination of categorical behavior. J Sens Stud 2010;25:54–66. [Google Scholar]
- 79.Schutz HG. Food action rating scale for measuring food acceptance. J Food Sci 1965;30:365–74. 10.1111/j.1365-2621.1965.tb00316.x [DOI] [Google Scholar]
- 80.Cappelleri JC, Bushmakin AG, Gerber RA, et al. Evaluating the power of food scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes 2009;33:913–22. 10.1038/ijo.2009.107 [DOI] [PubMed] [Google Scholar]
- 81.Cepeda-Benito A, Gleaves DH, Fernández MC, et al. The development and validation of Spanish versions of the state and trait food cravings questionnaires. Behav Res Ther 2000;38:1125–38. 10.1016/S0005-7967(99)00141-2 [DOI] [PubMed] [Google Scholar]
- 82.Garner DM, Garfinkel PE. The eating attitudes test: an index of the symptoms of anorexia nervosa. Psychol Med 1979;9:273–9. 10.1017/S0033291700030762 [DOI] [PubMed] [Google Scholar]
- 83.Raudenbush B, van der Klaauw NJ, Frank RA. The contribution of psychological and sensory factors to food preference patterns as measured by the food attitudes survey (Fas). Appetite 1995;25:1–15. 10.1006/appe.1995.0037 [DOI] [PubMed] [Google Scholar]
- 84.Dalton M, Finlayson G, Hill A, et al. Preliminary validation and principal components analysis of the control of eating questionnaire (CoEQ) for the experience of food craving. Eur J Clin Nutr 2015;69:1313–7. 10.1038/ejcn.2015.57 [DOI] [PubMed] [Google Scholar]
- 85.McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Services, 1971. [Google Scholar]
- 86.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 87.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70. 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 88.Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Pers Individ Dif 1985;6:21–9. 10.1016/0191-8869(85)90026-1 [DOI] [Google Scholar]
- 89.Stephenson MT, Hoyle RH, Palmgreen P, et al. Brief measures of sensation seeking for screening and large-scale surveys. Drug Alcohol Depend 2003;72:279–86. 10.1016/j.drugalcdep.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 90.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 91.Zhu K, Kerr DA, Meng X, et al. Two-Year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J Nutr 2015;145:2520–6. 10.3945/jn.115.218297 [DOI] [PubMed] [Google Scholar]
- 92.Melzack R. The short-form McGill pain questionnaire. Pain 1987;30:191–7. 10.1016/0304-3959(87)91074-8 [DOI] [PubMed] [Google Scholar]
- 93.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (vas pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CpGs), short Form-36 bodily pain scale (SF-36 BPs), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63(Suppl 11):S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 94.Sanson-Fisher RW, Perkins JJ. Adaptation and validation of the SF-36 health survey for use in Australia. J Clin Epidemiol 1998;51:961–7. 10.1016/S0895-4356(98)00087-0 [DOI] [PubMed] [Google Scholar]
- 95.McCallum J. The SF-36 in an Australian sample: validating a new, generic health status measure. Aust J Public Health 1995;19:160–6. 10.1111/j.1753-6405.1995.tb00367.x [DOI] [PubMed] [Google Scholar]
- 96.Choo JM, Leong LEX, Rogers GB. Sample storage conditions significantly influence faecal microbiome profiles. Sci Rep 2015;5:16350. 10.1038/srep16350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leong LEX, Taylor SL, Shivasami A, et al. Intestinal microbiota composition in sudden infant death syndrome and age-matched controls. J Pediatr 2017;191:63–8. 10.1016/j.jpeds.2017.08.070 [DOI] [PubMed] [Google Scholar]
- 98.Nielsen HB, Almeida M, Juncker AS, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol 2014;32:822–8. 10.1038/nbt.2939 [DOI] [PubMed] [Google Scholar]
- 99.Childs JT, Esterman AJ, Thoirs KA, et al. Ultrasound in the assessment of hepatomegaly: a simple technique to determine an enlarged liver using reliable and valid measurements. Sonography 2016;3:47–52. 10.1002/sono.12051 [DOI] [Google Scholar]
- 100.Ballestri S, Lonardo A, Romagnoli D, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int 2012;32:1242–52. 10.1111/j.1478-3231.2012.02804.x [DOI] [PubMed] [Google Scholar]
- 101.Gherlan GS. Liver ultrasound elastography: more than staging the disease. World J Hepatol 2015;7:1595–600. 10.4254/wjh.v7.i12.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dhillon J, Li Z, Ortiz RM. Almond snacking for 8 wk increases Alpha-Diversity of the gastrointestinal microbiome and decreases Bacteroides fragilis abundance compared with an isocaloric snack in college Freshmen. Curr Dev Nutr 2019;3:nzz079. 10.1093/cdn/nzz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown RC, Yong LC, Gray AR, et al. Perceptions and knowledge of nuts amongst health professionals in New Zealand. Nutrients 2017;9:220. [Google Scholar]
- 104.University of Oxford Diabetes Trial Unit [Internet]. Available: http://www.dtu.ox.ac.uk/homacalculator/index.php
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.