SUMMARY
Inhibition of anti-apoptotic proteins BCL-2 and MCL-1 to release pro-apoptotic protein BIM and reactivate cell death could potentially be an efficient strategy for the treatment of leukemia. Here, we show that a lncRNA, MORRBID, a selective transcriptional repressor of BIM, is overexpressed in human acute myeloid leukemia (AML), which is associated with poor overall survival. In both human and animal models, MORRBID hyperactivation correlates with two recurrent AML drivers, TET2 and FLT3ITD. Mice with individual mutations of Tet2 or Flt3ITD develop features of chronic myelomonocytic leukemia (CMML) and myeloproliferative neoplasm (MPN), respectively, and combined presence results in AML. We observe increased levels of Morrbid in murine models of CMML, MPN, and AML. Functionally, loss of Morrbid in these models induces increased expression of Bim and cell death in immature and mature myeloid cells, which results in reduced infiltration of leukemic cells in tissues and prolongs the survival of AML mice.
Graphical Abstract

In Brief
Cai et al. report that MORRBID/Morrbid expression is aberrantly increased in human AML patients and mouse models for CMML, MPN, and AML. Genetic loss of Morrbid makes leukemic cells vulnerable to apoptosis and mitigates the progression of myeloid neoplasms. High expression of MORRBID in humans is associated with poor survival of AML patients.
INTRODUCTION
One of the well-recognized hallmarks of cancer, including acute myeloid leukemia (AML), is its capability to evade cell death, which may also act as an underlying mechanism of drug resistance and/or tumor relapse in certain cancer therapies (Hanahan and Weinberg, 2011; McBride et al., 2019; Merino et al., 2018). It has been almost 30 years since the identification of BCL-2 and its family members in regulating cell apoptosis (anti-apoptosis: BCL-2, BCL-lnctnXL, and MCL-1; pro-apoptosis: BIM, BID, BAX, and BAK). The anti-apoptosis proteins of the BCL-2 family execute their function by sequestering pro-apoptosis proteins and preventing the creation of pores in the mitochondrial outer membrane via protein-protein interactions (Ashkenazi et al., 2017; Huang et al., 2019; Yang et al., 2019). Repressing the expression of anti-apoptosis protein via gene silencing or inhibiting such protein-protein interaction via BH3 mimetics are therefore emerging as novel targeting treatments for cancer, including several hematological malignancies in which BCL-2 and/or MCL-1 are aberrantly activated in leukemic stem cells (LSCs) or leukemic blasts. Indeed, venetoclax (ABT-199), an oral BCL-2 inhibitor, has been approved for the treatment of chronic lymphocytic leukemia and elderly patients with AML (DiNardo et al., 2019). Similarly, inhibition of MCL-1 via S64315 or AMG-176 as a single-agent therapy or in combination with BCL-2 inhibitor and other drugs is in clinical trials for AML treatment (Anstee et al., 2019; Caenepeel et al., 2018; Ramsey et al., 2018; Teh et al., 2018). Although BCL-2 itself is an important player in tumorigenesis and represents an important therapeutic target, in vivo stimulation of the expression of pro-apoptosis proteins in AML treatment (e.g., BIM) has not been studied in preclinical models (Shukla et al., 2017).
AML is a genetically and cellularly heterogenous clonal blood cancer caused by driver mutations that are able to transform hematopoietic stem cells (HSCs) to LSCs. The most prevailing ones include acute myelomonocytic leukemia (French-American-British [FAB] classification, M4) and acute monocytic leukemia (M5), where both mature myeloid cells and progenitors are leukemic and redundant. The recurrent mutations in M4 and M5 AML include genes encoding components of the signaling pathway and epigenetic regulations, such as the gain-of-function mutation FLT3ITD/+ and the loss-of-function mutation TET2+/−. Indeed, using murine models, recent studies suggest that mutations in Tet2 define a preleukemic condition in HSCs and may induce clonal hematopoiesis of indeterminate potential (CHIP), which is a strong risk factor for blood cancer (Jaiswal and Ebert, 2019). Acquisition of additional mutations such as Flt3ITD/+ in preleukemic cells bearing loss of Tet2 results in the development of full-blown AML (Shih et al., 2015). Furthermore, the Tet2−/−; Flt3ITD/ITD model of AML, the preleukemic model bearing Tet2 loss, and a murine model expressing a knockin allele of Flt3ITD manifest several cardinal features of human AML, chronic myelomonocytic leukemia (CMML), and myeloproliferative neoplasm (MPN), respectively (Chu et al., 2012; Moran-Crusio et al., 2011; Shih et al., 2015). Use of these clinically relevant models of myeloid neoplasm allow for validation of targets that are likely to provide best insight into human leukemia and its genetic drivers.
We have recently shown that the evolutionarily conserved novel long non-coding RNA (lncRNA) Morrbid is specifically expressed in myeloid cells and uniquely represses the expression of the pro-apoptotic gene Bim via DNA loop in cis to regulate the lifespan of myeloid cells (Kotzin et al., 2016). Morrbid−/− mice manifest inefficient production of innate immune cells (~50% of wild-type [WT] levels) but have normal activity and lifespan (Kotzin et al., 2016). Since Morrbid is specifically expressed in myeloid cells (i.e., neutrophils [NEs], monocytes [MOs], and eosinophils), but not in other cell types under physiologic conditions, the uniqueness of its expression pattern and function offers a great advantage and opportunity to test if it is required for the onset and progression of myeloid-related diseases, including various myeloid neoplasms. In the present study, we have characterized the role of lncRNA MORRBID/Morrbid and its target, BIM, in regulating the survival of preleukemic and leukemic cells.
RESULTS AND DISCUSSION
Role of Morrbid in Murine Models of CMML and MPN
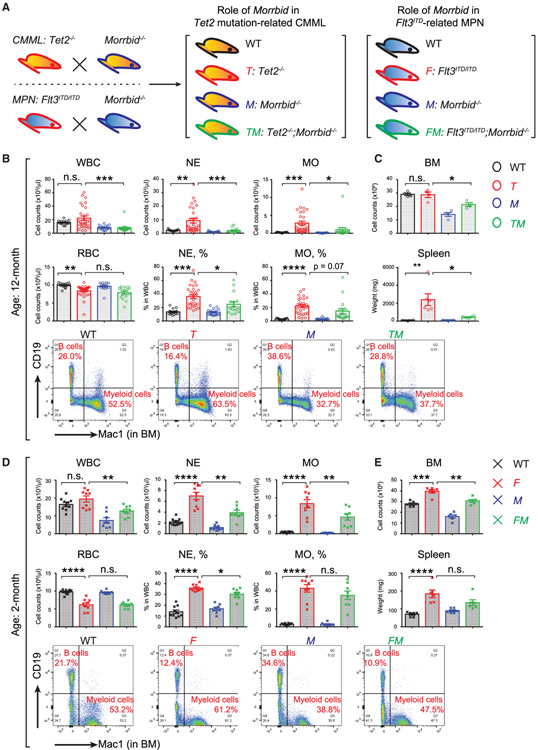
Our recent study has defined the role of Morrbid in inflammation and Tet2-mutation induced aberrant emergency granulopoiesis and clonal hematopoiesis (Cai et al., 2018). We here first compared the role of Morrbid in driving Tet2-mutation-induced CMML and Flt3ITD-mutation-induced MPN over an adequate lifetime, since CMML is a chronic disease in the elderly population (Figure 1A; Chu et al., 2012; Lee et al., 2007; Mead et al., 2013; Mead et al., 2017; Moran-Crusio et al., 2011). We generated Tet2−/−; Morrbid−/− double homozygous mice along with all the controls. A large cohort of these mice was allowed to age and subsequently analyzed for peripheral blood (PB) changes. Consistent with previous studies, as seen in Figure 1B, although the average count of white blood cells (WBCs) in aged Tet2−/− mice is higher than that in WT controls, the counts of WBCs in each Tet2−/− individual mouse are highly variable relative to controls, suggesting that the onset and penetration of the CMML-like disease in each individual is different in the large cohort. However, the increase in average value of WBC counts observed in Tet2 deficient mice was normalized and not highly variable in Tet2−/−; Morrbid−/− mice (Figure 1B). Importantly, a similar and significant reversal in the increase in absolute number of NEs and MOs was observed in Tet2−/−; Morrbid−/− mice compared to Tet2−/− mice. Likewise, the increase in the frequency of NEs and MOs observed in Tet2−/− mice was also rescued in the absence of Morrbid, along with the decrease in bone marrow (BM) cellularity and spleen weight (Figure 1B). Overall, while the percentages of NEs and MOs in Tet2−/−; Morrbid−/− mice remained higher than WT controls, they were significantly lower than that observed in Tet2−/− mice (Figure 1B). In contrast, no improvement in red blood cell (RBC) counts was observed in Tet2−/−; Morrbid−/− mice, suggesting that Morrbid does not impact the reduction in RBCs observed in aged Tet2−/− mice (Figure 1B).
Figure 1. Morrbid’s Role in Tet2-Mutation-Induced CMML and Flt3ITD-induced MPN.
(A) Schematic for generating Tet2−/−; Morrbid−/− (TM) and Flt3ITD/ITD; Morrbid−/− (FM) compound mutant mice along with controls.
(B and C) Examination of peripheral blood (PB), bone marrow (BM), and spleen in the four experimental groups: wild type (WT), Tet2−/− (T), Morrbid−/− (M), and TM (age, 12 months).
(D and E) Examination of PB, BM, and spleen in the four experimental groups: WT, Flt3ITD/ITD (F), Morrbid−/− (M), and FM (age, 2 months).
(B and D) Plots of PB cell counts (upper panel) and representative profiles of flow cytometry of mature myeloid cells (Mac1+) and B cells (CD19+) from BM (lower panel).
(C and, E) quantification of BM cellullarity and spleen weight.
n = ~4–19. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Compared to Flt3ITD/+ mice, Flt3ITD/ITD mice develop an early onset and full penetration of MPN-like phenotype via activation of STAT signaling, which eventually results in the activation of anti-apoptotic proteins BCL-2 or MCL-1 (Chu et al., 2012; Kikushige et al., 2008; Mead et al., 2013, 2017; Naganna et al., 2019). These mice develop several cardinal features of human MPN-like disease, including enhanced NE and MO counts, splenomegaly, and hyperceullular BM (Figures 1D and 1E). To determine the extent to which these changes are regulated by Morrbid-induced Bim repression, we generated mice lacking Morrbid and expressing Flt3ITD/ITD (Flt3ITD/ITD; Morrbid−/−). Similar to the results seen in the Tet2-deficiency-driven CMML model lacking Morrbid, we observed that loss of Morrbid in the setting of Flt3ITD/ITD expression rescued the abnormal phenotypes observed in Flt3ITD/ITD, including high NE and MO counts in PB (Figure 1D). Interestingly, while RBC and spleen size were not significantly corrected in the Flt3ITD/ITD mice lacking Morrbid, BM hypercellularity is completely rescued (Figures 1D and 1E). Taken together, these results suggest that Morrbid loss is able to partially correct the CMML and MPN phenotype in the setting of two distinct AML driver mutations, Tet2 and Flt3ITD.
Role of MORRBID/Morrbid in Human and Murine AML
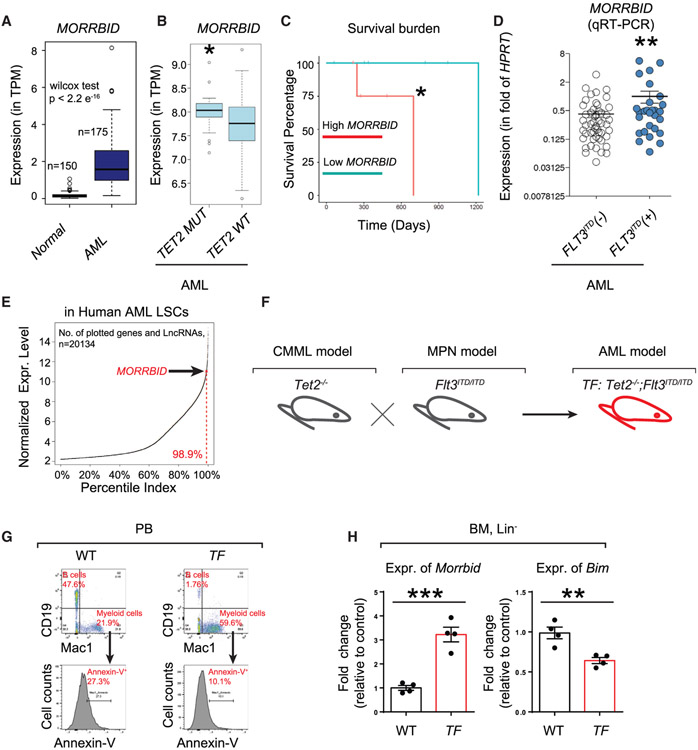
We next aimed to extend these findings to assess the role of Morrbid in an aggressive form of AML driven by a combination of loss of Tet2 and expression of Flt3ITD/ITD (i.e., Tet2−/−; Flt3ITD/ITD). We first mined the human AML gene expression database (unclassified, various subtypes included) as well as primary patient-derived AML samples to determine the contribution of MORRBID in human AML. As seen in Figure 2A, the presence of MORRBID is significantly higher in unclassified AML patients compared to normal control individuals. Furthermore, we also observed that a subset of AML patients bearing TET2 mutations show significantly higher expression of MORRBID (p = 0.048 by Mann-Whitney test) and worse overall survival compared to AML patients lacking TET2 mutations (p = 0.047 by log-rank test) from The Cancer Genome Atlas (TCGA) data (Figures 2B and 2C). Likewise, in AML samples collected from our own institution, AML patients with FLT3ITD mutations manifest ~3-fold higher expression of MORRBID compared to AML patients without FLT3ITD mutations (Figure 2D). Analysis on another independent dataset with expression of 20,134 genes, including lncRNAs, further confirmed that MORRBID is expressed in human leukemic stem cell (LSC) samples and its expression level is among top 1.1% percentile (Figure 2E). Taken together, these results suggest that hyperactivation of MORRBID is highly associated with TET2 and FLT3ITD mutations in human AML (Figures 2B-2D) or even unclassified AML (Figures 2A and 2E).
Figure 2. Highly Conserved Mammalian lncRNA MORRBID/Morrbid Is Hyperactivated in Human and Murine Models of AML.
(A) Boxplot showing the expression levels of the MORRBID transcript in 150 normal whole-blood samples from the Genotype-Tissue Expression (GTEx) project compared against its expression in 175 AML samples from TCGA project. *p < 2.2e−16, Wilcoxon test.
(B) Boxplot showing the expression levels of MORRBID in BM samples of AML patients with TET2 mutation (left) or WT TET2 (right) from TCGA gene expression data. y axis represents the log-normalized gene expression level. p = 0.048, Mann-Whitney test.
(C) Overall survival of TET2 mutated patients with high/low expression of MORRBID. A significant integrative effect of high expression MORRBID and TET2 mutation in determining patient’s overall survival was observed compared to low expression of MORRBID. *p = 0.047.
(D) Increased expression of MORRBID in AML cells with FLT3ITD mutation (FLT3ITD(+)) compared to AML cells without FLT3ITD mutation (FLT3ITD(−)). Mononuclear cells derived from AML patients negative for FLT3ITD (left) or positive for FLT3ITD (right) were subjected to qRT-PCR for MORRBID expression. n = 29 FLT3ITD(+) AML and 63 FLT3ITD(−) AML. **p < 0.01, Student’s t test.
(E) Scatterplot of normalized gene expression level showing the top 1.1% percentile of MORRBID expression level among 20,134 genes and lncRNAs in nine bulk human AML stem cell samples.
(F) Murine model of AML used in the present study. Flt3ITD/ITD mice (F) were bred with Tet2−/− mice (T) to generate Tet2−/−; Flt3ITD/ITD mice (TF).
(G) Representative apoptosis profiles in myeloid cells (Mac1+) of WT and TF mice determined by Annexin-V staining (n = 4).
(H) Expression of Morrbid and Bim in Lin− cells analyzed by qRT-PCR. **p < 0.01; ***p < 0.001.
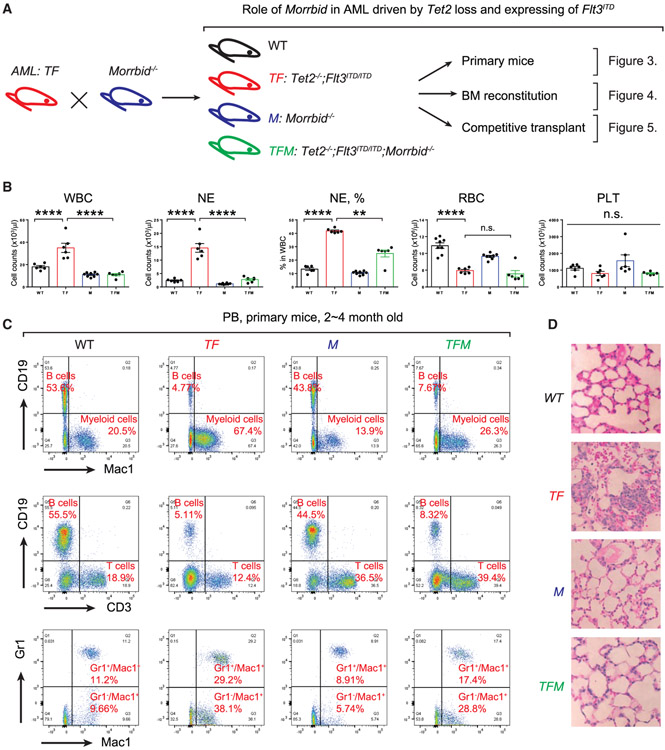
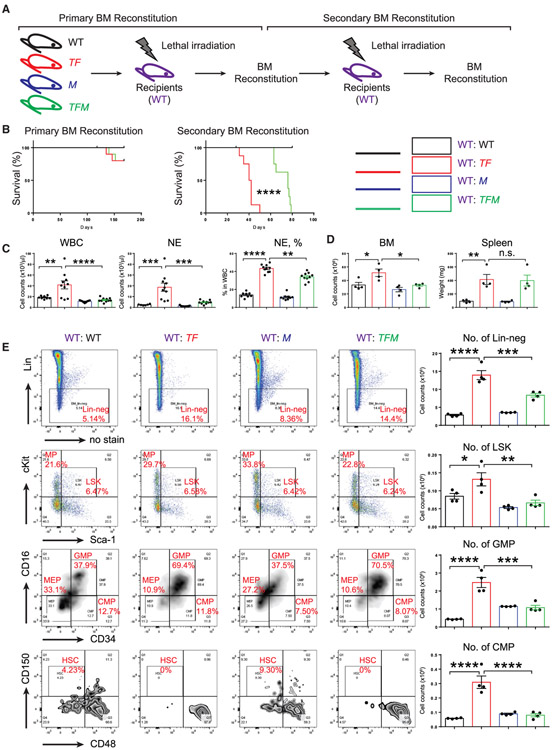
To evaluate the potential functional significance of MORRBID in AML, we next utilized a murine model of AML induced by the loss of Tet2 and expression of Flt3ITD (Tet2−/−;Flt3ITD/ITD, TF) (Figure 2F). Consistent with a previous report (Shih et al., 2015), TF mice manifest full penetration of abnormalities and high percentage of myeloid cells in the circulation (percentage of Mac1+; 21.9% in WT versus 59.6% in TF), while B cell development is repressed (percentage of CD19+: 47.6% in WT versus 1.76% in TF). Additionally, flow cytometry analysis with apoptosis marker Annexin-V showed that Mac1+ cells in TF mice are resistant to cell death (percentage of Annexin-V+: 27.3% in WT versus 10.1% in TF) (Figure 2G). Importantly, expression of Morrbid is significantly greater in BM Lin− cells derived from TF mice compared to WT mice, and expression of the gene encoding pro-apoptotic protein Bim (also known as Bcl2l11) is significantly reduced in TF Lin− cells (Figure 2H). Given these findings, we hypothesized that TF model of AML is suitable to study the functional consequences of Bim induction in the absence of Morrbid. We therefore generated mutants of TF lacking Morrbid (Tet2−/−;Flt3ITD/ITD;Morrbid−/−,TFM) and all the necessary controls (Figure 3A). We conducted three different experiments to define the functional requirement of Morrbid in induction and prevention of AML driven by TF in (1) primary TF and TFM mice, (2) BM-derived recipient animals with TF or TFM donors, and (3) BM-reconstituted recipients with competitor donors (BoyJ WT donor’s versus TF or TFM donors) (Figures 3A, 4A, and 5A). In all three scenarios, TFM mice or recipient mice with TFM donors manifest reduced WBC, NE and MO counts compared to controls (Figures 3B and 4C). Similar to our findings described earlier in TM and FM mutant mice (Figures 1 and 2), loss of Morrbid was unable to rescue the defects associated with RBCs in TFM mice (Figure 3B). In contrast, loss of Morrbid in TF-driven AML, reduced the leukemic cell percentage in PB and mitigated their infiltration into the lung of TFM mice (Figures 3C and 3D). Importantly, loss of Morrbid in the setting of TF cells extended the survival of BM-reconstituted animals with TF donors relative to controls in secondary transplant recipients (Figure 4B). Furthermore, while not changing the differentiation abnormalities in Lin−-Sca1+-cKit+ cells (LSKs), common myeloid progenitors (CMPs), and granulocyte-monocyte progenitors (GMPs), loss of Morrbid reduced the BM cellularity and absolute number of progenitor cells, including Lin−,LSK, GMP, and CMP cells, in the BM of recipient mice transplanted with TFM donor cells compared to controls (Figures 4D and 4E). In experiments involving competitive transplantation, loss of Morrbid in the setting of TF also mitigated the clonal expansion observed using TF cells in PB and BM (Figures 5B, 5C, and 5E). Loss of Morrbid in the setting of TF was also slightly but significantly able to increase the portion of B cells (Figure 5G). Additionally, the increase in spleen size associated with TF donors was completely rescued when cells were transplanted using TFM donors under a competitive setting using BoyJ cells as competitors (Figure 5F), further supporting the notion that loss of Morrbid represents a novel therapeutic strategy for AML treatment.
Figure 3. Genetic Loss of Morrbid in the Context of Tet2−/−; Flt3ITD/ITD Mice Rescues Myeloid Cell Abnormalities.
(A) A schematic describing various experimental approaches utilized to study the role of Morrbid in driving AML. TF mice were bred with Morrbid−/− mice to generate Tet2−/−; Flt3ITD/ITD; Morrbid−/− mice, indicated as TFM. The impact of Morrbid deficiency was analyzed in the original TF mice (primary mice) by utilizing BM cells derived from these mice and transplanting them into WT recipients and conducting competitive transplant experiments utilizing donors from TF and TFM mice along with normal WT competitors.
(B) Examination of PB parameters in the four experimental groups: WT, TF, TFM, and M. Shown are WBC, NE, RBC, and PLT counts as well as the frequency of NEs in various experimental groups. NE, neutrophil. PLT, platelet. RBC, red blood cell. WBC, white blood cell.
(C) Representative flow cytometry plots from PB of the four genotypes. Cells were stained with a combination of antibodies recognizing CD19 (B cells), Mac1 (myeloid cells), CD3 (T cells), and Gr1 (myeloid cells) markers. The percentage of various subsets are indicated in each quadrant in red.
(D) Lung histology from the four experimental groups. Infiltration of leukemic cells in the lung of TFM mice was reduced compared to TF mice.
n = 4–10; **p < 0.01; ****p < 0.0001.
Figure 4. Increased Survival of Mice Transplanted with TFM Donor Cells Compared to TF Cells.
(A) A schematic of recipient mice undergoing primary and secondary transplantation using BM derived donor cells from the four genotypes.
(B) Enhanced survival of recipient mice transplanted with TFM donor cells in secondary recipients compared to TF donors.
(C and D) Loss of Morrbid in the setting of TF cells rescues WBC and NE counts as well as NE frequency and BM cellularity, but not spleen size. (C) Samples from PB. D, samples from BM and spleen.
(E) Morrbid deficiency in the context of TF cells rescues the absolute number of Lin− cells, LSK cells, granulocyte-macrophage progenitors (GMPs), and common myeloid progenitors (CMPs) to near-WT levels.
n = 4–10; *p < 0.05; **p <0.01; ***p < 0.001; ****p < 0.0001.
Figure 5. Growth Advantage of TF Cells Was Mitigated When Morrbid Is Depleted.
(A) Donor cells from TF or TFM mice along with WT and Morrbid−/− controls were mixed with BoyJ cells in identical cell number (0.5 × 106 versus 0.5 × 106) for a competitive transplantation assay.
(B) Chimerism analysis of PB, BM, and spleen samples from chimeric mice at the indicated time points post transplantation.
(C and E) Representative chimerism profiles in BM (C) and spleen samples (E).
(D and F) BM cellularity (D) and spleen weight (F) were examined at 6 months post-transplantation.
(G) In chimeric mice, the portion of B cells is partially corrected in recipient mice transplanted with TFM donors.
Student’s t test (n = 4–10); *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
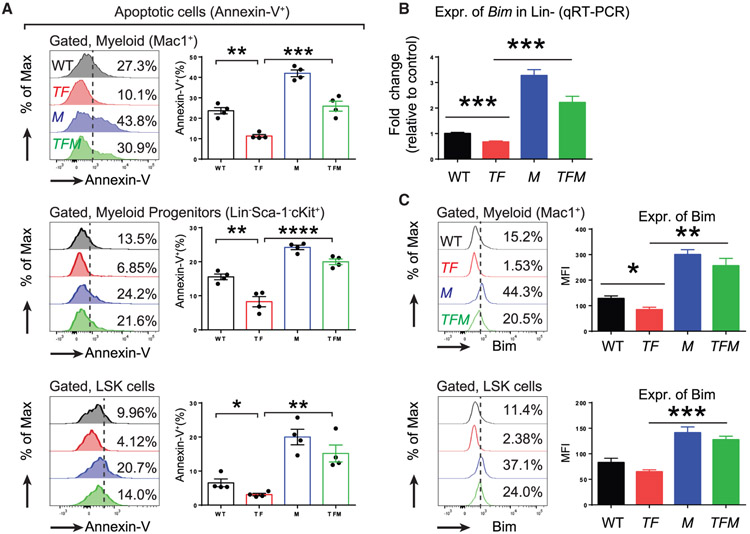
Given the significant correction of phenotype observed in TFM mice, we next sought to determine the extent to which loss of Morrbid impacts apoptosis in various compartments of the BM, including Mac1+ cells, myeloid progenitor (MP) cells (Lin−-cKit+-Sca1−), and LSK cells. As seen in Figure 6A, the reduction in apoptosis observed in Mac1+, MP, and LSK cells associated with TF mice was partially reversed in the TFM mice. Consistently, an increase in the expression of Bim in TFM cells was determined by both qRT-PCR and mean fluorescence intensity (MFI) assays (Figures 6B and 6C). Taken together, these results suggest that targeting Morrbid induces apoptosis in leukemic cells. Although the mechanism of transcriptional repression of Bim by Morrbid via DNA loop in cis was initially addressed in our previous studies (Kotzin et al., 2016), future studies are required to assess the extent to which Morrbid itself, rather than the act of its transcription, may be the primary mediator of Bim regulation.
Figure 6. Morrbid Loss Induces Increased Expression of Bim and Apoptosis in Leukemic Cells, Including Mature Myeloid Cells (Mac1+), Myeloid Progenitor Cells (Lin−Sca-1−cKit+), and LSK Cells.
(A) Level of apoptosis as determined by Annexin-V staining.
(B) Expression of Bim in Lin− cells as analyzed by qRT-PCR.
(C) Expression of Bim in myeloid cells and LSK cells as analyzed by intracellular staining of Bim and quantified by mean fluorescence intensity (MFI).
n = 4; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
To evaluate if there is any substantial functional difference between BCL-2/MCL-1 inhibition and Morrbid targeting, we treated TF mice with single-agent ABT-199 (a selective inhibitor of BCL-2), S63845 (a selective inhibitor of MCL-1), or 5-azacytidine (an effective drug for TET2-mutation-related myelodysplastic syndromes [MDS]) or with various combinations (Figure 7A; DiNardo et al., 2019; Pollyea et al., 2018). Although a high dose of ABT-199 and 5-azacytidine is toxic to the animal (data not shown), TF mice are resistant to treatment with any of the single agents at low dose or with a combination treatment of low dose ABT-199 and S63845 (Figures 7A and 7B). A drug regimen with a low dose of all three drugs (ABT-199 + S63845 + 5-azacytidine) is sufficient to reduce WBCs, NEs, and MOs in TF mice after three cycles of treatment (Figure 7B). However, this treatment failed to improve RBC counts (Figure 7B). Although the ratio of mature myeloid cells and B cells was slightly altered in the PB, BM, and spleen (Figure 7C), analysis of hematopoiesis on hematopoietic progenitors such as LSKs, CMPs and GMPs in the drug treated TF mice showed that this treatment failed to change the differentiation abnormalities associated with TF mice in the BM and spleen (data not shown). Future experiments are required to compare the impact of Bcl-2 repression via ABT-199 in TFM mice and TF mice to assess whether direct repression of Bcl-2 in TFM mice further improves the AML phenotype.
Figure 7. Treatment of TF AML Mice with BCL-2/MCL-1 Inhibitors and 5-Azacytidine.
(A) Scheme for six different drug regimens.
(B) Alteration of PB parameters for each individual mouse from six different cohort.
(C) Representative profiles of myeloid cells and B cells in PB, BM, and spleen after treatments.
n = 4; *p< 0.05; ***p < 0.001; ****p < 0.0001.
In summary, we have defined a critical role for a novel myeloid regulator Morrbid in a spectrum of myeloid neoplasms, including CMML, MPN, and AML. Our results show that Morrbid is a myeloid-specific cell survival regulator not only in physiologic conditions but also in leukemic conditions.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Reuben Kapur (rkapur@iupui.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate any unique datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
MICE
All the animal experiments were approved and maintained by Laboratory Animal Resource Center at Indiana University School of Medicine. C57/B6 mice (CD45.2+) were procured from IUSM core facility and used as wild-type controls. For transplantation studies, congenic BoyJ mice (CD45.1+) or F1 mice (CD45.1+CD45.2+) were procured from IUSM core facility and used as wild-type controls. CMML model mice (Tet2−/−), MPN model mice (Flt3ITD/+) and Morrbid deficient mice (Morrbid−/−) have been described (Kotzin et al., 2016; Lee et al., 2007; Li et al., 2011) and were bred for generating the compound mutants. Age matched mice (both male and female) were analyzed: for CMML analysis, mouse sample were collected at old age (~12 month old); for MPN analysis, mouse sample were collected at young age (~2 month old); for AML analysis, mouse sample were collected at large-range of age as indicated (2~6 month old or 2~6 month post the transplantation).
Human Blood Samples
Peripheral blood (PB) samples of human AML patients (both male and female) enrolled in our affiliated hospital were obtained from our blood bank with approved protocol.
METHOD DETAILS
Mouse Experimental Procedures
Analysis procedures involving mouse experiments (i.e., flow cytometry strategies and Annexin-V gating and intracellular staining for anti-Bim) have been in described in detail in our recent study (Cai et al., 2018).
qRT-PCR analysis of MORRBID using human AML blood samples
Peripheral blood of AML patients enrolled in our affiliated hospitals were sorted for monocytes and then analyzed by qRT-PCR for determining the association between MORRBID expression and FLT3ITD mutation. Expression of HPRT was used as internal control. Genomic DNA from blood samples was also purified and FLT3ITD mutation was determined by PCR genotyping.
Bioinformatic analysis of MORRBID and survival burden in human AML
To identify which lncRNAs potentially associated with AML, we downloaded raw RNA-seq data for 175 AML samples from TCGA, along with the pertaining patient clinical data. The downloaded data was aligned to the human reference genome (GRCh38) using HISAT2 and transcript abundance was computed using StringTie. Microarray data of the TCGA AML samples were also downloaded and analyzed. Genome scale survival analysis was performed over the AML expression and clinical data obtained from TCGA, using survival R package (https://github.com/therneau/survival), to obtain the survival statistics for all human lncRNAs obtained from Gencode v24. The top 20 (sorted ascending by FDR) lncRNAs including MORRBID were extracted for further analysis. Simultaneously, we downloaded 150 normal whole blood gene abundance data from the GTEx project to observe and compare lncRNA expression levels across AML and normal whole blood. Log-rank test was used to test the prognostic association of MORRBID in the AML patients with TET2 mutations. 17 TET2 mutated samples with both gene expression and mutation were analyzed. High and low expression level of MORRBID was determined by higher than 65% quantile and lower than 35% quantile of its expression level. To demonstrate the significant expression of MORRBID in human AML leukemic stem cells, we retrieved the dataset GSE17054 from NCBI GEO database (Majeti et al., 2009), which contains 9 samples of bulk leukemic stem cell (LSC) samples collected from AML patients. We compared the averaged expression of MORRBID versus other genes in the 9 AML LSC samples. Our analysis suggests MORRBID is among the top 1.1% expressed genes and lncRNA in all the measured probes.
QUANTIFICATION AND STATISTICAL ANALYSIS
All experimental procedures on mice were run in parallel with proper controls (sex and age matched littermate controls when possible) for observing experiment variabilities. Analysis of grouped data was not blinded and no samples were excluded. If not specified, P value was calculated using an unpaired t test for comparing means of two groups (GraphPad Prism 6.0). Error bars indicate the standard error of mean (s.e.m.).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Mouse: Tet2−/− | Li et al., 2011 | N/A |
| Mouse: Ft3ITD/+ | Lee et al., 2007 | N/A |
| Mouse: Morrbid−/− | Kotzin et al., 2016 | N/A |
| Mouse: C57/B6 | Jackson Laboratory | Cat #000664 |
| Mouse: BoyJ | Jackson Laboratory | Cat #002014 |
| Critical Commercial Assays | ||
| EasySep Mouse Hematopoietic Progenitor Cell Isolation Kit | StemCell | #19856 |
| Rneasy Mini Kit | QIAGEN | #74104 |
| SuperScript II Reverse Transcriptase | Fisher Scientific | #18064014 |
| SYBR Green master mix | Life Technologies | #4385612 |
| Antibodies for Flow Cytometry | ||
| TER-119, PE | BioLegend | #116208 |
| Gr1, PE | BioLegend | #108408 |
| Mac1, PE | BioLegend | #101208 |
| B220, PE | BioLegend | #103208 |
| CD3, PE | BioLegend | #100206 |
| CD4, PE | BioLegend | #116006 |
| CD8a, PE | BioLegend | #100708 |
| c-Kit, APC | BioLegend | #105812 |
| Sca-1, APC/Cy7 | BioLegend | #108126 |
| CD150, PE/Cy5 | BioLegend | #115912 |
| CD48, PE/Cy7 | BioLegend | #103424 |
| CD127, PE/Cy5 | BioLegend | #135016 |
| CD16/32, PE/Cy7 | bioLegend | #101318 |
| CD34, FITC | eBioscience | #11-0341-85 |
| Annexin V, FITC | BioLegend | #640906 |
| 7-AAD | BioLegend | #79993 |
| Bim mAb (C34C5), PE | Cell Signaling Tech | #12816S |
| Bim mAb (C34C5), AF488 | Cell Signaling Tech | #94805S |
| CD45.2, PerCP/Cy5.5 | BioLegend | #109928 |
| CD45.1, PE/Cy7 | BioLegend | #110730 |
| CD19, APC | BioLegend | #115512 |
| CD71, APC | eBioscience | #17-0711-82 |
| Oligonucleotides | ||
| Mouse_Actin-b, Forward: GACGGCCAGGTCATCACTATTG | Cai et al., 2018 | N/A |
| Mouse_Actin-b, Reverse: AGGAAGGCTGGAAAAGAGCC | Cai et al., 2018 | N/A |
| Mouse_Morrbid, Forward: TCTGAGAATGAGGGGACTGG | Kotzin et al., 2016 | N/A |
| Mouse_Morrbid, Reverse: TGTGCTGTGAAGATCCCAAG | Kotzin et al., 2016 | N/A |
| Human_HPRT, Forward: GCTATAAATTCTTTGCTGACCTGCTG | Kotzin et al., 2016 | N/A |
| Human_HPRT, Reverse: AATTACTTTTATGTCCCCTGTTGACTGG | Kotzin et al., 2016 | N/A |
| Human_MORRBID, Forward: ACTGGATGGTCGCTGCTTTT | Kotzin et al., 2016 | N/A |
| Human_MORRBID, Reverse: CTTCCCAGGAACTGTGCTGT | Kotzin et al., 2016 | N/A |
| Software and Algorithms | ||
| FlowJo | FlowJo | V10.2 |
| Prism | GraphPad Software | V6.0 |
| Adobe Illustrator | Adobe | CC-2015 |
Highlights.
Hyperactivation of Morrbid and reduced apoptosis in Tet2−/−;Flt3ITD/ITD AML
Morrbid modulates Tet2−/− CMML, Flt3ITD/ITD MPN, and Tet2−/−;Flt3ITD/ITD AML
Genetic loss of Morrbid makes leukemic cells vulnerable to apoptosis
Increased expression of MORRBID in human AML correlates with poor survival
ACKNOWLEDGMENTS
We thank our colleagues for technical support, critically reading our manuscript, and their suggestions to improve the manuscript. We would also like to thank Ms. Tracy Winkle for her administrative support. This work was supported in part by grants from the National Institutes of Health (R01-CA134777, R01-HL140961, R01-HL146137, and R01-CA173852 to R.K.) and by funds to R.K. from the Riley Children’s Foundation. Z.C. is supported by National Institutes of Health grant T32HL007910.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Anstee NS, Bilardi RA, Ng AP, Xu Z, Robati M, Vandenberg CJ, and Cory S (2019). Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice. Cell Death Differ. 26, 1316–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Fairbrother WJ, Leverson JD, and Souers AJ (2017). From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov 16, 273–284. [DOI] [PubMed] [Google Scholar]
- Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui D, Whittington DA, Huang X, Poppe L, Cheng AC, et al. (2018). AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 8, 1582–1597. [DOI] [PubMed] [Google Scholar]
- Cai Z, Kotzin JJ, Ramdas B, Chen S, Nelanuthala S, Palam LR, Pandey R, Mali RS, Liu Y, Kelley MR, et al. (2018). Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 23, 833–849.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Heiser D, Li L, Kaplan I, Collector M, Huso D, Sharkis SJ, Civin C, and Small D (2012). FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm. Cell Stem Cell 11, 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, et al. (2019). Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Huang K, O’Neill KL, Li J, Zhou W, Han N, Pang X, Wu W, Struble L, Borgstahl G, Liu Z, et al. (2019). BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis. Cell Res. 29, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, and Ebert B (2019). Clonal hematopoiesis in human aging and disease. Science 366 (6465), eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikushige Y, Yoshimoto G, Miyamoto T, Iino T, Mori Y, Iwasaki H, Niiro H, Takenaka K, Nagafuji K, Harada M, et al. (2008). Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J. Immunol. 180, 7358–7367. [DOI] [PubMed] [Google Scholar]
- Kotzin JJ, Spencer SP, McCright SJ, Kumar DBU, Collet MA, Mowel WK, Elliott EN, Uyar A, Makiya MA, Dunagin MC, et al. (2016). The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Fröhling S, Huntly BJ, et al. (2007). FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell 12, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cai X, Cai C-L, Wang J, Zhang W, Petersen BE, Yang F-C, and Xu M (2011). Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 118, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Becker MW, Tian Q, Lee T-LM, Yan X, Liu R, Chiang J-H, Hood L, Clarke MF, and Weissman IL (2009). Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc. Natl. Acad. Sci. USA 106, 3396–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Houtmann S, Wilde L, Vigil C, Eischen CM, Kasner M, and Palmisiano N (2019). The role of inhibition of apoptosis in acute leukemias and myelodysplastic syndrome. Front. Oncol 9, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AJ, Kharazi S, Atkinson D, Macaulay I, Pecquet C, Loughran S, Lutteropp M, Woll P, Chowdhury O, Luc S, et al. (2013). FLT3-ITDs instruct a myeloid differentiation and transformation bias in lymphomyeloid multipotent progenitors. Cell Rep. 3, 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AJ, Neo WH, Barkas N, Matsuoka S, Giustacchini A, Facchini R, Thongjuea S, Jamieson L, Booth CAG, Fordham N, et al. (2017). Niche-mediated depletion of the normal hematopoietic stem cell reservoir by Flt3-ITD-induced myeloproliferation. J. Exp. Med 214, 2005–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, and Strasser A (2018). BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer Cell 34, 879–891. [DOI] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. (2011). Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganna N, Opoku-Temeng C, Choi EY, Larocque E, Chang ET, Carter-Cooper BA, Wang M, Torregrosa-Allen SE, Elzey BD, Lapidus RG, and Sintim HO (2019). Amino alkynylisoquinoline and alkynylnaphthyridine compounds potently inhibit acute myeloid leukemia proliferation in mice. EBioMedicine 40, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, D’Alessandro A, Culp-Hill R, Riemondy KA, Gillen AE, et al. (2018). Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med 24, 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, Boyd KL, Strickland SA, Sensintaffar J, Hogdal LJ, et al. (2018). A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 8, 1566–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AH, Jiang Y, Meydan C, Shank K, Pandey S, Barreyro L, Antony-Debre I, Viale A, Socci N, Sun Y, et al. (2015). Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell 27, 502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Saxena S, Singh BK, and Kakkar P (2017). BH3-only protein BIM: an emerging target in chemotherapy. Eur. J. Cell Biol 96, 728–738. [DOI] [PubMed] [Google Scholar]
- Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, Jabbour A, Cummins K, Lackovic K, Blombery P, et al. (2018). Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia 32, 303–312. [DOI] [PubMed] [Google Scholar]
- Yang S, Mao Y, Zhang H, Xu Y, An J, and Huang Z (2019). The chemical biology of apoptosis: Revisited after 17 years. Eur. J. Med. Chem 177, 63–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.