Abstract
Background
Laparoscopic sleeve gastrectomy (SG) has surpassed Roux-en-Y gastric bypass (RYGB) as the most prevalent bariatric procedure worldwide. While RYGB and SG demonstrate equivalent short-term weight loss, long-term weight loss tends to be greater after RYGB. Differences in the effect of these procedures on gastrointestinal hormones that regulate energy homeostasis are felt to partially underlie differences in outcomes. The objective of this study was to prospectively quantify blood levels of gut hormones of energy and glucose homeostasis at one year follow up to delineate possible reasons for greater efficacy of RYGB over SG in achieving weight loss.
Methods
Patients undergoing SG (n = 19) and RYGB (n = 40) were studied before surgery and at 2,12, 26, and 52 weeks postoperatively. Blood samples drawn in the fasted state and after a liquid mixed meal were assayed at baseline, 26, and 52 weeks for peptide YY (PYY), glucagon-like peptide-1 (GLP-1), ghrelin, insulin, glucose, and leptin. Fasting and postprandial appetitive sensations were assessed by visual analog scale.
Results
At 1 year there was greater weight loss in RYGB compared with SG patients (30% vs 27%; P = 0.03). Area under the curve (AUC) after the mixed meal for PYY was greater in RYGB patients (P<0.001). RYGB patients had significant increases in GLP-1 AUC compared to baseline (P = 0.002). Ghrelin levels decreased only after SG compared to baseline (P<0.001) but were not significantly different from RYGB. There was a trend toward decreased sweet cravings in RYGB patients (P = 0.056).
Conclusions
Differences in gastrointestinal hormones that regulate energy and glucose homeostasis are a possible mechanism for greater efficacy of RYGB compared to SG.
Introduction
Obesity is a major health problem with increasing prevalence and is associated with increased mortality [1–3]. Bariatric surgery produces greater weight loss, improvement in comorbidities, and survival compared to non-surgical treatment for obesity [4–6]. There has been a 20-fold increase in procedures performed annually over the past 20 years in the United States. In the past decade, there has been a rapid shift in procedure type performed. Roux-en-Y gastric bypass (RYGB) predominated until the late-2000s, then was surpassed by sleeve gastrectomy (SG), now the most commonly performed procedure in the US and worldwide [7, 8]. While prior trials were not powered to compare groups [9, 10], long-term data comparing the efficacy of the surgeries has emerged favoring RYGB. A 65,000-patient retrospective multicenter cohort demonstrated 6.7% greater total weight loss after RYGB compared to SG at 5 year follow up [11]. In a longitudinal study, mean weight loss at 7 years follow up was significantly higher in RYGB (30.4%) vs SG (23.6%) [12]. A meta-analysis of over 5000 patients showed superior weight loss after RYGB with a mean difference of 17% at greater than 5 years follow up [13]. Additionally, RYGB has been shown to be superior to SG in achievement of type 2 diabetes remission. In a single center triple-blind randomized trial, diabetes remission was achieved in 75% of RYGB vs. 48% of SG subjects at 1 year [14]. In a 9700-patient retrospective multicenter cohort the diabetes remission rate was approximately 10% higher in patients who had RYGB compared to SG and they experienced significantly lower relapse rates at 5 years after surgery [15]. Several prior trials also showed more favorable remission rates after RYGB compared to SG [4, 9, 10].
Changes in gut hormones of energy and glucose homeostasis following these procedures are felt to partially underlie differences in outcomes [16–19]. A variety of hormones secreted from the gastrointestinal tract communicate with peripheral tissues and the central nervous system to regulate glucose homeostasis and energy balance. The gut hormones of interest in this report are peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and ghrelin. PYY and GLP-1 are secreted by distal intestinal L cells to decrease appetite, increase satiety, and slow gut motility. [20]. Additionally, PYY improves insulin sensitivity [21, 22] and GLP-1 functions as an incretin to potentiate glucose-stimulated insulin release. Ghrelin is an appetite stimulating neuropeptide secreted by the gastric fundus and proximal small intestine to increase food intake, gut motility, and decrease insulin secretion [23, 24]. Ghrelin levels increase during fasting, are suppressed following meal intake, and increase in response to diet-induced weight loss or adjustable gastric banding [25–27].
The objective of this study was to quantify blood levels of GI tract hormones at one year follow up to delineate possible reasons for greater efficacy of RYGB over SG in achieving weight loss and metabolic improvements.
Materials and methods
Protocol
We certify that all applicable institution and government regulations concerning the ethical use of human volunteers was obeyed during this study. The study was approved by the Columbia University Institutional Review Board and written informed consent was obtained from all subjects. Patients were recruited if they were above the age of 21, scheduled to undergo either RYGB or SG, and did not use weight loss medications within 90 days prior to enrollment. This cohort consists of 59 subjects, recruited from April 2003 to September 2017, who underwent either RYGB (n = 40) or SG (n = 19) and had 1 year of follow up data. Sample size was based on prior and ongoing work comparing RYGB and laparoscopic adjustable gastric banding (LAGB) in which differences in gut hormone levels were observed in cross-sectional and prospective studies [27–29].
The choice of bariatric procedure was based on patient and surgeon preference. RYGB entailed creation of a 15-30ml gastric pouch (divided from the proximal lesser curvature of the stomach and excluded the fundus) and anastomosed to a Roux limb of jejunum created by division of the jejunum 50-100cm distal to the ligament of Treitz and anastomosing the afferent biliopancreatic limb of the jejunum 100-150cm distally. Division of the vagus nerve and its branches was avoided. SG was performed with a 40Fr bougie as a template aligned along the lesser curvature. Gastric transection was performed beginning 5cm proximal to the pylorus and extended to the Angle of His.
Participants were seen at baseline, 2, 12, 26, and 52 weeks for measurement of body weight and waist circumference. At baseline, week 26, and week 52 blood was drawn in a fasted state followed by consumption of a liquid test meal over a 15 minute period (Optifast, Novartis, Minneapolis, MN, USA; 474 ml, 320 kcal, 50% carbohydrate, 35% protein, 15% fat). Venous blood was then drawn at 15, 30, 60, 90, and 120 minutes from the end of the meal. After centrifugation at 4ºC, both serum and plasma were stored at -80ºC. Subjects completed a validated visual analog scale (VAS) questionnaire [30, 31] in the fasted state and at 30, 60, 90, and 120 minutes after the test-meal. The VAS consisted of 100-mm lines with words anchored at each end describing extreme sensations of hunger, satiety, sweet cravings, and nausea or abdominal comfort. Subjects were asked to make a vertical mark across the line corresponding to their feelings. Quantification was performed by measuring the distance from the left end of the line to the mark.
Hormone assays
Total PYY was measured by ELISA (Millipore, MO, USA) with a sensitivity of 10pg/ml. Total GLP-1 was measured by RIA (Millipore, MO, USA) after alcohol extraction according to manufacturer’s protocol with a sensitivity of 3pM and recovery in each assay tested by parallel extraction of standards. Leptin was measured with a human RIA kit (LINCO Research, Inc, St. Charles, MO) using a 125I-iodinated human leptin tracer. Total plasma immunoreactive ghrelin was measured by a RIA kit (Phoenix Pharmaceuticals, Belmont, CA) using 125I-iodinated ghrelin tracer and a rabbit polyclonal antibody against full-length, octanolyated human ghrelin that recognizes the acyl and des-acyl forms of the hormone, with the lower limit of detection of 20 pg/ml. Plasma glucose was measured by the hexokinase method. Plasma insulin was measured with the Immulite Analyzer (Diagnostic Products Corp., Los Angeles, CA) with the lower limit of detection of 2ulU/ml. An aliquot from a pool of plasma was included in each assay to ensure that there was no change over time. All samples were assayed in duplicate.
Statistical analysis
Statistical model estimated means and standard errors are presented.
Linear mixed effects models were used to analyze the outcome variables. Specifically, the main predictors were procedure (RYGB versus SG), time (in week, treated as categorical for possible exploration of nonlinear temporal trend), and their interactions. Random intercept effects were included in the model to account for between-subject variation and within-subject correlation, which was equivalent to a compound symmetric covariance structure. No other covariance structures were explored due to limited sample size. The estimated mean and standard error at each time point was reported from the model fit and compared using a Wald test. A significant interaction effect was considered as a confirmation of procedure effect. The linear mixed model was chosen to assess the procedure effect for multiple reasons. First, compared with generalized estimating equation, another main approach to modeling longitudinal data, the mixed model is a conditional model and hence is able to capture the subject specific features, which matches with the purpose of clinical trials. Second, the mixed model is a likelihood-based model, and hence would remain appropriate even if there are missing data. In fact, it is theoretically shown that using all available data (i.e., even those subjects with partial data) the mixed model would yield correct parameter estimations under the missing at random (MAR) assumption, one which was deemed reasonable in practice and its violation could not be confirmed in such a small sample size. A P < .05 was considered statistically significant. SAS version 9.4 was used in the analysis. Total area under the curve (AUC) was calculated using the trapezoidal rule from 0–120 min, with the exception of GLP-1, which was calculated from 0–60 min. Insulin resistance was calculated using the homeostatic model of assessment (HOMA-IR) [32]. The Pearson correlation analysis was used for HOMA-IR and percentage weight loss.
Results
Study subjects
There were 59 subjects enrolled consisting of 63% Hispanic, 37% non-Hispanic, 22% African American, and 78% Caucasian adults; 40 subjects underwent RYGB (8M/32F) and 19 underwent SG (4M/15F).
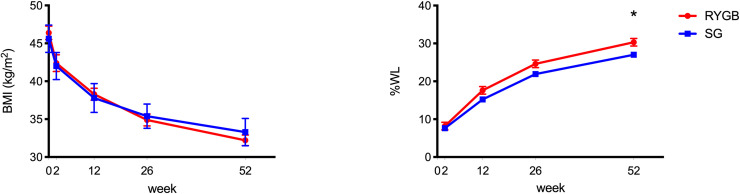
Baseline characteristics were similar for age (RYGB 43.6± 1.6 years; SG 44.5 ± 1.8 years P = 0.34) and BMI (RYGB 46.4 ± 5.5 kg/m2; SG 45.6 ± 8 kg/m2 P = 0.32). Baseline characteristics and anthropometric data are presented in Table 1. While weight was similar at baseline (RYGB 123.6 ± 19.2 kg; SG 124 ± 26.9 kg P = 0.47), there was a significantly higher percentage weight loss after RYGB compared to SG (30.3% vs 27.0%, P = 0.03) at 52 weeks (Fig 1).
Table 1. Baseline characteristics of participants.
| Sleeve Gastrectomy (n = 19) | Roux-en-Y Gastric Bypass (n = 40) | |
|---|---|---|
| Age--yr | ||
| Mean | 44.5 ± 1.8 | 43.6 ± 1.6 |
| Range | 27–57 | 22–64 |
| Sex | ||
| Female—no. (%) | 15 (78.9) | 32 (80.0) |
| Male—no. (%) | 4 (21.1) | 8 (20.0) |
| Race | ||
| White—no. (%) | 12 (63.2) | 34 (85.0) |
| Black—no. (%) | 7 (36.8) | 6 (15.0) |
| Ethnicity | ||
| Hispanic—no. (%) | 12 (63.2) | 25 (62.5) |
| Non-Hispanic—no. (%) | 7 (36.8) | 15 (37.5) |
| Weight/BMI | ||
| Weight (kg) | 124.0 ± 4.4 | 123.6 ± 3.0 |
| Waist (cm) | 124.4 ± 3.0 | 125.2 ± 2.0 |
| BMI (kg/ m2) | 45.6 ± 1.4 | 46.4 ± 0.9 |
Results are presented as mean ± SEM unless indicated otherwise.
Fig 1. BMI and percentage weight loss (%WL) after SG and RYGB.
Values are reported as mean ± SEM. *P <0.05 between group difference.
Hormone and glucose levels
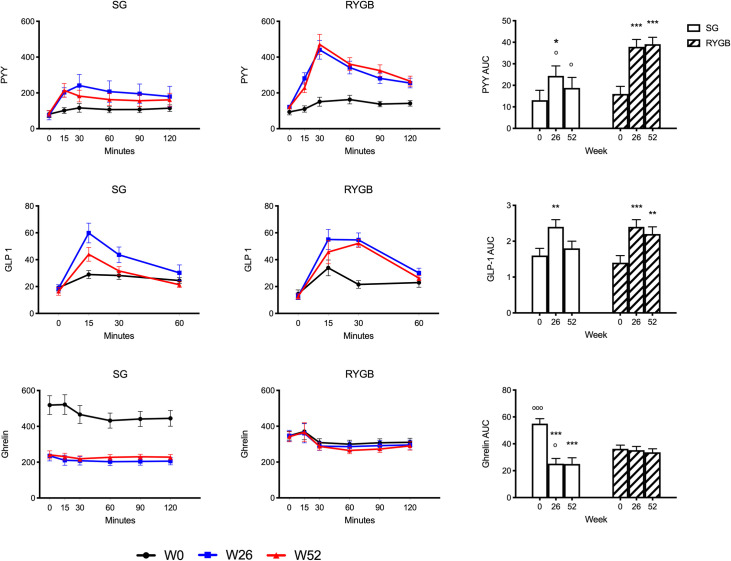
Values of blood hormone levels are presented in Table 2 and Fig 2. Fasting levels of PYY and GLP-1 did not change after RYGB or SG. Postprandial PYY increased significantly after RYGB at 52 weeks, with AUC levels approximately two-fold greater than baseline. Postprandial PYY increased to a lesser degree after SG and AUC levels were significantly lower compared with RYGB. While PYY AUC increased after SG compared to baseline at 26 weeks, significance was not maintained at 52 weeks. GLP-1 levels increased in both groups. Similar to PYY, GLP-1 AUC following SG was significantly higher than baseline at 26 weeks, but was not maintained at 52 weeks. GLP-1 AUC increased significantly after RYGB from baseline at 26 weeks and persisted at 52 weeks. At baseline, ghrelin levels were greater in the SG group compared with RYGB. However, ghrelin levels drastically decreased after SG such that levels became significantly lower post-surgery compared with baseline and compared with the same time-points post-RYGB. In contrast, there was no significant change in ghrelin levels from baseline after RYGB. Data were further analyzed as percent change in AUC from baseline: at 26 weeks percent change was -46.6% for SG and 11.3% for RYGB (P <0.01); and at 52 weeks percent change was -45.2% for SG and 13.7% for RYGB (P <0.01). Leptin levels decreased significantly in both groups and were consistently lower at baseline and post-surgery in the RYGB group.
Table 2. Baseline characteristics and changes over time in body weight, glucose and plasma hormone levels after SG and RYGB.
| Sleeve Gastrectomy | Roux-en-Y Gastric Bypass | |||||
|---|---|---|---|---|---|---|
| Week 0 | Week 26 | Week 52 | Week 0 | Week 26 | Week 52 | |
| Wt/BMI | ||||||
| Wt (kg) | 124.0 ± 4.4 | 96.8 ± 4.4*** | 90.4 ± 4.4*** | 123.6 ± 3.0 | 92.9 ± 3.1*** | 85.8 ± 3.1*** |
| Waist (cm) | 124.4± 3.0 | 102.1 ± 3.0*** | 97.5 ± 3.0*** | 125.2 ± 2.0 | 104.9 ± 2.0*** | 98.3 ± 2.0*** |
| BMI (kg/m2) | 45.6 ± 1.4 | 35.4 ± 1.4*** | 33.3 ± 1.4*** | 46.4 ± 0.9 | 34.9 ± 0.9*** | 32.3 ± 0.9*** |
| WL (%) | n/a | 21.9 ± 1.3*** | 27.0 ± 1.3*** | n/a | 24.6 ± 1.0*** | 30.3 ± 1.0***O |
| Glucose(mg/dl) | ||||||
| Fasting | 102.4 ± 5.4 | 81.7 ± 5.4*** | 81.0 ± 5.6*** | 112.3 ± 3.7 | 91.8 ± 3.8*** | 86.3 ± 3.7*** |
| AUC x 103 | 14.2 ± 0.9 | 10.3 ± 0.9*** | 9.6 ± 1.0*** | 15.0 ± 0.7 | 11.3 ± 0.7*** | 10.6 ± 0.7*** |
| Insulin (uIU/ml) | ||||||
| Fasting | 17.0 ± 4.1 | 12.6 ± 4.1 | 8.5 ± 4.4 | 20.7 ± 2.8 | 14.7 ± 3.0 | 7.0 ± 2.8*** |
| AUC x 103 | 8.3 ± 0.8 | 5.5 ± 0.8*** | 5.2 ± 0.9*** | 6.8 ± 0.6 | 4.5 ± 0.6*** | 4.0 ± 0.6*** |
| HOMA-IR | ||||||
| 4.5 ± 1.1 | 2.5 ± 1.1 | 1.7 ± 1.3* | 6.0 ± 0.8 | 3.2 ± 0.7** | 1.5 ± 0.8*** | |
| PYY (pg/ml) | ||||||
| Fasting | 81.1 ± 21.5 | 75.8 ± 21.5 | 75.0 ± 22.6 | 92.6 ± 14.9 | 123.5 ± 15.5* | 119.7 ± 14.9 |
| AUC x 103 | 13.1 ± 4.6 | 24.4 ± 4.6* | 18.8 ± 4.9 | 16.0 ± 3.5 | 37.9 ± 3.4***O | 39.1 ±3.2***OOO |
| GLP-1 (pmol/l) | ||||||
| Fasting | 19.1 ± 3.1 | 18.1 ± 3.2 | 16.5 ± 3.1 | 14.5 ± 2.3 | 13.5 ± 2.3 | 13.1 ± 2.2 |
| AUC x 103 | 1.6 ± 0.2 | 2.4 ± 0.2** | 1.8 ± 0.2 | 1.4 ± 0.2 | 2.4 ± 0.2*** | 2.2 ± 0.2** |
| Ghrelin (pg/ml) | ||||||
| Fasting | 518.6 ± 35.2 | 230.8 ± 36.7*** | 224.9 ± 43.5*** | 347.1 ±24.2OOO | 347.4 ± 25.0 OO | 347.1 ± 24.7 O |
| AUC x 103 | 55.0 ± 3.7 | 25.2 ± 3.9*** | 25.0 ± 4.6*** | 36.3 ± 2.7OOO | 35.3 ± 2.7O | 33.7 ± 2.6 |
| Leptin (ng/ml) | ||||||
| Fasting | 56.7 ± 3.7 | 27.0 ± 3.7*** | 26.0 ± 3.9*** | 43.2 ± 2.5 OO | 17.2 ± 2.6***O | 15.6 ± 2.5*** O |
Values presented are linear mixed model mean ± SEM. P value compared with Week 0 within-group
*P < 0.05
**P < 0.01
***P < 0.001. P value compared with SG values at same time point: P < 0.05
ººP < 0.01
ºººP < 0.001. HOMA-IR units: (mmol x μIU x l-2). AUC x 103 are integrated over 0–120 min with the exception of AUC for GLP-1 which was determined from 0–60 min.
Fig 2. Fasting and postprandial gut hormone levels after SG and RYGB.
Values are reported as model estimated mean ± SEM. *P <0.05, **P <0.01, ***P <0.001 within group difference; oP <0.05, ooP <0.01, oooP <0.001 between group difference.
Both groups had a similar percentage of subjects with type 2 diabetes mellitus (21% SG, 27% RYGB, P = 0.59). Combined data from subjects with and without diabetes are presented (Table 2). Fasting and postprandial glucose decreased in both groups without significant differences between procedures. Insulin also decreased, with both groups demonstrating early peak levels at 15 minutes post-test meal followed by a rapid decline such that insulin AUC decreased significantly in each (Table 2 and Fig 3). HOMA-IR decreased in both groups. While there was a significant negative correlation between HOMA-IR and percent weight loss in SG (r = -0.65, P = 0.008) there was no correlation in RYGB (r = -0.15, P = 0.4) (Fig 4). Data were similar when subjects with diabetes were excluded from the correlation analysis; there remained a significant negative correlation between HOMA-IR and percent weight loss in SG (r = -0.59, P = 0.04) and no correlation in RYGB (r = -0.25, P = 0.19).
Fig 3. Fasting and postprandial glucose and insulin.
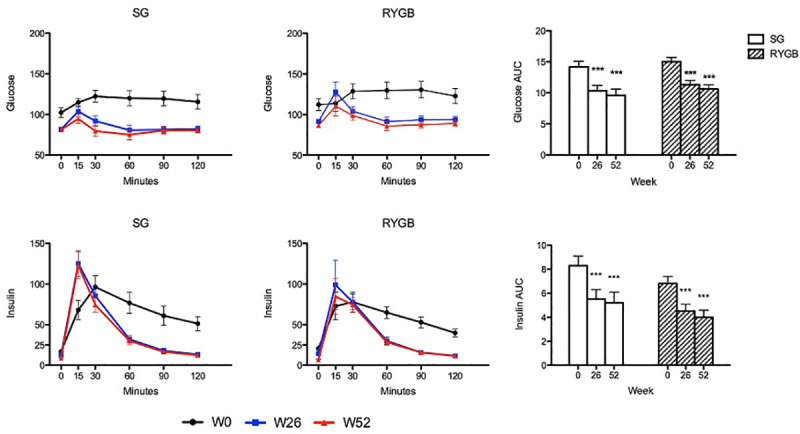
Values are reported as model estimated mean ± SEM. *P <0.05, **P <0.01, ***P <0.001 within group difference.
Fig 4. HOMA-IR and correlation with percentage Weight Loss (%WL) after SG and RYGB.
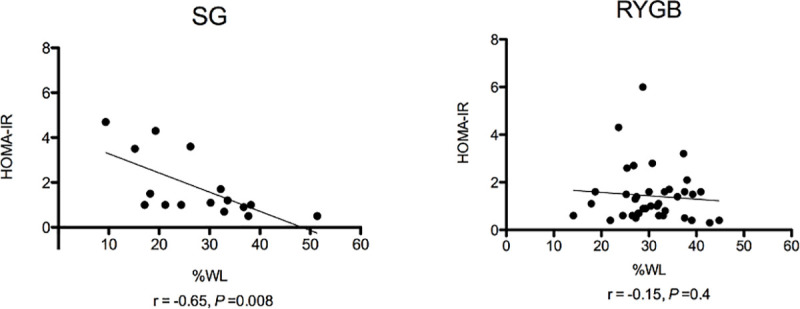
Visual analog scale analysis
VAS ratings for appetitive sensations were similar in both groups at baseline. There were decreases in hunger after SG at 26 weeks (P = 0.03) that were not maintained at 1 year, whereas the opposite trend was observed after RYGB with a decrease in hunger at 1 year (P = 0.09). Both groups showed increases in fullness. There were no differences in abdominal discomfort. When asked “How much do you crave something sweet right now?” there was a trend towards decreased sweet cravings in RYGB compared to baseline (P = 0.07) and compared with SG (P = 0.056) at 52 weeks (Fig 5), whereas there were no significant changes in sweet cravings after SG.
Fig 5. Visual analog scale results of appetitive ratings.
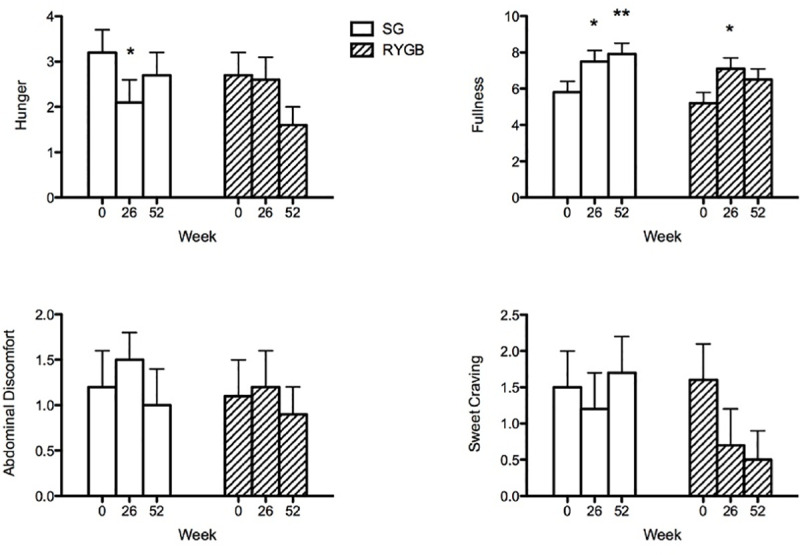
Values are AUC 0–120 min. *P <0.05, **P <0.01, ***P <0.001 within group difference.
Discussion
This study demonstrates significant changes after bariatric surgery in levels of gut hormones that regulate glucose and energy homeostasis and shows differences in these levels between RYGB and SG. Increases in postprandial PYY and GLP-1 levels in the blood were observed primarily after RYGB and transiently after SG. Anatomic changes after RYGB that circumvent the gastric pylorus accelerates nutrient delivery to the distal intestine and likely stimulates secretion of these L cell hormones. Chronically high gastric emptying rates after RYGB have also been shown to induce adaptive changes such as an increase in enteroendocrine cell number, villous height, and surface area [33–35]. In SG there is increased gastric pressure, lack of receptive relaxation in the tubular stomach remnant, accelerated gastric emptying and small bowel transit to induce early and prolonged secretion of L-cell hormones from the distal intestine [36–39]. RYGB has been shown to induce higher and sustained postprandial PYY and GLP-1 compared to SG up to 1 year follow up [40–43]. We observed increases in PYY and GLP-1 in SG at 26 weeks which were not maintained at 52 weeks. Sleeve dilatation after SG does occur in a majority of patients at one year follow up [44]. We postulate this could slow gastric emptying and subsequent GI hormone secretion though this has not been studied.
Changes in GLP-1 and PYY may underlie improvements in glucose homeostasis. Increases in postprandial insulin levels were seen in both groups, as early as 15 minutes following consumption of the test meal. Early postprandial peaks in insulin secretion, proportional to glucose, have been observed in patients with Type 2 diabetes undergoing RYGB, that correlated with improved hemoglobin A1C [45]. In addition to rapid nutrient transport and an early glycemic stimulus, GLP-1 contributes significantly to insulin secretion [46]. GLP-1 receptor blockade after RYGB significantly reduces insulin secretion and this reduction is greater than that seen in non-surgical controls [47]. The increase in PYY may also contribute to improved insulin sensitivity [21, 22].
Interestingly, HOMA-IR correlated with percentage weight loss only after SG, suggesting that improvement in insulin resistance is mediated, at least in part, by the amount of weight loss. In contrast, there was not a significant correlation between HOMA-IR and weight loss after RYGB. Thus, it is possible that weight loss independent changes, such as the increases in GLP-1 and PYY, are driving much of the improvement in HOMA-IR, with weight loss playing a somewhat lesser role. In conjunction with greater weight loss, additional gut hormones and changes in other factors not measured in this study such as metabolites, bile acids and the microbiome may promote improvement in glucose homeostasis which most studies show is greater after RYGB compared with SG in patients with type 2 diabetes mellitus [19, 43].
Both procedures result in a dysregulation of ghrelin when compared with non-surgical weight loss or laparoscopic adjustable gastric banding (LAGB), which result in a rise in fasting ghrelin and maintenance of meal-related decreases [25, 26]. In contrast, there was no significant change in ghrelin levels after RYGB, although it should be noted that by two years and later after RYGB, ghrelin levels do increase [26] and there was a large decrease after SG. In our study, pre-operative ghrelin levels were greater in the SG group compared with RYGB for unclear reasons. There was a wide range of fasting ghrelin values (SG, 226–960 pg/ml; RYGB, 110–904 pg/ml); this inter-individual variability and the smaller sample size in the SG group likely underlie the difference in baseline values. We cannot rule out the possibility that some of the variability in levels was due to the assay used which measures both acyl-ghrelin, considered the active form of ghrelin, plus des-acyl ghrelin. Other characteristics associated with differences in ghrelin levels, such as BMI, insulin resistance, and sex [48, 49] were similar between groups. Racial differences in ghrelin have been observed: African Americans had higher fasting levels and impaired postprandial suppression[50–52]. In our study, there was a larger proportion of African American subjects in the SG group compared to RYGB (36.8% and 15.0%, respectively), but there was no difference in fasting ghrelin between African American and Caucasian SG subjects (P = 0.56). Despite differences in ghrelin at baseline, the statistical analysis of change in ghrelin with time took into account baseline levels. Additionally, the percent reduction in AUC persisted at 52 weeks after SG. The large decrease in ghrelin is most likely due to resection of a large portion of the ghrelin-secreting cells of the gastric fundus and is consistent with the findings of recent studies [41, 42].
The mean fasting leptin level was also lower in RYGB compared to SG despite similar BMI at baseline. Body composition analysis was not performed thus, we are unable to decipher if differences in leptin were due to differences in adipose tissue mass or distribution. Menopause is known to be associated with decreased leptin and there was a higher proportion of women above the age of 50 in the RYGB group compared to SG (38% and 20%, respectively) which may have contributed somewhat to lower leptin levels. Given that the relationship between leptin and ghrelin is complex, [53] we are unable to draw inferences about whether differences in leptin levels between groups contributed to differences in ghrelin.
Paradoxically, decreases in post-prandial hunger were not maintained at 52 weeks despite marked decreases in ghrelin after SG. In addition, fullness increased significantly after SG despite lower postprandial PYY and GLP-1 levels compared to RYGB. In a study of 12 subjects who underwent SG, there were similar VAS scores for hunger and satiety at 3 months follow up compared to baseline despite increases in postprandial PYY [54]. Other mediators of fullness and satiety related to the mechanical changes after SG, such as increases in gastric pressure and vagal firing, could contribute to these findings. Interestingly, there was a trend toward decreased sweet cravings after RYGB, which recapitulates the findings of a prior study comparing LAGB with RYGB where LAGB patients had no change in sweet cravings and RYGB patients had significantly lower fasting and postprandial sweet cravings [26]. GLP-1 and PYY are potential mediators of this effect as they have been shown to enhance the ability to taste sweet flavors and heighten aversion to sweet taste [55, 56]. The presence of oral sweet taste receptors in the intestinal tract are known to enhance the intake and preference for sugar-rich foods and mediate GLP-1 release [57]. Our finding of increases in GLP-1 and PYY associated with RYGB support this mechanism and highlight the potential role of the gut-brain axis in mediating sugar preference recently described in rodents [58].
There was a significantly higher mean percentage weight loss after RYGB at 52 weeks. In line with long-term data, these differences in weight loss are anticipated to become more pronounced over time [11]. A subset of subjects was followed out to two years with weight data available for 74% of SG (14/19) and 70% of RYGB (28/40) demonstrating a lack of continued weight loss in SG and continued weight loss in RYGB of 26% and 32%, respectively.
A limitation of this study is the lack of randomization. There are also other gastrointestinal hormones of energy and glucose homeostasis of interest which were not measured [59, 60]. Additionally, only one type of meal stimulus was used. A solid meal stimulus or a different macronutrient composition, administered at different times of the day or at different rates, could have elicited different effects on hormone secretion and appetitive sensation. Rates of feeding have the ability to evoke higher sensations of satiety without corresponding changes in GLP-1 or PYY, which makes it possible other mechanisms unrelated to gut hormone secretion might underlie the success of surgery [54].
In conclusion, our results demonstrate that differences in gastrointestinal hormones of energy homeostasis and changes in sweet cravings are possible mechanisms for greater weight loss after RYGB compared to SG. Additionally, changes in these hormones may mediate weight-independent improvement in insulin resistance after RYGB. Continued research may further identify different factors that contribute to the metabolic benefits of SG and RYGB and the differences between these procedures.
Supporting information
(XLSX)
Acknowledgments
We would like to thank Irene Conwell, Princess Swan and Mya Pugh for their expert technical assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Institutes of Health [DK072011 to JK, T32 DK07271 to Dr. Domenico Accili, UL1TR001873 and UL1TR000040 to Dr. Muredach Reilly]. RA was supported in part by the National Institutes of Health (T32 DK 007559-29). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. 10.1056/NEJMoa055643 [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom LV. Mortality of severely obese subjects. Am J Clin Nutr. 1992;55(2 Suppl):516S–23S. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N Engl J Med. 2017;376(7):641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961 10.1136/bmj.g3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond). 2008;32 Suppl 7:S93–7. [DOI] [PubMed] [Google Scholar]
- 7.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–32. 10.1007/s11695-015-1657-z [DOI] [PubMed] [Google Scholar]
- 8.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27(9):2279–89. 10.1007/s11695-017-2666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA. 2018;319(3):241–54. 10.1001/jama.2017.20313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kroll D, Borbely Y, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA. 2018;319(3):255–65. 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, et al. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann Intern Med. 2018;169(11):741–50. 10.7326/M17-2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed B, King WC, Gourash W, Belle SH, Hinerman A, Pomp A, et al. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery. 2018;164(4):774–83. 10.1016/j.surg.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis. 2017;13(2):170–80. 10.1016/j.soard.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Hofso D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(12):912–24. 10.1016/S2213-8587(19)30344-4 [DOI] [PubMed] [Google Scholar]
- 15.McTigue KM, Wellman R, Nauman E, Anau J, Coley RY, Odor A, et al. Comparing the 5-Year Diabetes Outcomes of Sleeve Gastrectomy and Gastric Bypass: The National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA Surg. 2020:e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R15–27. 10.1152/ajpregu.00038.2011 [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care. 2016;39(6):893–901. 10.2337/dc16-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab. 2010;21(6):337–44. 10.1016/j.tem.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14(5):481 10.1007/s11892-014-0481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf). 2008;69(2):173–9. [DOI] [PubMed] [Google Scholar]
- 21.Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3–36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R367–75. 10.1152/ajpregu.00726.2005 [DOI] [PubMed] [Google Scholar]
- 22.Guida C, Stephen SD, Watson M, Dempster N, Larraufie P, Marjot T, et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine. 2019;40:67–76. 10.1016/j.ebiom.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab. 2015;4(6):437–60. 10.1016/j.molmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–51. 10.2337/db10-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30. 10.1056/NEJMoa012908 [DOI] [PubMed] [Google Scholar]
- 26.Tsouristakis AI, Febres G, McMahon DJ, Tchang B, Conwell IM, Tsang AJ, et al. Long-Term Modulation of Appetitive Hormones and Sweet Cravings After Adjustable Gastric Banding and Roux-en-Y Gastric Bypass. Obes Surg. 2019;29(11):3698–705. 10.1007/s11695-019-04111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33(7):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring). 2006;14(9):1553–61. [DOI] [PubMed] [Google Scholar]
- 29.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. 10.1016/j.soard.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. 10.1038/sj.ijo.0801083 [DOI] [PubMed] [Google Scholar]
- 31.Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–15. 10.1017/s0007114500001719 [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 33.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25(1):e70–9. 10.1111/nmo.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6. 10.1097/SLA.0b013e3181d3d21f [DOI] [PubMed] [Google Scholar]
- 35.Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, et al. Differences in Alimentary Glucose Absorption and Intestinal Disposal of Blood Glucose After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology. 2016;150(2):454–64 e9. 10.1053/j.gastro.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 36.Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21. 10.1007/s11695-009-9954-z [DOI] [PubMed] [Google Scholar]
- 37.Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258(6):976–82. 10.1097/SLA.0b013e3182774522 [DOI] [PubMed] [Google Scholar]
- 38.Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer AO, et al. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg. 2011;21(1):95–101. 10.1007/s11695-010-0317-6 [DOI] [PubMed] [Google Scholar]
- 39.Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18(9):1083–8. 10.1007/s11695-008-9576-x [DOI] [PubMed] [Google Scholar]
- 40.Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–52. 10.1007/s11695-013-1066-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svane MS, Bojsen-Moller KN, Martinussen C, Dirksen C, Madsen JL, Reitelseder S, et al. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology. 2019;156(6):1627–41 e1. 10.1053/j.gastro.2019.01.262 [DOI] [PubMed] [Google Scholar]
- 42.Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, et al. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: an 18-Month Prospective Study. Obes Surg. 2017;27(6):1563–72. 10.1007/s11695-016-2505-5 [DOI] [PubMed] [Google Scholar]
- 43.Casajoana A, Pujol J, Garcia A, Elvira J, Virgili N, de Oca FJ, et al. Predictive Value of Gut Peptides in T2D Remission: Randomized Controlled Trial Comparing Metabolic Gastric Bypass, Sleeve Gastrectomy and Greater Curvature Plication. Obes Surg. 2017;27(9):2235–45. 10.1007/s11695-017-2669-7 [DOI] [PubMed] [Google Scholar]
- 44.Disse E, Pasquer A, Pelascini E, Valette PJ, Betry C, Laville M, et al. Dilatation of Sleeve Gastrectomy: Myth or Reality? Obes Surg. 2017;27(1):30–7. 10.1007/s11695-016-2261-6 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen KT, Billington CJ, Vella A, Wang Q, Ahmed L, Bantle JP, et al. Preserved Insulin Secretory Capacity and Weight Loss Are the Predominant Predictors of Glycemic Control in Patients With Type 2 Diabetes Randomized to Roux-en-Y Gastric Bypass. Diabetes. 2015;64(9):3104–10. 10.2337/db14-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larraufie P, Roberts GP, McGavigan AK, Kay RG, Li J, Leiter A, et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019;26(6):1399–408 e6. 10.1016/j.celrep.2019.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–80 e2. 10.1053/j.gastro.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriano-Guillen L, Ortega L, Navarro P, Riestra P, Gavela-Perez T, Garces C. Sex-related differences in the association of ghrelin levels with obesity in adolescents. Clin Chem Lab Med. 2016;54(8):1371–6. 10.1515/cclm-2015-0555 [DOI] [PubMed] [Google Scholar]
- 49.Sakao Y, Ohashi N, Sugimoto M, Ichikawa H, Sahara S, Tsuji T, et al. Gender Differences in Plasma Ghrelin Levels in Hemodialysis Patients. Ther Apher Dial. 2019;23(1):65–72. 10.1111/1744-9987.12764 [DOI] [PubMed] [Google Scholar]
- 50.Araneta MR, Barrett-Connor E. Adiponectin and ghrelin levels and body size in normoglycemic Filipino, African-American, and white women. Obesity (Silver Spring). 2007;15(10):2454–62. [DOI] [PubMed] [Google Scholar]
- 51.Brownley KA, Light KC, Grewen KM, Bragdon EE, Hinderliter AL, West SG. Postprandial ghrelin is elevated in black compared with white women. J Clin Endocrinol Metab. 2004;89(9):4457–63. 10.1210/jc.2004-0607 [DOI] [PubMed] [Google Scholar]
- 52.Fluitt MB, Gambhir KK, Nunlee-Bland G, Odonkor W. Fasting plasma ghrelin levels are reduced, but not suppressed during OGTT in obese African American adolescents. Ethn Dis. 2013;23(4):436–40. [PMC free article] [PubMed] [Google Scholar]
- 53.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003;124(5):1532–5. 10.1016/s0016-5085(03)00350-0 [DOI] [PubMed] [Google Scholar]
- 54.Rigamonti AE, Bini S, Rocco MC, Giardini V, Massimini D, Crippa MG, et al. Post-prandial anorexigenic gut peptide, appetite and glucometabolic responses at different eating rates in obese patients undergoing laparoscopic sleeve gastrectomy. Endocrine. 2017;55(1):113–23. 10.1007/s12020-016-0933-6 [DOI] [PubMed] [Google Scholar]
- 55.Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107(4):476–83. 10.1016/j.physbeh.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 56.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104(5):709–21. 10.1016/j.physbeh.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 57.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1119–33. 10.1152/ajpregu.00038.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, et al. The gut-brain axis mediates sugar preference. Nature. 2020;580(7804):511–6. 10.1038/s41586-020-2199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2019;42(2):117–28. 10.1007/s40618-018-0892-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dimitriadis GK, Randeva MS, Miras AD. Potential Hormone Mechanisms of Bariatric Surgery. Curr Obes Rep. 2017;6(3):253–65. 10.1007/s13679-017-0276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]