Abstract
Prostate cancer (PCa) is the most frequently diagnosed non-cutaneous malignancy and leading cause of cancer mortality in men. At the initial stages, prostate cancer is dependent upon androgens for their growth and hence effectively combated by androgen deprivation therapy (ADT). However, most patients eventually recur with an androgen deprivation-resistant phenotype, referred to as castration-resistant prostate cancer (CRPC), a more aggressive form for which there is no effective therapy presently available. The current review is an attempt to cover and establish an understanding of some major signaling pathways implicated in prostate cancer development and castration-resistance, besides addressing therapeutic strategies that targets the key signaling mechanisms.
Keywords: Prostate cancer, Androgen, Signaling pathways, Castration resistant prostate cancer
1. Introduction
Prostate cancer (PCa) is the most common cancer among men with worldwide detection of more than 8,90,000 new cases and over 2,58,000 deaths each year [1]. Genetic preferences, inflammation, and increased cell proliferation are some of the predeterminant factors for PCa initiation. The occurrence of these processes in the epithelium of normal prostate initiates a cascade of events that lead to the formation of lesions, which can either directly progress to primary PCa or proliferative inflammatory atrophy (PIA) or induce an intermediate stage called prostatic intraepithelial neoplasia (PIN), in which basal cell layers lose their proliferation capacity resulting in increased activity of luminal secretory cells [2]. Molecular and pathological analysis of human PCa samples and studies with PCa animal models have shown that infectious agents, estrogenic hormones to dietary carcinogens, age, race, genetics and other occupational factors can cause damage to the prostate epithelium and elicit inflammatory responses leading to chronic or recurrent condition of PCa [2].
Androgen deprivation therapy (ADT) via chronic administration of gonadotropin-releasing hormone analogs, anti-androgens or their combination is considered as the standard choice of treatment for men with de novo or recurrent metastatic disease [3]. However, most patients invariably relapse and develop castration resistant PCa (CRPC) within 18–24 months despite maintenance of castrate testosterone serum levels, due to amplification or mutations in androgen receptors allowing their activation by progesterone, estrogens and androgen antagonists, generation of alternative splicing variants or to androgen neosynthesis in prostate tumor or adrenals. Besides, a host of molecular alterations also contribute to androgen-independence of PCa cells thereby stimulating disease progression [4,5].
2. In vitro and in vivo reports signifying the involvement of intracellular signaling cascades in PCa development/progression
The role of AR signaling in PCa development and progression has been well established [6–10]. In addition, several in vitro and in vivo studies have established the role of other signaling cascades in PCa development and progression. Earlier in 2002, Gasparian et al. [11] reported constitutive activation of nuclear factor kappa- light chain- enhancer of activated B cells (NF-κB) ‘survival signaling’ pathway in androgen-independent PCa cells. Studies by Zhang et al. demonstrated that activation of NF-κB pathway and subsequent downstream targets contributes to the progression and metastasis of PCa [12]. Huang et al. showed that blockade of NF-κB in human PCa cells is associated with suppression of angiogenesis, invasion, and metastasis [13]. Recently Jin et al. [14] demonstrated that activation of NF-κB signaling increases the expression of androgen receptor splicing variants (ARVs) in PCa cells and converts androgen-sensitive PCa cells to become androgen-insensitive, whereas downregulation of NF-κB signaling inhibits ARV expression and restores responsiveness of CRPC to anti-androgen therapy. In addition to NF-κB, constitutive activation of phosphoinositide-3-kinase/AKT (PI3K/Akt) has also been documented during PCa progression in autochthonous transgenic mouse model [15]. Shukla et al. [16] reported that treatment of human PCa cells LNCaP, PC-3 and DU145 with PI3K pharmacological inhibitor, LY294002, potentially suppressed the invasive properties in each of these cell lines. Chen et al. discussed that epidermal growth factor (EGF) and its receptor (EGFR)/Akt or EGFR/mitogen-activated protein kinase (MAPK) signaling path- ways are critical for maintaining cell survival in PCa [17]. Gan et al. studied the effects of extracellular signal-regulated kinases (ERK) and Akt pathways on EGF-mediated EGFR signaling, trafficking and cell motility in DU145 and PC3 cell lines and confirmed the significance of the EGFR signaling in PCa via ERK as well as Akt-dependent down streaming signaling [18]. Studies with NSK01105, a sorafenib derivative in human PCa cells and xenograft model indicated that this compound inhibited tumor growth and neovascularization by disrupting the EGFR activation [19]. Lorenzo et al. demonstrated the involvement of growth factor receptors of EGFR family in PCa development and progression to androgen independence [20]. Recent studies by whang et al., on lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor [21] gave a new shape to the EGFR signaling concept in the PCa that revealed the potent role of cross-talk of growth factors and their correlation with androgen-mediated development of PCa [22]. Drake et al. experimented on mouse model and projected that elevated tyrosine kinase signaling in advanced PCa [23]. Studies with antagonist of growth hormone inhibitors have also been shown to down-regulate PCa progression via down-regulation and inactivation of Akt and ERK kinases [24]. Deregulation in the expression of downstream effectors of PI3K/and RAS/MAPK pathways, specifically MNK have not only been documented during PCa but are also reported to contribute significantly to PCa growth and development [25–27]. Atala et al. showed that phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), a key downstream target of MNK promotes PCa development and progression [28]. Studies by Feng et al. established that targeting fibroblast growth factor receptor (FGFR) signaling could be a promising approach for treating aggressive PCa [29]. Muñoz-Moreno et al. demonstrated treatment of PC-3 cells with Growth Hormone-Releasing Hormone (GHRH) antagonists significantly decreased the level of gonadotropin-releasing hormone (GnRH); vascular endothelial growth factor (VEGF); and hypoxia-inducible factor 1-alpha (HIF-1a) expressions [30]. Yamamoto et al. identified that Wnt5, a critical ligand that activates the β-catenin-independent pathway in Wnt signaling promotes the rapid proliferation and metastasis of PCa [31]. Recently Valkenburg et al. demonstrated that activation of Wnt/β-catenin signaling is sufficient to produce high-grade prostate intraepithelial neoplasia (HGPIN) in hormonally normal mice [32]. Heidegger et al. demonstrated that overexpression of insulin receptor IGF1R promoted tumor growth and enhanced angiogenesis in human PCa cells [33]. Zengerling et al. reported that inhibition of IGF-1R diminishes transcriptional activity of the androgen receptor and its constitutively active, C-terminally truncated counterparts Q640X and AR-V7 via down-regulation of ARΔLBD signaling [34]. Shodeinde et al. disclosed that PCa growth development depend upon activated signal transducer and activator of transcription-3 (STAT3) [35]. Martin et al. showed that STAT3 activation drives CRPC progression via mediating loss of p53, a key tumor repressor [36]. Tyrosine phosphorylation of AR protein by non-receptor tyrosine kinases Src and Ack1 (activated cdc42-associated kinase) has been reported to activate AR even in low androgen environment, thereby promoting CRPC development [37,38].
3. Key molecular pathways promoting PCa development and progression
From the above studies, it is evident that besides AR signaling multiple intracellular signaling circuits play critical roles in the development and progression of PCa (Fig. 1), essentially influencing the transition of PCa cells from androgen dependent to castration resistant (CR) stage. This section summarizes alterations in key intracellular signaling pathways that promote PCa progression and the mechanisms by which they may influence therapeutic action of drugs targeting them.
Fig. 1.
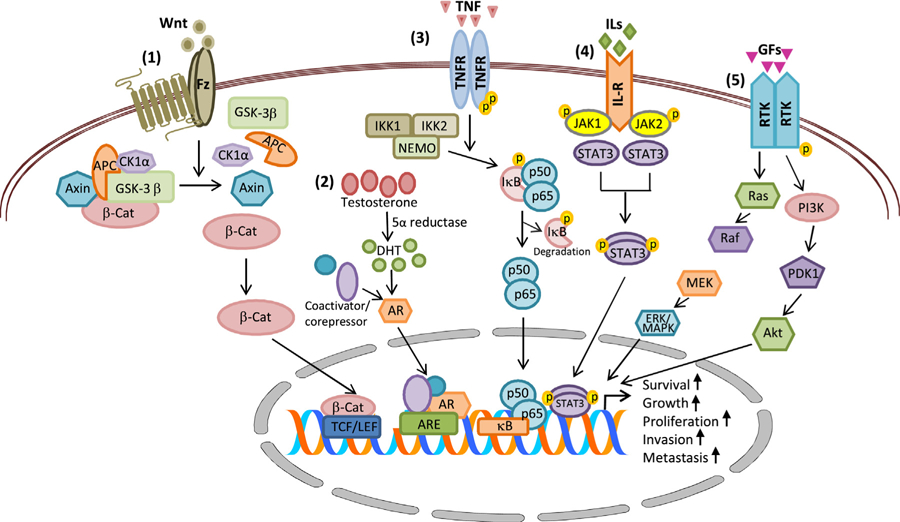
Chief signaling pathways involved in PCa development and progression. (1) Wnt/β-catenin signaling, (2) AR signaling, (3) NF-κB signaling, (4) JAK/STAT signaling and (5) receptor tyrosine kinase signaling. Abbreviations: AKT, akt serine/threonine kinase; AR, androgen receptor; ARE, androgen responsive elements; β-Cat, beta-catenin; DHT, dihydrotestosterone; GF, growth factor; Fz, Frizzled receptor; IκB, inhibitor of kappa B; ILs, interleukins; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa B; PI3K, phosphoinositide-3-kinase; Ras, rat sarcoma protein; RTK, receptor tyrosine kinase; STAT, signal transducers and activators of transcription; TCF/LEF-1, t-cell specific transcription factor/lymphoid enhancer-binding factor; Wnt, wnt ligands.
3.1. Androgen receptor (AR) mediated signaling pathway
The AR signaling is vital for normal functioning of the prostate, and initiation and maintenance of spermatogenesis [6]. AR is a member of the steroid hormone receptor family of ligand-activated nuclear transcription factors comprising of four distinct functional domains, a poorly conserved N-terminal domain (NTD) with transcriptional activation function; a highly conserved deoxyribonucleic acid (DNA)-binding domain (DBD) liable for DNA binding specificity and dimerization/stabilization of AR-DNA complex; a moderately conserved ligand-binding domain (LBD) that facilitates binding to steroid hormones. A short amino acid sequence called the ‘hinge region’ separates the LBD from the DBD and also contains part of a bipartite ligand-dependent nuclear localization signal (NLS) for AR nuclear transport [6,39].
Androgenic steroids are 19-carbon steroids with testosterone being the prototype. Testosterone is produced primarily by the testes with a small contribution from the adrenal glands. Testosterone is converted to DHT (highly expressed in prostate and genital tissues) by the action of the cytochrome P450 enzyme, 5α-reductase. Under physiological conditions, both testosterone and DHT can bind to and activate AR signaling.
In the absence of ligand, AR resides primarily in the cytoplasm in association with heat shock proteins (HSPs), cytoskeletal proteins and other chaperones.
Binding of ligand to the AR induces conformational changes in the LBD facilitating intramolecular and intermolecular interaction between N-terminal and C-terminal domains. This subsequently results in AR homo-dimerization, phosphorylation and nuclear translocation. In the nucleus, ligand bound AR binds to specific recognition sequences known as “androgen response elements” (AREs) in the promoter and enhancer regions of target genes along with its coregulators thereby modulating gene expression [6,39].
Deregulated AR signaling is common during PCa development and CRPC progression due to overexpression of AR arising due to amplification/mutations, co-activator and co-repressor modifications, aberrant activation/post-translational modification, altered steroidogenesis, and generation of AR splice variants [40]. Mutations in the AR although rare during initial stages of PCa are common during CRPC. These mutations permit continued androgen-axis activation even in the presence of low levels of androgen in the prostate microenvironment [41,42]. In addition, point mutations in AR besides facilitating increased AR activity in the prostate environment also broaden the ligand pool to which AR responds. In advanced PCa, growth factors such as transforming growth factor β (TGF-β), bone morphogenetic proteins (BMPs), insulin-like growth factor-1 (IGF-1), EGF, VEGF, fibroblast growth factor (FGF), interleukins (ILs), and other cytokines also act to promote synergistic activities of the androgen receptor [43,44]. Besides, more than 200 molecules have been identified as co-activators and co-repressors to the AR. Mutations in the various components of the co-regulator complex have also been reported to improve androgen-stimulated AR activation and disease progression of disease [45,46]. Aberrant activation of AR also occurs via alterations in the steroidogenesis pathways which permits PCa cells to bypass testosterone, and utilize adrenal androgens to generate functionally potent DHT via the 5α-dione pathway [47,48]. ARVs, the splice variants of full length AR generated as a result of alternative splicing such as exon skipping, cryptic exon inclusion, and cryptic splicing donor or acceptor usage also play a crucial role in promoting PCa progression. These splice variants although retain NTD and DBD of full-length AR, lack LBD ensuing in acquisition of hormone-independent activity to the AR. Besides localization in the nucleus, some AR variants also exhibit exclusive cytoplasmic function that is sufficient enough for transcriptional effects. In addition, ARVs can freely enter the nucleus without association with the Hsp90 chaperone complex [49–51]. Aside from the above described mechanisms that induce changes in the activity of AR, various transcription factors such as the proto-oncogene Myc, c-Jun, Sp1, FOXO3a, lymphoid enhancer binding factor 1 (LEF1), sterol regulatory element-binding proteins (SREBPs), cAMP response element-binding (CREB), NF-κB and twist-1 also have a crucial role in promoting AR expression via gene regulation [52].
3.2. NF-κB signaling
NF-κB is a protein complex that regulates expression of key genes required for innate and adaptive immunity, cell proliferation and survival, and lymphoid organ development. In humans, the NF-κB family comprises of five proteins namely p65 (RelA), RelB, p105/p50 (NF-κB1), p100/p52 (NF-κB2) and c-Rel. These proteins associate with each other to form homo- or heterodimeric complexes that are transcriptionally active. Although 15 different Rel dimer complexes can be made, the p50/65 heterodimer is the most abundant one and occurs in almost all cell types. The Rel proteins are activated by divergent stimuli including pro-inflammatory cytokines, T-and B-cell mitogens, bacteria and lipopolysaccharides, viruses, viral proteins, double stranded RNA, and physical and chemical stressors. In the unstimulated state, NF-κB dimers are retained in the cytosol as inactive proteins bound to IκB (inhibitor of kappa) proteins. In the canonical pathway of activation, degradation of the IκB inhibitory protein occurs through phosphorylation at specific serine residues (Ser 177/181 or Ser 176/180) by IκB kinase complex (IKKα or IKKβ). As a consequence, free NF-κB dimer enters the nucleus, binds to κB enhancer sites in the DNA and activates transcription of a wide array of genes participating in the immune and inflammatory response, cell growth, adhesion, metastasis, and apoptosis evasion. In contrast, the non-canonical NF-κB pathway activated by stimuli such as lymphotoxin-β (LTβ) and B cell-activating factor (BAFF) involves IKKα-dependent p100 processing instead of IκB degradation [53,54].
In prostate tumor cells, NF-κB is found frequently stimulated due to augmented levels of receptors such as tumor necrosis factor (TNF) that radically increase IκB degradation [55]. In androgen-independent prostate tumors, NF-κB expression is increased at both mRNA and protein level due to increased IL-6 expression that occurs as a result of constitutive NF-κB activation promoted by signal transduction via NF-κB inducing kinase (NIK) and IKK [56]. NF-κB also targets a transcription regulatory element of the prostate specific antigen PSA, a vital marker for development and progression of PCa [57,58]. NF-κB signaling in PCa cells also correlates with cancer progression, chemoresistance, and PSA recurrence [59]. Reports also indicate that NF-κB activation contributes to soft-tissue or bone metastasis in PCa [60,61]. TNF-α, a proinflammatory cytokine and prototypical NF-κB inducer along with its receptors TNFR1 and TNFR2 are found to be highly expressed in PCa. In Pca cells, amplified TNF-α expression has been correlated with increased proliferation and survival, angiogenesis, metastasis, and resistance to chemotherapeutic agents [62,63]. Recent studies have also demonstrated the existence of cross-talk between NF-κB and AR signaling. NF-κB expression has been shown to augment with increased AR expression and activity in androgen-independent xenografts. The p65/RelA subunit of NF-κB activity is also reported to increase both the gene and protein expression of AR. Furthermore, p65 of NF-κB could increase endogenous AR expression and its associated downstream target genes enhancing growth and survival in human PCa cells [64,65].
3.3. Growth factor signaling
PCa progression is often interrelated with deregulation of specific growth factors and their respective signaling pathways [66]. Growth factors are of three categories: positive growth factors, which promote growth and proliferation; negative growth factors, which regulate and inhibit cell growth/proliferation and induce apoptosis, and angiogenic growth factors, that provide growth factors necessary to build vascular and oxygen supplies necessary for tissue growth and survival. Growth factor receptors have receptor tyrosine kinase (RTK) activity. Ligand binding to growth factor receptors, triggers signaling pathways resulting in the activation of transcription factors and altered expression of numerous genes responsible for cell growth, proliferation and survival. While IGF-1 and its associated signaling pathway functions as a positive growth-promoting signal transduction pathway, FGF family of growth factors plays the role of both a positive growth factor and an angiogenic growth factor. Transforming growth factor-β (TGF-β) functions as a negative growth factor thereby regulating cell differentiation and proliferation [66,67]. Table 1 summarizes the role of various growth factors in PCa development and progression.
Table 1.
Role of growth factors in prostate cancer development and progress.
| Growth Factor | Role in prostate cancer |
|---|---|
| Adrenomedullin | Role in hormone-independent tumor growth and angiogenesis |
| Angiopoietin | Regulator of angiogenesis |
| Autocrine Motility Factor (AMF) | Cell motility and metastatic dissemination |
| Bone Morphogenetic Protein (BMPs) | Bone metastasis |
| Brain Derived Neurotrophic Factor (BDNF) | Apoptosis evasion |
| Epidermal Growth Factor (EGF) | Promotes cell migration, adhesion, and proliferation |
| Erythropoietin | Growth regulation |
| Fibroblast Growth Factor (FGF) | Bone metastasis |
| Growth Differentiation Factor (GDF) | Promotes cell adhesion and motility |
| Hepatocyte Growth Factor (HDGF) | Promote cell invasion and epithelial to mesenchymal transition |
| Insulin-like Growth Factors (IGFs) | Vital roles in cell proliferation, apoptosis evasion and invasion |
| Nerve Growth Factors (NGF) and other neurotrophins | Cancer cell proliferation and apoptosis evasion |
| Platelet-Derived Growth Factors (PDGF) | Promoter of tumor angiogenesis and bone metastasis |
| Transforming Growth Factor-β (TGF-β) | Promotes extracellular matrix production, induces angiogenesis, and inhibits host immune function |
| Tumour Necrosis Factor-α (TNF-α) | Inflammatory processes in tumor promotion |
| Vascular Endothelial Growth Factor (VEGF) | Plays a positive role in promoting angiogenesis |
| Placental Growth Factor (PlGF) | Promotes neovascularization |
During ADT, increase in autocrine and paracrine growth factor loops results in deregulation of essential growth and survival pathways. EGF, TGF-α, IGF-1, keratinocyte growth factor (KGF) or basic fibroblast growth factor (bFGF) as well as their corresponding receptors are found to be overexpressed in CRPC [68–71]. IGF-1 is commonly overexpressed in the prostatic stroma and exerts mitogenic action on prostatic epithelial cells in a paracrine manner. High serum levels of IGF-1 is considered as a predictor for PCa and increased risk of malignancy [71,72]. In addition, bFGF such as FGF-2, FGF-7, and FGF-8 are overexpressed in both benign and malignant prostate cells and are indicative of uncontrolled proliferation, tumor metastasis, and exceedingly low survival rates [73,74]. TGF-β1 is often augmented in the serum of PCa patients and is related with bone metastasis and poor clinical outcome [75–77]. Numerous other studies have also recognized that changes in the levels of TGF-β and its pathway components contribute to PCa progression and cellular responses [78,79]. Overexpression of the EGF receptor (EGFR, ErbB-1) has also been documented during PCa and strongly correlates with biochemical relapse [80,81]. RTK Her-2/neu (ErbB-2) is also reported to be overexpressed in androgen-independently growing cell lines as well as in sublines that have been xenografted into castrated mice [82,83]. This in turn leads to activation of AR related genes through the Akt pathway resulting in enhanced metastatic and angiogenetic potential [84,85].
3.4. Phosphoinositide-3-kinase/AKT signaling
PI3K/AKT pathway, a chief intracellular signal transduction mechanism that links diverse classes of membrane receptors essentially plays a central role in cellular quiescence, cell growth, proliferation, differentiation, motility, survival and angiogenesis. In humans, PI3K exists as a heterodimer of 110 kDa catalytic subunit and a 85 kDa regulatory subunit. Following stimulation by tyrosine kinase growth factor receptors such as EGFR; IGF-1R; G-protein-coupled receptors (GPCRs); cell adhesion molecules or oncogenic Ras, PI3K induces conscription and stimulation of the serine/threonine-specific protein kinase AKT via phosphorylation of the D3 position of phosphoinosities generating biologically active phosphatidylinositol (4,5) bisphosphate (PIP2) into phosphatidylinositol (3,4,5) trisphosphate (PIP3). PIP3 subsequently binds to AKT resulting in membrane translocation and its activation via phosphorylation. Activated AKT in turn phosphorylates and galvanizes several other proteins including mammalian target of rapamycin (mTOR) ultimately inducing and regulating a wide array of cellular processes [86]. Besides this, activation of mTOR by AKT can phosphorylate and inactivate 4E-BP1 (for eIF4E-Binding Proteins), a family of small acidic proteins that function as translational repressors of eIF4E. Phosphorylation of 4E-BP1 by mTOR releases 4E-BP1 from eIF4E thereby activating eIF4E involved translation-initiation complex and consequently the translational repertoire of a cell toward malignancy [87].
In 30%–50% of PCa patients, PI3K/AKT pathway is often augmented due to the loss of tumor suppressor PTEN (phosphatase and tensin homolog) that negatively regulates PI3K/AKT signaling by dephosphorylating PIP3 to PIP2 [86] [88]. In PCa cells, aberrant PI3K/AKT pathway disturbs the action of ERKs thereby favoring AR-independent growth. Congruently, AR target genes might impede PI3K/AKT pathway to favor AR-dependent growth in PCa cells [89,90]. Several mechanisms of cross-talk have been reported between AR and PI3K/AKT signaling. AKT can phosphorylate AR directly and inhibit activation of AR target genes, or PI3K/AKT signaling can regulate AR transcription via mechanisms other than AR phosphorylation [91,92]. Aberrations in PI3K pathway are also likely to constrain Ras/MEK/ERK pathway via amplified AKT activation. Besides, interrelationship between AKT and IGF signaling has also been reported in PCa cells. Upregulation of IGF, an upstream effector on AKT promotes PCa development in vivo [93,94]. Myc, a downstream PI3K/AKT target also interacts with AKT to endorse PCa development and progression. PI3K/AKT pathway can also act alongside with other proteins such as MST1, acetate Kinase (Ack1) and Bmi1 increasing their oncogenic potential [95,96]. PI3K/AKT is also known to increase the expression of MT1-MMP which are metalloproteinase receptors, thereby favoring PCa invasion and metastasis [90].
3.5. Janus Kinase/signal transducers and activators of transcription (JAK/STAT) signaling
JAK/STAT pathway is an imperative and pleiotropic membrane-to-nucleus cascade that transduces multitude of signals for normal development, cellular homeostasis, cell proliferation, differentiation, migration and apoptosis following stimulation by a wide variety of stimuli including reactive oxygen species, cytokines, and growth factors [97,98]. Briefly, activation of JAK/STAT pathway occurs when ligand binding induces multimerization of receptor subunits resulting in signal propagation via phosphorylation of receptor-associated JAK tyrosine kinases (JAK1, JAK2, JAK 3 and Tyk2). Principally, JAK activation occurs only upon receptor oligomerization as two JAKs are in close proximity ensuring trans-phosphorylation. Activated JAKs subsequently induce phosphorylation of other additional targets comprising both the receptors and STAT proteins. STATs reside in the cytosol as dormant transcription factors and become activated following phosphorylation by JAKs at conserved C-terminal tyrosine residue. Phosphorylation in turn induces dimerization of STATs through conserved SH2 domain subsequently allowing their entry into the nucleus through importin α-5 and the Ran nuclear import pathway. In the nucleus, STATs bind to specific sequences in the DNA to stimulate or suppress transcription of target genes [99].
Inhibition of JAK/STAT3 signaling is reported to suppress PCa cell growth and induces apoptosis [100]. The DNA repair gene BRAC1 can induce cell proliferation and inhibit apoptotic cell death in PCa cells through interaction with JAK1/2 and STAT3 phosphorylation [101]. AR can also associate with STAT3 and activate JAK/STAT pathway to stimulate cell proliferation and antiapoptotic effects. In addition, activation of STAT3 in PCa cells also stimulates various other genes that are associated with cell cycle progression, anti-apoptosis, angiogenesis and tumor invasion [102–104]. Besides, JAK2 can activate STAT5a/b in PCa cells via phosphorylation on conserved tyrosine residues Y694Stat5a and Y699Stat5b. This results in STAT5a/b dimerization and ensuing nuclear translocation where the dimer binding to specific response elements of target genes promotes prostate cancer growth, tumor progression and distant metastases [104–107]. Components of JAK-STAT pathway specifically pJAK-1 and pSTAT-3 function as predictors of biochemical relapse and poor prognosis of PCa [100].
3.6. MAPK pathway
MAPKs are serine-threonine kinases comprising of three distinct groups specifically ERKs, Jun N-terminal kinases (JNKs), and p38 isoforms. MAPK signaling links extracellular signals to the machinery that controls fundamental cellular processes such as growth, proliferation, differentiation, migration, apoptosis and transformation. Each MAPK signaling axis comprises of a three-tier kinase module: a MAPK kinase kinase (MAP3K), a MAPK kinase (MAP2K), and a MAPK. MAP3Ks phosphorylate and activate MAP2Ks, which in turn phosphorylate and activate MAPKs [108–110]. MAPK pathways are activated either by a sequence of binary interactions between the kinase components or via formation of a signaling complex containing multiple kinases guided by a scaffold protein. While kinase suppressor of Ras-1 (KSR) and MEK partner 1 (MP1) function as scaffold proteins for the ERK pathway, JNK-interacting proteins (JIPs) serve as scaffold proteins for the JNK pathway. β-Arrestin 2 acts as a scaffold protein for both the ERK and JNK signaling pathway [111]. Upon activation, the MAPKs phosphorylate various substrate proteins including transcription factors such as c-Jun, c-Fos, ATF2, and p53. Erk or p38 MAPKs can also activate MAPK interacting protein kinases 1 and 2 (MNK1 and MNK2) that play important roles in controlling signals involved in mRNA translation [112]. Phosphorylation of MNKs 1 and MNK2) by MAPK activates its kinase activity as well as to enhances its binding to the eukaryotic initiation factor 4G (eIF4G) which functions as a scaffolding protein. Additionally MNK mediated phosphorylation of eIF4E regulates its release from eIF4G which along with its binding partners and the small ribosomal subunits constitute vital components of the 48S initiation complex required for cap-dependent translation initiation. Activation of the eIF4E by MNKs promote the translation of a subset of mRNAs are referred to as ‘eIF4E-sensitive’ most of which includes proliferation and survival-promoting proteins such as cyclin D1 and D3, c-Myc, MDM2 (mouse double minute 2), VEGF, survivin and Bcl-2 (B-cell lymphoma 2) [113,114].
Overexpression of EGF, FGF, IGF, and KGFs in PCa frequently results in activation of endogenous Ras and MAPK pathways [115–117]. Overexpression of Ras and its mutant form also promotes castration resistance in LNCaP cells endorsing tumorigenicity [118]. p38 signaling chiefly activated at later stages of PCa increases the expression of aquaporins which are pore-forming proteins thereby enabling PCa cells survive through hypoxia [119,120]. Besides, MNKs and p-eIF4E are also found to be highly overexpressed in PCas [25–27] [25–27]. The Oncomine database documents that MNK2 is overexpressed 1.5 to 4.4-fold in hormone resistant and metastatic prostate tumors [121]. Increased MNK activity is documented to promote proliferation in PCa cells [25–27,122].
3.7. Wnt/β-catenin signaling
Wnt/β-catenin pathway is a highly conserved developmental signaling pathway comprising of secreted glycoproteins that play a vital role in tissue homeostasis, cell proliferation, differentiation, migration, and epithelial-mesenchymal communications, polarity and asymmetric cell division [123]. Based on the ability to stabilize the multifunction protein β-catenin, Wnt signaling is subdivided into canonical pathway and the noncanonical (planar cell polarity or Wnt/calcium) pathway. β-catenin predominantly exists in the cytosol in complex with adenomatous polyposis coli (APC), axin, casein kinase 1 (CK1), and glycogen synthase kinase 3β (GSK-3β). In the absence of Wnt signal, GSK-3β and CK1, phosphorylate β-catenin at specific serine/threonine residues leading to its ubiquitination and proteasomal degradation via F-box/WD repeat-containing protein 1A (FBXW1A)/S-phase kinase-associated protein (SKP) complex. In contrast, binding of Wnt ligands to frizzled receptors (FZD) hyperphosphorylates and activates disheveled proteins (DVL). Activated Dvl in its turn displaces GSK-3β from the β-catenin complex thereby averting GSK-3β-mediated phosphorylation and consequent degradation of β-catenin. As a result, free β-catenin accumulates in the perinuclear region forming a pool of free signaling molecules which ultimately interact with lymphoid enhancer factor/T cell factor (LEF/TCF) in the DNA to stimulate transcription of various target genes including that of c-Myc, p300, Foxo, Bcl9–2, c-Jun, CtBP, and cyclin D1 through displacement of groucho-HDAC co-repressors [124–128].
Increased expression of β-catenin occurs quite commonly in PCa due aberrant AKT signaling which results in phosphorylation and inactivation of GSK3β which favours formation of uncomplexed cytosolic β-catenin [129]. Besides, mutant forms of β-catenin have also been discovered in PCa. The mutant forms of β-catenin surpass normal mechanisms of phosphorylation by GSK3b and further degradation via the ubiquitin pathway [130,131]. During PCa, β-catenin is also found to associate with AR thereby enhancing expression of various growth and proliferative genes [132]. Expression of Wnt ligands is high in PCa and correlates with disease progression, higher Gleason scores, augmented PSA, and metastasis [133–135]. In addition to Wnts, frizzled receptors are also upregulated during PCa. Specifically, FZD4 overexpression is common in PCa and favours epithelial-to-mesenchymal transition [135,136].
4. Novel therapeutics targeting key signalling pathways in PCa
So far, Food and Drug Administration (FDA) has approved 19 agents for PCa therapy and treatment [137–158]. Fig. 2 represents the chemical structures of some of the drugs approved and in advanced development for PCa treatment. The majority of these agents are hormonal modulators targeting the androgen pathway. The gonadotropin-releasing agonists such as leuprolide (Lupron), goserelin (Zoladex), and triptorelin (Trelstar) that block the release of LHRH, and anti-androgens such as bicalutamide (Casodex), flutamide (Eulexin), and nilutamide (Nilandron) that help block the action of testosterone in PCa cells have been the backbone of PCa treatment [137–143]. However, for advanced PCa specifically CRPC limited treatment options exist providing avenues for the development of newer agents targeting AR and other major signaling cascades.
Fig. 2.
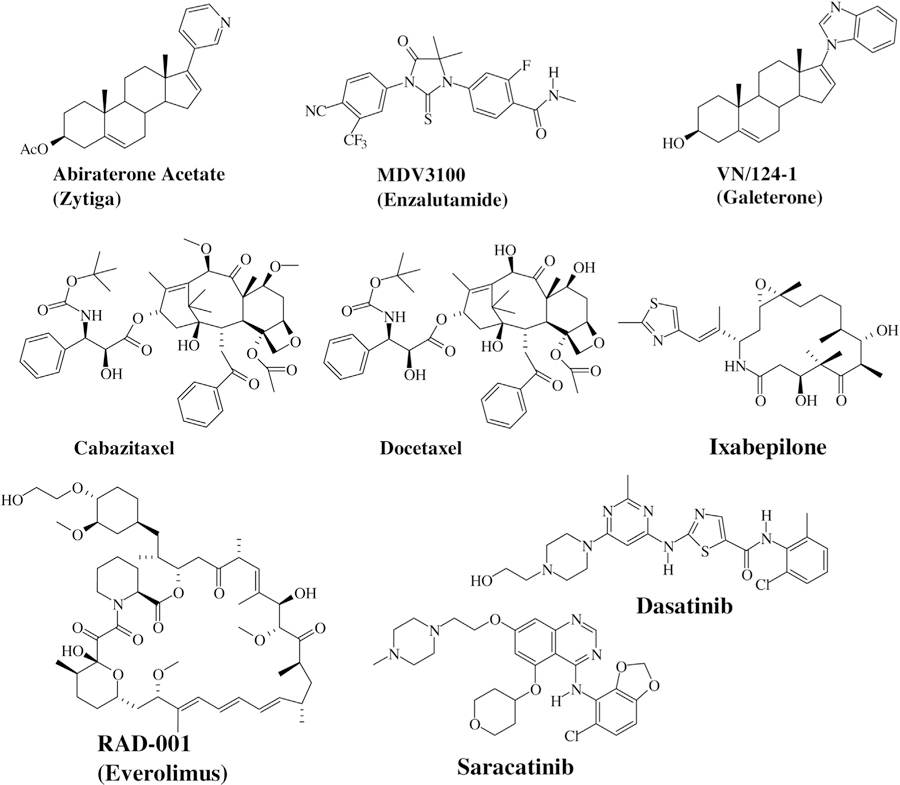
Chemical Structures of some of the approved/in development Prostate Cancer Drugs.
4.1. Inhibitors of AR signaling
In depth understanding of AR signaling and the mechanisms of castration resistance in PCa has resulted in the development of novel agents that can more efficiently retract AR signaling. Abiraterone acetate (Zytiga) is a selective, oral agent that can irreversibly inhibit the enzymatic activity of CYP17A1, a key member of the cytochrome p450 family with dual functions of 17a-hydroxylase and C17,20-lyase activity. It catalyzes the 17α-hydroxylation of steroid intermediates that is critical in testosterone biosynthesis. The blockade of CYP17A1 by Abiraterone thus decreases androgen synthesis in adrenal glands, testes, and tumor cells. Abiraterone has been shown to reduce serum testosterone levels to below a detection threshold of 1 ng/dL [159]. Abiraterone is 10- to 30-fold more potent CYP17 inhibitor than ketoconazole, a broad spectrum antifungal agent that has been extensively used as second-line hormonal therapy for PCa due to its ability to inhibit 11-β hydroxylation, cholesterol side chain cleavage to pregnenolone and CYP17 [160,161]. MDV3100 (Enzalutamide) is a potent oral antagonist. As opposed to firstgeneration anti-androgens, MDV3100 is an anti-androgen with multiple effects on AR- it is a competitive inhibitor of the C-terminus ligand-binding domain, besides it also prevents AR nuclear translocation, AR binding to DNA, and co-activator recruitment. MDV3100 exhibits a 5- to 8-fold greater activity than bicalutamide and only 2- to 3-fold reduced activity compared with the native ligand dihydrotestosterone [162–164]. While abiraterone and enzalutamide show survival benefit in castrate-resistant disease, PCa cells eventually develop resistance [165–167]. Galeterone (VN/124–1, TOK-001) developed by Tokai Pharmaceuticals is a 17-heteroazole steroidal analogue currently in pivotal phase 3 clinical trial for men with metastatic, castration-resistant PCa or CRPC, whose prostate tumor cells express the AR- V7 splice variant [168]. Galeterone disrupts the androgen receptor signaling by functioning in 3 ways: (i) androgen receptor degradation, which reduces the amount of androgen receptor in tumor cells (ii) CYP17 enzyme inhibition, which blocks the synthesis of testosterone (iii) androgen receptor inhibition, which blocks the binding of testosterone or DHT with the androgen receptor [169–171].
4.2. Inhibitors of growth factor signaling, RTK and PI3K/AKT/mTOR pathway
Several inhibitors of key intracellular signaling pathways that are aberrant in PCa such as IGF, RTK and PI3K/AKT/mTOR signaling are also being actively investigated either as single agents or in combination for PCa treatment and therapy. Drugs targeting IGF-1R signaling are broadly classified as (A) neutralizing antibodies and (B) small molecule inhibitors of the IGF-1R tyrosine kinase activity (eg: BMS-754807, NVP-ADW742, NVP-AEW541, OSI-906, XL228). Besides blocking IGF-1R activity, the neutralizing antibodies (AMG-479, cixutumumab or IMC-A12, figitumumab or CP751,871, MK0646, R-1507, Sch-717454) also down-regulate IGF-1R overtime by promoting receptor internalization [172]. Inhibitors of PI3K/Akt pathway are also in clinical development. They are grouped into four main classes as PI3K inhibitors, Akt inhibitors, mTOR inhibitors and dual PI3K–mTOR inhibitors. The PI3K inhibitors are further divided into isoform-specific inhibitors or pan-PI3K inhibitors and include XL147, PX866, GDC0941, BKM120, and CAL101. Akt inhibitors comprise of ATP mimetics and non-catalytic site inhibitors. Examples of Akt inhibitors include perifosine, GSK690693, VQD002 and MK2206. Dual PI3K–mTOR inhibitors target the p110α, β and δ isoforms, mTORC1 and mTORC2 as the p110 subunits of PI3K and mTOR share similar structures. Examples of this class of inhibitors include BEZ235, BGT226, XL765, SF1126 and GSK1059615. mTOR inhibitors directly inhibit catalytic site of mTOR including mTORC1 and mTORC2. Examples of this kind include OSI027, AZD8055, CCI-779, and RAD-001 (Everolimus) [173–176].
Several small molecule inhibitors of Src family of kinases (SFKs), a group of non-receptor tyrosine kinases that play crucial role in PCa development, progression and metastasis have been developed and are under clinical testing. Of these, Dasatinib is the first FDA approved SFK/ABL dual inhibitor. Dasatinib in addition to inhibiting Fyn, Yes, Src, and Lyk- the Src family of kinases, also obstructs BCR-ABL, EphA2, platelet-derived growth factor receptor, c-Kit, MAPKs and RTKs resulting in suppression of cell adhesion, migration and invasion. Other SFK inhibitors under clinical testing include saracatinib (AZD0530) and bosutinib (SKI-606) [177–181]. A phase II trial of AZD0530 induced durable PSA responses in patients with advanced CRPC [182].
4.3. RANKL inhibitors
Preclinical studies in prostate cancer xenografts have firmly established that blockade of receptor activator of nuclear factor-κB (RANK) signal instigated by binding of RANK ligands (RANK-L) impairs the establishment and progression of bone metastasis [183]. Denosumab, a fully human monoclonal antibody is an FDA approved drug for treating metastasis in men with PCa. It binds specifically binds to and antagonizes RANK-L function thus alleviating bone reabsorption. Besides, Denosumab can also directly inhibit osteoclasts to abrogate bone reabsorption [183,184].
4.4. Taxanes and epothilones
Taxanes [Paclitaxel (Taxol) and Docetaxel (Taxotere)] encompass a class of cytotoxic chemotherapeutic agents that has been shown to provide a survival benefit in advanced PCa, inhibiting tumor development and decreasing prostate-specific antigen (PSA) levels. The mechanism of action of taxanes involves the stabilization of microtubules. Taxanes block cell cycle progression through centrosomal impairment, stimulation of abnormal spindle formation and destruction of spindle microtubule dynamics. Taxanes are also suggested to influence AR function by reducing the expression of AR-activated genes [185]. Tubulin mutations and overexpression of ATP-dependent drug efflux pump P-glycoprotein are key factors chemoresistance to taxanes [186]. Efforts to overawe resistance to taxanes have resulted in the development of epothilones and cabazitaxel, which are novel tubulin-binding agents [187,188]. Epothilones are macrolide antibiotics which are structurally different from taxanes in their tubulin-binding site. They induce stabilization of microtubules via binding near the taxane-binding site resulting in cell death and tumor regression [187]. Ixabepilone, a semisynthetic derivative of the natural epothilone B is reported to be active against metastatic CRPC in chemo-naive and docetaxel-refractory CRPC [189]. Cabazitaxel (Jevtana) is a potent, second-generation, semisynthetic taxane. Although it functions as a microtubule inhibitor similar to docetaxel and paclitaxel and binds to tubulin, leading to microtubule stabilization, inhibition of mitosis and cell death, it exhibits poor affinity for P-glycoprotein-mediated efflux pumps. Hence cabazitaxel benefits patients with demonstrated taxane resistance. Besides it can also penetrate the blood brain barrier to a greater extent than docetaxel and paclitaxel, further augmenting its ability to target metastatic brain lesions [188]. Table 2 summarizes the mechanism of actions of some of the approved/ in development PCa drugs.
Table 2.
Mechanism of action of some of the approved/in development PCa drugs.
| S.No. | Name/Trade name | Mechanism of action/target |
|---|---|---|
| 1. | Abiraterone actate (Zytiga) | CYP17 inhibitor |
| 2. | MDV3100 (Enzalutamide) | Androgen-receptor (AR) antagonist |
| 3. | VN/124-1 (Galaterone) | Androgen receptor antagonist, AR degradation, CYP17 inhibition |
| 4. | Cabazitaxel | Microtubule inhibitor |
| 5. | Docetaxel | Microtubule inhibitor |
| 6. | Ixabepilone | Antimicrotubular agent |
| 7. | RAD-001 (Everolimus) | mTOR inhibitor |
| 8. | Dasatinib | Tyrosine kinase inhibitor |
| 9. | Saracatinib | Dual kinase inhibitor (Src and Bcr-Abl tyrosine-kinase inhibitor) |
| 10. | Denosumab | RANKL inhibitor |
5. Conclusions and prospects
A search of clinicaltrials.gov for new PCa protocols registered during the past 3 years identified 783 protocols (search criteria: search term = PCa; study type = interventional; first received = from 01/01/2013 to 01/28/2016). Of these, drug interventional studies total to 452, of which 45 protocols were for CRPC [190]. Hence a great deal of effort has been made in the past few years to develop new therapeutic options for PCa, in particular CRPC. A constellation of “potential PCa targets” have been uncovered in recent years resulting in a large number of new agents specifically belonging to the innovative classes of targeted therapy. However, it is still not sure whether a multi-targeted approach that concurrently inhibits diverse signaling cascades including their feedback activation will result in a well-tolerated additive/synergistic therapeutic effect. Besides, another major challenge is the identification of appropriate biomarkers that will predict clinical response from targeted novel therapeutics in heterogeneous PCa patients. Hence, it is foremost to identify biomarkers that correlate well with overall survival and promising antitumor activity in conjunction with the development of targeted agents. Thus, with the development of effective targeted therapeutics that block key signaling cascades aberrant during PCa and its progression, and identification of biomarkers that accurately validates the efficacy of these therapeutics, we anticipate that survival and well-being in patients with PCa will improve significantly in the years ahead.
Acknowledgments
This work was supported in part by a grant from NIH and NCI (RO1CA129379 and R21CA195694) and start-up funds from University of Maryland School of Medicine, the Center for Biomolecular Therapeutics (CBT), and Marlene and Stewart Greenebaum Cancer Center (Philanthropic Funds), Baltimore, USA to Professor Vincent C.O. Njar; and by the Office of the Assistant Secretary of Defense for Health Affairs, through the Prostate Cancer Research Program under Award No. W81XWH-15-1-0586 to Vidya P Ramamurthy. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
References
- [1].Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F, International variation in prostate cancer incidence and mortality rates, Eur. Urol 61 (6) (2012) 1079–1092. [DOI] [PubMed] [Google Scholar]
- [2].Nelson WG, Sfanos KS, DeMarzo AM, Yegnasubramanian S, Prostate inflammation and prostate cancer, Management of Prostate Cancer, Springer, 2013, pp. 103e115. [Google Scholar]
- [3].Saad F, Fizazi K, Androgen deprivation therapy and secondary hormone therapy in the management of hormone-sensitive and castration-resistant prostate cancer, Urology 86 (5) (2015) 852–861. [DOI] [PubMed] [Google Scholar]
- [4].Katzenwadel A, Wolf P, Androgen deprivation of prostate cancer: leading to a therapeutic dead end, Cancer Lett 367 (1) (2015) 12–17. [DOI] [PubMed] [Google Scholar]
- [5].Harris WP, Mostaghel EA, Nelson PS, Montgomery B, Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion, Nat. Clin. Pract. Urol 6 (2) (2009) 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lonergan PE, Tindall DJ, Androgen receptor signaling in prostate cancer development and progression, J. Carcinog 10 (2011) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Culig Z, Santer FR, Androgen receptor signaling in prostate cancer, Cancer Metastasis Rev 33 (2–3) (2014) 413–427. [DOI] [PubMed] [Google Scholar]
- [8].Debes JD, Tindall DJ, The role of androgens and the androgen receptor in prostate cancer, Cancer Lett 187 (1–2) (2002) 1–7. [DOI] [PubMed] [Google Scholar]
- [9].Tan MH, Li J, Xu HE, Melcher K, Yong EL, Androgen receptor: structure, role in prostate cancer and drug discovery, Acta Pharmacol. Sin 36 (1) (2015) 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suzuki H, Ueda T, Ichikawa T, Ito H, Androgen receptor involvement in the progression of prostate cancer, Endocr. Relat. Cancer 10 (2) (2003) 209–216. [DOI] [PubMed] [Google Scholar]
- [11].Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV, The role of IKK in constitutive activation of NF-kappaB transcription factor in prostatecarcinoma cells, J. Cell Sci 115 (Pt 1) (2002) 141–151. [DOI] [PubMed] [Google Scholar]
- [12].Zhang Y, Huang H, Zhou H, Du T, Zeng L, Cao Y, Chen J, Lai Y, Li J, Wang G, Guo Z, Activation of nuclear factor (B pathway and downstream targets survivin and livin by SHARPIN contributes to the progression and metastasis of prostate cancer, Cancer 120 (20) (2014) 3208–3218. [DOI] [PubMed] [Google Scholar]
- [13].Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ, Blockade of NF- kappaB activity in human prostate cancer cells is associated withsuppression of angiogenesis, invasion, and metastasis, Oncogene 20 (31) (2001) 4188–4197. [DOI] [PubMed] [Google Scholar]
- [14].Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, Hayward SW, Matusik RJ, Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression, Oncogene 34 (28) (2015) 3700–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S, Constitutive activation of PI3K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model, Prostate 64 (3) (2005) 224–239. [DOI] [PubMed] [Google Scholar]
- [16].Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S, Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion, Int. J. Cancer 121 (7) (2007) 1424–1432. [DOI] [PubMed] [Google Scholar]
- [17].Chen W, Xie D, Hou J, Long H, Li G, Pu J, Ouyang J, Wu Y, Inhibition of EGFR signaling in prostate cancer treated with EGFR siRNA and Gefitinib, Life Sci. J 9 (2) (2012) 544–552. [Google Scholar]
- [18].Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y, Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells, Oncogene 29 (35) (2010) 4947–4958. [DOI] [PubMed] [Google Scholar]
- [19].Yu P, Ye L, Wang H, Du G, Zhang J, Zuo Y, Zhang J, Tian J, NSK-01105, a novel sorafenib derivative, inhibits human prostate tumor growth via suppression of VEGFR2/EGFR-mediated angiogenesis, PLoS One 9 (12) (2014) e115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lorenzo GD, Bianco R, Tortora G, Ciardiello F, Involvement of growth factor receptors of the epidermal growth factor receptor family in prostate cancer development and progression to androgen independence, Clin. Prostate Cancer 2 (1) (2003) 50–57. [DOI] [PubMed] [Google Scholar]
- [21].Whang YE, Armstrong AJ, Rathmell WK, Godley PA, Kim WY, Pruthi RS, Wallen EM, Crane JM, Moore DT, Grigson G, Morris K, Watkins CP, George DJ, A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patientswith castration-resistant prostate cancer, Urol. Oncol 31 (1) (2013) 82–86. [DOI] [PubMed] [Google Scholar]
- [22].Ren J, Bollu LR, Su F, Gao G, Xu L, Huang WC, Hung MC, Weihua Z, EGFR- SGLT1 interaction does not respond to EGFR modulators, but inhibition of SGLT1 sensitizes prostate cancer cells to EGFR tyrosine kinase inhibitors, Prostate 73 (13) (2013) 1453–1461. [DOI] [PubMed] [Google Scholar]
- [23].Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON, Oncogene-specific activation of tyrosine kinase networks during prostate cancerprogression, Proc. Natl. Acad. Sci. U. S. A 109 (5) (2012) 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rick FG, Schally AV, Szalontay L, Block NL, Szepeshazi K, Nadji M, Zarandi M, Hohla F, Buchholz S, Seitz S, Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases, Proc. Natl. Acad. Sci. U. S. A 109 (5) (2012) 1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bianchini A, Loiarro M, Bielli P, Busà R, Paronetto MP, Loreni F, Geremia R, Sette C, Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells, Carcinogenesis 29 (12) (2008) 2279–2288. [DOI] [PubMed] [Google Scholar]
- [26].Ramamurthy VP, Ramalingam S, Gediya L, Kwegyir-Afful AK, Njar VC, Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines, Oncotarget 6 (5) (2015) 3195–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mbatia HW, Ramalingam S, Ramamurthy VP, Martin MS, Kwegyir-Afful AK, Njar VC, Novel C-4 heteroaryl 13-cis-retinamide Mnk/AR degrading agents inhibit cell proliferation and migration and induce apoptosis in human breast and prostate cancer cells and suppress growth of MDA-MB-231 human breast and CWR22Rv1 human prostate tumor xenografts in mice, J. Med. Chem 58 (4) (2015) 1900–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Re Atala A., eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression, J. Urol 185 (4) (2011) 1533. [DOI] [PubMed] [Google Scholar]
- [29].Feng S, Shao L, Yu W, Gavine P, Ittmann M, Targeting fibroblast growth factor receptor signaling inhibits prostate cancer progression, Clin. Cancer Res 18 (14) (2012) 3880–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muñoz-Moreno L, Arenas MI, Schally AV, Fernández-Martínez AB, Zarka E, González-Santander M, Carmena MJ, Vacas E, Prieto JC, Bajo AM, Inhibitory effects of antagonists of growth hormone- releasing hormone on growth andinvasiveness of PC3 human prostate cancer, Int. J. Cancer 132 (4) (2013) 755–765. [DOI] [PubMed] [Google Scholar]
- [31].Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A, Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression ofmetalloproteinase, Oncogene 29 (14) (2010) 2036–2046. [DOI] [PubMed] [Google Scholar]
- [32].Valkenburg KC, Yu X, De Marzo AM, Spiering TJ, Matusik RJ, Williams BO, Activation of Wnt/β-catenin signaling in a subpopulation of murine prostate luminal epithelial cells induces high grade prostate intraepithelial neoplasia, Prostate 74 (15) (2014) 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heidegger I, Kern J, Ofer P, Klocker H, Massoner P, Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis, Oncotarget 5 (9) (2014) 2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zengerling F, Azoitei A, Herweg A, Jentzmik F, Cronauer MV, Inhibition of IGF-1R diminishes transcriptional activity of the androgen receptor and its constitutively active, C-terminally truncated counterparts Q640X and AR-V7, World J. Urol (2015) in press). [DOI] [PubMed]
- [35].Shodeinde AL, Barton BE, Potential use of STAT3 inhibitors in targeted prostate cancer therapy: future prospects, Onco Targets Ther 5 (2012) 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martin P, Liu YN, Pierce R, Abou-Kheir W, Casey O, Seng V, Camacho D, Simpson RM, Kelly K, Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition, Am. J. Pathol 179 (1) (2011) 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ, Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor, Cancer Res 66 (2006) 11047–11054. [DOI] [PubMed] [Google Scholar]
- [38].Mahajan NP, Whang YE, Mohler JL, Earp HS, Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox, Cancer Res 65 (2005) 10514–10523. [DOI] [PubMed] [Google Scholar]
- [39].Green SM, Mostaghel EA, Nelson PS, Androgen action and metabolism in prostate cancer, Mol. Cell. Endocrinol 360 (1–2) (2012) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chandrasekar T, Yang JC, Gao AC, Evans CP, Targeting molecular resistance in castration-resistant prostate cancer, BMC Med 13 (2015) 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu W, Xie CC, Zhu Y, Li T, Sun J, Cheng Y, Ewing CM, Dalrymple S, Turner AR, Sun J, Isaacs JT, Chang BL, Zheng SL, Isaacs WB, Xu J, Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer, Neoplasia 10 (8) (2008) 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL, Integrative genomic profiling of human prostate cancer, Cancer Cell 18 (1) (2010) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ, Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance, Cancer Res 68 (13) (2008) 5469–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hara T, Kouno J, Nakamura K, Kusaka M, Yamaoka M, Possible role of adaptive mutation in resistance to antiandrogen in prostate cancer cells, Prostate 65 (3) (2005) 268–275. [DOI] [PubMed] [Google Scholar]
- [45].Heemers HV, Tindall DJ, Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex, Endocr. Rev 28 (7) (2007) 778–808. [DOI] [PubMed] [Google Scholar]
- [46].Wolf IM, Heitzer MD, Grubisha M, DeFranco DB, Coactivators and nuclear receptor transactivation, J. Cell. Biochem 104 (5) (2008) 1580–1586. [DOI] [PubMed] [Google Scholar]
- [47].Chang KH, Ercole CE, Sharifi N, Androgen metabolism in prostate cancer: from molecular mechanisms to clinical consequences, Br. J. Cancer 111 (7) (2014) 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N, Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer, Proc. Natl. Acad. Sci. U. S. A 108 (33) (2011) 13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J, Ligand-independent androgen receptor variants derived from splicing of cryptic exonssignify hormone-refractory prostate cancer, Cancer Res 69 (1) (2009) 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR, Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant, J. Clin. Invest 120 (8) (2010) 2715–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dehm SM, Tindall DJ, Alternatively spliced androgen receptor variants, Endocr. Relat. Cancer 18 (5) (2011) R183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shiota M, Yokomizo A, Naito S, Increased androgen receptor transcription: a cause of castration-resistant prostate cancer and a possible therapeutic target, J. Mol. Endocrinol 47 (1) (2011) R25–41. [DOI] [PubMed] [Google Scholar]
- [53].Hayden MS, Ghosh S, Shared principles in NF-kappaB signaling, Cell 132 (3) (2008) 344–362. [DOI] [PubMed] [Google Scholar]
- [54].Prasad S, Ravindran J, Aggarwal BB, NF-kappaB and cancer: how intimate is this relationship, Mol. Cell. Biochem 336 (1–2) (2010) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gélinas C, Rabson AB, Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells, Prostate 52 (3) (2002) 183–200. [DOI] [PubMed] [Google Scholar]
- [56].Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL, Co-operative functions between nuclear factors NFkappaB and CCAT/enhancer-binding protein-beta (C/EBP-beta) regulate the IL-6 promoter in autocrine human prostate cancer cells, Prostate 61 (4) (2004) 354–370. [DOI] [PubMed] [Google Scholar]
- [57].Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S, Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression, Neoplasia 6 (4) (2004) 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen CD, Sawyers C B.L.N.F-kappa, activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer, Mol. Cell. Biol 22 (8) (2002) 2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, Smith JA Jr., R.J. Matusik, NF-(B gene signature predicts prostate cancer progression, Cancer Res 74 (10) (2014) 2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee CH, Jeon YT, Kim SH, Song YS, NF-kappaB as a potential molecular target for cancer therapy, Biofactors 29 (1) (2007) 19–35. [DOI] [PubMed] [Google Scholar]
- [61].Jiang J, Slivova V, Jedinak A, Sliva D, Gossypol inhibits growth, invasiveness, and angiogenesis in human prostate cancer cellsby modulating NF-(B/AP-1 dependent- and independent-signaling, Clin. Exp. Metastasis 29 (2) (2012) 165–178. [DOI] [PubMed] [Google Scholar]
- [62].de Miguel MP, Royuela M, Bethencourt FR, Santamaría L, Fraile B, Paniagua R, Immunoexpression of tumour necrosis factor-alpha and its receptors 1 and 2 correlates with proliferation/apoptosis equilibrium in normal, hyperplasic and carcinomatous human prostate, Cytokine 12 (5) (2000) 535–538. [DOI] [PubMed] [Google Scholar]
- [63].Bouraoui Y, Ricote M, García-Tuñón I, Rodriguez-Berriguete G, Touffehi M, Rais NB, Fraile B, Paniagua R, Oueslati R, Royuela M, Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer, Cancer Detect. Prev 32 (1) (2008) 23–32. [DOI] [PubMed] [Google Scholar]
- [64].Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC, Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells, Cancer Res 70 (8) (2010) 3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, Scher HI, Gerald WL, NF-kappaB regulates androgen receptor expression and prostate cancer growth, Am. J. Pathol 175 (2) (2009) 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Djakiew D, Dysregulated expression of growth factors and their receptors in the development of prostate cancer, Prostate 42 (2) (2000) 150–160. [DOI] [PubMed] [Google Scholar]
- [67].Reynolds AR, Kyprianou N, Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting, Br. J. Pharmacol 147 (Suppl. 2) (2006) S144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Pompeo AC, Del Giglio A, Molecular oncogenesis of prostate adenocarcinoma: role of the human epidermal growth factor receptor 2 (HER-2/neu), Tumori 96 (5) (2010) 645–649. [DOI] [PubMed] [Google Scholar]
- [69].Traish AM, Morgentaler A, Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth, Br. J. Cancer 101 (12) (2009) 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M, In vivo progression of LAPC-’9 and LNCaP prostate cancer models to androgenindependence is associated with increased expression of insulin- like growth factor I (IGF-I) and IGF-I receptor (IGF-IR), Cancer Res 61 (16) (2001) 6276–6280. [PubMed] [Google Scholar]
- [71].Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y, Implications of insulin-like growth factor-I for prostate cancer therapies, Int. J. Urol 16 (2) (2009) 161–167. [DOI] [PubMed] [Google Scholar]
- [72].Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB, Baltimore Longitudinal Study on Aging Serum levels of insulin-like growth factor I (IGF- I), IGF-II, IGF-binding protein-3, andprostate-specific antigen as predictors of clinical prostate cancer, J. Clin. Endocrinol. Metab 85 (11) (2000) 4258–4265. [DOI] [PubMed] [Google Scholar]
- [73].Ropiquet F, Giri D, Lamb DJ, Ittmann M, FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation, J. Urol 162 (2) (1999) 595–599. [PubMed] [Google Scholar]
- [74].Gnanapragasam VJ, Robinson MC, Marsh C, Robson CN, Hamdy FC, Leung HY, FGF8 isoform b expression in human prostate cancer, Br. J. Cancer 88 (9) (2003) 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wikström P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A, Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer, Prostate 37 (1) (1998) 19–29. [DOI] [PubMed] [Google Scholar]
- [76].Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC, Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma, J. Urol 161 (1) (1999) 182–187. [PubMed] [Google Scholar]
- [77].Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM, Preoperative plasma levels of transforming growth factor beta (1) (TGF-beta(1)) stronglypredict progression in patients undergoing radical prostatectomy, J. Clin. Oncol 19 (11) (2001) 2856–2864. [DOI] [PubMed] [Google Scholar]
- [78].Derynck R, Akhurst RJ, Balmain A, TGF-beta signaling in tumor suppression and cancer progression, Nat. Genet 29 (2) (2001) 117–129. [DOI] [PubMed] [Google Scholar]
- [79].Wakefield LM, Roberts AB, TGF-beta signaling: positive and negative effects on tumorigenesis, Curr. Opin. Genet. Dev 12 (1) (2002) 22–29. [DOI] [PubMed] [Google Scholar]
- [80].Peraldo-Neia C, Migliardi G, Mello-Grand M, Montemurro F, Segir R, Pignochino Y, Cavalloni G, Torchio B, Mosso L, Chiorino G, Aglietta M, Epidermal Growth Factor Receptor (EGFR) mutation analysis, gene expression profilingand EGFR protein expression in primary prostate cancer, BMC Cancer 11 (2011) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, D’Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F, Expression of epidermal growth factor receptor correlates with disease relapse andprogression to androgen-independence in human prostate cancer, Clin. Cancer Res 8 (11) (2002) 3438–3444. [PubMed] [Google Scholar]
- [82].Craft N, Shostak Y, Carey M, Sawyers CL, A mechanism for hormone- independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase, Nat. Med 5 (3) (1999) 280–285. [DOI] [PubMed] [Google Scholar]
- [83].Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C, From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells, Proc. Natl. Acad. Sci. U. S. A 96 (10) (1999) 5458–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhau HY, Zhou J, Symmans WF, Chen BQ, Chang SM, Sikes RA, Chung LW, Transfected neu oncogene induces human prostate cancer metastasis, Prostate 28 (2) (1996) 73–83. [DOI] [PubMed] [Google Scholar]
- [85].Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML, CXCL12/ CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone, Mol. Cancer Res 6 (3) (2008) 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González- Barón M, PI3K/Akt signalling pathway and cancer, Cancer Treat. Rev 30 (2) (2004. April) 193–204. [DOI] [PubMed] [Google Scholar]
- [87].Manning BD, Cantley LC, AKT/PKB signaling: navigating downstream, Cell 129 (7) (2007) 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Morgan TM, Koreckij TD, Corey E, Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway, Curr. Cancer Drug Targets 9 (2) (2009) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gao N, Zhang Z, Jiang BH, Shi X, Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer, Biochem. Biophys. Res. Commun 310 (4) (2003) 1124–1132. [DOI] [PubMed] [Google Scholar]
- [90].Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA, Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy, Leukemia 17 (3) (2003) 590–603. [DOI] [PubMed] [Google Scholar]
- [91].Lee SH, Johnson D, Luong R, Sun Z, Crosstalking between androgen and PI3K/AKT signaling pathways in prostate cancer cells, J. Biol. Chem 290 (5) (2015. January 30) 2759–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL, Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer, Cancer Cell 19 (5) (2011) 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, Mukhtar H, Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease, Clin. Cancer Res 15 (6) (2009) 1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H, Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer, Cancer Res 64 (23) (2004) 8715–8722. [DOI] [PubMed] [Google Scholar]
- [95].Clegg NJ, Couto SS, Wongvipat J, Hieronymus H, Carver BS, Taylor BS, Ellwood-Yen K, Gerald WL, Sander C, Sawyers CL, MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors, PLoS One 6 (3) (2011) e17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N, Rubin MA, Yelick PC, Freeman MR, The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1, EMBO J 26 (21) (2007) 4523–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kiu H, Nicholson SE, Biology and significance of the JAK/STAT signalling pathways, Growth Factors 30 (2) (2012) 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Harrison DA, The Jak/STAT pathway, Cold Spring Harbor Perspect. Biol 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Li WX, Canonical and non-canonical JAK-STAT signaling, Trends Cell Biol 18 (11) (2008) 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu X, He Z, Li CH, Huang G, Ding C, Liu H, Correlation analysis of JAK-STAT pathway components on prognosis of patients with prostate cancer, Pathol. Oncol. Res 18 (1) (2012) 17–23. [DOI] [PubMed] [Google Scholar]
- [101].Gao B, Shen X, Kunos G, Meng Q, Goldberg ID, Rosen EM, Fan S, Constitutive activation of JAK-STAT3 signaling by BRCA1 in human prostate cancer cells, FEBS Lett 488 (3) (2001) 179–184. [DOI] [PubMed] [Google Scholar]
- [102].Zhu ML, Kyprianou N, Androgen receptor and growth factor signaling cross- talk in prostate cancer cells, Endocr. Relat. Cancer 15 (4) (2008) 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC, Stat3 activation in prostatic carcinomas, Prostate 51 (4) (2002) 241–246. [DOI] [PubMed] [Google Scholar]
- [104].Bishop JL, Thaper D, Zoubeidi A, The multifaceted roles of STAT3 signaling in the progression of prostate cancer, Cancers (Basel) 6 (2) (2014) 829–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ioannis Gkouveris I, Nikitakis N, Sauk J, STAT3 signaling in cancer, J. Cancer Ther 6 (2015) 709–726. [Google Scholar]
- [106].Kazansky AB, Kabotyanski EB, Wyszomierski SL, Mancini MA, Rosen JM, Differential effects of prolactin and src/abl kinases on the nuclear translocation of STAT5B and STAT5A, J. Biol. Chem 274 (1999) 22484–22492. [DOI] [PubMed] [Google Scholar]
- [107].Wen Z, Zhong Z, Darnell JE, Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation, Cell 82 (1995) 241–250. [DOI] [PubMed] [Google Scholar]
- [108].Dhillon AS, Hagan S, Rath O, Kolch W, MAP kinase signalling pathways in cancer, Oncogene 26 (22) (2007) 3279–3290. [DOI] [PubMed] [Google Scholar]
- [109].Wagner EF, Nebreda AR, Signal integration by JNK and p38 MAPK pathways in cancer development, Nat. Rev. Cancer 9 (8) (2009) 537–549. [DOI] [PubMed] [Google Scholar]
- [110].Koul HK, Pal M, Koul S, Role of p38 MAP kinase signal transduction in solid tumors, Genes Cancer 4 (9–10) (2013) 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Katz M, Amit I, Yarden Y, Regulation of MAPKs by growth factors and receptor tyrosine kinases, Biochim. Biophys. Acta 1773 (8) (2007) 1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cargnello M, Roux PP, Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases, Microbiol. Mol. Biol. Rev 75 (1) (2011) 50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Joshi S, Platanias LC, Mnk kinase pathway: cellular functions and biological outcomes, World J. Biol. Chem 5 (3) (2014) 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hou J, Lam F, Proud C, Wang S, Targeting Mnks for cancer therapy, Oncotarget 3 (2) (2012) 118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dorkin TJ, Robinson MC, Marsh C, Bjartell A, Neal DE, Leung HY, FGF8 over-expression in prostate cancer is associated with decreased patient survival and persists in androgen independent disease, Oncogene 18 (17) (1999) 2755–2761. [DOI] [PubMed] [Google Scholar]
- [116].Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z, Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associatedwith reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway, Am. J. Pathol 162 (2) (2003) 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bakin RE, Gioeli D, Bissonette EA, Weber MJ, Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4–2prostate cancer cells, Cancer Res 63 (8) (2003) 1975–1980. [PubMed] [Google Scholar]
- [118].Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H, Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor, Cancer Res 54 (20) (1994) 5474–5478. [PubMed] [Google Scholar]
- [119].Limami Y, Pinon A, Leger DY, Pinault E, Delage C, Beneytout JL, Simon A, Liagre B, The P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic acid-induced apoptosis in colorectal and prostate cancer cells, Biochimie 94 (8) (2012) 1754–1763. [DOI] [PubMed] [Google Scholar]
- [120].Katoh M, FGFR2 abnormalities underlie a spectrum of bone, skin, and cancer pathologies, J. Invest. Dermatol 129 (8) (2009) 1861–1867. [DOI] [PubMed] [Google Scholar]
- [121].Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM, Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression, Cancer Cell 8 (5) (2005) 393–406. [DOI] [PubMed] [Google Scholar]
- [122].Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA, Pandolfi PP, Saad F, Sonenberg N, eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression, Proc. Natl. Acad. Sci. U. S. A 107 (32) (2010) 14134–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kühl SJ, Kühl M, On the role of Wnt/β-catenin signaling in stem cells, Biochim. Biophys. Acta 1830 (2) (2013) 2297–2306. [DOI] [PubMed] [Google Scholar]
- [124].Curtin JC, Lorenzi MV, Drug discovery approaches to target Wnt signaling in cancer stem cells, Oncotarget 1 (7) (2010) 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Huelsken J, Behrens J, The wnt signalling pathway, J. Cell Sci 115 (Pt 21) (2002) 3977–3978. [DOI] [PubMed] [Google Scholar]
- [126].Mosimann C, Hausmann G, Basler K, Beta-catenin hits chromatin: regulation of Wnt target gene activation, Nat. Rev. Mol. Cell Biol 10 (4) (2009) 276–286. [DOI] [PubMed] [Google Scholar]
- [127].Zhao J, Yue W, Zhu MJ, Sreejayan N, Du M, AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of beta-catenin at Ser 552, Biochem. Biophys. Res. Commun 395 (1) (2010) 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gullick WJ, Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers, Br. Med. Bull 47 (1) (1991) 87–98. [DOI] [PubMed] [Google Scholar]
- [129].Sharma M, Chuang WW, Sun Z, Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation, J. Biol. Chem 277 (34) (2002) 30935–30941. [DOI] [PubMed] [Google Scholar]
- [130].Chesire DR, Isaacs WB, Beta-catenin signaling in prostate cancer: an early perspective, Endocr. Relat. Cancer 10 (4) (2003) 537–560. [DOI] [PubMed] [Google Scholar]
- [131].He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM, A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells, Neoplasia 6 (1) (2004) 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yardy GW, Brewster SF, Wnt signalling and prostate cancer, Prostate Cancer Prostatic Dis 8 (2) (2005) 119–126. [DOI] [PubMed] [Google Scholar]
- [133].Grigoryan T, Wend P, Klaus A, Birchmeier W, Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice, Genes Dev 22 (17) (2008) 2308–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET, Prostate cancer cells promote osteoblastic bone metastases through Wnts, Cancer Res 65 (17) (2005) 7554–7560. [DOI] [PubMed] [Google Scholar]
- [135].Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O, FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells, Cancer Res 70 (17) (2010) 6735–6745. [DOI] [PubMed] [Google Scholar]
- [136].Polakis P, The many ways of Wnt in cancer, Curr. Opin. Genet. Dev 17 (1) (2007) 45–51. [DOI] [PubMed] [Google Scholar]
- [137].Debruyne FM, Dijkman GA, Lee DC, Witjes WP, del Moral F, Karthaus HF, van der Mejden AP, Plasman JW, Pull HC, Kums JJ, Idema JG, Hoefakker JW, Heijbroek RP, Kil PJ, Khoe GS, A new long acting formulation of the luteinizing hormone-releasing hormone analogue goserelin: results of studies in prostate cancer, J. Urol 155 (4) (1996) 1352–1354. [PubMed] [Google Scholar]
- [138].Iacopino F, Lama G, Angelucci C, Sica G, Leuprorelin acetate affects ERK1/2 activity in prostate cancer cells, Int. J. Oncol 29 (1) (2006) 237–247. [PubMed] [Google Scholar]
- [139].Shore N, Cookson MS, Gittelman MC, Long-term efficacy and tolerability of once-yearly histrelin acetate subcutaneous implant in patients with advanced prostate cancer, BJU Int 109 (2) (2012) 226–232. [DOI] [PubMed] [Google Scholar]
- [140].Osguthorpe DJ, Hagler AT, Mechanism of androgen receptor antagonism by bicalutamide in the treatment of prostate cancer, Biochemistry 50 (19) (2011. May 17) 4105–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Flutamide approved for prostate cancer. Oncology (Williston Park) 1989. March;3(3):135. [PubMed] [Google Scholar]
- [142].Millar JL, Triptorelin approved for prostate cancer treatment, Am. J. Health. Syst. Pharm 57 (15) (2000) 1386. [DOI] [PubMed] [Google Scholar]
- [143].Steinberg M, Degarelix: a gonadotropin-releasing hormone antagonist for the management of prostate cancer, Clin. Ther 3 (Pt 2) (2009) 2312–2331. [DOI] [PubMed] [Google Scholar]
- [144].Ruch JM, Hussain MH, Evolving therapeutic paradigms for advanced prostate cancer, Oncology (Williston Park) 25 (6) (2011) 496–504, 508. [PubMed] [Google Scholar]
- [145].de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, COU-AA-301 Investigators: abiraterone and increased survival in metastatic prostate cancer, N. Engl. J. Med 364 (21) (2011) 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bahl A, Masson S, Birtle A, Chowdhury S, de Bono J, Second-line treatment options in metastatic castration-resistant prostate cancer: acomparison of key trials with recently approved agents, Cancer Treat Rev 40 (1) (2014) 170–177. [DOI] [PubMed] [Google Scholar]
- [147].Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, AFFIRM Investigators: increased survival with enzalutamide in prostate cancer after chemotherapy, N. Engl. J. Med 367 (13) (2012) 1187–1197. [DOI] [PubMed] [Google Scholar]
- [148].D’Amico AV, US Food and Drug Administration approval of drugs for the treatment of prostate cancer: a new era has begun, J. Clin. Oncol 32 (4) (2014) 362–364. [DOI] [PubMed] [Google Scholar]
- [149].Galsky MD, Small AC, Tsao CK, Oh WK, Clinical development of novel therapeutics for castration-resistant prostate cancer:historic challenges and recent successes, CA. Cancer J. Clin 62 (5) (2012) 299–308. [DOI] [PubMed] [Google Scholar]
- [150].Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators: docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer, N. Engl. J. Med 351 (15) (2004) 1502–1512. [DOI] [PubMed] [Google Scholar]
- [151].de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO, TROPIC Investigators: prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial, Lancet 376 (9747) (2010) [DOI] [PubMed] [Google Scholar]
- [152].Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, Sathyanarayana JR, Yakemchuk VN, Thomas GM, Erlich LE, et al. , Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant tolocal field external beam irradiation in the management of endocrine resistant metastaticprostate cancer, Int. J. Radiat. Oncol. Biol. Phys 25 (5) (1993) 805–813. [DOI] [PubMed] [Google Scholar]
- [153].Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzén L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O, ALSYMPCA Investigators: alpha emitter radium-223 and survival in metastatic prostate cancer, N. Engl. J. Med 369 (3) (2013) 213–223. [DOI] [PubMed] [Google Scholar]
- [154].Abi-Ghanem AS, McGrath MA, Jacene HA, Radionuclide therapy for osseous metastases in prostate cancer, Semin. Nucl. Med 45 (1) (2015) 66–80. [DOI] [PubMed] [Google Scholar]
- [155].Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C, Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study, Lancet 377 (9768) (2011) 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, Kotler JA, Freeman LM, Olivier P, Quadramet 424Sm10/11 Study Group: samarium-153- Lexidronam complex for treatment of painful bone metastases in hormone- refractory prostate cancer, Urology 63 (5) (2004) 940–945. [DOI] [PubMed] [Google Scholar]
- [157].Singh BH, Gulley JL, Therapeutic vaccines as a promising treatment modality against prostate cancer: rationale and recent advances, Ther. Adv. Vaccines 2 (5) (2014) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, IMPACT Study Investigators: sipuleucel-T immunotherapy for castration-resistant prostate cancer, N. Engl. J. Med 363 (5) (2010) 411–422. [DOI] [PubMed] [Google Scholar]
- [159].Vogelzang NJ, Words of wisdom. e: phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-esistant prostate cancer commonly remains hormonedriven. Attard G, Reid AH, Yap TA, et al. J. Clin. Oncol. 2008;26:4563–71, Eur. Urol 56 (1) (2009) 220. [DOI] [PubMed] [Google Scholar]
- [160].O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, Harland S, Robbins A, Halbert G, Nutley B, Jarman M, Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer, Br. J. Cancer 90 (12) (2004) 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ, Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583), J. Clin. Oncol 22 (6) (2004) 1025–1033. [DOI] [PubMed] [Google Scholar]
- [162].Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL, Prostate cancer Foundation/Department of defense prostate cancer clinical trials consortium: antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study, Lancet 375 (9724) (2010) 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL, Molecular determinants of resistance to antiandrogen therapy, Nat. Med 10 (1) (2004) 33–39. [DOI] [PubMed] [Google Scholar]
- [164].Vasaitis TS, Njar VC, Novel, potent anti-androgens of therapeutic potential: recent advances and promising developments, Future Med Chem 2 (4) (2010) 667–680. [DOI] [PubMed] [Google Scholar]
- [165].Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS, Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration- resistant prostate cancer, J. Clin. Oncol 27 (23) (2009) 3742–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Sternberg CN, de Bono JS, Chi KN, Fizazi K, Mulders P, Cerbone L, Hirmand M, Forer D, Scher HI, Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial, Ann. Oncol 25 (2) (2014) 429–434. [DOI] [PubMed] [Google Scholar]
- [167].Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J, AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer, N. Engl. J. Med 371 (11) (2014) 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Njar VC, Brodie AM, Discovery and development of Galeterone (TOK-001 or VN/124–1) for the treatment of all stages of prostate cancer, J. Med. Chem 58 (5) (2015) 2077–2087 (28). [DOI] [PubMed] [Google Scholar]
- [169].Kwegyir-Afful AK, Ramalingam S, Purushottamachar P, Ramamurthy VP, Njar VC, Galeterone and VNPT55 induce proteasomal degradation of AR/AR- V7, induce significantapoptosis via cytochrome c release and suppress growth of castration resistant prostatecancer xenografts in vivo, Oncotarget 6 (29) (2015) 27440–27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Yu Z, Cai C, Gao S, Simon NI, Shen HC, Balk SP, Galeterone prevents androgen receptor binding to chromatin and enhances degradation of mutant androgen receptor, Clin. Cancer Res 20 (15) (2014) 4075–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, Guo Z, Fang HB, Njar VC, Brodie AM, Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer, Mol. Cancer Ther 7 (8) (2008) 2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Gualberto A, Pollak M, Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions, Oncogene 28 (34) (2009) 3009–3021. [DOI] [PubMed] [Google Scholar]
- [173].Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, Lara PN Jr., The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase IICalifornia/Pittsburgh cancer consortium trial, Clin. Genitourin. Cancer 5 (7) (2007) 433–437. [DOI] [PubMed] [Google Scholar]
- [174].George DJ, Armstrong AJ, Creel P, A phase II study of RAD001 in men with hormone-refractory metastatic prostate cancer (HRPC), Genitourinary Cancers Symposium 2008 (2016) 181 abstract. [Google Scholar]
- [175].Ross RW, Manola J, Oh WK, et al. , Phase I trial of RA (R) and docetaxel (D) in castration resistant prostate cancer (CRPC) with FDG-PET assessment of RAD001 activity, J. Clin. Oncol 26 (May 20) (2008) (abstract 5069). [Google Scholar]
- [176].Bianchini D, Zivi A, Sandhu S, de Bono JS, Horizon scanning for novel therapeutics for the treatment of prostate cancer, Expert Opin. Investig. Drugs 19 (12) (2010) 1487–1502. [DOI] [PubMed] [Google Scholar]
- [177].Lara PN Jr., Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, Posadas E, Stadler W, Gandara DR, A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study, Anticancer Drugs 20 (3) (2009) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Morris MJ, Hudes G, Calabrò F, Cheng S, Trudel GC, Paliwal P, Sternberg CN, Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer, Clin. Cancer Res 15 (23) (2009) 7421–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Snyder DS, New approved dasatinib regimen available for clinical use, Expert Rev. Anticancer Ther 9 (3) (2009) 285–292. [DOI] [PubMed] [Google Scholar]
- [180].Cetnar JP, Rosen MA, Vaughn DJ, Phase II study of sorafenib and docetaxel in men with metastatic castration resistant prostate cancer (mCRPC), J. Clin. Oncol 27 (Suppl) (2009) (abstract e16055). [Google Scholar]
- [181].Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, Schell AJ, Taylor S, Hansen C, Gauthier I, Walsh W, Seymour L, A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer, Ann. Oncol 19 (4) (2008) 746–751. [DOI] [PubMed] [Google Scholar]
- [182].Lara PN Jr., Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, Posadas E, Stadler W, Gandara DR, A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advancedcastration-resistant prostate cancer: a California Cancer Consortium study, Anticancer Drugs 20 (3) (2009) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET, Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone, Cancer Res 63 (22) (2003) 7883–7890. [PubMed] [Google Scholar]
- [184].Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C, Denosumab HALT Prostate Cancer Study Group: denosumab in men receiving androgen- deprivation therapy for prostate cancer, N. Engl. J. Med 361 (8) (2009) 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Fauzee NJ, Taxanes: promising anti-cancer drugs, Asian Pac. J. Cancer Prev 12 (4) (2011) 837–851. [PubMed] [Google Scholar]
- [186].Wang H, Vo T, Hajar A, Li S, Chen X, Parissenti AM, Brindley DN, Wang Z, Multiple mechanisms underlying acquired resistance to taxanes in selecteddocetaxel-resistant MCF-7 breast cancer cells, BMC Cancer 14 (2014) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Trivedi M, Budihardjo I, Loureiro K, Reid TR, Ma JD, Epothilones: a novel class of microtubule-stabilizing drugs for the treatment of cancer, Future Oncol 4 (4) (2008) 483–500. [DOI] [PubMed] [Google Scholar]
- [188].Paller CJ, Antonarakis ES Cabazitaxel, a novel second-line treatment for metastatic castration-resistant prostate cancer, Drug Des. Dev. Ther 5 (2011) 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Denduluri N, Swain SM, Ixabepilone for the treatment of solid tumors: a review of clinical data, Expert Opin. Investig. Drugs 17 (3) (2008) 423–435. [DOI] [PubMed] [Google Scholar]
- [190].Clinicaltrials.gov, In: Bethesda MNLoM Clinicaltrials.gov, Bethesda, MD: National Library of Medicine; (accessed 01.28.16). [Google Scholar]


