In this issue of Circulation Research, investigators report that angiotensin II reduces hemoglobin’s affinity for oxygen, a protective compensatory response to reduce renal hypoxia and tubulointerstitial fibrosis, the hallmark of chronic kidney disease. This pathway is mediated by a sphingosine kinase 1-dependent induction of 2,3-biphosphoglycerate (2,3-BPG) which shifts the hemoglobin-oxygen dissociation curve to the right, allowing more oxygen to be released to the tissues. This commentary discusses the article’s findings in the broader context of renal injury and hypoxia.
The kidney functions as an important oxygen-sensing organ by stimulating the production of erythropoietin (EPO). Drops in the partial O2 pressure (pO2) lead to synthesis of EPO, the main stimulus for erythropoiesis in the bone marrow, by renal cortical interstitial cells1. In the injured kidney, these EPO-producing interstitial cells transform into myofibroblasts and lose their ability to produce EPO. This loss of EPO, coupled with other factors such as a chronic inflammatory state, result in anemia of chronic kidney disease. Conversely, hypoxia is implicated in the progression of chronic kidney disease (CKD). In severe acute kidney injury and CKD, injured peritubular capillaries and pericyte functional changes lead to vascular rarefaction, hypoxic renal tubules, and production of hypoxia inducible factor (HIF)-1α2. Both endothelial and tubular HIF-1α promote tubulointerstitial fibrosis in murine models of CKD through upregulation of collagen I and profibrotic growth factors3, 4. Many studies have shown the importance of hypoxia in CKD progression, but almost all focus on the injured endothelia and crosstalk with renal tubules or neighboring pericytes.
In this issue, Xie et al show an angiotensin II-dependent change in erythrocyte oxygen delivery that protects the kidney from hypoxia and fibrosis through augmented production of 2,3-bisphosphoglycerate (2,3-BPG), which enhances erythrocyte release of oxygen into tissues5. This group previously showed that high altitude induces greater oxygen release by erythrocytes through augmented production of 2,3-BPG mediated by the lipid signaling molecule sphingosine-1-phosphate (S1P)6. Sphingosine kinase 1 (SphK1), the only enzyme to produce S1P in erythrocytes, was selectively deleted in murine erythrocytes in the current study. Metabolomics revealed that angiotensin II treatment induces a shift in glucose metabolism to promote 2,3-BPG production via the Rapoport-Luebering shunt (Figure 1), as previously observed in hypoxic conditions. However, SphK1−/− erythrocytes failed to show the same angiotensin II-induced metabolic shift. The authors confirmed with glucose flux analyses using isotopic tracers that glucose metabolism shifts from the pentose phosphate pathway towards glycolysis and that metabolites in the Rapoport-Luebering shunt were increased by angiotensin II, but not in SphK1−/− erythrocytes. Consistent with this, angiotensin II treatment raised the release of oxygen by erythrocytes as measured by P50, the pO2 required to reach 50% of hemoglobin saturated with oxygen, but only in the presence of SphK1.
Figure 1.
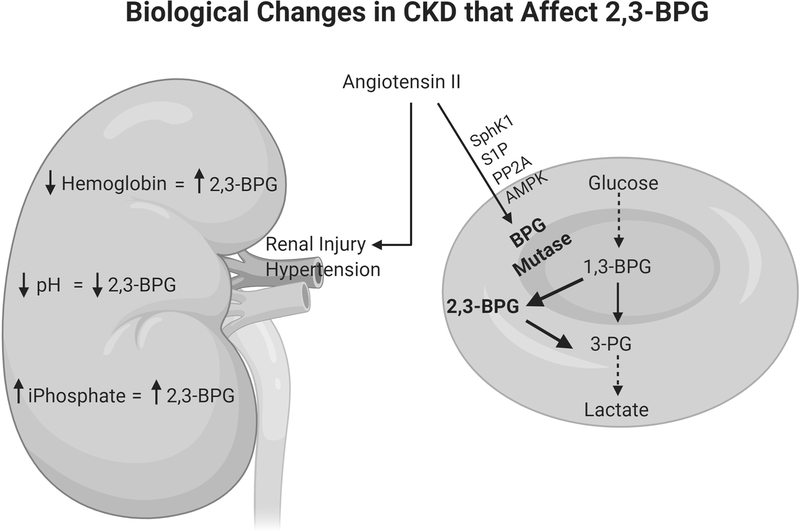
Chronic kidney disease (CKD) alters several biologic parameters (e.g. hemoglobin, acid/base status, mineral metabolism) that have direct effects on 2,3-bisphosphoglycerate (2,3-BPG). Angiotensin II is a potent inducer of hypertension and renal injury but can also induce a protective effect on erythrocytes’ affinity for oxygen. Angiotensin II increases 2,3-BPG production through the Rapoport-Luebering shunt (shown in bold) by a pathway regulated by sphingosine kinase 1 (SphK1). S1P, sphingosine 1-phosphate; PP2A, protein phosphatase 2A; AMPK, AMP kinase.
The authors dissected out the mechanisms by which angiotensin II leads to 2,3-BPG production in a SphK1-dependent manner (Figure 1). SphK1−/− erythrocytes failed to induce S1P after treatment with angiotensin II. This increased ratio of ceramide:S1P led to increased protein phosphatase 2A (PP2A), which is known to de-phosphorylate several proteins important in the response to injury. One of these targets is AMP-activated protein kinase (AMPK)-1α, and SphK1−/− erythrocytes had augmented PP2A activity as well as reduced active AMPK. AMPK augments the activity of BPG mutase, the enzyme that generates 2,3-BPG. This mechanism adds to previous work done by the same group showing that the adenosine receptor ADORA2B is critical for AMPK-dependent production of 2,3-BPG in angiotensin II murine model of CKD7. As this group previously linked the ADORA2B pathway to increased SphK1 activity8, it is plausible that angiotensin II-induced hypoxia leads to adenosine/ADORA2B signaling which augments the SphK1/S1P pathway leading to increased AMPK activity and reduced hypoxia through 2,3-BPG production.
Is this pathway relevant to renal hypoxia and models of CKD? The authors treat mice containing erythrocyte-specific SphK1 conditional deletion (eSphk1−/−) and floxed controls with angiotensin II. The eSphk1−/− mice have decreased renal function, increased renal fibrosis, and augmented hypoxia by hydroxyprobe compared to the control angiotensin II-treated mice5. One caveat is that the eSphk1−/− mice have significantly higher blood pressure which likely contributes to the augmented injury. In particular, the differences in albuminuria are likely explained by these blood pressure differences. However, the absolute levels of albuminuria are not very high and unlikely to contribute much to the tubulointerstitial fibrosis on trichrome staining. Treatment of the angiotensin II-injured eSphkl1−/− mice with either an AMPK activator (AICAR) or PP2A inhibitor augmented 2,3-BPG levels in erythrocytes and well as BPG mutase activity. Furthermore, these inhibitors reduced tissue fibrosis and fibrotic gene expression. In the case of AICAR, beneficial effects were seen in both control and eSphk1−/− mice treated with angiotensin II without altering the blood pressure. Both systemic treatments improved hypoxia assessed by hydroxyprobe, but these treatments likely had beneficial effects on cells other than erythrocytes (e.g. renal tubules, myofibroblasts) that contributed to the attenuated injury in both control and eSphk1−/− mice as the authors have discussed. However, the differences between injury in eSphk1−/− and control mice dissipated with AICAR and the PP2A inhibitor implying that the eSphk1−/− phenotype was due, in part, to a PP2A- and AMPK-dependent pathway.
One important question raised is whether this SphK1-dependent alteration in erythrocyte O2 delivery via 2,3-BPG is induced by CKD or angiotensin II or both. Xie et al build a strong case for angiotensin II-stimulated Sphk1-dependent 2,3-BPG production5, 7. The authors validated this pathway in CKD patients, but these patients had much higher blood pressures than did controls (average SBP of 156 versus 113mmHg). These CKD patients with significant hypertension likely have higher amounts of renin-angiotensin activity, thus obscuring the effect of angiotensin II versus CKD. In the future, examining a subpopulation of CKD without concurrent hypertension might better determine the role of CKD in 2,3-BPG levels.
CKD alters several biological variables that may independently affect levels of 2,3-BPG and O2 extraction. Many of these data come from physiology studies from the 1970’s using patients with advanced CKD or on renal replacement therapy (i.e. dialysis). Anemia, or low hemoglobin, such as noted in this study’s CKD population, also increases 2,3-BPG production. Anemia increases the levels of deoxyhemoglobin, which is a stronger base than oxyhemoglobin, and this intracellular alkalosis stimulates glycolysis and 2,3-BPG production leading to improved tissue oxygenation9. Acid/base status and plasma inorganic phosphate concentrations, both altered by CKD, can also impact hemoglobin saturation with oxygen. Many patients with advanced CKD have some degree of acidosis which can have two, divergent effects on the oxygen affinity of hemoglobin. A low pH quickly shifts the oxygen saturation of hemoglobin curve to the right, the Bohr effect, improving tissue delivery of oxygen. This is countered in chronic acidosis by suppression of 2,3-BPG10. As renal function declines, the serum concentration of inorganic phosphates rises which stimulates 2,3-BPG. Given the mixed effects of hemoglobin, acid/base status, and serum inorganic phosphates on hemoglobin saturation with oxygen in CKD patients, the P50 values become important to integrate these and better understand the net effect on oxygen affinity of hemoglobin. Older studies have shown that, while anemic CKD patients have elevated P50 levels, these increases are attenuated compared to those of anemic patients with normal renal function9. This suggests that there may be an opportunity to further optimize oxygen delivery in patients with CKD. However, it should be noted that the care of CKD patients in terms of anemia, bicarbonate treatment, and phosphorous control, has changed considerably since the original studies done almost 50 years ago, and some of these changes (e.g. erythropoietin) could also impact tissue delivery of oxygen.
The question remains whether CKD per se, or just angiotensin II causes this compensatory increase in 2,3-BPG and increased P50 in erythrocytes. This study convincingly provides the proof-of-concept that impairing an angiotensin II-induced compensatory increase in 2,3-BPG promotes renal hypoxia and fibrosis5. Future preclinical studies are needed to determine whether further augmenting 2,3-BPG levels in CKD models would provide additional protection from hypoxia and renal fibrosis. This manuscript nicely dissects out the molecular mechanisms whereby angiotensin II increases P50 through SphK1-mediated changes in erythrocyte metabolism leading to augmented 2,3-BPG. CKD causes many complex changes to hemoglobin’s affinity for oxygen and subsequent tissue oxygenation. Physiologic studies fifty years ago established some of these relationships between erythrocyte binding to oxygen and acidemia, anemia, and hyperphosphatemia. Studies by Xie’s group have extended our understanding of how angiotensin II affects hemoglobin’s binding to oxygen and the molecular pathways involved. Many studies support chronic hypoxia as, not just a consequence of injury, but also a mediator of kidney injury and renal fibrosis. The injured endothelial cells and maladaptive changes in pericytes have deservedly garnered much attention in chronic hypoxia, but perhaps the erythrocyte has been overlooked as another putative target.
Acknowledgment
This work was supported by VA Merit 1I01BX003425-01A21 (LG) and NIDDK R01DK108968-01 (LG).
Footnotes
COI: None.
References
- 1.Koury MJ and Haase VH. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nature reviews Nephrology. 2015;11:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatachalam MA, Weinberg JM, Kriz W and Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo R, Zhang W, Zhao C, Zhang Y, Wu H, Jin J, Zhang W, Grenz A, Eltzschig HK, Tao L, et al. Elevated Endothelial Hypoxia-Inducible Factor-1alpha Contributes to Glomerular Injury and Promotes Hypertensive Chronic Kidney Disease. Hypertension. 2015;66:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie T, Chen C, Peng Z, Brown BC, Reisz JA, Xu P, Zhou Z, Song A, Zhang Y, Bogdanov MV, et al. Erythrocyte Metabolic Reprogramming by Sphingosine 1-Phosphate in Chronic Kidney Disease and Therapies. Circ Res. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Sun K, Zhang Y, D’Alessandro A, Nemkov T, Song A, Wu H, Liu H, Adebiyi M, Huang A, Wen YE, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nature communications. 2016;7:12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Z, Luo R, Xie T, Zhang W, Liu H, Wang W, Tao L, Kellems RE, Xia Y. Erythrocyte Adenosine A2B Receptor-Mediated AMPK Activation: A Missing Component Counteracting CKD by Promoting Oxygen Delivery. J Am Soc Nephrol. 2019;30:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Zhang Y, Bogdanov MV, Wu H, Song A, Li J, Dowhan W, Idowu M, Juneja HS, Molina JG, e. Elevated adenosine signaling via adt alenosine A2B receptor induces normal and sickle erythrocyte sphingosine kinase 1 activity. Blood. 2015;125:1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtman MA, Murphy MS, Whitbeck AA and Kearney EA. Oxygen binding to haemoglobin in subjects with hypoproliferative anaemia, with and without chronic renal disease: role of pH. Br J Haematol. 1974;27:439–52. [DOI] [PubMed] [Google Scholar]
- 10.Astrup P, Rorth M and Thorshauge C. Dependency on acid-base status of oxyhemoglobin dissociation and 2,3-diphosphoglycerate level in human erythrocytes. II. In vivo studies. Scand J Clin Lab Invest. 1970;26:47–52. [DOI] [PubMed] [Google Scholar]