Abstract
Oncogene activation promotes an intrinsic inflammatory pathway that is crucial for cancer development. Here, we have investigated the actual effect of the inflammatory cytokine tumor necrosis factor (TNF) on the natural history of spontaneous mammary cancer in the HER2/neuT (NeuT) transgenic mouse model. Bone marrow transplantation from TNF knockout mice into NeuT recipients significantly impaired tumor growth, indicating that the source of TNF fostering tumor development was of bone marrow origin. We show that the absence of leukocyte-derived TNF disarranged the tumor vasculature, which lacked pericyte coverage and structural integrity, leading to diffuse vascular hemorrhage and stromal necrosis. In addition, tumor-associated Tie2-expressing monocytes were reduced and cytokine expression skewed from Th2 to Th1 type. Treatment of NeuT mice with anti-TNF antibody partially phenocopied the antitumor effect of TNF-deficient bone marrow cell transplantation, providing a strong preclinical background and rationale for the introduction of TNF antagonists in the treatment of human breast cancer, including basal-like samples for which consolidated targeted therapies do not exist.
Introduction
Exogenous (carcinogens, pathogens) as well as endogenous (oncogene-driven) inflammatory stimuli defining the extrinsic and intrinsic inflammatory pathways, respectively, concur in tumor development (1).
Both pathways converge on NF-κB transcription factor. Downstream NF-κB, a key cytokine often involved in tumorigenesis is the tumor necrosis factor (TNF). Although originally identified as an inducer of tumor necrosis (2, 3), it soon became evident that TNF could also act as a tumor promoter (4–6). High local doses of TNF induce tumor destruction, whereas low, chronic production of TNF sustains tumor growth contributing to tissue remodeling, stroma formation, and neoangiogenesis. In particular, TNF activity on endothelial cells (EC) and angiogenesis has been the subject of controversy: it has been reported that TNF is able to block EC proliferation and migration in vitro (7, 8) and to either down-modulate the activity and expression of VEGFR2 (9, 10) or upregulate VEGFR2 expression (11) and induce EC migration (12). In vivo, TNF induces angiogenesis in the cornea (7), but TNF receptor I knockout (TNFR-I KO) mice show enhanced angiogenesis in the same experimental model (13) and in wounded skin (14). Moreover, continuous administration of TNF could inhibit, whereas a 2- to 3-day pulse could stimulate, angiogenic sprouting (15).
A critical role for TNF in tumor promotion has been shown using TNF KO mice that are resistant to chemical skin carcinogenesis induced by 7,12-dimethylbenz(a)anthracene and 12-O-tetradecanoylphorbol-13-acetate or okadaic acid promotion (16, 17). Mice deficient for TNFR-I and, to a lesser extent, TNF receptor II (TNFR-II) are resistant to skin carcinogenesis (18). TNFR-I KO mice also develop less liver damage and cancer after exposure to hepatic carcinogens (19) and, when treated with azoxymethane and dextran sulfate sodium, showed reduced mucosal damage, inflammatory infiltration, and subsequent tumor formation (20). All these studies explored the extrinsic inflammatory pathway.
Although small amounts of TNF could be produced directly by malignant cells (21), its most relevant source within the tumor microenvironment is from infiltrating leukocytes (20, 22).
TNF has also been found expressed in a variety of human tumors, both as mRNA and protein, and its high level in the serum of cancer patients is often associated with a poor prognosis (23). Expression of TNF and its receptors has been shown in benign and malignant human breast tissues (24), but its role in mammary carcinogenesis and its involvement in the intrinsic inflammatory pathway has only been partially investigated (25). Reduced mammary tumorigenesis associated with decreased epithelial cell proliferation has been shown in TNF KO mice carrying the HER2/neu oncogene on a mixed FVBxC57BL/6 background (25). We also used the HER2/neu (p185) oncogene under the control of the mouse mammary tumor virus promoter to induce autochthonous mammary carcinomas from a different strain (26) and with the different aim of dissecting the actual role of leukocyte-produced TNF in mammary carcinogenesis. Using bone marrow transplantation (BMT) experiments, we show that TNF produced by BM-derived cells is necessary for pericyte coverage of tumor vessels and for Tie2-expressing monocyte (TEM) infiltration that significantly contribute to mammary tumor growth. Showing that anti-TNF antibody administration reduces tumor progression in the HER2/NeuT model, we offer a rational background for exploiting TNF antagonists in the treatment of human breast cancer.
Materials and Methods
Animal models
C57BL/6 mice were purchased from Charles River. TNF KO (B6;129S-Tnftm1Gkl/J), TNFR-I (B6.129-Tnfrsf1atm1Mak/J), and TNFR-II KO (B6;129S2-Tnfrsf1bTm1Mwm/J) mice were from The Jackson Laboratory. Male BALB/NeuT mice were described elsewhere (26, 27), and were mated with C57BL/6 females to obtain the F1 CxB6NeuT strain. The presence of the HER2/neu transgene has been checked by PCR on tail DNA as previously described (27). Mice were bred and maintained at the Fondazione IRCCS Istituto Nazionale Tumori and treated according to the European Union guidelines and to protocols approved by the Animal Ethical Committee appointed by the Fondazione IRCCS Istituto Nazionale Tumori.
Mammary gland histology
Whole mount preparations were performed as described on the UC Davis web site http://tgmouse.compmed.ucdavis.edu/HistoLab/wholmt1.htm. Digital photos were acquired with a Nikon Coolpix 995 (Nital SpA) mounted on a Nikon stereoscopic microscope (Nikon Instruments).
BMT
Seven- to eight-week-old mice were lethally γ-irradiated with 1,000 cGy and BMT performed as previously described (28). BMT engraftment was checked by staining with FITC-conjugated Kd and PE-conjugated Kb (from BD Biosciences) and subsequent fluorescence-activated cell sorting analysis. Two to three mice in each group of chimeras were sacrificed for whole mount analysis at 18 to 20 weeks, the others were kept in observation for tumor development. Tumors were measured with a caliper once a week, and tumor volume is calculated as d2 × D, where d is the smaller diameter and D is the longer one.
Histopathologic and immunophenotypical analyses on formalin-fixed paraffin-embedded samples
Histopathologic evaluation was performed on sections stained with H&E and Masson’s trichrome. Immunohistochemistry was performed using the streptavidin-biotin- peroxidase complex method as previously reported (29). Either aminoethylcarbazole (red signal) or 3,3′-diaminobenzidine (brown signal) were used as chromogenic substrates and sections were counterstained with hematoxylin. Slides were evaluated under a Leica DM3000 optical microscope and microphotographs were collected using a Leica DFC320 digital camera. Cell counts on immunostained slides were performed on five representative high-power fields (400× magnification) and the results were expressed as average ± SD. Mean vascular density was assessed by counting the absolute number of CD31+ vessels out of 10 high-power (400×) microscopic fields and was expressed as the mean value ± SD.
Immunohistochemistry on frozen sections
Tumor fragments were embedded in optimal cutting temperature compound, snap-frozen, and stored at −80°C. Slides were fixed in acetone and air-dried, rinsed in methanol/3% H2O2 to block endogenous peroxidase activity, washed in PBS, and blocked in 10% FCS. Slides were covered with the primary antibody for 1 hour (rat α-mouse CD31 from BD), washed three times in PBS 1× followed by incubation with the secondary antibody (HRP conjugated) and HRP-conjugated streptavidin (Sigma). Reactivity was revealed with 3,3′-diaminobenzidine. Sections were counterstained with Mayer’s hematoxylin, dehydrated in graded alcohol (70%, 95%, and 100% ethanol), and mounted in BDH mounting medium (Merck Eurolabs).
Double immunofluorescent staining and laser scanning confocal analyses
Acetone-fixed frozen sections were rehydrated in PBS, blocked in 10% FCS and incubated for 1 hour with the first primary antibody. Sections were then washed in PBS and incubated for 1 hour with the appropriate secondary antibody (anti-rabbit Alexa Fluor 546-conjugated; Molecular Probes). After washing, sections were incubated for 30 minutes with the second primary antibody, washed again, and incubated for 30 minutes with Alexa Fluor–conjugated secondary antibody (α-rat Alexa Fluor 488–conjugated). Primary antibodies used were the following: rabbit a-mouse Tie2, rat α-mouse CD31, rat α-mouse CD11b (from eBioscience), and rat α-mouse mannose receptor C-type 1 (MRC1/CD206). Slides were mounted in Prolong Antifade (Molecular Probes) and examined with a Zeiss LSM 510 Meta laser scanning confocal microscope. For intracellular staining of the cytokines, TNF, interleukin (IL)-10, and IL-12 sections were incubated for 10 minutes in a 1% bovine serum albumin, 0.1% Tween 20 solution before adding the primary antibody (all from BD). For immunohistochemical and immunofluorescence analysis images were digitally captured on a microscope (Nikon) equipped with a digital camera (DXM1200; Nikon) and analyzed using ACT1 software.
In vivo V1q treatment
CxB6NeuT mice were treated with 200 μg of rat a-mouse TNF V1q (kindly provided by D. Maennel, Germany), every 3 days, starting at 15, or at 19 weeks of age, for 4 weeks. Two to three mice in each group were sacrificed for whole mount analysis at 22 or at 24 weeks, whereas the others were kept in observation for tumor development.
Statistical analysis
Growth data were expressed as mean + SE. Differences between groups at different time points were analyzed for statistical significance by means of unpaired t test at 30 or 35 weeks of age, with P < 0.05 as significant cutoff.
Results
TNF produced by cells of BM origin contribute to mammary tumor growth
To assess whether TNF was relevant for mammary carcinogenesis, we performed BMT experiments, reconstituting lethally irradiated mice with BM cells from either TNF KO or wild-type C57BL/6 (hereafter B6) mice. Mice were irradiated at 8 weeks of age, because at this time point the mouse mammary gland tree is completely formed but transformation is still at an early stage with initial epithelial hyperplasia (see Supplementary Fig. S1A).
Because the transgenic HER2/NeuT strain is on a BALB/c background, we crossed HER2–expressing BALB/c males with B6 females to obtain (C57BL/6xBALB/NeuT) F1 hybrids (hereafter CxB6NeuT) as recipients of BM cells from TNF KO mice, commercially available only on a B6 background. The CxB6NeuT strain develops mammary tumors with a kinetics slightly slower than the parental HER2/NeuT on the BALB/c background (Supplementary Fig. S1B).
Mice transplanted with TNF KO cells showed delayed onset and reduced tumor growth in comparison with mice receiving wild-type BM. Whole mount analysis of mammary glands from transplanted mice confirmed the result (Fig. 1A). To exclude the possibility that the lack of TNF could affect mammary gland organization, we performed whole mount analysis at 18 weeks of age from TNF KO mice, in comparison with B6 wild-type counterparts, and from TNF KO > CxB6 and B6 > CxB6 BM chimeras without finding any difference among them (Supplementary Fig. S1C).
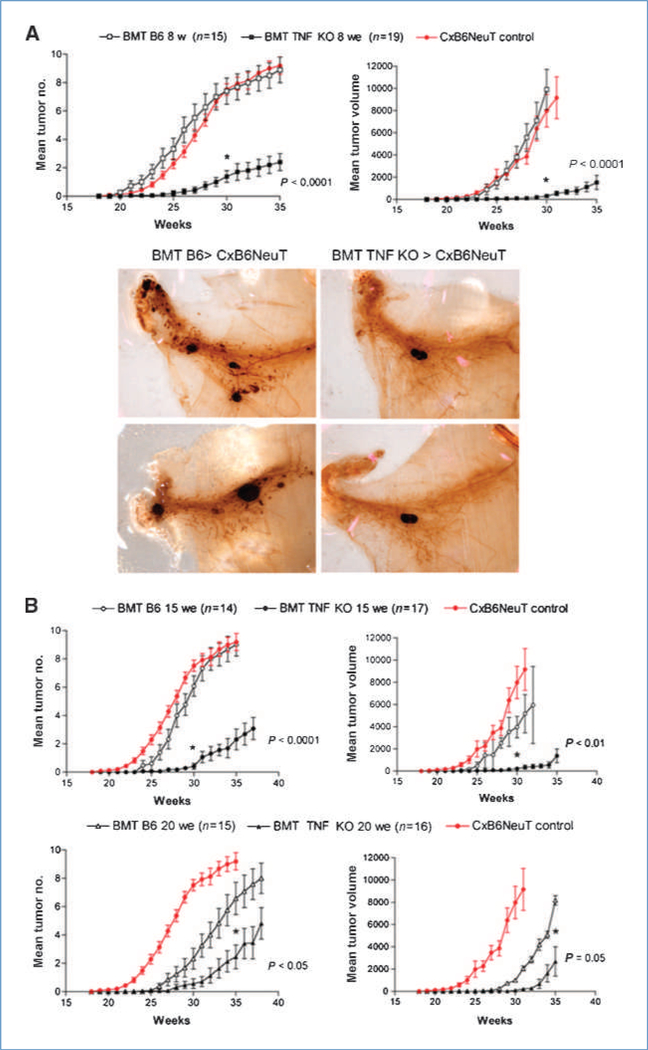
Figure 1.
BMT from TNF KO donors hampers NeuT mammary carcinogenesis. A, top, 8-wk-old CxB6NeuT mice were lethally irradiated and transplanted with wild-type B6 or TNF KO BM cells and kept in observation for tumor onset and progression. Tumor incidence is depicted as mean tumor number and mean tumor volume. Growth curve for CxB6NeuT mice is shown for reference. Mice are considered tumor-free until a palpable mass (>3 mm) is detected. Single tumor volumes in each animal are added together. Statistical difference from B6 and TNF KO chimeras is evaluated by paired t test (week 30), with P < 0.05 as significant cutoff. SE bars are shown (three separate experiments, each with five to seven mice per group; bottom). Whole mount analysis of mammary glands from mice transplanted with B6 and TNF KO BM. Two samples from different mice for each group are shown. B, mammary adenocarcinoma incidence of CxB6NeuT mice transplanted with wild-type B6 or TNF KO BM cells. BMT is performed at 15 (upper panels) and at 20 (lower panels) weeks of age (three separate experiments, each with five-seven mice per group). Statistical difference is calculated as above.
C, hematoxylin/eosin staining on paraffin-embedded sections of tumors from B6 wild-type NeuT chimeras in comparison with samples from TNF KO chimeric animals. Tumors are collected at 28–32 weeks, at a size between 8 × 8 and 12 × 12 mm. Bar, 500 μm. D, Masson’s trichrome staining of tumor sections from mice receiving wild-type B6 (left) or TNF KO (right) BM cells. Bar, 500 μm.
To test TNF effect at different stages of tumor progression, we carried out BMT experiments at 15 weeks of age, a stage at which in situ carcinomas are already present, and at 20 weeks, when invasive carcinomas are present in all mammary glands (see Supplementary Fig. S1A).
The results shown in Fig. 1B clearly indicate that a TNF protumorigenic role was required along with tumor progression: the effect of TNF withdrawal at 15 weeks of age was comparable with that seen when BMT was performed at 8 weeks, whereas the TNF effect was less evident, but still statistically significant, when BMT was delayed until 20 weeks of age.
It is worth noting that BMT performed at 20 weeks of age also causes a delay in tumor onset in mice receiving wild-type cells, likely because total body radiation affects the stroma cell components and/or directly the incipient tumor. Nevertheless, comparison of TNF KO with wild-type chimeras performed at the same time point also clearly confirmed the antitumoral effect of TNF removal in advanced stages of tumor progression.
TNF withdrawal during tumor progression significantly affects tumor stroma organization
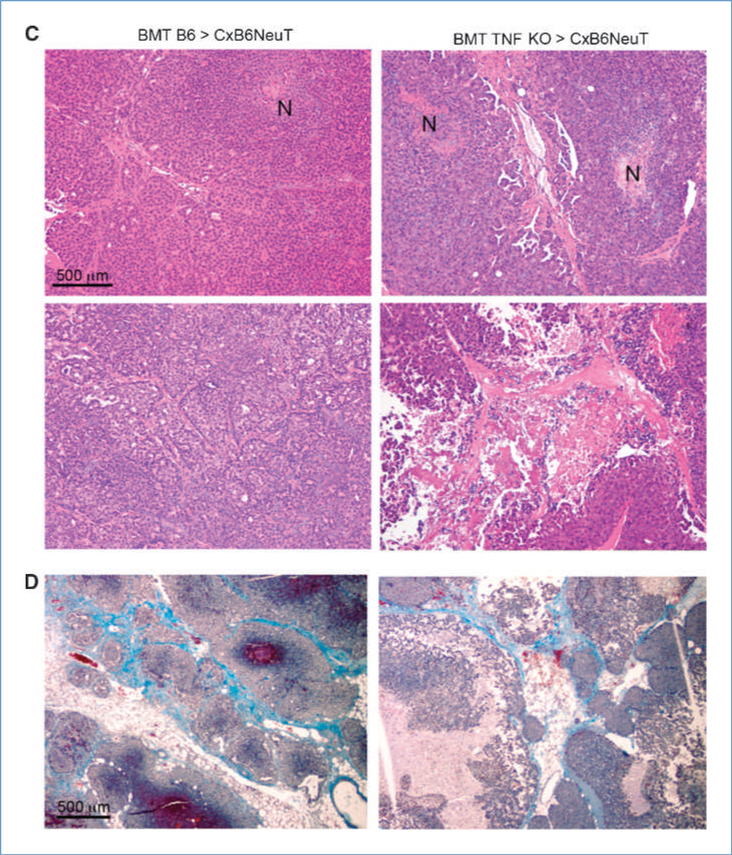
To study the mechanisms behind the effect of TNF removal, we analyzed tumor organization by H&E- and Masson’s trichrome-stained sections. Tumors from B6-transplanted chimeras were well differentiated, showing a nest-like growth pattern, with fine-branching fibrovascular stromal meshwork, and limited foci of epithelial necrosis. Tumors from TNF KO-transplanted mice showed residual epithelial nests with coarse morphology, gross stromal axes and extended necrosis, also involving stromal and perivascular areas, indicating a role for TNF in stromal axes organization and function (Fig. 1C–D).
The absence of TNF alters leukocyte infiltration and cytokine production at the tumor site
We tested whether the altered stroma organization occurring in the absence of TNF was due to impaired leukocyte recruitment and cytokine production at the tumor site.
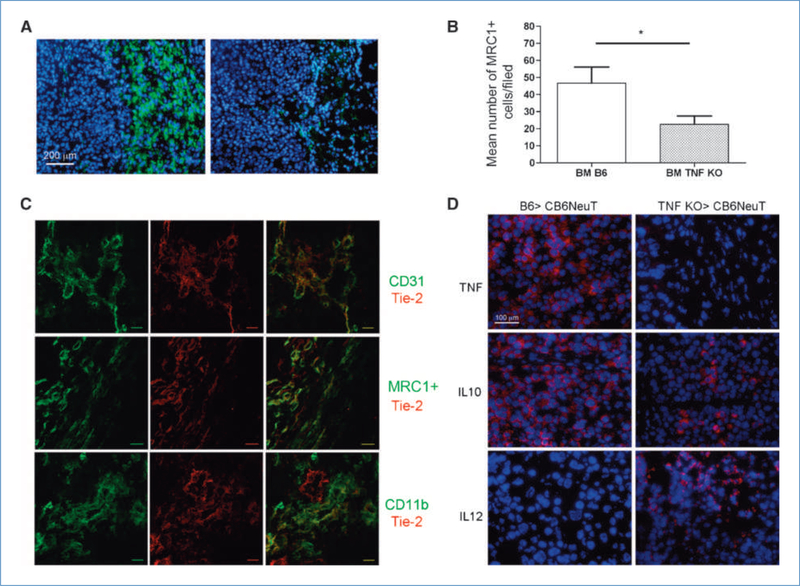
The percentage of infiltrating CD45+ leukocytes (FACS analysis) was higher in wild-type chimeras than in TNF KO chimeras (19.2 ± 4.8 and 13.2 ± 4.3, respectively), although the difference was not significant. However, the lack of TNF altered the number and localization of some leukocyte subtypes, in particular, MRC1+ cells that were highly represented in the capsular area of tumors from B6 chimeras were scarce in tumors from TNF KO chimeras (Fig. 2A and B). MRC1, a marker of M2/wound-healing macrophages (30), has recently been reported to be upregulated 5.3-fold in TEMs (31, 32) in comparison with tumor-associated macrophages (32). To better characterize these cells, we performed immunofluorescence staining with MRC1, CD11b, CD31, and Tie2. Results showed that Tie2 colocalizes with CD31, but also with some CD11b+ cells and a relevant percentage of MRC1+ cells, indicating that at least part of the MRC1+ cells in NeuT tumors were TEM (Fig. 2C). In agreement with immunohistologic data, FACS analysis shows a significantly higher percentage of Tie2/CD11b+ TEM (gated on CD45+ cells) in tumors from B6 compared with TNF KO BMT (5.76 ± 1.91 versus 1.43 ± 0.41).
Figure 2.
TNF withdrawal affects monocyte/macrophage infiltration of NeuT mammary adenocarcinomas and redirects cytokine profile of tumor-infiltrating leukocytes. A, immunofluorescence staining of tumor samples from B6 (left) and TNF KO (right) chimeric mice with MRC1+ antibody. Nuclei are stained with 4′,6-diamidino-2-phenylindole. Bar, 200 μm. B, mean number of MRC1+ cells infiltrating tumors from wt and TNF KO BM transplants. C, confocal immunofluorescence staining of Tie2 cells in tumor samples from B6 chimeras with CD31, CD11b, and MRC1+ markers. Bar, 5 μm. D, immunofluorescence analysis of tumor samples from B6 and TNF KO chimeric mice for TNF, IL-10, and IL-12 (red). Nuclei are stained with 4′,6-diamidino-2-phenylindole. Bar, 100 μm.
Immunofluorescence analysis of cytokines at the tumor site showed that in tumors from TNF KO chimeras, M1 (e.g., IL-12) and M2 cytokines (e.g., IL-10) were upregulated and downregulated, respectively (Fig. 2C). Phosphorylation of p65 NF-κB was also reduced in both infiltrating leukocytes and tumor cells from TNF KO in comparison with B6 chimeras (Supplementary Fig. S2). These results are consistent with a switch from M2 to M1 in tumor-associated macrophages of tumors from TNF-deficient chimeras.
Altered tumor vasculature in TNF-deficient BM chimeras
TNF withdrawal significantly changed tumor vascularization from organized vessels around the “nested” tumor architecture of B6 chimeras into abnormal vessels and vascular lacunae, with frequent perivascular erythrocyte extravasation (Fig. 3A–B). Staining with anti-CD31 antibody clearly marked tumor vessel outline in wild-type animals, whereas a discontinuous staining was characteristically observed in tumors from TNF KO chimeras (insets Fig. 3A). Although the vessel structure was dramatically altered, the microvascular density was not significantly different: 13.625 ± 2.722 in B6 > CxB6NeuT tumors versus 11.83 ± 5.344 in TNF KO > CxB6NeuT tumors. Remarkably, subendothelial mural cells/pericytes, characterized by staining with antibodies to NG2 (Fig. 3C), and further characterized by costaining of NG2 cells with antibodies to PDGFRβ or α-SMA (data not shown), were almost completely absent in tumors from TNF KO chimeras (Fig. 3C). The number of NG2+ pericytes was 23.5 ± 7.89 and 6.83 ± 2.85 in tumors from wild-type and TNF KO BM chimeras, respectively, confirming the qualitative immunohistologic data.
Figure 3.
Altered tumor vasculature in mice receiving TNF-deficient BM cells. A, immunostaining with CD31 antibody of tumor vessels in samples from B6 and TNF KO chimeric mice. Bar, 500 μm. B, erythrocyte extravasations in tumor samples from TNF KO chimeric mice (left, black arrows; Giemsa; bar, 100 μm). Hemosiderin deposits are also observed (right, yellow arrows; Giemsa; bar, 100 μm). C, immunostaining of tumor from B6 > CxB6NeuT and TNF KO > CxB6NeuT mice of pericytes with anti-NG2 antibody (top; N, necrosis; bar, 500 μm).
Searching for TNF targets: BMT experiments with TNFR-I KO donors
To identify which cells in the tumor sense TNF, we analyzed the expression of TNFR-I in combination with cell surface markers. Immunohistochemistry showed that subendothelial cells, endothelial cells, and stromal cells endowed with monocyte/macrophage morphology expressed TNFR-I (Supplementary Fig. S3). Immunofluorescence confirmed that cells expressing TNFR-I were also CD11b+, NG2+, or CD31+ (Supplementary Fig. S3). Pericytes constitute a heterogeneous population of cells whose ontogeny is not well-defined. Although the most accepted theory sustains their origin from mesenchymal cells, recent data have indicated that BM contains a reservoir of pericyte progenitors that can be recruited to angiogenic sites of tumors (33). To distinguish between host and donor origin of subendothelial cells in NeuT BM chimeras, we performed ad hoc experiments in which BALB-NeuT mice (MHC haplotype: Kd) were transplanted with a CxB6 BM (MHC haplotype Kd-Kb) to recognize donor pericytes by the gain of Kb on NG2 cells. Confocal microscopy analysis showed that NG2 cells stain mostly donor Kb antigens but also that some pericytes remain of host origin (Kb negative) thus suggesting the coexistence of donor and host pericytes (Supplementary Fig. S3C).
To show a possible distinction between donor and host TNF-responsive cells, we performed BMT experiments using TNFR-I KO donors into CxB6NeuT mice. Tumor outgrowth in such BM chimeras did not differ from their wild-type counterpart (Fig. 4A) and histologic analysis of tumors from TNFR-I KO chimeras showed a stroma organization very similar to that of B6 chimeras (Fig. 4B) and completely different from the disorganized and necrotic tumors of TNF KO chimeras. Immunostaining with anti-CD31 showed regular vessel structures and the presence of NG2+ pericytes around EC (Fig. 4C). In tumors from TNFR-I KO chimeras, NG2+ pericytes embraced Tie2+ vessels, as in wild-type chimeras (data not shown). Moreover, the presence of MRC1+Tie2+ cells in TNFR-I KO chimeric mice did not differ from control chimeras (Fig. 4D compared with Fig. 2C).
Figure 4.
TNF protumoral activity is exerted through host-derived cells. A, 8-wk-old CxB6NeuT mice are transplanted with B6 or TNFR-I-KO BM cells and kept in observation for tumor onset and progression. Mean tumor number and mean tumor volume are shown (pool of two separate experiments, each with seven mice per group. SE bars are shown; n.s., not significant). B, histologic analysis (H&E staining) of tumors from B6 wild-type NeuT chimeras in comparison with samples from TNFR-I KO chimeric animals. Bar, 500 μm. C, immunohistochemical staining of CD31 tumor vessels (top left; bar, 500 μm; inset, ×400 magnification) and NG2 pericytes (top right; bar, 500 μm) in TNFR-I KO BM chimeras. N, areas of necrosis. D, confocal immunofluorescence analysis of tumor samples from TNFR-I KO chimeras with Tie-2 and MRC1 markers. Bar, 5 μm.
Thus, the absence of TNFR-I on BM-derived cells did not change NeuT carcinogenesis, tumor vessel organization, or the presence of NG2+ pericytes and of MRC1+ cells. These and the above results indicate that TNF should come from donor-derived leukocytes whereas radioresistant cells of host origin sensing TNF are sufficient to recruit and/or sustain pericytes and MRC1+ accessory cells.
Anti-TNF antibody treatment partially recapitulated the result obtained following BMT with TNF-deficient mice
We evaluated whether treatment with anti-TNF blocking antibody was able to inhibit tumor growth. NeuT mice were treated with V1q, a rat anti-mouse TNF blocking antibody for 4 weeks (from weeks 15 to 18), and tumor growth was evaluated by whole mount analysis at 22 weeks of age. As shown in Fig. 5A, anti-TNF treatment halted tumor growth until 1 month after antibody treatment was ceased. Later time point analyses showed resumed tumor growth (Fig. 5B), along with recovery of TNF activity, an event that could not occur in the case of TNF KO BM chimeras, in which leukocyte-derived TNF is irreversibly removed. Although the differences in tumor onset and volume are not statistically significant in anti-TNF-treated mice, histology showed some vascular impairment resembling that occurring in TNF KO BM chimeras (Fig. 5C).
Figure 5.
Effect of treatment of NeuT mice with anti-TNF antibody. A, whole mount analysis of inguinal mammary glands from NeuT mice treated, or untreated, with V1q anti-TNF antibody, starting at 15 wk of age, for 4 wk. Mice are sacrificed at 22 wk of age; a single mammary gland for each mouse (n = 3) is shown. B, NeuT mice treated, or untreated, with V1q anti-TNF antibody, starting at 15 wk of age, for 4 wk. Tumor incidence is shown as mean tumor number and mean tumor volume. The graph shows a representative experiment, repeated twice, performed with seven mice per group. Statistical difference is evaluated by paired t test (week 30), with P < 0.05 as a significant cutoff; n.s., not significant. SE bars are shown. C, histologic analysis of tumors from CxB6NeuT mice treated with isotype control of V1q monoclonal antibodies. D, whole mount analysis of inguinal mammary glands from NeuT mice treated, or untreated, with V1q anti-TNF antibody, starting at 19 wk until 24 wk, when mice are sacrificed; two mammary glands from each mouse are shown (experiment repeated twice, with four mice each group). E, NeuT mice treated, or untreated, with V1q anti-TNF antibody, starting at 19 wk of age, for 4 consecutive weeks as for B.
We also treated NeuT mice in advanced stages of carcinogenesis, from weeks 19 to 23: whole mount analysis at week 24 showed that the late treatment is still able to partially delay tumor progression (Fig. 5D), but analysis at later time points showed no significant difference in tumor growth (Fig. 5E).
Frequency and distribution of NG2+ subendothelial cells in human breast cancers
Considering NG2+ pericytes a possible indirect functional target of TNF and the possibility of using TNF antagonists to treat human breast cancer, we investigated the presence and amount of NG2+ subendothelial cells in samples from human ductal invasive breast cancers, the human counterpart of mouse NeuT tumors. NG2+ cells were constantly found associated with vessels in the context of the intratumoral fibrovascular meshwork (Fig. 6). The amount of NG2+ cells was rather heterogeneous among tumor samples with the same histotype and seemed unrelated to histologic grade, hormone receptor status, or HER2 expression (Table S1). Notably, tumors rich in NG2+ cells were also identified among high-grade estrogen receptor-, progesteron receptor-, and HER2-(basal-like) cases. These findings allow us to speculate on the potential application of anti-TNF strategies to cases that cannot benefit from consolidated targeted therapies.
Figure 6.
Presence of NG2+ cells in human ductal breast carcinoma samples. Immunostaining with NG2 marker in samples from human ductal invasive breast cancers. Bar, 500 μm.
Discussion
BM-derived cells that are associated with epithelial transformation mostly belong to the immune/inflammatory lineage (34). Accordingly, the inflammatory NF-κB pathway is frequently implicated in tumor initiation and progression (22, 35, 36); its inhibitors, or that of its downstream signals, can modify stroma cell components and function, thus limiting tumor support.
Here, we evaluated the role of the prototypical pro-inflammatory cytokine TNF in the development and progression of autochthonous mammary carcinogenesis occurring in the NeuT transgenic mice. We showed that TNF withdrawal in BM-derived cells delayed tumor onset and reduced tumor number and volume. Differently from 7,12-dimethylbenz(a) anthracene-induced skin carcinogenesis, in which TNF has been shown to be critically needed during the initial phase of de novo carcinogenesis (16), in our mammary tumor model TNF was required until the progression reached the stage of local invasive carcinoma, as shown by BMT experiments performed at 15 and 20 weeks of age. Despite the improvements in the diagnosis of early breast cancers, a significant fraction of patients are still identified in advanced stages. Therefore, understanding the time window in which TNF deprivation is still effective in halting tumor progression would help in the design of therapeutic strategies for breast cancer, based on the use of TNF antagonists.
Histopathologic analysis revealed that TNF was required for organizing tumor stroma into nest-like structures, surrounded by a well-organized branching fibrovascular stroma meshwork that is characterized by CxB6NeuT tumors and is observed in some well-differentiated as well as invasive breast cancers. Tumors from mice receiving B6 BM maintained such an organization, whereas those from mice receiving TNF-deficient cells showed a disarranged stroma with large areas of necrosis involving the perivascular regions. In particular, subendothelial cells, identified by NG2 expression and stained for TNFR-I, were almost completely lost in tumors from TNF-deficient BM chimeras, thus explaining the disorganization and leakiness of tumor vessels in those tumors. Loss of pericytes could deprive tumor endothelial cells from essential survival factors, such as VEGF (33). Using BM chimeras, we observed that pericytes could be of both BM and host origin. Thus, testing TNFR-I KO donor into CxB6NeuT recipient chimeras, we found that tumor growth did not differ from wild-type control chimeras, and that tumors had the same NG2+ pericyte content and distribution of wild-type NeuT tumors. These findings indicate that although TNF should come from donor leukocytes, the radioresistant host compartment expressing TNFR-I, pericytes included, was sufficient to sustain tumor outgrowth. TNF may also behave as a growth and survival factor for some tumor cell lines (6). However, in our model, this is unlikely because NeuT-derived tumor cell lines treated with recombinant TNF neither proliferated nor underwent apoptosis, consistent with the undetectable expression of TNFRs on tumor cells (data not shown). This is in contrast with a recent report showing that erbB2 transgenic mice that were also knocked out for TNF had reduced cell growth, apparently because of the interrupted autocrine loop in which tumor produced TNF fuelled cell proliferation (25). Although in the presence of a similar reduction in tumor progression, TNF knockdown from embryonic life or from adulthood underscores major differences including the possible activation of compensatory mechanisms in the former versus the prompt withdrawal at different stages of tumor progression, dosing the time of BMT, in the latter case. Moreover, BMT allows a distinction between leukocyte and tumor source of TNF as the most relevant for the tumor to progress.
Besides altering stroma and vasculature organization, the lack of TNF modified the number and state of activation of infiltrating leukocytes. The edges of tumors from CxB6NeuT mice and wild-type chimeras were highly infiltrated by macrophages positive for MRC1+, a marker of M2/wound healing macrophages (30), whereas in tumors from TNF KO chimeras, their numbers were greatly reduced. Most of the MRC1+ cells in tumors from wild-type chimeras also expressed the Tie2 marker which defines the TEM population fostering tumor angiogenesis (31, 32). The paucity of these cells in tumors from TNF KO chimeras might contribute to their altered vasculature. Immunohistochemical staining of intracellular cytokines also suggested a switch to M1-secreted cytokines (higher IL-12 and lower IL-10) in the infiltrating leukocytes of tumors from TNF KO chimeras, a condition associated with tumor rejection and induced by local treatment with CCL16, CpG, and systemic IL-10 receptor neutralization (37).
We propose that TNF exerts protumor activity through at least two mechanisms. First, TNF is required for a correct stroma organization and for the presence of pericytes sustaining tumor vasculature. Second, TNF contributes to the creation of a tumor microenvironment characterized by the presence of tumor-associated macrophages with a M2 cytokine profile and of angiogenesis-promoting TEM. The observation that BMT from TNFR-I KO did not phenocopy TNF KO chimeras indicates that radioresistant cells, likely some pericytes, and very likely EC, sense TNF directly. Accordingly, tumors from TNFR-I KO chimeras were infiltrated by NG2+ and MRC1+ cells similarly to those from wild-type chimeras. TNFR triggering on host pericytes and EC may induce the secretion of mediators such as VEGF, PDGF-β, and angiopoietins that, in turn, may recruit and/or activate pericytes and tumor-associated monocytes, in particular TEM, even if TNFR-I KO. Altogether, these cells contribute to an efficient pro-angiogenic environment.
Although TNFR-I signaling is relatively well known, that of TNFR-II is still poorly characterized. TNFR-II primarily binds transmembrane TNF and it can modulate the activity of TNFR-I (4). Thus, although TNF operates mainly through TNFR-I, we could not exclude a priori a role for TNFR-II, selectively expressed on immune and endothelial cells. Although we have not been able to detect a clear staining for TNFR-II in our tumor samples, to rule out its possible role in cells of BM origin, we are performing BMT experiments with TNFR-II KO animals into CxB6NeuT mice. Preliminary data showing no difference in tumor growth from control chimeras seem to exclude such a role. A similar result has just been published for the ID8 ovarian cancer model in which BMT from TNFR-II does not change tumor growth (38).
Results from skin and liver carcinogenesis experiments in TNF and TNFR-KO mice have pointed out the involvement of the TNF signaling pathway in early stages of tumor development and progression (16–19), and prompted initial clinical studies testing TNF antagonists in cancer patients (39–41). The above-mentioned animal studies that were performed directly in TNF or TNFR-KO mice using a chemically induced carcinogenesis model were unsuitable for distinguishing between tumor- and host-derived TNF. On the contrary, our experimental setting, in addition to testing the relevance of TNF produced by BM-derived leukocytes for the growth of NeuT mammary carcinomas, allowed TNF depletion from the time of irradiation thus mimicking a clinical condition in which TNF activity could be precisely switched off.
Popivanova and colleagues recently reported that blocking TNF, using TNFR-I KO mice or by treating wild-type mice with Etanercept, an antagonist of both TNF and lymphotoxin, reduces azoxymethane and dextran sulfate sodium-induced colorectal carcinogenesis (20). Our work, on the other hand, corroborates the link between inflammation and carcinogenesis in the absence of external inflammatory stimuli (1) and confirms that tumor progression could be reversed by TNF antagonists.
To test the effect of TNF inhibition using methods other than BMT, we treated NeuT mice with a TNF blocking antibody for 4 weeks and evaluated tumor onset and progression. This treatment prevented tumor growth as long as the antibody was administered, a finding in line with the results of other studies (25). Because BMT with TNF KO donors permanently removed leukocyte-produced TNF, the antibody treatment would require continuous administration to be equally effective. Another difference between the two treatments is total body irradiation that is required for BMT and that might directly affect the tumor. Indeed, we have observed that irradiation of NeuT mice at late time points (15 and 20 wk) was sufficient to delay tumor onset, although concomitant deficiency of TNF in donor cells resulted in a more pronounced growth arrest. It has been recently reported that myelo-monocytic cells contribute to tumor regrowth after radiation by increasing vasculogenesis (42) and tumor stroma remodeling (43). This observation and our data support the idea of combining TNF antagonists with local radiotherapy.
Searching for a possible human counterpart that might benefit from TNF inhibition, we found that even human ductal invasive breast cancers devoid of conventional molecular targets like Her2 and estrogen receptor are rich in NG2+ subendothelial cells surrounding tumor vessels.
Considering that TNF antagonists, such as the chimeric monoclonal antibody infliximab, are currently, and successfully, employed in the treatment of autoimmune diseases, the transition of their use to cancer treatment would encounter few regulatory hurdles and would be poised for a rapid effect. The combination of TNF antagonists with local radiotherapy or, considering the role of TNF we have observed on tumor vasculature organization, with anti-VEGF strategies, might be particularly attractive.
Supplementary Material
Acknowledgments
The authors thank Dr. P. Casalini for her technical expertise in confocal acquisition and analysis; and M. Parenza, B. Cappetti, and I. Arioli for their technical assistance.
Grant Support
Associazione Italiana Ricerca sul Cancro and the Italian Ministry of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 2.Gray PW, Aggarwal BB, Benton CV, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature 1984;312:721–4. [DOI] [PubMed] [Google Scholar]
- 3.Old LJ. Tumor necrosis factor (TNF). Science 1985;230:630–2. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9:361–71. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F TNF-α in promotion and progression of cancer. Cancer Metastasis Rev 2006;25:409–16. [DOI] [PubMed] [Google Scholar]
- 6.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-α as a tumour promoter. Eur J Cancer 2006;42:745–50. [DOI] [PubMed] [Google Scholar]
- 7.Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A 1987;84:5277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato N, Goto T, Haranaka K, et al. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst 1986;76: 1113–21. [PubMed] [Google Scholar]
- 9.Patterson C, Perrella MA, Endege WO, Yoshizumi M, Lee ME, Haber E. Downregulation of vascular endothelial growth factor receptors by tumor necrosis factor-α in cultured human vascular endothelial cells. J Clin Invest 1996;98:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon C, Iyer M, Prabakaran I, Canter RJ, Lehr SC, Fraker DL. TNF-α downregulates vascular endothelial Flk-1 expression in human melanoma xenograft model. Am J Physiol Heart Circ Physiol 2003;284: H317–29. [DOI] [PubMed] [Google Scholar]
- 11.Giraudo E, Primo L, Audero E, et al. Tumor necrosis factor-α regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem 1998;273:22128–35. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Xu Y, Ekman N, et al. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem 2003;278:51267–76. [DOI] [PubMed] [Google Scholar]
- 13.Ilg RC, Davies MH, Powers MR. Altered retinal neovascularization in TNF receptor-deficient mice. Curr Eye Res 2005;30:1003–13. [DOI] [PubMed] [Google Scholar]
- 14.Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J 2002;16:963–74. [DOI] [PubMed] [Google Scholar]
- 15.Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008;111:4997–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med 1999;5:828–31. [DOI] [PubMed] [Google Scholar]
- 17.Scott KA, Moore RJ, Arnott CH, et al. An anti-tumor necrosis factor-α antibody inhibits the development of experimental skin tumors. Mol Cancer Ther 2003;2:445–51. [PubMed] [Google Scholar]
- 18.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-α receptor subtypes is essential for optimal skin tumour development. Oncogene 2004;23:1902–10. [DOI] [PubMed] [Google Scholar]
- 19.Knight B, Yeoh GC, Husk KL, et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med 2000;192:1809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008;118:560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulbe H, Thompson R, Wilson JL, et al. The inflammatory cytokine tumor necrosis factor-α generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res 2007;67:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pikarsky E, Porat RM, Stein I, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. [DOI] [PubMed] [Google Scholar]
- 23.Szlosarek PW, Balkwill FR. Tumour necrosis factor α: a potential target for the therapy of solid tumours. Lancet Oncol 2003;4:565–73. [DOI] [PubMed] [Google Scholar]
- 24.Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Expression of tumour necrosis factor (TNF α) and its receptors in benign and malignant breast tissue. Int J Cancer 1994;56: 777–82. [DOI] [PubMed] [Google Scholar]
- 25.Warren MA, Shoemaker SF, Shealy DJ, Bshar W, Ip MM. Tumor necrosis factor deficiency inhibits mammary tumorigenesis and a tumor necrosis factor neutralizing antibody decreases mammary tumor growth in neu/erbB2 transgenic mice. Mol Cancer Ther 2009;8:2655–63. [DOI] [PubMed] [Google Scholar]
- 26.Lucchini F, Sacco MG, Hu N, et al. Early and multifocal tumors in breast, salivary, Harderian and epididymal tissues developed in MMTY-Neu transgenic mice. Cancer Lett 1992;64:203–9. [DOI] [PubMed] [Google Scholar]
- 27.Boggio K, Nicoletti G, Di Carlo E, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med 1998;188:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR, Colombo MP. Leukocyte, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J Exp Med 2003;198:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripodo C, Di Bernardo A, Ternullo MP, et al. CD146(+) bone marrow osteoprogenitors increase in the advanced stages of primary myelofibrosis. Haematologica 2009;94:127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–26. [DOI] [PubMed] [Google Scholar]
- 32.Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes (TEMs), blood “resident” monocytes and embryonic macrophages suggests common functions and developmental relationships. Blood 2009; 114:901–14. [DOI] [PubMed] [Google Scholar]
- 33.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 2005;7:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211–7. [DOI] [PubMed] [Google Scholar]
- 35.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285–96. [DOI] [PubMed] [Google Scholar]
- 36.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004;6: 297–305. [DOI] [PubMed] [Google Scholar]
- 37.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 2005;65: 3437–46. [DOI] [PubMed] [Google Scholar]
- 38.Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest 2009;19:3011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madhusudan S, Foster M, Muthuramalingam SR, et al. A phase II study of etanercept (Enbrel), a tumor necrosis factor α inhibitor in patients with metastatic breast cancer. Clin Cancer Res 2004;10: 6528–34. [DOI] [PubMed] [Google Scholar]
- 40.Madhusudan S, Muthuramalingam SR, Braybrooke JP, et al. Study of etanercept, a tumor necrosis factor-α inhibitor, in recurrent ovarian cancer. J Clin Oncol 2005;23:5950–9. [DOI] [PubMed] [Google Scholar]
- 41.Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor α as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol 2007; 25:4542–9. [DOI] [PubMed] [Google Scholar]
- 42.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008;13:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn GO, Brown JM. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle 2009;8:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.