Abstract
In the last century, a global transformation of Earth’s surface has occurred due to human activity with extensive agriculture replacing natural ecosystems. Concomitant declines in wild and managed bees are occurring, largely due to a lack of floral resources and inadequate nutrition, caused by conversion to monoculture-based farming. Diversified fruit and vegetable farms may provide an enhanced variety of resources through crops and weedy plants, which have potential to sustain human and bee nutrition. We hypothesized fruit and vegetable farms can enhance honey bee (Hymenoptera: Apidae, Apis mellifera Linnaeus) colony growth and nutritional state over a soybean monoculture, as well as support a more diverse wild bee community. We tracked honey bee colony growth, nutritional state, and wild bee abundance, richness, and diversity in both farm types. Honey bees kept at diversified farms had increased colony weight and preoverwintering nutritional state. Regardless of colony location, precipitous declines in colony weight occurred during autumn and thus colonies were not completely buffered from the stressors of living in a matrix dominated with monocultures. Contrary to our hypothesis, wild bee diversity was greater in soybean, specifically in August, a time when fields are in bloom. These differences were largely driven by four common bee species that performed well in soybean. Overall, these results suggest fruit and vegetable farms provide some benefits for honey bees; however, they do not benefit wild bee communities. Thus, incorporation of natural habitat, rather than diversified farming, in these landscapes, may be a better choice for wild bee conservation efforts.
Keywords: wild bee, honey bee, Apis mellifera, diversified farming
Bees are an essential component of ecosystems providing a pivotal service through the pollination of a wide variety of plants, including economically important crops (Winfree et al. 2008, 2009; Potts et al. 2010a; Ollerton et al. 2011). However, wild bee populations have declined at local and regional scales (Banaszak 1992, Steffan-Dewenter et al. 2002, Kremen et al. 2004) and managed honey bees are also facing high colony losses (Aizen and Harder 2009, Potts et al. 2010b, Steinhauer et al. 2014).
Wild and managed bees are affected by interacting environmental stressors, such as diseases, inadequate nutrition, and exposure to pesticides as a result of agricultural intensification (Oldroyd 2007, Naug 2009, Goulson et al. 2015). Worldwide, habitat conversion due to transformation of landscapes into row-crop agricultural systems is cited as a primary driver of wild and managed bee declines (Koh et al. 2016, Sánchez-Bayo and Wyckhuys 2019). For example, conversion of natural habitat into extensive row-crop agriculture in the Midwestern United States is associated with reduced wild bee populations (Koh et al. 2016) and reduced habitat suitability for honey bees (Otto et al. 2016). In the United States, beekeepers lose up to 40% of their honey bee colonies annually. In the Midwest, these losses can exceed 60% (Seitz et al. 2016).
Land used for agriculture can reduce natural and seminatural habitat creating a scarcity in floral diversity and abundance that affects bee abundance (Kremen et al. 2007, Isaacs et al. 2009, Potts et al. 2010a) and health (Naug 2009, Winfree 2010). Although mass-flowering monocultures may provide transient forage for some bee species (Westphal et al. 2003, Jauker et al. 2012, Holzschuh et al. 2013, Todd et al. 2016), the simplified landscape and postcrop bloom results in a paucity of floral abundance (Kremen et al. 2002, Klein et al. 2007). Such loss of resource diversity can lead to suboptimal bee nutrition resulting in a compromised bee immune system and poor overall health (Dolezal et al. 2019b).
The Midwestern United States has been identified as a critical focus region for bee declines (Koh et al. 2016), due to the extreme simplification that came with the production of corn and soybean in monocultures. In the Midwestern state of Iowa, 85.5% of the landscape is committed to farming (NASS-USDA 2017), primarily to produce corn and soybean (Brown and Schulte 2011). Despite this, a diverse community of wild bees (at least 40 species of Apoidea) has been observed in corn and soybean within Iowa (Gill and O’Neal 2015, Wheelock and O’Neal 2016, Wheelock et al. 2016). Historical records of bee diversity in this region are limited; thus, it is unclear to what extent this community represents the possible bee diversity in this region. To date, 300 bee species have been reported to currently reside in the state of Iowa (DNR, Iowa. Department of Natural Resources 2018), suggesting a richer community is present. Given this extant community and the extreme form of agriculture practiced within Iowa, this is an ideal location to study bee responses to human-driven ecological change.
In general, pollinator responses to landscape complexity and noncrop resources have been a subject of several studies (Ricketts et al. 2008, Batáry et al. 2011, Garibaldi et al. 2011, Shackelford et al. 2013, Crist and Peters 2014). However, few studies have investigated the response of managed honey bees and wild bees within the same context (Shackelford et al. 2013, Mallinger et al. 2017). Although similar stressors affect wild and managed bees, wild bees encompass thousands of species with varying life histories (Michener 2007); therefore, their responses to stress may differ from managed bees depending on their individual foraging preferences within a specific landscape. Understanding how managed and wild bees cope with different types of agricultural landscapes is necessary for the creation of effective conservation plans.
Historically, farming included the production of several crops within a single parcel of land (Foley et al. 2005). The use of more diverse farming practices has the potential to not only produce human sustenance, but also enhance biodiversity, ecosystem services, and bee health (Garibaldi et al. 2017). In general, greater plant diversity increases bee diversity (Shackelford et al. 2013), and more noncrop habitat at the local and landscape level is associated with greater availability of floral and nesting resources that are, in turn, associated with greater bee richness (Ricketts et al. 2008, Shackelford et al. 2013). To what extent wild and managed bees utilize floral resources in more diversified farms (e.g., fruit and vegetable farms) is not well understood. Diverse farms that grow a multifarious mix of cultivars may provide more floral resources throughout a growing season than farms with a few large monocultures. Diversified farms are likely to be composed of exotic species of crops and weeds, which the introduced honey bees, generalists (Giannini et al. 2015) that forage on a variety of crops, may benefit from more readily than native, wild bees (Thapa 2006, Calderone 2012). Although some wild bees also forage on certain crops (Michener 2007), others are more specialized foragers with varying nesting site requirements and may not benefit from diversified farms. Our goal was to investigate the responses of wild and managed bees in a monoculture (i.e., soybeans) and more diverse farms (i.e., fruit and vegetables).
We incorporated a ‘landscape physiology’ approach (Alaux et al. 2017), measuring not only the response of managed honey bee colonies and the bee community, but also individual nutritional state in honey bees. We hypothesized that diverse farms would support increased honey bee colony growth (i.e., weight, capped brood production, and adult bee population), nutritional state (i.e., lipid content), and a more diverse community of wild bees compared with farms committed to soybean production. To test these hypotheses, we deployed sentinel honey bee colonies at selected farms in central Iowa that were either committed to conventional soybean production or produced fruits and vegetables. Regardless of the type of farm, all were embedded in the same landscape matrix consisting of corn and soybean production (Supp Fig. 1A and B [online only]). Throughout the growing season, we monitored honey bee colony growth and used pan traps to sample the wild bee community. Overall, our study aims to provide insights into the potential of diversified farming to foster bee abundance, diversity, and health.
Materials and Methods
Farm Selection
We identified farms as the experimental unit to test our hypotheses regarding the impact of farm diversity on honey bees and wild bees. We selected two types of farms located within central Iowa that either grew only soybean (Mono-SOY) or grew fruits and vegetables (Div-FV), in 2015 and 2016. From those farms, we randomly selected a subset of farms which did not have honey bee colonies placed within 1.6 km, and all sites were located at least 3.2 km or more from one another. We chose this distance to reduce resource competition with our sentinel colonies (see below), as studies indicate that on average, foragers do not often forage farther than 2 km from their hives (Couvillon et al. 2014, Seeley 2019, Carr-Markell et al. 2020). In total, 4 Div-FV and 10 Mono-SOY farms were selected in 2015 and 5 Div-FV and 10 Mono-SOY farms in 2016 (Supp Table 1 [online only]). All Mono-SOY and Div-FV farms were independent of each other with the exception of two Div-FV farms, which were visited in both years (Supp Table 1 [online only]). The number of crops produced on Div-FV farms ranged from 12 to 50 (29.86; ±4.91 SEM) and farms ranged in size from 1.2 to 16.2 ha (6.2 ha; ±2.42 SEM; Supp Table 2 [online only]). Two Div-FV farms were certified organic, and all participating Div-FV farmers reported use of the following pesticides that are approved for use in organic farms: foliar Bt, organic insecticidal soaps, and diatomaceous earth. On two of the nonorganic farms, the only nonorganic approved pesticide applied was glyphosate. On all Mono-SOY farms glyphosate was used for weed management and there were no applications of foliar insecticides. Soybean seed planted in the Mono-SOY farms were treated with only a fungicide (Fluopyram, ILeVO, Bayer, Pittsburgh PA).
Iowa is dedicated primarily to corn and soybean production (65.5% land area), producing a uniform landscape matrix (NASS-USDA 2018). However, other types of land cover exist within the state’s landscape and have been shown to affect insect diversity within soybean (Gardiner et al. 2009, 2010). To ensure the surrounding landscape matrix of each experimental farm was similar, we quantified the landscape surrounding each farm and used several aspects of land cover type as covariates to account for variation in the wild bee community and honey bee colony growth. In general, with the exception of the genus Bombus, wild bees forage around 500 m from their nest location (Gathmann and Tscharntke 2002; Zurbuchen et al. 2010a,b). Therefore, we quantified land cover at a 0.8 km radius centered on the pan trap location. To address variation in honey bee colony metrics, land cover within a 1.6 km radius centered on the honey bee colonies was measured. Land cover was quantified in ArcGIS, ArcMap 10.3.1 using the 2015 and 2016 USDA-NASS cropland data layer at a 30 m × 30 m resolution (https://nassgeodata.gmu.edu/CropScape/). The proportion of pixels associated with each land cover type were measured by using ‘isecpolyrst’ function in Geospatial Modeling Environment (Version 0.7.4.0). Land cover types were categorized into four groups (cropland, developed, grassland, and woodland). We only considered annual crops in the cropland category (i.e., soybean, corn, sweet corn, winter wheat, rye, oats, alfalfa, other hay, and other crops). The only perennial crops present in the landscape were apples, which were only present at one farm across the 2 yr and at <0.001% of the total land area. Because apples are a permanent woody feature of the landscape, we considered them in the woodland category along with deciduous, evergreen and mixed forest, shrub land, woody wetland, and herbaceous wetland. Grassland included clover/wildflower, fallow crop, and grass pasture. Developed land included developed open space, as well as low, medium, and high intensities developed land. The only land cover features excluded from the analyses were open water and barren land, as these are not likely to provide resources for bees. Using two-tailed t-tests with Satterthwaite variance, we compared each landscape category between Div-FV and Mono-SOY. We found no differences in proportion cropland or developed land between farm types at a 0.8 km radius; however, there were significantly higher proportions of grassland and woodland surrounding Div-FV compared with Mono-SOY farms (Supp Fig. 1A [online only]). At a 1.6 km radius, we observed no difference in the proportions of cropland, developed, grassland, or woodland between Div-FV and Mono-SOY farms (Supp Fig. 1B [online only]).
Comparing Bee Communities between Farm Type
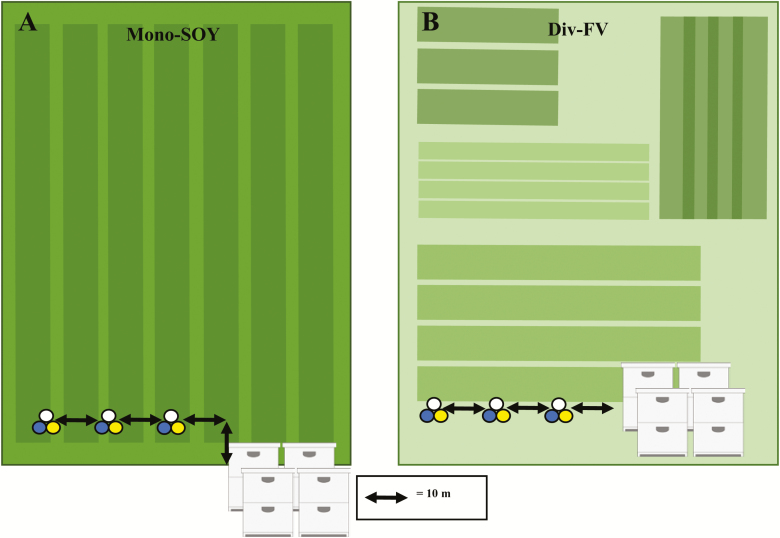
We used modified pan traps (bee-bowls) to measure bee abundance and richness in Div-FV and Mono-SOY farms. Traps were deployed on posts (Droege et al. 2010, Gill and O’Neal 2015), to hold three 3.2 oz. bowls (Solo brand). Because different colors have varying levels of attractiveness to distinctive bee species (Droege 2006), we used bowls painted fluorescent yellow, blue, or left white to maximize the diversity captured. Because each farm was considered an experimental unit, individual bee-bowls at a farm were subsamples. Each field had three bowls of each color (total of nine bowls), deployed on three posts, and each post was placed 10 m apart from each other. In Mono-SOY farms, traps were placed parallel to honey bee colonies and 10 m inside of the field (Fig. 1A). To avoid disturbing the cropping area in Div-FV farms, traps were placed in an area planted with grass directly adjacent to the crops and parallel to honey bee colonies (Fig. 1B).
Fig. 1.
Placement of honey bee colonies and bee-bowl pan traps in Mono-SOY (A) and Div-FV (B) farms in central Iowa in 2015 and 2016. In Mono-SOY farms, traps were placed parallel to honey bee colonies and 10 m inside of the field. In Div-FV farms, traps were placed in an area planted with grass directly adjacent to the crops and also parallel to honey bee colonies. Honey bee colonies were placed in the exterior grassy perimeter, 3 m from the edge of the crop and 10 m from the bee-bowls.
The sampling period spanned 13 wk each year, from 1 July through 24 September in 2015, and 15 June through 9 September in 2016. Collections were made on a biweekly basis. Because the growth and type of foliage near the traps varied between farms and across the season, we standardized trap height by adjusting bee-bowls on the post, so that their height was level with the adjacent plant canopy during each collection (Wheelock and O’Neal 2016). A collection was made by filling each bowl one quarter full with soapy water solution made from a 0.2% aqueous soap solution (Dawn brand). Bee-bowls were deployed when weather conditions were considered favorable to bee foraging behavior and remained in the field for 24 h. When collecting samples, all bowls for a field at a specific date were combined resulting in one collection for each time point.
Specimens were processed using methodologies by Droege et al. (2010) prior to identification. Specimens were identified following standardized methods for monitoring bee populations (LeBuhn et al. 2003), where bees were identified to species or the lowest taxonomic unit possible, with the exception of the genus Lasioglossum, which were identified to subgenus and then morphotyped. Individuals were identified to genus using the dichotomous key ‘The Bee Genera of North and Central America’ (Michener 1994) and to species using the online dichotomous key ‘Discover Life’ (Ascher and Pickering 2015) and ‘Key to Pollinators of the Midwest’ (Arduser 2016). Specimens were verified against a vouchered reference collection deposited in the ISU insect collection (Science II Hall, Ames, IA). From the identified specimens, a subset was processed and fully curated and are deposited as a voucher collection in M.E.O.’s Laboratory at Iowa State University in Ames, IA.
Comparing Honey Bee Response between Farm Type
In 2015, all honey bee colonies were started from commercially obtained packages of Italian (Apis mellifera ligustica) honey bees provided by C.F. Koehnen & Sons LLC, Northern California. Packages contained 0.9 kg of adult bees and a queen. All packages were initiated on bare plastic foundation in a standard 10-frame Langstroth hive and were installed at the Bee and Wasp Research Farm, Ames, IA, on 24 April 2015. After 4 wk, colonies were inspected and ranked by size based on weight. Colonies were randomly selected to go to farms such that each farm had the same average weight of colonies (6.66 kg ±0.35 SEM). On 6 June 2015, colonies were transported to farms within a single day. All colonies were placed in the exterior grassy perimeter, 3 m from the edge of the soybean or fruit/vegetable and 10 m from the bee-bowls (Fig. 1A and B). In 2015, four honey bee colonies were placed at Mono-SOY farms and two were placed at Div-FV farms, totaling 48 colonies. All colonies were left at these farms until 15 October 2015, after which they were returned to the Bee and Wasp Research Farm, where they were kept through the winter.
In 2016, this protocol was repeated with the exception that colonies were derived from those used in 2015 that survived the winter. Colonies were created on 3 May 2016 on fully drawn comb from the previous year rather than bare foundation. Before colonies were randomly assigned a farm, each was manipulated so that they had the same weight, adult bee, and brood populations as colonies of 24 April 2015. Each colony was provided with a new A. mellifera ligustica queen purchased from the same source as 2015. Colonies were initiated in fields on 22 May 2016. We created enough colonies to increase the number at both Div-FV and Mono-SOY farms to four, for a total of 60 colonies.
All colonies were managed using the following practices. Every month, Varroa destructor populations were monitored within each colony using the alcohol wash method (Shimanuki 1991). At the beginning of the experiment (6 June 2015 and 22 May 2016), the mite load (estimated based on mites per 300 bees) in colonies was zero. Although mite levels throughout the season remained below the 10% of adult bees infested threshold (Lee et al. 2010), thymol (Apilife Var; Mann Lake, LTD, Hackensack, MN) was applied per label instructions beginning the last week in month August 2015 and 2016 to prevent mite infestation from confounding the effects of landscape (Dolezal et al. 2016). During the experiment, if no queen or sign of queen presence (recently laid eggs) was observed, a new A. mellifera ligustica queen was introduced.
Honey Bee Colony Growth
In 2015, colonies were inspected on a biweekly basis from June through August and monthly from September through October. Colony growth was quantified by measuring weight; an indicator of colony honey and pollen stores (Klein et al. 2019) and overall colony productivity. Hive equipment was weighed prior to the experiment allowing the calculation of added mass only. Additional hive boxes were added to the colonies when those present reached approximately 75% capacity. Colony inspections occurred on alternate weeks as bee-bowl sampling to reduce the influence that disturbed honey bee colonies may pose for estimating bee activity/density. In 2016, inspections followed the same practice as 2015 with the exception that we maintained a biweekly inspection regime starting 22 May through 18 October resulting in four additional seasonal measurements. In addition to colony weight, we estimated the area of capped brood (i.e., pupae) and adult bee population, and collected bees for measurements of nutritional state (see below). Capped brood was quantified with a Plexiglas grid screen (Delaplane et al. 2013), allowing calculation of brood area in cm2. Adult bee population was estimated in terms of frame sides of bees, i.e., fractional estimates of the sides of a frame covered in bees (Delaplane et al. 2013). Because farm is the experimental unit, colonies are subsamples, and all metrics of colony growth are reported at the farm level.
Honey Bee Nutritional State
In 2016, we evaluated changes in nutritional state of honey bees by estimating whole body lipid content. At each inspection date a 15-ml tube of putative nurse bees (i.e., worker bees collected from frames of open larvae) was collected and stored at −80°C until assayed. Lipid content was measured via the protocol of Toth and Robinson (2005) as modified in Dolezal et al. (2016). Fifty nurse bees were homogenized; from this, 0.25 g of homogenate was subsampled and weighed. Lipid content was quantified via sulphophospho-vanillin spectrophotometric assay, mg lipid per sample was calculated based on a standard curve of pure cholesterol, and lipid content was calculated as percent of total bee mass.
Statistical Analysis
We had unequal sample size between farm treatment types. To determine whether our sampling effort was sufficient to compare species richness between each farm type, we constructed coverage-based rarefaction and extrapolation curves as well as species richness-based rarefaction/extrapolation curves in R 3.4.1 (Chao and Jost 2012, R Core Team 2017). We used the vegan package (Community Ecology Package V2.4–6; Oksanen et al. 2018), the SpadeR package (Species-Richness Prediction and Diversity Estimation with R V1.1.1; Chao et al. 2016), and the INext package (Interpolation and Extrapolation for Species Diversity V2.0.12, 2016; Hsieh et al. 2016) from bee-bowl data. Our data contained many singleton and doubleton species, therefore, we used the Chao2 estimator to derive a lower bound of undetected species richness (Chao 1984, 1987; Colwell and Coddington 1994). The Chao2 estimator reports species richness with respect to incidence of species occurrence rather than sample size (Chao and Chiu 2016). Incidence-based rarefaction and extrapolation curves represent an estimator of the expected species accumulation, which depicts richness with respect to incidence per sample (Chao and Chiu 2016). Curves were reported by farm type (Div-FV vs. Mono-SOY) for 2015 and 2016 combined.
We used nonmetric multidimensional scaling (NMDS) to visually represent the bee community from both farm types using the ‘metaMDS’ function (Oksanen et al. 2018). We used Bray-Curtis metric in NMDS scaling because it takes into consideration abundance rather than just presence/absence. The output from NMDS was used to create a 2D plot indicating the dissimilarity of the counts for Div-FV versus Mono-SOY for 2015 and 2016 combined. The resulting stress value of less than 0.1 confirmed that this analysis maintained the dissimilarities observed in the original data in the reduced dimensions (Buja et al. 2008).
We used permutated multivariate analysis of variance (MANOVA) to test for significance between the bee communities in Div-FV and Mono-SOY farms by creating a model with farming type, year, and the interaction as predictor variables. In some cases, permutated MANOVA can report false significances between treatments if there is a strong lack of homogeneity in the variability of the data between groups. We wanted to examine the variability of these data in the different farm types. To check this assumption, we used the ‘betadisper’ function (Oksanen et al. 2018). The results of this test indicated that there was not a significant difference in the homogeneity between Div-FV and Mono-SOY (F1, 27 = 0.54; P = 0.47); therefore, we are confident in the results of the pMANOVA.
Because bee community sampling took place at different time points across the 2 yr, to include all sampling dates, we binned sampling points by month to test if there was a difference in abundance, richness, and diversity (as measured by Shannon–Wiener Index; Shannon et al. 1950) of bees between Div-FV and Mono-SOY. We created a repeated-measure mixed effect model (PROC GLIMMIX) in SAS 9.4 with farm type, month, farm type by month interaction, and year as predictor variables and site as a random variable. To account for variation in bee community that could potentially be a result of land cover surrounding each farm, we used proportion cover of several landscape categories from the 0.8 km radius as covariates in the model. Cropland correlated heavily with other landscape categories (woodland, developed, and grassland) and was therefore not included in the model (Supp Fig. 2B [online only]), but proportions of all other landscape categories were. Least square means comparisons with Tukey adjustments were used to look for differences in abundance, richness, and diversity of the wild bee community between farm types on specific months.
To further explore our bee community data, after noting that a subset of bee species was collected in extremely high abundance relative to other species across the farm types, we performed an exploratory analysis in which the most common bee species were identified and analyzed separately from the remainder of the community. To identify which bees were common, we assessed relative abundance, which identifies how abundant a species is relative to the other species in the community (Preston 1948). The underlying assumption is that an even community of bees will have equal proportion of all species. We created a boundary for relative abundance of 1/S where S was the total number of species captured across all sites (Wilsey et al. 2005). For our study, we collected 81 species, indicating that species occurring at a relative abundance of >1.2% were classified as ‘common’. Of these individual common species, we then examined abundance between farm types across months. To determine whether common bee species that exhibited a positive response to Mono-SOY were drivers of variation in the bee community, species with a positive association with Mono-SOY were removed from the dataset. We then reanalyzed the community abundance, richness, and diversity using the analysis described above.
We determined if honey bee colony weight varied by farm type by combining data taken in both sampling years by using dates in 2016 that aligned with those from 2015. We created a repeated-measure mixed effect model with year, date, and farm type as predictor variables and colony and site as random variables in SAS. To account for variation in honey bee colonies which may be a result of the surrounding land cover, we used the proportion woodland, developed, and grassland land cover categories at the 1.6 km radius as covariates in the model (Supp Fig. 2B [online only]). Using post hoc paired comparisons of least square means with Tukey adjustment for multiple comparison, we compared all dates to look for significance between farm types at each time point. Because we increased our sampling effort in 2016 to include more time points and additional measures of colony growth, we also analyzed the 2016 colony data (weight, brood, adult bees, and lipid content) separately using the same model as above.
Results
Bee Community
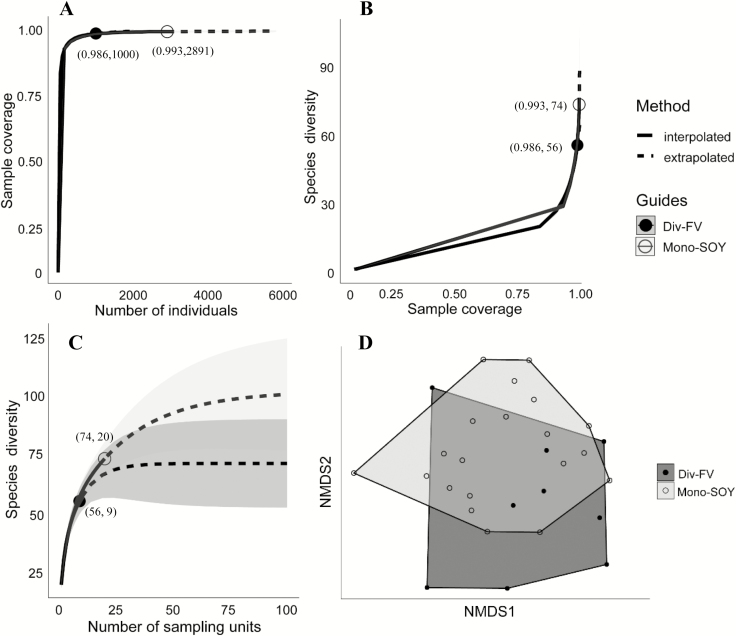
In total, 3,891 bees were collected across all farms for a total of 81 species from 24 genera (Supp Table 3 [online only]). Thirty-five species were classified as morphospecies of the genus Lasioglossum (Curtis) (Hymenoptera: Halictidae), which accounted for 64% of all bees collected (Supp Table 3 [online only]). Based on species coverage curves generated from these data, our sampling efforts produced a coverage of 99.3% in Mono-SOY at 2,891 individuals (Fig. 2A) and 74 species (Fig. 2B). We had a coverage of 98.6% in Div-FV farms at 1,000 individuals (Fig. 2A) and 56 species (Fig. 2B). The two farm types yielded nearly identical sample coverage values (~99%), indicating they are equally complete (Chao and Jost 2012). Therefore, the bee communities sampled between the farm types can be reliably compared. At our sampling effort, Mono-SOY farms had a ratio of 1.32 times more species than Div-FV farms. Based on our species rarefaction curves, increased sampling effort to the point of an asymptote in species would result in a species ratio of 1.35 times higher in Mono-SOY compared with Div-FV (Fig. 2C).
Fig. 2.
Coverage-based rarefaction/extrapolation curves with 95% confidence intervals (shaded areas, based on a bootstrap method with 200 replications) comparing wild bee species abundance (A) and richness (B) collected from bee-bowls for data of two farming types (Div-FV and Mono-SOY) in central Iowa in 2015 and 2016. Incidence-based rarefaction (solid lines) and extrapolation (dashed lines) sampling curves with 95% confidence intervals (shaded areas, based on a bootstrap method with 200 replications) comparing wild bee species richness (C). Observed samples are denoted by the solid circle (Div-FV) and open circle (Mono-SOY). Nonmetric, multidimensional scaling plot of the pollinator community found in Div-FV and Mono-SOY farms in central Iowa in 2015 and 2016 (D). The hulls (closed circles for Div-FV and open circles for Mono-SOY) are constructed from a representation of the pollinator community found at each of the 9 Div-FV and 20 Mono-SOY farms across the 2 yr.
Within the NMDS plots, we produced polygons (i.e., hulls) connecting the perimeter distributions of sites constructed from the bees collected inside Div-FV and Mono-SOY farms (Fig. 2D). The pMANOVA indicated no significant difference in bee communities between the farm types (F1, 25 = 1.71; P = 0.09; Fig. 2D), although several species were unique to the different farm types. There was a significant difference in the bee communities across years (F1, 25 = 4.36; P = 0.001). There was no interaction between farm type and year (F1, 25 = 0.96; P = 0.45). Species that were collected in only one farm type were often rare species (i.e., singletons or doubletons). We observed 49 taxa shared between the farm types, meaning they appeared in at least one site in each farm type (Supp Table 3 [online only]). Shared taxa consisted primarily of solitary, ground nesting bees. Twenty-six taxa were collected exclusively in Mono-SOY, whereas only six taxa were exclusively collected in Div-FV farms (Supp Table 3 [online only]).
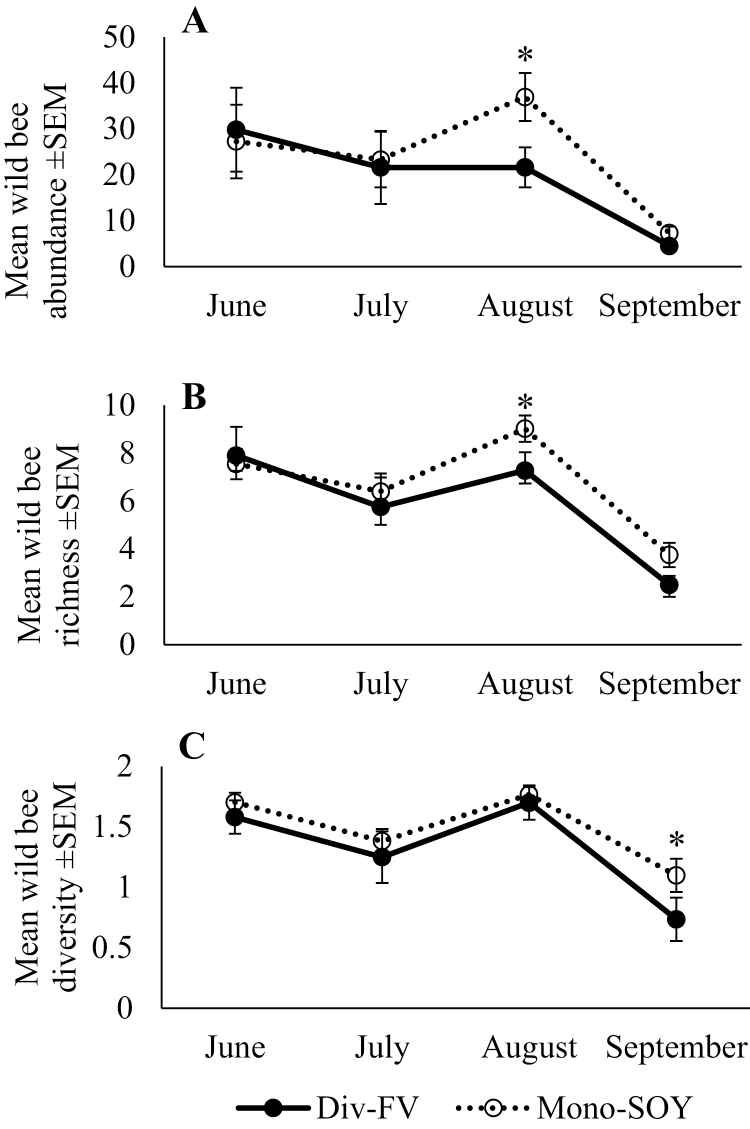
Abundance, richness, and diversity of the wild bee community all varied significantly by month (F3, 125 = 4.27; P = 0.006; F3, 125.5 = 14.58; P < 0.0001; and F3, 125.7 = 14.41; P ≤ 0.0001 for abundance, richness, and diversity, respectively; Supp Table 4 [online only]). There were no significant interactions of farm type and month with bee abundance, richness, or diversity (F3, 129.5 = 1.09; P = 0.36; F3, 130.5 = 0.82; P = 0.48; and F3, 132.1 = 0.42; P = 0.74 for abundance, richness, and diversity, respectively; Supp Table 4 [online only]). There were no observable differences in the total abundance (F1, 24.9 = 0.96; P = 0.34; Supp Table 4 [online only]) of the wild bee community; however, during the month of August, wild bee abundance was significantly greater in Mono-SOY farms compared with Div-FV (T52.24 = 2.05; P = 0.05; Fig. 3A; Supp Table 5 [online only]). On average, we collected 2.1 bees per pan trap per site per sampling day, an amount that is consistent with other studies which have sampled bees in this region (range of 1.1–2.1 bees/trap/site/d; Hendrix et al. 2010, Gill and O’Neal 2015, Wheelock et al. 2016, Wheelock and O’Neal 2016). Richness of wild bees was marginally higher in Mono-SOY farms compared with Div-FV (F1, 25.39 = 3.78; P = 0.06; Supp Table 4 [online only]), these differences were most notable during the month of August (T59.29 = 2.51; P = 0.01; Fig. 3B; Supp Table 5 [online only]). Diversity of the wild bee community was observed to be greater in Mono-SOY farms compared with Div-FV farms (F1, 25.23 = 6.46; P = 0.02; Supp Table 4 [online only]), especially during the month of September (T138.6 = 2.1; P = 0.04; Fig. 3C; Supp Table 5 [online only]). Across the entire season, an average of 144.55 individuals (±24.72 SEM) for an average of 20.85 species (±1.40 SEM) per farm were collected in Mono-SOY. Within Div-FV, an average of 111.11 individuals (±26.1 SEM) for an average of 20.44 species (±1.63 SEM) were collected per farm. Bee abundance did not vary by year (F1, 28.03 = 1.72; P = 0.20; Supp Table 4 [online only]); however, richness and diversity of the bee community did vary by year (richness, F1, 29.32 = 8.87; P = 0.006; diversity, F1, 32.88 = 7.03; P = 0.01; Supp Table 4 [online only]).
Fig. 3.
Mean abundance (A), richness (B), and diversity (Shannon–Wiener Index; C) of the wild bee community observed in Div-FV (solid line and closed circles) and Mono-SOY (dotted line and open circles) in central Iowa across the season during 2015 and 2016. Error bars represent 1 SEM. Values represent the mean of bees collected at each farm per month. Asterisks signify post hoc least squares means comparison for differences between farm types at each time point.
We identified common bees within the community based on relative abundance. Sixteen bees were classified as common (i.e., >1.2% relative abundance), as follows: Agapostemon virescens (Fabricius) (Hymenoptera: Halictidae), A. texanus (Cresson) (Hymenoptera: Halictidae), Melissodes bimaculata (Lepeletier) (Hymenoptera: Apidae), Halictus ligatus (Say) (Hymenoptera: Halictidae), H. confusus (Smith) (Hymenoptera: Halictidae), H. parallelus (Say) (Hymenoptera: Halictidae), Lasioglossum (Dialictus) morphospecies #1, 2, 6, 9, 12, 14, 18, and 25, L. (Evylaeus) morphospecies #1. Of those species, most species did not vary in abundance between faming type across the season (see Supp Table 6 [online only] complete results for year, month, and farm type interaction) nor at any specific time point (Supp Table 7 [online only]). There were positive effects of Mono-SOY on the abundance of M. bimaculata across the season (F1, 146 = 3.95, P = 0.05; Supp Table 6 [online only]). There were marginally more H. ligatus in Mono-SOY across the season (F1, 23.44 = 3.78, P = 0.06; Supp Table 6 [online only]). Post hoc analysis at each sampling month revealed that A. virescens (T72.85 = 1.98, P = 0.05), M. bimaculata (T146 = 3.25, P = 0.001), H. ligatus (T61.17 = 2.67, P = 0.01), and L. (Dialictus) morphospecies #2 (T63.32 = 2.27, P = 0.03) were significantly more abundant in Mono-SOY during the month of August (Supp Table 7 [online only]). Lasioglossum (Dialictus) morphospecies #6 (T146 = 2.03, P = 0.04) and L. (Evylaeus) morphospecies #1 (T109.5 = 2.53, P = 0.01) were more abundant in Div-FV farms during the month of June compared with Mono-SOY (Supp Table 7 [online only]). Lasioglossum (Dialictus) morphospecies #12 were more abundant in Div-FV farms in August compared with Mono-SOY (T70.58 = 2.01, P = 0.05; Supp Table 7 [online only]).
When the four common bee species with a positive response to Mono-SOY were removed from the overall bee community in our exploratory analysis, the abundance, richness, and diversity of the remaining bee community (77 species) no longer varied between the farm types (Supp Table 8 [online only]). Specifically, when the common four bees were removed, we observed no difference in the overall abundance (F1, 25.15 = 0.18, P = 0.68), richness (F1, 25.37 = 1.42, P = 0.25), or diversity (F1, 24.48 = 2.35, P = 0.14) of the wild bee community between Mono-SOY and Div-FV (Supp Table 8 [online only]). Furthermore, we observed no differences in the abundance or richness of the bee community between farm types during any sampling months (Supp Table 9 [online only]). Diversity of the wild bee community remained significantly higher in Mono-SOY compared with Div-FV farms in September (T129.2 = 2.24, P = 0.03; Supp Table 9 [online only]). Abundance, richness, and diversity did vary by year (F1, 28.3 = 6.13, P = 0.02, F1, 30.04 = 16.42, P = 0.0003, F1, 30.05 = 11.64, P = 0.002; Supp Table 8 [online only] for abundance, richness, and diversity, respectively) and by month (F3, 125.2 = 3.91, P = 0.01, F3, 125.5 = 11.93, P ≤ 0.0001, F3, 124.9 = 13.35, P ≤ 0.0001; Supp Table 8 [online only] for abundance, richness, and diversity, respectively). There were no interactions of farm type by month for abundance, richness, or diversity of the wild bee community (Supp Table 8 [online only]).
Honey Bee Colony Growth
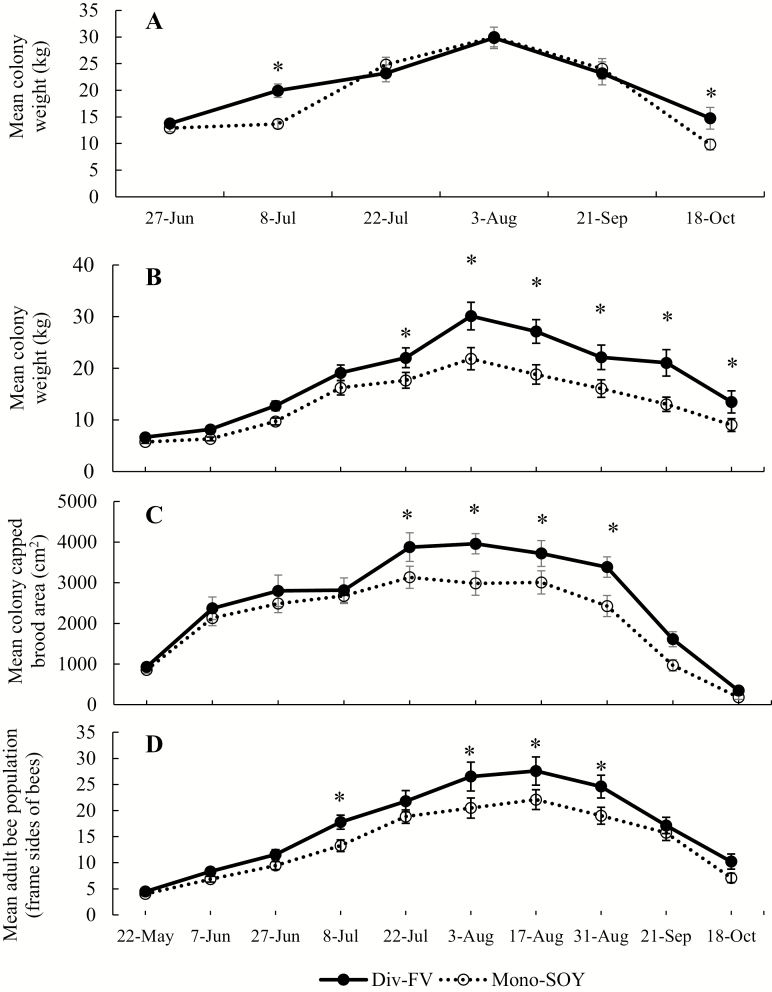
Colony weight did not vary significantly by farm type (F1, 21.03 = 2.39; P = 0.14; Supp Table 10 [online only]). Colony weight varied significantly by date, year, and all interactions of date, year, and farm type for data combined from both years, except for a farm type by year interaction (Supp Table 10 [online only]). The farm type by date interaction was observed on 8 July when colonies were significantly heavier when kept at Div-FV farms (T45.7 = 3.07; P = 0.003; Fig. 4A; Supp Table 11 [online only]) and on 18 October (T50.88 = 2.82; P = 0.006; Fig. 4A; Supp Table 11 [online only]). The heavier weight in Div-FV prior to overwintering sparked an interest in investigating the late season changes in colonies more closely, as we may have overlooked subtle changes by only using a subset of the colony data from 2016 in this combined analysis. Therefore, we analyzed the 2016 data separately and observed significant differences in several honey bee colony metrics between the two farm types. Colonies kept at Div-FV farms had higher weight (F1, 10 = 6.87; P = 0.03; Fig. 4B; Supp Table 12 [online only]), with weight varying significantly by date (F9, 522 = 74.63; P < 0.0001; Fig. 4B; Supp Table 12 [online only]). Colonies were significantly heavier in Div-FV farms compared with Mono-SOY starting 22 July and remained heavier than Mono-SOY through 18 October (Fig. 4B; Supp Table 13 [online only]). No overall difference in brood production between the farm types was observed (F1, 10 = 3.86; P = 0.08; Fig. 4C; Supp Table 12 [online only]); however, brood production varied by date (F9, 522 = 56.75; P < 0.0001; Fig. 4C; Supp Table 12 [online only]). Brood production was significantly higher in colonies kept at Div-FV farms compared with Mono-SOY on 22 July, 3 August, 17 August, and 31 August (Fig. 4C; Supp Table 13 [online only]). There were no interactions of date and farm type with brood production (Supp Table 12 [online only]). Adult bee population was greater in colonies kept at Div-FV farms (F1, 10 = 4.74; P = 0.05; Supp Table 12 [online only]) and varied by date (F9, 520.9 = 72.04; P < 0.0001; Fig. 4D; Supp Table 12 [online only]), with no interactions of date and farm type (Supp Table 12 [online only]). Colonies kept at Div-FV farms had greater populations of adult bees on 8 July and from 3 August through 31 August (Fig. 4D; Supp Table 13 [online only]).
Fig. 4.
Honey bee colony weight in Div-FV (solid line and closed circles) and Mono-SOY (dotted line and open circles) farms in central Iowa for 2015 and 2016 combined (A). Honey bee colony weight (B), capped brood area (C), and adult bee population (i.e., frame sides of honey bees; D) in colonies in Div-FV and Mono-SOY farms in central Iowa for 2016 only. Error bars represent 1 SEM. Asterisks signify post hoc least squares means comparison for differences between farm types at each time point. Results based on repeated-measure linear mixed effect model.
Honey Bee Nutritional State
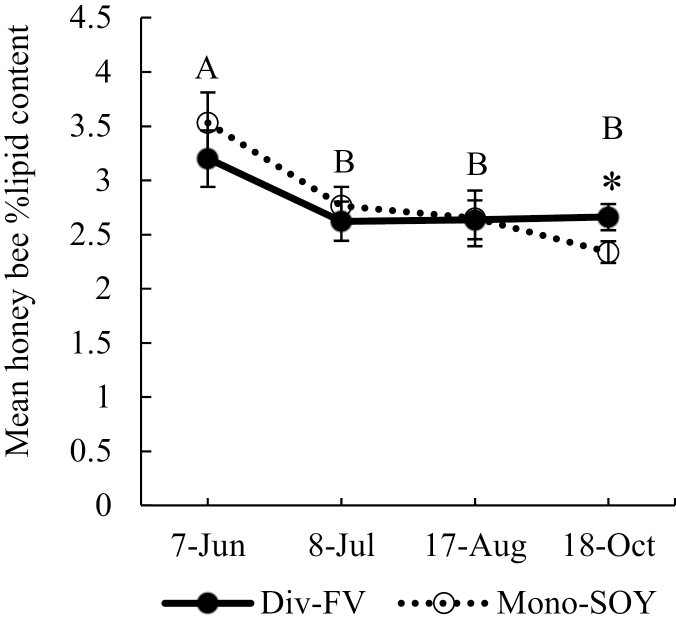
We did not observe a difference in total lipid content of honey bees between colonies kept at Mono-SOY and Div-FV farms in 2016 (F1, 12.97 = 0.02; P = 0.88; Fig. 5; Supp Table 12 [online only]); however, on one date (18 October), lipid content was significantly higher in honey bees from colonies kept at Div-FV farms (T24.28 = 1.97; P = 0.05; Fig. 5; Supp Table 13 [online only]). Honey bee lipid content varied by date (F3, 100.4 = 8.5; P < 0.0001; Supp Table 12 [online only]) with lipids highest at the start of the season (7 June) and then decreasing on 8 July with no change throughout the remainder of the season regardless of farm type (Fig. 5; Supp Table 14 [online only]). There were no interactions of treatment and date (F3, 100.4 = 1.52; P = 0.21; Supp Table 12 [online only]).
Fig. 5.
Mean percent honey bee lipid content (lipid mg bee-mass/mg) for colonies in Div-FV (solid line and closed circles) and Mono-SOY (dotted line and open circles) farm types in central Iowa in 2016. Error bars represent 1 SEM. Asterisks signify post hoc least squares means comparison for differences between farm types at each time point. Letters signify post hoc least squares means comparison for differences between sampling dates. Results based on repeated-measures linear mixed effect model.
Discussion
This study presents novel insights into whether there is potential for bee conservation through diverse farming in a monoculture landscape. Our data support the hypothesis that diversified fruit and vegetable farms, even when found in a landscape that consists of extensive monoculture crops, can benefit honey bee health. Specifically, our results show Div-FV farms supported increased colony growth and individual nutritional state from honey bees collected from within a managed colony (Figs. 4A–D and 5). Because the fruit and vegetable farms we studied are characterized by increased plant diversity and abundance throughout the season (in the form of both crops and weedy plants), our data suggest that diversified farming may benefit honey bees through increased forage availability.
Although we observed positive effects on managed honey bees in fruit and vegetable farms over soybean grown in a monoculture, we observed the opposite effect with the wild bee community. During the month of August, soybean rather than diversified fruit and vegetable farms housed a higher abundance and richness of wild bees (Fig. 3A and B). Furthermore, we observed overall diversity of the wild bee community to be greater in soybean compared with diversified fruit and vegetable farms (Fig. 4C). Specifically, soybean contained 77% more unique species (26 species) compared with diversified farms (six unique species). As sample size increased, it is expected that more individuals will be captured (Chao and Jost 2012). The increased collection of unique species may have been a result of the increased sample size of soybean compared with the fruit and vegetable farms.
Although there were more unique species in soybean farms, we found the increase in abundance and richness of bees in soybean, particularly in August, to be driven largely by four bee species Agapostemon virescens, Melissodes bimaculata, Lasioglossum (Dialictus) sp. 2, and Halictus ligatus, which accounted for 9.5, 8, 6.2, and 4.3% of the total community, respectively. These species are all considered generalist and have previously been documented in corn and soybean fields of Iowa (Gill and O’Neal 2015, Wheelock and O’Neal 2016, Wheelock et al. 2016). With the exception of M. bimaculata, the abundance of these common species individually did not vary by farm type (Supp Table 6 [online only]); however, each was significantly more abundant in soybean during the month of August (Supp Table 7 [online only]). When we removed these four species from our overall analysis of abundance and richness, all positive effects of soybean on the overall bee community, especially during August, disappeared (Supp Tables S8 and S9 [online only]). Thus, the positive trends for wild bees in soybean fields appear to be driven by a few, extremely common species. These data suggest that a small subset of bee species may be well adapted to living in highly disturbed landscapes and thrive on resources available in agricultural systems. In August, blooms in soybean fields and other potentially valuable resources in field edges, such as clover, are highly abundant in Iowa (Dolezal et al. 2019a). This increase in floral availability, rather than diversity, may sufficiently boost the abundance of a subset of generalist bees that are capable of opportunistic foraging on these highly abundant resources, a phenomenon that has been observed in other monoculture cropping systems (Westphal et al. 2003; Holzschuh et al. 2011, 2013). This is further supported by the fact that these bees are regularly found in surveys conducted in the United States and are often associated with landscapes impacted by human disturbance (Kremen et al. 2002; Winfree et al. 2007, 2008; Pardee and Philpott 2014).
Although these farms support a community of wild bees, increased plant diversity through cropping fruit and vegetables did not support an increase in abundance, richness, or diversity of most species of wild bees. In landscapes dominated by production of just a few commodity crops, like Iowa (NASS-USDA 2018), the addition of diversity through agriculture may not be enough to significantly support a diverse bee community. The response of arthropods to conservation efforts may vary depending on the surrounding landscape matrix (Isaacs et al. 2009), per the Intermediate Disturbance Hypothesis. Central Iowa may be such a disturbed environment that the floral resources found in the form of fruits and vegetables are not sufficient to improve upon the abundance and diversity of the wild bee community. Fruit and vegetable farms, although more diverse than monocultures of soybean, are still predominately comprised of a few crop species (12–50 in our study), as well as exotic or weedy species. Production of agricultural crops may not provide the resources to support more sensitive or specialized ecotone species that require noncrop habitat for nesting or forage at some point in their development (Duelli and Obrist 2003).
It is also possible that a lack of a positive effect in diversified farms could be a result of the use of pan traps as the sole method of assessing the wild bee community. Pan traps are a robust method for sampling bees though there are limitations to their usage. Pan traps can be biased toward collecting more bees when floral diversity and abundance is low (Baum and Wallen 2011, Popic et al. 2013, Adhikari et al. 2019). Low plant diversity of soybean fields and short-term bloom period in comparison to diversified farms could have inflated bee captures in our study, particularly in August when soybeans are in bloom. Pan traps can also yield a different community of bees compared with other methods (Roulston et al. 2007). Specifically, pan traps are biased toward collecting smaller bees, often from the family Halictidae (Roulston et al. 2007, Baum and Wallen 2011, Gonçalves et al. 2012), which we have observed to be the dominant bee types in our study. Pan traps are a commonly used method to assess bee communities in agricultural systems (Hall and Ascher 2011, Mallinger et al. 2016, Happe et al. 2018, Adhikari et al. 2019); however, the use other sampling methods may aid in a more accurate understanding of the relationship between bee communities in diversified fruit and vegetable farms compared with monoculture.
With respect to managed honey bees, our data suggest on-farm diversity can have subtle, but significant impact on the health and fitness of honey bees. Across both years, honey bee colonies in Div-FV farms had higher colony weight at the end of the season prior to overwintering (Fig. 4A). In 2016, colonies in Div-FV farms had higher colony weight, bee populations throughout much of the season, and also produced more brood at individual dates throughout the season (Fig. 4B–D). In addition to the abundant resources (e.g., soybean and clover nectar; Dolezal et al. 2019a) provided by the surrounding agricultural landscape, these data suggest honey bees benefit from being housed in the Div-FV farms. The morphology and behavior of honey bees allows them to more readily utilize many plant species as forage, making them a ‘supergeneralist’ compared with other bee species (Giannini et al. 2015). Unlike many wild bee species which only forage roughly 500 m from their nesting site (Gathmann and Tscharntke 2002; Zurbuchen et al. 2010a,b), honey bees are capable of foraging long distances (Beekman and Ratnieks 2000) and utilizing both the resources directly available from the diversity of Div-FV farms and in the soybean surrounding those farms. Taken together with the results from wild bees, this study suggests that farm practices that benefit honey bees are not necessarily a good indicator of how wild bee communities in general will respond. As other studies have previously suggested, honey bees cannot be indiscriminately used as an ‘indicator species’ to extrapolate wild bee response to anthropogenic landscape change (Heard et al. 2017), especially in areas such as the United States where honey bees are exotic to the landscape and probably utilizing different foraging resources compared with many wild bees.
Although honey bees gained some measurable benefits from being in Div-FV farms compared with Mono-SOY, there was nonetheless a precipitous decline in weight of colonies in the late summer regardless of farm type (Fig. 4A and B). This resulted in colonies from both farm types entering the winter with honey stores below what is considered adequate to sustain them (Brodschneider and Crailsheim 2010, Caron and Conner 2013). An additional challenge for honey bees kept at either farm type is indicated by our lipid analysis. Honey bee workers in preparation for overwintering invest energy in accumulation of fat body lipids (Fluri et al. 1977, Döke et al. 2015); therefore, lipid stores of bees in the colony may be an indicator of colony overwintering potential (Dolezal et al. 2016). Although honey bee total lipid content was higher in Div-FV farms than Mono-SOY preoverwintering (Fig. 5), even the highest lipid levels observed were below what would be considered adequate for successful overwintering (Dolezal et al. 2016), indicating that neither farm type is ideal for long-term success of honey bee colonies.
Overall, this study indicates that fruit and vegetable farms can result in a measurable, though modest, improvement of some key health indicators for honey bees over monoculture soybean farms. However, more diverse crop production did not have a positive impact on the wild bee community. For greater benefits to be realized, the land area in diversified farming and type of resources provided may need to be more extensive and pollinator targeted. If declines in bee populations continue as they have in recent years (Kremen et al. 2002, Steinhauer et al. 2014), the future of crop pollination success will probably depend on incorporating both wild and managed bees into pollination management plans (Greenleaf and Kremen 2006, Garibaldi et al. 2013). In extensively cultivated agricultural systems, where much of the natural landscape has been displaced by monoculture farms, there has been interest in supporting bee populations through both diversification of the agricultural system itself, as well as increasing the amount of seminatural or natural landscape within or surrounding agriculture. Our results suggest increased crop diversity through fruit and vegetable farming alone is not sufficient for increasing bee biodiversity beyond common agricultural species, nor for optimal long-term honey bee colony health. Alternatively, studies investigating agricultural landscapes that incorporate diversity through the addition of hedgerows and/or buffer strips of seminatural or native habitat have shown the potential to increase wild bee abundance and richness (Morandin and Kremen 2013, Schulte et al. 2017, Sutter et al. 2017). In the region of this study, native plantings in the form of prairie have been shown to provide valuable resources for honey bees in the late season (Carr-Markell et al. 2020), to sustain colony growth, and to enhance individual honey bee lipid levels (Dolezal et al. 2019a). We suggest an increase in more native, perennial habitat may be a better option to support both wild and managed bee pollinators in extensive agricultural systems.
Supplementary Material
Acknowledgments
We thank the many farmers who allowed us to conduct experiments in their fields and to Iowa State University, Practical Farmers of Iowa, and Blomgren Seed Company for helping us connect with them. Thanks to Edward Hsieh, David Stein, Zoe Pritchard, and members of the Toth and O’Neal labs for helping with field and lab assistance. This research was supported with funding from the United Soybean Board (grant number 1520-732-7225) and Leopold Center for Sustainable Agriculture (grant number E2015-06). Funding sources were not involved in the design, collection, interpretation, or writing of this article.
Data Availability
Upon publication, all data will be made available.
References Cited
- Adhikari S., Burkle L. A., O’Neill K. M., Weaver D. K., Delphia C. M., and Menalled F. D.. . 2019. Dryland organic farming partially offsets negative effects of highly simplified agricultural landscapes on forbs, bees, and bee-flower networks. Environ. Entomol. 48: 826–835. [DOI] [PubMed] [Google Scholar]
- Aizen M. A., and Harder L. D.. . 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19: 915–918. [DOI] [PubMed] [Google Scholar]
- Alaux C., Allier F., Decourtye A., Odoux J. F., Tamic T., Chabirand M., Delestra E., Decugis F., Le Conte Y., and Henry M.. . 2017. A ‘landscape physiology’ approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 7: 40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduser, M. 2016. Key to the genera and species of the Midwestern United States with an emphasis on the Tallgrass Prairie region. (http://www.pwrc.usgs.gov/nativebees/Keys.html).
- Ascher J. S., and Pickering J.. . 2015. Discover life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila) (http://www.discoverlife.org./mp/20q?guide=Apoidea_species).
- Banaszak J. 1992. Strategy for conservation of wild bees in an agricultural landscape. Agric. Ecosyst. Environ. 40: 179–192. [Google Scholar]
- Batáry P., Báldi A., Kleijn D., and Tscharntke T.. . 2011. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc. Biol. Sci. 278: 1894–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum K. A., and Wallen K. E.. . 2011. Potential bias in pan trapping as a function of floral abundance. J. Kansas Entomol. Soc. 84: 155–159. [Google Scholar]
- Beekman M., and Ratnieks F. L. W.. . 2000. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 14: 490–496. [Google Scholar]
- Brodschneider R., and Crailsheim K.. . 2010. Nutrition and health in honey bees. Apidologie 41: 278–294. [Google Scholar]
- Brown P. W., and Schulte L. A.. . 2011. Agricultural landscape change (1937–2002) in three townships in Iowa, USA. Landsc. Urban Plan. 100: 202–212. [Google Scholar]
- Buja A., Swayne D., Littman M., Dean N., Hofmann H., and Chen L.. . 2008. Data visualization with multidimensional scaling. J. Comput. Graph. Stat. 17: 444–472. [Google Scholar]
- Calderone N. W. 2012. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS One 7: e37235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. M., and Conner L. J.. . 2013. Honey bee biology and beekeeping. Wicwas Press, Pittsburgh, PA. [Google Scholar]
- Carr-Markell M. K., Demler C. M., Couvillon M. J., Schürch R., and Spivak M.. . 2020. Do honey bee (Apis mellifera) foragers recruit their nestmates to native forbs in reconstructed prairie habitats? PLoS One 15: e0228169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. 1984. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11: 265–270. [Google Scholar]
- Chao A. 1987. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43: 783–791. [PubMed] [Google Scholar]
- Chao A., and Chiu C. H.. . 2016. Species richness: estimation and comparison. Wiley StatsRef: Statistics Reference Online. 1–26. doi: 10.1002/9781118445112.stat03432.pub2 [DOI] [Google Scholar]
- Chao A., and Jost L.. . 2012. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93: 2533–2547. [DOI] [PubMed] [Google Scholar]
- Chao A., Ma K. H., Hsieh T. C., and Chiu C.-H.. . 2016. SpadeR: species-richness prediction and diversity estimation with R. R package version 0.1.1. (https://rdrr.io/cran/SpadeR/).
- Colwell R. K., and Coddington J. A.. . 1994. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 345: 101–118. [DOI] [PubMed] [Google Scholar]
- Couvillon M. J., Schürch R., and Ratnieks F. L.. . 2014. Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS One 9: e93495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist T. O., and Peters V. E.. . 2014. Landscape and local controls of insect biodiversity in conservation grasslands: implications for the conservation of ecosystem service providers in agricultural environments. Land 3: 693–718. [Google Scholar]
- Delaplane K. S., van Der Steen J., and Guzman-Novoa E.. . 2013. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apicult. Res. 52: 1–12. [Google Scholar]
- DNR, Iowa. Department of Natural Resources 2018. Pollinators. (http://www.iowadnr.gov/Conservation/Iowas-Wildlife/Pollinators) (Assessed 19 August 2019).
- Döke M. A., Frazier M., and Grozinger C. M.. . 2015. Overwintering honey bees: biology and management. Curr. Opin. Insect Sci. 10: 185–193. [DOI] [PubMed] [Google Scholar]
- Dolezal A. G., Carrillo-Tripp J., Miller W. A., Bonning B. C., and Toth A. L.. . 2016. Intensively cultivated landscape and Varroa mite infestation are associated with reduced honey bee nutritional state. PLoS One 11: e0153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal A. G., St Clair A. L., Zhang G., Toth A. L., and O’Neal M. E.. . 2019a. Native habitat mitigates feast-famine conditions faced by honey bees in an agricultural landscape. Proc. Natl. Acad. Sci. USA 116: 25147–25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal A. G., Carrillo-Tripp J., Judd T. M., Allen Miller W., Bonning B. C., and Toth A. L.. . 2019b. Interacting stressors matter: diet quality and virus infection in honeybee health. R. Soc. Open Sci. 6: 181803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droege S. 2006. Impact of color and size of bowl trap on numbers of bees captured. (http://online.sfsu.edu/~beeplot/pdfs/color%20and%20size.pdf) (Accessed 8 November 2019).
- Droege S., Tepedino V., Lebuhn G., Link W., Minckley R., Chen Q., and Conrad C.. . 2010. Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv. Diver. 3: 15–23. [Google Scholar]
- Duelli P., and Obrist M. K.. . 2003. Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl. Ecol. 4: 129–138. [Google Scholar]
- Fluri P., Wille H., Gerig L., and Lüscher M.. . 1977. Juvenile hormone, vitellogenin and haemocyte composition in winter worker honeybees (Apis mellifera). Experientia 33: 1240–1241. [Google Scholar]
- Foley J. A., Defries R., Asner G. P., Barford C., Bonan G., Carpenter S. R., Chapin F. S., Coe M. T., Daily G. C., Gibbs H. K., . et al. 2005. Global consequences of land use. Science 309: 570–574. [DOI] [PubMed] [Google Scholar]
- Gardiner M., Landis D., Gratton C., Schmidt N., O’Neal M., Mueller E., Chacon J., Heimpel G., and Difonzo C.. . 2009. Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 15: 554–564. [Google Scholar]
- Gardiner M. M., Landis D., Gratton C., Schmidt N., O’Neal M., Mueller E., Chacon J., and Heimpel G.. . 2010. Landscape composition influences the activity density of Carabidae and Arachnida in soybean fields. Biol. Control 55: 11–19. [Google Scholar]
- Garibaldi L. A., Steffan-Dewenter I., Kremen C., Morales J. M., Bommarco R., Cunningham S. A., Carvalheiro L. G., Chacoff N. P., Dudenhöffer J. H., Greenleaf S. S., . et al. 2011. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 14: 1062–1072. [DOI] [PubMed] [Google Scholar]
- Garibaldi L. A., Steffan-Dewenter I., Winfree R., Aizen M. A., Bommarco R., Cunningham S. A., Kremen C., Carvalheiro L. G., Harder L. D., Afik O., . et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339: 1608–1611. [DOI] [PubMed] [Google Scholar]
- Garibaldi L. A., Gemmill-Herren B., D’Annolfo R., Graeub B. E., Cunningham S. A., and Breeze T. D.. . 2017. Farming approaches for greater biodiversity, livelihoods, and food security. Trends Ecol. Evol. 32: 68–80. [DOI] [PubMed] [Google Scholar]
- Gathmann A., and Tscharntke T.. . 2002. Foraging ranges of solitary bees. J. Anim. Ecol. 71: 757–764. [Google Scholar]
- Giannini T. C., Garibaldi L. A., Acosta A. L., Silva J. S., Maia K. P., Saraiva A. M., Guimarães P. R. Jr., and Kleinert A. M.. . 2015. Native and non-native supergeneralist bee species have different effects on plant-bee networks. PLoS One 10: e0137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill K. A., and O’Neal M. E.. . 2015. Survey of soybean insect pollinators: community identification and sampling method analysis. Environ. Entomol. 44: 488–498. [DOI] [PubMed] [Google Scholar]
- Gonçalves R., Santos E., and Scott-Santos C.. . 2012. Bees (Hymenoptera: Apoidea: Apidae) captured with Malaise and pan traps along an altitudinal gradient in the Parque Estadual da Serra do Mar, Ubatuba, São Paulo, Brazil. Check List 8: 53–56. [Google Scholar]
- Goulson D., Nicholls E., Botías C., and Rotheray E. L.. . 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347: 1255957. [DOI] [PubMed] [Google Scholar]
- Greenleaf S. S., and Kremen C.. . 2006. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 103: 13890–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H. G., and Ascher J. S.. . 2011. Surveys of wild bees (Hymenoptera: Apoidea: Anthophila) in organic farms of Alachua County in North-Central Florida. Fla. Entomol. 94: 539–552. [Google Scholar]
- Happe A., Riesch F., Rösch V., Gallé R., Tscharntke T., and Batáry P.. . 2018. Small-scale agricultural landscapes and organic management support wild bee communities of cereal field boundaries. Agric. Ecosyst. Environ. 254: 92–98. [Google Scholar]
- Heard M. S., Baas J., Dorne J. L., Lahive E., Robinson A. G., Rortais A., Spurgeon D. J., Svendsen C., and Hesketh H.. . 2017. Comparative toxicity of pesticides and environmental contaminants in bees: are honey bees a useful proxy for wild bee species? Sci. Total Environ. 578: 357–365. [DOI] [PubMed] [Google Scholar]
- Hendrix S.D., Kwaiser K. S., and Heard S. B.. . 2010. Bee communities (Hymenoptera: Apoidea) of small Iowa hill prairies are as diverse and rich as those of large prairie preserves. Biodivers. Conserv. 19: 1699–1709. [Google Scholar]
- Holzschuh A., Dormann C. F., Tscharntke T., and Steffan-Dewenter I.. . 2011. Expansion of mass-flowering crops leads to transient pollinator dilution and reduced wild plant pollination. Proc. Biol. Sci. 278: 3444–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh A., Dormann C. F., Tscharntke T., and Steffan-Dewenter I.. . 2013. Mass-flowering crops enhance wild bee abundance. Oecologia 172: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. C., Ma K. H., Chao A., and McInerny G.. . 2016. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7: 1451–1456. [Google Scholar]
- Isaacs R., Tuell J., Fiedler A., Gardiner M., and Landis D.. . 2009. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: the role of native plants. Front. Ecol. Environ. 7: 196–203. [Google Scholar]
- Jauker F., Peter F., Wolters V., and Diekötter T.. . 2012. Early reproductive benefits of mass-flowering crops to the solitary bee Osmia rufa outbalance post-flowering disadvantages. Basic Appl. Ecol. 13: 268–276. [Google Scholar]
- Klein A. M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., and Tscharntke T.. . 2007. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Pasquaretta C., He X. J., Perry C., Søvik E., Devaud J. M., Barron A. B., and Lihoreau M.. . 2019. Honey bees increase their foraging performance and frequency of pollen trips through experience. Sci. Rep. 9: 6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh I., Lonsdorf E. V., Williams N. M., Brittain C., Isaacs R., Gibbs J., and Ricketts T. H.. . 2016. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. USA 113: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C., Williams N. M., and Thorp R. W.. . 2002. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 99: 16812–16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C., Williams N., Bugg R., Fay J. P., and Thorp R. W.. . 2004. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecol. Lett. 7: 1109–1119. [Google Scholar]
- Kremen C., Williams N. M., Aizen M. A., Gemmill-Herren B., LeBuhn G., Minckley R., Packer L., Potts S. G., Roulston T., Steffan-Dewenter I., . et al. 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol. Lett. 10: 299–314. [DOI] [PubMed] [Google Scholar]
- LeBuhn G., Griswold T., Minckley R., Droege S., Roulston T. A., Cane J., Parker F., Buchmann S., Tepedino V., Williams N., . et al. 2003. A standardized method for monitoring bee populations – the bee inventory (BI) plot. (http://online.sfsu.edu/beeplot/pdfs/Bee%20Plot%202003.pdf) (Accessed 5 February 2020).
- Lee K. V., Moon R. D., Burkness E. C., Hutchison W. D., and Spivak M.. . 2010. Practical sampling plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies and apiaries. J. Econ. Entomol. 103: 1039–1050. [DOI] [PubMed] [Google Scholar]
- Mallinger R., Gibbs J. E., and Gratton C.. . 2016. Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc. Ecol. 31: 1523–1535. [Google Scholar]
- Mallinger R. E., Gaines-Day H. R., and Gratton C.. . 2017. Do managed bees have negative effects on wild bees? A systematic review of the literature. PLoS One 12: e0189268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C. D. 1994. The bee genera of North and Central America (Hymenoptera: Apoidea). Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Michener C. D. 2007. The bees of the world, 2nd ed. Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Morandin L. A., and Kremen C.. . 2013. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol. Appl. 23: 829–839. [DOI] [PubMed] [Google Scholar]
- (NASS-USDA) National Agricultural Statistics Survey-United States Department of Agriculture 2017. State agricultural overview (https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=IOWA) (Accessed 20 November 2019).
- (NASS-USDA) National Agricultural Statistics Survey-United States Department of Agriculture 2018. State agricultural overview (https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=IOWA) (Accessed 25 November 2019).
- Naug D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142: 2369–2372. [Google Scholar]
- Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P. R., O’Hara R. B., Simpson G. L., Solymos P., . et al. 2018. Community Ecology Package. R package version 2.4–6. (https://CRAN.R-project.org/package=vegan).
- Oldroyd B. P. 2007. What’s killing American honey bees? PLoS Biol. 5: e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J., Winfree R., and Tarrant S.. . 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Otto C. R., Roth C. L., Carlson B. L., and Smart M. D.. . 2016. Land-use change reduces habitat suitability for supporting managed honey bee colonies in the Northern Great Plains. Proc. Natl. Acad. Sci. USA 113: 10430–10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee G., and Philpott S.. . 2014. Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosys. 17: 641–659. [Google Scholar]
- Popic T. J., Davila Y. C., and Wardle G. M.. . 2013. Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps. PLoS One 8: e66665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., and Kunin W. E.. . 2010a. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Potts S. G., Roberts S., Dean R., Marris G., Brown M. A., Jones R., Neumann P., and Settele J.. . 2010b. Declines of managed honey bees and beekeepers in Europe. J. Apicult. Res. 49: 15–22. [Google Scholar]
- Preston F. 1948. The commonness, and rarity, of species. Ecology 29: 254–283. [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. R Core Team, Vienna, Austria. [Google Scholar]
- Ricketts T. H., Regetz J., Steffan-Dewenter I., Cunningham S. A., Kremen C., Bogdanski A., Gemmill-Herren B., Greenleaf S. S., Klein A. M., Mayfield M. M., . et al. 2008. Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11: 499–515. [DOI] [PubMed] [Google Scholar]
- Roulston T. A. H., Smith S. A., and Brewster A. L.. . 2007. A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J. Kansas Entomol. Soc. 80: 179–181. [Google Scholar]
- Sánchez-Bayo F., and Wyckhuys K. A. G.. . 2019. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232: 8–27. [Google Scholar]
- Schulte L. A., Niemi J., Helmers M. J., Liebman M., Arbuckle J. G., James D. E., Kolka R. K., O’Neal M. E., Tomer M. D., Tyndall J. C., . et al. 2017. Prairie strips improve biodiversity and the delivery of multiple ecosystem services from corn-soybean croplands. Proc. Natl. Acad. Sci. USA 114: 11247–11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley T. 2019. The lives of bees: the untold story of the honey bee in the wild. Princeton University Press, Princeton, NJ. [Google Scholar]
- Seitz N., Traynor K. S., Steinhauer N., Rennich K., Wilson M. E., Ellis J. D., Rose R., Tarpy D., Sagili R., Caron D. M., . et al. 2016. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apicult. Res. 54: 292–304. [Google Scholar]
- Shackelford G., Steward P. R., Benton T. G., Kunin W. E., Potts S. G., Biesmeijer J. C., and Sait S. M.. . 2013. Comparison of pollinators and natural enemies: a meta-analysis of landscape and local effects on abundance and richness in crops. Biol. Rev. Camb. Philos. Soc. 88: 1002–1021. [DOI] [PubMed] [Google Scholar]
- Shannon C. E., Weaver W., and Wiener N.. . 1950. The mathematical theory of communication. Phys. Today 3: 31–32. [Google Scholar]
- Shimanuki H. 1991. Diagnosis of honey bee diseases. Beltsville, MD: U.S. Dept. of Agriculture, Agricultural Research Service; Available from National Technical Information Service, Springfield, VA. (https://www.ars.usda.gov/is/np/honeybeediseases/honeybeediseases.pdf) (Accessed 15 October 2019). [Google Scholar]
- Steffan-Dewenter I., Munzenberg U., Burger C., Thies C., and Tscharntke T.. . 2002. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83: 1421–1432. [Google Scholar]
- Steinhauer N., Rennich K., Wilson M. E., Caron D. M., Lengerich E., Pettis J., Rose R., Skinner J., Tarpy D., Wilkes J., . et al. 2014. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the Bee Informed Partnership. J. Apicult. Res. 53: 1–18. [Google Scholar]
- Sutter L., Jeanneret P., Bartual A., Bocci G., Albrecht M., and Macivor S.. . 2017. Enhancing plant diversity in agricultural landscapes promotes both rare bees and dominant crop-pollinating bees through complementary increase in key floral resources. J. Appl. Ecol. 54: 1856–1864. [Google Scholar]
- Thapa R. B. 2006. Honeybees and other insect pollinators of cultivated plants: a review. J. Inst. Agric. Anim. Sci. 27: 1–23. [Google Scholar]
- Todd K. J., Gardiner M. M., and Lindquist E. D.. . 2016. Mass flowering crops as a conservation resource for wild pollinators (Hymenoptera: Apoidea). J. Kansas Entomol. Soc. 89: 158–167. [Google Scholar]
- Toth A. L., and Robinson G. E.. . 2005. Worker nutrition and division of labour in honeybees. 69: 427–435. [Google Scholar]
- Westphal C., Steffan-Dewenter I., and Tscharntke T.. . 2003. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 6: 961–965. [Google Scholar]
- Wheelock M. J., and O’Neal M. E.. . 2016. Insect pollinators in Iowa cornfields: community identification and trapping method analysis. PLoS One 11: e0143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Rey K. P., and O’Neal M. E.. . 2016. Defining the insect pollinator community found in Iowa corn and soybean fields: implications for pollinator conservation. Environ. Entomol. 45: 1099–1106. [DOI] [PubMed] [Google Scholar]
- Wilsey B. J., Chalcraft D. R., Bowles C. M., and Willig M. R.. . 2005. Relationships among indices suggest that richness is an incomplete surrogate for grassland biodiversity. Ecology 86: 1178–1184. [Google Scholar]
- Winfree R. 2010. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 1195: 169–197. [DOI] [PubMed] [Google Scholar]
- Winfree R., Griswold T., and Kremen C.. . 2007. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 21: 213–223. [DOI] [PubMed] [Google Scholar]
- Winfree R., Williams N. M., Gaines H., Ascher J. S., and Kremen C.. . 2008. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 45: 793–802. [Google Scholar]
- Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., and Aizen M. A.. . 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
- Zurbuchen A., Cheesman S., Klaiber J., Müller A., Hein S., and Dorn S.. . 2010a. Long foraging distances impose high costs on offspring production in solitary bees. J. Anim. Ecol. 79: 674–681. [DOI] [PubMed] [Google Scholar]
- Zurbuchen A., Landert L., Klaiber J., Müller A., Hein S., and Dorn S.. . 2010b. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol. Conserv. 143: 669–676. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon publication, all data will be made available.