Abstract
Context
Statins have been linked to the development of diabetes and atherosclerotic plaque calcification in patients with cardiac disease.
Objective
To determine the association between statin use and statin characteristics and insulin resistance and abdominal aortic calcification (AAC) in participants of the Canadian Multicentre Osteoporosis Study (CaMos).
Design
Observational study.
Setting
General community.
Participants
Nondiabetic participants of the Kingston CaMos site
Intervention
Insulin resistance and AAC in statin users and nonstatin users were compared with and without the inclusion of a propensity score (PS) to be on a statin. The covariates of hypertension, sex, body mass index, smoking, kidney stones, and age that were included in the PS were selected based on clinical judgment confirmed by the statistical analysis of a difference between statin users and nonstatin users.
Main Outcome Measures
Insulin resistance measured by the homeostasis model assessment (HOMA-IR) and AAC assessed on lateral spine radiographs using the Framingham methodology.
Results
Using a general linear model, statin use was associated with higher levels of HOMA-IR after stratified PS adjustment (β = 1.52, [1.18–1.95], P < 0.01). Hydrophilic statin users (n = 9) and lipophilic statins users (n = 30) had higher HOMA-IR compared to nonstatin users (n = 125) ([β = 2.29, (1.43–3.68), P < 0.001] and [β = 1.36, (1.04–1.78), P < 0.05]), respectively, in general linear models after stratified PS adjustment. Statin use was associated with AAC without stratifying by PS in the Wilcoxon test, but was no longer significant when stratified by PS.
Conclusions
Statins, widely prescribed drugs to lower cholesterol, may have unintended consequences related to glucose homeostasis that could be relevant in healthy aging.
Keywords: statins, insulin resistance, calcification, CaMoS
Hypercholesterolemia is a major cardiovascular risk factor and an important therapeutic target. Statins, or 3-hydroxy-3-methyl-glutaryl coenzyme-A (HMG-CoA) reductase inhibitors, are a first-line therapy to lower cholesterol levels and, thus, are widely prescribed drugs. Analysis of the drug dispensing patterns of a seniors pharmacare program in Canada showed a dramatic increase in statin prescription, from 5% to 20%, between the years 2000 and 2013 [1].
Statins have been shown to consistently reduce cardiovascular events in the general population and are thus amongst the first-line therapies for patients at high risk for cardiovascular disease. Statins reduce atherosclerosis by decreasing low-density lipoprotein (LDL) cholesterol and by improving endothelial function [2, 3]. However, statins may also have less desirable pleiotropic actions, including a reduction in insulin secretion and worsening of insulin resistance [4–6]. Some, but not all, large trials of primary prevention have reported an increased incidence of diabetes with statins [7, 8]. From a pooled analysis of randomized trials, factors associated with the development of diabetes in statin users included elevated triglycerides, elevated body mass index (BMI), and a history of hypertension [9]. However, these studies continued to demonstrate a reduction in cardiovascular endpoints despite the increased incidence of diabetes; thus, no change in clinical practice has occurred.
Several factors are proposed to contribute to the pleiotropic effects of statins. First, the pleiotropic actions of statins may differ based on the lipophilicity versus the hydrophilicity of the particular statin drug [10, 11], suggesting that certain statins may decrease cardiovascular risk without increasing the risk of diabetes. Pravastatin and rosuvastatin are hydrophilic statins, whereas atorvastatin, fluvastatin, lovastatin, and simvastatin are lipophilic. Hydrophilic statins require carrier-mediated uptake, while lipophilic statins may diffuse passively through the hepatocellular membrane; thus, lipophilic statins tend to have been implicated in the development of insulin resistance. The second factor contributing to pleiotropic effects of statins is potency. Rosuvastatin is transported with greater affinity than lipophilic statins (despite being a hydrophilic drug) and is the most potent statin drug to reduce LDL-cholesterol levels. Potency is a second consideration when comparing pleiotropic effects of different statins. In one meta-analysis, rosuvastatin carried the highest risk for the development of type 2 diabetes [12].
Statin drugs have not consistently shown a similar level of cardiovascular benefit in patients with low kidney function, and an increased risk of stroke was observed to be associated with statins in 1 large, well-conducted, randomized, controlled trial [13]. Evidence from 1 study of patients with reduced kidney function demonstrated that statin drug use was associated with greater severity of coronary artery calcification (CAC) at baseline and greater progression of calcification over 1.5 years [14]. A critical tissue-based inhibitor of vascular calcification is matrix Gla protein, 1 of several vitamin K–dependent proteins in the body [15, 16]. There is emerging data to suggest that by inhibiting the production of intermediates of cholesterol biosynthesis, statins also inhibit the mevalonate pathway and impede the production of vitamin K2 in peripheral tissues [17, 18]. There is growing evidence to suggest that vitamin K2 plays a key role in glucose homeostasis [19–22] as well as vascular calcification [23–27]. On this background, we hypothesized that statin use would be associated with both insulin resistance and vascular calcification in community-dwelling participants of a large longitudinal study of osteoporosis.
Our primary objective was, firstly, to evaluate the association between statin use and insulin resistance assessed by the homeostasis model assessment (HOMA-IR) in nondiabetic participants at year 10 from the Kingston center of the population-based observational Canadian Multicenter Osteoporosis Study (CaMos) [28] and, secondly, to evaluate the association between statin use and abdominal aortic calcification (AAC) assessed by the Framingham method in participants at year 10 of the CaMos study. Our secondary objective was to explore the impact of the hydrophilicity versus lipophilicity of the specific statin drug on the outcomes.
Materials and Methods
Cohort demographics
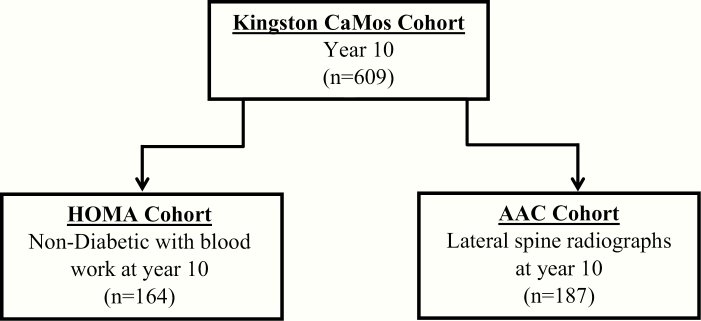
Statin use and specific type was assessed by direct interview at year 10 (2005–2007) in CaMos participants of the Kingston site. Of the original 1075 participants, 609 were still being followed at year 10. As shown in Fig. 1, the sample size for the various analyses varied from 164 to 187. The covariates of age, sex, BMI, and history of hypertension, diabetes, osteoporosis, smoking, and kidney stones were also collected from the interview data.
Figure 1.
Flow chart of study cohorts at the Kingston CaMos site. Abbreviations: AAC, abdominal aortic calcification; CaMos, Canadian Multicentre Osteoporosis Study; HOMA, homeostasis model assessment.
Insulin resistance
Insulin resistance was assessed by HOMA-IR at year 10 in nondiabetic participants only. The HOMA-IR sample was thus comprised of 164 nondiabetic participants (from the self-reported comorbidity list) who had blood work drawn at year 10 (Fig. 1). Fasting glucose and serum insulin were measured at year 10 by methods described by Langsetmo [29]. Briefly, an enzymatic colorimetric assay (Roche Diagnostics GmbH; Mannheim, Germany) was used to measure serum glucose and a chemiluminescent assay was used to measure serum insulin. The HOMA-IR was calculated using the equation: (glucose(mM)*insulin(µIU/mL))/22.5.
AAC analysis
Abdominal aortic calcification was assessed on lateral spine radiographs using the Framingham method [30]. The AAC sample consisted of 187 participants who had radiographs performed at year 10 (Fig. 1).
HOMA-IR and AAC analyses with propensity score to be prescribed a statin
HOMA-IR and AAC in statin users and nonstatin users were compared with and without the inclusion of a propensity score (PS) to be on a statin in the statistical analysis [31]. The covariates of hypertension, sex, BMI, smoking, kidney stones, and age were included in the PS. These variables were selected based on our own clinical judgment, a previous study identifying risk factors for the development of diabetes with statin use [9], and was confirmed by statistical analysis of a difference between statin users and nonstatin users.
Database design, creation, and queries
MySQL Workbench version 6.3.9 CE (Oracle Corporation and/or its affiliates, Redwood Shores, CA) for Windows was used as the graphical user interface. Result sets returned from queries were exported as csv files and imported into SAS.
Statistical analysis
SAS version 9.4 (SAS Institute, Inc., Cary) for Windows was used for the statistical analysis and an α level of 0.05 was used to indicate statistical significance. Normality of data distribution was assessed from frequency distribution (histogram) plots, Q-Q plots, and normality tests. Median and interquartile range were determined for non-normally distributed data. A general linear model was used to compare normally distributed continuous variables (log-transformed HOMA) in statin users and nonstatin users. Continuous variables that were not normally distributed (AAC, age, BMI) were compared using Wilcoxon tests.
Chi-square tests were used to compare binary variables (hypertension, diabetes, sex, history of kidney stones, and history of smoking [ever smoked]). Logistic regression was used to create a PS to be on a statin. Participants were grouped into 5 strata according to PS. The stratified PS was used in the general linear model (log-transformed HOMA) or Wilcoxon test (AAC) to give a stratified comparison between statin users and nonstatin users. The number of participants on a statin in each strata used in the PS analysis varied from 7–8 for the HOMA-IR and 11–12 for the AAC assessment.
Results
Differences between statin users and nonstatin users in HOMA-IR, AAC, and covariates predicted to influence statin use
Table 1 demonstrates demographic and clinical variables at year 10 of follow-up in the Kingston site cohort overall (n = 609) as well as stratified by statin users (n = 152) versus nonusers (n = 457). Participants had a median age of 71, the majority (74%) were female, 33% percent had diabetes, more than half had smoked at some point, and 44% had a history of hypertension. Compared to nonstatin users, statin users were significantly older with a greater BMI and were more likely to be male and have hypertension, diabetes, and a history of smoking and kidney stones. Statin users had significantly higher HOMA-IR levels (Table 1, Fig. 2) (2.6 [1.9–4.4] vs 1.7 [1–2.9], P < 0.001). The AAC score was also significantly higher in statin users.
Table 1.
Demographic, clinical variables, HOMA, and AAC at year 10 in CaMos participants at the Kingston site overall and stratified by statin use
| Variable | All (n = 609) | Statin User (n = 152) | Nonstatin User (n = 457) | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Sex - Female, n (%) | 451 (74.0) | 99 (65) | 352 (77) | 0.004 |
| Age, years, median [IQR] | 70.6 [61.9–77.5] | 73.5 [67.2–78.3] | 69.3 [60.1–77.2] | <0.001 |
| BMI, kg/m2, median [IQR] | 27.2 [24.2–31.0] | 28.4 [25.1–32.0] | 27.0 [23.9–30.8] | 0.007 |
| Medical history | ||||
| Osteoporosis, n (%) | 55 (9.0) | 17 (11.2) | 38 (8.3) | ns |
| Diabetes, n (%) | 202 (33.2) | 61 (40.1) | 141 (30.9) | 0.035 |
| Hypertension, n (%) | 265 (43.5) | 86 (56.6) | 179 (39.2) | <0.001 |
| Kidney stones, n (%) | 30 (4.9) | 16 (10.5) | 14 (3.1) | <0.001 |
| Past/current smoker, n (%) | 319 (52.4) | 91 (59.9) | 228 (49.9) | 0.03 |
| Clinical measures/calculations | ||||
| n = 164 | n = 39 | n = 125 | ||
| HOMA, median [IQR] | 2.0 [1.1–3.1] | 2.6 [1.9–4.4] | 1.7 [1–2.9]) | <0.001 |
| n = 187 | n = 50 | n = 127 | ||
| AAC, median [IQR] | 3.0 [0.0–6.0] | 4.5 [1.0–8.0] | 2.0 [0.0–6.0] | 0.015 |
Note: Kruskal-Wallis test comparing statin users and nonstatin users.
Abbreviations: AAC, abdominal aortic calcification; BMI, body mass index; CaMos, Canadian Multicentre Osteoporosis Study; HOMA, homeostasis model assessment; IQR, interquartile range.
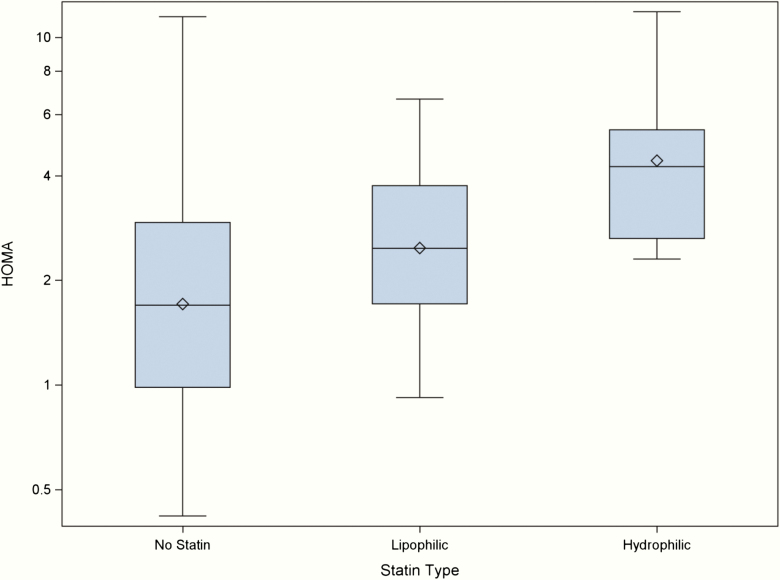
Figure 2.
HOMA-IR varies with statin type in CaMOS participants. N values as follows: no statin (n = 125), lipophilic statin (n = 30), hydrophilic statin (n = 9).
Adjustment for propensity to be on a statin when comparing HOMA-IR and AAC in statin users and non-statin users
Statin users were compared to non-statin users with and without the inclusion of a PS to be on a statin included in the statistical analysis. Variables included in the PS included age, sex, hypertension, BMI, smoking, and kidney stones. HOMA-IR and AAC were higher in statin users with and without the PS included in the statistical analysis (Table 2). HOMA-IR was significantly higher in statin users in the general linear model with log-transformed HOMA unadjusted for the PS (exp(β) = 1.64 (1.29, 2.08), P < 0.001) and with adjustment for the PS (exp(β) = 1.52, (1.18, 1.95, P < 0.01). Statin use was associated with higher AAC without the stratifying by PS in the Wilcoxon test, but was no longer significant when stratified by PS.
Table 2.
HOMA and AAC in statin users compared to nonstatin users, unadjusted and adjusted for the PS
| Unadjusted for PS | Adjusted for PS | |||||
|---|---|---|---|---|---|---|
| Variable | n | Exp(β) (95% CI) | p-value | n(statin)/strata | Exp(β), 95% CI | p-value |
| HOMAa | 163 | 1.64 (1.29–2.08) | <0.001 | 7–8 | 1.52 (1.18–1.95) | <0.01 |
| AACb | 183 | - | <0.05 | 11–12 | - | >0.05 |
Abbreviations: AAC, abdominal aortic calcification; CI, confidence interval; HOMA, homeostasis model assessment; PS, propensity score.
a General linear model with log-transformed variable.
b Wilcoxon (Van Elteren) test.
Influence of statin type on HOMA-IR, AAC and covariates predicted to influence statin use
We examined participant characteristics, demographics, HOMA-IR and AAC based on the hydrophilicity/lipophilicity of the particular statin drug. As demonstrated in Table 3, users of hydrophilic statins were slightly older with lower BMI but were more likely to be male, have hypertension or have kidney stones. HOMA-IR was significantly higher in hydrophilic statin users compared to lipophilic statin users (exp(β) = 1.79, (1.15, 2.79), P < 0.05). Compared to non-statin users, HOMA-IR was higher in those on hydrophilic statins with (exp(β) = 2.29, (1.43–3.68), P < 0.001) and without (exp(β) = 2.60, (1.63–4.14), P < 0.001) PS stratification, as well as in those on lipophilic statins with (exp(β) = 1.36, (1.04–1.78), P < 0.05) and without (exp(β) = 1.45, (1.12–1.88), P < 0.01) PS stratification(Table 4). We examined rosuvastatin users separately due to the inherent potency of this particular statin. Compared to lipophilic statin users, HOMA-IR was higher in rosuvastatin users with (exp(β) = 2.42, (1.45–4.03), P < 0.001) and without (exp(β) = 2.80, (1.7–4.61), P < 0.001) PS stratification.
Table 3.
Demographic and clinical variables in all participants and stratified by hydrophilic and lipophilic statin use
| Nonstatin Users (n = 457) | Hydrophilic Statin Users (n = 37) | Hydrophilic P-Value | Lipophilic Statin Users (n = 115) | Lipophilic P-value | Total | Overall Difference P-Value | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Gender | 0.095 | 0.009 | 0.015 | ||||
| F | 352 (77.0%) | 24 (64.9%) | 75 (65.2%) | 451 (74.1%) | |||
| M | 105 (23.0%) | 13 (35.1%) | 40 (34.8%) | 158 (25.9%) | |||
| Age (years) | 0.02 | 0.005 | 0.002 | ||||
| Median [IQR] | 69.3 [60.1 to 77.2] | 74.7 [68.3 to 78.6] | 73.5 [66.6 to 77.8] | ||||
| BMI | 0.536 | 0.004 | 0.015 | ||||
| Median [IQR] | 27.0 [23.9 to 30.8] | 27.5 [24.7 to 31.2] | 28.6 [25.5 to 32.0] | ||||
| Medical history | |||||||
| Osteoporosis | 0.601 | 0.315 | 0.562 | ||||
| No | 419 (91.7%) | 33 (89.2%) | 102 (88.7%) | 554 (91.0%) | |||
| Yes | 38 (8.3%) | 4 (10.8%) | 13 (11.3%) | 55 (9.0%) | |||
| Diabetes | 0.12 | 0.09 | 0.098 | ||||
| No | 316 (69.1%) | 21 (56.8%) | 70 (60.9%) | 407 (66.8%) | |||
| Yes | 141 (30.9%) | 16 (43.2%) | 45 (39.1%) | 202 (33.2%) | |||
| Hypertension | <0.001 | 0.018 | <0.001 | ||||
| No | 278 (60.8%) | 10 (27.0%) | 56 (48.7%) | 344 (56.5%) | |||
| Yes | 179 (39.2%) | 27 (73.0%) | 59 (51.3%) | 265 (43.5%) | |||
| Kidney Stones | <0.001 | 0.002 | <0.001 | ||||
| Missing | 3 (0.7%) | 3 (8.1%) | 4 (3.5%) | 10 (1.6%) | |||
| No | 440 (96.3%) | 29 (78.4%) | 100 (87.0%) | 569 (93.4%) | |||
| Yes | 14 (3.1%) | 5 (13.5%) | 11 (9.6%) | 30 (4.9%) | |||
| Past/current smoker | 0.151 | 0.076 | 0.098 | ||||
| No | 229 (50.1%) | 14 (37.8%) | 47 (40.9%) | 290 (47.6%) | |||
| Yes | 228 (49.9%) | 23 (62.2%) | 68 (59.1%) | 319 (52.4%) | |||
| Clinical measures | |||||||
| HOMA | N = 125 | N = 9 | <0.001 | N = 30 | 0.004 | <0.001 | |
| Median [IQR] | 1.7 [1.0 to 2.9] | 4.2 [2.6 to 5.4] | 2.5 [1.7 to 3.7] | ||||
| AAC | N = 127 | N = 11 | 0.12 | N = 49 | 0.034 | 0.047 | |
| Median [IQR] | 2.0 [0.0 to 6.0] | 6.0 [2.0 to 10.0] | 4.0 [1.0 to 8.0] |
Comparisons made using the Kruskal-Wallis test.
Abbreviations: AAC, abdominal aortic calcification; BMI, body mass index; HOMA, homeostasis model assessment; IQR, interquartile range.
Table 4.
HOMA and AAC in hydrophilic and lipophilic statin users compared to nonstatin users (not stratified by propensity scores)
| Unadjusted for PS | |||||
|---|---|---|---|---|---|
| Hydrophilic Statin User | Lipophilic Statin User | ||||
| Variable | Exp(β) (95% CI) | P-value | Exp(β), 95% CI | P-value | |
| HOMAa | n = 163 | 2.60 (1.63–4.14) | <0.001 | 1.45 (1.12–1.88) | <0.01 |
| AACb | n = 183 | - | >0.05 | - | <0.05 |
| Variable | Adjusted for PS | ||||
| Hydrophilic Statin User | Lipophilic Statin User | ||||
| Exp(β) (95% CI) | P-value | β, 95% CI | P-value | ||
| HOMAa | n = 163 | 2.29 (1.43–3.68) | <0.001 | 1.36 (1.04–1.78) | <0.05 |
| AACb | n = 183 | - | >0.05 | - | >0.05 |
Abbreviations: AAC, abdominal aortic calcification; CI, confidence interval; HOMA, homeostasis model assessment; PS, propensity score.
a General linear model with log transformed variable.
b Wilcoxon test.
Discussion
In this cohort of community-dwelling participants, users of lipophilic and hydrophilic statins had higher levels of insulin resistance compared to non-statin users with and without a PS adjustment. Insulin resistance was greater in hydrophilic statin users compared to lipophilic statin users. Although previous studies have implicated lipophilicity as a risk factor for this pleiotropic effect of statins, 73% of the hydrophilic statins in use in this study were rosuvastatin, a high potency statin that has been shown in one other study to have the highest risk for the development of diabetes [12]. With respect to calcification, statin users had higher AAC but this was no longer significant after the PS adjustment.
Although not a disease, insulin resistance appears to be associated with the development of cardiovascular disease based on a meta-analysis of published data from 20 studies [32]. The development of impaired fasting glucose resulted in a progressively higher risk of developing myocardial infarction, cardiovascular disease, and mortality in a large observational study of Korean patients [33]. Over time, insulin resistance can lead to type 2 diabetes as the pancreas fails to keep up with the body’s increasing demands for insulin. These metabolic abnormalities predate the development of diabetes by more than 10 years [34]. Although many risk factors for insulin resistance have been identified, obesity remains the most important.
With regards to statins, the balance between the benefits of cardiovascular risk reduction versus the cardiovascular risk associated with the development of insulin resistance is not known. Previous studies have implicated statins in the development of diabetes. In a meta-analysis of 17 randomized, controlled trials, 12 trials involved studies of secondary prevention, while the remaining trials studied patients with baseline risk factors [12]. Treatment with rosuvastatin had the highest incidence of new-onset diabetes mellitus (DM) (25% increase), while pravastatin was deemed the “safest.” The risk for developing DM was not influenced by the different abilities of statins to reduce cholesterol. In the JUPITER trial, a trial of primary prevention, there was a 27% increase in relative risk for physician-reported DM in rosuvasatin-treated patients compared to placebo [7]. However, despite this apparent risk for diabetes, rosuvastatin significantly reduced the incidence of major cardiovascular events. The Treating to New Targets and the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trials determined that the overall diabetogenic impact of atorvastatin treatment was modest. However, it was accentuated dramatically by an increase in BMI and levels of fasting plasma glucose and triglycerides. This trial was conducted in patients with coronary artery disease [35]. Taken together, in previous studies high potency and lipophilic statins appear to increase the risk of developing type 2 diabetes and, in our study, were associated with increasing insulin resistance. The data suggest that it might be prudent to monitor the glycemic status in those at greatest risk for diabetes and consider lower risk statins in those with risk factors. Further consideration for other pharmacological and nonpharmacological options might also be considered.
Several mechanisms have been proposed to explain the association between statins and new-onset diabetes, as reviewed by Brault et al [36]. Statins may impact on calcium channels in pancreatic β-cells where an increase in intracellular calcium concentration stimulates insulin secretion. In vitro work suggests that statins block calcium channels, suggesting that this is a direct, rather than an indirect, impact of statin drugs. Reduced translocation of glucose transporter 4 has also been implicated, suggesting that statins may decrease glucose uptake and increase insulin resistance in adipose tissue, muscle, and liver. The impact of statins on adipocyte maturation and differentiation has been evaluated primarily in vitro and in preclinical models. An 8-day incubation of 3T3-L1 cells with various statins showed a concentration-dependent inhibition of adipocyte differentiation that may be mediated by inhibition of the transcription factor PPAR-γ [37, 38]. As preadipocytes do not secrete insulin-sensitizing hormone, the accumulation of undifferentiated adipocytes could contribute to insulin resistance. Preclinical studies have also demonstrated that statins have a selective effect on the secretion of adiponectin, an insulin-sensitizing adipokine [39]. However, studies in vivo have demonstrated both an increase in subcutaneous adipose tissue in obese rats as well as a reduction in adiposity, suggesting that the relationship between statin therapy and changes in adiposity is uncertain and requires further study [40, 41].
By inhibiting HMG-CoA reductase, there are statin-associated downstream effects on the production of other products of the cholesterol biosynthetic pathway, including coenzyme 10, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, and dolichol. Depletion of these substrates may lead to a downstream reduction of intracellular signalling. Coenzyme Q10 supplementation has been shown to improve glucose homeostasis in various patient populations. In an 8-week trial of simvastatin-treated patients, Coenzyme Q10 did not change muscle GLUT4 content, insulin sensitivity, or secretory capacity. However, hepatic insulin sensitivity appeared to improve [42]. Geranylgeranyl pyrophosphate is a key intermediate in the conversion of dietary vitamin K1 to MK-4. While vitamin K1 is the predominant vitamin K form measured in blood, liver, bone, and heart, MK-4 (1 form of vitamin K2) is the form primarily measured in the pancreas. The function of MK-4 in the pancreas is not clear; however, it might act as a potent amplifier of the incretin effect [20]. Novel data support a role of statins in modifying vitamin K status. Harshman et al recently demonstrated for the first time in vivo that statins reduce endogenous production of MK-4 in mouse kidney by approximately 40% [43]. There is emerging data in support of a role for vitamin K in glucose homeostasis [19, 44]. In patients with diabetes, vitamin K2 supplementation improved insulin sensitivity [44]. However, in healthy people, vitamin K supplementation had no effect on glycemic indices. The uncarboxylated form of osteocalcin (ucOC), a bone-derived vitamin K–dependent protein that functions as a hormone, has also been implicated in regulating insulin secretion and sensitivity in mice, possibly via GPRC6A, a receptor for ucOC [45]. Taken together, emerging evidence suggests a role for vitamin K in energy metabolism that may be modified by a statin-induced decrease in MK-4 production in the pancreas.
In this study of community-dwelling people, we did not find an association between statin use and AAC after PS adjustment. Previous studies examining the impact of statins on calcification have evaluated patients with established atherosclerotic disease. In a post hoc patient-level analysis of 8 prospective randomized trials that employed serial coronary intravascular ultrasound, serial changes in the coronary percentage of atheroma volume and calcium were measured in patients with established coronary artery disease [46]. Independent of their plaque-regressive effects, statins promoted coronary artery atheroma calcification, suggesting a potential role for statins in stabilizing plaque. Our method of calcification measurement does not distinguish between medial and intimal calcification. One study pooled data from 2 clinical trials involving atorvastatin and a placebo, and examined CAC scores assessed by computed tomography at baseline, 2 years and at 4 to 6 years. After 2 years of follow-up, a similar increase in CAC score was noted between placebo and low-dose atorvastatin. However, at the later time point, atorvastatin use was associated with a greater progression of CAC compared to placebo. However, this change did not appear to be clinically significant, as the increase in CAC did not translate into more clinical events. Whether an absence of clinical impact would also apply to patients with a propensity to calcify, such as those with reduced kidney function, is not known. The potential tipping point between a beneficial effect of statins on plaque calcification and stabilization versus the impact on progressive arterial medial calcification and vessel stiffening on outcomes, including tissue perfusion and cardiomyopathy, is unknown.
The percentage of total Kingston CaMos participants taking statins closely mirrors the percentage of seniors taking statins at the same time period in a Canadian seniors pharmacare program [1]. The sex difference in statin use likely reflects the greater proportion of men that would be classified as high cardiovascular disease risk using the American College of Cardiology, American Heart Association, and Canadian Cardiovascular Society guidelines. This higher percentage of male compared to female statin users was also seen in the Canadian Health Measures Survey 2007–2011 [47].
There are limitations to our study. The CaMos cohort was a random sample of community-living individuals. However, the sampling framework was developed to give greater representation to older women, given the focus on osteoporosis. This may restrict generalizability to other groups. Secondly, the study is cross-sectional; long-term studies would be necessary to resolve the temporal relationship between statin use and the development and progression of insulin resistance. Although we used PS matching in an attempt to eliminate confounding by indication, there is still room for bias based on unobserved or inaccurately measured confounders. Finally, we do not have cholesterol levels or statin doses in these participants. However, a meta-analysis that included 17 randomized, controlled trials concluded that the risk for developing DM was not influenced by the degree to which the statin reduced cholesterol [12]. The small sample size in our study limited the analysis of statin type (hydrophilic vs. lipophilic) on calcification severity and progression.
In summary, in the Kingston CaMos cohort, statin users had higher indices of insulin resistance. Users of hydrophilic statins had greater HOMA-IR levels; however, the majority of the participants were taking the high-potency statin rosuvastatin. Statins, widely prescribed drugs to lower cholesterol, may have unintended consequences related to glucose homeostasis that could be relevant in healthy aging. In those individuals with risk factors for diabetes, consideration for choosing nonlipophilic statins and avoidance of rosuvasatin and lipophilic statins may provide the intended cardiovascular protection without the increased incidence of insulin resistance.
Acknowledgments
Financial Support: Award: Canadian Frailty Network summer studentship to Karen Rees-Milton. The Canadian Multicentre Osteoporosis Study (CaMos) is currently funded by the Canadian Institutes of Health Research (CIHR) and Amgen Canada Inc. CaMos has received support from the Canadian Institutes of Health Research (CIHR); Amgen Canada Inc.; Actavis Pharma Inc. (previously Warner Chilcott Canada Co.); Dairy Farmers of Canada; Eli Lilly Canada Inc.: Eli Lilly and Company; GE Lunar; Hologic Inc.; Merck Frosst Canada Ltd.; Novartis Pharmaceuticals Canada Inc.; P&G Pharmaceuticals Canada Inc.; Pfizer Canada Inc.; Roche (F. Hoffmann-La Roche Ltd.); Sanofi-Aventis Canada Inc. (previously Aventis Pharma Inc.); Servier Canada Inc.; and The Arthritis Society.
Additional Information
Disclosure Summary: K.R.M., P.N., C.B., M.H., M.T., C.B., T.A., W.H., and W.P. have nothing to declare. R.H. and M.A. have received grant support from the Canadian Institute for Health Research, OPKO Renal, and Sanofi Inc.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Minard LV, Corkum A, Sketris I, Fisher J, Zhang Y, Saleh A. Trends in statin use in seniors 1999 to 2013: time series analysis. PLoS ONE. 2016;11(7):e0158608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez-Gonzalez J, Badimon L. Influence of statin use on endothelial function: from bench to clinics. Curr Pharm Des. 2007;13(17):1771–1786. [DOI] [PubMed] [Google Scholar]
- 3. Volpe M, Costanzi V. Patient at intermediate cardiovascular risk: statins, yes! - antihypertensive therapy, maybe. J Cardiovasc Med (Hagerstown). 2018;19Suppl 1:e130–e132. [DOI] [PubMed] [Google Scholar]
- 4. .Morville T, Dohlmann T, Kuhlman AB, et al. . Glucose homeostasis in statin users-The LIFESTAT study. Diabetes Metab Res Rev. 2019;35(3):e3110. [DOI] [PubMed] [Google Scholar]
- 5. Thomson SR, Chogtu B, Shetty R, Devasia T. Analysis of glycemic status in diabetes-naïve patients on statins: a hospital-based cross-sectional study. Indian J Pharmacol. 2018;50(6):320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hori E, Kikuchi C, Imaeda K, Okayama N, Suzuki T, Matsunaga T. [Effect of statins on glycemic status and plasma adiponectin concentrations in patients with type 2 diabetes mellitus and hypercholesterolemia]. Yakugaku Zasshi. 2019;139(5):807–815. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. [DOI] [PubMed] [Google Scholar]
- 8. Freeman DJ, Norrie J, Sattar N, et al. . Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103(3):357–362. [DOI] [PubMed] [Google Scholar]
- 9. Waters DD, Ho JE, DeMicco DA, et al. . Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57(14):1535–1545. [DOI] [PubMed] [Google Scholar]
- 10. Real J, Miranda C, Olofsson CS, Smith PA. Lipophilicity predicts the ability of nonsulphonylurea drugs to block pancreatic beta-cell KATP channels and stimulate insulin secretion; statins as a test case. Endocrinol Diabetes Metab. 2018;1(2):e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth BD, Bocan TM, Blankley CJ, et al. . Relationship between tissue selectivity and lipophilicity for inhibitors of HMG-CoA reductase. J Med Chem. 1991;34(1):463–466. [DOI] [PubMed] [Google Scholar]
- 12. Navarese EP, Buffon A, Andreotti F, et al. . Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–1130. [DOI] [PubMed] [Google Scholar]
- 13. Wanner C, Krane V, März W, et al. ; German Diabetes and Dialysis Study Investigators Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Qureshi AR, Parini P, et al. . Doesa statins promote vascular calcification in chronic kidney disease? Eur J Clin Invest. 2017;47(2):137–148. [DOI] [PubMed] [Google Scholar]
- 15. Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100(4):593–603. [PubMed] [Google Scholar]
- 16. Spronk HM, Soute BA, Schurgers LJ, et al. . Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem Biophys Res Commun. 2001;289(2):485–490. [DOI] [PubMed] [Google Scholar]
- 17. Chen L, Ma MY, Sun M, et al. . Endogenous sterol intermediates of the mevalonate pathway regulate HMGCR degradation and SREBP-2 processing. J Lipid Res. 2019;60(10):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirota Y, Nakagawa K, Sawada N, et al. . Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. Plos One. 2015;10(4):e0125737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dihingia A, Ozah D, Ghosh S, et al. . Vitamin K1 inversely correlates with glycemia and insulin resistance in patients with type 2 diabetes (T2D) and positively regulates SIRT1/AMPK pathway of glucose metabolism in liver of T2D mice and hepatocytes cultured in high glucose. J Nutr Biochem. 2018;52(2018):103–114. [DOI] [PubMed] [Google Scholar]
- 20. Ho HJ, Shirakawa H, Hirahara K, Sone H, Kamiyama S, Komai M. Menaquinone-4 amplified glucose-stimulated insulin secretion in isolated mouse pancreatic islets and INS-1 rat insulinoma cells. Int J Mol Sci. 2019;20(8):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. .Hussein AG, Mohamed RH, Shalaby SM, Abd El Motteleb DM. Vitamin K2 alleviates type 2 diabetes in rats by induction of osteocalcin gene expression. Nutrition. 2018;47(2018):33–38. [DOI] [PubMed] [Google Scholar]
- 22. Bourron O, Phan F. Vitamin K: a nutrient which plays a little-known role in glucose metabolism. Curr Opin Clin Nutr Metab Care. 2019;22(2):174–181. [DOI] [PubMed] [Google Scholar]
- 23. Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V. Association of the inactive circulating matrix gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: a review. Int J Mol Sci. 2019;20(3):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruderman I, Holt SG, Hewitson TD, Smith ER, Toussaint ND. Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis. Semin Dial. 2018;31(5):487–499. [DOI] [PubMed] [Google Scholar]
- 25. Shea MK, Booth SL, Miller ME, et al. . Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98(1):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villa JKD, Diaz MAN, Pizziolo VR, Martino HSD. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit Rev Food Sci Nutr. 2017;57(18):3959–3970. [DOI] [PubMed] [Google Scholar]
- 27. Hou YC, Lu CL, Zheng CM, et al. . Emerging role of vitamins D and K in modulating uremic vascular calcification: the aspect of passive calcification. Nutrients. 2019;11(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kreiger N. Tenenhouse A; Mackenzie JL PS, Brown JP, Prior JC: The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging. 1999;18(3):376–387. [Google Scholar]
- 29. Langsetmo L, Barr SI, Dasgupta K, et al. . Dietary patterns in men and women are simultaneously determinants of altered glucose metabolism and bone metabolism. Nutr Res. 2016;36(4):328–336. [DOI] [PubMed] [Google Scholar]
- 30. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):24–250. [DOI] [PubMed] [Google Scholar]
- 31. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int. 2016;113(35–36):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233–240. [DOI] [PubMed] [Google Scholar]
- 33. Lee G, Kim SM, Choi S, et al. . The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: a nationwide cohort study. Cardiovasc Diabetol. 2018;17(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kohli P, Knowles JW, Sarraju A, Waters DD, Reaven G. Metabolic markers to predict incident diabetes mellitus in statin-treated patients (from the treating to new targets and the stroke prevention by aggressive reduction in cholesterol levels trials). Am J Cardiol. 2016;118(9):1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63(6):735–745. [DOI] [PubMed] [Google Scholar]
- 37. Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49(8):1881–1892. [DOI] [PubMed] [Google Scholar]
- 38. Nicholson AC, Hajjar DP, Zhou X, He W, Gotto AM Jr, Han J. Anti-adipogenic action of pitavastatin occurs through the coordinate regulation of PPARgamma and Pref-1 expression. Br J Pharmacol. 2007;151(6):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan T, Hamilton MP, Mundy DI, Chua SC, Scherer PE. Impact of simvastatin on adipose tissue: pleiotropic effects in vivo. Endocrinology. 2009;150(12):5262–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aguirre L, Hijona E, Macarulla MT, et al. . Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol. 2013;64(3):281–288. [PubMed] [Google Scholar]
- 41. Fraulob JC, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Beneficial effects of rosuvastatin on insulin resistance, adiposity, inflammatory markers and non-alcoholic fatty liver disease in mice fed on a high-fat diet. Clin Sci (Lond). 2012;123(4):259–270. [DOI] [PubMed] [Google Scholar]
- 42. Kuhlman AB, Morville T, Dohlmann TL, et al. . Coenzyme Q10 does not improve peripheral insulin sensitivity in statin-treated men and women: the LIFESTAT study. Appl Physiol Nutr Metab. 2019;44(5):485–492. [DOI] [PubMed] [Google Scholar]
- 43. Harshman SG, Shea MK, Fu X, et al. . Atorvastatin decreases renal menaquinone-4 formation in C57Bl6 male mice. J Nutr. in press, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y, Chen JP, Duan L, Li S. Effect of vitamin K2 on type 2 diabetes mellitus: a review. Diabetes Res Clin Pract. 2018;136(2018):39–51. [DOI] [PubMed] [Google Scholar]
- 45. Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10(7):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puri R, Nicholls SJ, Shao M, et al. . Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273–1282. [DOI] [PubMed] [Google Scholar]
- 47. Hennessy DA, Bushnik T, Manuel DG, Anderson TJ. Comparing guidelines for statin treatment in Canada and the United States. J Am Heart Assoc. 2015;4(7):e001758. [DOI] [PMC free article] [PubMed] [Google Scholar]