Abstract
Provocative mouse studies and observational human data have generated considerable enthusiasm for modulating follicle-stimulating hormone (FSH) action in humans to prevent bone loss and, in addition, to treat obesity. This perspective summarizes the strengths and potential weaknesses of the mouse studies examining the skeletal phenotype of FSHβ or FSH receptor null mice, as well as more recent studies using FSH neutralizing antibodies. Although human observational studies do demonstrate correlation of serum FSH levels with postmenopausal bone loss, these studies cannot distinguish whether serum FSH is simply a better biomarker than estradiol or causally related to the bone loss. Establishing causality requires direct interventional studies either suppressing or infusing FSH in humans and to date, such studies have uniformly failed to demonstrate an effect of FSH on bone turnover independent of changes in sex steroid levels. In addition, suppression of FSH is unable to prevent increases in body fat following the induction of sex steroid deficiency, at least in men. Thus, although the preclinical mouse and human observational data are intriguing, there is currently no direct evidence from interventional studies that FSH regulates bone or fat metabolism in vivo in humans.
Keywords: bone, sex steroids, follicle-stimulating hormone, fat
One of the most provocative hypotheses in recent years is that follicle-stimulating hormone (FSH) has important extragonadal actions, particularly on bone (1). This is supported by substantial evidence in rodents, although some findings remain conflicting. In addition, observational human data are consistent with an important role for rising FSH levels in the peri- and postmenopausal period potentially driving the bone loss previously attributed to estrogen deficiency (1). Recent studies in mice also indicate effects of FSH in regulating adiposity (2). This has led to enthusiasm for using FSH neutralization (e.g., FSH antibodies) to simultaneously treat the age-related comorbidities of osteoporosis and obesity (1). This perspective briefly reviews the rodent and human observational data regarding FSH regulation of bone metabolism; however, since findings in mouse models may not always translate to humans, and correlation does not prove causality, a key focus of this review is on direct interventional studies rigorously testing the hypothesis that FSH is a physiologically or clinically relevant regulator of bone metabolism in humans. As some of these studies also provide insights into FSH regulation of adipose tissue in humans, these findings are summarized where appropriate.
Evidence From Mouse Models
Sun et al from the Zaidi laboratory demonstrated in 2006 that neither FSHβ nor FSH receptor (FSHR) null mice had bone loss despite marked estrogen deficiency (3). Moreover, bone mass was increased in FSHβ +/- mice which had a 50% reduction in serum FSH levels and normal ovarian function, suggesting that the skeletal action of FSH was estrogen independent. Based on these observations, the investigators made the admittedly bold assertion that “high circulating FSH causes hypogonadal bone loss.” These findings and conclusions received widespread attention, as they had the potential to completely redefine our thinking regarding the role of estrogen deficiency in mediating bone loss in women (4) and indeed, given the key role of estrogen in regulating bone metabolism in men (5), these data also had major implications for our understanding of the role of sex steroids versus FSH in regulating the male skeleton.
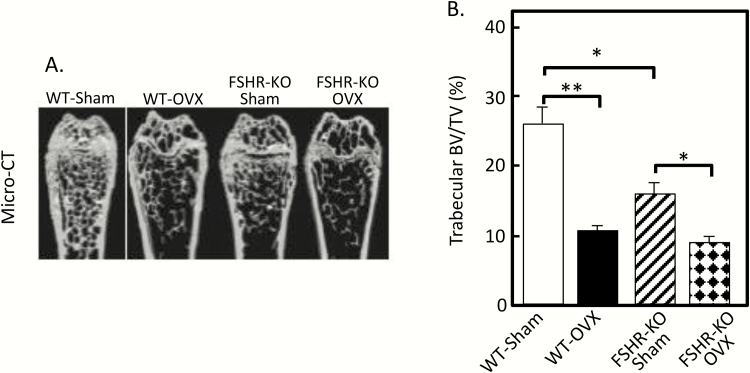
A subsequent letter to the editor by Seibel and colleagues (6) highlighted some important concerns regarding the mouse models used by Sun et al (3), concerns which were at least partially validated in a later publication from the Goltzman laboratory (7). Seibel et al (6) noted that both the FSHβ and FSHR null mice have important hormonal abnormalities, specifically increased luteinizing hormone (LH) and testosterone levels (by up to 10-fold in female mice) (8-10), as a secondary consequence of FSH loss, that could explain the preservation of bone mass without invoking a role for FSH in skeletal regulation. Indeed, the key study that was not done in the original paper from the Zaidi laboratory (3) was ovariectomy: if the skeletal preservation was truly due to lack of FSH, or lack of FSH action, then ovariectomy would not cause bone loss in either the FSHβ or the FSHR null mice. Conversely, if ovarian steroids, specifically androgens, were responsible for the preservation of bone mass, then ovariectomy would result in loss of the ovarian steroids and subsequent bone loss. Precisely this study was, in fact, done in a subsequent paper by Gao et al (7) from the Goltzman laboratory. As shown in Fig. 1, contrary to the findings of Sun et al (3), these investigators found that the FSHR null mice had reduced (not preserved) trabecular bone volume/tissue volume (BV/TV) at baseline; more importantly, the FSHR null mice clearly lost BV/TV following ovariectomy, indicating that it was ovarian factors (presumably androgens, although other ovarian growth factors may also be involved, see below), rather than lack of FSH, that was responsible for the relative preservation of bone mass in the setting of FSHR deletion and the concomitant estrogen deficiency. Importantly, as shown in Fig. 1B, trabecular BV/TV was identical in the wild-type (WT) and FSHR null mice following ovariectomy despite the fact that both groups of mice had low gonadal steroid levels but high FSH levels post ovariectomy; however, only the WT mice were responsive to FSH. This would indicate that FSH action had no effect on bone distinct from its effects on ovarian steroids—i.e., if the FSH/bone hypothesis were true, then removal of the gonads should have resulted in lower BV/TV following ovariectomy in the WT mice (due to the high FSH levels) as compared to the FSHR null mice (which would be protected from the high FSH levels). In a recent review (1), Zaidi and colleagues dismiss these findings by noting that mice lacking the aromatase enzyme have equivalent increases in serum testosterone levels as found in the FSHβ or FSHR null mice but have bone loss (11). The problem with this argument is that the FSHβ and FSHR null mice are able to locally (e.g., in bone) aromatize testosterone to estrogen (12), whereas the aromatase-deficient mice are not, and this inability to convert androgens to estrogens is the more likely explanation for the bone loss identified in the aromatase-deficient mice and the relative lack of bone loss in the FSHβ or FSHR null mice.
Figure 1.
(A) MicroCT images of the distal femur in wild type (WT) and FSHR null sham and ovariectomized (ovx) mice; (B) Trabecular BV/TV in WT and FSHR null sham and ovx’d mice. *P < 0.05; **P < 0.01; ***P < 0.001. Reproduced from Gao et al (7), with permission.
In a subsequent paper, the Seibel laboratory went further and created transgenic female mice expressing human FSH (TgFSH) and found that TgFSH dose-dependently increased bone mass, even in hpg mice lacking gonadotropin-releasing hormone (GnRH) and therefore endogenous FSH and LH secretion (13). Moreover, ovariectomy abolished the increase in bone mass in the TgFSH hpg mice, demonstrating again the dependence of FSH on ovarian factors (e.g., increased testosterone or other factors) in mediating the effects of FSH in not reducing, but rather increasing bone mass. Here it is important to note that in addition to androgens, both inhibins (14) and activins (15) produced by the ovaries have been shown to suppress bone resorption and increase bone formation, adding further to the complexity of interpreting the findings from the FSHβ, FSHR, and TgFSH mice as these growth factors are also altered in each of these mouse models.
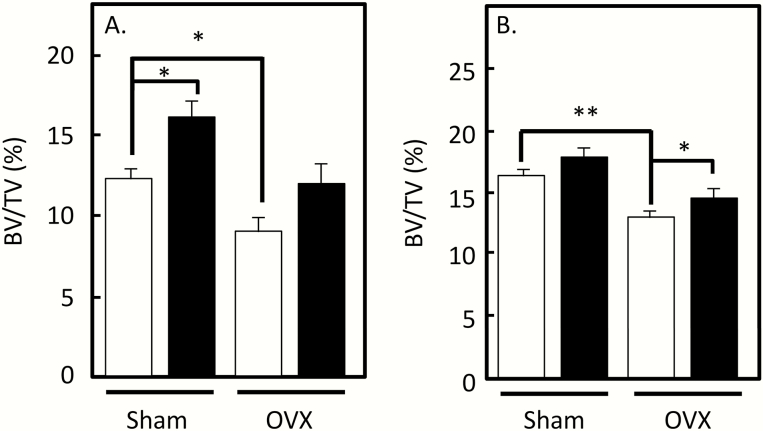
It is clear that the collective evidence from multiple laboratories on the skeletal phenotype of the FSHβ or FSHR null mice indicates that to the extent that bone mass is preserved in these estrogen-deficient mice, this is likely driven by ovarian factors, including androgens that act directly on bone and are also aromatized locally to estrogens (16). That said, in a more recent study from the Zaidi laboratory (2), a polyclonal antibody that targeted the β-subunit of FSH increased bone mass and, interestingly, also reduced adipose tissue in mice; similar findings were subsequently reported by this group using monoclonal antibodies (17). However, although these studies measured serum estrogen levels in the mice (which are extremely low when assessed by mass spectroscopy even in female mice and are generally unreliable when assessed using immunoassays in mice (18)), none of the studies using FSH antibodies report on LH or testosterone levels in the mice. Thus, the same concerns regarding inhibiting FSH signaling with resultant increases in LH/androgen levels (or alterations in other ovarian factors) noted above for the genetic models would be applicable here, at least in gonadally intact mice. This could explain the modest increase in BV/TV with the FSH antibodies in sham-operated mice (Fig. 2A and 2B; data are shown for the monoclonal antibodies (17)). However, these antibodies did at least partially prevent the reduction in BV/TV seen following ovariectomy (Fig. 2A and 2B), and this prevention of bone loss following ovariectomy cannot be explained by any residual ovarian steroid action. Thus, assuming these antibodies are truly specific for FSH, this would seem to be the most compelling evidence to date in support of the FSH/bone hypothesis as these ovariectomized models are not confounded by ovarian steroid/other factor changes known to be present in the global FSHβ or FSHR null mice, and also likely to occur with FSH neutralization in intact mice. Nonetheless, these provocative findings would be further supported by tissue-specific (i.e., osteoblast- or osteoclast-specific) deletion of the FSHR, as recently suggested by Kumar (19). Such models would not have alterations in LH or sex steroid/other ovarian factor levels as occur in the global knock outs and would provide the key causal link, at least in mice, for direct FSH regulation of bone metabolism in vivo.
Figure 2.
(A) Effects of the monoclonal anti-FSH antibody, Hf2, on spine BV/TV in sham and ovx mice. Open bars are mice treated with control antibody and solid bars are mice treated with Hf2. (B) Effects of the monoclonal anti-FSH antibody, Mf4, on spine BV/TV in sham and ovx mice. Open bars are mice treated with control antibody and solid bars are mice treated with Hf2. *P ≤ 0.05; **P ≤ 0.01. Reproduced from Ji et al (17), with permission.
Observational Studies in Humans
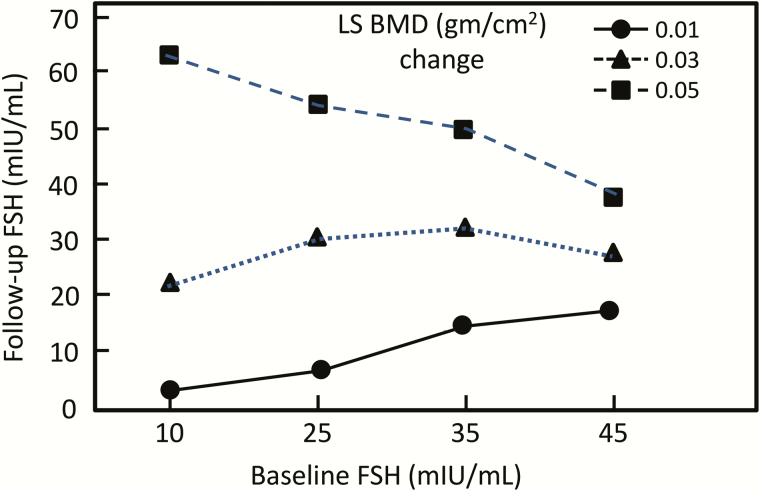
Proponents of the FSH/bone hypothesis appropriately cite the now extensive observational human data (well summarized in reference (1)) linking baseline FSH levels as well as changes in FSH levels during the menopause to menopausal bone loss as supporting an important role for FSH in the regulation of bone metabolism in humans. The most widely cited evidence is from the Study of Women’s Health Across the Nation (SWAN) where the investigators conducted a longitudinal (5 annual examinations) multiple site cohort study assessing annual dual-energy x-ray absorptiometry (DXA) measures of the lumbar spine and total hip bone mineral density (BMD) along with menstrual histories and reproductive hormonal profiles (20). The key finding of the study was that baseline FSH concentrations, subsequent FSH levels, and their interaction predicted 4-year BMD loss. Thus, as shown in Fig. 3, the combination of the baseline and 4-year follow-up FSH levels were predictive of BMD loss, whereas measures of baseline estradiol (E2) and its 4-year variation were poor predictors of incremental BMD change. However, significant lumbar spine BMD loss was associated with E2 levels below 35 pg/mL, regardless of the baseline E2 levels.
Figure 3.
Fitted lines representing projections of 4-year spine BMD loss in premenopausal and early perimenopausal women based on initial FSH concentrations, subsequent annual follow-up FSH concentrations, and their interaction. Reproduced from Sowers et al (20), with permission.
As Sowers and colleagues acknowledged in their paper, these data would indeed be supportive of possible direct effects of FSH on bone (20). However, these investigators were also careful to note that “FSH may serve as a proxy measure of ovarian dynamics involving E2. During the menopausal transition, FSH values may better characterize ovarian status than do E2 values because of the cyclic interaction of E2 and progesterone in the luteal phase represented by progressively irregular menstrual cycles” (20). In addition, peri- and early-menopausal alterations in luteal phase progesterone levels (21) may also contribute to the observed bone loss during this period, which is characterized by relatively normal E2 but rising FSH levels (22). Finally, Perrien et al (23) have shown that serum inhibin levels significantly inversely correlated with markers of bone formation and resorption in pre- and perimenopausal women and with markers of bone formation in postmenopausal women. Moreover, serum inhibin levels predicted bone formation and resorption markers in premenopausal women better than either FSH or bioavailable E2 levels. In short, the fundamental problem with all of the available observational human data linking FSH to menopausal bone loss is that these studies cannot distinguish whether FSH is simply an excellent biomarker for the disrupted ovarian dynamics of the perimenopausal period or is causally related to the bone loss.
Additional observational evidence in support of the FSH/bone hypothesis comes from 2 studies using a candidate gene approach linking polymorphisms in the FSHR gene to BMD (24, 25). However, by current standards, these studies were relatively small (n = 289 (24) and 1980 (25) subjects), and a more comprehensive genome-wide meta-analysis involving 32 961 individuals of European and East Asian ancestry identified loci associated with BMD/fracture risk at a genome-wide significant level which notably did not include the FSHR (or FSHB) gene, but did include the ESR1 gene (encoding ERα) (26).
Other observational data have included a relatively small clinical study comparing women with either hypergonadotropic (n = 7; mean FSH, 85 ± 41 IU/L) versus hypogonadotropic (n = 15; FSH 10 ± 6 IU/L) amenorrhea (27). In this study, the women with elevated FSH levels did have lower spine BMD, but no differences in femoral neck BMD. Although supportive of a role for FSH in bone metabolism, the relatively small number of subjects, differences in age (the hypergonadotropic women were approximately 7 years older than the hypogonadotropic women), and differences in other confounders underlying the clinical conditions leading to the amenorrhea make it difficult to draw meaningful conclusions.
In summary, the observational studies summarized above and elsewhere (1) could lead to the conclusion that FSH plays a physiologically or clinically meaningful role in regulating bone metabolism in women. However, each of these of these studies has potential alternative explanations for their findings and suffers from the fact that correlation simply cannot prove causality; rather, the gold standard for proving causality is interventional studies.
Human Interventional Studies
As endocrinologists, the fundamental clinical approach to evaluating our patients involves not just static hormonal measures, but classical suppression or stimulation tests which typically amplify the underlying pathology (28). Thus, a baseline cortisol level cannot definitively diagnose Cushing’s disease; rather, cortisol suppression tests are needed for an accurate diagnosis. Conversely, stimulation tests are often necessary to diagnose adrenal insufficiency. Similar suppression and stimulation tests have been used to test the FSH/bone hypothesis in humans, as summarized below.
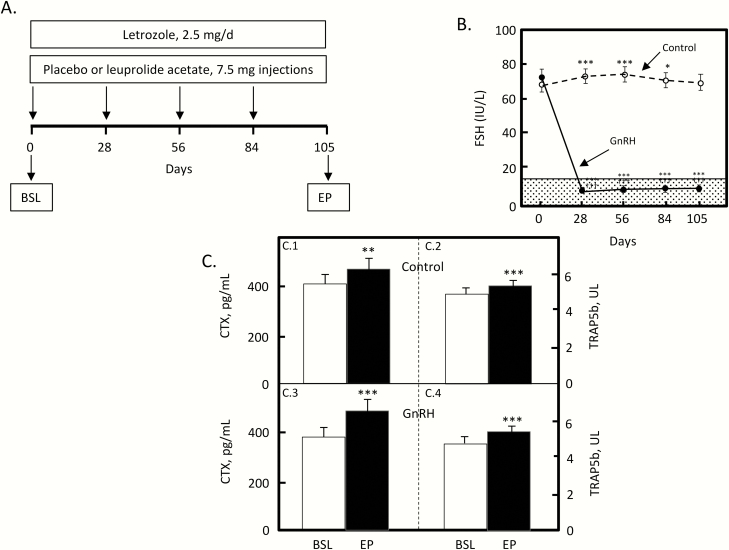
The first approach used a GnRH analog to suppress endogenous FSH secretion in postmenopausal women (29). As shown in Fig. 4A, 46 postmenopausal women were randomized into a double-blind, placebo-controlled study to receive either a GnRH analog (leuprolide acetate) or placebo injections every 28 days for 105 days (15 weeks). In order to avoid confounding by differing endogenous estrogen levels in the women receiving GnRH versus placebo, both groups were also treated with an aromatase blocker (letrozole) to eliminate even the low postmenopausal estrogen levels in these women. Fig. 4B shows the changes in FSH levels in the 2 groups: FSH did not change in the placebo group, but declined by ~90% (well into the normal premenopausal range) in the GnRH group. Due to the aromatase blocker, serum E2 declined from the low postmenopausal levels in these women (~5 pg/mL) to below the level of detection of the assay in both groups. This experimental design, then, created a head-to-head conflict: if bone turnover were being driven principally by estrogen—even the very low postmenopausal estrogen levels in these women—then further suppression of those low estrogen levels would lead to an increase in bone resorption markers. If, on the other hand, FSH were playing a significant role in human bone metabolism, then the marked (~90%) suppression of FSH levels would lead to a decrease in bone resorption markers in the GnRH-treated women, but not the placebo-treated. As shown in Fig. 4C, the results were unequivocal. Markers of bone resorption (serum C-terminal telopeptide of type I collagen [CTX] and tartrate-resistant acid phosphatase 5b [TRAP5b]) increased identically in both the placebo- and GnRH-treated women. Thus, in the GnRH-treated women, reduction of even the very low postmenopausal estrogen levels led to increased CTX and TRAP5b levels despite the marked suppression of FSH—specifically, there was no evidence in this model of any FSH effects on bone. Conceptually, these human data are very similar to the findings noted earlier in the ovariectomized WT versus FSHR null mice (Fig. 1B), where in the absence of gonadal steroids, BV/TV was identical in the 2 groups of mice, in that case regardless of the ability to respond to FSH.
Figure 4.
(A) Overview of the study design for FSH suppression in postmenopausal women. Abbreviations: BSL, baseline; EP, endpoint. (B) Changes in serum FSH over time in the control and GnRH groups. The shaded region represents the premenopausal reference range. (C) Serum CTX (C0.1) and serum TRAP5b (C0.2) in the control women; serum CTX (C0.3) and serum TRAP5b (C0.4) in the GnRH-treated women. *P < 0.05, ***P < 0.001 vs day 0; †††P < 0.001 for comparison with the control group at the specific time point. Reproduced from Drake et al (29), with permission.
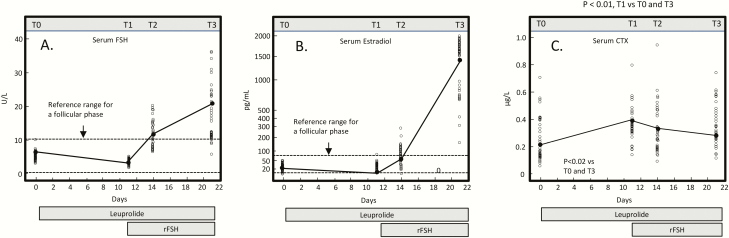
The converse study, a stimulation test, has also been done. Here, Omodei et al (30) induced pharmacological suppression of endogenous gonadotropin and E2 production using a GnRH analog (leuprolide) followed by stimulation with recombinant FSH (rFSH) in 29 premenopausal women. As shown in Fig. 5A, the GnRH analog initially led to a reduction in serum FSH levels, followed by a progressive increase during the second phase when rFSH was infused. Serum E2 initially decreased during the GnRH infusion, and then increased during the rFSH infusion (Fig. 5B). Importantly, changes in the bone resorption marker, CTX, tracked with changes in E2, not FSH. Thus, during the GnRH-alone phase, as E2 and FSH fell, CTX increased, demonstrating the dominant effect of the E2 decline over the FSH decline. Conversely, during the rFSH infusion, despite the marked increase in FSH, CTX decreased due to the rising E2 levels. A serum bone formation marker (osteocalcin) did not change during the study.
Figure 5.
Variation in serum FSH (A), E2 (B), and CTX (C) after leuprolide and rFSH administration in 29 women. Blood samples were drawn at 4 time points: T0 (at the beginning of leuprolide therapy), T1 (at the beginning of rFSH therapy), T2 (3 days after starting rFSH), and T3 (10 days after starting rFSH). Reproduced from Omodei et al (30), with permission.
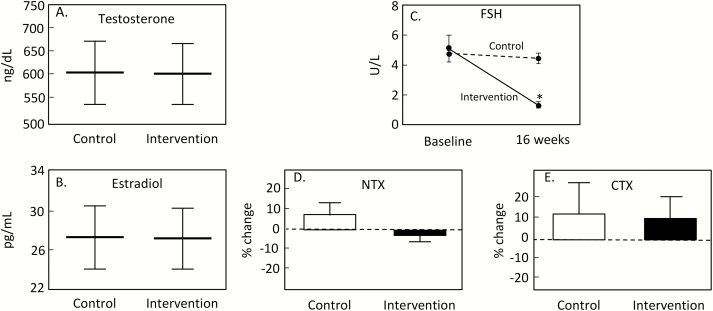
Collectively, these studies, which represent classical endocrine suppression and stimulation tests, failed to demonstrate any effects of FSH on bone turnover markers in humans. A limitation of both studies, however, is that in each case, both FSH and E2 levels were changing simultaneously. Thus, even though the effects of changes in E2 levels dominated in determining changes in bone turnover markers, one cannot formally exclude some effect of FSH on bone turnover in humans that was effectively “swamped” by the changes in E2 levels that also affected bone turnover. To address this issue, Uihlein et al (31) randomized 58 eugonadal men from 20 to 50 years of age to receive monthly GnRH analog injections plus daily topical testosterone gel (intervention group) or matching placebos (control group) for 16 weeks. In this experimental model, the GnRH agonist resulted in FSH suppression, but the testosterone replacement maintained normal testosterone and E2 levels (via aromatization of the testosterone). Thus, as shown in Fig. 6A and 6B these interventions resulted in identical testosterone and E2 levels in the control and intervention groups, but serum FSH levels decreased markedly in the intervention group (Fig. 6C). This elegant study design circumvented the problem in the 2 previous studies, where both FSH and E2 levels were changing. Here, testosterone and E2 were unchanged, but FSH was suppressed. Again, the outcome was clear: as shown in Fig. 6, changes in the bone resorption markers, N-telopeptide of type I collagen (NTX; Fig. 6D) and CTX (Fig. 6E), were no different between the groups. Changes in the bone formation marker, serum osteocalcin, also did not differ between groups (not shown).
Figure 6.
Mean serum testosterone (A) and E2 levels (B) with 95% confidence intervals during the study period; (C) change in serum FSH levels in the intervention and placebo groups; *Statistical significance of between-group differences in FSH from baseline to week 16, P < 0.0001. Change in serum NTX (D) and CTX levels (E) from baseline to study completion in the intervention and placebo groups. Reproduced from Uihlein et al (31), with permission.
In considering their findings in the context of the original studies with the FSHβ and FSHR null mice (3), Uihlein et al (31) acknowledged the possibility that their negative findings could be due to the fact that the magnitude of the reduction in FSH in their study may have been insufficient to affect bone turnover. However, they noted that in the original mouse study from the Zaidi laboratory, BMD was higher and bone resorption was lower in mice that were haploinsufficient for the β-subunit of FSH, which led to a 50% reduction in FSH levels (3). The human study of Uihlein et al (31) in fact achieved a 60% reduction in FSH levels without any effects on bone turnover; similarly, the earlier study in postmenopausal women described above achieved a 90% suppression of FSH levels (29), again without any effects on bone turnover markers. Thus, although the suggestion has been made that FSH effects on bone turnover in humans may require suppression of FSH to extremely low levels (32), the findings from the FSHβ +/- mice would argue against this possibility. Collectively, over a wide range of FSH concentrations, from low premenopausal to high postmenopausal FSH levels, no direct interventional study has been able to demonstrate an effect of FSH on bone metabolism in humans independent of the effects of sex steroids.
Finally, in addition to these studies, it is now well established that patients with prostate cancer undergoing long-term treatment with GnRH analogs who have suppressed FSH but also low testosterone and estrogen levels have marked ongoing bone loss (33) and increased fracture risk (34). Thus, in this clinical setting suppression of FSH is unable to prevent bone loss or fractures, raising the question of whether FSH neutralization in other sex steroid–deficient states (e.g., in postmenopausal women) will be able to overcome the dominant effects of sex steroid deficiency on bone.
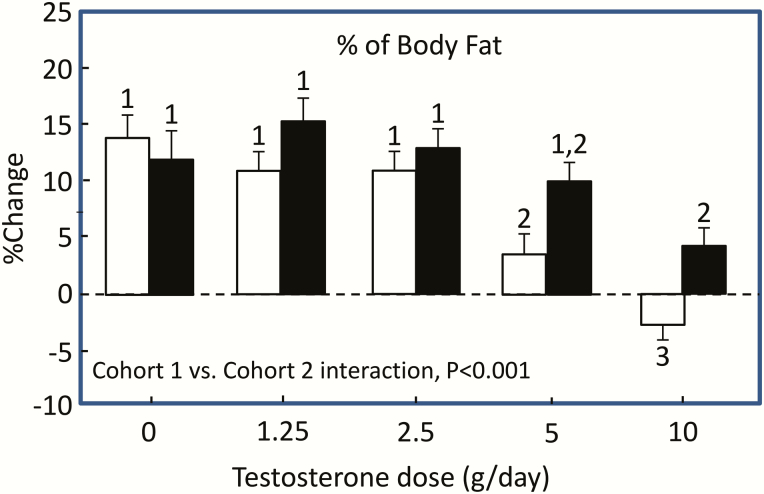
Even though the focus of this perspective is on FSH effects on bone metabolism in humans, it has been suggested that neutralizing FSH would lead to a concomitant increase in bone mass and reduction in adiposity due to the observed effects of FSH neutralization on adipose tissue in mice (1, 2). Although there is less evidence in humans directly testing the FSH/adiposity hypothesis, several studies relevant to this issue are worth noting. Thus, Finkelstein and colleagues (35) studied 198 healthy men aged 20 to 50 years who were treated with a GnRH agonist (goserelin acetate), which suppressed endogenous LH, FSH, and sex steroids, and randomly assigned them to receive increasing doses of testosterone gel (0, 1.25, 2.5, 5, or 10 g daily) for 16 weeks (cohort 1). Another 202 men received the GnRH agonist, doses of testosterone gel as above, and an aromatase blocker (anastrozole) to block the conversion of testosterone to E2 (cohort 2). Thus, both cohorts 1 and 2 had suppressed FSH levels (due to the GnRH agonist), were replaced with varying doses of testosterone which was either permitted to be aromatized to E2 (cohort 1) or not (cohort 2). With the induction of hypogonadism, and despite FSH suppression with the GnRH agonist, the men not receiving testosterone/E2 replacement (“0” dose) had significant increases in body fat (Fig. 7). Thus, as in the case of bone, suppression of FSH was unable to overcome the effects of induced sex steroid deficiency in increasing body fat. Interestingly, higher doses of testosterone replacement in cohort 1, where aromatization was present, prevented the increase in body fat following sex steroid deficiency, whereas blocking aromatization (cohort 2) led to increased body fat, indicating a dominant role for estrogen over testosterone (and over FSH) in regulating body fat even in men.
Figure 7.
Mean percent change from baseline in percentage of body fat. Open bars, cohort 1; filled bars, cohort 2. Within each cohort, bars with the same number indicate no significant difference between dose groups. The change in the percentage body fat did not differ significantly among the groups that received 0, 1.25, or 2.5 g of testosterone daily in cohort 1 (all labeled “1”). The change in each of those groups differed significantly from the change in the group that received 5 g per day (labeled “2”) and the change in the group that received 10 g per day (labeled “3”), and the change also differed significantly between these latter 2 groups. P values are for the cohort-testosterone dose interaction terms in analyses of variance comparing changes in percentage body fat between cohorts 1 and 2. Reproduced from Finkelstein et al (35), with permission.
In another study using a different design, Ostergren et al (36) reported body composition data on 58 men with advanced prostate cancer assigned to receive either orchiectomy (low sex steroids, high FSH) or a GnRH agonist (triptorelin; low sex steroids, low FSH). They found that, compared to the GnRH group, the men receiving orchiectomy had greater increases in total body and subcutaneous fat mass, but no differences in changes in visceral fat, consistent with a potential role for FSH in regulating at least peripheral fat depots in humans. A caveat to this study, however, is that orchiectomy likely results in more profound sex steroid deficiency than GnRH agonist therapy (37). Moreover, other studies have clearly demonstrated substantial increases in fat mass in men with prostate cancer treated long-term with GnRH analogs (38), arguing that FSH suppression, as in the case of bone loss, cannot overcome the effects of sex steroid deficiency in increasing fat mass.
Summary and Conclusions
Based on provocative mouse data and human observational studies, there is considerable interest in modulating FSH levels in humans to prevent bone loss and treat obesity (1). However, studies using global FSHβ or FSHR null mice are confounded by changes in LH, androgen, and potentially other ovarian growth factor levels 8-10, 14, 15), making it difficult to definitively assign a role for FSH in regulating bone metabolism in these models. The evidence from studies of FSH neutralizing antibodies (2, 17) also suffer to some extent from similar problems, given the interconnectedness of changes in FSH action and sex steroid levels. However, the effects of the polyclonal and monoclonal antibodies to at least modestly increase bone mass in gonadectomized mice do suggest that, in mice, FSH may have a role in bone mass regulation brought out in the setting of gonadectomy (2, 17), although concerns remain regarding the specificity of these antibodies as being exclusively for FSH. Clearly, tissue-specific (i.e., osteoblast-, osteoclast-, or adipocyte-specific) deletion of the FSHR would circumvent these problems and provide more definitive evidence for a role for FSH in regulating bone and/or fat metabolism, at least in mice. In addition, since the biological actions of FSH may well differ between rodents and humans, it would be of interest to perform studies defining FSH action on bone in other nonrodent species (e.g., sheep or goats) that might better model human reproductive and bone physiology.
The observational human data are certainly consistent with an important role for FSH in predicting bone loss (1); however, these studies cannot establish whether FSH is simply a superior biomarker relative to measuring E2 levels or is causally related to the bone loss. To date, direct interventional studies either suppressing or infusing FSH, even with keeping sex steroid levels constant, have not provided any evidence that FSH regulates bone turnover in humans independent of changes in sex steroid levels (29-31). In addition, the majority of the interventional evidence on FSH regulation of adipose tissue in humans indicates that suppression of FSH cannot prevent increases in body fat following sex steroid deficiency (35, 38). As such, there is at present no direct evidence that FSH regulates bone and/or fat metabolism in vivo in humans. Pending future positive findings, this leaves open the potentially unsatisfying possibility of species differences between mice and humans with respect to FSH effects on bone or fat metabolism.
Acknowledgments
Financial Support: This work was supported by NIH Grants AG004875, AG048792, and AR027065.
Glossary
Abbreviations
- BMD
bone mineral density
- BV/TV
bone volume/tissue volume
- CTX
C-terminal telopeptide of type I collagen
- E2
estradiol
- FSH
follicle-stimulating hormone
- FSHR
follicle-stimulating hormone receptor
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- NTX
N-telopeptide of type I collagen
- TgFSH
transgenic mice expressing human FSH
- TRAP5b
tartrate-resistant acid phosphatase 5b
- WT
wild-type
Additional Information
Disclosure Summary: The author has no relevant financial disclosures.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Zaidi M, Lizneva D, Kim SM, et al. . FSH, bone mass, body fat, and biological aging. Endocrinology. 2018;159(10):3503-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu P, Ji Y, Yuen T, et al. . Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112. 10.1038/nature22342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun L, Peng Y, Sharrow AC, et al. . FSH directly regulates bone mass. Cell. 2006;125(2):247-260. [DOI] [PubMed] [Google Scholar]
- 4. Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279-302. [DOI] [PubMed] [Google Scholar]
- 5. Khosla S, Melton LJ 3rd, Riggs BL. Clinical review 144: estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87(4):1443–1450. 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 6. Seibel MJ, Dunstan CR, Zhou H, Allan CM, Handelsman DJ. Sex steroids, not FSH, influence bone mass. Cell. 2006;127(6):1079; author reply 1080-1079; author reply 1081. [DOI] [PubMed] [Google Scholar]
- 7. Gao J, Tiwari-Pandey R, Samadfam R, et al. . Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148(6):2613-2621. [DOI] [PubMed] [Google Scholar]
- 8. Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHbeta subunit knockout mice. Reproduction. 2003;125(2):165-173. [DOI] [PubMed] [Google Scholar]
- 9. Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69(4):1281-1293. [DOI] [PubMed] [Google Scholar]
- 10. Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141(11):4295-4308. [DOI] [PubMed] [Google Scholar]
- 11. Oz OK, Hirasawa G, Lawson J, et al. . Bone phenotype of the aromatase deficient mouse. J Steroid Biochem Mol Biol. 2001;79(1–5):49-59. 10.1016/s0960-0760(01)00130-3 [DOI] [PubMed] [Google Scholar]
- 12. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86(3–5):225-230. 10.1016/s0960-0760(03)00360-1 [DOI] [PubMed] [Google Scholar]
- 13. Allan CM, Kalak R, Dunstan CR, et al. . Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107(52):22629-22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaddy D. Inhibin and the regulation of bone mass. Curr Osteoporos Rep. 2008;6(2):51-56. [DOI] [PubMed] [Google Scholar]
- 15. Lotinun S, Pearsall RS, Horne WC, Baron R. Activin receptor signaling: a potential therapeutic target for osteoporosis. Curr Mol Pharmacol. 2012;5(2):195-204. [DOI] [PubMed] [Google Scholar]
- 16. Almeida M, Laurent MR, Dubois V, et al. . Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev. 2017;97(1):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji Y, Liu P, Yuen T, et al. . Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci U S A. 2018;115(9):2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nilsson ME, Vandenput L, Tivesten Å, et al. . Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015;156(7):2492-2502. [DOI] [PubMed] [Google Scholar]
- 19. Kumar TR. Extragonadal Actions of FSH: A Critical Need for Novel Genetic Models. Endocrinology. 2018;159(1):2-8. 10.1210/en.2017-03118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sowers MR, Jannausch M, McConnell D, et al. . Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261-1267. [DOI] [PubMed] [Google Scholar]
- 21. Prior JC. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric. 2018;21(4):366-374. [DOI] [PubMed] [Google Scholar]
- 22. Sowers MR, Finkelstein JS, Ettinger B, et al. ; Study of Women’s Health Across the Nation The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14(1):44-52. [DOI] [PubMed] [Google Scholar]
- 23. Perrien DS, Achenbach SJ, Bledsoe SE, et al. . Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab. 2006;91(5):1848-1854. [DOI] [PubMed] [Google Scholar]
- 24. Rendina D, Gianfrancesco F, De Filippo G, et al. . FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163(1):165-172. [DOI] [PubMed] [Google Scholar]
- 25. Mendoza N, Quereda F, Presa J, et al. . Estrogen-related genes and postmenopausal osteoporosis risk. Climacteric. 2012;15(6):587-593. [DOI] [PubMed] [Google Scholar]
- 26. Estrada K, Styrkarsdottir U, Evangelou E, et al. . Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab. 2004;22(4):360-364. [DOI] [PubMed] [Google Scholar]
- 28. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drake MT, Srinivasan B, Mödder UI, et al. . Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5056-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Omodei U, Mazziotti G, Donarini G, et al. . Effects of recombinant follicle-stimulating hormone on bone turnover markers in infertile women undergoing in vitro fertilization procedure. J Clin Endocrinol Metab. 2013;98(1):330-336. [DOI] [PubMed] [Google Scholar]
- 31. Uihlein AV, Finkelstein JS, Lee H, Leder BZ. FSH suppression does not affect bone turnover in eugonadal men. J Clin Endocrinol Metab. 2014;99(7):2510-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woodruff TK, Khosla S. New hope for symptom management during natural and iatrogenic menopause transitions. Biol Reprod. 2017;97(2):177-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DK, Lee JY, Kim KJ, et al. . Effect of androgen-deprivation therapy on bone mineral density in patients with prostate cancer: a systematic review and meta-analysis. J Clin Med 2019;8(1):113 10.3390/jcm8010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu CT, Yang YH, Chen PC, Chen MF, Chen WC. Androgen deprivation increases the risk of fracture in prostate cancer patients: a population-based study in Chinese patients. Osteoporos Int. 2015;26(9):2281-2290. [DOI] [PubMed] [Google Scholar]
- 35. Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(25):2457. [DOI] [PubMed] [Google Scholar]
- 36. Østergren PB, Kistorp C, Fode M, Bennedbaek FN, Faber J, Sønksen J. Metabolic consequences of gonadotropin-releasing hormone agonists vs orchiectomy: a randomized clinical study. BJU Int. 2019;123(4):602-611. [DOI] [PubMed] [Google Scholar]
- 37. Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000;164(3 Pt 1): 726-729. [DOI] [PubMed] [Google Scholar]
- 38. Galvão DA, Spry NA, Taaffe DR, et al. . Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47. [DOI] [PubMed] [Google Scholar]