Abstract
Background & Aims:
We investigated antibody responses to hepatitis C virus (HCV) antigens E1 and E2 and the relevance of animal models for vaccine development. We compared antibody responses to vaccination with recombinant E1E2 complex in heathy volunteers, non-human primates (NHPs), and mice.
Methods:
We analyzed 519 serum samples from participants in a phase 1 vaccine trial (NCT00500747) and compared them with serum or plasma samples from C57BL/6J mice (n=28) and rhesus macaques (n=4) immunized with the same HCV E1E2 antigen. Blood samples were collected at different timepoints and analyzed for antibody binding, neutralizing activity, and epitope specificity. Monoclonal antibodies from the immunized NHPs were isolated from single plasmablasts and memory B cells, and their immunogenetic properties were characterized.
Results:
Antibody responses of the volunteers, NHPs, and mice, to the non-neutralizing epitopes on the E1 N-terminus and E2 hypervariable region 1 did not differ significantly. Antibodies from volunteers and NHPs that neutralized heterologous strains of HCV primarily interacted with epitopes in the antigen region 3. However, the neutralizing antibodies were not produced in sufficient levels for broad neutralization of diverse HCV isolates. Broadly neutralizing antibodies similar to the human VH1-69 class antibody specific for antigen region 3 were produced in the immunized NHPs.
Conclusions:
In an analysis of vaccinated volunteers, NHPs, and mice, we found that recombinant E1E2 vaccine antigen induces high-antibody titers that are insufficient to neutralize diverse HCV isolates. Antibodies from volunteers and NHPs bind to the same neutralizing epitopes for virus neutralization. NHPs can therefore be used as a preclinical model to develop HCV vaccines. These findings also provide useful baseline values for development of vaccines designed to induce production of neutralizing antibodies.
Keywords: HVR1, AR3, animal model, mAb
Graphical Abstract
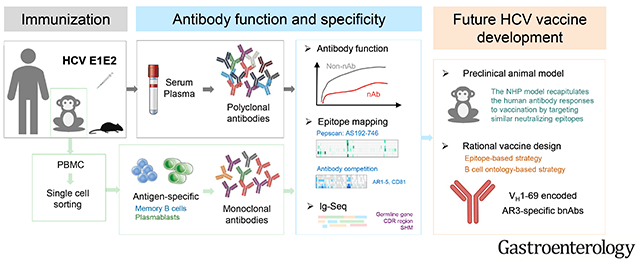
Lay Summary:
Non-human primates can be used to study vaccine candidates for hepatitis C virus infection.
Hepatitis C virus (HCV) chronically infects an estimated 71 million people worldwide, predisposing them to risk of developing life-threatening liver cirrhosis and hepatocellular carcinoma. Although direct-acting antiviral drugs to HCV have dramatically improved the rate of cure, treatment alone will unlikely be sufficient to achieve HCV elimination. Limited access to care, high cost of therapy, poor awareness of infection status, drug resistance, reinfection following cure and the rising rate of new infections illustrate a pressing need for a broadly effective vaccine to stop global HCV transmission.1,2
HCV vaccine development has been stymied by the extreme genetic diversity of circulating viruses, the numerous mechanisms through which HCV evades the immune response, and the lack of a preclinical animal model for vaccination-challenge trials. The chimpanzee is the only non-human species susceptible to persistent HCV infection, but financial, practical and especially ethical constraints have urged the exploration of alternative animal models. Nevertheless, cumulative evidence of protective cellular and humoral immunity during HCV infection suggests that vaccination to prevent viral persistence is feasible.3,4 Several strategies to elicit neutralizing antibodies (nAbs), T cell responses, or both, have demonstrated immunogenicity of vaccine antigens and, in some cases, even protective immunity in the chimpanzee model.5–9 Yet there remains an open question whether animals can faithfully recapitulate human immune responses to HCV vaccination. Two major promising vaccine candidates had previously been assessed in humans. The first one is based on recombinant full-length HCV envelope glycoproteins E1 and E2 derived from a genotype 1a isolate, HCV-1. In a phase I clinical trial (NCT00500747), immunization of healthy human volunteers with the E1E2 candidate elicited broadly nAbs (bnAbs) in only a few subjects.10 The second one composed of nonstructural proteins NS3-NS5 has been recently evaluated in phase I/II clinical trials (NCT01436357). According to the announcement by the National Institute of Allergy and Infectious Diseases (NIAID) in an HCV vaccine development workshop (https://www.niaid.nih.gov/news-events/trial-evaluating-experimental-hepatitis-c-vaccine-concludes) and the results recently released at ClinicalTrials.gov, it failed to elicit protective immunity in high-risk people who inject drugs, although high frequencies of virus-specific polyfunctional CD4 and CD8 T cells were elicited in healthy volunteers.11 The next-generation HCV vaccine strategies will likely require rationally designed antigens targeting conserved epitopes to overcome viral variability. A reliable animal model and a better understanding of vaccine-elicited immune responses will be essential to facilitate future HCV vaccine development.
In this study, we sought to gain a comprehensive insight of antibody response to HCV glycoproteins and to evaluate preclinical animal models for vaccine development. We took advantage of the recombinant E1E2 vaccine candidate utilized in the clinical trial. E1 and E2 are targets of HCV nAbs. Most potent and bnAbs isolated so far have been mapped to the E2 neutralizing face, a predominantly hydrophobic surface formed by the E2 front layer and the tip of the CD81 binding loop,12 and to the E1E2 heterodimer.13 Early immunization studies of the E1E2 candidate in animal models (chimpanzee, guinea pig and mouse) and humans have yielded various results on its ability to induce antiviral antibody responses, ranging from cross neutralizing,10,14,15 neutralizing,14, 16, 17 virus-enhancing18 to epitope-interfering19. The discrepancy could be a result of the variability of the assays and reagents used in different studies. Here, we immunized rhesus macaques and mice with the same antigens and compared their antibody responses directly to that of immunized humans.
Methods
Human serum samples
A total of 519 human serum samples were obtained from a completed phase I, placebo-controlled, dose-escalation clinical trial (DMID 01-002; ClinicalTrials.gov identifier NCT00500747), in which a candidate HCV vaccine constituting a recombinant E1E2 immunogen derived from a genotype 1a isolate (HCV-1) formulated with the MF59 adjuvant (Novartis Vaccines and Diagnostics) was tested for safety and immunogenicity in healthy human volunteers.16 All samples were heat-inactivated at 56 °C for 30 min prior to all assays to inactivate complement.
NHP immunization
Four male rhesus macaques (Macaca mulatta) of India origin, 4-5 years of age and weighted between 8-11 kg, designated 30734, 31782, 31859 and 31881, were housed at Southwest National Primate Research Center at Texas Biomedical Research Institute (TBRI). Animals were immunized intramuscularly five times at 0, 4, 12, 25 or 28, and 42 weeks with the same immunogen utilized in the clinical trial. Each immunization consisted of two intramuscular injections in the quadriceps of each leg with a total of 50 μg of HCV E1E2 formulated in AddaVax (InvivoGen) or Adjuplex (Advanced Bioadjuvants and Sigma-Aldrich) adjuvant. Whole blood was collected and processed for plasma and peripheral blood mononuclear cells (PBMCs) using Ficoll-Paque and Leucosep tubes according to manufacturers’ instructions. Plasma samples were heat-inactivated at 56 °C for 30 min prior to all assays. All procedures and experiments in NHPs were performed in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of TBRI and The Scripps Research Institute (TSRI).
Mouse immunization
Twenty-eight female mice (C57BL/6) around 6-8 weeks old were housed at TSRI. Animals were immunized subcutaneously four times in 4-week intervals with the same immunogen utilized in humans and NHPs and formulated in AddaVax or Adjuplex adjuvant (25 μg immunogens for prime immunization and 5 μg immunogens for boosters). Serum samples were collected at 0, 9 and 13 weeks, and heat-inactivated at 56 °C for 30 min before analyses. All procedures and experiments in mice were performed in accordance with protocols reviewed and approved by the IACUC of TSRI.
ELISA
Binding ELISA and competition ELISA were performed to assess antibody titers and to map discontinuous epitopes, respectively. See the Supplementary Materials for further details.
Pepscan analysis
A library of peptides consisting of 15 amino acids in length and overlapped by 12 residues spanning the full length of HCV-1 envelope glycoproteins was used in pepscan to map continuous epitopes. See the Supplementary Materials for further details.
HCV neutralization assays
HCV pseudoparticles (HCVpp) were generated by co-transfection of 293T cells with pNL4-3.lucR-E- and the corresponding expression plasmids encoding the E1E2 from isolate HCV-1 (genotype 1a), H77 (genotype 1a), UKN1B 12.6 (genotype 1b), J6 (genotype 2a), S52 (genotype 3a), UKN4.11.1 (genotype 1a) or SA13 (genotype 5a) at a 4:1 ratio by polyethylenimine (Polysciences). Neutralization was carried out on Huh-7 cells with diluted sera or mAbs as previously described.20 For HCV cell culture (HCVcc) neutralization, adapted HCV recombinant HCV-1, H77 and SA13(Core-NS2)/JFH1 were propagated in Huh-7.5.1 cells as described previously.21 Virus neutralization was determined by measurement of the percentage reduction of the number of infectious foci. See the Supplementary Materials for further details.
ELISPOT
E1E2-specific antibody-secreting cells (ASCs) were detected by ELISPOT assay on fresh PBMCs as previously described with modifications.22 See the Supplementary Materials for further details.
Flow cytometry and single B cell sorting
For analytical flow cytometry, PBMCs were surface stained with a panel of antibodies (Supplementary Table 1) for 30 min in the dark at 4 °C and washed twice with FACS buffer composed of 2% FBS and 2 mM EDTA in PBS. Samples were acquired with LSR-II flow cytometer (BD Bioscience) and Flowjo 10 (Tree Star) was used for analysis. For sorting, 25-50 million fresh PBMCs were stained and sorted on the BD FACSAria III (BD Biosciences). Single Plasmablasts (CD3−CD20−CD80+HLA-DR+) and E2-specific memory B cells (CD3− CD20+CD27+IgG+ E2+) were collected into 96-well PCR plates with 4 μl of lysis buffer containing of RNaseOUT (Invitrogen) and dithiothreitol. Plates were flash-frozen on dry ice and immediately transferred to −80 °C.
Generation of monoclonal antibodies (mAbs)
Immunoglobulin variable genes from single-sorted B cells were amplified by RT-PCR and nested PCR reactions as described previously.23 PCR products were sent for sequencing (Retrogen) before cloning into human Igγ1, Igκ and Igλ expression vectors.24 Plasmids containing paired antibody heavy and light chain genes were co-transfected (1:1 ratio) into HEK293T or ExpiCHO cells using polyethylenimine (Polysciences) or ExpiFectamine™ CHO Transfection Kit (Thermo Fisher Scientific), respectively. Antibody-containing supernatants were harvested 3-14 days after transfection. Antibodies produced in HEK293T cell were quantified by anti-human IgG Fc and used directly in neutralization assays for screening purposes. Antibody supernatants produced in ExpiCHO cells were purified over Protein A-Sepharose 4 Fast Flow (GE healthcare) columns per manufacture’s instruction.
Bioinformatics
Antibody sequences were submitted to IgBLAST (https://www.ncbi.nlm.nih.gov/igblast/) and ImMunoGeneTics information system (IMGT, http://www.imgt.org/) for gene identification and genetic assignment. Multiple-sequence alignments were performed using MegAlign Pro program in Lasergene 15.3 (DNAstar). Maximum-likelihood phylogenetic tree was constructed using MEGA X software.
Statistics
Graphpad Prism v8.02 was used for all statistical analyses. The significance of differences in antibody response between groups was calculated using unpaired, two-tailed Mann-Whitney U tests. Antibody responses by dose in immunized humans were compared using one-way ANOVA test. Correlations in data were assessed using a two-tailed Pearson correlation coefficient. r values between 0 and 0.3 indicate a negligible positive correlation, between 0.3 and 0.5 indicate a weak correlation, between 0.5 and 0.7 indicate a moderate correlation, while between 0.7 and 1.0 indicate a strong correlation. P values less than 0.05 were considered significant.
Results
Immunization with HCV E1E2 elicits comparable antibody responses in humans and animal models
NHP rhesus macaques (n = 4) and C57BL/6J mice (n = 28) were immunized with the same E1E2 antigen formulated in AddaVax,25 a squalene-based oil-in-water emulsion similar to MF59 that was utilized in the clinical trial, or Adjuplex,26,27 a non-oil-based adjuvant that has demonstrated superiority to alum, Freund and numerous other experimental adjuvants (Figure 1A). In parallel, longitudinal serum samples collected from 56 human subjects in the clinical trial were re-analyzed and compared to the animal samples (plasma for NHPs and serum for mice) using the same experimental methods and controls. Initial screening of the immune samples at a dilution of 1:50 demonstrated that all immunized subjects developed high titers of anti-E1E2 antibodies, with the peak endpoint titers ranging from 19,107-321,951, 108,710-397,754 and 12,255-604,246 for humans, NHPs and mice, respectively (Figure 1B and Supplementary Tables 2–4). Around 66% of humans and all animals displayed autologous serum neutralizing activity for at least one timepoint in the study (Supplementary Tables 2–4). There was no significant difference for different antigen doses in immunized humans except the autologous neutralization at week 26 (Supplementary Figure 1) and the results were consistent with a previous report.16 In animal models, the E1E2/Adjuplex formulation induced apparently stronger antibody responses than the E1E2/AddaVax formulation (Figure 1C and D).
Figure 1.
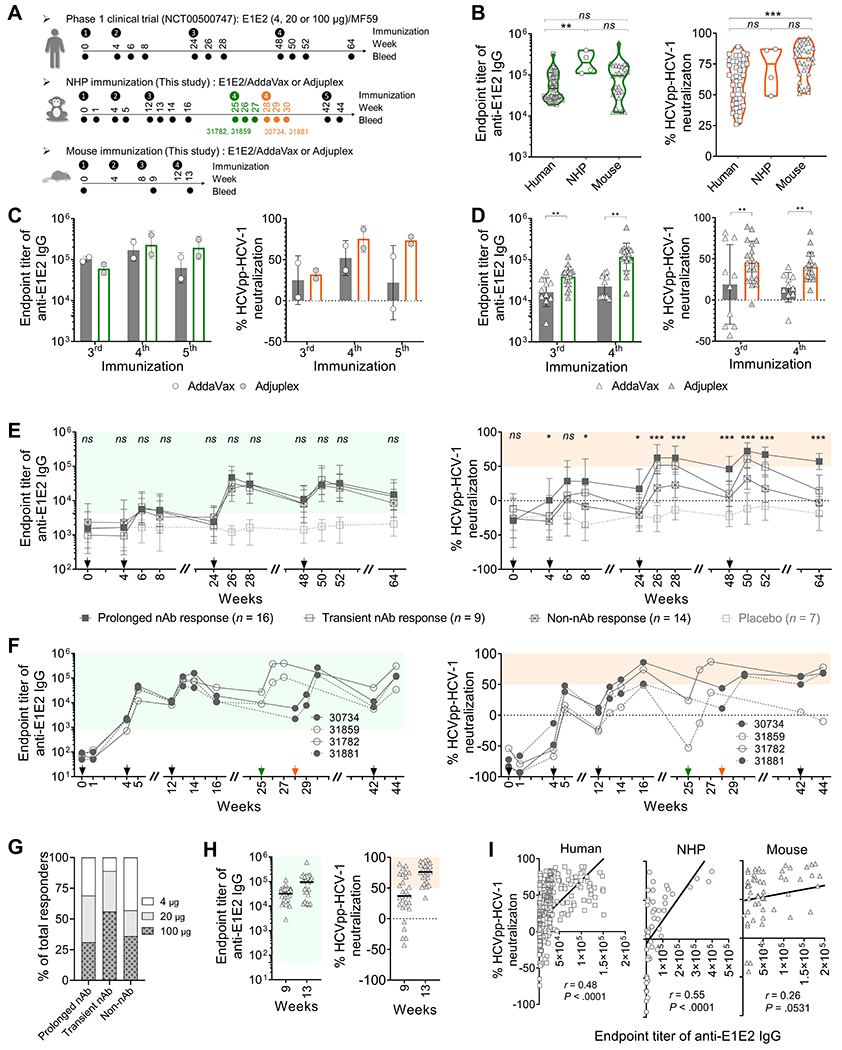
Antibody responses elicited by E1E2 immunization in humans and animal models. Immune samples were tested for endpoint titer of anti-E1E2 IgG and HCVpp neutralization against the autologous HCV-1 isolate at a dilution of 1:50. (A) Immunization and sampling schedules for humans, NHPs and mice. (B) Comparison of peak antibody responses in humans, NHPs and mice. P values were calculated by two-tailed Mann-Whitney test. (C and D) NHP (C) and mouse (D) antibody responses to E1E2 formulated with AddaVax or Adjuplex adjuvant. (E and F) Kinetics of antibody response in humans (E) and NHPs (F). Positive binding (green shading) was defined as binding titers 3 standard deviations above the mean of non-immune healthy human or NHP samples. Positive neutralization (orange shading) was defined as >50% neutralization by immune samples at 1:50. Arrows indicate immunization time points. Green and orange arrows indicate the 4th immunization on NHPs 31782 and 31859, and on NHPs 30734 and 31881, respectively. Human prolonged nAb responses, transient nAb responses and non-nAb responses were compared using one-way ANOVA tests. *P < .05, ***P < .0001, ns, not significant. (G) The proportion of human responders immunized with different E1E2 dosage. (H) Mouse antibody responses following the 3rd (week 9) and 4th (week 13) immunizations. (I) Correlation analysis (two-tailed Pearson correlation) between autologous binding and neutralization of human (n = 387), NHP (n = 50) and mouse (n = 56) immune sera. Placebo and pre-immunization human samples are excluded in this analysis.
Longitudinal analysis of the human and NHP antibody responses showed that most E1E2-specific antibodies appeared after the 2nd immunization and peaked after the 3rd immunization (Figure 1E and F). 41% of immunized humans and 3 of 4 NHPs (30734, 31782 and 31881) generated a prolonged autologous nAb response persisting for over three months (Figure 1 E and F, right). In contrast, 23% of immunized humans and NHP 31859 had a transient response that waned precipitously and became non-neutralizing at the final time point. No significant difference was found in the magnitude of the anti-E1E2 antibody titers among the prolonged neutralizers, transient neutralizers and non-neutralizers (Figure 1 E and F, left). There is no difference in the proportion of responders among the human dosage groups for prolonged nAb responses, but there appears to be a trend for more transient nAb responders in the 20-μg and 100-μg dosage groups than in the 4-μ dosage group (Figure 1G and Supplementary Figure 1B). In the mouse model, most animals reached peak antibody responses after the 4th immunization (Figure 1H). Overall, the animals mounted an antibody response that was comparable to, or even stronger than, that of the immunized humans (Figure 1B). An overall weak to moderate correlation between autologous E1E2-binding and nAb response was observed for humans (r = 0.48, P < .0001) and NHPs (r = 0.55, P < .0001), but not for mice (r = 0.26, P = .0531) (Figure 1I).
Human and NHP nAbs exhibit cross-binding reactivity and neutralization potential against heterologous viral strains
To assess the antibody functions, selected human, NHP and mouse immune samples with autologous neutralization were tested for cross-reactivity against a panel of viral isolates representing 6 major HCV genotypes at a dilution of 1:50. Similar profiles of cross-reactivity were observed for humans and NHPs (Figure 2). In both cases, all samples tested bound to native and denatured E1E2 from diverse HCV genotypes (Figure 2A). As expected, the highest reactivity was observed against E1E2 from the autologous genotype 1a isolate HCV-1, followed by viral strains H77 and UKN1B12.6 from the same genotype. Notably, the NHP plasma had a broader reactivity across the genotypes when compared to the human sera, likely caused by their higher binding titers.
Figure 2.
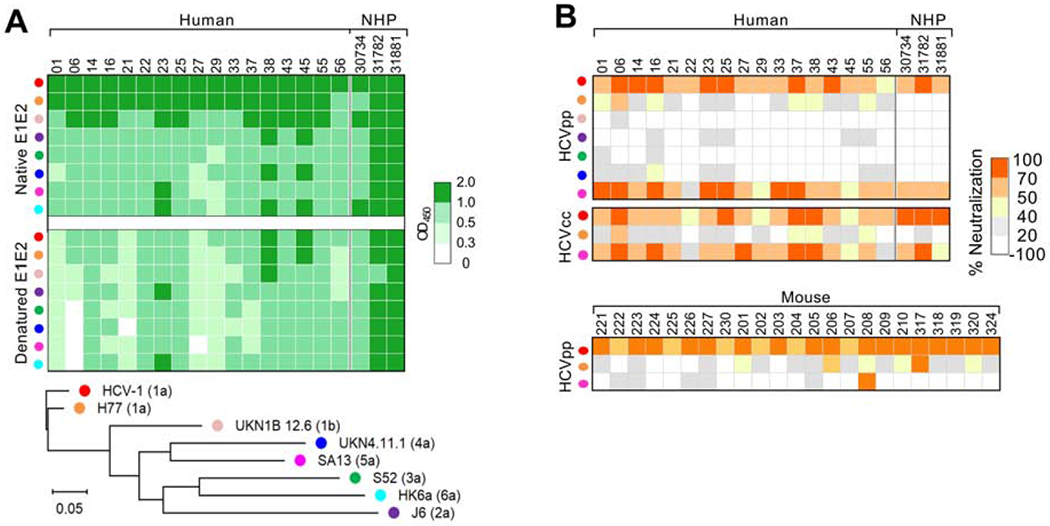
Cross-reactivity of serum nAbs. Selected autologous neutralizing samples from human (week 52), NHP (week 27 or 30) and mouse (week 13) were tested for cross-binding and -neutralization to a panel of HCV strains representing the major 6 genotypes at 1:50. (A) Binding of immune sera to native and denatured E1E2. The E1E2 amino-acid sequences of the HCV isolates (represented by different color dots) are provided in Supplementary Figure 2 and compared here in the maximum-likelihood phylogenic tree. The genotype of each isolate is shown in bracket. (B) Neutralization of HCVpp and HCVcc by the immune sera.
Most human and NHP autologous neutralizers also neutralized a heterologous genotype 5a strain, SA13, in both HCVpp and HCVcc systems (Figure 2B). Of note, a natural variation L442 was found in the E2 of SA13 (Supplementary Figure 2), which may render the virus more sensitive to antibody neutralization.28 Strikingly, the prototypic genotype 1a strain H77, which shares 95% of E1E2 amino acid identity with HCV-1 E1E2 (Supplementary Figure 3), was resistant to neutralization by the majority of human and NHP samples. In the mouse model, a weak heterologous nAb response against H77 was also elicited, but no strong response against SA13 was observed for most animals (Figure 2B). Together, these data indicate that nAb responses to E1E2 immunization were very similar in NHPs and humans but were qualitatively different in mice.
Mapping of antibody specificity
To understand the virus-neutralizing activities of antibody responses and the targeted epitopes, we performed pepscan and antibody competition analyses on selected neutralizing and non-neutralizing samples from the post 3rd or 4th immunization (at 1:50). At these time points, most immunized subjects reached peak nAb activities for epitope mapping. Due to the limited availability of the human sera at week 50 and mouse sera at week 13, the human week-52 and mouse week-9 sera were studied instead. In pepscan, a library of overlapping peptides covering the HCV-1 E1E2 sequence was used to map continuous epitopes. Here, continuous epitopes and antigen sites (AS) are named based on their first amino acid position on the HCV polypeptide, e.g. E2 antigenic site 412-423 is called AS412. In antibody competition, soluble large external loop of CD81 and five well-characterized human mAbs targeting distinct antigenic regions AR1-529–31 were used to map discontinuous epitopes. AR1 is proximal to the CD81 binding site. The AR1-specific mAbs only bind genotype 1 HCV and do not have significant neutralizing activity. AR2 is distal from the CD81 binding site and mAb AR2A can neutralize several HCV isolates. AR3, as well as the overlapping AS434, and the nearby AS412, cluster on an E2 antigenic surface collectively known as the E2 neutralizing face.12 Antibodies to AR3 (e.g., AR3A) usually exhibit broadly neutralizing activity against diverse HCV genotypes. AR4 and AR5 are present only on the E1E2 complex and are adjacent to each other. MAbs AR4A and AR5A also mediated cross-neutralization.
An overall similar set of immune epitopes were recognized by humans and NHPs (Figure 3A). In both cases, the most dominant continuous epitopes were mapped to the E2 HVR1 AS396 (100% responders for both humans and NHPs), followed by the E1 N-terminus AS192 (88% human and 100% NHP responders) and AS210 (69% human and 75% NHP responders). Antibody responses to the conserved AS412 and the E2 front layer AS434 were observed for 35% and 27% humans versus 25% and 75% NHPs, respectively. Although the polyclonal antibodies had less reactivity toward the discontinues epitopes on the AR1, AR2 and AR4, most human and NHP neutralizers competed with mAbs AR3A and AR5A, and blocked CD81 binding to E1E2. Compared to non-neutralizers, the neutralizers exhibited more frequent and stronger reactivities toward epitopes on AR3, AR5, the E2 variable region 2 (VR2) AS462 and stalk region AS654 (Figure 3B–D), indicating that the autologous nAbs likely targeted these regions rather than the well described immune decoy HVR1.32–34
Figure 3.
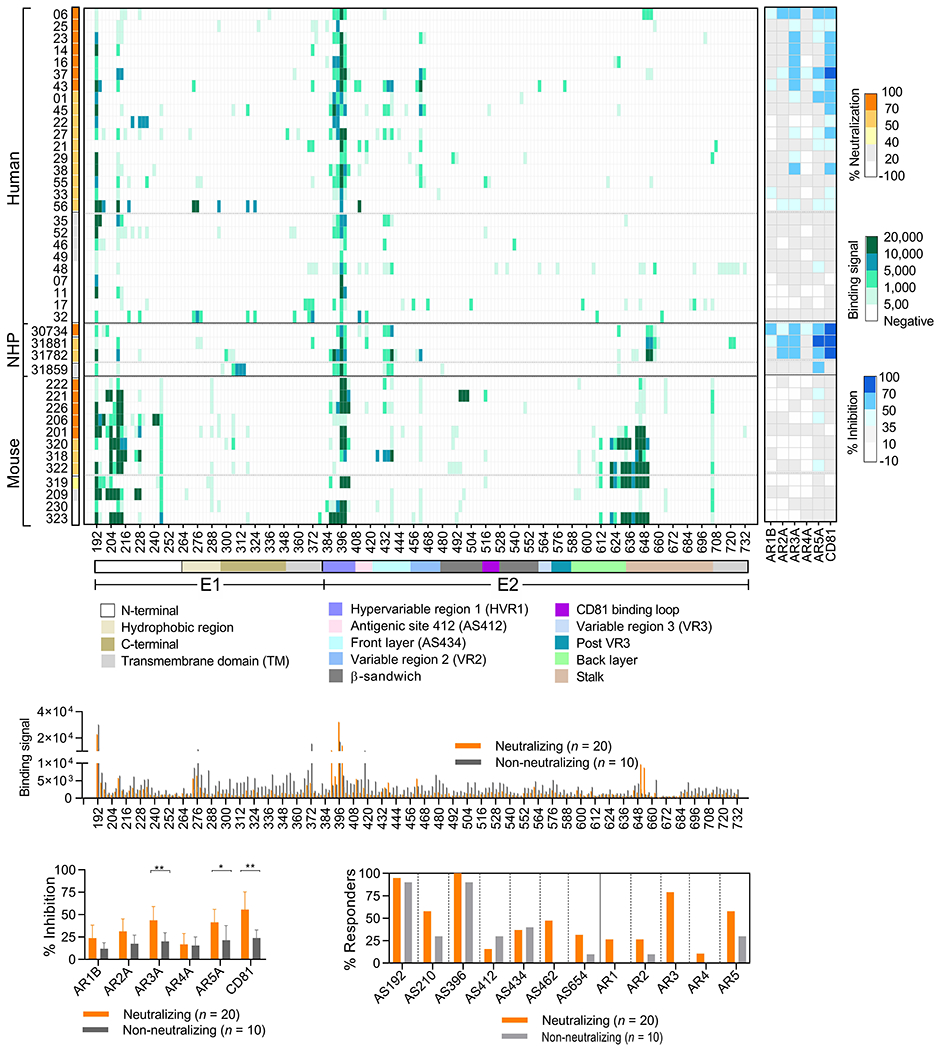
Epitope specificities of serum antibody responses. Selected neutralizing and non-neutralizing samples from human (week 52, n = 26), NHP (week 27 or 30, n = 4) and mouse (week 9, n = 12) were analyzed for specificity to continuous and discontinuous epitopes at 1:50. (A) Left, mapping of continuous epitopes by pepscan analysis. Neutralizing activity against HCVpp-HCV-1 of each sample is shown on the left. Right, mapping of discontinuous epitopes by antibody competition analysis. (B-D) Comparison of specificities of neutralizing (orange) and non-neutralizing (grey) responses in human and NHP samples. B, Binding to HCV-1 E1E2 peptides; C, Competition of binding to HCV-1 E1E2 by mAbs targeting AR1-5 and soluble large external loop of CD81. P values were calculated by two-tailed Mann-Whitney tests. *, P < .05. **, P < .001. D, Immunodominant epitopes targeted by neutralizers and non-neutralizers.
In the mouse model, the immunodominant epitopes were directed against the E1 N-terminus AS246, AS192, AS210 and AS204 (83-100% responders), E2 HVR1 AS396 (83% responders), as well as the stalk region AS645 and AS705 (100% responders) (Figure 3A, left). A few mice developed a weak response to AR5, but none competed strongly with mAb AR3A (Figure 3A, right). Collectively, E1E2 immunization elicited antibody responses primarily targeting the E1 N-terminus and E2 HVR1. Most human and NHP neutralizers, but not mice, generated a detectable AR3-specific antibody response.
Serum neutralization is more related to AR3- and AR5- instead of HVR1-specific antibody responses
To map the neutralizing epitopes, we carried out correlation analysis between neutralization and specificity of antibody responses to HVR1, AR3 and AR5 in humans and mice. NHP samples were not analyzed here due to the small number of animals studied. A weak correlation between autologous neutralization and HVR1-binding antibodies was observed in humans (r = 0.39, P = .048), but not in mice (r = 0.26, P = .4145) (Figure 4 A and B). However, the correlations between neutralization (both autologous and heterologous) and antibody responses to AR3 or AR5 in humans were significantly higher (r ≥ 0.68, P ≤ .0002, Figure 4A).
Figure 4.
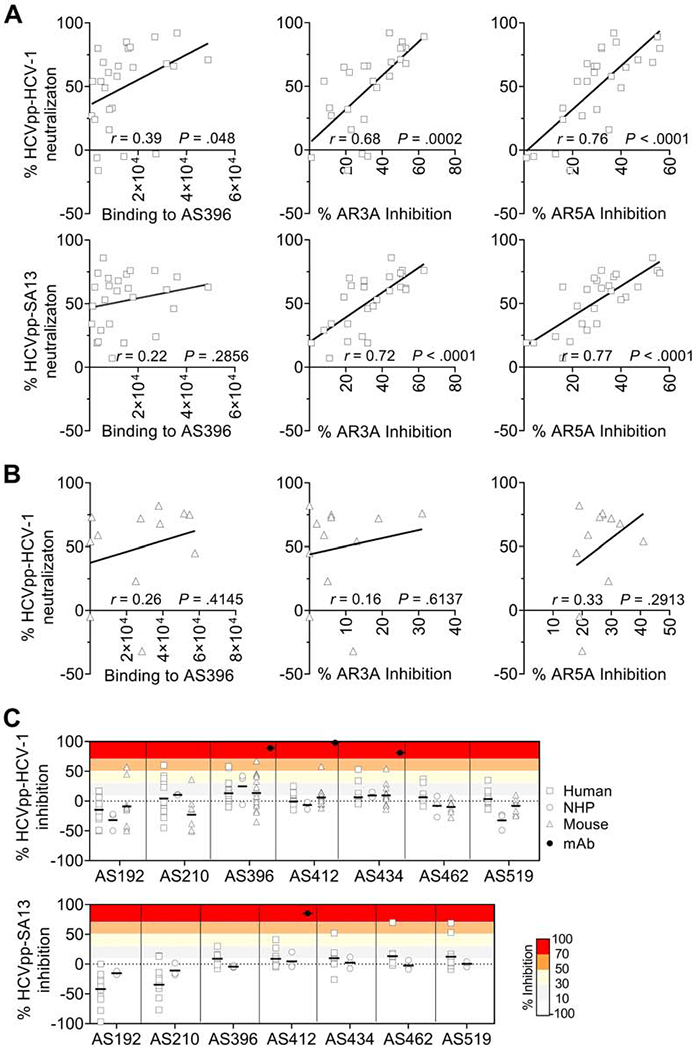
Neutralizing epitopes targeted by human, NHP and mouse immune sera. (A and B) Correlation between neutralization and antibody responses to HVR1, AR3 and AR5. A, Human antibody response. B, Mouse antibody response. P values were calculated using a two-tailed Pearson correlation. (C) Inhibition of neutralization against HCV-1 (genotype 1a) and SA13 (genotype 5a) by peptides corresponding to known continuous epitopes. See supplementary Table 5 for further information of the peptides.
Next, we performed virus neutralization with the neutralizing samples pre-complexed with a saturating concentration of peptides (Supplementary Table 5) to investigate nAb specificity (Figure 4C). The antigenic sites studied included the E1 N-terminus (AS192 and AS210), E2 HVR1 (AS396), AS412, front layer (AS434), VR2 (AS462) and CD81 binding loop (AS519). A few samples exhibited partial inhibition by peptide AS396, AS434, AS412, AS192 or AS210 in HCVpp-HCV-1, and by AS434, AS462 or AS519 in HCVpp-SA13. However, for most samples the presence of antibody-blocking peptides had a negligible impact on neutralizing activity. These data confirmed that, despite being immunodominant, HVR1-specific antibodies elicited by the recombinant E1E2 antigen were not the main factor responsible for autologous neutralization. Taken together, immunization of humans and the two animal models with E1E2 elicited dominant antibody responses against the E1 N-terminus and E2 HVR1 but they appeared to be mostly non-neutralizing.
Kinetics of antigen-specific B cell responses to E1E2 immunization
Given that immune cells are not available from the clinical trial, we investigated the antigen-specific B cell responses in the NHPs after the 4th immunization. With ELISPOT, we observed a rapid and robust antibody-secreting cell (ASC) response that peaked on day 4 and contracted rapidly on day 7 (Figure 5A). 70% of these cells were E1E2-specific, with IgG being the predominant isotype, followed by IgA and IgM. Similar dynamics of plasmablasts (CD3−CD20−CD80+HLA-DR+) were detected using flow cytometric analysis (Figure 5B, left). The responses are in line with previous findings from NHP studies35 and earlier than human ASC/plasmablast responses to influenza, which peaked around 7 days post vaccination.36,37
Figure 5.
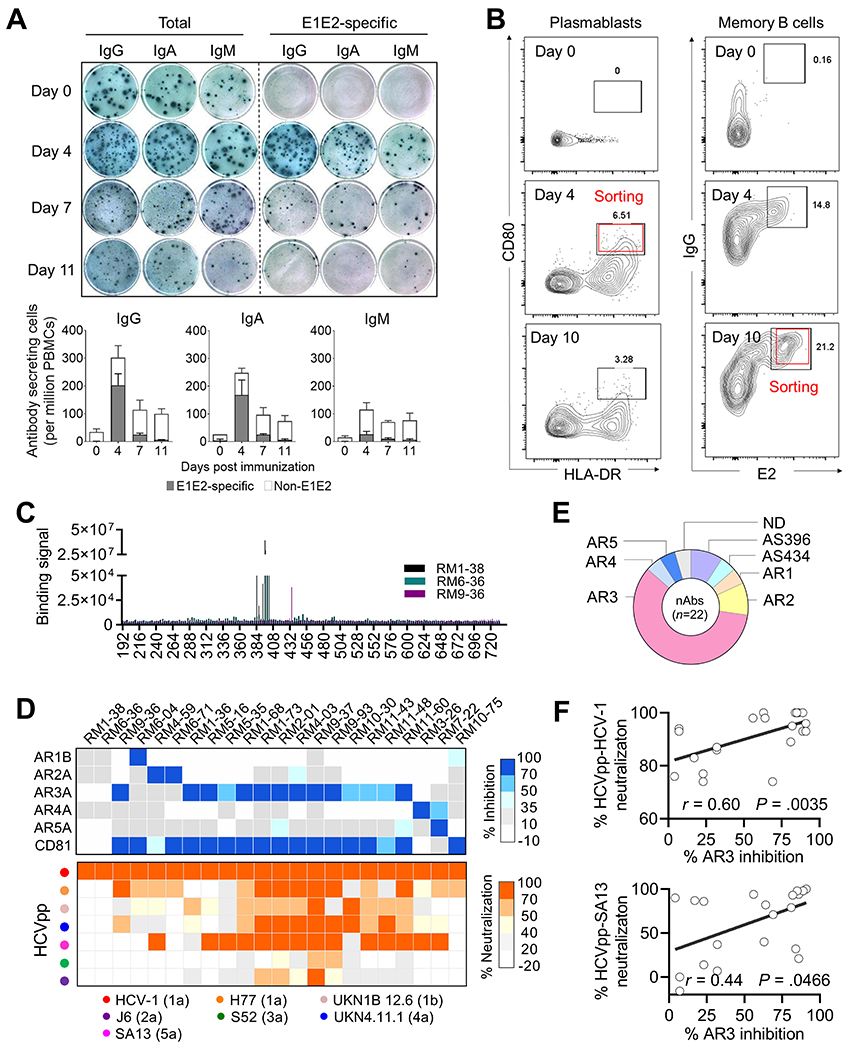
Characterization of NHP B cell responses. (A and B) Kinetics of antigen-specific B responses in NHPs following E1E2 immunization. A, Total and E1E2-specific antibody-secreting cell (ASC) responses measured by ELISPOT assay. The wells shown were plated with 5 × 105 PBMCs. Percentage of E1E2-specific ASCs for each antibody isotype is shown in the bar charts. B, Flow cytometry analysis of plasmablast (CD3−CD20−CD80+HLA-DR+) and E2-specific IgG+ memory B cell responses (CD3−CD20+CD27+IgG+ E2+). Cells from red boxes were sorted for generation of mAbs. (C-F) Characterization of neutralizing mAbs. C, Specificity of mAbs targeting the continuous epitopes measured by pepscan. D, Upper, Specificity of mAbs to discontinuous epitopes measured by antibody competition analysis. Lower, neutralization breadth and potency of mAbs using HCVpp. E, Distribution of nAb specificities. N.D. not defined. F, Correlation between autologous (HCV-1) or heterologous (SA13) neutralization and antibody response to AR3 (two-tailed Pearson correlation).
A rapid expansion of antigen-specific IgG+ memory B cells (CD3−CD20+CD27+IgG+ E2+) were also observed on day 4 and accounted for ~10% of total IgG+ memory B cells in circulation. The frequency reached approximately to 20% on day 10 post boost (Figure 5B, right). These findings indicate the optimal time points for cloning antibodies from plasmablasts and memory B cells from the immunized NHPs.
Identification and characterization of mAbs from NHPs
Ninety-three E1E2-reactive mAbs were isolated from single plasmablasts and memory B cells from NHPs 30734 and 31881. Of these, only 30% displayed autologous neutralizing activity in the preliminary screening. Using purified IgG, we determined the specificity and function of 22 neutralizing mAbs. Most nAbs (59%) targeted AR3, while the others targeted AR5, HVR1, AR2, AR4 or AS434 (Figure 5 C–E). Heterologous neutralization against SA13 (genotype 5a), H77 (genotype 1a), UKN4 11.1 (genotype 4a) and/or J6 (genotype 2a) were detected for all the nAbs (except for those targeting HVR1), albeit at relatively low potency (Figure 5D). Consistent with the plasma analysis data above, a moderate correlation was observed between autologous or heterologous neutralization and antibody response to AR3 (Figure 5F).
To explore genetic features underlying the nAbs, we investigated the heavy chain and light chain germline gene usage, complementarity-determining region (CDR) 3 length and somatic hypermutation (SHM) rate of the antibodies. Like human AR3-specific bnAbs, which are predominantly derived from an immunoglobulin heavy chain variable gene VH1-69 (mainly VH1-69*01 and VH1-69*06 alleles),30,38–42 most (90%) NHP AR3-specific cross-nAbs identified in this study were encoded by VH1.36 (Figure 6A), a rhesus VH gene that shares 90% amino acid identity and similar genetic features (e.g. the hydrophobic tip of CDRH2) with the human VH1-69 (Figure 6B). By contrast, the closest murine VH gene is VH1-81*01 which differs from human VH1-69 by 33% amino acid identity, and murine VH1-69 gene (VH1-69*01 and VH1-69*02) displays an even higher divergence (33-34%, Figure 6B). These data suggest that the NHP VH1.36 gene is highly homologous to human VH1-69 gene both genetically and functionally in response to HCV vaccination. Of the VH1.36 cross-neutralizing mAbs, RM2-01 and RM9-93 belong to the same clonal lineage, based on the V and J gene usage, CDR3 length and CDR3 nucleotide sequence identity (Figure 6A). In addition to the biased usage of VH gene, the NHP cross-nAbs tend to carry a longer CDRH3 loop but similar levels of SHM compared to strain-specific nAbs or nAbs with limited breadth (Figure 6C).
Figure 6.
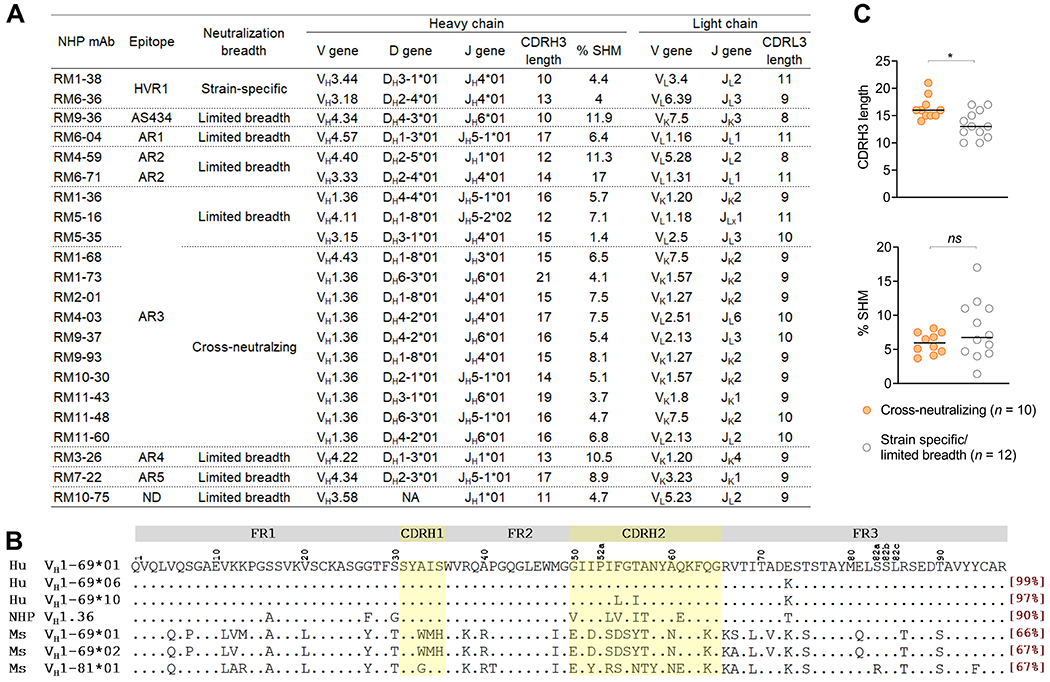
Summary of NHP neutralizing mAbs. (A) Specificity, function and genetic features of NHP neutralizing mAbs. Clonal lineages were assigned based on the following criteria: 1) matching of V and J gene usage, 2) identical CDR3 length, and 3) CDR3 nucleotide sequence homology >80%. CDR, complementarity-determining region. ND, not defined. NA, not available. CDR3 length is based on Kabat numbering, in which the CDRH3 is 2 amino acids shorter than in the IMGT definition. (B) Alignment of amino acid sequences of human VH1-69 gene and its homologues in NHPs and mice. Human VH1-69 sequences were exemplified by VH1-69*01 and *06, the most frequently used alleles by human HCV AR3-specific antibodies, and VH1-69*10, the closest human corresponding gene of to NHP VH1.36. The amino acid identity of each germline gene/allele compared to human VH1-69*01 allele is square-bracketed in red. FR, heavy chain framework. (C) Comparison of the CDRH3 length and SHM rate between cross-nAbs and strain-specific or limited breadth nAbs. P values were calculated by two-tailed Mann-Whitney test. *P < .05, ns, not significant.
Discussion
Overall, this comparative study provides important insights into antibody responses elicited by an E1E2-based subunit vaccine candidate. We demonstrated that all subjects developed an anti-E1E2 antibody response following immunization, but most antibodies induced are directed against epitopes that have no or low antiviral function (e.g. the E1 N-terminus). Surprisingly, serum antibodies to HVR1 are mostly non-neutralizing (Figure 4), implying that, instead of forming a flexible, exposed loop on the virus, the highly immunogenic HVR1 appears to be mostly inaccessible to nAbs, either by packing tightly on the viral surface, adopting a conformation not recognizable by most of the anti-HVR1 antibodies, or both. HVR1 has recently been reported to work in concert with N-glycans on E2 for antibody escape.34,43 In contrast, neutralizing antibody responses to the known conserved antigenic sites, e.g. AS412, AS434 and AR3, were relatively rare (Figure 3), indicating the intrinstically low immunogenicity of these regions, which could in part explain the failure of induction of sufficient bnAbs by this vaccine candidate. The failure may also be caused by the presence of immunodominant regions outcompeting conserved neutralizing epitopes on the same antigen to elicit bnAb response.34, 44 Future HCV vaccine development should include rational antigen design to direct the antibody responses from non-neutralizing immunodominant regions to conserved neutralizing epitopes.
In humans and NHPs, E1E2 immunization elicited nAbs predominantly focused on AR3. At the polyclonal level, the antibody responses are mostly against the autologous HCV-1 (1a) and heterologous SA13 (5a) isolates. At the monoclonal level, several AR3-specific mAbs also exhibited neutralization against a broad spectrum of heterologous strains H77 (1a), UKN1B 12.6 (1b), UKN4.11.1 (4a), and even J6 (2a) and S52 (3a), albeit at relatively low potency. These findings implicate that cross-nAbs targeting AR3 were able to be elicited by E1E2 immunization, but their levels in blood were insufficient for broad neutralization. We did not observe a strong AR3-specific antibody response in mice, although non-nAbs to E2 front layer and CD81 binding loop regions were isolated previously.20 Strikingly, of the isolated NHP mAbs, the majority of cross-nAbs were derived from rhesus macaque VH1.36 gene, the closest macaque orthologue to the human VH1-69 gene (90% homology). Such a preferential usage of a specific VH gene has previously been observed in antiviral antibody responses against HIV-1 and influenza.42 These antibodies share germline-encoded genetic and structural features to recognize the same antigenic site, and often develop broadly neutralizing activity through a similar pathway. Understanding the developmental pathways and structural biology of these antibodies will faciliate the B cell ontogeny vaccine strategy to elicit bnAbs.45
Chimpanzee is the best animal model for studies of HCV infection and vaccine development because of its ability to support persistent HCV infection and its close genetic relationship to humans.46,47 Immunization of chimpanzees with E1E2 has previously been shown to elicit nAbs and protective immunity in the animals.5,15,48 Intriguingly, chimpanzees immunized with HCV-like particles consisting of the viral core, E1 and E2 failed to generate antibodies to E1 and E2.7 This raises an important question regarding E1E2 immunogenicity when the antigens are presented on different platforms. With the moratorium on chimpanzee research, use of this animal model is no longer feasible.49 In this study, we demonstrate that the NHP rhesus macaque model produced nAbs sharing several important features with human nAbs, and thus, is potentially a useful preclinical model for studying HCV vaccine candidates designed to elicit nAbs. In addition, given the scarcity of human samples from the clinical trial, the NHP immune samples from this study may serve as a substitute of E1E2-immune sera for future vaccine studies.
A rational approach for HCV vaccine development is to design immunogens that focus the antibody responses on conserved neutralizing epitopes. Several studies have shown that AR3-targeting antibodies are relevant in natural clearance and protection against re-infection in HCV infected individuals.40,41,50 However, as shown here the subunit vaccine based on an authentic recombinant E1E2 complex elicited only weak to moderate nAb response in a fraction of the subjects. A broadly effective vaccine would require the production of potent and broad nAbs, particularly those targeting AR3. There are several technical challenges for such endeavor. First, the HCV E2 neutralizing face containing AR3 appears to be conformationally flexible on recombinant E2 antigens,51 potentially limiting the ability of the antigens to properly present the neutralizing epitopes to the immune system in vaccination. Second, as evidenced in this and other studies, not all binding antibodies to AR3 are broadly neutralizing (Figures 2 and 6).41, 52 This can be caused partly by E2 flexibility and genetic diversity within this conserved region (Supplementary Figure 2).53–56 A better understanding of the subtle difference between nAb-sensitive and nAb-resistant HCV isolates will be crucial in the development of a broad effective HCV vaccine. This study provides novel information on the antibody responses of humans, NHPs and mice immunized with the same E1E2 vaccine candidate. In addition to a general description of the antigen binding and virus neutralizing activities of the antibody responses, we have extensively mapped the antibody specificities and identified similarities and differences in the antibody responses between the different in vivo models to understand the virus neutralizing activities. The data offer a useful reference and baseline for studying nAb production and epitope specificity of future envelope glycoprotein-based vaccine antigens, particularly the development of epitope-focused vaccine designed to elicit bnAbs targeting conserved E1E2 neutralizing epitopes. We are currently studying the genetic and structural properties of the NHP neutralizing mAbs to understand how bnAbs are generated. The results will inform whether human and NHP use similar structural features to neutralize HCV. Nevertheless, here we demonstrated that AR3-targeting antibodies can be readily elicited in human and NHP immunization studies.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT:
Studies are needed of antibody responses to hepatitis C virus (HCV) antigens E1 and E2 in animal models and clinical trials.
NEW FINDINGS:
The authors compared antibody responses among volunteers, non-human primates (NHPs), and mice given the Chiron HCV vaccine candidate E1E2. These antigens induced high antibody titers, but were insufficient to neutralize diverse HCV isolates. The immunized NHPs used a similar antibody gene found in humans to produce cross-neutralizing antibodies.
LIMITATIONS:
B cells from participants in the phase 1 trial were unavailable for immunogenetic comparison with B cells from the NHPs.
IMPACT:
NHPs can be used as a model for development of HCV vaccines. These findings provide useful baseline values for development of vaccines designed to elicit neutralizing antibodies.
Acknowledgements:
We thank Rajen Koshy for help with clinical sample coordination, Philip Dormitzer, Timothy Pepini and Novartis Vaccines and Diagnostics for recombinant E1E2 antigen, Emily Carrow and Advanced Bioadjuvants for Adjuplex adjuvant, and Normal Blood Donor Service at The Scripps Research Institute for procurement of normal blood samples. This is manuscript 29802 from The Scripps Research Institute.
Grant support:
This work was funded by NIH Grants AI079031, Al106005 and AI123861. This investigation used resources that were supported by the Southwest National Primate Research Center (SNPRC) grant P51 OD011133 from the Office of Research Infrastructure Programs, NIH.
Abbreviations:
- AR
antigenic region
- AS
antigen site
- ASC
antibody-secreting cell
- bnAb
broadly neutralizing antibody
- CDRH
heavy chain complementarity-determining region
- HCV
hepatitis C virus
- HCVcc
HCV cell culture
- HCVpp
HCV pseudoparticles
- HVR1
hypervariable region 1
- mAb
monoclonal antibody
- nAb
neutralizing antibody
- NHP
non-human primate
- PBMC
peripheral blood mononuclear cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Conflict of interest statement: The authors have declared that no conflict of interest exists.
References
- 1.Li DK, Chung RT. Overview of direct-acting antiviral drugs and drug resistance of hepatitis C virus. Methods Mol Biol 2019;1911:3–32. [DOI] [PubMed] [Google Scholar]
- 2.Walker CM, Grakoui A. Hepatitis C virus: why do we need a vaccine to prevent a curable persistent infection? Curr Opin Immunol 2015;35:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman ZT, Cox AL. Lessons from nature: Understanding immunity to HCV to guide vaccine design. PLoS Pathog 2016;12:e1005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med 2013;19:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A 1994;91:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmowalid GA, Qiao M, Jeong SH, et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A 2007;104:8427–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folgori A, Capone S, Ruggeri L, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med 2006;12:190–197. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Shin EC, Capone S, et al. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology 2012;143:1048–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn JW, Hu YW, Tricoche N, et al. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol 2008;82:10896–10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law JL, Chen C, Wong J, et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One 2013;8:e59776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swadling L, Capone S, Antrobus RD, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med 2014;6:261ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzarum N, Wilson IA, Law M. The neutralizing face of hepatitis C virus E2 envelope glycoprotein. Front Immunol 2018;9:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinchen VJ, Cox AL, Bailey JR. Can broadly neutralizing monoclonal antibodies lead to a hepatitis C virus vaccine? Trends Microbiol 2018;26:854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamataki Z, Coates S, Evans MJ, et al. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine 2007;25:7773–7784. [DOI] [PubMed] [Google Scholar]
- 15.Meunier JC, Gottwein JM, Houghton M, et al. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis 2011. ;204:1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey SE, Houghton M, Coates S, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010;28:6367–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray R, Meyer K, Banerjee A, et al. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J Infect Dis 2010;202:862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer K, Banerjee A, Frey SE, et al. A weak neutralizing antibody response to hepatitis C virus envelope glycoprotein enhances virus infection. PLoS One 2011;6:e23699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachko A, Frey SE, Sirota L, et al. Antibodies to an interfering epitope in hepatitis C virus E2 can mask vaccine-induced neutralizing activity. Hepatology 2015;62:1670–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruwona TB, Giang E, Nieusma T, et al. Fine mapping of murine antibody responses to immunization with a novel soluble form of hepatitis C virus envelope glycoprotein complex. J Virol 2014;88:10459–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J, Gastaminza P, Cheng G, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 2005;102:9294–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith K, Garman L, Wrammert J, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc 2009;4:372–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundling C, Phad G, Douagi I, et al. Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J Immunol Methods 2012;386:85–93. [DOI] [PubMed] [Google Scholar]
- 24.Tiller T, Meffre E, Yurasov S, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 2008;329:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabro S, Tritto E, Pezzotti A, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013;31:3363–3369. [DOI] [PubMed] [Google Scholar]
- 26.Sastry M, Zhang B, Chen M, et al. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One 2017;12:e0186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T, Doria-Rose NA, Cheng C, et al. Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell Rep 2017;19:719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velazquez-Moctezuma R, Galli A, Law M, et al. Hepatitis C virus-escape studies for human monoclonal antibody AR4A reveal isolate-specific resistance and a high barrier to resistance. J Infect Dis 2019;219:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law M, Maruyama T, Lewis J, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 2008;14:25–27. [DOI] [PubMed] [Google Scholar]
- 30.Giang E, Dorner M, Prentoe JC, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 2012;109:6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopal R, Jackson K, Tzarum N, et al. Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis. PLoS Pathog 2017;13:e1006735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prentoe J, Velazquez-Moctezuma R, Foung SK, et al. Hypervariable region 1 shielding of hepatitis C virus is a main contributor to genotypic differences in neutralization sensitivity. Hepatology 2016;64:1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velazquez-Moctezuma R, Law M, Bukh J, et al. Applying antibody-sensitive hypervariable region 1-deleted hepatitis C virus to the study of escape pathways of neutralizing human monoclonal antibody AR5A. PLoS Pathog 2017;13:e1006214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentoe J, Bukh J. Hypervariable region 1 in envelope protein 2 of hepatitis C virus: A linchpin in neutralizing antibody evasion and viral entry. Front Immunol 2018;9:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira EL, Kasturi SP, Kovalenkov Y, et al. Vaccine-induced plasmablast responses in rhesus macaques: phenotypic characterization and a source for generating antigen-specific monoclonal antibodies. J Immunol Methods 2015;416:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008;453:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012;109:9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keck ZY, Saha A, Xia J, et al. Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J Virol 2011;85:10451–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keck ZY, Wang Y, Lau P, et al. Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice. Hepatology 2016;64:1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merat SJ, Molenkamp R, Wagner K, et al. Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS One 2016;11:e0165047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey JR, Flyak AI, Cohen VJ, et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017;2:e92872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen F, Tzarum N, Wilson IA, et al. VH1-69 antiviral broadly neutralizing antibodies: genetics, structures, and relevance to rational vaccine design. Curr Opin Virol 2019;34:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prentoe J, Velazquez-Moctezuma R, Augestad EH, et al. Hypervariable region 1 and N-linked glycans of hepatitis C regulate virion neutralization by modulating envelope conformations. Proc Natl Acad Sci U S A 2019;116:10039–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farci P, Shimoda A, Coiana A, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 2000;288:339–344. [DOI] [PubMed] [Google Scholar]
- 45.Kwong PD, Mascola JR. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 2018;48:855–871. [DOI] [PubMed] [Google Scholar]
- 46.Bukh J. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 2012;142:1279–1287. [DOI] [PubMed] [Google Scholar]
- 47.Burm R, Collignon L, Mesalam AA, et al. Animal models to study hepatitis C virus infection. Front Immunol 2018;9:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev 2011;239:99–108. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser J. Biomedical research. An end to U.S. chimp research. Science 2015;350:1013. [DOI] [PubMed] [Google Scholar]
- 50.Merat SJ, Bru C, van de Berg D, et al. Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J Hepatol 2019;71:14–24. [DOI] [PubMed] [Google Scholar]
- 51.Kong L, Lee DE, Kadam RU, et al. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proc Natl Acad Sci U S A 2016;113:12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinchen VJ, Zahid MN, Flyak AI, et al. Broadly neutralizing antibody mediated clearance of human hepatitis C virus infection. Cell Host Microbe 2018;24:717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarr AW, Urbanowicz RA, Hamed MR, et al. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol 2011. ;85:4246–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbanowicz RA, McClure CP, Brown RJ, et al. A diverse panel of hepatitis C virus glycoproteins for use in vaccine research reveals extremes of monoclonal antibody neutralization resistance. J Virol 2015;90:3288–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wasilewski LN, Ray SC, Bailey JR. Hepatitis C virus resistance to broadly neutralizing antibodies measured using replication-competent virus and pseudoparticles. J Gen Virol 2016;97:2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Diwany R, Cohen VJ, Mankowski MC, et al. Extra-epitopic hepatitis C virus polymorphisms confer resistance to broadly neutralizing antibodies by modulating binding to scavenger receptor B1. PLoS Pathog 2017;13:e1006235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


