Abstract
Background
Indeterminate pulmonary nodules (IPN) suspected for early stage lung cancer mandate accurate diagnosis. Both percutaneous needle biopsy (PCNB) and surgical biopsy (SB) are valuable options. The present study aimed to compare the efficacy and cost-effectiveness between PCNB and SB for IPN suspected for early stage lung cancer.
Methods
During January–November 2018, patients who underwent operation for IPN suspected for early stage lung cancer (SB group, n = 245) or operation after PCNB (PCNB group, n = 113) were included. Patient-level cost data were extracted from medical bills from the institution. Propensity score matching was performed between the two groups from a retrospectively-collected database.
Results
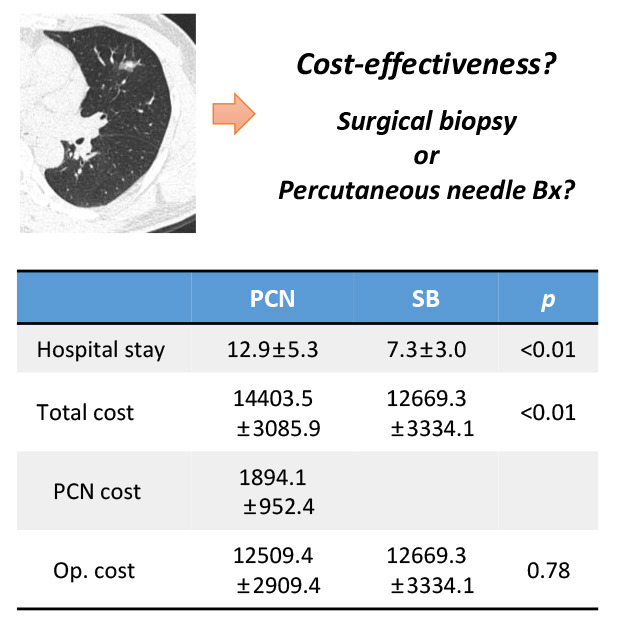
Fifteen patients (11.5%) had complications after PCNB; thirteen (11.5%) were not confirmed to have lung cancer through PCNB but underwent operation for IPN. In SB group, 172 (70.2%) and 7 (2.9%) patients underwent wedge resection and segmentectomy for SB, respectively; 66 patients (26.9%) underwent direct lobectomy without SB. After propensity score matching, 58 paired samples were produced. Most patients in PCNB group were admitted twice (n = 55, 94.8%). The average hospital stay was longer in PCNB group (12.9 ± 5.3 vs. 7.3 ± 3.0, P < 0.001). Though the cost of the operation was comparable (USD 12,509 ± 2,909 vs. 12,669 ± 3,334; P = 0.782), the total cost was higher for PCNB group (USD 14,403 ± 3,085 vs. 12,669 ± 3,334; P = 0.006). The average subcategory cost, which increases proportional to hospital stay, was higher in PCNB group, whereas the cost of operation and surgical materials were comparable between the two groups.
Conclusion
Lung cancer operation following SB for IPN was associated with lesser cost, shorter hospital stays, and lesser admission time than lung cancer operation after PCNB. The increased cost and longer hospital stay appear largely related to the admission for PCNB.
Keywords: Percutaneous Needle Biopsy, Surgical Biopsy, Lung Cancer, Cost-effectiveness
Graphical Abstract
INTRODUCTION
With the recent technological advances and widespread use of computed tomography (CT) in clinical settings, indeterminate pulmonary nodules (IPN), especially ground-glass opacity nodules (GGN), have become increasingly detectable. Reportedly, up to 40% of IPNs are malignant, particularly in high-risk populations.1,2,3 Moreover, persistent partly solid GGNs have a much higher probability of malignancy than solid IPNs do,4,5 especially when the partly solid GGNs have a solid component larger than 5 mm.6 Thus, one of the most important steps in managing IPN is to determine the probability of the IPN being malignant and decide the management plan for the lesion.
As the detection rate of IPNs has increased recently, several guidelines regarding the management of IPNs have been published and updated during the last two decades.7,8,9 The most current guidelines recommend nonsurgical and/or surgical biopsy (SB) for IPNs with a high probability of malignancy. Percutaneous needle biopsy (PCNB) is a traditional method for pathological confirmation of IPN and is still considered the first choice in several medical institutions.10 Nonetheless, SB followed by curative lung cancer operation has been one of the most valuable options for management of IPNs. This is because of the significantly decreased morbidity rate through adoption of minimally invasive thoracic surgery and improved diagnostic accuracy of CT-based assessment of IPN.6,7,8,9,11,12
Given the worsening financial challenges in the medical field, the cost-effectiveness and efficacy of diagnosis and treatment procedures are the focus of increasing interest. Investigating the economic effect of procedures is important for helping patients as well as doctors in choosing appropriate treatment and/or diagnostic procedures and for providing necessary information for framing health policies and resource allocation. Several previous studies have investigated the diagnostic accuracy and peri-procedural or peri-operative outcome of PCNB and SB. However, studies comparing the cost-effectiveness between the two methods for diagnosis and treatment of IPN suspected for early stage lung cancer are rare.
This study aimed to compare the efficacy and cost-effectiveness of lung cancer operation after PCNB and SB for IPNs suspected for early stage lung cancer. Propensity-score matched analysis was performed to minimize selection bias and account for heterogeneity between the groups.
METHODS
Patients
A retrospective review of the prospectively collected database was performed for 622 patients who had undergone lung resection operation for pathologically-confirmed lung cancer or a pulmonary nodule or mass suspected for lung cancer between January and November 2018 in our institution, Seoul National University Hospital. We excluded patients who had undergone PCNB at another hospital (n = 61), transbronchial biopsy for pulmonary nodules (n = 31), robotic lung operation (n = 22), combined operation for other diseases with lung resection operation (n = 9), PCNB during treatment for other diseases (n = 8), other previous treatment for lung cancer (n = 2), and patients who had lymph node metastasis and required an extensive lung operation (n = 32). We also excluded 9 patients who did not have available cost data for analysis. Among them, we included the patients who had IPN equal to or less than 30 mm in preoperative CT. As a result, 358 patients were included in the analysis: 113 patients in the PCNB group and 245 patients in the SB group. PCNB group is defined as patients who underwent lung cancer surgery after PCNB for IPN and SB group is defined as patients who underwent SB followed by lung cancer surgery for IPN without PCNB.
Cost data acquisition
The cost based on patient-level data was collected, and the average cost of each group was calculated. The medical cost data were extracted from the medical bills from our institution. The medical bills were classified into the Korea National Health Insurance covered payment and Korea National Health Insurance non-covered payment on specific items, such as physician service, hospitalization, injection, medication, anesthesia, procedure and operation, imaging studies, and laboratory test. We excluded the cost of imaging studies for lung cancer staging work up, such as brain magnetic resonance imaging and 18-Fluoro-deoxyglucose-positron emission tomography, as those studies were not performed identically in all the patients. We compared the total amount of Korea National Health Insurance covered and Korea National Health Insurance non-covered medical costs for the analysis.
Propensity score matching
For propensity score matching, we excluded 66 patients from the SB group who had undergone direct lobectomy without SB for various reasons. As these patients underwent direct lung cancer surgery without biopsy, we excluded them as the purpose of this study was to compare two different biopsy methods for IPN suspected for early stage lung cancer. Propensity scores were calculated using logistic regression modeling, including the following variables that were considered as determinant factors in selecting the methods for tissue diagnosis and operative strategies: age, sex, tumor size on CT, consolidation/tumor ratio, clinical T stage, history of redo-operation, and extent of the operation. We matched propensity scores one-to-one using the nearest neighbor methods without replacement, using a 0.15 caliper width. After the matching procedure, 58 patients were selected from each group for the analysis.
Statistical analysis
All statistical analyses were performed using the R software package, version 3.5.0 (http://www.R-project.org). Student's t-test and Wilcoxon rank-sum test were used to compare continuous variables, and χ2 test and Fischer's exact test were used to compare nominal variables for unmatched data. McNemar test and paired t-test were used for comparison between matched data. All statistical analyses were performed using the two-sided method. P values less than 0.05 were considered statistically significant.
Ethics statement
The study protocol was reviewed by the Institutional Review Board of Seoul National University Hospital and was approved as a minimal-risk retrospective study (approval No. H-1902-044-1008) that did not require individual informed consent according to the institutional guidelines for consent waivers.
RESULTS
Baseline characteristics
The demographic and clinical characteristics of the study population are summarized in Table 1. The PCNB group included the patients with a significantly large-sized tumor, high consolidation/tumor ratio, and advanced clinical T stage. The differences of baseline and clinical characteristics between the two groups were adjusted after the propensity score matching process. Fifteen patients (13.3%) developed complications after PCNB: multiple PCNBs for IPNs (n = 5), hemoptysis or pulmonary hemorrhage (n = 5), pneumothorax requiring supportive care (n = 5) and intervention (n = 3). Lung cancer was confirmed in 100 patients (88.5%) and 13 patients (11.5%) were not confirmed to have lung cancer but they underwent lung resection operation as the IPN was strongly suspected for early stage lung cancer.
Table 1. Demographic and clinical characteristics of the study population.
| Variables | Unmatched groups | Matched groups | |||||
|---|---|---|---|---|---|---|---|
| PCNB group (n = 113) | SB group (n = 245) | P value | PCNB group (n = 58) | SB group (n = 58) | P value | ||
| Age, yr | 65.6 ± 10.0 | 62.9 ± 10.1 | 0.019 | 64.7 ± 10.3 | 65.0 ± 8.7 | 0.833 | |
| Sex, male | 46 (40.7) | 96 (39.2) | 0.784 | 19 (32.8) | 18 (31.0) | 0.827 | |
| Smoking | 0.974 | 0.722 | |||||
| Never | 77 (68.1) | 168 (68.6) | 43 (74.1) | 45 (77.6) | |||
| Ex | 28 (24.8) | 60 (24.5) | 13 (22.4) | 10 (17.2) | |||
| Current | 8 (7.1) | 15 (6.1) | 2 (3.5) | 3 (5.2) | |||
| FEV1, % | 106.8 ± 17.6 | 108.2 ± 16.9 | 0.485 | 107.0 ± 19.1 | 108.3 ± 14.9 | 0.685 | |
| DLCO, % | 92.0 ± 16.2 | 94.8 ± 17.7 | 0.153 | 91.4 ± 15.3 | 93.7 ± 15.3 | 0.546 | |
| Size of tumor, mm | 20.0 ± 6.0 | 17.2 ± 6.3 | < 0.001 | 17.9 ± 5.9 | 17.4 ± 6.4 | 0.580 | |
| C/T ratio | 0.95 ± 0.13 | 0.60 ± 0.33 | < 0.001 | 0.90 ± 0.17 | 0.89 ± 0.18 | 0.621 | |
| Clinical T stage | < 0.001 | 0.706 | |||||
| cT1 | 93 (82.3) | 230 (93.9) | 52 (89.7) | 53 (91.4) | |||
| cT2 | 18 (15.9) | 15 (6.1) | 6 (10.3) | 5 (8.6) | |||
| cT3 | 2 (1.8) | 0 | 0 | 0 | |||
| Clinical N stage | NA | NA | |||||
| cN0 | 113 (100.0) | 245 (100.0) | 58 (100.0) | 58 (100.0) | |||
Data are presented as mean ± standard deviation or number (%).
PCNB = percutaneous needle biopsy, SB = surgical biopsy, FEV1 = forced expiratory volume in one second, DLCO = diffusing capacity of carbon monoxide, C/T ratio = consolidation/tumor ratio.
Operative and pathologic detail
In the SB group, 172 (70.2%) and 7 patients (2.9%) underwent wedge resection and segmentectomy for SB, respectively. Sixty-six patients (26.9%) underwent direct lobectomy for the IPN without SB. The proportion of patients who underwent limited resection was significantly higher in the SB group. Moreover, the SB group included more patients who underwent minimally invasive operation or redo-operation. Mediastinal lymph node dissection was performed more on patients in the PCNB group. After the matching process, the differences between the two groups were comparable. However, the number of cartridges used for the operation was higher in the SB group in the matched analysis (Table 2).
Table 2. Operative details.
| Variables | Unmatched groups | Matched groups | |||||
|---|---|---|---|---|---|---|---|
| PCNB group (n = 113) | SB group (n = 245) | P value | PCNB group (n = 58) | SB group (n = 58) | P value | ||
| Extent of operation | < 0.001 | 0.298 | |||||
| Wedge resection | 9 (8.0) | 62 (25.3) | 13 (22.4) | 9 (15.5) | |||
| Segmentectomy | 11 (9.7) | 48 (19.6) | 7 (12.1) | 6 (10.3) | |||
| Lobectomy | 93 (82.3) | 135 (55.1) | 38 (65.5) | 43 (74.1) | |||
| VATS | 105 (92.9) | 241 (98.4) | 0.022 | 57 (98.3) | 57 (98.3) | 1.000 | |
| Redo-operation | 1 (0.9) | 24 (9.8) | 0.001 | 1 (1.7) | 1 (1.7) | 1.000 | |
| Mediastinal LN dissection | 105 (92.9) | 193 (78.8) | < 0.001 | 48 (82.8) | 50 (86.2) | 0.593 | |
| No. of cartridges | 6.4 ± 2.1 | 7.5 ± 3.1 | < 0.001 | 6.3 ± 2.2 | 7.4 ± 3.0 | 0.030 | |
Data are presented as mean ± standard deviation or number (%).
PCNB = percutaneous needle biopsy, SB = surgical biopsy, VATS = video-assisted thoracic surgery, LN = lymph node.
Among 13 patients in the PCNB group who were not confirmed as having lung cancer by PCNB, 8 (61.5%) were diagnosed with cancer, and 5 (38.5%) were diagnosed with benign lesions. In the SB group, 19 patients (7.8%) were diagnosed with benign diseases after SB, and the other 226 patients (92.2%) were confirmed as having cancer. Most patients were diagnosed with lung adenocarcinoma in both groups (87.5% in the PCNB group, 90.2% in the SB group).
Hospital stays, admission, medical costs
The lengths of hospital stay for the operation were comparable between the two groups. However, the total length of hospital stays was significantly longer in the PCNB group, and this difference was mostly because of the hospital stays for PCNB (Table 3). The patients in the PCNB group were mostly admitted twice for PCNB and the operation (n = 55, 94.8%), whereas all patients in the SB group were admitted once for the operation.
Table 3. Length of stays and admission times.
| Variables | Unmatched groups | Matched groups | |||||
|---|---|---|---|---|---|---|---|
| PCNB group (n = 113) | SB group (n = 245) | P value | PCNB group (n = 58) | SB group (n = 58) | P value | ||
| Length of stay, day | 13.0 ± 4.6 | 6.9 ± 2.4 | < 0.001 | 12.9 ± 5.3 | 7.3 ± 3.0 | < 0.001 | |
| Admission for PCNB | 5.1 ± 2.0 | NA | NA | 4.8 ± 1.7 | NA | NA | |
| Admission for operation | 7.9 ± 4.2 | 6.9 ± 2.4 | 0.004 | 8.0 ± 5.1 | 7.3 ± 3.0 | 0.379 | |
| Admission | < 0.001 | < 0.001 | |||||
| Once | 6 (5.3) | 245 (100.0) | 3 (5.2) | 58 (100.0) | |||
| Twice | 107 (94.7) | 0 | 55 (94.8) | 0 | |||
Data are presented as mean ± standard deviation or number (%).
PCNB = percutaneous needle biopsy, SB = surgical biopsy, NA = not applicable.
The medical costs for the operation were comparable between the two groups. The total medical cost was significantly higher in the PCNB group, as the medical cost of PCNB was added to the medical cost of operation (Table 4). We analyzed the total medical cost between the two groups through subcategories. The medical costs related to the operation (costs for procedures and operation, surgical materials, and anesthesia) were comparable between the two groups. However, the medical costs which increase proportionally to the length of hospital stays (costs for laboratory test, injection, medication, hospitalization, doctor's fee, and others) were significantly higher in the PCNB group (Table 4).
Table 4. Comparison of the medical costs.
| Variables | Unmatched groups | Matched groups | |||||
|---|---|---|---|---|---|---|---|
| PCNB group (n = 113) | SB group (n = 245) | P value | PCNB group (n = 58) | SB group (n = 58) | P value | ||
| Cost attributable to PCNB, USD | 2,086.5 ± 1,220.6 | NA | NA | 1,894.1 ± 952.4 | NA | ||
| Cost attributable to operation, USD | 12,778.2 ± 2,817.4 | 12,380.7 ± 3,137.6 | 0.247 | 12,509.4 ± 2,909.4 | 12,669.3 ± 3,334.1 | 0.782 | |
| Total cost, USD | 14,864.6 ± 3,171.6 | 12,380.7 ± 3,137.6 | < 0.001 | 14,403.5 ± 3,085.9 | 12,669.3 ± 3,334.1 | 0.006 | |
| Laboratory test | 2,407.0 ± 893.7 | 1,265.2 ± 408.2 | < 0.001 | 2,226.6 ± 705.7 | 1,266.3 ± 392.4 | < 0.001 | |
| Surgical materials | 3,899.3 ± 823.9 | 4,176.3 ± 1,310.0 | 0.040 | 3,768.4 ± 864.6 | 4,110.5 ± 1,151.8 | 0.094 | |
| Procedures and surgery | 5,062.7 ± 1,145.7 | 4,283.2 ± 1,517.3 | < 0.001 | 4,814.8 ± 1,391.7 | 4,558.0 ± 1,654.6 | 0.328 | |
| Anesthesia | 722.0 ± 135.6 | 687.1 ± 128.0 | 0.019 | 699.8 ± 148.3 | 704.9 ± 135.9 | 0.843 | |
| Injection | 402.0 ± 107.3 | 353.2 ± 107.2 | < 0.001 | 405.8 ± 116.7 | 350.5 ± 61.9 | < 0.001 | |
| Medication | 145.5 ± 103.2 | 92.9 ± 76.1 | < 0.001 | 145.8 ± 104.8 | 93.1 ± 70.8 | 0.001 | |
| Hospitalization | 1,803.4 ± 1,115.5 | 1,215.9 ± 963.1 | < 0.001 | 1,778.0 ± 1,205.5 | 1,261.4 ± 1,072.8 | 0.024 | |
| Doctor's fee | 392.0 ± 161.5 | 214.1 ± 76.8 | < 0.001 | 401.8 ± 182.8 | 225.6 ± 102.3 | < 0.001 | |
| Others | 163.2 ± 92.2 | 94.0 ± 55.3 | < 0.001 | 162.5 ± 107.5 | 98.9 ± 61.5 | < 0.001 | |
Data are presented as mean ± standard deviation or number (%).
PCNB = percutaneous needle biopsy, SB = surgical biopsy, NA = not applicable, USD = the United States dollar.
DISCUSSION
According to the results of our study, SB is a more cost-effective strategy for diagnosis and treatment of IPN suspected for early stage lung cancer than PCNB. The total medical cost was significantly higher in the PCNB group because of the additional costs attributable to PCNB, whereas the cost of the operation was comparable between the two groups. The subcategory medical cost which increases proportionally to hospital stays were significantly higher in the PCNB group than in the SB group, whereas subcategory costs related to surgical procedures were comparable between the two groups. Conclusively, the difference of the cost between the two groups were mostly because of multiple admissions and longer hospital stays in the PCNB group. Though there have been many previous literature which reported the diagnostic accuracy of PCNB or SB for IPN suspected for lung cancer, our study is the first study that compared the cost-effectiveness of both procedures for the diagnosis and treatment of IPN.
Currently, both PCNB and SB are recommended for the diagnosis of IPN suspected for early stage lung cancer.7,8,9 Though the clinical significance of SB has increased during recent decades, PCNB is still regarded the primary option for the diagnosis of IPN. PCNB is an established diagnostic technique for IPN with high diagnostic yield.13,14 However, PCNB is not always feasible for IPN in clinical settings for its controversial role in the diagnosis of GGN, low sensitivity for small nodules, or difficult location of IPN.15,16 Moreover, PCNB could result in post-procedural complications, such as pneumothorax, hemothorax, and hemoptysis which have been reported to be as high as 38% in previous studies.17 SB is regarded a valuable option for the diagnosis of IPN and recommended by several guidelines from international societies.7,8,9 The evidence for these guidelines is: 1) The radiological characteristics of IPN, especially in GGN, are closely correlated with the pathological finding of IPN with the recent technological advancement of CT. Therefore, SB without preoperative tissue diagnosis is regarded a reasonable strategy for the evaluation of IPN suspected for early stage lung cancer. 2) SB could be used for both the diagnosis and treatment of IPN without unnecessary procedures and hospital admission. 3) The accurate pathological diagnosis of small nodules and GGNs is possible as the specimen including entire tumor lesion can be obtained using SB and it could lead to thorough histological evaluation of IPN.
In the National Lung Screening Trial, low-dose chest CT screening resulted in 20% decrease in lung cancer-specific mortality and 6.7% decrease in the overall mortality in high-risk patients compared with those resulting from conventional chest radiography.18 A Korean multi-society collaborative committee provided guidelines for lung cancer screening, which recommended annual, low-dose CT screening for high-risk patients and the pilot study demonstrated significant positive results.19,20 As thin-section, multi-detector CT raised the sensitivity for lung nodules detection, the requirement for evaluation of IPN has been increased in clinical settings.21 Therefore, it is important to analyze the cost-effectiveness of both PCNB and SB for evaluating IPNs to help doctors as well as patients choose appropriate diagnostic methods and allocate healthcare resources. According to the result of our study, the PCNB group showed significantly higher total medical costs than the SB group. Though the number of cartridges used for the operation was significantly higher in the SB group, the cost of the lung cancer operation was not different between the two groups. The largest driver of increased cost in the PCNB group is the price of admission for PCNB, and not for the PCNB procedure itself. Specifically, subcategory costs which increased proportionally to hospital stays were significantly higher in the PCNB group than in the SB group, and it largely contributed to the difference in medical costs between the two groups. Furthermore, the complications developed after PCNB result in prolonged health care system contact and can lead to a substantial increase in medical cost through longer hospital stays and procedures for treating complications.
Some recent studies demonstrated that CT-based assessment of IPN showed a high correlation with pathological findings and could even predict for histopathological invasiveness to differentiate lung adenocarcinoma subtypes.6,22,23 Lee et al.6 reported that CT-based resection of GGN with a solid component > 5 mm had 86.4% accuracy for lung cancer. They proposed that CT-based resection without biopsy could be a reasonable option in routine practice. In our study, the accuracy of the CT-based assessment of IPNs in the SB group was 92.2%. Moreover, thirteen patients (11.5%) in the PCNB group who were not confirmed as having lung cancer after PCNB eventually received operation, and 8 patients were diagnosed with lung cancer by SB. The result of our study was consistent with those of previous studies and demonstrated that approximately 10% of patients in the PCNB group underwent SB as clinicians could not exclude malignancy using radiological and clinical findings. Thus, our results also support that SB without preoperative tissue diagnosis for IPN is a reasonable option.
There are several limitations of this study that must be acknowledged. First, there might be a selection bias because this study was retrospective in nature and not randomized. Second, the cost data of both the groups only included cost incurred during admission, so the cost incurred at the outpatient clinic and the social cost were not included. Third, our study could not reflect the diversity of health care systems worldwide as we only included the cost data incurred in Korea. However, we think that our result can be universally applicable in other countries, as we revealed that the largest driver of the cost increase in the PCNB group was longer hospital stays because of multiple admissions.
Conclusively, we demonstrated that SB is associated with lower cost, shorter hospital stays, and lesser admission time. Moreover, increased cost and longer hospital stay appear largely related to the admission for PCNB. Given that CT-based assessment of IPN provides high accuracy in the diagnosis of malignancy, we suggest that direct SB is a cost-effective strategy for diagnosis and treatment of IPN suspected for early stage lung cancer.
Footnotes
Funding: This work was supported by the grant from the Seoul National University College of Medicine Research Fund (grant No. 800-20150269).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Na KJ, Park IK.
- Data curation: Na KJ.
- Formal analysis: Na KJ, Park IK.
- Investigation: Na KJ, Park IK, Park S, Kang CH, Kim YT.
- Methodology: Na KJ, Park IK.
- Software: Na KJ.
- Validation: Park IK, Park S, Kang CH, Kim YT.
- Writing - original draft: Na KJ, Park IK.
- Writing - review & editing: Na KJ, Park IK, Park S, Kang CH, Kim YT.
References
- 1.Kramer BS, Berg CD, Aberle DR, Prorok PC. Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST) J Med Screen. 2011;18(3):109–111. doi: 10.1258/jms.2011.011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YH, Lee KS, Primack SL, Kim H, Kwon OJ, Kim TS, et al. Small pulmonary nodules on CT accompanying surgically resectable lung cancer: likelihood of malignancy. J Thorac Imaging. 2002;17(1):40–46. doi: 10.1097/00005382-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Shin KE, Lee KS, Yi CA, Chung MJ, Shin MH, Choi YH. Subcentimeter lung nodules stable for 2 years at LDCT: long-term follow-up using volumetry. Respirology. 2014;19(6):921–928. doi: 10.1111/resp.12337. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178(5):1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Sone S, Abe H, Macmahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology. 2004;233(3):793–798. doi: 10.1148/radiol.2333031018. [DOI] [PubMed] [Google Scholar]
- 6.Lee SM, Park CM, Song YS, Kim H, Kim YT, Park YS, et al. CT assessment-based direct surgical resection of part-solid nodules with solid component larger than 5 mm without preoperative biopsy: experience at a single tertiary hospital. Eur Radiol. 2017;27(12):5119–5126. doi: 10.1007/s00330-017-4917-6. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon H, Naidich DP, Goo JM, Lee KS, Leung AN, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 10.Kim TJ, Lee JH, Lee CT, Jheon SH, Sung SW, Chung JH, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol. 2008;190(1):234–239. doi: 10.2214/AJR.07.2441. [DOI] [PubMed] [Google Scholar]
- 11.Jeon JH, Kang CH, Kim HS, Seong YW, Park IK, Kim YT, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg. 2014;45(4):640–645. doi: 10.1093/ejcts/ezt460. [DOI] [PubMed] [Google Scholar]
- 12.Nwogu CE, D'Cunha J, Pang H, Gu L, Wang X, Richards WG, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance) Ann Thorac Surg. 2015;99(2):399–405. doi: 10.1016/j.athoracsur.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol. 2000;175(1):239–243. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 14.vanSonnenberg E, Casola G, Ho M, Neff CC, Varney RR, Wittich GR, et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167(2):457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 15.Choo JY, Park CM, Lee NK, Lee SM, Lee HJ, Goo JM. Percutaneous transthoracic needle biopsy of small (≤ 1 cm) lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol. 2013;23(3):712–719. doi: 10.1007/s00330-012-2644-6. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz JM. Updates in percutaneous lung biopsy: new indications, techniques and controversies. Semin Intervent Radiol. 2012;29(4):319–324. doi: 10.1055/s-0032-1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27(1):138–148. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang SH, Sheen S, Kim HY, Yim HW, Park BY, Kim JW, et al. The Korean guideline for lung cancer screening. J Korean Med Assoc. 2015;58(4):291–301. [Google Scholar]
- 20.Lee JW, Kim HY, Goo JM, Kim EY, Lee SJ, Kim TJ, et al. Radiological report of pilot study for the Korean Lung Cancer Screening (K-LUCAS) project: feasibility of implementing lung imaging reporting and data system. Korean J Radiol. 2018;19(4):803–808. doi: 10.3348/kjr.2018.19.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischbach F, Knollmann F, Griesshaber V, Freund T, Akkol E, Felix R. Detection of pulmonary nodules by multislice computed tomography: improved detection rate with reduced slice thickness. Eur Radiol. 2003;13(10):2378–2383. doi: 10.1007/s00330-003-1915-7. [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa M, Kusumoto M, Johkoh T, Noguchi M, Minami Y, Sakai F, et al. Radiologic-pathologic correlation of solid portions on thin-section CT images in lung adenocarcinoma: a multicenter study. Clin Lung Cancer. 2018;19(3):e303–12. doi: 10.1016/j.cllc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Ko JP, Suh J, Ibidapo O, Escalon JG, Li J, Pass H, et al. Lung adenocarcinoma: correlation of quantitative CT findings with pathologic findings. Radiology. 2016;280(3):931–939. doi: 10.1148/radiol.2016142975. [DOI] [PubMed] [Google Scholar]


