Abstract
Background
Nonadherence to disease-modifying drugs (DMDs) for multiple sclerosis (MS) is associated with poorer clinical outcomes, including higher rates of relapse and disease progression, and higher medical resource use. A systematic review and quantification of adherence and persistence with oral DMDs would help clarify the extent of nonadherence and nonpersistence in patients with MS to help prescribers make informed treatment plans and optimize patient care.
The objectives were to: 1) conduct a systematic literature review to assess the availability and variability of oral DMD adherence and/or persistence rates across ‘real-world’ data sources; and 2) conduct meta-analyses of the rates of adherence and persistence for once- and twice-daily oral DMDs in patients with MS using real-world data.
Methods
A systematic review of studies published between January 2010 and April 2018 in the PubMed database was performed. Only studies assessing once- and twice-daily oral DMDs were available for inclusion in the analysis. Study quality was evaluated using a modified version of the Newcastle-Ottawa Scale, a tool for assessing quality of observational studies. The random effects model evaluated pooled summary estimates of nonadherence.
Results
From 510 abstracts, 31 studies comprising 16,398 patients with MS treated with daily oral DMDs were included. Overall 1-year mean medication possession ratio (MPR; n = 4 studies) was 83.3% (95% confidence interval [CI] 74.5–92.1%) and proportion of days covered (PDC; n = 4 studies) was 76.5% (95% CI 72.0–81.1%). Pooled 1-year MPR ≥80% adherence (n = 6) was 78.5% (95% CI 63.5–88.5%) and PDC ≥80% (n = 5 studies) was 71.8% (95% CI 59.1–81.9%). Pooled 1-year discontinuation (n = 20) was 25.4% (95% CI 21.6–29.7%).
Conclusions
Approximately one in five patients with MS do not adhere to, and one in four discontinue, daily oral DMDs before 1 year. Opportunities to improve adherence and ultimately patient outcomes, such as patient education, medication support/reminders, simplified dosing regimens, and reducing administration or monitoring requirements, remain. Implementation of efforts to improve adherence are essential to improving care of patients with MS.
Keywords: Adherence, Dimethyl fumarate, Discontinuation, Fingolimod, Meta-analysis, Persistence, Real-world, Teriflunomide
Background
Multiple sclerosis (MS) is a progressive, inflammatory, autoimmune, neurodegenerative disease of the central nervous system that often begins in early adult life. Guidelines recommend that clinicians should offer disease-modifying drugs (DMDs) to people diagnosed with relapsing forms of MS (RMS) [1, 2]. DMDs have been shown to reduce the rate of relapse, slow the rate of disease progression, [3–6] and improve long-term outcomes for patients with RMS [7, 8]. It is also recommended that clinicians monitor for medication adherence, adverse events, tolerability, safety, and effectiveness of the therapy in people with MS on DMDs [1].
Medication adherence and persistence are challenging for patients with MS [9, 10]. Nonadherence to or nonpersistence with DMD therapy for MS has been associated with poorer clinical outcomes, including higher rates of relapse and disease progression, and higher medical resource use [9, 11–14]. Although it has been hypothesized that the oral route of DMD administration may offer improved adherence to the injectable route of administration, recent studies have reported that real-world adherence to and persistence with the once- and twice-daily oral maintenance DMDs (i.e., fingolimod, dimethyl fumarate, and teriflunomide) may be similar to that of self-injectable DMDs [11, 15–18].
A systematic review and quantification of the real-world adherence to and persistence with oral DMDs would help clarify the extent of nonadherence and nonpersistence in patients with MS. Kantor et al. 2018 conducted a systematic review and meta-analysis of real-world persistence with fingolimod in patients with relapsing-remitting MS (RRMS) and reported a consensus 1-year persistence rate of 82% (95% confidence interval [CI] 79–85%) [19]. The current study aimed to expand on this previous meta-analysis to include other daily oral DMDs and to evaluate both adherence and persistence with oral DMDs. Specifically, the objectives of this study were to: 1) conduct a systematic literature review to assess the availability and variability of oral DMD adherence and/or persistence rates across ‘real-world’ data sources; and 2) conduct meta-analyses of the rates of adherence and persistence for all currently available oral DMDs in patients with MS.
Methods
Systematic literature review
A systematic literature search was performed of all studies published between January 2010 and April 2018 in the PubMed database that evaluated adherence or persistence to oral DMDs. The search strategy used the following terms: (Aubagio OR cladribine OR dimethyl fumarate OR fingolimod OR Gilenya OR Tecfidera OR teriflunomide OR oral OR disease modifying drug OR DMD OR disease modifying therapy OR DMT) AND multiple sclerosis AND (adherence OR compliance OR persistence OR discontinuation). A priori exclusion criteria were: lack of primary data; lack of primary real-world DMD adherence/persistence data; lack of oral DMD adherence/persistence data; pediatric studies; non-English studies; and abstract-only available. Two reviewers independently reviewed the search results and reference lists of selected articles to identify additional appropriate studies and carried out data extraction. Full search strategy and search results are provided in Additional File 1.
Information extracted from the screened articles included the type of study/data source; study population (baseline demographic and clinical characteristics); treatment arms; duration of follow-up after DMD initiation; sample size; outcomes evaluated (including definition of adherence and method of measurement); clinical results; secondary results; and strengths and limitations of the studies.
The quality of selected studies was evaluated using a modified version of the Newcastle-Ottawa Scale (NOS), a tool for assessing the quality of observational studies [20]. The NOS was modified as it was primarily designed to evaluate case-control and comparative studies. Study quality was evaluated with the modified NOS from the perspective of study design and patient selection as well as outcome to generate an overall assessment of the quality of the studies and their internal validity. Three criteria were evaluated under the NOS ‘study design and patient selection’ perspective: ascertainment of the intervention/validity of study design, patient selection, and outcome not present at the start of the study. Criteria evaluated under the ‘outcome evaluation’ perspective were appropriateness of the measure of adherence/persistence, adequacy or appropriateness of duration of follow-up, and thoroughness of follow-up for all patients. Each criterion was given a full-, partial-, or poor-quality score (Table 1). Per Cochrane Collaboration [21] and other recommendations [22] that meta-analyses not be adjusted on the basis of quality, the results of the study quality assessment were presented as standard tables and systematic narrative description and commentary about each of the elements.
Table 1.
Study quality as evaluated using a modified version of the Newcastle-Ottawa Scale
| Reference | Study design and patient selection | Outcome evaluation | ||||
|---|---|---|---|---|---|---|
| Ascertainment of intervention/validity of study design | Patient selection | Outcome not present at start | Appropriate measure of adherence/persistence | Adequate/appropriate duration of follow-up | All patients accounted for followed up | |
| Lanzillo R, et al. J Neurol. 2018;265:1174–1183 | ◉ | ● | ● | ● | ● | ● |
| Ferraro D, et al. Curr Med Res Opin. 2018;34:1803–1807 | ◉ | ● | ● | ● | ● | ● |
| Granqvist M, et al. JAMA Neurol. 2018;75:320–327 | ● | ● | ● | ● | ● | ● |
| Hua LH, et al. Mult Scler. 2018;1,352,458,518,765,656 | ◉ | ◉ | ● | ● | ◉ | ● |
| Eriksson I, et al. Eur J Clin Pharmacol. 2018;74:219–226 | ● | ● | ● | ● | ● | ● |
| Williams MJ, et al. Curr Med Res Opin. 2018;34:107–115 | ● | ◉ | ○ | ● | ● | ● |
| Ernst FR, et al. Curr Med Res Opin. 2017;33:2099–2106 | ◉ | ● | ● | ● | ◉ | ● |
| Lattanzi S, et al. J Neurol. 2017;264:2325–2329 | ◉ | ● | ● | ● | ● | ● |
| Gerber B, et al. Mult Scler Relat Disord. 2017;18:218–224 | ● | ◉ | ○ | ● | ● | ● |
| Zimmer A, et al. Patient Prefer Adherence. 2017;11:1815–1830 | ◉ | ● | ◉ | ◉ | ◉ | ● |
| Hersh CM, et al. Mult Scler J Exp Transl Clin. 2017;3:2055217317715485 | ◉ | ● | ● | ● | ● | ● |
| Vollmer B, et al. Mult Scler J Exp Transl Clin. 2017;3:2055217317725102 | ◉ | ◉ | ◉ | ● | ◉ | ● |
| Johnson KM, et al. J Manag Care Spec Pharm. 2017;23:844–852 | ● | ◉ | ● | ● | ● | ● |
| Smoot K, et al. Mult Scler. 2017;1,352,458,517,709,956 | ● | ● | ● | ● | ● | ● |
| Burks J, et al. Clinicoecon Outcomes Res. 2017;9:251–260 | ● | ◉ | ○ | ● | ● | ● |
| Munsell M, et al. Patient Prefer Adherence. 2016;11:55–62 | ● | ◉ | ○ | ● | ● | ● |
| Hersh CM, et al. Mult Scler Relat Disord. 2016;10:44–52 | ◉ | ◉ | ● | ● | ● | ● |
| Zhovtis L, et al. Ther Adv Neurol Disord. 2016;9:454–461 | ◉ | ● | ● | ● | ● | ● |
| Nazareth T, et al. BMC Neurol. 2016;16:187 | ● | ● | ● | ◉ | ● | ● |
| Wicks P, et al. BMC Res Notes. 2016;9:434 | ◉ | ● | ● | ● | ○ | ◉ |
| Warrender-Sparkes M, et al. Mult Scler. 2016;22:520–532 | ● | ◉ | ● | ● | ◉ | ● |
| Lapierre Y, et al. Can J Neurol Sci. 2016;43:278–283 (29) | ◉ | ○ | ◉ | ● | ● | ● |
| Braune S, et al. J Neurol. 2016;263:327–333 | ● | ● | ● | ● | ● | ◉ |
| Frisell T, et al. Mult Scler. 2016;22:85–93 | ● | ◉ | ● | ● | ● | ● |
| Longbrake EE, et al. Mult Scler J Exp Transl Clin. 2016;2 | ◉ | ◉ | ● | ● | ◉ | ● |
| He A, et al. JAMA Neurol. 2015;72:405–413 | ● | ● | ● | ◉ | ● | ● |
| Hersh CM, et al. Int J Neurosci. 2015;125:678–685 | ◉ | ● | ● | ● | ◉ | ● |
| Bergvall N, et al. J Med Econ. 2014;17:696–707 | ● | ◉ | ○ | ● | ● | ● |
| Al-Hashel J, et al. CNS Drugs. 2014;28:817–824 | ◉ | ● | ● | ◉ | ◉ | ● |
| Agashivala N, et al. BMC Neurol. 2013;13:138 | ● | ◉ | ○ | ● | ● | ● |
| Ontaneda D, et al. J Neurol Sci. 2012;323:167–172 | ◉ | ◉ | ● | ● | ○ | ● |
Abbreviations:RRMS relapsing-remitting multiple sclerosis, ● full-quality score, ◉ partial-quality score, ○ poor-quality score
For the ascertainment of the intervention/validity of study design criterion, if the study was a medical chart review, evidence that there was an effort made to validate reported data resulted in a full-quality score. Otherwise, the medical chart review or registry study was assigned a partial-quality score. Prospective cohort studies and administrative claims database evaluations were given a full-quality score for this criterion
For the patient selection criterion, studies were given a full-quality score if the patients were well characterized (i.e., age, sex, region, duration of MS diagnosis, MS severity, prior treatments, current treatment) and were representative of patients with RRMS. Studies were not penalized for including selected populations or for only evaluating a single center because it was felt that these studies were still valid cohort studies. Studies were given a poor-quality score if the patient population was not well-characterized and was not representative of patients with RRMS
For the outcome of interest not present at the start of the study criterion, studies were given a full-quality score if they attempted to capture patient prescription abandonment and thoroughly described how it was ascertained. Administrative claims database analyses were not able to ascertain this, and were therefore given a poor-quality score for this criterion
For the appropriate measurement of adherence/persistence criterion, studies with a full-quality score had to appropriately define and measure adherence and persistence and include and/or delineate switching for discontinuation
The adequate/appropriate duration of follow-up criterion required full-quality studies to measure adherence/persistence over 1 year as this was the most common time horizon used and enabled comparability
The all patients accounted at follow-up criterion required that all patients evaluated were followed up throughout the study and did not have missing data
Meta-analyses
The selection of endpoints was driven by the availability of data in the published, peer-reviewed literature. Adherence was evaluated using either the medication possession ratio (MPR) or the proportion of days covered (PDC). MPR was calculated as the total number of days of medication supply between the first prescription claim and the last prescription claim issued during the follow-up period divided by the total number of days in the follow-up period. A variable follow-up period was used for the MPR denominator (number of days between the index date and the last prescription dispensed inclusive of supply) [23]. PDC was calculated as the total number of days in the follow-up period in which medication was available (excluded overlapping days’ supply) divided by the duration of the observation period (i.e., 1 year in the case of oral DMD adherence studies). Adherence was calculated as means (mean MPR and mean PDC) and the proportion of patients attaining the 0.8 threshold, which is commonly considered an acceptable level of adherence [24]. Discontinuation was defined as the proportion of patients who either switched DMDs or discontinued DMD medications altogether. Analyses were conducted for 5 separate endpoints over a 1-year follow-up period: (1) mean MPR for patients overall (2); mean PDC for patients overall (3); proportion of patients ‘adherent’, defined as proportion with MPR ≥80% (4); proportion of patients ‘adherent’, defined as proportion with PDC ≥80%; and (5) proportion of patients who discontinued the initial treatment.
In line with published recommendations regarding the use of real-world data in meta-analyses, [25] statistical heterogeneity was evaluated using Cochran’s Q test and the I2 statistic, which provide a measure of the presence of heterogeneity and the share of dispersion across studies, respectively [26]. If Q was significant and I2 was > 50%, the random-effects model (REM) was used to calculate pooled summary estimates; otherwise, a fixed-effect model was used. The studies were weighted according to the extent of variation among the intervention effects. Egger’s test was used to detect publication bias [27]. The ‘metaprop’ and the ‘metamean’ functions in the R statistical programming language [28] were used to conduct the meta-analysis, and a p-value of < 0.05 was considered statistically significant. Subgroup analyses conducted included study location (i.e., US/ex-US studies) and study design (i.e., prospective cohort vs. retrospective chart review vs. administrative claims database evaluation). The impact of any single study on the overall results was assessed using a leave-one-out sensitivity analysis, in which each study was iteratively removed and the findings compared to the overall meta-analysis.
Results
Systematic literature review
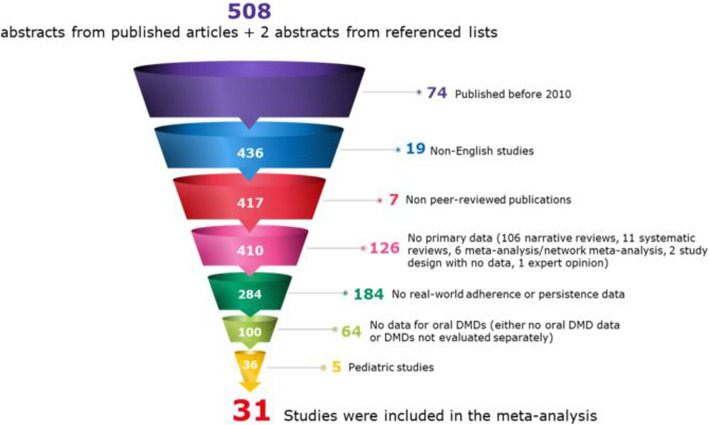
From a total of 510 abstracts identified, 31 studies comprising 16,398 patients were included in the systematic review after applying exclusion criteria (Fig. 1). Table 2 provides information about the individual studies. No studies evaluating cladribine tablets were identified due to its recent approval, therefore the analyses focused on once- and twice-daily oral maintenance DMDs. Siponimod was not available at the time of the study and is not included in the analyses.
Fig. 1.
Study selection flowchart. Abbreviations:DMD disease-modifying drug
Table 2.
| Reference | Study design | Geographical area | Sample size for oral DMD(s) studied | Study population | Outcomes evaluated |
|---|---|---|---|---|---|
| Agashivala N, et al. BMC Neurol. 2013;13:138 | Retrospective administrative claims database evaluation | USA | Fingolimod (n = 248) | Patients with MS | Mean MPR and PDC; MPR ≥80%; PDC ≥80%; discontinuation rate |
| mean age 46.4 (10.7) years; | |||||
| 79.0% female; | |||||
| Al-Hashel J, et al. CNS Drugs. 2014;28:817–824a | Retrospective evaluation of patient MS registry | Kuwait | Fingolimod (n = 175) | Patients with RRMS | % discontinuation |
| mean age 33.3 ± 9.2 years; | |||||
| 75.4% female | |||||
| Bergvall N, et al. J Med Econ. 2014;17:696–707 | Retrospective administrative claims database evaluation | USA | Fingolimod (n = 889) | 889 patients (age range NS) with MS initiating fingolimod | MPR ≥80%; PDC ≥80%; discontinuation rate |
| Braune S, et al. J Neurol. 2016;263:327–333 | Prospective, observational, multi-center cohort study | Germany | Fingolimod (n = 99) | Patients with RRMS (age range NS) with failure of earlier therapy with injectable DMT initiating fingolimod | Discontinuation rate |
| Burks J, et al. Clinicoecon Outcomes Res. 2017;9:251–260 | Retrospective administrative claims database study | USA | N = 1018 | Patients with MS (aged 18–65 years) initiating an oral DMD | Mean PDC; proportion of patients with PDC ≥80%; discontinuation rate |
| Teriflunomide, fingolimod, DMF | |||||
| mean age 44.41 (10.52); 72.1% female | |||||
| Eriksson I, et al. Eur J Clin Pharmacol. 2018;74:219–226 | Prospective cohort which includes retrospective claims and other health-related data analysis | Stockholm county, Sweden | DMF (n = 400) | 400 patients with RRMS (age range NS) initiating DMF | Discontinuation rate |
| Mean age ranged from 35.3–40.5 years | |||||
| 61% previously treated. | |||||
| Ernst FR, et al. Curr Med Res Opin. 2017;33:2099-2106a | Retrospective medical chart review | USA | DMF (n = 307) | 307 patients (aged ≥18 years) with RRMS initiating DMF | Discontinuation rate |
| Mean age 46.6 ± 11.8 years; | |||||
| 77.9% female; | |||||
| Ferraro D, et al. Curr Med Res Opin. 2018;34:1803–1807 | Prospective observational cohort study | Italy | N = 258 | Patients with RRMS (age range NS) initiating oral DMD | Discontinuation rate |
| Teriflunomide, DMF | |||||
| mean age 43 years, | |||||
| 29.8% female | |||||
| Frisell T, et al. Mult Scler. 2016;22:85–93 | Prospective, observational, multi-center cohort study | Sweden | Fingolimod (n = 876) | Patients with RRMS initiating fingolimod tx | Discontinuation rate |
| mean (SD) age 38 (10) years; | |||||
| 67% females | |||||
| Gerber B, et al. Mult Scler Relat Disord. 2017;18:218–224 | Retrospective administrative database evaluation | Alberta, Canada | N = 72 | 72 patients with MS (aged < 35–≥65 years) initiating an oral DMD | MPR ≥80%; discontinuation rate |
| Fingolimod, teriflunomide, DMF | |||||
| 61.7% aged 35–55 years; | |||||
| 73.8% female | |||||
| Granqvist M, et al. JAMA Neurol. 2018;75:320–327 | Retrospective medical chart review from MS registry | Sweden | N = 103 | Patients with RRMS initiating DMD tx | Discontinuation rate |
| DMF (n = 86), fingolimod (n = 17) | The median (interquartile) age 34.4 (27.4–43.4) years; | ||||
| 68% female | |||||
| He A, et al. JAMA Neurol. 2015;72:405–413 | Matched retrospective analysis of data collected prospectively from an international, observational cohort study | International | Fingolimod (n = 148) | Patients with MS (age range NS) switching to fingolimod tx | Discontinuation rate |
| Hersh CM, et al. Int J Neurosci. 2015;125:678–685a | Retrospective, single-center medical chart review | Cleveland, OH, USA | Fingolimod (n = 306) | Patients with MS (age range NS) initiating fingolimod | Discontinuation rate |
| 3.5% treatment naïve and 24.0% had remote DMT use prior to fingolimod | |||||
| Hersh CM, et al. Mult Scler J Exp Transl Clin. 2017;3:2055217317715485a | Retrospective, single-center medical chart review | Cleveland, OH, USA | Fingolimod (n = 264), DMF (n = 396) | Patients with MS (age range NS) being treated with DMD for ≥1 year | Discontinuation rate |
| Hersh CM, et al. Mult Scler Relat Disord. 2016;10:44–52 | Retrospective, single-center medical chart review | Cleveland, USA | Fingolimod (n = 317), DMF (n = 458) | Patients with MS initiating DMD tx | Discontinuation rate |
| mean age DMF 47.1 ± 11.2 years fingolimod 43.9 ± 9.2 years; | |||||
| RRMS DMF 73.5% fingolimod 81.7% | |||||
| Hua LH, et al. Mult Scler. 2018;1352458518765656a | Retrospective medical chart review | Cleveland, OH; Las Vegas, NV; Weston, FL, USA | Fingolimod (n = 10), DMF (n = 74), teriflunomide (n = 40) | Patients (aged over 60 years) with MS on DMD for ≥2 years | Discontinuation rate |
| Johnson KM, et al. J Manag Care Spec Pharm. 2017;23:844–852 | Retrospective administrative claims database evaluation | USA | Fingolimod (n = 195), DMF (n = 1160), teriflunomide (n = 143) | Patients with MS (aged ≥18 years) initiating DMD tx | MPR, MPR ≥80%, mean PDC, PDC ≥80%, discontinuation rate |
| Mean age range 44.4–53.2 years; 75.5–83.6% female | |||||
| Lanzillo R, et al. J Neurol. 2018;265:1174–1183 | Retrospective medical chart review | Italy | N = 1312 | Patients with RRMS (age range NS) initiating oral DMD | Discontinuation rate |
| Teriflunomide, DMF | |||||
| mean age 40.0 (11.2) year | |||||
| Lapierre Y, et al. Can J Neurol Sci. 2016;43:278–283a | Prospective, observational, multi-center cohort study | Canada | Fingolimod (n = 2399) | Patients with RRMS (age range NS) receiving fingolimod and participating in an education and support program | Discontinuation rate |
| Mean age was 41.2 years (range 18–75.5); 75.2% were female. | |||||
| Lattanzi S, et al. J Neurol. 2017;264:2325–2329 | Retrospective medical chart review | Italy | Fingolimod (n = 129), teriflunomide (n = 64), DMF (n = 114) | Patients with RRMS; | Discontinuation rate |
| mean age was 41.2 (10.3) years, 66.5% female | |||||
| Longbrake EE, et al. Mult Scler J Exp Transl Clin. 2016;2a | Retrospective, single-center medical chart review | USA | Teriflunomide (n = 83), fingolimod (n = 92), DMF (n = 254) | Patients (age range NS) with relapsing forms of MS initiating oral DMD tx | Discontinuation rate |
| mean age 39.8–49.4 years; 72.0–81.9% female | |||||
| Munsell M, et al. Patient Prefer Adherence. 2016;11:55–62 | Retrospective administrative claims database evaluation | USA | N = 1175 | Patients with MS (aged 18–64 years) initiating an oral DMD; mean age 44.9 years; 76.2% female | Mean MPR; proportion of patients with MPR ≥80%; discontinuation rate |
| Teriflunomide, fingolimod, DMF | |||||
| Nazareth T, et al. BMC Neurol. 2016;16:187 | Retrospective medical chart review | USA | Teriflunomide (n = 31), fingolimod (n = 55), DMF (n = 65) | Patients with MS (aged ≥18 years) initiating DMF; | Discontinuation rate |
| Age range (mean ± SD); 44.2 ± 10.7 to 50.6 ± 9.6 years. | |||||
| Ontaneda D, et al. J Neurol Sci. 2012;323:167–172a | Retrospective, single-center medical chart review | Cleveland, OH, USA | Fingolimod (n = 317) | Patients with MS (age group NS) prescribed fingolimod | Discontinuation rate |
| Smoot K, et al. Mult Scler. 2017;1,352,458,517,709,956 | Prospective registry at a single site | Oregon, USA | DMF (n = 417) | Patients (aged ≥18 years) with RMS initiating DMF tx; | Discontinuation rate |
| mean age 49.4 ± 12.0 years | |||||
| Vollmer B, et al. Mult Scler J Exp Transl Clin. 2017;3:2055217317725102 | Retrospective, single-center medical chart review | Colorado, USA | Fingolimod (n = 271), DMF (n = 342) | Patients with MS (age range NS) initiating DMD tx; | Discontinuation rate |
| mean age range 42.5–45.8 years; | |||||
| 69.6–72.0% female | |||||
| Warrender-Sparkes M, et al. Mult Scler. 2016;22:520–532a | Prospective, observational multi-center cohort study | International | Fingolimod (n = 426) | Patients with CIS or early RRMS (age range NS) initiating fingolimod tx | Discontinuation rate |
| Wicks P, et al. BMC Res Notes. 2016;9:434a | Online community patient survey | USA | Fingolimod (n = 93), DMF (n = 188) | Patients (aged ≥18 years) with RRMS with current or past experience of fingolimod or DMF tx | Discontinuation rate |
| mean age 46.2–51.8 years; % female 77.0–93.8%; | |||||
| Williams MJ, et al. Curr Med Res Opin. 2018;34:107–115 | Retrospective administrative claims database evaluation | USA | DMF (n = 133) | Patients (aged ≥18 years) with MS initiating DMD tx | Mean MPR; MPR ≥80%; mean PDC; PDC ≥80%; discontinuation rate |
| Zhovtis Ryerson L, et al. Ther Adv Neurol Disord. 2016;9(6):454–461 | Retrospective medical chart review in 2 tertiary MS clinics | New York/New Jersey, USA | DMF (n = 382)b | Patients (aged ≥18 years) with RRMS initiating DMF | Discontinuation rate |
| range across subgroups: mean age 42.5–47.4 years, 71–83% female | |||||
| Zimmer A, et al. Patient Prefer Adherence. 2017;11:1815–1830a | Prospective, observational, single-center cohort study | Basel, Switzerland | Fingolimod (n = 98) | Patients with relapsing MS (aged ≥18 years) initiating fingolimod; | Discontinuation rate; % nonadherent (pill count) |
| 80% female |
Note: a Study excluded from the meta-analysis
Dosage: DMF 120 mg PO BID for initial 7 days, increase to 240 mg PO BID; fingolimod 0.5 mg PO QD; teriflunomide 7 mg or 14 mg PO QD
Abbreviations:BID twice a day, CIS clinically isolated syndrome, DMD disease-modifying drug, DMF dimethyl fumarate, MPR medication possession ratio, MS multiple sclerosis, NS not specified, PO oral administration, PDC proportion of days covered, QD once a day, RRMS relapsing-remitting MS, tx therapy
Most studies (n = 18; 58.1%) were conducted in the US, one-quarter (25.8%; n = 8) were from Europe, two (6.5%) were multinational, two (6.5%) were from Canada, and one (3.2%) was from Kuwait (Table 2). Twelve studies (38.7%) were retrospective analyses of chart/electronic medical records; eight (25.8%) analyzed administrative claims databases; seven (22.6%) were prospective observational cohort studies; three (9.7%) used patient registries; and one (3.2%) was a patient survey (Table 2). The duration of follow-up for the studies ranged from 3 months to 3 years, with 21 studies (67.7%) reporting data at 1-year follow-up. All 31 studies evaluated treatment discontinuation for various follow-up periods. The 1-year treatment discontinuation range was 5.1–42.3% (n = 20 studies). For 1-year adherence, 4 studies reported the mean MPR, 6 studies reported MPR ≥80%, 4 studies reported the mean PDC, and 5 studies reported PDC ≥80%.
Quality assessments of the selected studies are presented in Table 1. For the ascertainment of the intervention/validity of study design criteria in the study design and patient selection perspective, approximately half of the studies had a full-quality score and half had a partial-quality score. One study (Lapierre et al. 2016) was assigned a poor-quality score for patient selection because the patient population was neither well-characterized nor representative of patients with RRMS [29]. For the criterion of outcome of interest not present at the start of the study, poor-quality scores were assigned to administrative claims database analyses (Williams et al. 2018; Gerber et al. 2017; Johnson et al. 2017; Burks et al. 2017; Munsell et al. 2016; Bergvall et al. 2014; Agashivala et al. 2013) as they were unable to ascertain patient prescription abandonment [5, 11, 12, 17, 30–32]. For the three criteria in the ‘outcome evaluation’ perspective (appropriateness of the measure of adherence/persistence, adequacy or appropriateness of duration of follow-up, and thoroughness of follow-up for all patients), most studies had full-quality scores. Two studies (Wicks et al. 2016; Ontaneda et al. 2012) were assigned poor-quality scores for the appropriateness of duration of follow-up criterion: Wicks et al. 2016 had a variable follow-up period across patients and Ontaneda et al. 2012 followed patients for only 3 months [33, 34].
Meta-analysis
A significant Cochran’s Q statistic and an I2 > 50% confirmed the requirement to use random effects models (REMs). A lack of significance on the Egger’s test suggested an absence of publication bias.
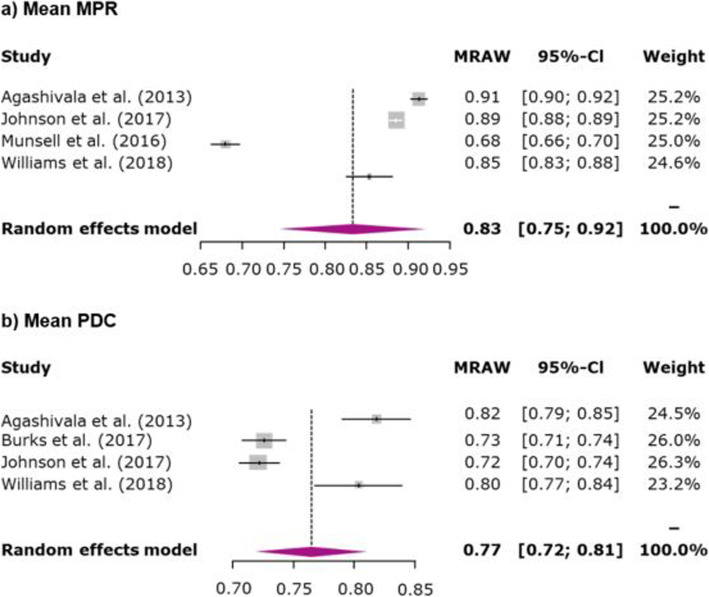
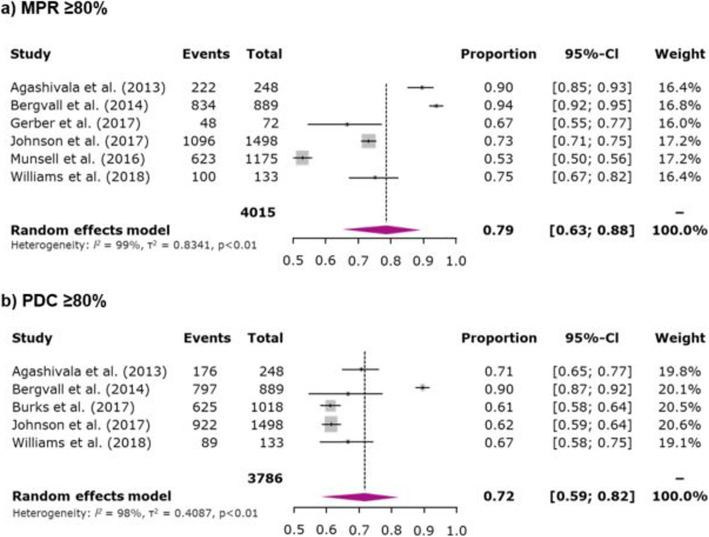
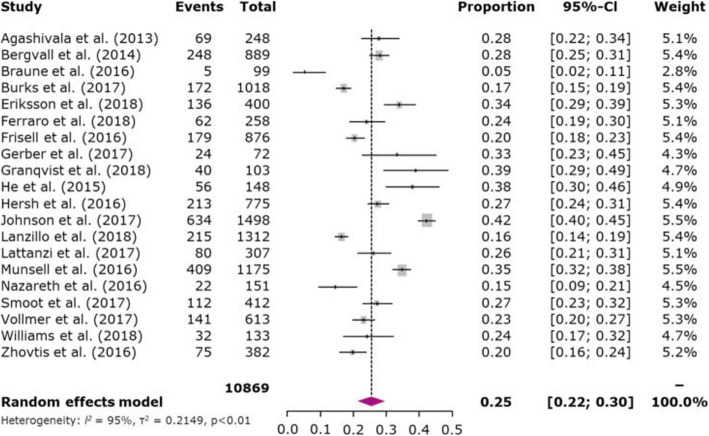
The overall mean MPR during the 1-year follow-up period for once- and twice-daily oral maintenance DMDs (4 studies) was 83.3% (95% CI 74.5–92.1%) (Fig. 2a) whereas the overall 1-year mean PDC (4 studies) was 76.5% (95% CI 72.0–81.1%) (Fig. 2b). The pooled MPR ≥80% adherence rate during the 1-year follow-up period across 6 studies was 78.5% (95% CI 63.5–88.5%) (Fig. 3a) and 1-year pooled PDC ≥80% adherence rate (5 studies) was 71.8% (95% CI 59.1–81.9%) (Fig. 3b). All 31 studies evaluated treatment discontinuation using various follow-up periods; the 1-year pooled discontinuation rate across 20 studies for oral maintenance DMDs was 25.4% (95% CI 21.6–29.7%) (Fig. 4).
Fig. 2.
Meta-analysis of mean adherence rate as determined by a) MPR or b) PDC. Note: For studies for which results for treatment-naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. The area of each grey square is proportional to the study’s weight in the meta-analysis. Weight values are rounded. Abbreviations: CI, confidence interval; DMD, disease-modifying drug; MPR, medication possession ratio; MRAW, raw mean; PDC, proportion of days covered
Fig. 3.
Meta-analysis of proportion of patients adherent to a DMD as determined by MPR or PDC. Note: For studies for which results for treatment-naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. The area of each grey square is proportional to the study’s weight in the meta-analysis. Weight values are rounded. Abbreviations: CI: confidence interval; DMD: disease-modifying drug; MPR: medication possession ratio; PDC: proportion of days covered
Fig. 4.
Meta-analysis of proportion of patients discontinuing a DMD. Note: For studies for which results for treatment-naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined; for Lattanzi et al., number of patients discontinuing at 12 months included patients for whom data were not available at 12 months because they stopped taking medications (n = 34); 34 + 46 = 80 of 307 or 26.05%; for Vollmer et al. and He et al., for which data were reported in Kaplan–Meier curves only, 1-year persistence rates were extracted from the curves using a digitizer (Guyot P et al. 2012); for Zhovtis et al. 2016, number of patients discontinuing at 12 months was derived from the reported 14-month rate (n = 76.4). The area of each grey square is proportional to the study’s weight in the meta-analysis. Weight values are rounded. Abbreviations: CI, confidence interval; DMD, disease-modifying drug
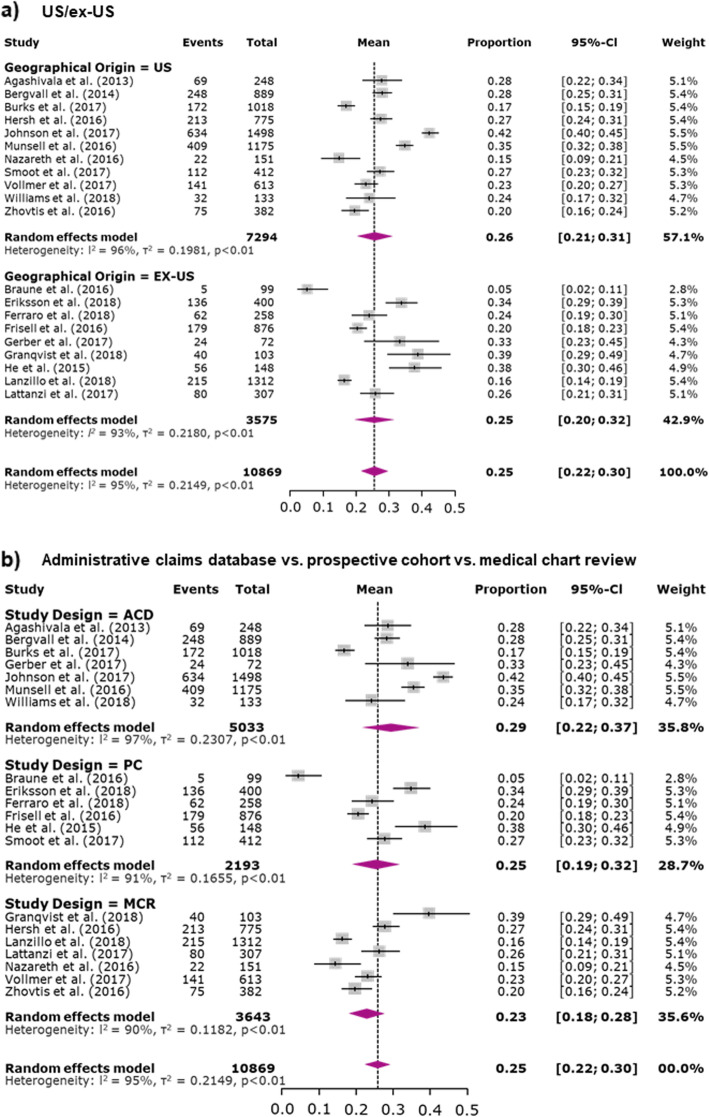
Subgroup analyses were only conducted for the outcome of discontinuation due to the small number of studies reporting MPR and PDC oral DMD adherence. When studies were analyzed by US and ex-US groupings, similar proportions of patients were found to discontinue DMD therapy (25.6% [95% CI 20.7–31.1%] vs. 25.3% [95% CI 19.6–31.9%], respectively) (Fig. 5a). The proportion of patients discontinuing DMD therapy was greatest for studies evaluating administrative claims databases (29.0, 95% CI 22.0–37.2%), followed by prospective cohort studies (24.7, 95% CI 18.7–31.9%) and medical chart reviews (22.9, 95% CI 18.4–28.1%) (Fig. 5b), though some overlapping of CIs was apparent. The results of the leave-one-out sensitivity analysis confirmed that removal of individual studies did not affect the results (Supplementary Figs. 1, 2a, and 2b).
Fig. 5.
Subgroup meta-analyses of proportion of patients discontinuing a DMD. Note: For studies for which results for treatment naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. The area of each grey square is proportional to the study’s weight in the meta-analysis. Weight values are rounded. Abbreviations: ACD, administrative claims database; CI, confidence interval; MCR, medical chart review; PC, prospective cohort
Discussion
This is the first meta-analysis to assess real-world adherence to and persistence with multiple oral maintenance DMDs in patients with MS. Results showed that one in five patients do not adhere to once- or twice-daily oral maintenance DMDs, and one in four patients discontinue the initially-prescribed oral DMDs before 1 year. Ninety-five percent CIs for the estimates of adherence and persistence were wide, reflecting the heterogeneity in rates of adherence and discontinuation.
Adherence to DMDs is an important aspect of optimizing patient care in MS as greater adherence has been shown to be associated with improvements in relapse outcomes and quality of life, fewer hospitalizations and emergency room visits, decrease in neuropsychological issues, fewer days of work lost, and lower MS-related medical costs [11–14, 54–57]. Compared with nonadherence, adherence to newly initiated DMDs (oral or injectable) over 1 year among patients in the US was associated with a decrease of 42% in the likelihood of relapse, 38% in emergency visits, and 52% in hospitalizations, as well as an average of 0.7 fewer outpatient visits annually (all p < 0.0001) [11]. Based on the differences in predicted US mean costs over the 12-month post-initiation period, adherence was estimated to decrease the total annual medical care costs by $5816 per patient (all costs are reported in 2015 US$), including outpatient visits ($2802), emergency visits ($171), and hospitalizations ($1953) compared with nonadherence [11]. Persistence with DMDs for MS is also important for achieving the best clinical outcomes, as DMD persistence has been shown to be associated with decreased likelihoods of inpatient admission or emergency room visits, [58] decreased relapses and disease progression, [59] and lower costs [60].
An increased understanding of barriers to DMD adherence and persistence and the implementation of efforts to improve adherence and persistence are important. Real-world data on adherence to DMDs in patients with MS can help inform therapeutic decision making [61]. Factors that generally influence adherence and persistence in chronic illness, particularly when cognition is a factor (as in MS), include the frequency and complexity of the dosing regimen, [62, 63] the need for ongoing safety monitoring, office visits, or waiting time associated with dosing, [64] prior treatment experience, [64] and the presence or absence of active symptoms at the time of dosing [64]. Studies in other therapeutic areas have shown that simpler and less frequent dosing produces greater adherence than more frequent administration [62, 63, 65]. A study of MS patient preferences for oral treatments also found that the most important driver of predicted nonadherence was frequency of daily dosing (17.4% out of 100%) [66].
Depression has also been shown to be associated with decreased DMD adherence and persistence in patients with MS. Gerber, et al. and Munsell, et al. both reported a significant relationship between comorbid depression and nonadherence, [12, 17] whereas Lattanzi, et al. reported a significant relationship between depression and persistence [35]. Previous studies have shown that patients with MS and comorbid depression are significantly more likely to be nonadherent to DMDs than patients with MS without comorbid depression [67, 68].
The rates of adherence and discontinuation may differ among oral DMDs due to several potential factors such as the dosing regimen (e.g. once- versus twice-daily), efficacy, tolerability, and adverse events [15, 35, 36]. Assessments of the specific impacts of these factors could not be conducted in the current study due to the small number of studies evaluating the individual oral DMDs’ adherence/persistence over 1 year (i.e., 7 studies evaluated dimethyl fumarate, 10 studies evaluated fingolimod, and only 2 studies evaluated teriflunomide) and due to the challenges in elucidating the specific reasons for any associations that might be observed among studies with significant heterogeneity.
This study was conducted in line with recommendations available in the literature for the use of real-world evidence in meta-analyses [25]. In our findings, 95% CIs showed a wide range of values, particularly for the proportion of patients who were adherent, but this is expected when pooling observational (real-world) data [25]. Heterogeneity is likely to arise because of differences in patient populations, treatments, study design, outcomes, and data quality [69]. This was evident in the results of our assessment of the quality of the studies, which highlighted how the appraisal of the studies needed to be adapted for their individual design (hence our modification of the NewCastle Ottawa Scale [NOS]). Study location, in and of itself, was not a source of heterogeneity in the current meta-analysis.
The I2 statistics obtained in this study ranged from 93.8 to 99.5%. These values are consistent with those noted in other published meta-analyses of medication adherence across indications [19, 70–73].
Subgroup analyses demonstrated that there were essentially no differences between US- and ex-US-based studies and support the need for a better understanding of why patients worldwide discontinue oral DMD treatment. The study design subgroup analyses showed overlapping CIs, indicating a lack of significant differences. However, numerical differences suggested that administrative claims data analyses may more fully capture discontinuation than prospective cohort studies and medical chart reviews. With prospective cohort studies and medical chart reviews, bias may arise from patient or investigator report, whereas administrative claims database analyses provide objective billing information for medication dispensed. Also, cohort studies may also inherently encourage patients to remain in the study and continue treatment. Chart reviews may not capture all discontinuations, depending upon the availability and quality of follow-up data. Further subgroup analyses were limited by the small number of real-world/observational studies, suggesting that more real-world research is needed.
This is the second published meta-analysis of real-world adherence to or persistence with oral DMDs in patients with MS. Kantor et al. 2018 conducted a systematic review and meta-analysis focusing on the real-world persistence with fingolimod in patients with RRMS [19]. In contrast, our meta-analysis evaluated both adherence and persistence in all once- and twice-daily oral maintenance DMDs. Unlike the Kantor et al. 2018 meta-analysis, which included conference posters as well as published studies, the current analysis restricted studies to those published in the peer-reviewed medical literature. Several published studies not captured in the Kantor et al. 2018 evaluation (which captured studies through 2015) were available for inclusion since the literature search was extended through April 2018. The consensus 1-year persistence rate of 82% (95% CI 79–85%) reported by Kantor et al. 2018 corresponds to a mean adherence rate of 77–83% of once- and twice-daily oral maintenance DMDs (depending on the method used to measure adherence) reported in this meta-analysis.
There are limitations to this study. Although nonrandomized cohort studies or observational studies may provide a more ‘real-world’ representation of outcomes, costs, and utilization, differences in baseline characteristics can introduce biases, and the influence of unmeasured factors cannot be ruled out. As described, the substantial proportion of heterogeneity found across the studies is also a limitation. The use of a variable follow-up period for the MPR denominator potentially contributed to an inflated mean MPR [23]. Additionally, MPR may overestimate medication adherence as compared to PDC, as it counts the total number of days of medication supply, which may be inflated by patients who fill their prescriptions early and gain extra supply within a period [74]. No studies evaluating cladribine tablets were identified due to its recent approval, therefore the analyses focused on once- and twice-daily oral maintenance DMDs. Siponimod was not available at the time of the study and is not included in the analyses. Finally, the limited number of studies restricted the ability to analyze MPR and PDC adherence in greater detail and the ability to perform subgroup analysis across adherence and persistence measures.
Conclusions
Adherence to treatment is an important issue for the management of patients with MS. This meta-analysis of real-world studies showed that approximately one in five patients with MS do not adhere to once- or twice-daily oral maintenance DMD treatment regimens, and one in four patients with MS discontinue once- or twice-daily oral maintenance DMDs before 1 year. Wide 95% CIs for the estimates of adherence and discontinuation reflect the heterogeneity in the rates that was observed. Opportunities to address barriers to DMD adherence in patients with MS remain, such as patient education efforts to manage expectations and to emphasize the importance of adherence, implementation of medication support/reminder techniques, simplification of dosing regimens, and reducing the need for specialized administration or monitoring requirements [61]. Implementation of efforts to improve adherence are essential to improving care of patients with MS.
Supplementary information
Additional file 1: Supplementary Methods: Electronic Search Strategy. Details of electronic literature search strategy, exclusion criteria and full list of search results (including abstract citation, title, text, digital objective identifier [DOI], PubMed ID [PMID], and author names and information).
Additional file 2: Supplementary Figure 1. Leave-one-out sensitivity analysis for the proportion of patients discontinuing a DMD. For studies for which results for treatment naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. Abbreviations: DMD, disease-modifying drug.
Additional file 3: Supplementary Figure 2. Leave-one-out sensitivity analysis for subgroup analyses for the proportion of patients discontinuing a DMD. For studies for which results for treatment naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. Abbreviations: DMD: disease-modifying drug.
Acknowledgments
Writing and editorial support for the preparation of this manuscript was provided by Jenna Steere, PhD and Nick White of Ashfield Healthcare (New York, NY); funding was provided by EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany. The authors had full editorial control, and provided their final approval of all content.
Abbreviations
- CI
Confidence interval
- DMD
Disease-modifying drug
- MPR
Medication possession ratio
- MS
Multiple sclerosis
- NOS
Newcastle-Ottawa Scale
- PDC
Proportion of days covered
- REM
Random-effects model
- RMS
Relapsing MS
- RRMS
Relapsing-remitting MS
Authors’ contributions
JN, NE, RE, AD, MG, and AP were involved in the study design, interpretation of the data, writing and reviewing the manuscript, and the final approval of the version of the manuscript to be published. AD, NE, and RE conducted the data analyses.
Funding
The design of this study, the collection and analysis of data, and medical writing and editorial support in development of this manuscript were sponsored by EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JN has received consulting fees for speaking, advising, and consulting from: Biogen Idec, EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany, Genzyme, Genentech, MSAA, MS World, and Novartis. She has received research grants from Genzyme, Biogen, and Novartis. NCE and RAE are employees of Health Services Consulting Corporation. AD is an employee of Fair Dynamics Consulting and worked on behalf of Health Services Consulting Corporation. Health Services Consulting Corporation received funding from EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany, to run the analysis. MG and ALP are employees of EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12883-020-01830-0.
References
- 1.Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis Report Guideline Development Dissemination Implement Subcommittee American Academy. Neurology. 2018;90(17):777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 2.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 3.Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI study group and the IFNB multiple sclerosis study group. Neurology. 1993;43(4):662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 4.Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. [PubMed] [Google Scholar]
- 5.Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study group. Neurology. 1995;45(7):1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 7.Rudick RA, Cutter GR, Baier M, Weinstock-Guttman B, Mass MK, Fisher E, et al. Estimating long-term effects of disease-modifying drug therapy in multiple sclerosis patients. Mult Scler. 2005;11(6):626–634. doi: 10.1191/1352458505ms1203oa. [DOI] [PubMed] [Google Scholar]
- 8.Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology. 2011;76(1 Suppl 1):S26–S34. doi: 10.1212/WNL.0b013e318205051d. [DOI] [PubMed] [Google Scholar]
- 9.Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19(1 Suppl A):S24–S40. doi: 10.18553/jmcp.2013.19.s1.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heesen C, Bruce J, Feys P, Sastre-Garriga J, Solari A, Eliasson L, et al. Adherence in multiple sclerosis (ADAMS): classification, relevance, and research needs. A meeting report. Mult Scler. 2014;20(13):1795–1798. doi: 10.1177/1352458514531348. [DOI] [PubMed] [Google Scholar]
- 11.Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clin Outcomes Res. 2017;9:251–260. doi: 10.2147/CEOR.S130334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber B, Cowling T, Chen G, Yeung M, Duquette P, Haddad P. The impact of treatment adherence on clinical and economic outcomes in multiple sclerosis: real world evidence from Alberta, Canada. Mult Scler Relat Disord. 2017;18:218–224. doi: 10.1016/j.msard.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Yermakov S, Davis M, Calnan M, Fay M, Cox-Buckley B, Sarda S, et al. Impact of increasing adherence to disease-modifying therapies on healthcare resource utilization and direct medical and indirect work loss costs for patients with multiple sclerosis. J Med Econ. 2015;18(9):711–720. doi: 10.3111/13696998.2015.1044276. [DOI] [PubMed] [Google Scholar]
- 14.Lizan L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Pref Adherence. 2014;8:1653–1664. doi: 10.2147/PPA.S67253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanzillo R, Prosperini L, Gasperini C, Moccia M, Fantozzi R, Tortorella C, et al. A multicentRE observational analysiS of PErsistenCe to treatment in the new multiple sclerosis era: the RESPECT study. J Neurol. 2018;265(5):1174–1183. doi: 10.1007/s00415-018-8831-x. [DOI] [PubMed] [Google Scholar]
- 16.Ferraro D, Camera V, Baldi E, Vacchiano V, Curti E, Guareschi A, et al. First-line disease-modifying drugs in relapsing-remitting multiple sclerosis: an Italian real-life multicenter study on persistence. Curr Med Res Opin. 2018;34(10):1803–1807. doi: 10.1080/03007995.2018.1451311. [DOI] [PubMed] [Google Scholar]
- 17.Munsell M, Frean M, Menzin J, Phillips AL. An evaluation of adherence in patients with multiple sclerosis newly initiating treatment with a self-injectable or an oral disease-modifying drug. Patient Prefer Adherence. 2016;11:55–62. doi: 10.2147/PPA.S118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longbrake EE, Cross AH, Salter A. Efficacy and tolerability of oral versus injectable disease-modifying therapies for multiple sclerosis in clinical practice. Mult Scler J Exp Transl Clin. 2016;2. [DOI] [PMC free article] [PubMed]
- 19.Kantor D, Johnson K, Vieira MC, Signorovitch J, Li N, Gao W, et al. Real-world persistence with fingolimod for the treatment of multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2018;388:168–174. doi: 10.1016/j.jns.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 21.Ryan RHS, Prictor M, McKenzie J. Cochrane consumers and communication review group. Stud Qual Guide. 2013. [DOI] [PubMed]
- 22.Herbison P, Hay-Smith J, Gillespie WJ. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J Clin Epidemiol. 2006;59(12):1249–1256. doi: 10.1016/j.jclinepi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Kozma CM, Dickson M, Phillips AL, Meletiche DM. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509–516. doi: 10.2147/PPA.S40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 25.Briere JB, Bowrin K, Taieb V, Millier A, Toumi M, Coleman C. Meta-analyses using real-world data to generate clinical and epidemiological evidence: a systematic literature review of existing recommendations. Curr Med Res Opin. 2018;34(12):2125–2130. doi: 10.1080/03007995.2018.1524751. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzer G. General package for meta-analysis version 4.9–4 2019 [Available from: https://cran.r-project.org/web/packages/meta/meta.pdf.
- 29.Lapierre Y, O'Connor P, Devonshire V, Freedman MS, Kremenchutzky M, Yeung M, et al. Canadian experience with Fingolimod: adherence to treatment and monitoring. Can J Neurol Sci. 2016;43(2):278–283. doi: 10.1017/cjn.2015.325. [DOI] [PubMed] [Google Scholar]
- 30.Williams MJ, Johnson K, Trenz HM, Korrer S, Halpern R, Park Y, et al. Adherence, persistence, and discontinuation among Hispanic and African American patients with multiple sclerosis treated with fingolimod or glatiramer acetate. Curr Med Res Opin. 2018;34(1):107–115. doi: 10.1080/03007995.2017.1374937. [DOI] [PubMed] [Google Scholar]
- 31.Bergvall N, Petrilla AA, Karkare SU, Lahoz R, Agashivala N, Pradhan A, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014;17(10):696–707. doi: 10.3111/13696998.2014.940422. [DOI] [PubMed] [Google Scholar]
- 32.Agashivala N, Wu N, Abouzaid S, Wu Y, Kim E, Boulanger L, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138. doi: 10.1186/1471-2377-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wicks P, Rasouliyan L, Katic B, Nafees B, Flood E, Sasane R. The real-world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis. BMC Res Notes. 2016;9(1):434. doi: 10.1186/s13104-016-2243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ontaneda D, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci. 2012;323(1–2):167–172. doi: 10.1016/j.jns.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lattanzi S, Danni M, Taffi R, Cerqua R, Carlini G, Pulcini A, et al. Persistence to oral disease-modifying therapies in multiple sclerosis patients. J Neurol. 2017;264(11):2325–2329. doi: 10.1007/s00415-017-8595-8. [DOI] [PubMed] [Google Scholar]
- 36.Vollmer B, Nair KV, Sillau SH, Corboy J, Vollmer T, Alvarez E. Comparison of fingolimod and dimethyl fumarate in the treatment of multiple sclerosis: two-year experience. Mult Scler J Exp Transl Clin. 2017;3(3):2055217317725102. doi: 10.1177/2055217317725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Hashel J, Ahmed SF, Behbehani R, Alroughani R. Real-world use of fingolimod in patients with relapsing remitting multiple sclerosis: a retrospective study using the national multiple sclerosis registry in Kuwait. CNS Drugs. 2014;28(9):817–824. doi: 10.1007/s40263-014-0185-z. [DOI] [PubMed] [Google Scholar]
- 38.Braune S, Lang M, Bergmann A, NeuroTransData SG. Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol. 2016;263(2):327–333. doi: 10.1007/s00415-015-7970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksson I, Cars T, Piehl F, Malmstrom RE, Wettermark B, von Euler M. Persistence with dimethyl fumarate in relapsing-remitting multiple sclerosis: a population-based cohort study. Eur J Clin Pharmacol. 2018;74(2):219–226. doi: 10.1007/s00228-017-2366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernst FR, Barr P, Elmor R, Wong SL. Relapse outcomes, safety, and treatment patterns in patients diagnosed with relapsing-remitting multiple sclerosis and initiated on subcutaneous interferon beta-1a or dimethyl fumarate: a real-world study. Curr Med Res Opin. 2017;33(12):2099–2106. doi: 10.1080/03007995.2017.1380616. [DOI] [PubMed] [Google Scholar]
- 41.Frisell T, Forsberg L, Nordin N, Kiesel C, Alfredsson L, Askling J, et al. Comparative analysis of first-year fingolimod and natalizumab drug discontinuation among Swedish patients with multiple sclerosis. Mult Scler. 2016;22(1):85–93. doi: 10.1177/1352458515579216. [DOI] [PubMed] [Google Scholar]
- 42.Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320–327. doi: 10.1001/jamaneurol.2017.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He A, Spelman T, Jokubaitis V, Havrdova E, Horakova D, Trojano M, et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol. 2015;72(4):405–413. doi: 10.1001/jamaneurol.2014.4147. [DOI] [PubMed] [Google Scholar]
- 44.Hersh CM, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA, Ontaneda D. Experience with fingolimod in clinical practice. Int J Neurosci. 2015;125(9):678–685. doi: 10.3109/00207454.2014.969839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hersh CM, Love TE, Bandyopadhyay A, Cohn S, Hara-Cleaver C, Bermel RA, et al. Comparative efficacy and discontinuation of dimethyl fumarate and fingolimod in clinical practice at 24-month follow-up. Mult Scler J Exp Transl Clin. 2017;3(3):2055217317715485. doi: 10.1177/2055217317715485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hersh CM, Love TE, Cohn S, Hara-Cleaver C, Bermel RA, Fox RJ, et al. Comparative efficacy and discontinuation of dimethyl fumarate and fingolimod in clinical practice at 12-month follow-up. Mult Scler Relat Disord. 2016;10:44–52. doi: 10.1016/j.msard.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Hua LH, Fan TH, Conway D, Thompson N, Kinzy TG. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler. 2019;25(5):699–708. doi: 10.1177/1352458518765656. [DOI] [PubMed] [Google Scholar]
- 48.Johnson KM, Zhou H, Lin F, Ko JJ, Herrera V. Real-world adherence and persistence to Oral disease-modifying therapies in multiple sclerosis patients over 1 year. J Manag Care Spec Pharm. 2017;23(8):844–852. doi: 10.18553/jmcp.2017.23.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazareth T, Friedman HS, Navaratnam P, Herriott DA, Ko JJ, Barr P, et al. Persistency, medication prescribing patterns, and medical resource use associated with multiple sclerosis patients receiving oral disease-modifying therapies: a retrospective medical record review. BMC Neurol. 2016;16(1):187. doi: 10.1186/s12883-016-0698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smoot K, Spinelli KJ, Stuchiner T, Lucas L, Chen C, Grote L, et al. Three-year clinical outcomes of relapsing multiple sclerosis patients treated with dimethyl fumarate in a United States community health center. Mult Scler. 2018;24(7):942–950. doi: 10.1177/1352458517709956. [DOI] [PubMed] [Google Scholar]
- 51.Warrender-Sparkes M, Spelman T, Izquierdo G, Trojano M, Lugaresi A, Grand'Maison F, et al. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler. 2016;22(4):520–532. doi: 10.1177/1352458515594041. [DOI] [PubMed] [Google Scholar]
- 52.Zhovtis Ryerson L, Green R, Confident G, Pandey K, Richter B, Bacon T, et al. Efficacy and tolerability of dimethyl fumarate in White-, African- and Hispanic- Americans with multiple sclerosis. Ther Adv Neurol Disord. 2016;9(6):454–461. doi: 10.1177/1756285616661929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmer A, Coslovsky M, Abraham I, Decard BF. Adherence to fingolimod in multiple sclerosis: an investigator-initiated, prospective, observational, single-center cohort study. Patient Prefer Adherence. 2017;11:1815–1830. doi: 10.2147/PPA.S140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devonshire V, Lapierre Y, Macdonell R, Ramo-Tello C, Patti F, Fontoura P, et al. The global adherence project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77. doi: 10.1111/j.1468-1331.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Invest. 2010;30(2):89–100. doi: 10.2165/11533330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Tan H, Yu J, Tabby D, Devries A, Singer J. Clinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort study. Mult Scler. 2010;16(8):956–963. doi: 10.1177/1352458510373487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan H, Cai Q, Agarwal S, Stephenson JJ, Kamat S. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther. 2011;28(1):51–61. doi: 10.1007/s12325-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 58.Thomas NP, Curkendall S, Farr AM, Yu E, Hurley D. The impact of persistence with therapy on inpatient admissions and emergency room visits in the US among patients with multiple sclerosis. J Med Econ. 2016;19(5):497–505. doi: 10.3111/13696998.2015.1134546. [DOI] [PubMed] [Google Scholar]
- 59.Nicholas J, Ko JJ, Park Y, Navaratnam P, Friedman HS, Ernst FR, et al. Assessment of treatment patterns associated with injectable disease-modifying therapy among relapsing-remitting multiple sclerosis patients. Mult Scler J Exp Transl Clin. 2017;3(1):2055217317696114. doi: 10.1177/2055217317696114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ. 2010;13(1):90–98. doi: 10.3111/13696990903579501. [DOI] [PubMed] [Google Scholar]
- 61.Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care. 2013;15(1):36–45. doi: 10.7224/1537-2073.2011-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–539. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–e33. [PubMed] [Google Scholar]
- 64.Bubalo J, Clark RK, Jr., Jiing SS, Johnson NB, Miller KA, Clemens-Shipman CJ, et al. Medication adherence: pharmacist perspective. J Am Pharm Assoc (2003). 2010;50(3):394–406. [DOI] [PubMed]
- 65.Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence. 2010;4:1–9. doi: 10.2147/ppa.s8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wicks P, Brandes D, Park J, Liakhovitski D, Koudinova T, Sasane R. Preferred features of oral treatments and predictors of non-adherence: two web-based choice experiments in multiple sclerosis patients. Interact J Med Res. 2015;4(1):e6. doi: 10.2196/ijmr.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarrants M, Oleen-Burkey M, Castelli-Haley J, Lage MJ. The impact of comorbid depression on adherence to therapy for multiple sclerosis. Mult Scler Int. 2011;2011:271321. doi: 10.1155/2011/271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higuera L, Carlin CS, Anderson S. Adherence to disease-modifying therapies for multiple sclerosis. J Manag Care Spec Pharm. 2016;22(12):1394–1401. doi: 10.18553/jmcp.2016.22.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 70.Durand H, Hayes P, Morrissey EC, Newell J, Casey M, Murphy AW, et al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35(12):2346–2357. doi: 10.1097/HJH.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 71.Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich) 2011;13(12):898–909. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheepers L, van Onna M, Stehouwer CDA, Singh JA, Arts ICW, Boonen A. Medication adherence among patients with gout: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;47(5):689–702. doi: 10.1016/j.semarthrit.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Shehab A, Bhagavathula AS, Abebe TB, Abegaz TM, Elnour AA, Sabbour HM, et al. Patient adherence to novel oral anticoagulants (NOACs) for the treatment of atrial fibrillation and occurrence of associated bleeding events: a systematic review and meta-analysis. Curr Vasc Pharmacol. 2019;17(4):341–349. doi: 10.2174/1570161116666180123111949. [DOI] [PubMed] [Google Scholar]
- 74.Canfield SL, Zuckerman A, Anguiano RH, Jolly JA, DeClercq J, Wascher M, et al. Navigating the wild west of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25(10):1073–1077. doi: 10.18553/jmcp.2019.25.10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Methods: Electronic Search Strategy. Details of electronic literature search strategy, exclusion criteria and full list of search results (including abstract citation, title, text, digital objective identifier [DOI], PubMed ID [PMID], and author names and information).
Additional file 2: Supplementary Figure 1. Leave-one-out sensitivity analysis for the proportion of patients discontinuing a DMD. For studies for which results for treatment naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. Abbreviations: DMD, disease-modifying drug.
Additional file 3: Supplementary Figure 2. Leave-one-out sensitivity analysis for subgroup analyses for the proportion of patients discontinuing a DMD. For studies for which results for treatment naive and treatment-experienced patients were reported separately (combined data were not available), data were combined; for studies reporting data for more than 1 oral DMD (combined data were not reported), data were combined; for studies reporting data for subgroups (combined data were not reported), data were combined. Abbreviations: DMD: disease-modifying drug.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.