Abstract
Objective
Comorbid conditions are associated with poor prognosis in COVID-19. Registry data show that patients with cirrhosis may be at high risk. However, outcome comparisons among patients with cirrhosis+COVID-19 versus patients with COVID-19 alone and cirrhosis alone are lacking. The aim of this study was to perform these comparisons.
Design
A multicentre study of inpatients with cirrhosis+COVID-19 compared with age/gender-matched patients with COVID-19 alone and cirrhosis alone was performed. COVID-19 and cirrhosis characteristics, development of organ failures and acute-on-chronic liver failure (ACLF) and mortality (inpatient death+hospice) were compared.
Results
37 patients with cirrhosis+COVID-19 were matched with 108 patients with COVID-19 and 127 patients with cirrhosis from seven sites. Race/ethnicity were similar. Patients with cirrhosis+COVID-19 had higher mortality compared with patients with COVID-19 (30% vs 13%, p=0.03) but not between patients with cirrhosis+COVID-19 and patients with cirrhosis (30% vs 20%, p=0.16). Patients with cirrhosis+COVID-19 versus patients with COVID-19 alone had equivalent respiratory symptoms, chest findings and rates of intensive care unit transfer and ventilation. However, patients with cirrhosis+COVID-19 had worse Charlson Comorbidity Index (CCI 6.5±3.1 vs 3.3±2.5, p<0.001), lower presenting GI symptoms and higher lactate. Patients with cirrhosis alone had higher cirrhosis-related complications, maximum model for end-stage liver disease (MELD) score and lower BiPAP/ventilation requirement compared with patients with cirrhosis+COVID-19, but CCI and ACLF rates were similar. In the entire group, CCI (OR 1.23, 95% CI 1.11 to 1.37, p<0.0001) was the only variable predictive of mortality on multivariable regression.
Conclusions
In this multicentre North American contemporaneously enrolled study, age/gender-matched patients with cirrhosis+COVID-19 had similar mortality compared with patients with cirrhosis alone but higher than patients with COVID-19 alone. CCI was the only independent mortality predictor in the entire matched cohort.
Keywords: cirrhosis, liver cirrhosis, chronic liver disease, sepsis, infectious disease
Significance of this study.
What is already known on this subject?
COVID-19 is associated with higher mortality in patients with comorbidities.
Whether COVID-19 adds to the mortality of hospitalised patients with cirrhosis and complications is unknown.
What are the new findings?
In a multicentre North American experience, inpatients with cirrhosis and COVID-19 had higher mortality risk than inpatients with COVID-19 infection alone.
The risk of mortality in hospitalised patients with cirrhosis+COVID-19 was not, however, significantly higher than those hospitalised with cirrhosis but without COVID-19.
Patients with cirrhosis hospitalised for COVID-19 are more likely to develop complications related to the viral infection rather than the complications related to cirrhosis observed in those with cirrhosis alone.
Patients with cirrhosis+COVID-19 are less likely to present with GI symptoms compared with those with COVID-19 alone.
How might it impact on clinical practice in the foreseeable future?
In patients with COVID-19, underlying cirrhosis should be considered a high-risk comorbid condition.
When patients with cirrhosis and COVID-19 infection are hospitalised, focus should not be taken away from prevention and early treatment of complications of cirrhosis.
Despite this pandemic, patients with cirrhosis without COVID-19 should not be discouraged from seeking essential medical care.
Introduction
Worldwide, severe acute respiratory syndrome coronavirus 2 infection causing COVID-19 has been associated with high mortality.1 While the predominant manifestations and mortality are mediated by pulmonary involvement, cirrhosis, due to an inherent immune dysfunction and altered gut–liver axis, is assumed to be a high-risk comorbid condition for severe COVID-19.2 Registry data in those with cirrhosis in the setting of COVID-19 note an association with a poor prognosis with an almost 40% mortality rate,3 4 which was mostly related to pulmonary followed by liver-related causes. In these registry data, the death rate increased with severity of cirrhosis, and this risk worsened in those with a background of liver transplant.5 However, the lack of a denominator comparing these data to patients with cirrhosis but without COVID-19 and those with COVID-19 and the absence of advanced liver disease make it challenging to assume the increased risk of severe outcomes in cirrhosis. A population-based claims data analysis showed that cirrhosis is associated with poor outcomes, which are largely based on billing data.6 Therefore, the impact of COVID-19 on in-hospital outcomes in patients with cirrhosis is unclear with respect to mortality and acute-on-chronic liver failure (ACLF) development. Studies including comparisons with control populations with and without cirrhosis and COVID-19 are limited.7 8
Therefore, the aim of our study was to determine the impact of COVID-19 on hospitalised patients with cirrhosis by matching them with cohorts with cirrhosis but without COVID-19 and those with COVID-19 without cirrhosis who were contemporaneously hospitalised in several North American centres.
Patients and methods
We reviewed all non-elective hospitalisations from all centres between 23 March and 21 May 2020 for all COVID-19-positive cases and all patients with cirrhosis. Cirrhosis was diagnosed through either a prior liver biopsy, evidence of frank hepatic decompensation, radiological evidence of a nodular liver and/or features of portal hypertension or endoscopic evidence of varices in patients with chronic liver disease. Diagnosis of COVID-19 required a positive nasopharyngeal swab as per the local site-specific diagnosis protocol. Patient groups with cirrhosis+COVID-19 and patient groups with COVID-19 alone and with cirrhosis alone (cirrhosis without COVID-19) were matched with each other with respect to age and gender in a 1:2 and 1:5 ratios. Our initial aim was to match at each site cirrhosis+COVID-19 to the other two groups in a 1:5 ratio. Because there was high variability in the number of COVID-19 cases across sites during the study time period, age and gender matching had to be altered and ranged from 1:2 to 1:5. We excluded subjects who had solid organ transplant, had HIV infection and those with unclear diagnoses of cirrhosis or COVID-19. For those admitted multiple times during this period, only the first admission was analysed.
Data recorded were demographics; comorbid conditions and Charlson Comorbidity Index (CCI); reasons for admission, hospital course, including complications related to COVID-19 and also those due to cirrhosis as applicable; ACLF occurrence; and outcome of the hospitalisation.9 ACLF was defined according to the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) criteria.7 10 Data were entered in a secure REDCap site, and the patients groups with cirrhosis+COVID-19 were compared with patient groups with COVID-19 without cirrhosis and cirrhosis without COVID-19 using analysis of variance, χ2 test and Kruskal-Wallis test as appropriate. Patients with cirrhosis+COVID-19 were individually compared with patients with cirrhosis alone and COVID-19 alone using χ2 tests, t-tests and Fisher exact tests as appropriate. Multivariable binary logistic regression using inpatient mortality for the entire cohort was performed using variables that were significant at the p<0.10 level on univariable analysis. Variable inputs were age, gender, ethnicity, race, COVID-19 status, cirrhosis status, CCI and other comorbid conditions.
Patient and public involvement
There was no patient or public involvement.
Results
Figure 1 shows the matching of cohorts and the three groups. The cohort of cirrhosis+COVID-19 included 37 patients, while 127 had cirrhosis alone and 108 with COVID-19 alone.
Figure 1.
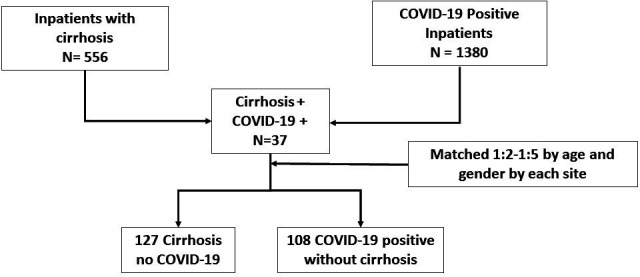
Flowchart of patient recruitment and matching.
Subject characteristics
No differences in age, gender, race and ethnicity were observed between groups (table 1). Cardiovascular and respiratory comorbid conditions were similar between groups, but patients with cirrhosis alone had lower hypertension rates. Patients with cirrhosis alone also had higher rates of current smoking and alcohol consumption compared with the other groups. CCI was significantly higher in cirrhosis regardless of COVID-19 status compared with patients with COVID-19 only. Of the 37 patients with cirrhosis+COVID-19, 10 were from Virginia Commonwealth University (VCU), 9 from Yale, 7 from Washington, 6 from Philadelphia, 2 each from Toronto and Central Virginia Veterans Healthcare System and 1 from Mayo Clinic.
Table 1.
Baseline comparison between the three groups
| COVID-19 alone (n=108) |
COVID-19+cirrhosis (n=37) |
Cirrhosis alone (n=127) |
P value for all groups* | |
| Age | 61.3±11.5 | 61.0±10.6 | 59.5±10.8 | 0.45 |
| Male gender | 37 (34%) | 10 (27%) | 43 (34%) | 0.69 |
| Race (white/non-white) | 55/53 | 19/18 | 79/49 | 0.23 |
| Hispanic ethnicity | 9 (8%) | 4 (10%) | 7 (6%) | 0.51 |
| Cirrhosis aetiology (HCV/Alc/HCV+Alc/NASH/others) |
– | 9/9/4/9/6 | 17/58/12/23/17 | 0.10 |
| Comorbid conditions | ||||
| Type 2 diabetes | 41 (38%) | 18 (49%) | 47 (37%) | 0.43 |
| Hypertension†‡ | 72 (67%) | 25 (67%) | 64 (50%) | 0.02 |
| Coronary artery disease | 15 (14%) | 4 (10%) | 10 (8%) | 0.33 |
| Congestive heart failure | 9 (8%) | 8 (22%) | 16 (13%) | 0.12 |
| Asthma | 9 (8%) | 3 (9%) | 13 (10%) | 0.86 |
| COPD | 11 (10%) | 7 (19%) | 20 (16%) | 0.31 |
| Other chronic lung disease | 6 (6%) | 3 (9%) | 2 (2%) | 0.12 |
| Chronic kidney disease | 17 (16%) | 8 (22%) | 28 (22%) | 0.45 |
| Hepatocellular cancer† | 0 (0%) | 3 (8%) | 6 (5%) | 0.03 |
| Other cancer | 11 (10%) | 4 (10%) | 6 (5%) | 0.21 |
| History of stroke‡ | 12 (11%) | 2 (5%) | 4 (3%) | 0.04 |
| Charlson Comorbidity Index†‡ | 3.3±2.5 | 6.5±3.1 | 5.9±2.5 | <0.0001 |
| Social history | ||||
| Current cigarette† | 7 (6%) | 8 (22%) | 49 (39%) | 0.001 |
| Current other tobacco | 0 (0%) | 1 (3%) | 3 (2%) | 0.50 |
| Current alcohol†‡ | 2 (2%) | 4 (10%) | 32 (25%) | <0.001 |
| Prior hospital visits in 6 months, median (IQR) | 0 (1) | 1 (2) | 2 (2) | <0.001 |
Data presented as raw number (%) or mean±SD unless otherwise stated. No significant differences in the CCI in cirrhosis+COVID-19 versus cirrhosis alone.
*Kruskal-Wallis, χ2 tests or analysis of variance as appropriate.
†P<0.05 between COVID-19+cirrhosis and cirrhosis alone using Fisher exact test, χ2 test or unpaired t-test.
‡P<0.05 between COVID-19 alone and COVID-19+cirrhosis.
Alc, alcohol-related; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; NASH, non-alcoholic steatohepatitis.
Mortality risk
There was a significantly higher death/hospice rate in the cirrhosis+COVID-19 group compared with the COVID-19-alone group (30% vs 13%, p=0.03), but this rate was statistically similar to that of the cirrhosis-alone group (30% vs 20%, p=0.11; figure 2 and table 2). Of the 40 patients with NACSELD-ACLF, 11 had cirrhosis+COVID-19 and 29 had cirrhosis alone. In those with NACSELD-ACLF, the mortality rate was similar regardless of COVID-19 (n=6, 55%, vs n=10, 36%, p=0.25).
Figure 2.
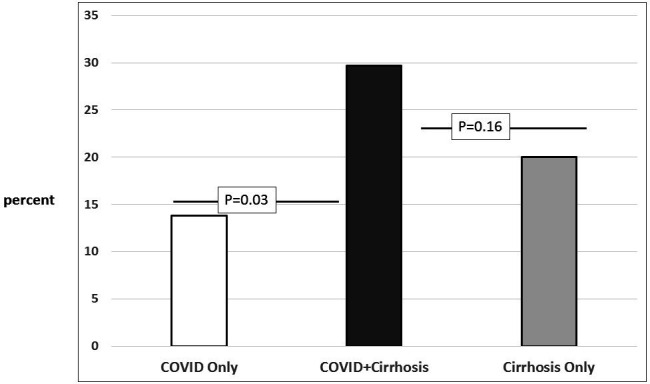
Mortality (in-hospital mortality and hospice) comparison between groups.
Table 2.
Hospital course and outcomes
| COVID-19 alone (n=108) | COVID-19+cirrhosis (n=37) | Cirrhosis alone (n=127) | P value for all groups* | |
| Infections | ||||
| Infection as reason for admission† | 108 (100%) | 33 (89%) | 29 (23%) | <0.0001 |
| Further infections | 15 (14%) | 5 (14%) | 32 (25%) | 0.05 |
| Hospital course | ||||
| Large-volume paracentesis | – | 3 (11%) | 26 (20%) | <0.0001 |
| Central line placement† | 24 (22%) | 10 (27%) | 14 (11%) | 0.07 |
| BiPAP†‡ | 11 (10%) | 10 (27%) | 3 (2%) | <0.0001 |
| Upper endoscopy | 0 (0%) | 3 (11%) | 21 (17%) | <0.0001 |
| GI bleeding‡ | 1 (1%) | 5 (14%) | 27 (21%) | <0.0001 |
| Variceal banding | – | 1 (3%) | 11 (9%) | 0.001 |
| Highest lactate‡ | 1.8±0.91 | 3.9±4.6 | 3.3±2.3 | 0.001 |
| Lowest sodium‡ | 134.0±5.3 | 132.9±5.1 | 132.6±6.5 | 0.31 |
| Lowest albumin‡ | 2.9±0.5 | 2.5.0±0.6 | 2.6±0.66 | 0.001 |
| Highest creatinine‡ | 1.9±2.4 | 2.53±2.1 | 2.3±2.4 | 0.38 |
| Highest MELD | – | 17.6±8.6 | 22.8±10.1 | 0.004 |
| Organ failures | ||||
| Mechanical ventilation† | 41 (39%) | 14 (38%) | 18 (14%) | <0.0001 |
| Renal replacement‡ | 7 (7%) | 7 (19%) | 15 (12%) | 0.12 |
| HE grade III/IV | – | 5 (14%) | 32 (25%) | <0.0001 |
| Shock† | 18 (17%) | 11 (30%) | 11 (9%) | 0.006 |
| Outcomes | ||||
| NACSELD-ACLF | – | 11 (30%) | 29 (23%) | 0.11 |
| ICU transfer† | 41 (38%) | 16 (43%) | 31 (24%) | 0.05 |
| Length of stay | 12.6±8.2 | 17.5±22.0 | 11.1±16.2 | 0.11 |
| Death/hospice‡ | 15 (13.8%) | 11 (30%) | 24 (19%) | 0.12 |
Data presented as raw number (%) or mean±SD unless otherwise stated.
*Kruskal-Wallis, χ2 tests or analysis of variance as appropriate.
†P<0.05 between COVID-19+cirrhosis and cirrhosis alone using Fisher exact test, χ2 test or unpaired t-test.
‡P<0.05 between COVID-19 only and COVID-19+cirrhosis.
ACLF, acute-on-chronic liver failure; BiPAP, bilevel positive airway pressure; HE, hepatic encephalopathy; ICU, intensive care unit; MELD, model for end-stage liver disease; NACSELD, North American Consortium for the Study of End-Stage Liver Disease.
Hospital course details
We found higher mechanical ventilation rates, BiPAP use, central line placement and intensive care unit (ICU) transfer in cirrhosis+COVID-19 as compared with the cirrhosis-alone group (table 2). BiPAP use was also higher in the cirrhosis+COVID-19 group than in the COVID-19-alone group. Cirrhosis-alone patients had higher rates of grade III/IV HE, developed a higher model for end-stage liver disease (MELD) score and required more endoscopic procedures, including variceal banding, and large-volume paracentesis, than the cirrhosis+COVID-19 group. Both cirrhosis groups had lower albumin and serum sodium and higher lactate and serum creatinine levels than patients with COVID-19-alone, but no specific differences were found between the two cirrhosis subgroups. NACSELD-ACLF was similar between the two cirrhosis groups. Three patients in the cirrhosis-alone group were transplanted while inpatients and were counted as alive.
COVID-19 details
All patients were diagnosed using nasopharyngeal swabs per criteria set out at local sites and were admitted due to symptoms pertaining to COVID-19 in all cases of patients with COVID-19 only, and in most patients with cirrhosis+COVID-19 (n=33, 89%). The remaining four patients with cirrhosis+COVID-19 were diagnosed within 2 days of admission. The most common symptoms were respiratory but as shown in table 3, patients with cirrhosis+COVID-19 were less likely to present with GI symptoms compared with those without COVID-19. Patients with COVID-19 with or without cirrhosis had similar chest imaging findings (table 3). When compared with patients with cirrhosis alone, chest imaging showed ground-glass opacities and pneumonia in more patients with COVID-19 regardless of cirrhosis (online supplementary table S1) and more compared with cirrhosis alone. Pleural effusions and pulmonary oedema rates were equivalent between the three groups, while a higher proportion of patients cirrhosis alone had normal chest imaging. The rate of chronic kidney disease and the renal replacement therapy was similar across all groups. Patients with COVID-19 with and without cirrhosis were on similar patterns of antibiotics, which were mostly beta-lactam antibiotics, macrolides and vancomycin (online supplementary table S2). A similar proportion of patients with cirrhosis+COVID-19 and COVID-19 alone were on non-steroidal anti-inflammatory drugs, acetaminophen, chloroquine/hydroxychloroquine and open-label remdesivir. A higher proportion of patients with COVID-19 alone were enrolled in placebo-controlled trials compared with those with cirrhosis+COVID-19.
Table 3.
COVID-19-specific questions
| COVID-19 alone (n=108) | COVID-19+cirrhosis (n=37) | |
| Symptoms of COVID-19 | ||
| Fever | 75 | 20 |
| Cough | 70 | 26 |
| Shortness of breath | 74 | 23 |
| Abdominal pain | 17 | 9 |
| Diarrhoea* | 29 | 3 |
| Nausea/vomiting* | 29 | 4 |
| Generalised fatigue | 44 | 12 |
| Body aches* | 31 | 4 |
| Headaches | 15 | 2 |
| Loss of taste | 5 | 1 |
| Loss of smell | 5 | 1 |
| Confusion | 8 | 4 |
| Chest pain | 19 | 3 |
| Syncope | 1 | 0 |
| Chest X-ray | ||
| Normal | 9 | 4 |
| Bilateral ground-glass opacities | 33 | 11 |
| Bilateral ground-glass consolidation | 5 | 1 |
| Pneumonia | 46 | 11 |
| Pulmonary oedema | 14 | 4 |
| Pleural effusion | 12 | 6 |
| CT scan | ||
| Normal | 0 | 0 |
| Bilateral ground-glass opacities | 23 | 7 |
| Bilateral ground-glass consolidation | 6 | 2 |
| Pneumonia | 19 | 6 |
| Pulmonary oedema | 2 | 3 |
| Pleural effusion | 6 | 2 |
Data presented as raw numbers.
Apart from diarrhoea and nausea/vomiting, all other comparisons were statistically similar.
*P<0.05 between the two groups.
gutjnl-2020-322118supp001.pdf (20.1KB, pdf)
Regression for mortality
On univariable analysis, only age, gender and CCI were significant for inpatient mortality. Cirrhosis alone, COVID-19 alone, hypertension and being in a clinical trial were not significant. On multivariable analysis, a higher CCI was associated with greater mortality (OR 1.23, 95% CI 1.11 to 1.37, p<0.0001).
Discussion
Our multicentre experience shows that age-matched and gender-matched patients with cirrhosis+COVID-19 had a higher mortality rate compared with those with COVID-19 alone, but this rate was not significantly higher than that in patients with cirrhosis alone. Most patients with COVID-19+cirrhosis were admitted with respiratory complaints and required similar rates of respiratory and intensive care compared with those admitted with COVID-19 alone. In contrast, patients with cirrhosis alone admitted during the same time period required care pertaining to cirrhosis-related complications.
These findings build on multiple case series and registry data that demonstrate an overall poor prognosis of patients with cirrhosis who develop COVID-19 and compare them to controls from both COVID-19 and cirrhosis cohorts.3 4 11 The focused time frame and dedicated controls with COVID-19 and comparing with cirrhosis alone are key strengths of this analysis.
Although the mortality in patients with cirrhosis, irrespective of COVID-19 status, was statistically similar, there were differences in the two cohorts from a liver disease severity perspective. Patients with cirrhosis and COVID-19 had lower MELD scores and a lower rate of liver disease complications. Thus, it is likely that COVID-19 contributed to the mortality in those with cirrhosis, which is further supported by a higher rate of need for mechanical ventilation and bilevel positive airway pressure (BiPAP) compared with patients with cirrhosis and without COVID-19. While the hospitalisations in patients with cirrhosis have decreased in a population-based Veterans-centred study, patients with cirrhosis who develop urgent issues continue to receive in-hospital care.12 It may be possible that, during the COVID-19 pandemic, only the sickest patients with cirrhosis were admitted and the added infection had no effect on their course. This was confirmed with a higher ACLF rate in our population with cirrhosis compared with historical experience in North America and reiterates the need for contemporaneous comparison rather than relying on historical death rates.7
Our results demonstrate that patients with cirrhosis alone were more likely to be admitted and treated with cirrhosis-specific interventions such as large-volume paracentesis, variceal banding and developed grade III/IV hepatic encephalopathy compared with patients with cirrhosis+COVID-19. This is despite likely isolation protocols that could have limited these case admissions unless emergent. Cirrhosis-related biochemical parameters such as albumin, sodium and creatinine were similarly impaired in both cirrhosis subgroups. Importantly, the ACLF rates were similar and likely due to patients with cirrhosis alone developing more grade III/IV hepatic encephalopathy and liver failure evidenced by a higher peak MELD score, while higher shock and respiratory failure rates were seen in the COVID-19+cirrhosis group. Ultimately, the similar outcomes within the cirrhosis groups and similar ACLF and ACLF-associated mortality rates reiterate that major risk of death is inherent in patients with cirrhosis who are hospitalised for non-elective reasons.
On the other hand, the rate of death in cirrhosis+COVID-19 was higher than that of patients with COVID-19 alone. It is interesting that patients with cirrhosis+COVID-19 behaved largely like patients with COVID-19 alone with respect to respiratory symptoms and complications and a higher rate of hypertension at baseline. Rates of ICU transfer, central line placement and mechanical ventilation were similar in patients with COVID-19+cirrhosis and COVID-19 alone. Of note is the rate of chronic kidney disease and renal replacement therapy was similar across all groups. This is of interest since both patients with COVID-19 alone and those with cirrhosis are known to have high rates of renal dysfunction either presenting as new event and/or a high mortality risk. Unexpectedly, the rates of acute kidney injury (AKI) and mortality associated with AKI were comparably similar between groups and were relatively low compared with prior cirrhosis and COVID-19 literature.13 14 BiPAP being offered more to patients with cirrhosis+COVID-19 is interesting as it may signify attempts to avoid intubation, often a harbinger of death in cirrhosis. Baseline pulmonary ‘compromise’ due to factors such as sarcopenia and poor respiratory excursion, ascites elevating the diaphragms and pleural effusions may be other reasons for the high use of BiPAP.15 16 Despite these efforts, more patients with cirrhosis+COVID-19 died than those with COVID-19 alone. A higher serum lactate in those with cirrhosis+COVID-19 could signal a greater anaerobic milieu, which has already been shown to be associated with high mortality in cirrhosis.17 Therefore, a lower hepatic reserve and worse CCI could predispose to this higher death rate. Indeed, the only significant predictor of mortality was the CCI. These findings extend a published claims-based study of higher risk of death in patients with cirrhosis+COVID-19 compared with those with COVID-19 alone into a multisite, individually curated setting.6
While most of our COVID-19-positive patients were either diagnosed at admission and the rest within 2 days of admission, the presentation is often varied. In our experience, patients with cirrhosis+COVID-19 were likely to present with similar respiratory, taste/smell and cardiovascular symptoms but relatively lower rate of GI symptoms.3 4 18 While the reasons for this are unclear, one could speculate that these differences could be due to the inherently higher rate of GI symptoms at baseline in those with cirrhosis.19 The use of lactulose may also mask these as symptoms heralding COVID-19 in patients with cirrhosis.
Since we had matched for age and gender based on the cirrhosis+COVID-19 group, the sites had varying numbers of patients in the complementary groups that could fit these criteria. Further, these patients are representative of the published literature both in COVID-19 and in cirrhosis, and these findings are unlikely to change even if all patients admitted in this period were included. The higher mortality in the cirrhosis+COVID-19 group supports the position that such patients should be excluded from all trials except those specifically enrolling patients with cirrhosis+COVID-19. Other determinants of prognosis, such as ethnicity and race, were also evenly distributed, as were most comorbid conditions.20 While more patients in the COVID-19-alone group were in a clinical trial compared with those in the cirrhosis+COVID-19 group, it is unlikely that these would make a major difference since these were placebo-controlled and due to the relatively low efficacy of most agents that are under trial. Further, systematic exclusion of patients with cirrhosis from most of the therapeutic trials may or may not have played a role in outcomes. We also only focused on cirrhosis already diagnosed as an outpatient rather than the broader chronic liver disease diagnosis, given the impact of COVID-19 on hepatic biochemical tests, which makes the diagnosis of chronic non-cirrhotic liver disease difficult during hospitalisation.21
The strengths of the study include the control groups with cirrhosis but without COVID-19 and the group with COVID-19 without cirrhosis to allow better characterisation of the impact of COVID-19 in patients with cirrhosis. In addition, this is a multicentre study involving centres with high levels of expertise in infectious diseases and hepatology. Patients were hospitalised during the identical period to better understand the impact of COVID-19. The weaknesses of the study include the relatively small number of patients with cirrhosis+COVID-19. To that end, our observation will need to be validated in larger cohorts. Further, the mortality in the cirrhosis+COVID-19 group might be even higher than that in the COVID-19-alone group when effective treatment is available for COVID-19 since patients with cirrhosis are likely to be denied such treatment until safety in cirrhosis is demonstrated.
We conclude in this multicentre study that patients hospitalised with COVID-19 in the setting of cirrhosis have an inpatient mortality rate that is similar to that of patients admitted due to cirrhosis alone but higher than those admitted with COVID-19 without cirrhosis. Thus, it is important to recognise that hospitalised patients with cirrhosis are inherently at high risk of mortality independent of COVID-19. Patients with cirrhosis but without COVID-19 who would otherwise require hospitalisation should be hospitalised whenever local protocols allow.11 Another important observation is that three patients in the uninfected cirrhosis cohort underwent liver transplant during this short period of follow-up, a favourable observation that transplants were done despite the changes in hospital policies around the pandemic. Lastly, these data are important to interpret the risk of COVID-19 in the setting of cirrhosis. While our data suggest that COVID-19 does not significantly increase mortality risk in cirrhosis, this finding needs to be further evaluated in larger cohorts. Further, the mortality in the cirrhosis+COVID-19 group might trend even higher than that in the COVID-19-alone group in the future since these patients are likely to be denied potentially effective pharmacological therapy treatment until safety in cirrhosis is demonstrated. In the meantime, patients with cirrhosis should not be discouraged from seeking essential medical care during this pandemic.
Footnotes
Contributors: JSB, GG-T, SB, PSK, FW and KRR conceptualised the project and helped with collection and data visualisation, and drafting and review of the manuscript; SMcG was responsible for the REDCap database. SM, JS, MP, MC, AF, RdRR, JW, AO, CT and SD were involved in data entry.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved by all institutional review boards (ID BAJAJ0015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med 2020;382:2012–22. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention People of any age with underlying medical conditions. Available: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html
- 3. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2020;5:gutjnl-2020-321666. 10.1136/gutjnl-2020-321666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol 2020. doi: 10.1016/j.jhep.2020.05.013. [Epub ahead of print: 21 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US EpiCenter. Am J Transplant 2020;20:1800–8. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh S, Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: a multi-center research network study. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.04.064. [Epub ahead of print: 03 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367–74. 10.1002/hep.29773 [DOI] [PubMed] [Google Scholar]
- 8. Bajaj JS, Moreau R, Kamath PS, et al. Acute-On-Chronic liver failure: getting ready for prime time? Hepatology 2018;68:1621–32. 10.1002/hep.30056 [DOI] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10. Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. 10.1002/hep.27077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bollipo S, Kapuria D, Rabiee A, et al. One world, one pandemic, many guidelines: management of liver diseases during COVID-19. Gut 2020;69:gutjnl-2020-321553. 10.1136/gutjnl-2020-321553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmud N, Hubbard RA, Kaplan DE, et al. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.05.005. [Epub ahead of print: 05 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong F. Diagnosing and treating renal disease in cirrhotic patients. Minerva Gastroenterol Dietol 2016;62:253–66. [PubMed] [Google Scholar]
- 14. Selby NM, Forni LG, Laing CM, et al. Covid-19 and acute kidney injury in hospital: summary of NICE guidelines. BMJ 2020;369:m1963. 10.1136/bmj.m1963 [DOI] [PubMed] [Google Scholar]
- 15. Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574–80. 10.1002/hep.28316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gustot T, Fernandez J, Garcia E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–52. 10.1002/hep.27849 [DOI] [PubMed] [Google Scholar]
- 17. Sarmast N, Ogola GO, Kouznetsova M, et al. Model for end-stage liver Disease-Lactate and prediction of inpatient mortality in patients with chronic liver disease. Hepatology 2020. doi: 10.1002/hep.31199. [Epub ahead of print: 21 Feb 2020]. [DOI] [PubMed] [Google Scholar]
- 18. Suresh Kumar VC, Mukherjee S, Harne PS, et al. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol 2020;7:e000417. 10.1136/bmjgast-2020-000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalaitzakis E, Simrén M, Olsson R, et al. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol 2006;41:1464–72. 10.1080/00365520600825117 [DOI] [PubMed] [Google Scholar]
- 20. Bowleg L. We're not all in this together: on COVID-19, intersectionality, and structural inequality. Am J Public Health 2020:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol 2020. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-322118supp001.pdf (20.1KB, pdf)


