Abstract
Objective:
To investigate elevation of anti-citrullinated protein antibodies (ACPA) before RA diagnosis and risks for chronic obstructive pulmonary disease (COPD) or asthma.
Methods:
We performed a matched cohort study nested within the Nurses’ Health Studies among women who donated blood. Women with incident RA after blood draw (self-reported then confirmed by medical records) were each matched to three controls by age, cohort, year, and menopausal factors. Pre-RA ACPA+ was defined as >99th percentile of control distribution by a research assay or by CCP2 in a subset. Incident COPD and asthma after index date (date of blood draw) were identified by questionnaires. Cox regression estimated HRs for incident COPD or asthma (in separate analyses) associated with pre-RA, pre-RA ACPA+, or pre-RA ACPA− phenotypes each compared to their matched non-RA controls.
Results:
We analyzed 283 pre-RA women and 842 controls; blood was donated mean of 9.7 years (SD 5.8) before RA diagnosis. Fifty-nine women (20.8%) were pre-RA ACPA+. There were 107 cases of incident COPD and 105 incident asthma cases during 21,489 person-years of follow-up. Pre-RA ACPA+ was associated with increased COPD risk (HR 3.04, 95%CI 1.33,7.00) after adjusting for covariates including smoking pack-years. Pre-RA ACPA+ had a HR for asthma of 1.74 (multivariable 95%CI 0.72,4.24), similar to the risk of asthma for pre-RA ACPA− (HR 1.65, 95%CI 1.11,2.46).
Conclusion:
Women with elevated ACPA before RA diagnosis had increased risk for developing COPD compared to controls. Women who later developed RA were more likely to develop asthma, regardless of pre-RA ACPA status.
Keywords: COPD, asthma, obstruction, anti-citrullinated protein antibody, rheumatoid arthritis
INTRODUCTION
Elevation of anti-citrullinated protein antibodies (ACPA) is strongly associated with future risk of developing rheumatoid arthritis (RA)(1-5). Smoking is a known RA risk factor, perhaps in part due to increasing pulmonary mucosal inflammation leading to ACPA production particularly for individuals with the HLA-DRB1 shared epitope(2, 6-11). ACPA may originate at mucosal surfaces in the airways(12), and is central to RA pathogenesis. Inflammation, citrullination, ACPA formation, and autoimmunity leading to clinical RA may be important in the development of pulmonary abnormalities(13). Therefore, individuals with ACPA elevation may be more likely to develop obstructive lung diseases, even prior to clinical RA onset. This association has not been previously investigated.
Prior studies have demonstrated a relationship between RA and subsequent COPD risk(13-25). Several case-control studies and retrospective cohorts were limited by the inability to investigate serologic status(18, 22) or account for smoking(14-17, 21, 24), a strong risk factor for both COPD and RA. Some studies found that this association is more pronounced in seropositive RA(13, 25) and independent of smoking(13, 18, 22). Therefore, we investigated whether ACPA elevation before clinical RA onset could lead to COPD, particularly immediately prior to clinical RA diagnosis.
Asthma may also have a bi-directional association with RA, as it may be both a risk factor for RA and more likely to occur among patients with RA. Prior studies have also investigated the association between RA and asthma(13, 16, 17, 21, 26-28), with some showing that RA increased asthma risk, but these studies lacked data on ACPA status(26-28) or smoking(17, 27). A previous study suggested that airway abnormalities are more common in patients with pre-RA seropositivity than controls but was cross-sectional and did not study clinically diagnosed obstructive lung diseases(29).
Therefore, we investigated whether ACPA elevation in pre-RA banked blood was associated with subsequent risk for COPD or asthma. We measured ACPA in blood drawn prior to date of RA diagnosis and performed a cohort study to identify incident COPD or asthma occurring after blood draw. We aimed to determine whether pre-RA ACPA elevation was associated with subsequent risk of obstructive lung diseases. We hypothesized that elevated pre-RA ACPA would increase risks for COPD or asthma independent of smoking, particularly in the pre-RA period when lung inflammation may precede joint involvement.
METHODS
Study design and population
We conducted a matched cohort study nested within the Nurses’ Health Study (NHS) and NHSII, two large prospective cohorts of female registered nurses. The NHS is composed of 121,700 women aged 30-55 at time of baseline in 1976. The NHSII enrolled 116,429 women who were 25-42 at enrollment in 1989. Data on lifestyle, diseases, family history, and medications were obtained on questionnaires every two years during follow-up. This study was approved by the Partners HealthCare Institutional Review Board.
NHS and NHSII participants were asked to donate blood samples for research purposes that have been stored in aliquots at −70°C. In the NHS, 27% of women donated blood between 1989-90; in the NHSII, 25% of women donated blood between 1996-99.
Identification of women with RA
We previously reported details on the methods for RA identification(30). Women who self-reported RA were mailed a supplemental connective tissue disease screening questionnaire(31). For those who screened positive, medical records were obtained and reviewed independently by two rheumatologists. All met the 1987 ACR (American College of Rheumatology) or 2010 ACR/European League Against Rheumatism criteria for RA(32, 33). Date of clinical RA diagnosis as well as laboratory testing results for cyclic citrullinated peptide (CCP) or rheumatoid factor (RF) around the time of diagnosis were obtained from medical records. Some women with incident RA were missing CCP status since this was not measured in routine clinical care prior to the early 2000s.
For this analysis, we analyzed women who donated blood and were subsequently diagnosed with RA. We required at least three months between blood donation and the date of initial RA symptoms to ensure that all assays were performed on blood obtained before clinical RA onset.
Matched controls
For each woman with RA, we chose three controls who had never reported RA or other connective tissue diseases and had donated blood. We matched each woman with incident RA to controls by age at blood draw (within one year), cohort, time of day of blood collection, fasting status, menopausal status, and postmenopausal hormone use.
Measurement of ACPA
We used two separate laboratory assays to test for ACPA: a research multiplex assay and a commercial CCP2 assay.
Research multiplex ACPA assay.
The research ACPA test was performed at Stanford University (Palo Alto, CA, USA) using a bead-based multiplex assay(5, 34-36). Synovium-specific citrullinated and non-citrullinated protein antigens were conjugated to spectrally distinct beads using the Bio-Plex multiplex assay platform (Bio-Rad Laboratories, Hercules, CA, USA) and were analyzed using the Luminex 200 (Luminex, Austin, TX, USA). Each plate used established samples with no, low, medium, or high reactivity as internal controls.
Plasma from each subject was added to the bead mix, and the reactivity was measured in raw fluorescent intensity units. For these analyses, we only considered antibodies against citrullinated proteins that passed quality control. There were three separate batches sent for testing. All batches tested for citrullinated antibodies against epitopes on the following proteins: apolipoprotein A, apolipoprotein E, biglycan, clusterin, enolase, fibrinogen, fibronectin, filaggrin, histone 2A, histone 2B, and vimentin. There were multiple epitopes targeted for apolipoprotein E, clusterin, fibrinogen, histone 2A, histone 2B, and vimentin. The composition of each batch of the research ACPA assay varied slightly; a small number of citrullinated antigens were added to the assay while a small number of others were no longer tested. The assay also included a research test for CCP. We were underpowered to perform statistical analyses based on ACPA specifically targeting particular proteins since there were relatively few pre-RA women with ACPA elevation for any given protein.
Since these research assays did not have a clinical cutpoint for ACPA positivity, we determined this using the distribution among non-RA controls. Elevated ACPA was defined as >99th percentile of the control distribution for each ACPA epitope, separately for each NHS cohort (to account for possible differences by age or storage), and separately in each batch. We defined ACPA positivity on the research assay as two or more ACPA targeting different proteins (or one ACPA targeting a specific protein and research CCP positivity). The research ACPA assay was performed on a total of n=1,125.
Commercial CCP2 assay.
Stored plasma was tested for CCP autoantibodies using the second-generation Diastat enzyme-linked immunosorbent assay (Axis-Shield Diagnostics Limited, Dundee, UK). Analysis was performed at the Clinical Immunology Laboratory at Brigham and Women’s Hospital (Boston, MA, USA). At the time, the manufacturer suggested a threshold of >5 units/mL as a positive test(37). The commercial CCP2 assay was performed on a total of n=447. Of n=166 pre-RA women, 40 (24.0%) were positive by CCP2. Only one out of 281 matched controls tested (0.4%) had CCP2 >5 units/mL.
For those who had both research and commercial ACPA assays performed, we defined elevation in ACPA as positivity on either the research assay or positive on the CCP2 assay. Among the 167 pre-RA women that had both the research ACPA and commercial CCP2 assays performed, the kappa statistic was 0.82 with overall concordance of 93%, indicating good agreement (contingency table shown in Supplemental Table 1).
Exposure variables: RA by pre-RA ACPA status vs. matched controls
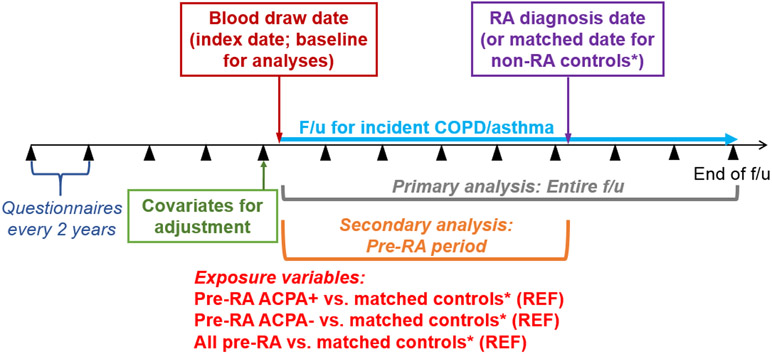
We hypothesized that women with ACPA positivity in blood banked prior to RA onset were more likely to develop obstructive lung diseases. Therefore, the primary exposure was pre-RA ACPA+ vs. their matched controls. The baseline for the cohort analysis was the date of blood draw (see Figure for study design). Controls were matched on time from blood draw to index date, so the pre-RA period was identical for women who developed RA and their matched controls in these analyses. We also investigated all RA vs. their matched controls as well as pre-RA APCA− vs. their matched controls.
Figure.
Study design for matched cohort study nested in the Nurses’ Health Studies performed among women who donated blood for research purposes, investigating pre-RA ACPA status and risks for incident chronic obstructive pulmonary disease or asthma. ACPA, anti-citrullinated protein antibodies; RA, rheumatoid arthritis.
*Each woman with RA was matched to 3 controls by age, time from blood draw to index date, cohort, calendar year, fasting status/time of day blood draw, menopausal status, and postmenopausal hormone use
Outcome variables: Incident COPD or asthma after blood draw date
All participants were asked whether they had ever been diagnosed with asthma by a physician, starting on the 1988 questionnaire in the NHS and the 1991 questionnaire in the NHSII. Similarly, physician-diagnosed chronic bronchitis/emphysema was asked on questionnaires starting in 1988 in the NHS and 1999 in the NHSII. Previous validation studies found that self-report of asthma and COPD by registered nurses had high validity compared to the gold standards determined by medical record review(38, 39).
Covariates
We considered potential confounders which were previously shown to be related to RA or asthma/COPD risk(40-42). Time-varying covariates were assessed using biennial questionnaires. Age and race (dichotomized as white/non-white) were self-reported. Smoking pack-years were calculated using self-report of duration and number cigarette packs smoked to obtain a continuous value. We also categorized smoking as: never, >0 to 10, >10 to 20, or >20 pack-years. As an assessment for passive smoking, women reported whether either of their parents smoked at home during their childhood and whether they ever lived with a smoker for >1 year. Continuous body mass index (BMI) was calculated using self-reported height and weight. As a proxy for socioeconomic status, we used area-level median household income at ZIP code level and categorized in quartiles.
Statistical analysis
We reported descriptive statistics for women with RA based on presence or absence of elevated ACPA as well as their matched controls. We tested for differences between pre-RA women and their matched controls using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. Among those who developed RA, we also reported the proportion that were CCP+ or RF+ at diagnosis from clinical testing.
We performed three sets of analyses for each outcome (COPD or asthma), comparing all pre-RA, pre-RA ACPA+, and pre-RA ACPA− to their respective matched controls. The baseline for all analyses was the date of blood collection. In each analysis, women with prevalent COPD or asthma at time of blood donation were excluded. Censoring variables were: outcome (COPD or asthma), loss to follow-up, death, or end of study (June 1, 2014 for the NHS or June 1, 2015 for the NHSII), whichever date was first. Hazard ratios (HRs) and 95% confidence intervals (CIs) for COPD or asthma were estimated using Cox regression. We initially performed unadjusted analyses. Since controls were matched by age at blood draw, time from blood draw to index date, cohort, fasting status, menopausal status, and postmenopausal hormone use. The unadjusted analyses effectively controlled for these variables since the proportions of categorical variable were identical between pre-RA and controls and continuous variables were nearly identical on inspection using descriptive statistics. We then performed multivariable analyses by adjusting for time-varying continuous smoking pack-years, continuous BMI, and median household income. We also adjusted for presence/absence of asthma at blood donation date in the COPD analysis. While our primary hypothesis was related to only the group with pre-RA ACPA+ and risk for obstructive lung diseases, we also tested for differences between pre-RA/control status and ACPA status and risk for each obstructive lung disease by including an interaction term and reporting the p values.
To investigate whether the pre-RA period imparted higher risks for COPD or asthma before RA onset, we performed secondary analyses where the follow-up time was restricted to the pre-RA period. For this analysis, the date of RA diagnosis (or matched date for controls) was also included as a censoring variable. We used the same modeling strategy as the primary analyses.
We performed several sensitivity analyses to evaluate the classification of pre-RA ACPA status, using alternative exposure groups, and evaluating for possible effect modification by smoking. First, we examined the pre-RA ACPA status and status of CCP and RF using the results obtained from medical record review at the time of RA diagnosis. Second, since ACPA+ may first develop within 5 years of clinical diagnosis, it is possible that some women we classified as pre-RA ACPA− in blood tested many years prior to RA diagnosis may have later seroconverted. Therefore, we analyzed ACPA− RA within 5 years of clinical diagnosis as an alternative to the primary analyses. Due to limited sample size in that group, we could only analyze this group for risk of incident COPD and incident asthma during the entire follow-up. Third, our primary analyses compare pre-RA ACPA+ or pre-RA ACPA− to controls since RA arises from the general population. However, we performed sensitivity analyses directly comparing pre-RA ACPA+ women to those that were pre-RA ACPA−. Fourth, we performed additional multivariable analyses additionally adjusting for smoking status and adding a quadratic term for continuous smoking pack-years in case there may have been residual confounding from smoking not adjusted for by continuous smoking pack-year that were in our primary models. Fifth, we performed an analysis among those without COPD or asthma and evaluated for incident COPD or asthma in a single analysis. Sixth, we examined for effect modification by smoking by performing stratified analyses by ever and never smoking for asthma. Since there were too few non-smokers who developed COPD, we only performed a subgroup analysis among ever smokers.
We defined two-sided p<0.05 as statistically significant. We tested the proportional hazards assumption by including an interaction term between time after baseline and the outcomes of COPD or asthma and verifying no statistical interaction for all analyses (p>0.05). All analyses were performed using SAS v.9.4 (Cary, NC, USA).
RESULTS
Characteristics of study sample
Table 1 shows the characteristics of the study samples for the COPD analysis (n=1,125) and the asthma analysis (n=951) at time of blood draw based on ACPA presence or absence before RA diagnosis and each of their matched controls. Among pre-RA ACPA+ women and their matched controls, the mean age was 51 years and there was a mean of 6.4 years (median 4.8 years) between blood draw and diagnosis/matched date. For pre-RA ACPA− women and controls, the mean age was 52 years and mean time from blood draw to index date was 10.6 years (median 10.6 years). Pre-RA ACPA+ had numerically higher smoking pack-years than matched controls (mean 21.5 vs. 19.3), but this did not reach statistical significance likely due to limited sample size.
Table 1.
Characteristics at time of blood draw according to pre-RA ACPA results for women who developed RA and their matched controls in the Nurses’ Health Studies.
| COPD analysis (n=1,125) | Pre-RA ACPA+ (n=59) |
Matched controls (n=176) |
p value |
Pre-RA ACPA− (n=224) |
Matched controls (n=666) |
p value |
|---|---|---|---|---|---|---|
| Mean age, years (SD) | 51.5 (7.6) | 51.2 (7.8) | 0.93 | 51.5 (8.0) | 51.5 (7.9) | 0.87 |
| Mean time to RA diagnosis or matched index date for controls, years (SD) | 6.4 (5.3) | 6.4 (5.3) | 0.92 | 10.6 (5.6) | 10.6 (5.6) | 0.96 |
| Median time to RA diagnosis of matched index date for controls, years (IQR) | 4.8 (2.2, 9.6) | 4.8 (2.2, 9.6) | 0.92 | 10.5 (6.1, 13.9) | 10.6 (6.1, 13.9) | 0.96 |
| CCP+ at diagnosis by medical record review, % | 81.6 | N/A | N/A | 8.7 | N/A | N/A |
| RF+ at diagnosis by medical record review, % | 76.3 | N/A | N/A | 54.0 | N/A | N/A |
| CCP+ or RF+ by medical record review at diagnosis, % | 88.1 | N/A | N/A | 55.4 | N/A | N/A |
| White race, % | 98.3 | 97.2 | 1.00 | 92.9 | 96.6 | 0.02 |
| Mean household income, $USD (SD) | 59,775 (20,555) | 62,154 (22,195) | 0.58 | 63,778 (25,889) | 63,886 (23,575) | 0.51 |
| Mean body mass index, kg/m2 (SD) | 26.8 (5.9) | 24.8 (4.3) | 0.02 | 25.5 (4.5) | 25.1 (4.6) | 0.10 |
| Mean pack-years (SD) | 12.7 (16.1) | 11.2 (16.5) | 0.49 | 11.5 (15.5) | 8.9 (15.2) | 0.01 |
| Mean pack-years among smokers (SD) | 21.5 (15.8) | 19.3 (17.7) | 0.26 | 21.6 (15.4) | 19.6 (17.4) | 0.06 |
| Smoking pack-year category, % | ||||||
| Never | 40.7 | 42.1 | 0.43 | 46.9 | 54.8 | 0.01 |
| >0 to 10 | 17.0 | 25.6 | 14.7 | 18.3 | ||
| >10 to 20 | 15.3 | 10.8 | 14.3 | 9.3 | ||
| >20 | 27.1 | 21.6 | 24.1 | 17.6 | ||
| Parent(s) smoked at home during childhood, % | 74.6 | 68.8 | 0.40 | 62.1 | 63.1 | 0.79 |
| Ever lived with a smoker, % | 67.8 | 55.1 | 0.09 | 58.0 | 56.8 | 0.74 |
| Asthma analysis (n=951) | Pre-RA ACPA+ (n=49) |
Matched controls (n=136) |
p value |
Pre-RA ACPA− (n=203) |
Matched controls (n=563) |
p value |
| Mean age, years (SD) | 52.6 (7.7) | 52.5 (8.0) | 0.89 | 51.9 (8.0) | 52.1 (7.9) | 0.75 |
| Mean time to RA diagnosis or matched index date for controls, years (SD) | 6.3 (5.3) | 6.3 (5.3) | 0.96 | 10.8 (5.6) | 10.8 (5.7) | 0.98 |
| Median time to RA diagnosis of matched index date for controls, years (IQR) | 4.4 (1.6, 9.6) | 4.4 (1.9, 9.6) | 0.96 | 11.1 (6.1, 14.2) | 11.0 (6.0, 14.4) | 0.98 |
| CCP+ at diagnosis by medical record review, % | 81.0 | N/A | N/A | 9.4 | N/A | N/A |
| RF+ at diagnosis by medical record review, % | 75.5 | N/A | N/A | 53.2 | N/A | N/A |
| CCP+ or RF+ by medical record review at diagnosis, % | 87.8 | N/A | N/A | 54.7 | N/A | N/A |
| White race, % | 98.0 | 96.3 | 1.00 | 93.6 | 97.7 | 0.01 |
| Mean household income, $USD (SD) | 61,867 (20,872) | 63,101 (23,406) | 0.93 | 64,446 (26,508) | 63,377 (23,079) | 0.90 |
| Mean body mass index, kg/m2 (SD) | 25.4 (4.2) | 25.0 (4.3) | 0.31 | 25.3 (4.4) | 25.0 (4.4) | 0.32 |
| Mean pack-years (SD) | 13.5 (17.0) | 12.9 (18.6) | 0.61 | 11.4 (15.8) | 8.9 (15.4) | 0.02 |
| Mean pack-years among smokers (SD) | 22.1 (16.8) | 21.9 (19.8) | 0.61 | 21.8 (15.8) | 19.8 (17.7) | 0.09 |
| Smoking pack-year category, % | ||||||
| Never | 38.8 | 41.2 | 0.29 | 47.8 | 55.2 | 0.05 |
| >0 to 10 | 16.3 | 25.0 | 14.8 | 17.8 | ||
| >10 to 20 | 16.3 | 8.1 | 13.3 | 9.2 | ||
| >20 | 28.6 | 25.7 | 24.1 | 17.8 | ||
| Parent(s) smoked at home during childhood, % | 71.4 | 67.7 | 0.62 | 61.6 | 63.6 | 0.61 |
| Ever lived with a smoker, % | 67.4 | 58.8 | 0.29 | 56.2 | 57.4 | 0.76 |
ACPA, anti-citrullinated protein antibodies (research tested); CCP, cyclic citrullinated peptide (clinically tested); COPD, chronic obstructive pulmonary disease; IQR, interquartile range; N/A; not applicable; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation.
Since women who had a lengthy time between blood donation and clinical diagnosis may have later seroconverted, we also showed the subgroups of women who had blood drawn within 5 years of clinical diagnosis. Of 59 pre-RA ACPA, 48 were CCP+ (81.4%), 1 was CCP− (1.7%), and 10 were missing CCP status at diagnosis. Of 224 pre-RA ACPA−, 139 (58.5%) remained CCP−, 19 (8.5%) became CCP+, and 74 (33%) were missing CCP status at diagnosis. Limiting to <5 years to diagnosis, of 40 pre-RA ACPA−, 36 remained CCP− (90%), and 4 became CCP+ (10%). Therefore, relatively few women seroreverted or seroconverted considering CCP status at clinical presentation. For pre-RA ACPA+, only 1.7-3.3% were CCP− at clinical diagnosis (seroreverters). For pre-RA ACPA−, 8.5-10.0% were CCP+ at clinical diagnosis (seroconverters), with a similar proportion in the subgroup restricted to blood donation within 5 years of clinical diagnosis.
Description of incident COPD and asthma cases
There were 107 cases of incident COPD during 21,489 person-years of follow-up. Mean age at COPD diagnosis was 64.5 years (SD 11.4) and 74.8% were smokers. Among women with pre-RA ACPA+, 13/59 (22.0%) developed COPD, and 46.2% of these COPD cases occurred prior to RA diagnosis. There were 105 cases of incident asthma during 18,365 person-years. Mean age at asthma diagnosis was 59.4 years (SD 9.5). There were n=34 who reported both COPD and asthma, n=73 that reported only COPD, n=71 that reported only asthma, and n=153 that developed either COPD or asthma (or both).
Incident COPD at any time after blood draw
Table 2 shows the results of the primary analysis for incident COPD for pre-RA ACPA+ and pre-RA ACPA− analyses. Pre-RA ACPA+ was significantly associated with incident COPD compared to matched controls in the model adjusting only for matching factors (HR 3.02, 95%CI 1.42,6.43) as well as in the multivariable model (HR 3.04, 95%CI 1.33,7.00) additionally adjusting for smoking pack-years, BMI, income, and asthma. Results were similar when additionally adjusting for smoking status and also including a quadratic term for continuous smoking pack-years (HR 2.91, 95%CI 1.28,6.64). Pre-RA ACPA− RA was not associated with COPD (HR 1.07, 95%CI 0.65,1.75). When restricted to pre-RA ACPA− RA within 5 years of blood draw, there was also no association with COPD (HR 0.49, 95%CI 0.10,2.39). There was also no association of all pre-RA women with COPD (HR 1.39, 95%CI 0.92,2.09). There was no statistical interaction between pre-RA/control status and ACPA status for COPD risk. In the pre-RA-only analysis, we also found that pre-RA ACPA+ was significantly associated with increased risk for incident COPD compared to pre-RA ACPA− (HR 2.22, 95%CI 1.12,4.41, Supplemental Table 2).
Table 2.
Hazard ratios for incident COPD after blood draw date, comparing women with RA to their matched controls in the Nurses’ Health Studies (n=1,125).
| Incident COPD cases/person-years |
HR (95%CI) controlling for matching factors* |
Multivariable** HR (95%CI) |
|
|---|---|---|---|
| Pre-RA ACPA+ (n=59) | 13/1,030 | 3.02 (1.42, 6.43) | 3.04 (1.33, 7.00) |
| Matched controls (n=176) | 14/3,375 | 1.00 (Ref) | 1.00 (Ref) |
| Pre-RA ACPA− (n=224) | 23/4,117 | 1.27 (0.78, 2.06) | 1.07 (0.65, 1.75) |
| Matched controls (n=666) | 57/12,967 | 1.00 (Ref) | 1.00 (Ref) |
| All pre-RA (n=283) | 36/5,147 | 1.61 (1.08, 2.40) | 1.39 (0.92, 2.09) |
| Matched controls (n=842) | 71/16,342 | 1.00 (Ref) | 1.00 (Ref) |
P for pre-RA/control status-ACPA status interaction in unadjusted analysis: 0.71
P for pre-RA/control status-ACPA status interaction in adjusted analysis: 0.27
Each woman with RA was matched to 3 controls by age, time from blood draw to index date, cohort, calendar year, fasting status/time of day at blood draw, menopausal status, and postmenopausal hormone use.
Additionally adjusted for smoking (continuous pack-years), body mass index (continuous, kg/m2), median household income (quartile), and asthma (yes/no).
ACPA, anti-citrullinated protein antibodies; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; RA, rheumatoid arthritis.
Incident COPD only in the pre-RA period
Table 3 shows the results of the secondary analysis for incident COPD restricting to time before clinical RA onset or matched date for controls. Pre-RA ACPA+ had multivariable HR of 10.84 (95%CI 1.13,104.08) for incident COPD compared to controls, with only one matched control developing COPD in this secondary analysis. Pre-RA ACPA− also had increased risk for COPD (HR 1.94, 95%CI 1.06,3.54).
Table 3.
Hazard ratios for incident COPD after blood draw date and before date of RA diagnosis (or matched date for matched controls), comparing women with RA to their matched controls in the Nurses’ Health Studies (n=1,125).
| Incident COPD cases/person-years |
HR (95%CI) controlling for matching factors* |
Multivariable** HR (95%CI) |
|
|---|---|---|---|
| Pre-RA ACPA+ (n=59) | 6/357 | 18.49 (2.22, 153.71) | 10.84 (1.13, 104.08) |
| Matched controls (n=176) | 1/1,115 | 1.00 (Ref) | 1.00 (Ref) |
| Pre-RA ACPA− (n=224) | 19/2,251 | 2.22 (1.23, 4.02) | 1.94 (1.06, 3.54) |
| Matched controls (n=666) | 26/6,854 | 1.00 (Ref) | 1.00 (Ref) |
| All pre-RA (n=283) | 25/2,608 | 2.82 (1.64, 4.87) | 2.33 (1.34, 4.04) |
| Matched controls (n=842) | 27/7,969 | 1.00 (Ref) | 1.00 (Ref) |
P for pre-RA/control status-ACPA status interaction in unadjusted analysis: 0.54
P for pre-RA/control status-ACPA status interaction in adjusted analysis: 0.16
Each woman with RA was matched to 3 controls by age, time from blood draw to index date, cohort, calendar year, fasting status/time of day at blood draw, menopausal status, and postmenopausal hormone use.
Additionally adjusted for smoking (continuous pack-years), body mass index (continuous, kg/m2), median household income (quartile), and asthma (yes/no).
ACPA, anti-citrullinated protein antibodies; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; RA, rheumatoid arthritis.
Incident asthma at any time after blood draw
The results of the primary analysis for asthma risk are shown in Table 4 based on pre-RA ACPA presence or absence. Compared to controls without RA, women who went on to develop RA had increased asthma risk (multivariable HR 1.65, 95%CI 1.11,2.46). Pre-RA ACPA− also had increased risk for asthma (HR 1.62, 95%CI 1.04,2.55) compared to controls. While pre-RA ACPA+ was not statistically associated with asthma, the effect size estimate was similar (HR 1.74, 95%CI 0.72,4.24). However, when restricted to pre-RA ACPA− RA within 5 years of blood draw, there was no association with asthma, but there were few outcomes in this period (HR 0.62, 95%CI 0.17,2.31). There was no statistical interaction between pre-RA/control status and ACPA status for asthma risk. In the pre-RA-only analysis, there was no difference between pre-RA ACPA+ and pre-RA ACPA− for risk of incident asthma (Supplemental Table 3).
Table 4.
Hazard ratios for incident asthma after blood draw date, comparing women with RA to their matched controls in the Nurses’ Health Studies (n=951).
| Incident asthma cases/person-years |
HR (95%CI) controlling for matching factors* |
Multivariable** HR (95%CI) |
|
|---|---|---|---|
| Pre-RA ACPA+ (n=49) | 8/890 | 1.71 (0.72, 4.07) | 1.74 (0.72, 4.24) |
| Matched controls (n=136) | 14/2,660 | 1.00 (Ref) | 1.00 (Ref) |
| Pre-RA ACPA− (n=203) | 31/3,777 | 1.73 (1.11, 2.70) | 1.62 (1.04, 2.55) |
| Matched controls (n=563) | 52/11,039 | 1.00 (Ref) | 1.00 (Ref) |
| All pre-RA (n=252) | 39/4,667 | 1.72 (1.16, 2.56) | 1.65 (1.11, 2.46) |
| Matched controls (n=699) | 66/13,698 | 1.00 (Ref) | 1.00 (Ref) |
P for pre-RA/control status-ACPA status interaction in unadjusted analysis: 0.20
P for pre-RA/control status-ACPA status interaction in adjusted analysis: 0.23
Each woman with RA was matched to 3 controls by age, time from blood draw to index date, cohort, calendar year, fasting status/time of day at blood draw, menopausal status, and postmenopausal hormone use.
Additionally adjusted for smoking (continuous pack-years), body mass index (continuous, kg/m2), and median household income (quartile).
ACPA, anti-citrullinated protein antibodies; CI, confidence interval; HR, hazard ratio; RA, rheumatoid arthritis.
Incident asthma only in the pre-RA period
Table 5 shows the HRs for the secondary analysis of incident asthma restricted to the pre-RA period. Pre-RA ACPA+ and pre-RA ACPA− had similar associations with asthma risk and neither reached statistical significance. However, all pre-RA had increased risk for asthma (HR 1.70, 95%CI 1.01,2.88) compared to controls.
Table 5.
Hazard ratios for incident asthma after blood draw date and before date of RA diagnosis (or matched date for matched controls), comparing women with RA to their matched controls in the Nurses’ Health Studies (n=951).
| Incident asthma cases/person-years |
HR (95%CI) controlling for matching factors* |
Multivariable** HR (95%CI) |
|
|---|---|---|---|
| Pre-RA ACPA+ (n=49) | 4/296 | 1.96 (0.55, 6.95) | 1.31 (0.35, 4.86) |
| Matched controls (n=136) | 6/835 | 1.00 (Ref) | 1.00 (Ref) |
| Pre-RA ACPA− (n=203) | 19/2,025 | 1.76 (0.99, 3.12) | 1.65 (0.93, 2.95) |
| Matched controls (n=563) | 31/5,836 | 1.00 (Ref) | 1.00 (Ref) |
| All pre-RA (n=252) | 23/2,321 | 1.78 (1.06, 3.00) | 1.70 (1.01, 2.88) |
| Matched controls (n=699) | 37/6,671 | 1.00 (Ref) | 1.00 (Ref) |
P for pre-RA/control status-ACPA status interaction in unadjusted analysis: 0.56
P for pre-RA/control status-ACPA status interaction in adjusted analysis: 0.62
Each woman with RA was matched to 3 controls by age, time from blood draw to index date, cohort, calendar year, fasting status/time of day at blood draw, menopausal status, and postmenopausal hormone use.
Additionally adjusted for smoking (continuous pack-years), body mass index (continuous, kg/m2), and median household income (quartile), and asthma (yes/no).
ACPA, anti-citrullinated protein antibodies; CI, confidence interval; HR, hazard ratio; RA, rheumatoid arthritis.
Incident COPD or asthma
Over the entire follow-up period, pre-RA ACPA+ (HR 2.28, 95%CI 1.08,4.81), pre-RA ACPA− (HR 1.58, 95%CI 1.08,2.29), and all pre-RA (HR 1.69, 95%CI 1.21,2.35) were significantly associated with incident COPD or asthma, each compared to matched controls.
Analyses among never and ever smoker subgroups
Analyses stratified by smoking status were generally consistent with the primary results. Among only ever smokers, pre-RA ACPA+ remained significantly associated with incident COPD compared to matched controls (HR 2.77, 95%CI 1.11,6.95, Supplemental Table 4). Among never smokers, all pre-RA was associated with increased incident asthma risk (HR 1.97, 95%CI 1.08,3.60, Supplemental Table 5).
DISCUSSION
We found a strong association between elevated ACPA in blood banked prior to RA onset and COPD, even after adjusting for smoking, and this was most pronounced in the period before RA diagnosis. These results suggest that biologic processes related to ACPA formation in airways could predispose patients to COPD development beyond the effect of smoking. We found that women who went on to develop RA also had increased asthma risk. However, there was no additional risk imparted by pre-RA ACPA positivity. These findings underscore that patients who go on to develop RA are more susceptible to respiratory morbidities beyond the effect of smoking and that this risk may commence even prior to the articular onset of RA.
Ours is the first to study elevation of ACPA in the pre-RA period and risk for chronic airway diseases, but prior studies have suggested that patients with RA have an increased risk of subsequent COPD development compared to non-RA controls(13-25). A meta-analysis found that RA increased COPD risk with a RR of 1.99 (95%CI 1.61,2.45)(20). Case-control studies found an association between RA and COPD(14, 15, 17), but did not investigate by ACPA status and did not adjust for smoking, a strong risk factor for both diseases as well as ACPA positivity(14, 15, 17). A population-based cohort study in British Columbia as well as several retrospective cohorts similarly found an association between RA and COPD but lacked data on serologic status(16, 18, 21, 24). A retrospective cohort found a similar association after adjustment for ever vs. never smokers, however these results may have residual confounding from smoking as they did not have data on smoking pack-years, which provides more detail on the duration and intensity of smoking(22). Further, a phenome-wide association study found an association between seropositive RA and COPD in comparison to seronegative RA(25). A previous prospective study by our group using the NHS consisting of n=843 women with incident RA and n=8,399 matched comparators found that overall RA, seropositive RA (defined as either ACPA or RF positivity at clinical diagnosis), and seronegative RA were associated with increased risk of COPD compared to controls after date of RA diagnosis or matched date for comparators(13). These findings were independent of time-updated covariates, including smoking. The present study extends these findings by focusing on pre-RA ACPA status, including younger women in the NHSII, and also analyzing the pre-RA period. We found even more pronounced associations of elevated pre-RA ACPA and COPD risk, particularly prior to clinical RA diagnosis. We found similar results of pre-RA ACPA+ increasing COPD risk when comparing to pre-RA ACPA− instead of non-RA controls. Our study design allowed for analysis of the pre-RA period to understand the role of ACPA in development of COPD before and after clinical RA onset, thus providing insight into both pathogenesis and outcomes. Our results demonstrate that ACPA elevation prior to RA diagnosis may be related to COPD risk and suggest that the pre-RA period may be a crucial time for COPD development related to ACPA elevation. However, we also found that women with pre-RA ACPA− had significantly increased risk for COPD in the secondary analysis restricted to the pre-RA/index period. This may be due to there being a much longer pre-RA period for women who tested as pre-RA ACPA−, providing more opportunity to accrue incident lung diseases. It is also possible that some women later seroconverted to ACPA+ after the blood donation but before RA onset. We found no incident COPD cases among the subgroup of pre-RA ACPA− who donated within 5 years of clinical RA diagnosis. Therefore, we suspect the lengthy duration prior to RA and some seroconversion may explain the increased risk for incident COPD also observed in the pre-RA ACPA− group, but this could also be explained by chance or healthcare utilization. While our primary hypothesis was related to only the pre-RA ACPA+ group and risk for obstructive lung disease, we found no statistical interaction between pre-RA/control status and ACPA status for COPD, likely due to limited sample size and wide confidence intervals despite statistical significance for incident COPD only in the pre-RA ACPA+ group. Therefore, larger studies are needed to confirm our findings in other populations.
Prior studies have also analyzed the association between RA and subsequent risk for asthma compared to controls without RA(13, 16, 17, 21, 26-28). A case-control study found that RA increased asthma risk, but did not have data on serostatus(17, 26). A retrospective cohort study found increased hospitalizations for asthma exacerbations among RA patients, but also lacked serostatus(28). Three retrospective studies had similar findings and limitations(16, 21, 27). The previous prospective cohort study in the NHS by our group did not find an association of RA with asthma after adjustment for time-varying confounders including smoking, but did not include younger women in the NHSII unlike the present study(13). Since older-onset asthma is uncommon, this may explain the null findings for RA and asthma risk in that previous study. Alternatively, the differences may be related to residual unmeasured confounding or generalizability since the women that donated blood for this study were relatively healthier than the overall cohorts. In the present study, we found that women who went on to be diagnosed with RA were more likely to develop asthma regardless of pre-RA ACPA status, contrary to the hypothesis that pre-RA ACPA+ RA would have higher risk. It is possible that some who were pre-RA ACPA− went on to develop ACPA after the blood draw, or that other factors such as RF may have been important for asthma risk among women with RA. Regardless of the biologic mechanism, our findings add to the growing literature of a bi-directional link between asthma and RA where each disease increases risk of the other. Future research is needed to better understand this relationship.
ACPA has been found in sputum of individuals who are at-risk for RA years prior to diagnosis and in lung biopsy tissue of newly diagnosed RA patients(43). In comparison to individuals who do not have RA, early RA patients prior to treatment are also significantly more likely to have elevated ACPA and other RA-related autoantibodies in the sputum and bronchoalveolar lavage(43-45). Citrullinated proteins are formed in inflamed tissues in a process catalyzed by peptidylarginine deiminase (PAD) enzymes(46). ACPA is thought to form as an immune response to citrullinated proteins at inflamed surfaces(47), a hypothesis which is underscored by the composition of lungs in ACPA+ RA patients, which show elevated levels of citrullinated proteins, such as PAD2 and PAD4(48). Biopsies from seropositive RA patients have shown enriched lymphoid aggregates which may lead to immune activation and lung changes(43). Seropositive individuals are more likely to progress to RA(49). A previous study of CCP-positive individuals presenting to a pulmonary specialty clinic also described a high proportion of patients with airway diseases, particularly COPD(50). Thus, ACPA may confer additional risk for developing respiratory diseases since it may be generated at mucosal surfaces in the airways and could create structural pulmonary abnormalities that clinically resembles obstructive lung disease.
Our study has several strengths. The NHS and NHSII consist of decades of prospective follow-up. The study sample is large and there is high follow-up. ACPA was tested from banked blood collected before RA diagnosis, allowing for analysis restricted to the pre-RA period to determine the timing of ACPA elevation and development of obstructive lung diseases. Our study design is unique because we analyzed blood collected before RA diagnosis, allowing us to perform a matched cohort analysis nested within the larger study. All women with RA met research criteria, confirmed by medical record review and there were many non-RA controls allowing for fine matching on important RA risk factors. Detailed data on adjustment variables including BMI, smoking pack-years, and passive smoking were collected at time of blood draw and prior to RA onset, minimizing potential for recall bias.
Our study also has potential limitations. We measured ACPA on blood samples using a composite of two different assays which may have resulted in misclassification. However, the research ACPA and CCP2 assays were highly concordant in the subset that had both tested. Only 20.8% of women who went on to be diagnosed with RA were ACPA+ and about two-thirds of patients with RA are ACPA+. This difference is likely explained by the lengthy duration of time between when blood was donated and when RA was diagnosed. When we compared the research ACPA assay to a commercial CCP test, we found strong agreement. Those with pre-RA ACPA+ were nearly all CCP-positive according to results from routine clinical care. We did not test the blood for other RA biomarkers, such as RF, for research purposes so it is unclear how other biomarkers would have affected results. Further, we measured ACPA on a single blood test so some pre-RA ACPA− individuals may have later seroconverted in the years between blood donation and RA diagnosis. Those who tested pre-RA ACPA− had longer time to RA diagnosis, so may have subsequently seroconverted. Therefore, we also examined the group that was ACPA− within 5 years of clinical diagnosis. We found these groups to have similar rates of seronegativity at clinical diagnosis. Our sensitivity analyses using this subgroup had overall similar results, so find this possible misclassification of pre-RA ACPA− unlikely to explain our results. COPD and asthma were collected by self-report using biennial questionnaires rather than using medical records or in-person assessments. However, the questionnaires were collected prospectively prior to RA onset and completed by nurses and had high validity, so we find this unlikely to explain the associations that we report(38). We were unable to phenotype COPD and asthma based on severity, subtype, and treatment. Since adult-onset asthma is uncommon, it is possible that these women had an overlap with COPD. However, relatively few women reported both COPD and asthma and the different results for each outcome argue that women were reporting different entities. When analyzing a composite outcome of COPD or asthma, we also found associations based on pre-RA ACPA status. Future studies are needed to understand how the disease course of COPD and asthma may contribute to RA-related autoantibody production and articular RA onset and course.
While the NHS cohorts have large sample size, there were limited numbers in this subset analysis of women who donated blood and had ACPA measured. In particular, some of the secondary analyses had few outcomes resulting in wide confidence intervals. Further study is required to understand how RA confers risk on different phenotypes of lung diseases. The study sample was nurses who were mostly healthy, educated, and working at baseline, so the results may not be generalizable to other populations. Further, only about 25% of women in both cohorts donated blood and may have been healthier than those who did not, further limiting generalizability. However, since RA arises from the healthy general population and we measured blood in the pre-RA period, our results still add to the literature related to understanding the timeline of ACPA development, pulmonary inflammation, and articular RA onset. We performed a cohort analysis using data collected for a nested case-control study so the results may not be representative of the source study population. As in all observational studies, there is potential for unmeasured confounders explaining the results, but the associations we report were independent of important known confounders including smoking pack-years.
In conclusion, ACPA positivity prior to RA onset was significantly associated with increased COPD risk, particularly in the pre-RA period. This may be due to loss of immune tolerance in the lungs from increased citrullination and ACPA production occurring before RA onset that pre-disposes to chronic airway disease and not explained by smoking. Women who went on to develop RA were also more likely to develop asthma compared to matched controls, however this was risk was not related to pre-RA ACPA status. Overall, our results emphasize that clinicians should closely monitor seropositive patients for airway abnormalities, even prior to RA onset. Future work is needed to elucidate the bi-directional link between RA and chronic airway diseases.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
We tested blood for anti-citrullinated protein antibodies (ACPA) on 283 women aged a mean of 9.7 years prior to rheumatoid arthritis (RA) diagnosis and 842 matched controls to investigate whether pre-RA ACPA positivity was associated with risk for incident chronic obstructive pulmonary disease (COPD) or asthma.
We found that pre-RA ACPA positivity was associated with 3-fold increased risk of incident COPD, independent of smoking and other confounders.
We found that women with incident RA onset were more likely to develop asthma than matched controls, regardless of pre-RA ACPA status.
Our findings suggest that ACPA-positive individuals should be monitored for COPD in addition to RA.
ACKNOWLEDGEMENTS
We thank the participants in the NHS and NHSII cohorts for their dedication and continued participation in these longitudinal studies, as well as the staff in the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School for their assistance with this project.
Funding/Support: This work was supported by the National Institutes of Health (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, R01 AR049880, K24 AR066109, UM1 CA186107, R01 CA049449, T32 AR007530, R01 AR 057327, R01 AR059073, UM1 CA176726, R01 CA067262, P30 AR070253, and P30 AR072577). Dr. Sparks is also funded by the Rheumatology Research Foundation K Supplement and the Brigham Research Institute. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Disclosures: Dr. Sokolove is currently employed by GlaxoSmithKline.
REFERENCES
- 1.Johansson L, Pratesi F, Brink M, Arlestig L, D’Amato C, Bartaloni D, et al. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res Ther. 2016;18(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. [DOI] [PubMed] [Google Scholar]
- 3.Brink M, Hansson M, Mathsson-Alm L, Wijayatunga P, Verheul MK, Trouw LA, et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res Ther. 2016;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lingampalli N, Sokolove J, Lahey LJ, Edison JD, Gilliland WR, Holers VM, et al. Combination of anti-citrullinated protein antibodies and rheumatoid factor is associated with increased systemic inflammatory mediators and more rapid progression from preclinical to clinical rheumatoid arthritis. Clin Immunol. 2018;195:119–26. [DOI] [PubMed] [Google Scholar]
- 5.Arkema EV, Goldstein BL, Robinson W, Sokolove J, Wagner CA, Malspeis S, et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: a nested case-control study. Arthritis Res Ther. 2013;15(5):R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Too CL, Yahya A, Murad S, Dhaliwal JS, Larsson PT, Muhamad NA, et al. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA). Arthritis Res Ther. 2012;14(2):R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50(10):3085–92. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Khalili H, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56(6):1745–53. [DOI] [PubMed] [Google Scholar]
- 11.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67(10):1488–92. [DOI] [PubMed] [Google Scholar]
- 12.Cafaro G, Alunno A, Valentini V, Leone MC, Marcucci E, Bartoloni E, et al. The onset site of rheumatoid arthritis: the joints or the lung? Reumatismo. 2016;68(4):167–75. [DOI] [PubMed] [Google Scholar]
- 13.Sparks JA, Lin TC, Camargo CA Jr., Barbhaiya M, Tedeschi SK, Costenbader KH, et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheum. 2018;47(5):639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhital R, Basnet S, Paudel P, Acharya YP, Poudel DR. Prevalence of chronic obstructive pulmonary disease (COPD) among rheumatoid arthritis: results from national inpatient database. J Community Hosp Intern Med Perspect. 2018;8(4):211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieber V, Cohen AD, Freud T, Agmon-Levin N, Gertel S, Amital H. Autoimmune smoke and fire--coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross-sectional analysis. Immunol Res. 2013;56(2-3):261–6. [DOI] [PubMed] [Google Scholar]
- 16.Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM. 2014;107(7):537–43. [DOI] [PubMed] [Google Scholar]
- 17.Ursum J, Nielen MM, Twisk JW, Peters MJ, Schellevis FG, Nurmohamed MT, et al. Increased risk for chronic comorbid disorders in patients with inflammatory arthritis: a population based study. BMC Fam Pract. 2013;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire K, Avina-Zubieta JA, Esdaile JM, Sadatsafavi M, Sayre EC, Abrahamowicz M, et al. Risk of Incident Chronic Obstructive Pulmonary Disease in Rheumatoid Arthritis: A Population-Based Cohort Study. Arthritis Care Res (Hoboken). 2019;71(5):602–10. [DOI] [PubMed] [Google Scholar]
- 19.Westreich D, Cole SR, Tien PC, Chmiel JS, Kingsley L, Funk MJ, et al. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol. 2010;171(6):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungprasert P, Srivali N, Cheungpasitporn W, Davis Iii JM. Risk of incident chronic obstructive pulmonary disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. Joint Bone Spine. 2016;83(3):290–4. [DOI] [PubMed] [Google Scholar]
- 21.Hemminki K, Liu X, Forsti A, Ji J, Sundquist J, Sundquist K. Subsequent leukaemia in autoimmune disease patients. Br J Haematol. 2013;161(5):677–87. [DOI] [PubMed] [Google Scholar]
- 22.Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). 2013;65(8):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemminki K, Liu X, Ji J, Sundquist K, Sundquist J. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J. 2011;37(2):463–5. [DOI] [PubMed] [Google Scholar]
- 25.Doss J, Mo H, Carroll RJ, Crofford LJ, Denny JC. Phenome-Wide Association Study of Rheumatoid Arthritis Subgroups Identifies Association Between Seronegative Disease and Fibromyalgia. Arthritis Rheumatol. 2017;69(2):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Min C, Oh DJ, Choi HG. Increased risk of asthma in patients with rheumatoid arthritis: A longitudinal follow-up study using a national sample cohort. Sci Rep. 2019;9(1):6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen TC, Lin CL, Wei CC, Tu CY, Li YF. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM. 2014;107(6):435–42. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Fan X, Jiang C, Arevalo Molina AB, Salgado M, Xu J. Rheumatoid arthritis is associated with increased in-hospital mortality in asthma exacerbations: a nationwide study. Clin Rheumatol. 2018;37(7):1971–6. [DOI] [PubMed] [Google Scholar]
- 29.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Tedeschi SK, Lu B, Zaccardelli A, Speyer CB, Costenbader KH, et al. Long-Term Physical Activity and Subsequent Risk for Rheumatoid Arthritis Among Women: A Prospective Cohort Study. Arthritis Rheumatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. [DOI] [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 33.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. [DOI] [PubMed] [Google Scholar]
- 34.Tedeschi SK, Cui J, Arkema EV, Robinson WH, Sokolove J, Lingampalli N, et al. Elevated BMI and antibodies to citrullinated proteins interact to increase rheumatoid arthritis risk and shorten time to diagnosis: A nested case-control study of women in the Nurses’ Health Studies. Semin Arthritis Rheum. 2017;46(6):692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36(4):706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camargo CA Jr., Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159(21):2582–8. [DOI] [PubMed] [Google Scholar]
- 39.Barr RG, Herbstman J, Speizer FE, Camargo CA Jr. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–71. [DOI] [PubMed] [Google Scholar]
- 40.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seror R, Henry J, Gusto G, Aubin HJ, Boutron-Ruault MC, Mariette X. Passive smoking in childhood increases the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2019;58(7):1154–62. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. [DOI] [PubMed] [Google Scholar]
- 43.Reynisdottir G, Olsen H, Joshua V, Engstrom M, Forsslund H, Karimi R, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–7. [DOI] [PubMed] [Google Scholar]
- 44.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-Citrullinated Protein Antibodies Are Associated With Neutrophil Extracellular Traps in the Sputum in Relatives of Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2017;69(6):1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14(6):490–7. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugli EB, Correia RE, Fischer R, Lundberg K, Bracke KR, Montgomery AB, et al. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res Ther. 2015;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford JA, Liu X, Marshall AA, Zaccardelli A, Prado MG, Wiyarand C, et al. Impact of Cyclic Citrullinated Peptide Antibody Level on Progression to Rheumatoid Arthritis in Clinically Tested CCP-Positive Patients Without RA. Arthritis Care Res (Hoboken). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer A, Solomon JJ, du Bois RM, Deane KD, Olson AL, Fernandez-Perez ER, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106(7):1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.