Abstract
Background:
The prognostic impact of increased beta-2 microglobulin (B2M) in patients with light chain (AL) amyloidosis undergoing autologous stem cell transplantation (ASCT) is unknown. The Mayo 2012 stage and increased bone marrow plasma cell (BMPC) percentage are known predictors for survival. Increased beta-2 microglobulin (B2M) predicts survival in patients with multiple myeloma. We evaluated the prognostic role of B2M in newly diagnosed AL patients undergoing ASCT.
Methods:
We retrospectively reviewed patients who had a diagnosis of AL amyloidosis and were treated with ASCT between July-1996 and September-2017. Patients with creatinine >1.2 mg/dL were excluded, as that affects B2M levels. The receiver operator curve was used to determine the best cutoff for B2M done before ASCT in predicting survival and was 2.5 μg/ml, which was also the upper limit of normal in our lab. Baseline characteristics were compared between patients with B2M >2.5 and ≤2.5. Progression-free survival (PFS) was defined as time from ASCT to relapse or death, whichever occurred first. Overall survival (OS) was calculated from ASCT to death of any cause. Univariate and multivariate analysis were done for OS.
Results:
Five-hundred patients were identified and 222 (44%) had a B2M >2.5. These patients were more likely to be >60 years old (54% vs. 37%, P=0.0002), have Mayo 2012 stage III/IV (33% vs. 8%, P<0.0001), have more than 2 organs involved (25% vs. 14%, P=0.001), and have ≥10% BMPCs (56% vs. 40%, P=0.0002) compared to patients with B2M ≤2.5. The median PFS and OS were shorter in patients with B2M >2.5 (median PFS: 64 vs. 80 months, P=0.03); (median OS: 104.9 vs. 175.5 months, P<0.0001). On univariate analysis, predictors for OS included age >60 (HR: 1.61, P=0.001), Mayo 2012 stage III/IV (HR: 3.36, P<0.0001), more than 2 organs involved (HR: 1.36, P=0.07), ≥10% BMPCs (HR: 1.5, P=0.005), melphalan conditioning 200mg/m2 (HR: 0.29, P<0.0001), B2M >2.5 (HR: 1.82, P<0.0001), and transplant period during or after 2010 (HR: 0.4, P=0.0006). In a multivariate analysis, only Mayo 2012 stage III/IV (HR: 1.89, P=0.005), melphalan conditioning 200mg/m2 (HR: 0.39, P<0.0001), B2M >2.5 (HR: 1.84, P=0.003), and transplant period during or after 2010 (HR:0.58, P=0.03) remained independent predictors of OS.
Conclusion:
Beta-2 microglobulin >2.5 mg/dL before ASCT is an independent predictor for OS in AL amyloidosis patients with normal kidney function and should be routinely measured.
Introduction:
The prognostic impact of beta-2 microglobulin (B2M) in patients with light chain amyloidosis (AL) undergoing autologous stem cell transplant (ASCT) is not clear. In 1990, Gertz et al. evaluated newly diagnosed AL patients based on the B2M levels (1). Patients with B2M > 2.7 μg/ml had a median OS of 10.8 months compared to 32.9 months in patients with B2M ≤ 2.7 μg/ml(1). Zerbini, et al evaluated 80 patients with AL amyloidosis undergoing treatment with melphalan and prednisone in 1998 and concluded that patients with B2M >4 μg/ml have shorter survival (2). However, patients with higher B2M were more likely to have higher creatinine levels and the sample size was small. The 2012 Mayo stage is wildly used to stage newly diagnosed AL amyloidosis patients (3). During the construction of the model in the original cohort, B2M was a predictor for OS in the univariable but not multivariable analysis that included serum free light chains (FLC) and cardiac biomarkers. However, the median creatinine was 1.1 mg/dl with the range being (0.8–2.2). The increased creatinine in some patients might have affected the results, as B2M is excreted by the kidneys. We evaluated the prognostic effect of B2M in newly diagnosed AL amyloidosis patients undergoing ASCT with normal creatinine.
Methods:
Patients with a diagnosis of AL amyloidosis with organ involvement based on the defined criteria (4), who had ASCT between July-1996 and September-2017 at Mayo Clinic, Rochester, and had a creatinine ≤ 1.2 mg/dl were included. Excluding patients with high creatinine was required, as B2M is excreted by the kidneys and having high creatinine levels would lead to falsely high B2M levels in some patients. The Mayo Clinic Institutional Review Board approved the study. If possible, the Mayo 2012 stage (3) was applied before ASCT and the transplant physician decided the need for induction treatment before ASCT, as well as, the dose of melphalan conditioning (200 vs. 140mg/m2). Hematological response assessment was done at day 100 post ASCT (5). None of the patients had end organ damage defining multiple myeloma (MM) (e.g. hypercalcemia, cast nephropathy, or bone lesions).
The receiver operator curve (ROC) was utilized to determine the best B2M levels before ASCT in predicting survival (dead vs. alive), which was 2.5 μg/ml. The area under the curve was 0.53, with a sensitivity of 50% and a specificity of 60%. Also, the upper limit of normal in our lab is 2.5 μg/ml and the median B2M level in our data set was 2.4 μg/ml. Hence, we evaluated the baseline characteristics and outcomes between patients with B2M >2.5 and ≤2.5 μg/ml. The Wilcoxon rank-sum test was used for continuous variables, as most of the continuous data was not normally distributed. The chi-square test was used for categorical data as the cell counts were appropriate and Fisher’s exact test was not needed.
Progression-free survival (PFS) was defined as time from ASCT to hematological relapse(6) or death, whichever occurred first and overall survival (OS) was calculated from ASCT to death of any cause. Survival analysis was done by the Kaplan-Meier method. The following variables were tested for univariate analysis for PFS and OS: Age> 60, Mayo 2012 stage (stage III/IV vs. II/I), bone marrow plasma cell (BMPC) percentage (≥10% vs <10%), B2M (>2.5 and ≤2.5 μg/ml), number of organs involved (more than 2 vs. less than 2), conditioning melphalan dose (200 vs. 140mg/m2), and transplant period during or after 2010 compared to before 2010. Only statistically significant variables in the univariate analysis (P<0.1) were included in the multivariate analysis using cox regression. Two multivariate analyses for OS were done, one including all variables that were statistically significant in the univariate analysis (Model 1) and another model (Model 2) that included only disease related biological factors (Mayo 2012 stage, BMPC%, and B2M levels). All tests were 2 sided and P <0.05 was considered statistically significant. Statistical analysis was done using JMP software (SAS Institute, Cary, NC).
Results:
We identified 510 patients and 222 (44%) had B2M >2.5 μg/ml before ASCT. The baseline characteristics are displayed in Table 1. Patients with B2M >2.5 μg/ml were older (median age 61 vs. 57, P=0.0002), and had more advanced Mayo 2012 stage (33% vs. 8%, P<0.0001) compared to patients with B2M ≤2.5 μg/ml. These patients were also more likely to have cardiac involvement, as well as, more than 2 organs involved (51% vs. 39%, P=0.005), and (25% vs. 14%, P=0.001), respectively. There was no difference between both groups for liver (P=0.5) and kidney involvement (P=0.38). More patients with B2M >2.5 μg/ml had BMPC ≥10% (56% vs. 40%, P=0.0002) and there was no difference between the two groups for having t (11;14) by FISH (P=0.5).
Table 1.
Baseline Characteristics.
| Variable | B2M >2.5 μg/ml (N=222) | B2M ≤ 2.5 μg/ml (N=288) |
|---|---|---|
| Age (years), median (IQR)* | 61 (54–66) | 57 (51.4–63.4) |
| Male, n (%) | 118 (53%) | 164 (57%) |
| Mayo 2012 stage (III/IV), n (%)* | 60/183** (33%) | 17/204** (8%) |
| Organs involved >2, n (%)* | 56 (25%) | 41 (14%) |
| Cardiac involvement, n (%)* | 113 (51%) | 111(39%) |
| NT-proBNP (pg/ml), median (IQR)*# | 692.5 (228–2641) | 194.5 (85–877) |
| Troponin (μg/L), median (IQR)*# | 0.01 (0.01–0.03) | 0.01 (0.01–0.01) |
| dFLC (mg/dl), median (IQR)* | 22 (8.8–52) | 9 (4–30) |
| Kidney involvement, n (%) | 133 (60%) | 161 (56%) |
| Liver involved, n (%) | 26 (12%) | 29 (10%) |
| FISH: t(11;14), n (%) | 56/164** (34%) | 71/191** (37%) |
| BMPC ≥ 10%, n (%)* | 125 (56%) | 114 (40%) |
Difference was statistically significant.
Data was not available in all patients. Percentage is from all patients where the test could be done.
Normal range for NT-proBNP (5–50 pg/ml), normal range for Troponin (≤15 μg/L)
Abbreviations: B2M: beta-2 microglobulin, IQR: interquartile range, NT-ProBNP: N-terminal pro brain natriuretic peptide, dFLC: difference in free light chain FISH: Fluorescence in situ hybridization, BMPC: bone marrow plasma cells.
Receiving induction chemotherapy before ASCT was done more frequently in patients with B2M >2.5 μg/ml (51% vs. 36%, P=0.0008), but the type of induction used and time from diagnosis to ASCT did not differ between both groups (P=0.3 and P=0.1, respectively) (Table 2). On the other hand, less patients received conditioning melphalan 200mg/m2 in the B2M >2.5 μg/ml group (73% vs. 85%, P=0.001). The 100 day transplant related mortality (TRM) was more in patients with the high B2M (9% vs. 3%, P=0.0026). All patients with TRM died from complications related to ASCT, except one patient who died from progression to multiple myeloma. Also, rates of overall response (partial response or better) and complete response (CR) were lower in this group (79% vs. 85%, P=0.05) and (35% vs. 43%, P=0.04), respectively.
Table 2.
Transplant variables and outcomes.
| Variable | B2M >2.5 μg/ml (N=222) | B2M ≤ 2.5 μg/ml (N=288) | P |
|---|---|---|---|
| Received induction before ASCT, n (%) | 114 (51%) | 105 (36%) | 0.0008 |
| Steroids/others | 39 (34%) | 40 (38%) | |
| Time from diagnosis to ASCT (months), median (IQR) | 3.9 (2.8–6.3) | 3.9 (2.7–6.5) | 0.1 |
| Conditioning melphalan 200mg/m2, n (%) | 163 (73%) | 241 (85%) | 0.001 |
| Mortality 100 day, n (%) | 21 (9%) | 9 (3%) | 0.0026 |
| (CR) | 77 (35%) | 125 (43%) | 0.04 |
Abbreviations: B2M: beta-2 microglobulin, ASCT: autologous stem cell transplant, IMiD: immunomodulatory drug, PI: proteasome inhibitor, PR: partial response, CR: complete response.
For the whole cohort, the median PFS and OS were 74 and 137.9 months respectively. The median PFS was shorter for patients with B2M >2.5 μg/ml compared to B2M ≤2.5 μg/ml (64 vs. 80 months, P=0.03) (Figure 1, A). This also translated into worse OS in the high B2M group (median OS: 104.9 vs. 175.5 months, P<0.0001) (Figure 1,B). We also evaluated the OS in patients based on their BMPC % and B2M levels. Patients with BMPC <10% and increased B2M had worse OS compared to patients with BMPC <10% and decreased B2M (median 120.8 months vs. 193.1 months, P=0.003) (Figure 2, A). Also, patients with BMPC ≥10 and increased B2M had a median OS of 85.4 months compared to 121.3 months in patients with BMPC ≥10 and low B2M levels (P=0.006) (Figure 2, B).
Figure 1.
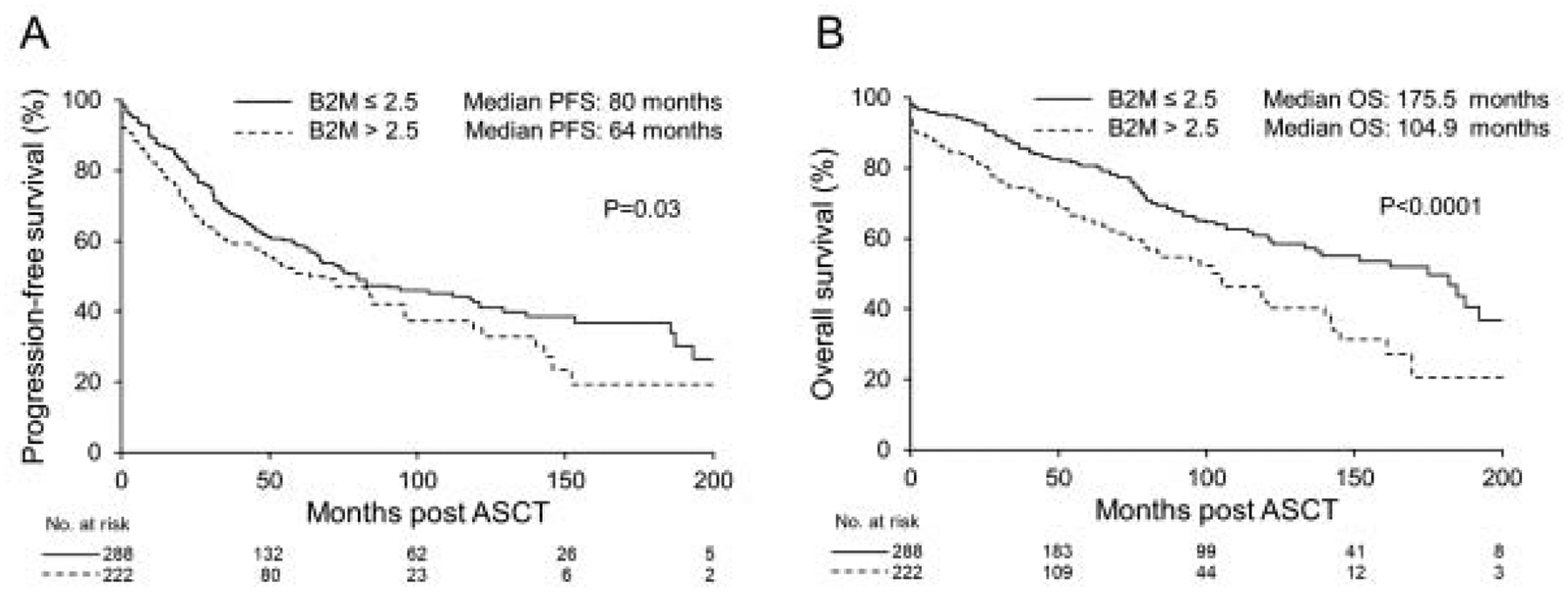
A) PFS according to B2M level. B) OS according to B2M level.
PFS: progression-free survival, OS: Overall survival, B2M: beta-2 microglobulin.
Figure 2.
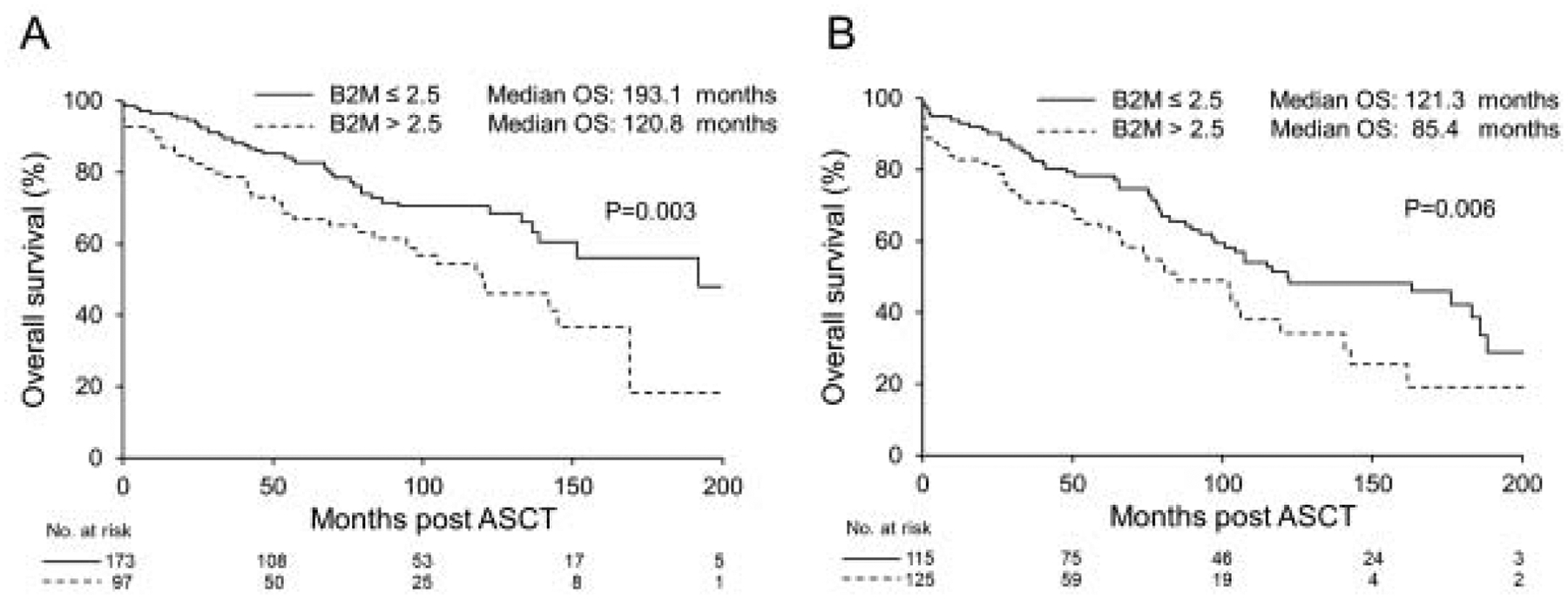
A) OS in patients with BMPC ≥10% based on the B2M level. B) OS in patients with BMPC <10% based on B2M level. OS: Overall survival, B2M: beta-2 microglobulin. BMPC: bone marrow plasma cell.
On univariate analysis, predictors for PFS included B2M >2.5 μg/ml (hazard ratio (HR): 1.31, P=0.03), Mayo 2012 stage (III/IV) (HR: 2.5, P<0.0001), BMPC ≥ 10% (HR: 1.53, P=0.0007), having more than 2 organs involved (HR: 1.52, P=0.006), using melphalan 200mg/m2 for conditioning (HR:0.38, P<0.0001), and transplant period during or after 2010 (HR: 0.35, P<0.0001) (Supplementary Table 1). However, on multivariate analysis, only Mayo 2012 stage (III/IV) (HR: 1.82, P=0.003), using melphalan 200mg/m2 for conditioning (HR:0.53, P=0.002), and transplant period during or after 2010 (0.37, P<0.0001) were predictive (Supplementary Table 2). For OS, predictors on the univariate analysis included age >60 (HR: 1.61, P=0.001), B2M >2.5 μg/ml (HR: 1.82, P<0.0001), Mayo 2012 stage (III/IV) (HR: 3.36, P<0.0001), BMPC ≥ 10% (HR: 1.5, P=0.005), having more than 2 organs involved (HR: 1.36, P=0.07), using melphalan 200mg/m2 for conditioning (HR:0.29, P<0.0001), and transplant period during or after 2010 (HR: 0.4, P=0.0006) (Supplementary Table 2). In Model 1, only B2M >2.5 (HR: 1.84, P=0.003), Mayo 2012 stage (III/IV) (HR: 1.89, P=0.005), melphalan conditioning 200mg/m2 (HR: 0.39, P<0.0001), and transplant period during or after 2010 (HR:0.58, P=0.03) remained independent predictors of OS. (Supplementary Table 2). Using Model 2, only B2M > 2.5 μg/ml (HR: 1.88, P=0.002) and Mayo 2012 stage (III/IV) (HR: 2.67, P<0.0001) were predictive for OS (Supplementary Table 2).
Discussion:
In our cohort we showed that having B2M >2.5 μg/ml before ASCT was an independent predictor of OS in patients undergoing ASCT for AL amyloidosis. The median OS was 70.6 months shorter in patients with high B2M levels. Overall, these patients were 80% more likely to die compared to patients with low B2M levels. The B2M is a well-known prognostic marker in MM, both in the original and revised international staging system (7, 8). It likely reflects tumor burden as the median BMPC % was higher in the high B2M group compared to the low group. Also more patients with the high B2M levels had more organs involved and more had advanced 2012 Mayo stage. Increased B2M was a significant predictor for OS even after adjusting for other known prognostic markers. It did not predict PFS and this reflects that increased B2M represents more an increase of overall burden of disease, not chemo sensitivity and relapse after response.
To our knowledge, the prognostic impact of increased B2M in AL amyloidosis was previously evaluated in two small retrospective studies (1, 2). B2M >2.7 μg/ml had a median OS that was 22 months less than patients with B2M ≤ 2.7 μg/ml(1). Increased B2M was still a predictor in the multivariate analysis that included congestive heart failure (CHF) and age. Increased creatinine causes an increase in B2M, which can affect its prognostic effect. All patients with creatinine ≥2 mg/dl had an abnormal B2M(1). The median survival was 9 months for patients with increased B2M and normal creatinine, compared to 14 months in patients who had an increased B2M and creatinine, highlighting that B2M level is more prognostic if the creatinine is normal(1). Interestingly, patients who had CHF and increased B2M had worse survival that patients with CHF and decreased B2M. In the other retrospective analysis, the median OS was 8 months less for patients with B2M >4 μg/ml vs. ≤ 4 μg/ml (P=0.04)(2). Again, the average creatinine in patients with increased B2M was almost doubled compared to patients with decreased B2M (2.67 vs. 1.43 mg/dl, P=0.019)(2). Increased B2M was more predictive of OS if it was increased with normal creatinine (median: 6.1 vs. 13.9 months) compared to increased B2M and increased creatinine, again highlighting the effect of increased creatinine on B2M levels. However, increased B2M was not predictive for OS when adjusting for cardiac involvement. This was most likely related to the small sample size of patients (n=80). From these 2 studies, it is clear that B2M is affected by the kidney function. B2M has been used to predict glomerular filtration rate and end stage renal disease (ESRD)(9–11), being an independent prognostic marker for ESRD. In our analysis, we evaluated more patients and excluded those with abnormal creatinine to better assess the effect of increased B2M on survival.
The Mayo 2012 stage for AL amyloidosis is a strong predictor for survival (3). The B2M level was evaluated during the construction of the model. Initially, 810 patients were evaluated for the development of the stage. The median creatinine was 1.1 mg/dl with a range of (0.8–2.2). B2M >3 μg/ml was a significant predictor for outcomes when evaluated with other plasma cell related factors. When included with cardiac biomarkers, it was not predictive. The discrepancy between this and our results is multifactorial. We strictly included patients with normal creatinine to evaluate the prognostic impact of B2M. The increased creatinine in some patients during the Mayo 2012 construction could be a reason for B2M losing its significance in the multivariate analysis. Also, our patient population is different, as we evaluated patients with newly diagnosed AL who underwent ASCT, in contrast to the original cohort of the Mayo stage, which included all patients. When the Mayo 2012 stage was validated in the transplanted patients, only 29% had stage III/IV(3), emphasizing the selection of patients with less advanced disease for ASCT. In our analysis, we also included age and number of organs (12), which are known prognostic factors for survival in AL.
The BMPC burden in AL amyloidosis is low as the median BMPC% is around 5–10% (13, 14). The BMPC% was not predictive for OS in our multivariable models. Also, the B2M was prognostic across patients with BMPC≥10 and <10%. This indicates that the burden of the plasma cells can be assessed by using the B2M level in conjunction with the BMPC%. Patients with increased B2M levels had more advanced Mayo 2012 stage, more organs involved, and were less likely to achieve CR post ASCT, highlighting the more aggressive nature of the plasma cell clone. It is well documented that increased B2M is associated with worse outcomes in MM, as B2M is included in staging system (7, 8), and we suggest its use in AL amyloidosis as well.
Our study has some limitations. We included patients with normal creatinine and the prognostic effect of B2M could not apply to patients with increased creatinine. We also included patients who were only eligible for ASCT. Finally, patients received treatment with ASCT over a long period of time. Despite this, we show that B2M level >2.5 μg/ml before ASCT is an independent and a strong prognostic marker in patients with AL amyloidosis undergoing ASCT with normal kidney function. It is easy to be measured and we recommend including it in the evaluation of patients with AL amyloidosis.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest related to the manuscript: None
References:
- 1.Gertz MA, Kyle RA, Greipp PR, Katzmann JA, O’Fallon WM. Beta 2-microglobulin predicts survival in primary systemic amyloidosis. Am J Med. 1990;89(5):609–14. [DOI] [PubMed] [Google Scholar]
- 2.Zerbini CA, Anderson JJ, Kane KA, Ju ST, Campistol JM, Simms RW, et al. Beta 2 microglobulin serum levels and prediction of survival in AL amyloidosis. Amyloid. 2002;9(4):242–6. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–28. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9. [DOI] [PubMed] [Google Scholar]
- 6.Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26(11):2317–25. [DOI] [PubMed] [Google Scholar]
- 7.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster MC, Coresh J, Hsu CY, Xie D, Levey AS, Nelson RG, et al. Serum beta-Trace Protein and beta2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59(5):653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muchtar E, Gertz MA, Lacy MQ, Go RS, Buadi FK, Dingli D, et al. Ten-year survivors in AL amyloidosis: characteristics and treatment pattern. Br J Haematol. 2019;187(5):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muchtar E, Gertz MA, Kourelis TV, Sidana S, Go RS, Lacy MQ, et al. Bone marrow plasma cells 20% or greater discriminate presentation, response, and survival in AL amyloidosis. Leukemia. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Tovar N, Rodriguez-Lobato LG, Cibeira MT, Magnano L, Isola I, Rosinol L, et al. Bone marrow plasma cell infiltration in light chain amyloidosis: impact on organ involvement and outcome. Amyloid. 2018;25(2):79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


