Abstract
Mentalizing, or thinking about others’ mental states, plays a key role in shaping human social interactions. Numerous studies suggest that older adults (OA) have reduced mentalizing capacities that are reflected by lower medial prefrontal cortex (mPFC) activation during person perception. Although it has been commonly argued that this lower mPFC activation reflects less spontaneous mentalizing during person perception, this has not been empirically established. The current study addresses this assertion in OA. While undergoing fMRI, younger adults (YA) and OA completed a person perception task in which they passively viewed ingroup White and outgroup Black and Asian faces. They also completed a well-validated fMRI-based mentalizing task in which they considered others’ mental states based on short vignettes. Outside of the scanner, they completed a different mentalizing task in which they inferred mental states from faces. Using a region in mPFC that was defined by the fMRI-based mentalizing task, we had two key findings: 1) OA had lower mPFC activity than YA during face perception, and 2) OA’ mPFC activity toward faces positively related to their mentalizing performance outside of the scanner. This finding suggests that although OA engage in less mentalizing than YA during face perception, the extent of their mPFC engagement may depend on their actual detection of mental states in faces. Finally, we found that whereas YA’ mPFC activity distinguished between different outgroups, OA’ mPFC activity did not. This finding suggests that OA’ lower mentalizing-related mPFC activity may reduce their ability to individuate outgroup members.
Keywords: mentalizing, person perception, medial prefrontal cortex, race
Thinking about others’ mental states, or mentalizing, is a critical aspect of human interaction (for reviews, see Leslie, 1987; Moran, 2013). The ability to represent others’ mental states allows people to predict how others might behave and humanize others (e.g., Jack, Dawson, & Norr, 2013). Numerous studies suggest that older adults (OA) mentalize less than do younger adults (YA; for a meta-analysis, see Henry, Phillips, Ruffman, & Bailey, 2013), which negatively affects OA’ social participation (Bailey, Henry, & von Hippel, 2008). In turn, lower social participation negatively affects OA’ physical and mental well-being (e.g., poorer mental and physical health; Cornwell & Waite, 2009). A limitation of extant work on age-related reductions in mentalizing is that OA’ mentalizing has primarily been assessed using directed, or explicit, mentalizing tasks (e.g., Laillier et al., 2019). By contrast, little is known about how mentalizing spontaneously engages in OA. Spontaneously thinking about others’ mental states is a key component of person perception that occurs whenever people see another face (Todorov, Said, Engell, & Oosterhof, 2008). Research in YA has shown that spontaneous mentalizing is associated with activation in the same brain regions as those involved in directed mentalizing (e.g., Amodio & Frith, 2006; Harris & Fiske, 2006). Spontaneous mentalizing in YA also predicts behavioral changes (e.g., adapting behavior to shifting expressions; Kringelbach & Rolls, 2003) and positively relates to social participation (Powers, Chavez, & Heatherton, 2015). Characterizing OA’ spontaneous mentalizing toward faces is important because it may reveal a baseline difference in OA’ mentalizing, in a context ubiquitous to everyday life, that potentially contributes to their directed mentalizing and their well-being. The present study addressed this gap in the literature.
Medial prefrontal cortex (mPFC) activity plays a central role in YA’ directed and spontaneous mentalizing (Gallagher & Frith, 2003; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Spiers & Maguire, 2006; Van Overwalle & Vandekerckhove, 2013). MPFC is also a key region in the brain’s “default network” (Mars et al., 2012), which comprises a functionally connected set of regions whose activation is proposed to reflect socio-cognitive thought in the absence of tasks (i.e., at rest; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008). A growing body work has shown that OA, relative to YA, have lower mPFC activity during mentalizing tasks (e.g., Moran, Jolly, & Mitchell, 2012), which may contribute to their reduced performance on these tasks (e.g., Moran, 2013). Moreover, OA have lower mPFC activity than YA when forming (Cassidy, Leshikar, Shih, Aizenman, & Gutchess, 2013) and updating (Suzuki et al., 2019) impressions. These data suggest that OA are less likely to mentalize about incoming social cues, which may reduce the quality of their social interactions (e.g., T. Lee et al., 2010).
Beyond lower mPFC activity during tasks where people are directed to consider others’ mental states, OA also have lower mPFC activity than YA when passively viewing shapes making animate (which elicits mentalizing) versus mechanical movements (Moran, Jolly, & Mitchell, 2012). These findings suggest age deficits in mPFC activity emerge across directed and spontaneous mentalizing tasks. They also suggest that, like YA (e.g., Van Overwalle & Vandekerckhove, 2013), OA similarly draw on mPFC activity across mentalizing tasks. We tested for this possibility by examining if OA’ spontaneous mPFC activity when merely perceiving faces positively corresponded to their performance on an independent measure, the Reading the Mind in the Eyes task (RME; Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001), in which their direct mentalizing performance during face perception was measured. We expected OA’ spontaneous mPFC activity to positively relate to their directed mentalizing performance (Hypothesis 1). Relatedly, we expected lower mPFC activity in OA versus YA during when perceiving faces (Hypothesis 2), which would reflect less spontaneous mentalizing in OA.
An additional, albeit exploratory, goal of this study was to characterize how OA activate mPFC toward faces belonging to different social groups (i.e., ingroup and outgroup faces). This is an important consideration because mentalizing negatively relates to expressed prejudice toward racial outgroups (Todd, Bodenhausen, Richeson, & Galinsky, 2011) as well as behaviors toward specific outgroup members (Shih, Wang, Bucher, & Stotzer, 2009). Because OA often express more bias against outgroup members (e.g., Cassidy, Lee, & Krendl, 2016), characterizing OA’ spontaneous mentalizing toward racial ingroup and outgroup faces may inform future work examining precursors to OA’ higher bias.
Relevant here, YA more accurately infer the mental states of racial ingroup members than of outgroup members (Adams et al., 2009; Mathur, Harada, & Chiao, 2011). This could be, at least in part, because they engage mentalizing more when perceiving ingroup versus outgroup faces (e.g., Xu, Zuo, Wang, & Han, 2009). Lower mPFC activity reflecting less mentalizing, however, is not universal when perceiving all outgroups (Harris & Fiske, 2007). One proposed function of mPFC activation toward different outgroups is that higher activation reflects more mentalizing to behave accordingly with norms (Amodio, 2014; Li, Cardenas-Iniguez, Correll, & Cloutier, 2016). Some racial outgroups have more positive stereotypes associated with them than others, however (S. Lee, Wong, & Alvarez, 2009). People often stereotype Asian individuals, for example, as being more trustworthy than Black individuals (Cassidy et al., 2017). Based on past work (e.g., Li et al., 2016), it could be that YA engage mPFC more toward racial outgroup faces for whom negative stereotypes (that conflict with norms) are more prominent. We hypothesized that YA might have higher mPFC activity toward ingroup White and outgroup Black faces relative to outgroup Asian faces (Hypothesis 3a).
A key benefit of examining mPFC activity toward ingroup and outgroup faces is that it can disentangle whether OA have broad or more specific deficits in mPFC activity toward faces. Because OA have lower mPFC activity and mentalizing than YA in tasks in which group membership is irrelevant (Moran et al., 2012; Slessor, Phillips, & Bull, 2007), it suggests that OA might have lower mPFC activity than YA regardless of group membership. Conversely, because OA express more anti-Black racial bias than YA do (e.g., Gonsalkorale, Sherman, & Klauer, 2009), it is also possible that OA might still engage mentalizing more toward racial ingroup than some outgroup faces. Here, we explored whether OA’ overall lower mPFC activity (relative to YA) toward faces differed by race by assessing if OA’ mPFC activity was higher for ingroup White than for outgroup Black and for outgroup Asian faces (Hypothesis 3b).
In the present study, YA and OA completed a person perception task where they perceived ingroup White and outgroup Black and Asian faces during fMRI. Because the person perception task had no instructions related to mentalizing and because people spontaneously mentalize about social stimuli (Powers et al., 2015), this task measured spontaneous mentalizing. We verified this measurement by having YA and OA also complete explicit mentalizing tasks inside and outside of the scanner. We used an explicit mentalizing task in the scanner to identify mPFC activation involved in directed mentalizing (the false belief task; Saxe & Kanwisher, 2003; Zaitchik, 1990). We then provided converging evidence that this activation is involved in directed mentalizing by relating it to performance on the directed mentalizing task outside the scanner. This mPFC region was then used to characterize mentalizing-related mPFC activation during the person perception task. This methodology allowed us to examine if mPFC activation defined by directed mentalizing exhibited lower activation in OA versus YA when perceiving faces.
Method
Participants
Forty YA (Mage=21.58 years, SD=2.81, age range=18–33, 25 female) and 35 OA (Mage=71.66 years, SD=6.09, age range=61–86, 22 female) adults recruited from Indiana University and the surrounding community participated as part of a larger study on aging and social cognition that took place over two sessions. The first session consisted of a series of social and cognitive behavioral measures (relevant measures described below) and an fMRI screening. The second was the fMRI study. Participants self-identified as White, were right-handed, did not have conditions potentially impacting cognitive function or brain activity, and provided written informed consent. The sample sizes of YA and OA were selected to be larger than samples used in recent work on aging and mentalizing (e.g., Suzuki et al., 2019). The Indiana University IRB approved this study.
OA had more years of education than YA and had higher vocabulary scores than YA (Shipley, 1986). YA had faster processing speed than OA, as measured by digit comparison (Hedden et al., 2002), and had higher working memory, as measured by scores on a shortened version of the operation span task (for details, see Oswald, McAbee, Redick, & Hambrick, 2015). YA and OA did not differ on MMSE scores (Folstein, Folstein, & McHugh, 1975). See Table 1 for statistics.
Table 1.
Main effect of Belief Response from the directed mentalizing task. Corrected p<.05 (FWE-correction, k=20) with MNI coordinates of peak activations.
| Region | BA | k | F | MNI coordinates |
|---|---|---|---|---|
| Frontal lobe | ||||
| L superior frontal gyrus | 8 | 406 | 39.88 | −9, 33, 57 |
| L superior frontal gyrus | 9 | * | 5.81 | −6, 48, 45 |
| R medial prefrontal cortex | 10 | * | 5.75 | 6, 54, 21 |
| R superior frontal gyrus/middle frontal gyrus | 8/9 | 23 | 31.74 | 27, 36, 48 |
| R superior frontal gyrus | 8 | * | 4.89 | 24, 30, 54 |
| L medial prefrontal cortex | 10/11 | 24 | 29.77 | 0, 54, −12 |
| Temporal lobe | ||||
| L middle temporal gyrus/L temporal pole | 21/38 | 1095 | 142.99 | −54, 6, −24 |
| L middle temporal gyrus | 21 | * | 107.56 | −60, −9, −12 |
| L middle temporal gyrus | 20 | * | 85.99 | −60, −24, −9 |
| R middle temporal gyrus/R temporal pole | 21/38 | 1019 | 141.28 | 57, 3, −24 |
| R temporal pole | 38 | * | 135.23 | 51, 15, −30 |
| R middle temporal gyrus | 20 | * | 124.10 | 57, −12, −15 |
| L inferior temporal gyrus/ fusiform gyrus | 37/20 | 87 | 47.94 | −54, −54, −9 |
| Parietal lobe | ||||
| R precuneus | 31 | 703 | 168.86 | 3, −57, 33 |
| R temporoparietal junction | 39/40/22 | 671 | 157.14 | 54, −51, 27 |
| L temporoparietal junction | 39 | 692 | 143.12 | −51, −57, 27 |
| Occipital lobe/other | ||||
| R cerebellum crus 1 | 68 | 72.54 | 27, −84, −30 | |
| L cerebellum crus 1 | 91 | 71.41 | −21, −84, −33 |
Note.
sub-cluster of above-listed region
Behavioral testing session
Mentalizing.
To directly measure mentalizing toward faces, participants completed a computer version of the RME (for details, see Baron-Cohen et al., 2001). The RME was self-paced and consisted of 36 trials. On each trial, participants saw a set of eyes at the center of the screen and four different trait adjectives, one at each corner of the screen. Participants were given a handout with definitions for every word used in the task. Participants selected the word that best described what the person in the picture was thinking or feeling. The maximum score on the RME is 36. Higher scores reflect better ability to detect others’ mental states. Although larger age deficits on the RME emerge for decoding negative versus positive mental states (Franklin Jr. & Zebrowitz, 2016), we used a score combining across mental states in the below analyses. We did this because the faces in the person perception task (see below) had neutral expressions, and because our hypotheses focused on mentalizing as a broad process (that is, spontaneous mentalizing may include decoding both negative and positive mental states).
Scanning session
Participants completed three tasks in a counterbalanced order. Two (described below) were relevant here. The other was an unrelated mental health attitudes task.
Person perception task.
Sixty Black and sixty White young male faces with neutral expressions were drawn from the Eberhardt Face Database (https://web.stanford.edu/group/mcslab/cgi-bin/wordpress/examine-the-research/). This database, which has been used in fMRI studies on race perception (e.g., Cassidy & Krendl, 2016), includes ratings on attractiveness for each face. Sixty Asian young male faces with neutral expressions were drawn from the CAS-PEAL database (Gao et al., 2008). Fifteen Indiana University undergraduates rated these faces for attractiveness using the same 7-point scale (1=not at all attractive, 7=very much attractive) used in the Eberhardt Face Database. An ANOVA showed that the Black, Asian, and White faces did not differ in attractiveness, F(2,177)=.27, p=.77, ηp2<.01. To verify that expected patterns in mPFC activity were specific to faces and did not generalize across objects, 120 cars (60 black and 60 white) were selected from online image searches and cropped to remove any background. All stimuli were presented in greyscale.
The task was modeled as an event-related design over two runs each lasting three minutes and 44 seconds (four 2s dummy scans followed by 108 scan-related TRs at 2s each). Participants viewed images (30 of each race and 30 of each car color in each run) for 1s each. Images were randomly presented. All conditions were equally represented in both runs. The order of stimuli and fixations were created using a random number generator. No two images of the same type appeared twice in succession.
Half of the images appeared on the right side of the display, and half on the left. It was equally probable that images from all conditions would appear on either side across runs. Participants indicated via button press on which side of the display images appeared (as in Cassidy et al., 2016; Cunningham et al., 2004). Responses were monitored to ensure attention during the task. On average, participants responded to 294.96 (SD=7.02) of the 300 trials for a response rate of 98.32% (SD=2.34%) with 99.24% (SD=.09%) accuracy. There were no age differences in response rate (MYA=98.56%, SD=2.34%; MOA=98.05%, SD=2.35%, t(73)=.94, p=.35, d=.22) or accuracy (MYA=99.34%, SD=.06%; MOA=99.12%, SD=1.15%, t(73)=1.05, p=.30, d=.28).
Jitter, in the form of a fixation cross at the center of the display, ranged from 1s to 7s and were pseudorandomly presented throughout each run. There were seven 1s fixations, three 3s fixations, three 5s fixations, and two 7s fixations in each run (Mjitter=3s, SD=2.27) with 10s of fixation at the beginning and 11s of fixation at the end, for a total of 66s of fixation and 150s of stimulus presentation.
Directed mentalizing localizer.
We used the false belief task (Saxe & Kanwisher, 2003; Zaitchik, 1990) to identify a mPFC region associated with directed mentalizing. The false belief task has been used as a mentalizing localizer for numerous social cognition tasks (e.g., Young, Camprodon, Hauser, Pascual-Leone, & Saxe, 2010). This independently defined mPFC region was then used to measure neural activity in the person perception task (i.e., spontaneous mentalizing). Of interest was how this region responded when YA and OA perceived racial ingroup and outgroup faces.
Participants viewed stories referring to either a person’s beliefs (i.e., mental trials) or to physical representations (i.e., physical trials), followed by a statement about those stories that they evaluated as being true or false. As an example of a mental trial, participants saw the following story: “When Lisa left Jacob, he was deep asleep on the beach. A few minutes later, a wave woke him. Seeing Lisa was gone, Jacob decided to go swimming,” which was followed by the statement “Lisa now believes that Jacob is sleeping.” An example of the physical story is, “When the picture was taken of the house, it was one story tall. Since then, the renovators added an additional story and a garage,” and was followed by the statement “In the picture, the house is two stories tall and has a garage”.
Twelve stories of each type were presented in two runs lasting five minutes and 28 seconds each, with six of each type in each run. The order of mental and physical trials was determined via a random number generator. Run order was counterbalanced between participants. Each trial began with a story presented for 10s. The story was followed by a variable delay of 0–6s in the form of a fixation cross at the center of the display. Finally, a statement that was true or false was presented for 6s (for detailed behavioral results, see Hughes et al., 2019). In each run, there were three 0s delays, three 2s delays, three 4s delays, and three 6s delays (Mdelay=3s, SD=2.34), with 8s of fixation at the beginning of the run and 10s of fixation at the end, for a total of 128s of fixation and 192s of stimulus presentation.
fMRI data acquisition.
Whole-brain imaging was performed on a Siemens 3.0T Prisma MRI scanner using a 20-channel phase arrayed head coil at the Indiana University Imaging Research Facility in Bloomington, Indiana. Stimuli were presented using a back projector and behavioral data were collected on a Dell laptop running Windows 7. The scanner was synced to the data collection equipment via scanner TTL.
Anatomical images were collected prior to the functional tasks in one run lasting three minutes and 52 seconds. These images were acquired with high-resolution 3-D magnetization prepared rapid gradient echo sequence (sagittal rotation; 160 slices, TE = 2.7ms, TR = 1800ms, TI = 900ms, flip angle = 9 degrees, 1.0mm isotropic voxels; with no fat suppression).
For the mentalizing localizer and the person perception task, functional images were collected using simultaneous multi-slice scanning, for which 54 slices 2.2mm thick were acquired with an echo-planar image (EPI) sequence sensitive to blood oxygen level dependent contrast (T2*; TE=30ms, TR=2000ms, flip angle=52 degrees, 2.2mm isotropic voxels, FOV=242mm, in-plane matrix size=110×110, A/P phase encoding direction). Slices were 2.2mm thick with no gap and collected in an interleaved order (multi-band acceleration factor=2). These slices provided partial-brain coverage (i.e., the entire cortex with partial cerebellum, but not brainstem). Four dummy scans were included at the start of each run to allow for stabilization of the scanner signal. Dummy scans were excluded from analyses.
fMRI data preprocessing and analyses.
Preprocessing and analyses of functional data were conducted in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were realigned to correct for motion, normalized to the MNI (Montreal Neurological Institute) template, and smoothed using an 8-mm FWHM isotropic Gaussian kernel. Data were resampled to 3mm-isotropic voxels.
For the person perception task, a GLM with the task conditions (Black face, Asian face, White face, black car, and white car) and covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) computed parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each participant. Relevant parameter estimates were included in a group level analysis, treating participants as a random effect.
For the mentalizing localizer, a GLM with four conditions (Mental Trial/Read Story, Physical Trial/Read Story, Mental Trial/Belief Response, Physical Trial/Belief Response) and covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) computed parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each participant. We localized activity specific to mentalizing using the main effect of Belief Response from a 2 (Age: YA, OA)×2 (Belief Response: mental trial, physical trial) whole-brain ANOVA, FWE-corrected p<.05. As expected, the main effect of belief response was associated with heightened activation in neural regions associated with mentalizing (see Table 1). The selected mPFC region was verified to be specific to mentalizing by examining the t-contrast of [mental trial>physical trial]. MPFC activation can reflect a variety of functions associated with mentalizing (Van Overwalle, 2009), making it difficult to ensure that all mPFC activations reflect the same function. It is thus important to characterize mPFC activation among YA and OA using the same mPFC region. For this reason, we examined an mPFC region from the main effect of Belief Response rather than age-specific mPFC regions. Notably, YA and OA each had mPFC activation similar to the activation emerging from the main effect of Belief Response in the mentalizing localizer (see Table 2). These patterns make examining YA’ and OA’ mPFC activation defined by directed mentalizing when they are spontaneously mentalizing appropriate and unlikely to be driven by an absence of mentalizing-related activity altogether among OA.
Table 2.
Interaction between Age Group and Belief Response from the directed mentalizing task, (p<.001 uncorrected and k=88 for a corrected p<.05).
| Region | BA | k | F | MNI coordinates |
|---|---|---|---|---|
| Interaction | ||||
| R temporal pole | 38/20 | 214 | 26.27 | 48, 18, −33 |
| R temporal pole | 21 | * | 24.39 | 63, 0, −33 |
| R temporal pole | 20 | * | 20.88 | 51, 3, −33 |
| L temporal pole | 21/20 | 233 | 22.40 | −63, −6, −30 |
| L temporal pole | 21 | * | 21.23 | −57, 9, −36 |
| L temporal pole | 38 | * | 21.23 | −45, 24, −33 |
| R precuneus | 30/23 | 194 | 21.75 | 3, −48, 15 |
| R precuneus / posterior cingulate cortex | 23 | * | 19.97 | 12, −48, 30 |
| L precuneus | 23 | * | 17.14 | −9, −51, 30 |
| Region | BA | k | t | MNI coordinates |
| YA false belief > false photo | ||||
| R middle temporal gyrus /R temporal pole | 38/21 | 2748 | 10.83 | 51, 15, −30 |
| R temporoparietal junction | 22 | * | 10.53 | 60, −57, 24 |
| R temporal pole | 21 | * | 10.41 | 54, 3, −30 |
| Precuneus | 23 | 1184 | 10.67 | 0, −54, 33 |
| R precuneus | 23 | * | 10.47 | 9, −51, 33 |
| L precuneus | 27 | * | 3.87 | −9, −30, 6 |
| L middle temporal gyrus/L temporal pole | 21/20 | 1816 | 9.99 | −54, 6, −24 |
| L temporal pole | 20 | * | 9.53 | −51, 9, −36 |
| L temporal pole | 38 | * | 8.98 | −42, 24, −30 |
| L temporoparietal junction | 39 | 1109 | 9.74 | −51, −60, 27 |
| L temporoparietal junction | 22 | * | 4.01 | −72, −39, 9 |
| L cerebellum crus 1 | 141 | 6.93 | −21, −84, −33 | |
| R cerebellum crus 1 | 107 | 6.89 | 27, −84, −33 | |
| R superior frontal gyrus/middle frontal gyrus/medial prefrontal cortex | 10/32 | 2790 | 6.13 | 3, 54, 24 |
| L superior frontal gyrus | 9 | * | 5.92 | −6, 51, 45 |
| R medial prefrontal cortex | 11 | * | 5.84 | 0, 51, −12 |
| L paracentral lobule | 4 | 177 | 4.60 | −3, −33, 69 |
| L precuneus | 5 | * | 3.95 | −9, −45, 84 |
| L precuneus | 5 | * | 3.73 | −6, −51, 75 |
| YA false photo > false belief | ||||
| L inferior temporal gyrus | 37 | 189 | 6.32 | −51, −54, −9 |
| L interior frontal gyrus | 45 | 131 | 5.02 | −48, 38, 12 |
| OA false belief > false photo | ||||
| R precuneus | 23 | 541 | 8.09 | 3, −57, 33 |
| R temporoparietal junction | 22/39 | 485 | 7.94 | 54, −51, 27 |
| R middle temporal gyrus/R temporal pole | 20/21 | 868 | 7.93 | 57, −12, −15 |
| R temporal pole | 21 | * | 7.00 | 57, 3, −24 |
| R temporal pole | 38 | * | 3.29 | 33, 24, −36 |
| L middle temporal gyrus/L temporal pole | 21/20/38 | 859 | 7.53 | −51, 3, −24 |
| L middle temporal gyrus | 22 | * | 6.76 | −57, −9, −12 |
| L middle temporal gyrus | 21 | * | 6.58 | −63, −24, −6 |
| L temporoparietal junction | 39 | 570 | 7.35 | −51, −57, 27 |
| R cerebellum crus 1 | 106 | 5.64 | 27, −81, −30 | |
| L superior frontal gyrus | 6/8 | 108 | 4.37 | −9, 30, 60 |
| L middle frontal gyrus | 8 | * | 4.31 | −6, 21, 66 |
| L medial prefrontal cortex | 10 | 147 | 4.28 | −6, 63, −3 |
| L orbitofrontal cortex | 11 | * | 3.88 | −15, 63, −18 |
| R orbitofrontal cortex | 11 | * | 3.84 | 6, 66, −9 |
| OA false photo > false belief | ||||
| no significant clusters | ||||
Note. An interaction emerged in mPFC (BA 10, k=115, F=17.73, peak MNI coordinates: −3, 63, 24) using the same thresholding as tasks where age deficits in mPFC activity have been previously detected (p<.005, k=56; Moran et al., 2012).
sub-cluster of above-listed region
Exploratory whole-brain analyses probed brain regions emerging in an Age × Belief Response interaction (see Table 2). We corrected for Type I error using 3dClustSim in AFNI. Smoothness estimates entered into 3dClustSim were spatial autocorrelation function (acf) parameters as calculated by 3dFWHMxyz using the –acf flag. Monte Carlo simulations determined that a voxel-wise threshold of p<.001 combined with a spatial extent threshold of 88 voxels corresponded to a p<.05 FWE correction.
Region of interest (ROI) analyses (see Poldrack, 2007) were conducted with Marsbar (http://marsbar.sourceforge.net/). We used a peak mPFC coordinate (0, 54, −12), defined by the highest mPFC activation in the mentalizing localizer, to create an independently defined mPFC ROI that was related to mentalizing. This ROI consisted of a 6mm sphere surrounding the peak coordinate. Average parameter estimates from the ROI were obtained by extracting values from relevant contrasts. A 2 (Age: YA, OA)×3 (Race: Black, Asian, White) ANOVA assessed mPFC activity related to mentalizing during the person perception task.
Results
RME Performance
YA and OA did not differ in RME performance (see Table 3), although means were in the direction of OA having lower RME performance than YA.1 Because the OA sample comprised a wide age range, we examined whether participant age negatively predicted RME performance. OA’ age negatively related to their RME performance, r(33)=−.38, p=.02 (Figure 1a). YA’ age was not significantly related to their RME performance, r(38)=.12, p=.48. A Fisher r-to-z comparison showed that this relationship was stronger among OA than among YA, z=2.17, p=.03.
Table 3.
Means (standard deviations) for demographic and behavioral data in YA and OA.
| YA | OA | t | p | Cohen’s d | |
|---|---|---|---|---|---|
| Years of Education | 15.24 (1.88) | 16.96 (2.19) | 3.66 | <.001 | .84 |
| Vocabulary | 31.25 (4.48) | 36.63 (2.32) | 6.40 | <.001 | 1.46 |
| Processing speed | 79.00 (14.69) | 61.03 (11.69) | 5.81 | <.001 | 1.33 |
| Operation span (absolute) | 20.75 (7.10) | 12.66 (8.94) | 4.37 | <.001 | 1.00 |
| Operation span (partial) | 25.28 (4.78) | 18.49 (8.15) | 4.46 | <.001 | 1.02 |
| Mini-Mental State Examination | 29.38 (.95) | 28.80 (1.62) | 1.90 | .06 | .44 |
| Reading the Mind in the Eyes (RME) | 27.05 (3.06) | 26.91 (4.05) | .17 | .87 | .03 |
Figure 1.
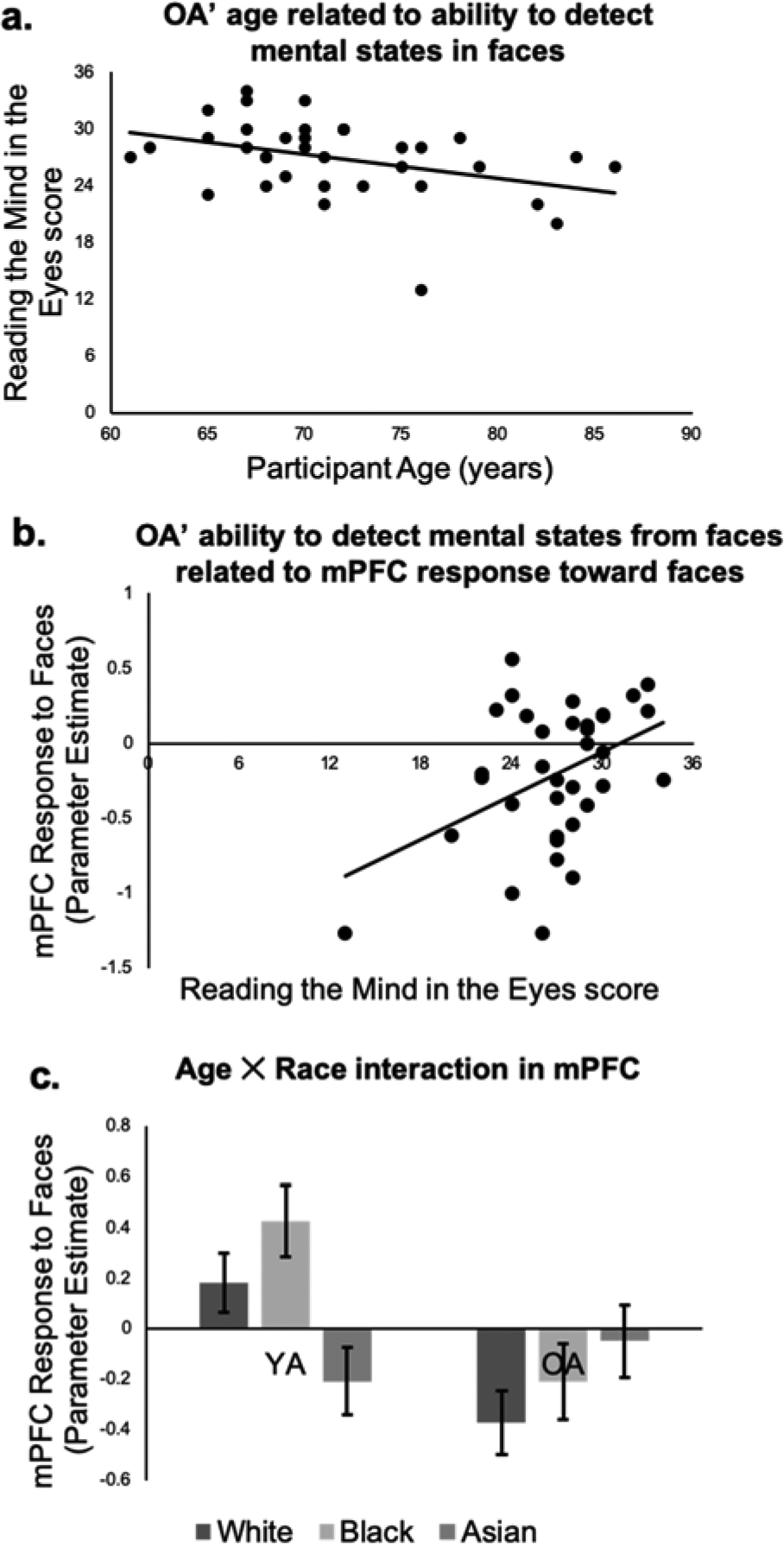
OA’ age negatively related to their detection of mental states in faces (measured by RME performance; a). OA’ performance on a directed mentalizing task toward faces positively related to their spontaneous mPFC response to faces (b). YA had higher spontaneous mPFC activity than OA overall. YA had higher mPFC activity toward White and Black versus Asian faces, and no difference between White and Black faces. OA had no difference between White and Black faces, no difference between Black and Asian faces, and higher activity toward Asian than White faces (b).
Hypothesis 1: OA’ spontaneous mPFC activation toward faces will positively relate to their directed mentalizing in response to faces.
A first goal was to show that similar to YA (Ma, Vandekerckhove, Van Overwalle, Seurinck, & Fias, 2011; Van Overwalle & Vandekerckhove, 2013), OA’ spontaneous and directed mentalizing toward faces similarly draws on mPFC activation. We correlated average mPFC response to faces collected during the person perception task (which measured spontaneous mentalizing) from the above-described independent mPFC ROI defined by the directed mentalizing task, with RME performance (which measured directed mentalizing in response to faces). Supporting Hypothesis 1, OA’ mPFC activity toward faces positively correlated with their RME performance, r(33)=.42, p=.01 (Figure 1b).2 One possibility is that the relatively brief presentation of each face (1s) might have been disproportionately burdensome for OA versus YA. In other words, task demands, and not mentalizing, might have accounted for age differences in mPFC activation. To explore this possibility, we examined whether OA’ processing speed (measured by scores on the digit comparison task) or working memory (measured by partial operation span scores) related to their mPFC response toward faces. If so, it would suggest that OA’ mPFC response might have been confounded by task demands. Neither OA’ processing speed nor working memory significantly, however, related to their mPFC response toward faces (Table 4a). Moreover, the correlation between OA’ RME performance and their mPFC response toward faces remained significant when controlling for processing speed and working memory (Table 4b). These findings support the assertion that OA’ reduced mentalizing capacities, rather than task demands, related to their mPFC response toward faces. The correlation between RME performance and mPFC response toward was not significant in YA, r(38)=−.10, p=.52. A Fisher r-to-z transformation revealed this correlation to be stronger in OA versus YA, z=2.29, p=.02.
Table 4.
Intercorrelations between mPFC activity toward faces and behavior in YA and OA.
| A. Measure | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. mPFC response toward faces | -- | −.10 | −.18 | −.16 |
| 2. Reading the Mind in the Eyes (RME) | .42* | -- | −.03 | −.05 |
| 3. Processing speed | .24 | .17 | -- | .18 |
| 4. Operation span (partial) | .16 | .36* | .39* | -- |
| B. Controlling for processing speed and operation span (partial) | RME | |||
| YA | OA | |||
| mPFC response toward faces | −.12 | .40* | ||
Note. Intercorrelations for YA are presented above the diagonal, and intercorrelations for OA are presented below the diagonal.
p<.05.
We next examined whether age moderated the effect of RME performance on mPFC activity toward faces. To do this, we used Model 1 in PROCESS for SPSS (Hayes, 2012), and dummy coded age such that 0=YA and 1=OA. The model was significant, F(3, 71)=4.96, p=.004. Age negatively related to mPFC response to faces, b=−2.15, SE=.91, t=−2.37, p=.02, suggesting that OA had a lower mPFC response than did YA (see below). RME performance did not relate to mPFC response toward faces, b=−.02, SE=.03, t=−.71, p=.48. Consistent with the above-described analyses, an interaction between age and RME performance emerged, b=.07, SE=.03, t=2.02, p=.047. RME performance positively related to OA’ mPFC response toward faces, b=.05, SE=.02, t=2.32, p=.02, but not YA’ mPFC response toward faces, b=−.02, SE=.03, t=−.71, p=.48.
Hypotheses 2–3: Characterizing Mentalizing-Related mPFC Activation to Faces in Aging
We next tested if age differences in mPFC activity during directed mentalizing tasks (Moran et al., 2012; Suzuki et al., 2019) extend to spontaneous mentalizing tasks (e.g., when merely viewing faces). We also characterized mPFC activity across racial ingroup and outgroup faces. Here, we entered parameter estimates extracted from the same mPFC ROI defined by the directed mentalizing task (peak coordinate: 0, 54, −12) into a 2 (Age: YA, OA)×3 (Race: Black, Asian, White) ANOVA (Table 2)
Hypothesis 2: OA will have less mentalizing-related mPFC activity than YA.
Supporting Hypothesis 2, a main effect of Age emerged in mPFC, F(1, 73)=8.53, p=.005, ηp2=.11, such that OA had lower spontaneous mPFC activation toward faces than did YA (Figure 1c). This finding extended work showing that OA have lower mPFC activity than YA during directed mentalizing tasks (Moran et al., 2012) to spontaneous mentalizing toward faces.
Support for Hypotheses 3a: YA’ spontaneous mPFC activity will vary toward racial ingroup and outgroup faces.
Supporting Hypothesis 3a, there was an interaction between Age and Race, F(2, 146)=5.50, p=.005, ηp2=.07 (Figure 1c). YA had higher spontaneous mPFC activation toward Black, t(39)=2.64, p=.01, and White, t(39)=2.04 p=.048, versus Asian faces. YA’ spontaneous mPFC activation toward Black and White faces did not differ, t(39)=1.31 p=.20. OA’ mPFC activation toward Black and White faces, t(34)=1.15 p=.26, and toward Black and Asian faces, t(34)=.90, p=.37, did not differ. Unexpectedly, OA had more activation toward Asian than White faces, t(34)=2.39, p=.02. There was no main effect of Race, F(2, 146)=1.90, p=.15, ηp2=.03.
Verifying face-specific effects.
To determine whether the above-described effects were unique to face perception (and did not generalize to other objects), we conducted a 2 (Age: YA, OA)×2 (Car Color: black, white) ANOVA on mPFC activation from the same independently defined ROI. There was no main effect of Age, F(1, 73)=1.74, p=.19, ηp2=.02, no main effect of Car Color, F(1, 73)=1.15, p=.29, ηp2=.02, and no interaction, F(1, 73)=.14 p=.71, ηp2=.002. One possibility for our finding that the age effects in mPFC emerged for faces, and not cars, is that mPFC was unresponsive in the car condition for both age groups. To rule out this possibility, we compared average mPFC response to faces to average mPFC response to cars. There was no difference in mPFC response overall, t(74)=1.74 p=.09, or separately among YA, t(39)=1.76 p=.09, or OA, t(34)=.57 p=.57.
Discussion
There were three key findings in this study. First, OA’ spontaneous mPFC activity toward faces positively related to their directed mentalizing toward faces. Second, OA had lower mPFC activity than YA when spontaneously mentalizing toward faces. This finding replicates work showing OA’ lower mPFC during explicit mentalizing tasks (Moran et al., 2012), and suggests age differences extend to merely perceiving faces. Third, exploratory analyses showed that whereas YA’ mPFC activity distinguished between different racial outgroups, OA’ mPFC activity did not.
OA’ spontaneous mPFC activity toward faces positively related to their RME performance, which measures directed mentalizing through the explicit detection of mental states from faces. The spontaneous mPFC activity characterized here was defined by an explicit mentalizing task, suggesting it reflected mentalizing. This finding extends the literature by showing that OA’ spontaneous and more directed mentalizing toward faces draw on similar mPFC activity. This finding is important because mentalizing-related mPFC activity is positively related to key aspects of social interaction, such as prosociality (Declerck & Bogaert, 2008; Telzer, Masten, Berkman, Lieberman, & Fuligni, 2011). This finding also highlights a nuance of OA’ lower overall mPFC response relative to YA. Not all OA have lower activity related to mentalizing toward faces as may be expected by past work (Moran et al., 2012). Instead, age differences may be larger if OA do not detect mental states from faces. OA who have lower RME performance, and also lower mPFC activity, may be most likely to face negative consequences of reduced mentalizing.
Because faces were shown for one second each, it is possible that the person perception task was burdensome for OA in ways that affected their mPFC response. Indeed, OA had slower processing speed and worse working memory than YA, as is widely found in the literature (e.g., Park et al., 2002). However, the positive relationship between OA’ RME performance and their mPFC response toward faces was significant even after controlling for OA’ processing speed and working memory. This relationship is also unlikely to be due to structural variation associated with cognitive decline, as medial prefrontal regions are less susceptible to age-related cortical thinning than other regions (Salat et al., 2004). Together, these findings support the claim that OA’ reduced mentalizing capacities, and not impaired working memory or processing speed, account for the correlation between their RME performance and mPFC activation during face perception.
OA’, but not YA’, RME performance positively related to their mPFC response toward faces. These data suggest a role of RME performance in OA’ mPFC response toward faces, but do not suggest that this relationship should emerge in all age groups. One possibility is that OA might have more variable mPFC response to faces than YA that better predicts their behavior. Another possibility is that YA’ RME performance might relate to connectivity between regions involved in mentalizing. Indeed, spontaneous mentalizing elicits increased connectivity within YA’ default mode network, which includes mPFC (Gottlich, Ye, Rodriguez-Fornells, Munte, & Kramer, 2017). The default mode network is less connected in OA versus YA, with decreases in connectivity at rest associated with age deficits in theory of mind (Hughes et al., 2019). Less connectivity within OA’ default mode network might strengthen the relationship that specific regions (e.g., mPFC) have with mentalizing-related behavior. Future work should examine this possibility.
YA and OA had similar RME performance.1 The lack of an overall behavioral difference does not mean that these YA and OA were equated in their mentalizing capacities. Indeed, YA had higher mentalizing-related mPFC response toward faces than OA. Further supporting this possibility, the extent of OA’ mental state decoding negatively correlated with their age despite similar performance as YA overall. These data suggest that age deficits in mentalizing might not emerge similarly across tasks. In other words, age deficits in mental state decoding, and thus mPFC response toward faces, might be stronger in some OA than others. Indeed, the same YA also showed enhanced theory of mind versus OA in the mentalizing localizer task (for details, see Hughes et al., 2019). This finding supports the assertion that the YA and OA in the current study were not equated in their mentalizing capacities. Finally, YA had more activation in brain regions that have been previously implicated in mentalizing (e.g., right temporoparietal junction, mPFC; Frith & Frith, 2006) during the mentalizing localizer than did OA (Table 2).
The present work revealed an overall age deficit in mPFC activity related to mentalizing when perceiving faces. This finding adds to the literature (e.g., Moran, 2013; Moran et al., 2012) by suggesting that OA, relative to YA, exhibit broadly lower mPFC activation even when mentalizing is spontaneously engaged. Because an age difference emerged when perceiving faces, but not when perceiving cars, it suggests that OA’ lower mPFC activity related to mentalizing does not reflect effects of task demands (see Henry et al., 2013). Our results suggest that OA’ relatively lower mPFC activity is specific to stimuli that engage mentalizing (e.g., faces), and does not reflect a broader metabolic change that would result in lower mPFC activity for OA across all stimuli types (e.g., Samanez-Larkin, Wagner, & Knutson, 2011).
The overall age deficit in mPFC activity during face perception was observed in the context of perceiving ingroup White and outgroup Black and Asian faces. YA’ mPFC activity toward ingroup and outgroup faces largely reflected past work. Specifically, YA had higher mPFC activation toward White than Asian faces. This pattern is consistent with work showing that YA mentalize more about ingroup than outgroup members (e.g., Adams et al., 2009). YA also had higher mPFC activation toward Black than Asian faces. This pattern reflects work showing that mPFC is not similarly activated toward all outgroup members (Harris & Fiske, 2006, 2007). No difference, however, emerged in mPFC activity toward White and Black faces.
One possibility is that this finding reflects efforts to resolve inconsistency between historically negative group stereotypes and current egalitarian norms through mentalizing, as has been found in prior work (Li et al., 2016). Speculatively, because Asian individuals are often positively stereotyped (S. Lee et al., 2009), perceiving outgroup Asian faces might not engage mPFC to the same extent as outgroup Black faces because the inconsistency between historically negative group stereotypes and egalitarian norms may be less applicable.
By contrast, outgroup race did not modulate OA’ mPFC activity. OA had similarly lower mPFC activity toward White versus Black and toward Black versus Asian faces. These findings are consistent with work showing that OA making fewer distinctions among faces than YA (e.g., Franklin Jr. & Zebrowitz, 2017; Ng, Zebrowitz, & Franklin Jr., 2014). This pattern also suggests that beyond having lower mPFC activity toward faces, OA had more uniform mPFC activation toward faces than YA. Unexpectedly, we found that OA had higher mPFC activity toward Asian versus White faces, which could suggest that OA engage in more spontaneously mentalizing about this group. Although we can only speculate as to why that might be, one possibility is that beyond potential social norm effects on mPFC activity, OA might be less familiar with Asian faces than they are with Black or White faces because Asian faces are underrepresented among what they might encounter each day (for example, in real-world interactions or through media depictions) (e.g., Taylor, Lee, & Stern, 1995). Speculatively, this possibility could make Asian faces less well understood to OA, eliciting more mentalizing toward them. Indeed, people engage mPFC when processing unexpected events (Dungan, Stepanovic, & Young, 2016), with OA especially sensitive to novelty (Richardson, Bucks, & Hogan, 2011). Future research can further explore this possibility.
A hallmark of the aging brain is less specificity, or dedifferentiation, in activation during cognitive tasks (e.g., Goh, Zuzuki, & Park, 2010). This dedifferentiated activation has been posited to reflect OA’ relative difficulty recruiting specialized neural mechanisms, thus resulting in more uniform activation to different stimulus categories (e.g., faces and objects). Less modulation of OA’ mPFC activity by race suggests that the dedifferentiation of face-selective mechanisms in OA might extend from regions involved in basic face processing (Zebrowitz, Ward, Boshyan, Gutchess, & Hadjikhani, 2016) to regions involved in mentalizing toward faces. This finding also raises the possibility that OA’ lower mPFC response toward faces than YA emerged because OA had less differentiation in the mentalizing localizer. OA indeed had lower activation than YA in some regions involved in mentalizing (e.g., Frith & Frith, 2006). Comparing the mentalizing to the control condition in the localizer, however, revealed many activations present in YA were also present in OA (see Table 2). This finding suggests that OA may have differentiated mPFC activation during mentalizing to some extent. OA may simply have less mPFC activation than YA depending on their mentalizing capacities. Specific within-category distinctions, however, might be less apparent in OA’ mPFC activation. It will be important for future work specifically designed to examine both within- and between category distinctions to better understand this finding.
Because only younger faces were used in the person perception task, it allows for the possibility that OA’ lower mPFC activity versus YA might be because all faces, with respect to age, were outgroup members to OA. Indeed, age and race are both primary categorizations that people make when perceiving faces (Messick & Mackie, 1989). Yet, numerous tasks (e.g., Moran et al., 2012) using stimuli where age is irrelevant has shown that OA have lower mPFC activity than do YA. Further, OA’ mPFC activity maintained some sensitivity to race (e.g., Asian faces). Taken together, these findings suggest that the overall age deficit in mPFC activity was not due to the faces being young. It will be important for future work to manipulate both the age and group membership of faces to directly address how other types of group membership affects OA’ mPFC activity.
Because people are not instructed to mentalize as they move through everyday life, characterizing how aging affects spontaneous mPFC activity related to mentalizing is important given the serious consequences of reduced mentalizing for interpersonal (Pettigrew & Tropp, 2008) and physical (Cornwell & Waite, 2009) well-being. Relatedly, showing OA’ lower mPFC activity across outgroup faces is important because lower spontaneous mentalizing may reduce the likelihood of the mentalizing toward outgroup members that reduces prejudicial behavior and improves social interaction (e.g., Galinsky & Todd, 2014). Together, these findings characterize reductions in a key aspect of OA’ social cognition that has the potential to affect their social behavior in a variety of interpersonal contexts.
Acknowledgments
This research was supported in part by NIMH grant T32MH103213 to C.H., and grant numbers KL2TR002530 and UL1TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award to A.C.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
Footnotes
OA’ worse RME performance than YA has been shown to be driven by performance on negative items (Franklin Jr. & Zebrowitz, 2016). To explore this pattern in our data, we categorized RME items as positive (8 items), neutral (16 items), and negative (12 items) based on past work (Harkness, Sabbagh, Jacobson, Chowdrey, & Chen, 2005). We then entered RME accuracy into a 2 (Age: YA, OA)×3 (Valence: positive, neutral, negative) ANOVA. There was a main effect of Valence, F(2, 146)=7.60, p=.001, ηp2=.09, but no main effect of Age, F(1, 73)=.59, p=.59, ηp2<.01, and no interaction, F(2, 146)=1.27, p=.28, ηp2=.02. The main effect of Valence was driven by higher accuracy for positive (M=.79, SD=.17) versus negative (M=.71, SD=.15), F(1, 73)=11.11, p=.001, ηp2=.13, and neutral (M=.76, SD=.12) versus negative, F(1, 73)=11.96, p=.001, ηp2=.14, items. Accuracy did not differ between positive and neutral items, F(1, 73)=1.59, p=.21, ηp2=.02. Note that we present these data with caution because age differences for negative items have emerged with larger sample sizes and when treating valence as a continuous variable.
Because the RME task used White faces, exploratory analyses identified whether mPFC activity positively related to RME performance irrespective of race. OA’ mPFC activity toward White faces, r(33)=.34, p=.04, and toward Black faces, r(33)=.44, p=.008, positively related to RME performance. No significant correlation emerged for Asian faces, r(33)=.02, p=.92. YA’ RME mPFC activity toward White, r(38)=−.09, p=.59, Asian, r(38)=.05, p=.77, or Black, r(38)=−.15, p=.36, faces did not significantly relate to RME performance.
References
- Adams R, Rule N, Franklin R Jr., Wang E, Stevenson M, Yoshikawa S, … Ambady N (2009). Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience, 22(1), 97–108. [DOI] [PubMed] [Google Scholar]
- Amodio D (2014). The neuroscience of prejudice and stereotyping. Nature Reviews Neuroscience, 15(10), 670–682. [DOI] [PubMed] [Google Scholar]
- Amodio D, & Frith C (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–277. [DOI] [PubMed] [Google Scholar]
- Bailey P, Henry J, & von Hippel W (2008). Empathy and social functioning in late adulthood. Aging & Mental Health, 12(4), 499–503. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, & Plumb I (2001). The “reading the mind in the eyes” test revised version: A study with normal adults, and adults with asperger syndrome or high-functioning autism. Journal of Psychology and Psychiatry, 42(241–251). [PubMed] [Google Scholar]
- Cassidy B, & Krendl A (2016). Dynamic neural mechanisms underlie race disparities in social cognition. NeuroImage, 132, 238–246. [DOI] [PubMed] [Google Scholar]
- Cassidy B, Krendl A, Stanko K, Rydell R, Young S, & Hugenberg K (2017). Configural face processing impacts race disparities in humanization and trust. Journal of Experimental Social Psychology, 73, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy B, Lee E, & Krendl A (2016). Age and executive ability impact the neural correlates of race perception. Social Cognitive and Affective Neuroscience, 11(11), 1752–1761. doi: 10.1093/scan/nsw081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy B, Leshikar E, Shih J, Aizenman A, & Gutchess A (2013). Valence-based age differences in medial prefrontal activity during impression formation. Social Neuroscience, 8(5), 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell E, & Waite L (2009). Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior, 50(1), 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W, Johnson M, Raye C, Gatenby J, Gore J, & Banaji M (2004). Separable neural components in the processing of black and white faces. Psychological Science, 15(12), 806–813. [DOI] [PubMed] [Google Scholar]
- Declerck C, & Bogaert S (2008). Social value orientation: Related to empathy and the ability to read the mind in the eyes. The Journal of Social Psychology, 148(711–726). [DOI] [PubMed] [Google Scholar]
- Dungan J, Stepanovic M, & Young L (2016). Theory of mind for processing unexpected events across contexts. Social Cognitive and Affective Neuroscience, 11(8), 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, & McHugh P (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Jounral of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Franklin R Jr., & Zebrowitz L (2016). Aging-related changes in decoding negative complex mental states from faces. Experimental Aging Research, 42(5), 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R Jr., & Zebrowitz L (2017). Age differences in emotion recognition: Task demands or perceptual dedifferentiation? Experimental Aging Research, 43(5), 453–466. [DOI] [PubMed] [Google Scholar]
- Frith C, & Frith U (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534. [DOI] [PubMed] [Google Scholar]
- Galinsky A, & Todd A (2014). Perspective-taking as a strategy for improving intergroup relations: Evidence, mechanisms, and qualifications. Social and Personality Psychology Compass, 8, 374–387. [Google Scholar]
- Gallagher H, & Frith C (2003). Functional imaging of “theory of mind”. Trends in Cognitive Sciences, 7(2), 77–82. [DOI] [PubMed] [Google Scholar]
- Gao W, Cao B, Shan S, Chen X, Zhou D, Zhang X, & Zhao D (2008). The CAS-PEAL large-scale Chinese face database and baseline evaluations. IEEE Transactions on Systems, Man, and Cybernetics Part A: Systems and Humans, 38(1), 149–161. [Google Scholar]
- Goh J, Zuzuki A, & Park D (2010). Reduced neural selectivity increases FMRI adaptation with age during face discrimination. NeuroImage, 51, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalkorale K, Sherman J, & Klauer K (2009). Aging and prejudice: Diminished regulation of automatic race bias among older adults. Journal of Experimental Social Psychology, 45, 410–414. doi: 10.1016/j.jesp.2008.11.004 [DOI] [Google Scholar]
- Gottlich M, Ye Z, Rodriguez-Fornells A, Munte T, & Kramer U (2017). Viewing socio-affective stimuli increases connectivity within an extended default mode network. NeuroImage, 148, 8–19. [DOI] [PubMed] [Google Scholar]
- Harkness K, Sabbagh M, Jacobson J, Chowdrey N, & Chen T (2005). Enhanced accuracy of mental state decoding in dysphoric college students. Cognition & Emotion, 19(7), 999–1025. [Google Scholar]
- Harris L, & Fiske S (2006). Dehumanizing the lowest of the low: neuroimaging responses to extreme outgroups. Psychological Science, 17, 847–853. [DOI] [PubMed] [Google Scholar]
- Harris L, & Fiske S (2007). Social groups that elicit disgust are differentially processed in mPFC. Social Cognitive and Affective Neuroscience, 2(1), 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. Retrieved from http://www.afhayes.com/public/process2012.pdf
- Hedden T, Park D, Nisbett R, Ji L, Jing Q, & Jiao S (2002). Cultural variation in verbal versus spatial neuropsychological function across the lifespan. Neuropsychology, 16, 65–73. [DOI] [PubMed] [Google Scholar]
- Henry J, Phillips L, Ruffman T, & Bailey P (2013). A meta-analytic review of age differences in theory of mind. Psychology and Aging, 28(3), 826–839. [DOI] [PubMed] [Google Scholar]
- Hughes C, Cassidy B, Faskowitz J, Avena-Koenigsberger A, Sporns O, & Krendl A (2019). Changes in neural connections within the default mode network underlie age-related deficits in theory of mind. NeuroImage, 191, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A, Dawson A, & Norr M (2013). Seeing human: distinct and overlapping neural signatures associated with two forms of dehumanization. NeuroImage, 79, 313–328. [DOI] [PubMed] [Google Scholar]
- Kringelbach M, & Rolls E (2003). Neural correlates of rapid reversal learning in a simple model of human social interaction. NeuroImage, 20, 1371–1383. [DOI] [PubMed] [Google Scholar]
- Laillier R, Viard A, Caillaud M, Duclos H, Bejanin A, de La Sayette V, … Laisney M (2019). Neurocognitive determinants of theory of mind across the adult lifespan. Brain and Cognition, 136, 103588. [DOI] [PubMed] [Google Scholar]
- Lee S, Wong N, & Alvarez A (2009). The model minority and the perpetual foreigner: Stereotypes of Asian Americans In Tewari N & Alvarez A (Eds.), Asian American Psychology: Current Perspectives (pp. 69–84). New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Lee T, Ip A, Wang K, Xi C, Hu P, Mak H, … Chan C (2010). Faux pas deficits in people with medial prefrontal lesions as related to impaired understanding of a speaker’s mental state. Neuropsychologia, 48(6), 1670–1676. [DOI] [PubMed] [Google Scholar]
- Leslie A (1987). Pretense and representation: the origins of “theory of mind”. Psychological Review, 94(4), 412–426. [Google Scholar]
- Li T, Cardenas-Iniguez C, Correll J, & Cloutier J (2016). The impact of motivation on race-based impression formation. NeuroImage, 124, 1–7. [DOI] [PubMed] [Google Scholar]
- Ma N, Vandekerckhove M, Van Overwalle F, Seurinck R, & Fias W (2011). Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: Spontaneous inferences activate only its core areas. Social Neuroscience, 6(2), 123–138. [DOI] [PubMed] [Google Scholar]
- Mars R, Neubert F, Noonan M, Sallet J, Toni I, & Rushworth M (2012). On the relationship between the “default mode network” and the “social brain”. Frontiers in Human Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur V, Harada T, & Chiao J (2011). Racial identification modulates default network activity for same and other races. Human Brain Mapping, 33(8), 1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messick D, & Mackie D (1989). Intergroup relations. Annual Review of Psychology, 40, 45–81. [DOI] [PubMed] [Google Scholar]
- Moran J (2013). Lifespan development: the effects of typical aging on theory of mind. Behavioural Brain Research, 237, 32–40. [DOI] [PubMed] [Google Scholar]
- Moran J, Jolly E, & Mitchell J (2012). Social-cognitive deficits in normal aging. The Journal of Neuroscience, 32(16), 5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S, Zebrowitz L, & Franklin R Jr. (2014). Age differences in the differentiation of trait impressions from faces. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald F, McAbee S, Redick T, & Hambrick D (2015). The development of a short domain-general measure of working memory capacity. Behavior Research Methods, 47(4), 1343–1355. [DOI] [PubMed] [Google Scholar]
- Park D, Lautenschlager G, Hedden T, Davidson N, Smith A, & Smith P (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. [PubMed] [Google Scholar]
- Pettigrew T, & Tropp L (2008). How does intergroup contact reduce prejudice? Meta-analytic tests of three mediators. European Journal of Social Psychology, 38(6), 922–934. [Google Scholar]
- Poldrack R (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2(1), 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers K, Chavez R, & Heatherton T (2015). Individual differences in response of dorsomedial prefrontal cortex predict daily social behavior. Social Cognitive and Affective Neuroscience, 11(1), 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C, Bucks R, & Hogan A (2011). Effects of aging on habituation to novelty: an ERP study. International Journal of Psychophysiology, 79(2), 97–105. [DOI] [PubMed] [Google Scholar]
- Salat D, Buckner R, Snyder A, Greve D, Deskikan R, Busa E, … Fischl B (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G, Wagner A, & Knutson B (2011). Expected value information improves financial risk taking across the adult life span. Social Cognitive and Affective Neuroscience, 6(2), 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, & Kanwisher N (2003). People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage, 19(4), 1835–1842. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff S, Rotarska-Jagiela A, Fink G, & Vogeley K (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17, 457–467. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Shih M, Wang E, Bucher A, & Stotzer R (2009). Perspective taking: reducing prejudice toward general outgroups and specific individuals. Group Processes and Intergroup Relations, 12(5), 565–577. [Google Scholar]
- Shipley W (1986). Shipley Institute of Living Scale. In. Los Angeles: Western Psychological Services. [Google Scholar]
- Slessor G, Phillips L, & Bull R (2007). Exploring the specificity of age-related differences in theory of mind tasks. Psychology and Aging, 22(3), 639–643. [DOI] [PubMed] [Google Scholar]
- Spiers H, & Maguire E (2006). Spontaneous mentalizing during an interactive real world task: an fMRI study. Neuropsychologia, 44(10), 1674–1682. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno M, Ishikawa K, Kobayashi A, Okubo M, & Nakai T (2019). Age-related differences in the activation of the mentalizing- and reward-related brain regions durign the learning of others’ true trustworthiness. Neurobiology of Aging, 73, 1–8. [DOI] [PubMed] [Google Scholar]
- Taylor C, Lee J, & Stern B (1995). Portrayals of African, Hispanic, and Asian Americans in magazine advertising. American Behavioral Scientist, 38(4), 608–621. [Google Scholar]
- Telzer E, Masten C, Berkman E, Lieberman M, & Fuligni A (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage, 58(1), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A, Bodenhausen G, Richeson J, & Galinsky A (2011). Perspective taking combats automatic expressions of racial bias. Journal of Personality and Social Psychology, 100(6), 1027–1042. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said C, Engell A, & Oosterhof N (2008). Understanding evaluation of faces on social dimensions. Trends in Cognitive Sciences, 12(12), 455–460. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, & Vandekerckhove M (2013). Implicit and explicit social mentalizing: dual processes driven by a shared neural network. Frontiers in Human Neuroscience, 7, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, & Han S (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience, 29, 8525–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Camprodon J, Hauser M, Pascual-Leone A, & Saxe R (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimululation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107(15), 6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitchik D (1990). When representations conflict with reality: The preschooler’s problem with false beliefs and “false” photographs. Cognition, 35(1), 41–68. [DOI] [PubMed] [Google Scholar]
- Zebrowitz L, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2016). Dedifferentiated face processing in older adults is linked to lower resting state metabolic activity in fusiform face area. Brain Research, 1644, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


