Abstract
Substantial evidence has accumulated that “original antigenic sin” is a central factor shaping immune responses against influenza viruses. Here, we argue that this propensity of initial influenza virus exposure to establish a lifelong immunological imprint presents a remarkable opportunity: Immunization of infants prior to their initial, natural viral exposure could circumvent narrow immunological imprinting directed toward a single viral strain. Simultaneous initial exposure to antigens from multiple influenza strains via vaccination holds the promise of extending immunological imprinting across all currently circulating strains and against potential pandemic strains of influenza A virus, potentially providing a readily accessible form of universal protection against severe disease from both pandemic and seasonal influenza.
In a remarkable essay published in 1960, Tom Francis, Jr. introduced the concept of “original antigenic sin” (OAS); the idea that antibody responses to early childhood influenza virus infections are preferentially recalled later in life upon exposure to antigenically distinct viral strains (Francis 1960). Francis made clear that he thought the real “sin” was that humans are infected with only one influenza virus strain in early childhood, leading to narrower immunological memory than multiple influenza virus strains would deliver. Francis dreamed of vaccines that would educate infant immune systems to respond effectively to diverse influenza virus strains later in life. Accordingly, he concluded his essay with these prescient words:
The gaps in [children's] immunity should be eliminated by providing early in life the antigenic stimuli to meet the known or anticipated recurrent strains. Natural exposures would then serve to enhance the broad immunity laid down by vaccination. It is our hope that such vaccines can be made from pools of chemically purified antigens—or even with strains experimentally devised. In this manner the original sin of infection could be replaced by an initial blessing of induced immunity.
In the intervening decades (Davenport et al. 1964; Webster 1966; Webster et al. 1976), and especially over the last few years (Kim et al. 2009; Lessler et al. 2012; Li et al. 2013; Miller et al. 2013; Fonville et al. 2014; Worobey et al. 2014; Gostic et al. 2016; Linderman and Hensley 2016), evidence has accumulated that OAS is a crucial factor shaping immune responses against influenza viruses both within and between subtypes. The time has come to carefully consider the challenges and opportunities presented by OAS for current and future vaccines and vaccine policy. Vaccines that are capable of granting an “initial blessing” of broad immunity should be an explicit goal of the influenza vaccine enterprise and indeed might already be at our fingertips. Vaccines delivered prior to initial infection offer a route toward universal protection by laying down a broad “immunological imprint” that could form the foundation of a lifetime of protective responses against a range of seasonal and pandemic influenza strains.
INITIAL CHILDHOOD INFLUENZA VIRUS EXPOSURES ESTABLISH LONG-LIVED IMMUNOLOGICAL IMPRINTS
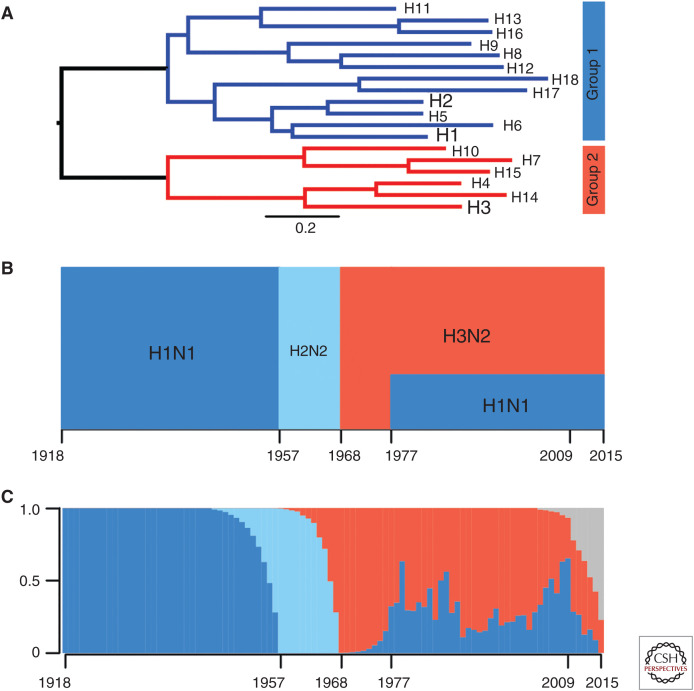
Influenza A viruses can have 18 different hemagglutinins (HAs) and 11 different neuraminidases (NAs) (Krammer et al. 2018). Different HA subtypes are antigenically distinct, but they can be phylogenically split into two general groups that share some conserved epitopes, including epitopes in the HA stalk (Fig. 1A). H1N1, H2N2, and H3N2 viruses have circulated in humans at different times over the past 101 years (Fig. 1B). H1N1 and H2N2 viruses both have “group 1” HAs, while H3N2 viruses have a more distant “group 2” HA.
Figure 1.
Hemagglutinin (HA) phylogenetic groups, circulation history, and imprinting patterns. (A) Phylogenetic tree of the 18 known influenza A virus (IAV) HA subtypes and the two HA groups they fall into. Seasonal human subtypes are depicted in larger font. (B) Circulation history of seasonal IAV subtypes, with group 1 strains in shades of blue and group 2 H3N2 in red. (C) Reconstruction of the percentage of each birth year cohort with initial IAV infection with each subtype. The color scheme follows B with the addition of gray, which represents the percentage of infants who remained naive to both H1N1 and H3N2 as of 2015. (B, C, From Gostic et al. 2016; adapted, with permission, from The American Association for the Advancement of Science © 2016.)
Most humans are infected with influenza viruses by 3 years of age (Bodewes et al. 2011). Because different influenza subtypes circulate each year (Fig. 1B), an individual's birth year largely predicts which type of virus they initially encountered early in life. For example, most older individuals were likely initially exposed to H1N1 in childhood as only H1N1 viruses circulated between 1918 and 1957, whereas individuals born after 1968 were more likely to be initially exposed to H3N2 in childhood (Fig. 1C). It is difficult to infer precise immune histories of individuals born after the mid-1970s, as both H1N1 and H3N2 have cocirculated since the reemergence of H1N1 in 1977.
Evidence suggests that individuals that first encounter “group 1” HAs in childhood have different immunological memory and viral susceptibility compared to individuals that first encounter “group 2” HAs (Worobey et al. 2014; Gostic et al. 2016, 2019; Arevalo et al. 2019). For example, H5N1 and H7N9 susceptibility appears to be highly dependent on an individual's birth year (Gostic et al. 2016). Although the immunological basis of this is incompletely understood, it is thought that initial childhood infection with H1N1 or H2N2 (group 1 viruses) provides lifelong immunity against H5N1 (also a group 1 virus), whereas initial infection with H3N2 (group 2 virus) provides lifelong immunity against H7N9 viruses (also a group 2 virus). Childhood infection with a putative H3N8 virus (group 2), mismatched to the 1918 H1N1 virus (group 1), was also strongly associated with high mortality among young adults during the 1918 pandemic, possibly as a result of OAS-mediated recall of unprotective anti-H3 HA stalk responses (Worobey et al. 2014). Recent studies suggest that early childhood influenza virus infections also influence susceptibility to seasonal H1N1 and H3N2 influenza virus strains later in life (Arevalo et al. 2019; Gostic et al. 2019). Thus, there appears to be something special about early life childhood influenza virus exposures—and the very first exposure in particular—that leads to lifelong immunity against antigenically related influenza virus strains. Unfortunately, most humans first encounter influenza virus antigens in the form of an infection with a single viral subtype and therefore are only immunologically imprinted with a limited number of antigens.
IMMUNOLOGICAL BASIS OF OAS
What is so special about initial influenza virus exposures? Two factors are likely to be critical for antibody responses to influenza viruses. First, the sheer amount of antigen available to naive B cells is likely highest during the first exposure because of relatively unfettered viral replication. Anamnestic responses during all subsequent infections—cross-reactive neutralizing antibodies, antibody-dependent cellular cytotoxicity, cytotoxic T cells—will diminish the total antigen available to naive B cells reactive to the secondary strain's epitopes.
Second, and probably more importantly, naive B cells potentially reactive to new epitopes of a secondary strain must compete on a severely tilted playing field against memory B cells archived from the first infection: Affinity-matured memory B cells have vastly higher avidity for antigen than naive B cells and an overall lower threshold of activation. Although a germinal center, the site of B-cell maturation, may be seeded by approximately 100 B cells, by the time it achieves its maximum size it is often composed of descendants of only one or a few (Mesin et al. 2016). Those “winner” B cells are the ones that achieve the most signaling from antigen and from T follicular helper (TFH) cells. If antigen is limited, memory B cells likely act as antigen hogs, reducing opportunities for naive B cells to acquire antigen and thereby receive survival and proliferation signals. Memory B cells also express higher levels of major histocompatibility complex (MHC) class II molecules and the costimulatory ligand CD80 compared to naive B cells (Bar-Or et al. 2001), making them more efficient than naive B cells at presenting influenza virus peptides to TFH cells and attaining TFH help.
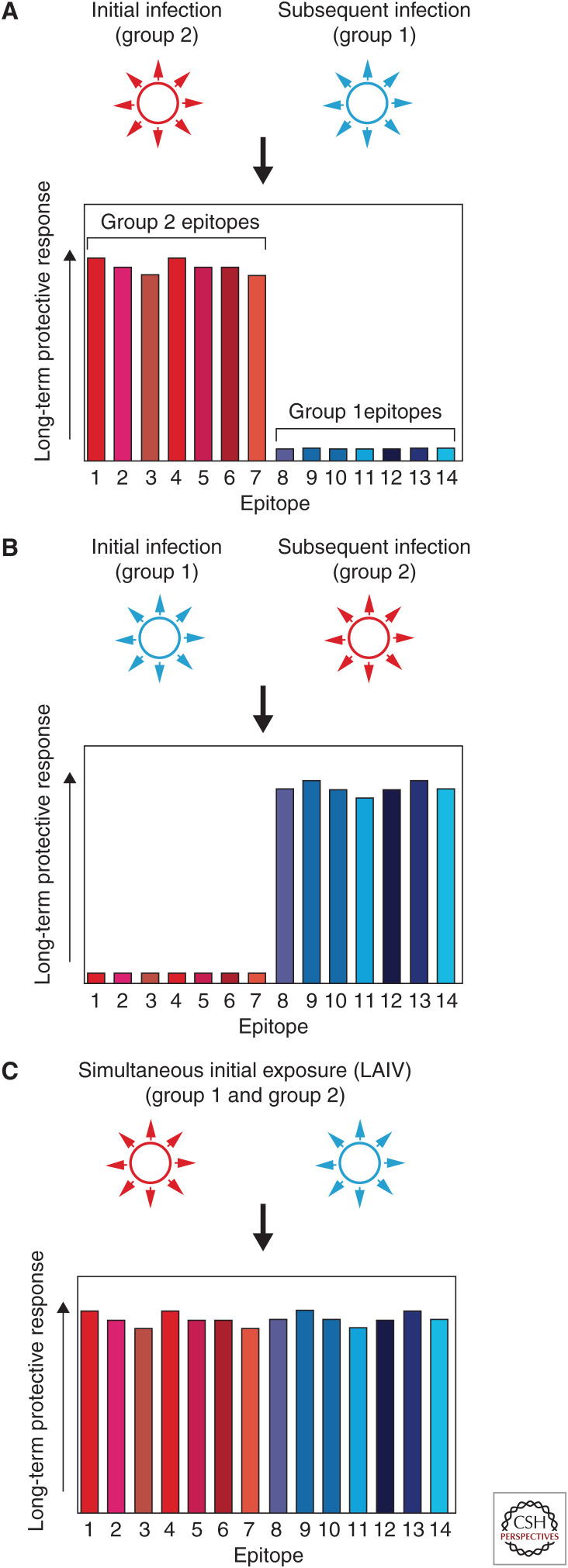
All this will act to disfavor primary responses to epitopes unique to the secondary strain, instead focusing responses on secondary strain epitopes shared with the first strain, whether or not they provide protection against the secondary strain. Later exposure to strains somewhat similar to the second strain is thus met with weaker immune responses than would have occurred had the first exposure never happened, or had the primary and secondary strains been encountered in reverse order (Fig. 2). In this way, OAS differs from “run-of-the-mill” immunological memory.
Figure 2.
Hypothetical protection patterns under different influenza A virus antigen exposure histories. (A) Initial natural infection with a group 2 hemagglutinin (HA) virus skews long-term protective responses toward epitopes present on the HA of that strain (epitopes 1–7, in shades of red). Subsequent infection with a group 1 virus results in weaker responses to that strain's epitopes (8–14, in shades of blue) because of diminished antigen levels and of competition between naive B cells reactive to the group 1 strain's epitopes and memory B cells reactive to the initial strain's epitopes. (B) Initial natural infection with a group 1 virus skews long-term protective responses toward group 1 epitopes. (C) Simultaneous delivery of group 1 and group 2 antigens during initial exposure, via live attenuated influenza vaccine (LAIV), allows for a broad immunological imprint and strong long-term protective responses against epitopes present on the HAs of both group 1 and group 2 viruses.
OAS AND VACCINATING YOUNG CHILDREN
But what if that first exposure to influenza virus antigens is not to a natural infection but to a vaccine, one that delivers multiple strains’ epitopes simultaneously? Since 2004, inactivated influenza vaccines have been recommended for children as young as 6 months of age in the United States (American Academy of Pediatrics Committee on Infectious Diseases 2004). Therefore, it is virtually certain that some children's first exposure now occurs via vaccination with inactivated antigens. Does this fulfill Francis's dream of laying down broadly protective immunity to antigenically distinct viral strains? The short answer is that we do not know. Perhaps these vaccines establish long-lasting immunological memory against group 1 and group 2 influenza A viruses as well as influenza B viruses. But it is unknown whether inactivated antigens provide an “immunological imprint” comparable to that induced by natural infection. Do inactivated vaccines deliver enough antigen and sustained immunological signaling to elicit robust memory B and T cell responses that provide some level of cross protection that limits severe disease, even without necessarily blocking infection? Attaining such protection in individual humans—across both HA groups—is highly desirable and is probably achievable by well-timed, early vaccination.
It is likely that stronger immunological imprints can be delivered by vaccines composed of live viruses. We suspect that live attenuated influenza vaccines (LAIVs), which are already in use and recommended broadly for children (Harper et al. 2003; Haber et al. 2015), are better able to mimic natural influenza virus infections. Similar to conventional inactivated vaccines, currently licensed LAIVs are composed of antigens from H1N1, H3N2, and influenza B viruses. The immunologic advantages of LAIVs include the induction of tissue resident memory B and cytotoxic T cells, the stimulation of local secretory IgA responses (Petukhova et al. 2012), and potential induction of antibodies to the HA stalk antigens that may be the key to long-lived, severity-lowering, broadly protective responses. However, because these vaccines are only recommended above the age of 2 years, it is likely that very few children receive their first influenza exposure through an LAIV.
LAIVs are not recommended for children younger than 2 yr of age because of an excess of wheezing in this age group in studies of the original U.S. LAIV strains (Belshe et al. 2007). However, it might be possible to further attenuate LAIV strains. And, given the new evidence of how profoundly initial exposure shapes the severity of subsequent influenza infections, it is worth reevaluating whether LAIVs could be safely used in younger children now. Importantly, the excess of wheezing reactions was seen mainly in infants younger than 10 months old and the authors concluded that LAIV was safe for children 12 months of age and older (Belshe et al. 2007). Other early studies of LAIV detected only mild reactogenicity events in infants 6 weeks to <24 weeks old (Vesikari et al. 2008). So it might already be possible to use LAIV safely in children who are younger than 2. If the recommended age of LAIV could be lowered from 2 years of age to 1, many more vaccinees could receive their first influenza exposure via LAIV. And focused efforts to develop LAIVs that are demonstrably safe in even younger infants, or to evaluate current LAIVs to see whether they may already be safe for use in infants younger than 12 months of age, might make Francis's aspiration a reality.
THE FUTURE
We believe it is imperative to test whether LAIVs indeed establish broader, more durable immunological imprints compared to inactivated vaccines in young children, and compared to natural infections by single strains. Previous studies suggest that whereas ∼80% of 3-year-olds have already seen their first influenza A infection, about half of 2-year-olds likely remain naive (Bodewes et al. 2011). Hence, even without lowering the age at which LAIVs are currently used, it would be feasible to evaluate relevant immune responses in individuals who receive LAIV prior to natural infection (e.g., memory B-cell repertoires, responses to subsequent vaccination, severity of subsequent natural infections). If there are imprinting-related benefits of LAIV use in naive infants, it will then be important to weigh these effects against the possible risks associated with an increased chance of wheezing if these vaccines were to be used in younger age groups. Longitudinal birth cohort studies, like those that are currently being initiated by the National Institute of Allergies and Infectious Diseases (NIAID) (Butler 2019), should help tease out whether LAIV and inactivated vaccines elicit fundamentally different types of immunological imprints compared to natural infections.
A fortunate byproduct of the current cocirculation of H3N2 and H1N1 is that current seasonal flu vaccines already contain antigens from both a group 2 (H3N2) and a group 1 (H1N1) influenza A virus. Hence, while fulfilling the intended goal of protecting against seasonal influenza, these vaccines, if delivered prior to natural infection, hold out the possibility of inducing broadly protective immunity across both influenza A virus HA groups, which could be recalled later in life, potentially protecting against severe outcomes from any influenza A virus subtype, seasonal or pandemic (Fig. 2). In other words, currently licensed vaccines against seasonal influenza may exhibit properties akin to aspirational universal influenza vaccines when used in the very young.
Although our central idea is that any vaccine that simultaneously delivers at least one group 1 HA and one group 2 HA prior to natural infection is likely to provide protection against any potential influenza A subtype, in the future we should also consider adding antigens from other viral strains to childhood vaccines. For example, besides H1N1 and H3N2, the only other influenza A virus strain with sustained circulation among humans over the past 100 years is H2N2 (Fig. 1). Although H1N1 early-life priming likely provides some protection against H2N2, it would be reasonable to include H2 antigens in next-generation vaccines that are designed to elicit broad immunological imprints in children. It may be worth adding other HAs as well, especially from subtypes like H5N1 and H7N9 that represent pandemic risks today.
CONCLUDING REMARKS
The landscape of human influenza could be dramatically altered by vaccines that deliver strong immunological imprints against distinct influenza strains in childhood. Given the apparent robust but HA group–restricted lifelong protection against severe disease that the first natural infection can deliver, intentionally broadening that immunity across both influenza A virus HA groups via early use of multivalent vaccines is an exciting prospect. It should be emphasized that such a priming vaccination would not obviate the need to immunize each year with vaccines that match the circulating strains, but that severe influenza disease and devastating pandemics caused by new strains might thus be prevented; increasing herd immunity to both HA groups over time is expected to act as an obstacle to a new strain achieving efficient transmission in humans or causing severe disease if it does.
Although our focus here is on LAIVs, inasmuch as inactivated influenza vaccines are used in infants as young as 6 months, consideration should be given to use of inactivated antigens or adjuvanted inactivated antigens as primers, on the condition that they are produced in cell culture or recombinant technologies to avoid adaptive HA mutations (Vesikari et al. 2018). Whether adjuvanted vaccines or novel immunization platforms like messenger RNA (mRNA) vaccination (Pardi et al. 2018) could successfully overwrite OAS from prior natural childhood infection is an important question, as almost every adult now living on Earth has already been infected with an influenza virus and therefore has a skewed immunological imprint. But it is important to recognize that early vaccination of children not yet exposed to influenza virus may be the one clear shot we have to establish broad immunity across both HA groups (Fig. 2). It thus offers an appealing solution to the challenge of providing universal protection against severe disease while simultaneously delivering standard-of-care protection against current seasonal influenza strains. Intensive preinfection vaccination could be a game-changer for combating influenza, providing readily accessible, broadly effective protection.
ACKNOWLEDGMENTS
M.W. was supported by a grant from the David and Lucile Packard Foundation. S.E.H. was supported by National Institutes of Health (NIH) 1R01AI113047, NIH 1R01AI108686, and HHSN272201400005C. S.E.H. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. All authors conceived and wrote the paper. S.P. has been a paid consultant to Sanofi Pasteur, GlaxoSmithKline, Merck, Pfizer, and Astra Zeneca for work unrelated to this report. S.E.H. has received consultancy fees from Lumen, Novavax, and Merck for work unrelated to this report.
This article has been made freely available online courtesy of TAUNS Laboratories.
Footnotes
Editors: Gabriele Neumann and Yoshihiro Kawaoka
Additional Perspectives on Influenza: The Cutting Edge available at www.perspectivesinmedicine.org
REFERENCES
- American Academy of Pediatrics Committee on Infectious Diseases. 2004. Recommendations for influenza immunization of children. Pediatrics 113: 1441–1447. 10.1542/peds.113.5.1441 [DOI] [PubMed] [Google Scholar]
- Arevalo P, McLean HQ, Belongia EA, Cobey S. 2019. Earliest infections predict the age distribution of seasonal influenza A cases. medRxiv 10.1101/19001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, Oliveira EM, Anderson DE, Krieger JI, Duddy M, O'Connor KC, Hafler DA. 2001. Immunological memory: contribution of memory B cells expressing costimulatory molecules in the resting state. J Immunol 167: 5669–5677. 10.4049/jimmunol.167.10.5669 [DOI] [PubMed] [Google Scholar]
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM, CAIV-T Comparative Efficacy Study Group. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. New Engl J Med 356: 685–696. 10.1056/NEJMoa065368 [DOI] [PubMed] [Google Scholar]
- Bodewes R, de Mutsert G, van der Klis FR, Ventresca M, Wilks S, Smith DJ, Koopmans M, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2011. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol 18: 469–476. 10.1128/CVI.00396-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. 2019. Long-term studies will track indelible marks of first flu. Nature 10.1038/d41586-019-01524-9 [DOI] [PubMed] [Google Scholar]
- Davenport FM, Hennessy AV, Drescher J, Mulder J, Francis T Jr. 1964. Further observations on the relevance of serologic recapitulations of human infection with influenza viruses. J Exp Med 120: 1087–1097. 10.1084/jem.120.6.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NMH, Pham QT, et al. 2014. Antibody landscapes after influenza virus infection or vaccination. Science 346: 996–1000. 10.1126/science.1256427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T. 1960. On the doctrine of original antigenic sin. Proc Am Philos Soc 104: 572. [Google Scholar]
- Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. 2016. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354: 722–726. 10.1126/science.aag1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostic KM, Bridge R, Brady S, Viboud C, Worobey M, Lloyd-Smith JO. 2019. Childhood immune imprinting to influenza A shapes birth year–specific risk during seasonal H1N1 and H3N2 epidemics. PLOS Pathogens (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber P, Moro PL, Cano M, Vellozzi C, Lewis P, Woo EJ, Broder K. 2015. Post-licensure surveillance of trivalent live-attenuated influenza vaccine in children aged 2–18 years, vaccine adverse event reporting system, United States, July 2005–June 2012. J Pediatric Infect Dis Soc 4: 205–213. 10.1093/jpids/piu034 [DOI] [PubMed] [Google Scholar]
- Harper SA, Fukuda K, Cox NJ, Bridges CB, Advisory Committee on Immunization Practices. 2003. Using live, attenuated influenza vaccine for prevention and control of influenza: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 52: 1–8. [PubMed] [Google Scholar]
- Kim JH, Skountzou I, Compans R, Jacob J. 2009. Original antigenic sin responses to influenza viruses. J Immunol 183: 3294–3301. 10.4049/jimmunol.0900398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, et al. 2018. Influenza. Nat Rev Dis Primers 4: 3 10.1038/s41572-018-0002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJ, Guan Y, Jiang CQ, Cummings DA. 2012. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog 8: e1002802 10.1371/journal.ppat.1002802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Myers JL, Bostick DL, Sullivan CB, Madara J, Linderman SL, Liu Q, Carter DM, Wrammert J, Esposito S, et al. 2013. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J Exp Med 210: 1493–1500. 10.1084/jem.20130212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderman SL, Hensley SE. 2016. Antibodies with “original antigenic sin” properties are valuable components of secondary immune responses to influenza viruses. PLoS Pathog 12: e1005806 10.1371/journal.ppat.1005806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Ersching J, Victora GD. 2016. Germinal center B cell dynamics. Immunity 45: 471–482. 10.1016/j.immuni.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. 2013. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 5: 198ra107 10.1126/scitranslmed.3006637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Karikó K, Barbosa CJ, Madden TD, et al. 2018. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun 9: 3361 10.1038/s41467-018-05482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova G, Korenkov D, Chirkova T, Donina S, Rudenko L, Naykhin A. 2012. B- and T-cell memory elicited by a seasonal live attenuated reassortant influenza vaccine: assessment of local antibody avidity and virus-specific memory T-cells using trogocytosis-based method. Influenza Other Respir Viruses 6: 119–126. 10.1111/j.1750-2659.2011.00279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T, Karvonen A, Smith HM, Dunning A, Razmpour A, Saville MK, Gruber WC, Forrest BD. 2008. Safety and tolerability of cold-adapted influenza vaccine, trivalent, in infants younger than 6 months of age. Pediatrics 121: e568–e573. 10.1542/peds.2007-1405 [DOI] [PubMed] [Google Scholar]
- Vesikari T, Kirstein J, Devota Go G, Leav B, Ruzycky ME, Isakov L, de Bruijn M, Oberye J, Heijnen E. 2018. Efficacy, immunogenicity, and safety evaluation of an MF59-adjuvanted quadrivalent influenza virus vaccine compared with non-adjuvanted influenza vaccine in children: a multicentre, randomised controlled, observer-blinded, phase 3 trial. Lancet Respir Med 6: 345–356. 10.1016/S2213-2600(18)30108-5 [DOI] [PubMed] [Google Scholar]
- Webster RG. 1966. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J Immunol 97: 177–183. [PubMed] [Google Scholar]
- Webster RG, Kasel JA, Couch RB, Laver WG. 1976. Influenza virus subunit vaccines. II: immunogenicity and original antigenic sin in humans. J Infect Dis 134: 48–58. [DOI] [PubMed] [Google Scholar]
- Worobey M, Han GZ, Rambaut A. 2014. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc Natl Acad Sci 111: 8107–8112. 10.1073/pnas.1324197111 [DOI] [PMC free article] [PubMed] [Google Scholar]