Abstract
Aging significantly changes the ability to respond to vaccinations and infections. In this review, we summarize published results on age-relared changes in the response to infection with the influenza virus and on the factors known to increase influenza risk infection leading to organ failure and death. We also summarize how aging affects the response to the influenza vaccine with a special focus on B cells, which have been shown to be less responsive in the elderly. We show the cellular and molecular mechanisms contributing to the dysfunctional immune response of the elderly to the vaccine against influenza. These include a defective interaction of helper T cells (CD4+) with B cells in Germinal Centers, changes in the microenvironment, and the generation of immune cells with a senescence-associated phenotype. Finally, we discuss the effects of aging on metabolic pathways and we show how metabolic complications associated with aging lead to immune dysfunction.
Keywords: Aging, B cells, T cells, vaccine responses, immunometabolism
1|. The impact of aging on humoral immunity to vaccination
1.1. Aging and influenza infection
Aging induces a progressive reduction of immune function (immunosenescence). Humoral immune responses are impaired by aging, and elderly individuals become more prone to viral and bacterial infections1,2. Hospitalization following infection with the influenza virus is higher in the elderly than in younger individuals and this represents a significant contributor to the reduction in function and development of disability1.
The Centers for Disease Control and Prevention (CDC) performs evaluations of influenza infections and associated hospitalizations through the Influenza Hospitalization Surveillance Network (FluSurv-NET), which covers approximately 27 million people, representing the 9% of the U.S. population (https://www.cdc.gov/mmwr/volumes/68/wr/mm6824a3.htm). Data from the 2018–2019 season have shown that, similar to previous seasons, hospitalization rates were higher among individuals >65 years of age and older, who accounted for a large percentage (almost 50%) of reported hospitalizations due to influenza infection. Among all influenza-associated hospitalizations, 95% were associated with influenza A virus, 4% with influenza B virus and only few with influenza A and B virus coinfection. Among those with influenza A virus, almost 50% were with the A/H3N2. The A/H3N2 virus, since its introduction in 1968, has undergone genetic and antigenic changes leading to several seasonal epidemics3. A/H3N2 is the most common subtype affecting elderly individuals4, leading to the highest hospitalization rates and death5.
Influenza infection is controlled by an initial antibody response needed to allow the development of T cell-mediated immune responses. With regards to T cells, CD8+ T cells are more effective than CD4+ T cells in terms of ability to clear virus6–9. Infection induces local pulmonary inflammation and then influenza-specific CD8+ T cell immune responses, which are needed for the clearance of influenza virus10–13. Viral clearance occurs through killing of infected pulmonary cells via mechanisms mediated by perforin13, Fas13, and/or TRAIL14.
Not only age, but also other factors increase the risk of influenza infection leading to organ failure and death. These include chronic lung and cardiac disease, metabolic disease, immunosuppression, obesity, and neuromuscular disorders15. Frailty is a measure of decrease in health and physical function, and increase in vulnerability. Frailty is a significant predictor of health outcomes and is another risk factor for influenza infection16. Frailty has been shown to be associated with an overall increased mortality risk and decline in functional status among older adults. Importantly, influenza infection and associated complications have been correlated with frailty in hospitalized elderly individuals7,17,18.
1.2. Aging and influenza vaccination
Although antiviral drugs are effective in protecting from influenza infection, vaccination is still the best way to prevent infection with the influenza virus19. Due to the increase in the aging world population, prevention of infection in the elderly represents an important public health concern. The influenza vaccine induces initially specific antiviral B and T cells that result in protective humoral and cellular immune responses, respectively20. The antibody response to the vaccine represents the initial line of protection from subsequent infection. However, age-related decreases in humoral immune responses have been reported and these are responsible for reduced responses of the elderly to vaccination1,2,21–26. Not only the production but also the time of duration of protective humoral and cellular immune responses following vaccination decrease with age27. Older adults (≥65 years of age) account for more than 90% of seasonal influenza-related deaths and hospitalizations. For this reason, yearly influenza vaccination is strongly recommended for these vulnerable individuals to protect them from infection and associated complications. Vaccination has been shown to significantly reduce disease burden and transmission of the infection to individuals living within the community.
In general, young individuals have more robust antibody responses as compared to elderly individuals when vaccinated for the first time, but after subsequent vaccinations the difference between the two age groups is reduced, suggesting the importance of previous vaccination and/or infection28. Repeated vaccinations with the same vaccine results in a significant increase in specific antibodies and serum titers in both young and elderly individuals29. However, influenza vaccine-specific antibodies do not reliably persist year-round in older adults, suggesting that alternative vaccination protocols providing better clinical benefits are needed30. Elderly individuals who have been vaccinated, however, can still become infected with severe additional complications that lead to hospitalization, catastrophic disability, deterioration of underlying medical conditions and death1,22,25. But there is evidence that those immunized have a less severe illness.
Several causes have been described as factors determining the limited success of influenza vaccination among elderly adults. In addition to age, history of previous vaccinations, individual’s genetic background, and chronic underlying conditions may compromise the capacity of older adults to generate protective responses after influenza vaccination31,32.
Seasonal influenza vaccines aim to produce high affinity neutralizing antibodies that protect from infection. Antibody responses are the gold standard to measure influenza vaccine-specific protective responses. This is significantly decreased in the elderly, in part due to decreased generation of specific protective antibodies4,20,33–36, switched memory B cells34,35,37–39 and long-lived plasma cells40,41. The majority of high affinity neutralizing antibodies are specific for the head domain of the strain-specific viral hemagglutinin (HA) molecule but some may also be directed toward the more conserved HA stalk region42–44. These high affinity antibodies are generated following many rounds of somatic hypermutation (SHM) of the immunoglobulin (Ig) variable regions genes in Germinal Centers (GCs)45. The GC is a microstructure that develops in secondary lymphoid tissue during an immune response and is responsible for the generation of plasma cells that secrete specific antibodies as well as memory B cells. The generation of a robust GC response is critical for the production of high affinity antibodies, since this is where SHM and affinity maturation of Ig genes occurs46. Age-associated defects in the ability to generate high affinity antibodies in response to influenza vaccination have been reported in both mice47–49 and humans50,51, and has been correlated with age-related dysfunctional innate and adaptive immune cells.
The reduced response of the elderly to influenza vaccination has been associated with the age-decrease in T cell function27,52,53, decreases in naïve T cells and parallel increases in memory/effector T cells54, loss of CD28 expression55, and increased cytomegalovirus (CMV) seropositivity56. In most studies, CMV-positive individuals that are ≥60 years of age, as compared to age-matched CMV-negative individuals, have lower influenza vaccine-specific antibody responses57–60, and this is associated with increased frequencies of senescent CD4+ T cells with a terminally-differentiated phenotype (CD45RA+CCR7-CD27-CD28-). These cells produce significant amounts of IL-107, a cytokine known to suppress the activation of cytotoxic T lymphocytes and to down-regulate the expression of costimulatory molecules on antigen-presenting cells61. High IL-10, together with reduced IFN-γ production, also reduces the generation of memory T cells in response to influenza vaccination7,62.
A recent study has shown that human effector memory CD8+ T cells, known to increase in frequency in the blood of elderly individuals, include two different subsets characterized by low and high expression of the IL-7Rα receptor63. The two subsets show different expression of effector molecules, transcription factors, and DNA methylation profiles, with the IL-7Rαlow subset expressing high levels of perforin, granzyme B, IFN-γ, TNF-α and the senescence-associated marker CD57, and being highly cytotoxic and pro-inflammatory. This subset was also characterized by increased expression of the chemokine receptors CX3CR1 and CXCR164,65. It was found that elderly individuals who are responders to the influenza vaccine have increased frequencies of the IL-7Rαlow subset as compared to elderly non-responders. The authors speculate that the increased expression of chemokine receptors may have induced a faster migration of these cells to the sites of vaccine injection where they may have been responsible for higher secretion of pro-inflammatory cytokines and better immune responses through the release of cytotoxic mediators63.
B cells also undergo profound age-related changes. These include a redistribution of B cell subsets in the peripheral blood with significant increased frequencies and numbers of pro-inflammatory B cells; decreases in the expression of molecules involved in Ig class switch recombination (CSR) and SHM, two processes leading to the generation of high affinity protective antibodies, as well as GC formation; and decreases in repertoire diversity. In vitro experiments have characterized age-defects in B cells and shown that mitogen-stimulated human B cells from elderly individuals have decreased expression of activation-induced cytidine deaminase (AID), the enzyme that regulates CSR and SHM, and secrete less IgG as compared to those from young individuals. Influenza vaccine-specific antibody responses are reduced in the elderly, mainly due to intrinsic defects in B cells but also to defects in antigen presentation and T cell help. AID is a B cell biomarker of antibody responses which is not only associated with, but also predicts the quality of the antibody response to the influenza vaccine, as it correlates with serum antibodies and with the generation of high affinity antibodies specific for the novel pandemic (p)H1N1 influenza vaccine34,51,66,67.
As opposed to serum antibodies, memory B cells generated in response to the influenza vaccine are maintained in aged humans68, suggesting an intrinsic defect of memory B cells to differentiate into plasma cells. The generation of vaccine-specific antibody responses is negatively associated with inflammation, measured by serum TNF-α69. Human B cells isolated from the peripheral blood of elderly people express higher levels of TNF-α mRNA than those from young individuals. B cell intrinsic TNF-α positively correlates with serum TNF-α and both negatively correlate with B cell function, measured by AID. Only memory B cell subsets express TNF-α mRNA and more in elderly than in young individuals70.
Age-related decreases in immune responses of dendritic cells (DCs) and monocytes include a reduced recruitment and function, such as reduced TLR-induced cytokine production, phagocytosis, granule release and microbial activity. Myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) from elderly individuals secrete significantly less pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12 (p40) in response to the stimulation with TLR agonists and these defects have been correlated with low responses to the influenza vaccine71. Classical monocytes (CD14+CD16-) are induced after influenza vaccination in both young and elderly individuals72, but those from older individuals secrete less TNF-α and IL-6, and more IL-10, after in vivo stimulation with the vaccine73. This cytokine secretion profile is associated with the reduced production of influenza vaccine-specific antibodies in elderly individuals72.
2 |. The impact of the aged / senescent environment on the humoral response to vaccination
While age impacts the B cell response to the influenza vaccine in both human and mouse models, most mechanistic insights have come from the examination of what occurs in mice and it is well documented that the B cell response to vaccination in aged mice is different and less good than that in young mice. The major causes of the age-associated decrease in vaccine responses are summarized in Fig. 1.
Figure 1. B cell intrinsic and extrinsic factors responsible for reduced vaccine-specific humoral immunity with age.
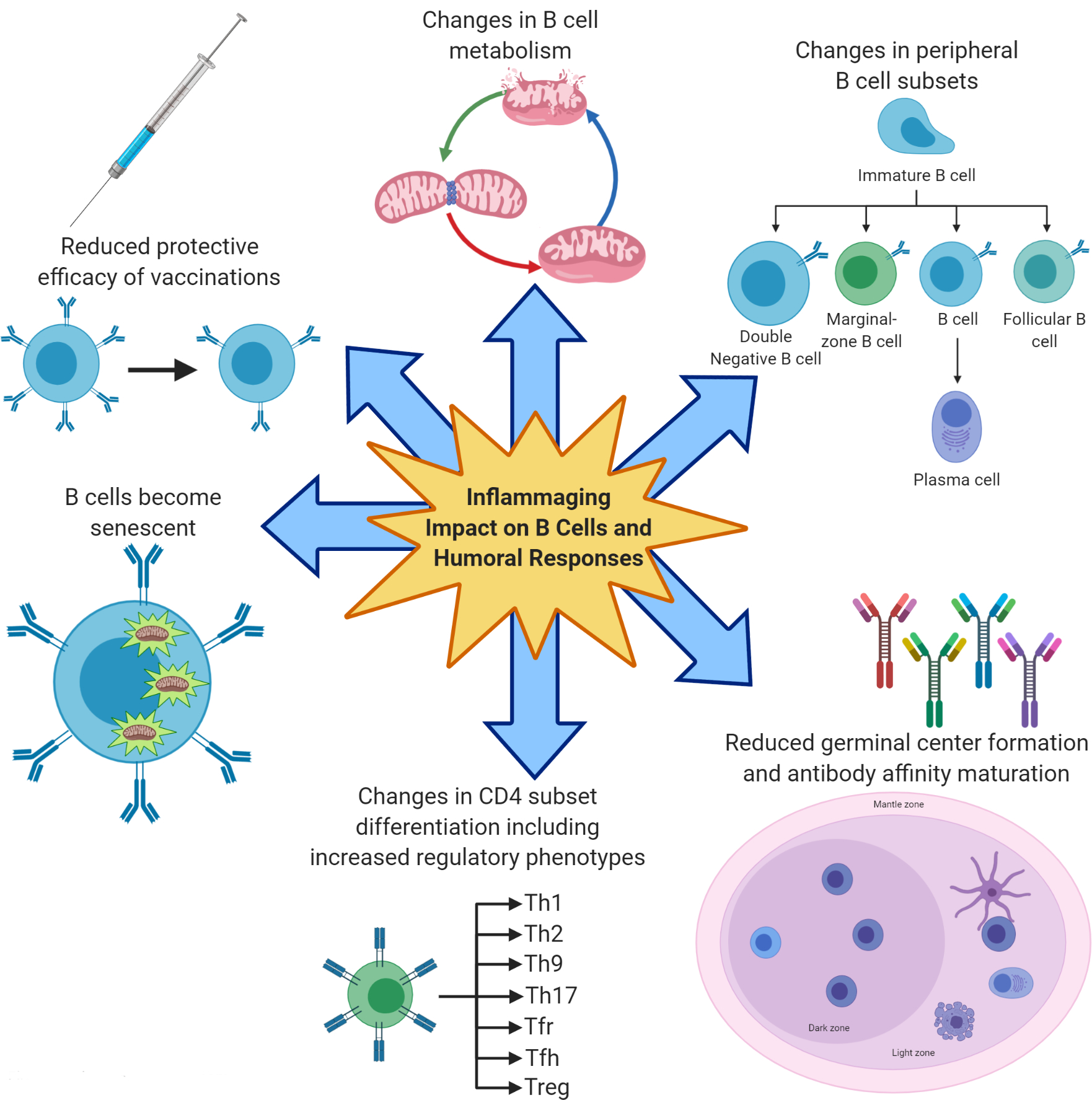
Reduced humoral responses of aged mice and humans to vaccination are associated with increased inflammaging, the chronic low grade inflammatory status of old age. Inflammaging induces B cell senescence and redistribution of the circulating B cell subsets with an increase in the pro-inflammatory B cell subset called Double Negative, and also impairs the differentiation of naïve CD4+ T cells into effector Tfh cells in the GC leading to changes in the relative proportions of CD4+ subsets with an increase in regulatory subsets. Changes in B cell metabolism with aging also occur and these lead to immune dysfunction.
The secretion of high affinity antibodies depends on the efficient interaction of antigen-specific B cells and CD4 T helper (Th) cells within the GC. One of the critical components necessary for a robust GC response is the proper differentiation of CD4 T cells. Naïve CD4 T cells can differentiate into various helper subsets such as Th1, Th2, Th9, Th17, T follicular helper (Tfh), and regulatory T cells (Treg) following antigenic stimulation by DCs in the presence of environmental cytokines74. Each of these subsets has a distinct role in an immune response, which has been tailored for the situation prompting the response: for example, IL-4 induces the generation of Th2 cells which are effective at clearing parasites such as helminth worms, while IL-12 and IFN-γ induce the generation of Th1 cells which are effective at clearing intracellular pathogens such as viruses. Important for this discussion, Tfh cells are vital for a robust GC response and high affinity antibody production from the GC cannot proceed in the absence of Tfh75.
2.1. Impact of the aged environment
With aging, there are reduced levels of the chemokine CXCL13, a B cell chemoattractant chemokine 13 in the B cell follicles76,77 and since this chemokine is important for the proper trafficking of CD4 Tfh cells to the B cell follicle during an immune response78, the CD4 T cell help available to B cells declines with age. In addition, the aged environment also impairs naïve CD4 T cell differentiation into Tfh cells by an unknown mechanism, so that there are fewer T cells available to help a B cell response76. Important for this discussion are T follicular regulatory cells (Tfr), which can be generated de novo from Foxp3+ natural regulatory T cells (Treg)79. These Tfr can work to limit B cell help, B cell responses and germinal center formation, thus reducing the robustness of an ongoing humoral response79,80. The critical factor during a GC response which determines the magnitude of the antibody response is the ratio of Tfh:Tfr81,82. Importantly, more Tfr cells accumulate in old versus young mice83, ultimately resulting in fewer GC B cells and reduced high affinity antibody production following vaccination of aged mice. It is also noteworthy that not only is the Tfh:Tfr ratio impacted by aging, other CD4 T cell subsets that are infrequent in young mice, such as activated regulatory T cells, exhausted and cytotoxic subsets, co-emerge in old mice84.
Adoptive transfer experiments performed in mice have shown that the aged environment plays a crucial role in many of these age-related changes in humoral immunity. When young donor CD4 T cells are transferred into old immunized hosts, the young donor T cells do not differentiate to a Tfh phenotype as readily as they do in young hosts76. In addition, TGF-β in the aged environment promotes the expression of the transcription factor forkhead box P3 (FOXP3) in the responding Tfh cells, thus driving them to differentiate to Tfr, which further limits the humoral response85. The end result of fewer Tfh cells and more Tfr cells is that there are fewer and smaller germinal centers in the aged hosts receiving young T cells when compared to young hosts. This reduced germinal center formation can then result in less affinity maturation of antibodies and induction of an overall less protective humoral response.
2.2. How senescence can impact the humoral response
What is different about the aging environment that can drive these age-related differences in CD4 T cell differentiation and negatively impact the humoral response to vaccination? One of the main differences between young and aged individuals lies in the presence of the senescent cells in the aged tissue microenvironment. Aging increases the number of cells characterized by a senescent phenotype86 and this can have a dramatic impact both systemically and in local tissues. A senescent cell is defined by cell cycle arrest and the inability to proliferate upon stimulation with a mitogenic challenge, which can occur in response to various cellular stresses. A variety of different stressors have been shown to induce senescence. These include telomeric and non telomeric DNA damage, such as telomere shortening after several cell divisions (replicative senescence), oxidative stress, mitochondrial deterioration, and oncogene expression87. While there is not a single identifying biomarker for senescence, some of the most common ones include expression of senescence-associated beta-galactosidase, elevated expression of p16INK4A, hypophosphorylated retinoblastoma protein, telomere damage, senescence associated heterochromatic foci, and several soluble factors that constitute the senescence associated secretory phenotype (SASP)88. The SASP is especially important in vivo since it is composed of soluble factors that can become systemic including cytokines, chemokines, bradykines, prostenoids, micro RNAs (miRNA), and damage associated molecular pattern proteins (DAMPs)89–91. One of the key factors in senescence is that while a senescent cell does undergo cell cycle arrest, it can still be transcriptionally active and secrete numerous inflammatory molecules of the SASP. When the SASP factors are released by senescent cells, they can impact cells within the local tissue and/or systemically, as well as cells of the immune system. This point is particularly important since cytokines and chemokines are two of the main components of the SASP and have the potential to impact the function of innate and adaptive immune cells. The cytokines that are often components of the SASP include IL-6, IL-8, IL-1α, TGF-β and GM-CSF92. TGF-β is of special importance in this list since it is one of the main inducers of CD4 T cell differentiation, inducing a Treg phenotype characterized by FOXP3 expression93. These induced Tregs (iTregs), much like thymus generated Tregs, can act to constrain an adaptive immune response and limit antibody production94 and Treg populations have been shown to be increased with both murine95–98 and human99,100 aging. IL-6 is another SASP component that plays an important role in CD4 T cell differentiation. It is involved in generation of both Tfh and Th17 subsets101,102 and, in fact, Th17 cells and IL-17 production by CD4 T cells is elevated with aging103–105. Thus, CD4 T cells that undergo differentiation within a senescent environment are likely to produce T helper subsets that are distinct from those generated in a young non-senescent environment and this has the potential to impact the adaptive immune response in older individuals.
Also, the chemokines CXCL1 (GROα), CXCL2 (GROβ), CCL2 (MCP-1), CCL5 (RANTES), CCL20 (MIP-3α) and CCL26 (eotaxin-3) can be notable components of the SASP92. These chemokines can act to enhance inflammation, trafficking and recruitment of cells of the innate and/or adaptive immune systems, thus contributing to age-related inflammation both locally and systemically. This is especially problematic when senescent cells develop in organs like the lungs and recruit responding T cells to an inflammatory environment where their differentiation program may not be similar to what occurs in a young, non-inflammatory environment.
2.3. B cell senescence
In addition to the senescent environment impacting the generation of robust humoral responses, B cells themselves can begin to exhibit senescent traits in older individuals. In mice, percentages and numbers of splenic B cells are maintained with age but there is a shift in the proportions of the different B cell subsets, characterized by increasing numbers of age-associated B Cells (ABCs), at the expense of the Follicular (FO) B cell subset, so that the total number of splenic B cells does not change significantly106–108. In humans, conversely, B cell percentages and numbers are significantly and progressively decreased with age109–113 and there is also a shift in the proportions of the different B cell subsets with a decrease in the percentage of switched memory B cells, no change in IgM memory and a significant increase in the percentage of naïve and the subset called Double Negative (DN) B cells70,110. DN B cells have been shown to be similar to mouse ABCs, generated from conventional mature B cell subsets (naïve in humans, FO in mice) after in vivo or in vitro stimulation with TLRs. These cells have previously been called late/exhausted memory or tissuelike memory B cells, and they represent the most pro-inflammatory B cell subset, which has been reported to be also increased in the blood of patients with autoimmune114–116 and infectious diseases117–119. These observations have suggested that DN B cells may accumulate in vivo in inflammatory conditions and in the presence of chronic stimulation with self antigens or viral/parasitic antigens, and may secrete autoimmune or protective antibodies, respectively. This occurs in autoimmune diseases in which B cells are chronically exposed to self antigens and in infectious diseases in which B cells are continuously exposed to and stimulated by viral or parasitic antigens, as it occurs in HIV or malaria, respectively. In both examples, DN B cells represent a terminally differentiated B cell subset that has undergone class switch in response to chronic stimulation and cannot be restimulated in vivo and/or in vitro.
DN B cells are transcriptionally and metabolically active and secrete several pro-inflammatory factors such as cytokines (TNF-α, IL-6), chemokines (IL-8) and micro-RNAs (microRNA-16, 155, 93), which are all components of the SASP70. DN B cells are therefore able to sustain and propagate systemic inflammation70. DN B cells show no proliferation and antibody secretion in response to “new” antigens (influenza vaccine), even in individuals previously vaccinated, but they secrete autoimmune antibodies in both mice120 and humans121. They do so because they express the membrane phenotype CD95+CD21-CD11c+ and they also spontaneously express the transcription factor T-bet, both associated with autoimmunity110,121. The frequency of this B cell population in the blood is negatively correlated with induction of a protective response following influenza vaccination. As opposed to human DN, mouse ABCs have specificity for a live influenza virus (A/PR8/34) and this occurs only in mice infected and not in naïve mice. Similar to human DN B cells, mouse ABCs do not have specificity for the influenza vaccine. The influenza-specific ABC response is non-follicular and helper T cell-independent, but requires the presence of the virus. Influenza-specific ABCs differentiate into specific antibody-secreting cells, some of which home to bone marrow and lungs, and persist for long periods of time after infection (>4 weeks), suggesting their role in providing significant protection122.
3 |. changes in B cell metabolism WITH AGE
3.1. Introduction to immunometabolism
Metabolism and immunity have been considered for a long time to be two independent systems, with the metabolism regulating transformation and assimilation of nutrients and the immune system regulating innate and adaptive immune responses against pathogens and vaccines. Ongoing research however shows that these two systems work together controlling the individual’s response to stress. Metabolic studies represent a rapidly emerging and evolving field of research. Lymphocyte metabolism is now recognized as a crucial regulator of cellular homeostasis and function123.
Effector cell function is intrinsically linked to cellular metabolism and it has been shown, at least for T cells124,125 and macrophages126,127, that several metabolic enzymes and their regulators can also have a direct effect on the regulation of cell function. Cell activation is critically supported by metabolic shifts that produce energy for cell activation and differentiation, suggesting the exciting possibility that cell function in various conditions could be regulated by an intervention targeting cell’s metabolism.
The major pathways utilized to generate energy are: 1) glycolysis, in which glucose is incompletely oxidized in the cytosol (anaerobic glycolysis), producing lactate as the final product. It is fast but energy inefficient; 2) oxidative phosphorylation (OXPHOS) in which carbon substrates such as glucose-derived pyruvate, Fatty Acids (FAs) and glutamine are oxidized in the mitochondria to generate ATP; 3) the pentose phosphate pathway, know to produce NADPH upon a shunt of glycolysis, a crucial pathway for the maintenance of cell redox balance and nucleotides. In general, glycolysis is used by cells involved in robust proliferation and secretion because it provides the biosynthetic precursors needed for nucleotide, amino acid, lipid synthesis [reviewed in128]. Intermediates of the glycolytic pathway provide carbon that provides energy for several biosynthetic pathways. Therefore, glycolysis provides all the components needed for proliferation and the synthesis of effector molecules. A better understanding of fuel utilization by immune cells from both mice and humans has gained significant support by extracellular flux analysis, which measures oxygen consumption rates (OCR) and extra-cellular acidification rates (ECAR), as indicators of OXPHOS or anaerobic glycolysis, respectively.
Studies conducted on T cells have shown that resting T cells are quiescent and require almost exclusively the production of adenosine triphosphate (ATP) for basal cell functions129. After stimulation, T cells enter the cell cycle, rapidly divide and require both ATP and biosynthetic precursors to support proliferation129–131. Memory T cells no longer need to proliferate and therefore decrease their glycolytic metabolism132. The transition of T cells from oxidative to glycolytic and vice versa has been shown to regulate not only cell survival but also the expansion of antigen-specific T cell clones and the competitive selection of high-affinity clones133,134.
It is not known if changes in the metabolic requirements of lymphocytes during immune responses are uniform, or if different stimuli induce different metabolic pathways to drive specific cell functions. In a pro-inflammatory microenvironment, activated CD4+ T cells differentiate into Th1 and Th17 cells, whereas in an anti-inflammatory microenvironment T regulatory (TREG) cells prevail135,136. Th1 and Th17 CD4+ T cells express high Glut1 levels and activate glycolysis, whereas TREG express lower levels of Glut1 and rely on OXPHOS and lipid oxidation. Metabolic analysis of CD4+ and CD8+ human T cells has shown that CD4+ T cells have larger mitochondria than CD8+ T cells and almost exclusively engage in OXPHOS to support their effector function. Conversely, CD8+ T cells are more glycolytic, leading to higher proliferation and faster growth137. Moreover, human CD8+ EMRA (Effector Memory reacquiring the RA marker) T cells, the subset with pro-inflammatory characteristics that increase with age, and have dysfunctional mitochondrial function but CD4+EMRA T cells have more functional mitochondria able to generate the energy requirements for function138.
Macrophage M1 and M2 subsets also show different patterns, with inflammatory M1 macrophages being mainly glycolytic, whereas anti-inflammatory M2 macrophages use lipid oxidation139.
3.2. Metabolic requirements of B cell responses
As opposed to T cells, B cell metabolic requirements for effective cell function have not been thoroughly investigated. It is not well known how metabolic requirements of B cells change after stimulation. Also the metabolic requirements of protective versus autoimmune antibody responses are not well known.
Similar to other immune cells, unstimulated B cells use glucose and fatty acids as sources of energy. B cells stimulated through the B cell receptor (BCR) in both mice and humans upregulate the expression of the glucose transporter Glut1 and primarily activate glycolysis and to a lesser extent OXPHOS to support their demands of energy for antibody production140,141. Published studies have shown that up-regulated expression of Glut1 depends on the activation of mechanisms dependent on the cell cycle regulator c-Myc and phosphatidylinositol-3-OH kinase (PI3K)141,142. Glycolytic inhibition or deletion of Glut1 significantly inhibits B cell proliferation and antibody secretion both in vivo and in vitro, suggesting that the importance of glucose transporters in the metabolic reprogramming of cells undergoing proliferation and antibody production.
In the absence of a second co-stimulatory signal, B cells are unable to perform glycolysis and OXPHOS and die, as a consequence of mitochondrial dysfunction resulting from accumulation of intracellular calcium through calcium response-activated calcium channels. The presence of T cells providing a second signal, or the simulation with TLR agonists, prevents cell death, suggesting that BCR signaling activates a metabolic program that controls survival or cell death, depending on the presence or absence of a second signal, respectively140. Using RNA-based next-generation sequencing (RNA-seq) to measure changes in the expression of genes encoding enzymes associated with cellular metabolism, it was found that the key glycolytic enzymes hexokinase 2 (HK2) and lactate dehydrogenase (LDHA) were up-regulated, consistent with the increase in glycolysis, whereas genes encoding subunits of the enzyme pyruvate dehydrogenase (PDHX), which catalyzes pyruvate to acetyl CoA, were less up-regulated, confirming that in response to BCR stimulation B cells primarily activate glycolysis and to a lesser extent OXPHOS140.
Metabolic reprogramming is suppressed in anergic B cells. However, B cells chronically exposed to high levels of BAFF show enhanced and more rapid metabolic reprogramming in response to TLR4 stimulation, with glycolysis being increased rapidly and OXPHOS also increased, but at a slower rate141. These results suggest that failure to induce tolerance redirects B cells to a program that is essential for the secretion of (autoimmune) antibodies.
3.3. Age-associated metabolic changes
Aging is associated with several metabolic changes such as enhanced insulin resistance143,144, reduced mitochondrial function145,146 and dysregulated nutrient uptake147. Each of the nine hallmarks of aging (genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication) is associated with metabolic dysfunction and it has been shown that both hyper-nutrition and sedentary lifestyle can accelerate aging and can have catastrophic metabolic consequences148. Similarly, seven pillars of aging were put forward by the Geroscience Interest Group (GSIG) and also include metabolism and inflammation (metabolism, macromolecular damage, epigenetics, inflammation, adaptation to stress, proteostasis, stem cells and regeneration). A study that has systemically integrated in vivo phenotyping with gene expression, biochemical analysis, and metabolomics was performed in young and old mice. Results have allowed the identification of a metabolic footprint of aging, based on altered metabolites in the plasma of young and old mice, that can be used as biomarkers of aging and healthspan149. Many of these plasma metabolites have been positively associated with inflammaging150–153, the chronic inflammatory status of the elderly154, and negatively associated with immune function155–157. Although several studies have investigated how plasma metabolites fuel inflammaging and vice versa, very few studies have investigated mouse and human metabolic changes in immune cells.
3.3.1. Age-dependent changes in mouse B cell metabolism
One of the few published studies on age-related changes in B cell metabolic pathways has shown that aging induces defects in glucose-induced energy production, leading to decreased OXPHOS. In this study, antibody-secreting cells (ASCs) from the bone marrow of young and old mice were analyzed and whole genome expression arrays were performed158. Results showed different expression of 1500 genes involved in both immune and metabolic regulation of cell function. The age-dependent reduction in OXPHOS was associated with decreased expression of both PDHX and LDHA genes, indicating that ASCs from old mice preferentially activate glycolysis rather than OXPHOS for energy production. The activation of glycolysis, moreover, was associated with increased levels of reactive oxygen species (ROS), suggesting higher levels of oxidative stress in ASCs from old as compared to those from young mice. These results provide a metabolic-associated mechanism to support the age-associated increase of ROS with aging, responsible for higher chromosomal instability and DNA mutations, as previously shown159. ROS are produced in the cell and in multiple cellular organelles [mitochondria, peroxisomes, endoplasmic reticulum (ER)] in response to stress. Genes regulating mitochondrial respiratory chain proteins known to affect mitochondrial morphology160 were found higher in ASCs from old versus young mice. Genes regulating peroxisome assembly and size161, peroxisome metabolism162 and peroxisome receptors for acyl-CoA esters163 were also found up-regulated in ASCs from old versus young mice.
B cells infiltrate the obese adipose tissue (AT), recruited by chemokines secreted by the adipocytes and by the immune cells that have infiltrated the AT, for which they express the corresponding receptors106,164. A recent publication in mice has shown that aging induces the expansion of AT resident B cells that are highly inflammatory, and their expansion is dependent on the activation of the NLRP3 inflammasome, likely due to AT-associated metabolic and mitochondria dysfunction and increased production of mitochondrial ROS165,166. Inhibition of Nlrp3 activation by blocking IL-1 signaling, or intra-AT removal of B cells with anti-CD20 antibodies, inhibits the NLRP3-dependent B cell accumulation and rescues the metabolic dysfunction of the aging AT164. These results demonstrate that the NLRP3 inflammasome, a major regulator of inflammaging and age-associated metabolic disorders, may be effectively targeted to reduce AT inflammation and associated complications.
3.3.2. Age-dependent changes in human B cell metabolism
Studies in humans have also shown different expression of metabolic markers in peripheral B cells from young and elderly individuals167. In particular, it was found that blood-derived ASCs from elderly individuals had higher mitochondrial mass and mitochondrial ROS and lower Sirtuin1 (SIRT1), an anti-inflammatory marker involved in DNA damage responses and cell metabolism168. SIRT1 levels were higher in ASCs from both young and elderly individuals that responded better to vaccination producing higher amounts of H1N1- and H3N2-specific IgG antibodies. SIRT1 is an anti-aging and anti-inflammatory molecule known to protect from viral infections, including influenza virus infection. When naïve B cells were isolated from the peripheral blood of young and elderly individuals and analyzed by Seahorse to measure OXPHOS by OCR and glycolysis by ECAR, unstimulated B cells from young individuals were found to be higher in both meaures as compared to B cells from elderly individuals. After culture with polyclonal stimuli, both OXPHOS and glycolysis increased as compared to unstimulated B cells and significant defects in OXPHOS, and mild defects in glycolysis, were observed in B cells from elderly versus young individuals. Transcriptome analyses showed additional defects in one-carbon metabolism, a pathway that makes one-carbon moiety (methyl group) available for nucleotide synthesis and methylation169.
A mitochondrial signature of young and old influenza vaccine responders has identified genes and proteins controlling mitochondrial biogenesis and OXPHOS170. Briefly, OXPHOS pathways and crucial genes involved in cellular respiration, mitochondrial DNA transcription and regulation, and heme biosynthesis were found up-regulated in vaccine responders, the majority of which were young. Although this study was performed on PBMCs and therefore it is not known which cell type is responsible for the activation of OXPHOS pathways, it represents the first genome-wide transcriptional analysis of age-associated metabolic measures performed before and after influenza vaccination, showing the crucial role of mitochondrial pathways in human vaccine responses.
We recently compared frequencies and metabolic requirements of DN B cells in the blood of healthy individuals of different ages and in the blood and in the AT of individuals with obesity. Our published results121 confirmed that DN B cell frequencies significantly increase in the blood of elderly versus young individuals, as we have previously reported70,110. Our initial observation on obese individuals was also confirmed as we showed that the frequencies of DN B cells were increased in the blood of obese versus lean young individuals, suggesting that obesity, similar to aging, induces higher frequencies of these pro-inflammatory B cells. In the immune B cell fraction of the AT, DN frequencies are the highest and we observed that some individuals had percentages as high as 50% of the total B cell pool. As to their metabolic requirements, we showed that DN B cells from young individuals show only basal activation levels of OXPHOS, aerobic glycolysis and fatty acid oxidation, whereas DN B cells from elderly and obese individuals show higher activation levels of OXPHOS and glycolysis to support their function. DN B cells from the AT have the highest levels of activation of metabolic pathways as they enroll in OXPHOS, glycolysis and fatty acid oxidation. When we measured the spontaneous secretion of antibodies specific for autoantigens that increase with age, such as dsDNA and MDA (malondealdehyde) in DN and naïve B cells, we found that DN B cells make dsDNA- and MDA-specific autoantibodies whereas naïve do not121. MDA is a marker of oxidative stress and product of lipid peroxidation171, both of which increase with age. Anti-MDA antibodies are also present in the serum of patients with lupus systemic erythematosus172. We also found that DN B cells, but not naïve B cells, make autoantibodies specific for AT-derived antigens, in agreement with the observation that fat mass increases with age in humans173. The secretion of these autoantibodies occurs without any exogenous stimulation. Therefore, the increased glucose consumption and the activation of OXPHOS, glycolysis and fatty acid oxidation pathways that we observed in AT DN cells is needed to support their function, i.e. the production of autoimmune antibodies.
DN B cells from the AT are also highly oxidative, and express the highest levels of intracellular ROS as compared to DN B cells from the blood from young and elderly lean individuals and from obese individuals. ROS is a marker of oxidative stress which is sensed by cellular anti-stress proteins such as AMPK (5’-AMP activated kinase) and Sestrin 1, able to reduce both stress and cell death. DN B cells show the highest levels of activated AMPK (phospho-AMPK) and Sestrin 1. AMPK is an enzyme able to sense nutrient changes and is a key metabolic regulator expressed in all mammalian cells174. The metabolic advantage of DN B cells in the AT, as compared to DN B cells from the blood drives their increased survival in the hostile pro-inflammatory milieu of the AT. Our results altogether highlight the impact of stress sensing pathways on DN B cell survival and also highlight possible mechanisms leading to the secretion of autoimmune antibodies. Further understanding of the regulation of these metabolic pathways is needed to provide new directions to control the secretion of autoimmune antibodies during aging, obesity and other inflammatory conditions.
CONCLUSIONS
The response to infection and vaccination is dramatically influenced by aging. Since older adults are often targeted for vaccination, this is an important clinical issue. B cells and humoral immunity are crucial components of the immune response and the generation of protective antibodies is the basis for most vaccinations, including influenza vaccines. Aging significantly changes B cell responses including alterations in B cell subpopulations, metabolism and differentiation following vaccination. In addition, B cell extrinsic factors, including the aging senescent environment, also have a negative impact on the generation of a robust humoral response. All of these factors must be considered in order to develop improved strategies for production of novel vaccines for older adults.
ACKNOWLEDGEMENTS
Study supported by NIH AG059719 (DF), AG023717 (BBB), AG021600 and AG060389 (LH).
Footnotes
CONFLICT OF INTEREST:
None
REFERENCES
- 1.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277(9):728–734. [PubMed] [Google Scholar]
- 2.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JD, Ross TM. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum Vaccin Immunother. 2018;14(8):1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhaney JE, Beran J, Devaster JM, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13(6):485–496. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. [DOI] [PubMed] [Google Scholar]
- 6.McElhaney JE, Ewen C, Zhou X, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27(18):2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30(12):2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309(1):13–17. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29(11):2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179(1):64–70. [DOI] [PubMed] [Google Scholar]
- 11.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160(3):814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 2004;17(2):197–209. [DOI] [PubMed] [Google Scholar]
- 13.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–5200. [PubMed] [Google Scholar]
- 14.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181(7):4918–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. [DOI] [PubMed] [Google Scholar]
- 16.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew MK, Shinde V, Ye L, et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J Infect Dis. 2017;216(4):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29(31):5015–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finco O, Rappuoli R. Designing vaccines for the twenty-first century society. Front Immunol. 2014;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murasko DM, Bernstein ED, Gardner EM, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–439. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. [DOI] [PubMed] [Google Scholar]
- 22.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518–527. [DOI] [PubMed] [Google Scholar]
- 23.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7(10):658–666. [DOI] [PubMed] [Google Scholar]
- 26.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25(16):3066–3069. [DOI] [PubMed] [Google Scholar]
- 27.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. [DOI] [PubMed] [Google Scholar]
- 28.Mosterin Hopping A, McElhaney J, Fonville JM, Powers DC, Beyer WEP, Smith DJ. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine. 2016;34(4):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, Wilson PC. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol. 2015;89(6):3308–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, MC IC. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine. 2017;35(2):212–221. [DOI] [PubMed] [Google Scholar]
- 31.Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccin Immunother. 2018;14(3):637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhakal S, Klein SL. Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. J Virol. 2019;93(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. [DOI] [PubMed] [Google Scholar]
- 34.Frasca D, Diaz A, Romero M, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28(51):8077–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frasca D, Diaz A, Romero M, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012;24(3):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Essen GA, Beran J, Devaster JM, et al. Influenza symptoms and their impact on elderly adults: randomised trial of AS03-adjuvanted or non-adjuvanted inactivated trivalent seasonal influenza vaccines. Influenza Other Respir Viruses. 2014;8(4):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. [DOI] [PubMed] [Google Scholar]
- 38.Frasca D, Diaz A, Romero M, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. 2013;31(35):3603–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3(8):e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritz T, Lair J, Ban M, et al. Plasma cell numbers decrease in bone marrow of old patients. European journal of immunology. 2015;45(3):738–746. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. The Journal of clinical investigation. 2011;121(8):3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews SF, Huang Y, Kaur K, et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med. 2015;7(316):316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corti D, Suguitan AL Jr., Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120(5):1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014;2(5):381–392. [DOI] [PubMed] [Google Scholar]
- 46.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. [DOI] [PubMed] [Google Scholar]
- 47.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182(10):6129–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller C, Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J Immunol. 1995;155(7):3377–3384. [PubMed] [Google Scholar]
- 49.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183(3):959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry C, Zheng NY, Huang M, et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe. 2019;25(3):357–366 e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 2012;8(9):e1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. Journal of virology. 2001;75(24):12182–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert reviews in molecular medicine. 2007;9(3):1–17. [DOI] [PubMed] [Google Scholar]
- 54.Pawelec G, Barnett Y, Forsey R, et al. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–1183. [DOI] [PubMed] [Google Scholar]
- 55.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. [DOI] [PubMed] [Google Scholar]
- 56.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Reviews in medical virology. 2009;19(1):47–56. [DOI] [PubMed] [Google Scholar]
- 57.Derhovanessian E, Maier AB, Hahnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol. 2014;193(7):3624–3631. [DOI] [PubMed] [Google Scholar]
- 58.Derhovanessian E, Theeten H, Hahnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31(4):685–690. [DOI] [PubMed] [Google Scholar]
- 59.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine. 2015;33(12):1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trzonkowski P, Mysliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21(25–26):3826–3836. [DOI] [PubMed] [Google Scholar]
- 61.van Duin D, Allore HG, Mohanty S, et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195(11):1590–1597. [DOI] [PubMed] [Google Scholar]
- 62.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21(4):440–445. [DOI] [PubMed] [Google Scholar]
- 63.Park HJ, Shin MS, Kim M, et al. Transcriptomic analysis of human IL-7 receptor alpha (low) and (high) effector memory CD8(+) T cells reveals an age-associated signature linked to influenza vaccine response in older adults. Aging Cell. 2019;18(4):e12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7Ralpha expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107(7):2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim HW, Kim CH. Loss of IL-7 receptor alpha on CD4+ T cells defines terminally differentiated B cell-helping effector T cells in a B cell-rich lymphoid tissue. J Immunol. 2007;179(11):7448–7456. [DOI] [PubMed] [Google Scholar]
- 66.Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Blomberg BB. Effects of age on H1N1-specific serum IgG1 and IgG3 levels evaluated during the 2011–2012 influenza vaccine season. Immun Ageing. 2013;10(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frasca D, Diaz A, Romero M, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frasca D, Diaz A, Romero M, Blomberg BB. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine. 2016;34(25):2834–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. High TNF-alpha levels in resting B cells negatively correlate with their response. Exp Gerontol. 2014;54:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frasca D, Diaz A, Romero M, Blomberg BB. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol. 2017;87(Pt A):113–120. [DOI] [PubMed] [Google Scholar]
- 71.Panda A, Qian F, Mohanty S, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. Journal of immunology. 2010;184(5):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohanty S, Joshi SR, Ueda I, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;211(7):1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metcalf TU, Wilkinson PA, Cameron MJ, et al. Human Monocyte Subsets Are Transcriptionally and Functionally Altered in Aging in Response to Pattern Recognition Receptor Agonists. J Immunol. 2017;199(4):1405–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang S, Dong C. A complex issue on CD4(+) T-cell subsets. Immunol Rev. 2013;252(1):5–11. [DOI] [PubMed] [Google Scholar]
- 75.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell. 2012;11(5):732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wols HA, Johnson KM, Ippolito JA, et al. Migration of immature and mature B cells in the aged microenvironment. Immunology. 2010;129(2):278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crotty S Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 79.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124(12):5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH Cell Function and Increased TFR Cells Contribute to Defective Antibody Production in Aging. Cell Rep. 2015;12(2):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elyahu Y, Hekselman I, Eizenberg-Magar I, et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci Adv. 2019;5(8):eaaw8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016;6:25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. [DOI] [PubMed] [Google Scholar]
- 89.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281(40):29568–29574. [DOI] [PubMed] [Google Scholar]
- 90.Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Naturae. 2018;10(1):4–14. [PMC free article] [PubMed] [Google Scholar]
- 93.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179(7):4685–4693. [DOI] [PubMed] [Google Scholar]
- 95.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128(11–12):618–627. [DOI] [PubMed] [Google Scholar]
- 96.Lages CS, Suffia I, Velilla PA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24(4):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177(12):8348–8355. [DOI] [PubMed] [Google Scholar]
- 99.Gottenberg JE, Lavie F, Abbed K, et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren’s syndrome. J Autoimmun. 2005;24(3):235–242. [DOI] [PubMed] [Google Scholar]
- 100.Gregg R, Smith CM, Clark FJ, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong C TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337–348. [DOI] [PubMed] [Google Scholar]
- 102.Suto A, Kashiwakuma D, Kagami S, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205(6):1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JS, Lee WW, Kim SH, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim MA, Lee J, Park JS, et al. Increased Th17 differentiation in aged mice is significantly associated with high IL-1beta level and low IL-2 expression. Exp Gerontol. 2014;49:55–62. [DOI] [PubMed] [Google Scholar]
- 105.Ouyang X, Yang Z, Zhang R, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev. 2017;162:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging Cell. 2013;12(2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology. 2010;11(2):125–137. [DOI] [PubMed] [Google Scholar]
- 110.Frasca D, Diaz A, Romero M, D’Eramo F, Blomberg BB. Aging effects on T-bet expression in human B cell subsets. Cell Immunol. 2017;321:68–73. [DOI] [PubMed] [Google Scholar]
- 111.Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring). 2016;24(3):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frasca D, Landin AM, Lechner SC, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180(8):5283–5290. [DOI] [PubMed] [Google Scholar]
- 113.Wikby A, Nilsson BO, Forsey R, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127(8):695–704. [DOI] [PubMed] [Google Scholar]
- 114.Adlowitz DG, Barnard J, Biear JN, et al. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PLoS One. 2015;10(6):e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Claes N, Fraussen J, Vanheusden M, et al. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol. 2016;197(12):4576–4583. [DOI] [PubMed] [Google Scholar]
- 116.Wehr C, Eibel H, Masilamani M, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113(2):161–171. [DOI] [PubMed] [Google Scholar]
- 117.Chang LY, Li Y, Kaplan DE. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Illingworth J, Butler NS, Roetynck S, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190(3):1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PLoS One. 2019;14(7):e0219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swain SL, Kugler-Umana O, Kuang Y, Zhang W. The properties of the unique age-associated B cell subset reveal a shift in strategy of immune response with age. Cell Immunol. 2017;321:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rathmell JC. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol Rev. 2012;249(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28(5):514–524. [DOI] [PubMed] [Google Scholar]
- 125.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diskin C, Palsson-McDermott EM. Metabolic Modulation in Macrophage Effector Function. Front Immunol. 2018;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stunault MI, Bories G, Guinamard RR, Ivanov S. Metabolism Plays a Key Role during Macrophage Activation. Mediators Inflamm. 2018;2018:2426138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68(2 Pt C):513–519. [DOI] [PubMed] [Google Scholar]
- 129.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84(4):949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27(2):173–178. [DOI] [PubMed] [Google Scholar]
- 131.Krauss S, Brand MD, Buttgereit F. Signaling takes a breath--new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15(4):497–502. [DOI] [PubMed] [Google Scholar]
- 132.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coloff JL, Mason EF, Altman BJ, et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J Biol Chem. 2011;286(7):5921–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wensveen FM, van Gisbergen KP, Derks IA, et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity. 2010;32(6):754–765. [DOI] [PubMed] [Google Scholar]
- 135.Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao Y, Rathmell JC, Macintyre AN. Metabolic reprogramming towards aerobic glycolysis correlates with greater proliferative ability and resistance to metabolic inhibition in CD8 versus CD4 T cells. PLoS One. 2014;9(8):e104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Callender LA, Carroll EC, Bober EA, Akbar AN, Solito E, Henson SM. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell. 2019:e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akkaya M, Traba J, Roesler AS, et al. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol. 2018;19(8):871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caro-Maldonado A, Wang R, Nichols AG, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192(8):3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Doughty CA, Bleiman BF, Wagner DJ, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107(11):4458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71(6):1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30(5):327–346. [DOI] [PubMed] [Google Scholar]
- 145.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61(5):654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Woudstra T, Thomson AB. Nutrient absorption and intestinal adaptation with ageing. Best Pract Res Clin Gastroenterol. 2002;16(1):1–15. [DOI] [PubMed] [Google Scholar]
- 148.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Houtkooper RH, Argmann C, Houten SM, et al. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Einstein FH, Huffman DM, Fishman S, et al. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci. 2010;65(8):800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nicholas DA, Zhang K, Hung C, et al. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1beta. PLoS One. 2017;12(5):e0176793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perreault M, Roke K, Badawi A, et al. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids. 2014;49(3):255–263. [DOI] [PubMed] [Google Scholar]
- 153.Stelzner K, Herbert D, Popkova Y, et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur J Immunol. 2016;46(8):2043–2053. [DOI] [PubMed] [Google Scholar]
- 154.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 155.Michelet X, Dyck L, Hogan A, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19(12):1330–1340. [DOI] [PubMed] [Google Scholar]
- 156.Sanchez HN, Moroney JB, Gan H, et al. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zurier RB, Rossetti RG, Seiler CM, Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1999;60(5–6):371–375. [DOI] [PubMed] [Google Scholar]
- 158.Kannan S, Dawany N, Kurupati R, Showe LC, Ertl HC. Age-related changes in the transcriptome of antibody-secreting cells. Oncotarget. 2016;7(12):13340–13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kirkwood TB. DNA, mutations and aging. Mutat Res. 1989;219(1):1–7. [DOI] [PubMed] [Google Scholar]
- 160.Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117(Pt 7):1201–1210. [DOI] [PubMed] [Google Scholar]
- 161.Murakami K, Ichinohe Y, Koike M, et al. VCP Is an integral component of a novel feedback mechanism that controls intracellular localization of catalase and H2O2 Levels. PLoS One. 2013;8(2):e56012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Giordano Attianese GM, Desvergne B. Integrative and systemic approaches for evaluating PPARbeta/delta (PPARD) function. Nucl Recept Signal. 2015;13:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nazarko TY. Atg37 regulates the assembly of the pexophagic receptor protein complex. Autophagy. 2014;10(7):1348–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Camell CD, Gunther P, Lee A, et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019;30(6):1024–1039 e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao AW, Canto C, Houtkooper RH. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol Med. 2014;6(5):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. [DOI] [PubMed] [Google Scholar]
- 167.Kurupati RK, Haut LH, Schmader KE, Ertl HC. Age-related changes in B cell metabolism. Aging (Albany NY). 2019;11(13):4367–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu Q, Dong L, Li Y, Liu G. SIRT1 and HIF1alpha signaling in metabolism and immune responses. Cancer Lett. 2018;418:20–26. [DOI] [PubMed] [Google Scholar]
- 169.Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thakar J, Mohanty S, West AP, et al. Aging-dependent alterations in gene expression and a mitochondrial signature of responsiveness to human influenza vaccination. Aging (Albany NY). 2015;7(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. The Journal of biological chemistry. 2003;278(33):31426–31433. [DOI] [PubMed] [Google Scholar]
- 172.Hardt U, Larsson A, Gunnarsson I, et al. Autoimmune reactivity to malondialdehyde adducts in systemic lupus erythematosus is associated with disease activity and nephritis. Arthritis Res Ther. 2018;20(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zamboni M, Rossi AP, Fantin F, et al. Adipose tissue, diet and aging. Mech Ageing Dev. 2014;136–137:129–137. [DOI] [PubMed] [Google Scholar]
- 174.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3(4):340–351. [DOI] [PubMed] [Google Scholar]


