Abstract
Understanding how the integrity of the nuclear membranes are protected against internal and external stresses is an emergent challenge. Work reviewed here investigated the mechanisms by which losses of nuclear-cytoplasmic compartmentalization are sensed and ameliorated. Fundamental to these is spatial control over interactions between the ESCRT machinery and LEM family inner nuclear membrane proteins, which together promote nuclear membrane sealing in interphase and at the end of mitosis. We suggest that the size of the nuclear envelope hole dictates the mechanism of its repair, with larger holes requiring BAF and the potential triggering of a post-mitotic nuclear envelope reassembly pathway in interphase. We also consider why these mechanisms fail at ruptured micronuclei. Together, this work re-emphasizes the need to understand how membrane flow and local lipid metabolism help ensure that the nuclear envelope is refractory to mechanical rupture yet fluid enough to allow its essential dynamics.
Over the past few decades, there has been considerable interest in determining the mechanisms that define membrane-bound organellar compartmentalization. These efforts have led to a remarkable understanding of the protein and lipid transport mechanisms that establish the biochemical identity, morphology, and function of specific organelles. What was perhaps underappreciated was the extent to which both extra-and intracellular stresses can disrupt the integrity of these organelles and/or their biochemical transport mechanisms, and the downstream consequences of these malfunctions. In this way, the nucleus has served as a model, as there are now many described pathological and physiological events that lead to the breakdown of nuclear-cytoplasmic compartmentalization [1–5]. What is most exciting is that cells also have the ability to monitor the integrity of the nucleus (and other organelles [6–9]), trigger downstream repair and, in some cases, clearance pathways [10]. Here, we will explore emerging themes across recent literature describing the mechanisms responsible for the continued maintenance of nuclear-cytoplasmic compartmentalization in the face of cell intrinsic and extrinsic insults.
Nuclear-cytoplasmic compartmentalization is established by the physical segregation of the genome by the two nuclear envelope membranes (the inner and outer nuclear membranes; INM and ONM) and the selective-permeability of nuclear pore complexes (NPCs) [11,12]. A key challenge for this membrane system is to maintain a permeability barrier in the context of dynamic membrane remodeling events that occur throughout interphase like de novo NPC assembly but also, under changing environmental conditions, nuclear autophagy [13–15]. Most dramatically, the nuclear envelope must be competently reassembled at the end of an open mitosis [11]. Over the last few years, several key studies have implicated the endosomal sorting complexes required for transport (ESCRT) machinery in ensuring the maintenance of a stable nuclear compartment in the face of these physiological membrane remodeling events. For example, ESCRTs play key roles in monitoring NPC assembly [16–18], NPC turnover in some quiescent cells [19], and mitotic reassembly [20–24]. Further, ESCRTs help to seal nuclear envelope ruptures caused by mechanical forces imposed as cells migrate through constrictions [25,26] or other mechanical perturbations [27,28]. ESCRTs may also protect the integrity of micronuclei [29]. Whether the molecular mechanisms that contribute to ESCRT-mediated protection in the context of these physiological and pathological scenarios proceeds through similar mechanisms remains to be fully understood. Nonetheless, key advances have been made in understanding how disruptions in the nuclear envelope barrier are sensed in both yeast and mammalian systems, and new players have been introduced that help seal the barrier. To facilitate navigating these studies, we present an overview of the key proteins—with a focus on LEM domain and ESCRT orthologues—that have been implicated in nuclear envelope-specific mechanisms across species in Table 1.
Table 1. The role(s) of LEM domain, ESCRT, and other select proteins at the nuclear envelope.
Abbreviations: BAF, Barrier-to-Autointegration Factor; Ce, Caenorhabditis elegans; CHMP/Chm/Cmp, Charged Multivesicular Body Protein; CNEP-1, CTD Nuclear Envelope Phosphatase 1 Homolog; Elo, Elongation Of Fatty Acids Protein; ESCRT, Endosomal Sorting Complexes Required for Transport; Heh, helix-extension-helix; Hs, Homo sapiens; INM, inner nuclear membrane; LEM, LAP2, Emerin, MAN1; NPC, nuclear pore complex; Nur, Nuclear rim protein; Sj, Schizosaccharomyces japonicus; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe; Vps, Vacuolar protein sorting; WH, winged helix; Xpo1, Exportin 1
| Protein | Species | Role(s) at nuclear envelope related to nuclear envelope surveillance/repair and post-mitotic reassembly |
|---|---|---|
| LEM-domain proteins | ||
| LEM2 | Sc (Hehl/Srcl) | – Required to recruit Chm7 to nuclear envelope upon genetic perturbation of NPC assembly [16] |
| – Cytosolic exposure of WH domain is sufficient to recruit and activate Chm7 at ER membranes [18] | ||
| Sp (Heh1/Lem2) | – Required for maintaining nuclear-cytoplasmic compartmentalization [69] | |
| – Helps control nucleus size, possibly by regulating membrane flow [68] | ||
| Sj | – Required for efficient nuclear envelope reformation after mitosis [70,71] | |
| Ce (LEM-2) | – Recruited to sites of nuclear rupture with ESCRT machinery [27] | |
| Hs | – Required for efficient nuclear envelope formation after mitosis, recruits CHMP7 [21] | |
| – Recruited to nuclear envelope ruptures by BAF; simultaneous deletion of LEM2, Emerin, and Ankle2 inhibits repair [28] | ||
| – WH domain forms co-polymer with CHMP7 [39] | ||
| Ankle2/LEM4 | Ce (LEM-4L) | – Required for nuclear envelope reassembly [72] |
| Hs | – Recruited to nuclear envelope ruptures by BAF; supports repair with LEM2 and Emerin [28] | |
| Emerin | Hs | – Recruited to nuclear envelope ruptures by BAF; supports repair with LEM2 and Ankle2 [28] |
| MAN1 | Sc (Heh2) | – N-terminal LEM-containing domain directly binds to Chm7 and Snf7 [16] |
| Sj | – Required for efficient disassembly and reformation of the nuclear envelope after mitosis [73] | |
| ESCRTs | ||
| CHMP7 | Sc (Chm7) | – Recruited to nuclear envelope upon perturbation of NPC assembly [16] |
| – Required to maintain nuclear envelope integrity in the context of nuclear envelope herniations [18] | ||
| – Exported from the nucleus by Crm1/Xpo1 [18] | ||
| – Hyperactivation by binding to Heh1 causes INM remodeling with extensions into the nucleus [18] | ||
| Sp (Cmp7) | – Acts to remodel nuclear envelope, suppressed by Vps4 [21] | |
| Sj (Cmp7) | – Required for efficient nuclear envelope reformation after mitosis; may play a role in releasing Lem2 from heterochromatin [71] | |
| Hs | – Directly interacts with membranes [22]; forms a co-polymer with LEM2 [39] | |
| – Required for efficient nuclear envelope reformation after mitosis [21,24] and for repair following mechanical disruption [25] | ||
| – Maintains stability of intact micronuclei [29]; hyperactivation drives membrane deformation and correlates with DNA damage [29,33] | ||
| – Exported from the nucleus by CRM1/XPO1 [33] | ||
| CHMP4A-C | Sc (Snf7) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| Sj (Vps32) | – Required for efficient nuclear envelope reformation after mitosis; may play a role in releasing Lem2 from heterochromatin [71] | |
| Ce (Vps32) | – Recruited to sites of nuclear rupture with LEM-2 [27] | |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [23,24] and for repair following mechanical disruption [25,26] | |
| – Recruited to forming micronuclei [29,51] | ||
| CHMP1A,B | Sc (Did2) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| CHMP2A,B | Sc (Vps2) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [23,24] and for repair following mechanical disruption [25] | |
| CHMP3 | Sc (Vps24) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [23,24] and for repair following mechanical disruption [26] | |
| CHMP5 | Sc (Vps60) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| IST1 | Sc (Ist1) | – Required for recruitment of Vps4 to sites of Chm7 activation [18] |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [24] | |
| VPS4 | Sc | – Functionally interacts with NPC biogenesis/quality control [16,17] |
| – Likely inhibits Chm7 hyperactivation [18] | ||
| Sp | – Likely inhibits Cmp7 hyperactivation [21] | |
| Sj | – Required for efficient nuclear envelope reformation after mitosis; may play a role in releasing Lem2 from heterochromatin [71] | |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [23,24] and for repair following mechanical disruption [25] | |
| – Inhibits CHMP7 hyperactivation, likely resolves filaments to seal membranes [29] | ||
| Other | ||
| Nur1 | Sc | – Supports activation of Chm7, likely through Heh1 [18] |
| Sj | – Required for efficient nuclear envelope reformation after mitosis; may play a role in releasing Lem2 from heterochromatin [71] | |
| Spastin | Hs | – Required for efficient nuclear envelope reformation after mitosis, likely to sever microtubules [24] |
| CC2D1B | Hs | – Required for efficient nuclear envelope reformation after mitosis by orchestrating ESCRT recruitment [20] |
| BAF | Ce | – Required for efficient nuclear envelope reformation after mitosis [47] |
| Hs | – Required for efficient nuclear envelope reformation after mitosis [48] and for sealing large ruptures [28] | |
| Elo2 | Sp | – Produces long-chain fatty acids essential for nuclear envelope stability [53] |
| CNEP-1 | Ce | – Promotes production of phospholipids required for nuclear envelope reformation in meiosis [61] |
How is a perturbed nuclear envelope barrier surveilled?
Much of our current understanding of the surveillance mechanism of the nuclear envelope barrier comes from work in S. cerevisiae. The principal components of this system are a unique ESCRT, Chm7, and its LEM domain-containing INM binding partner, Heh1/Src1 (Figure 1A; orthologue of LEM2, Table 1). While at ~70 kD Chm7 is small enough to diffuse (albeit slowly [30,31]) across the NPC, it contains nuclear export sequences (NESs) that are recognized by the major export nuclear transport receptor (NTR), Crm1/Xpo1, which rapidly expunge it from the nucleus should it enter [18]. Furthermore, efficient Heh1 targeting to the INM also requires NTRs and the RanGTPase system [32]. Thus, at steady-state these proteins are in a poised state, physically separated on either side of the nuclear envelope [18] (Figure 1A). Importantly, work in preprint shows that human CHMP7 also contains an NES, emphasizing the general conservation of this mechanism [33]. A clear prediction, then, is that any disruption to nuclear-cytoplasmic compartmentalization would lead to the physical association of Heh1 and Chm7 and the activation of a repair mechanism (Figure 1B–D). Critically, it is the exposure of the INM that is ultimately detected by Chm7 [18]. We suggest that such a system could operate on a local level, such as at small tears in the nuclear membranes when spindle forces are applied to the nucleus during mitosis, sites of defective NPC biogenesis [16–18], or in scenarios in which NPCs lose functionality. In the latter cases, the function of Chm7 is correlated with the presence of double-membrane seals over NPCs and/or NPC assembly intermediates ([16], see discussion in [34]). It is possible that a sealing mechanism may precede clearance of NPCs to ensure the barrier is preserved during their removal [19].
Figure 1. Surveillance and repair of nuclear pores and small nuclear envelope ruptures.

A: The surveillance system is poised as Chm7 and Heh1 are segregated on either side of the nuclear envelope by NTRs and NPCs. Cartoon models of Chm7 (orange) and Heh1 (green) depicting predicted winged helix (WH) domains and a microtubule interacting and transport (MIT) domain interacting motif (MIM) alongside established LAP2-Emerin-MAN1 (LEM) and nuclear export sequences (NES). B: Disruption of the nuclear envelope caused by defective nuclear pore biogenesis or nuclear envelope rupture results in the local meeting Chm7 and Heh1. Binding of Heh1 to Chm7 leads to its activation, which likely triggers polymer formation (depicted by an opening of the Chm7 structure). C: A co-polymer of Chm7 and Heh1 (orange/green) helps to seal the membrane likely with help from other ESCRT proteins including Vps4 (not shown, see Table 1). D: The nuclear envelope is repaired, and nuclear-cytoplasmic compartmentalization is reestablished. E: Unregulated surveillance leads to a hyper-activation of Chm7/CHMP7, which drives aberrant membrane expansion at micronuclei and at the INM and might directly cause DNA damage. In all panels: nuclear pore complex, blue; outer and inner nuclear membranes, brown; cytoplasm, yellow; nucleoplasm, purple.
How does the triggering of the surveillance system lead to nuclear membrane repair?
While the spatial segregation of Heh1/LEM2 and Chm7/CHMP7 provides an elegant mechanism to sense local perturbations in the nuclear envelope barrier, how the meeting of these two proteins leads to nuclear membrane sealing remains to be fully defined. Clues may be drawn from other ESCRT-mediated membrane fusion mechanisms where there is a local “activation” of ESCRT-III proteins, which transforms soluble monomeric ESCRT-III subunits into polymers by displacing autoinhibitory structural elements [35,36]. Interestingly, this is often achieved through the binding of winged helix (WH) domain containing ESCRT-II subunits, a common structural fold also predicted to be found at the N-terminus of Chm7/CHMP7 [22,37] and the C-terminus of Heh1/LEM2. This domain in Heh1/LEM2 is invariantly referred to as the MAN1-SRC1-C (MSC) or MAN1 C-terminal homology domain (MCTD) due to its sequence similarity to the WH domain of MAN1, the structure of which has been determined [38]. In vitro studies support that the LEM2 WH domain directly binds to CHMP7 [21]. While it remains to be fully determined whether this interaction is direct in budding yeast, there is nonetheless in vivo evidence to support that binding of the Heh1 WH domain (with a membrane anchor) to Chm7 leads to its activation [18] (Figure 1B). Data from a recent preprint drives this point home with a cross-linking mass spectrometry analysis suggesting conformational changes in CHMP7 upon LEM2 binding and the observation of a CHMP7-LEM2 ring-shaped co-polymer with a ~50 nm diameter [39].
A major challenge remains: How does formation of a CHMP7-LEM2 polymer translate into a membrane fusion event? If other ESCRT-mediated membrane fusion mechanisms are any guide, it is likely that additional ESCRTs like yeast Snf7 and Vps2/Vps24 are ultimately recruited to the nuclear envelope alongside the AAA ATPase, Vps4 [35] (Table 1). Vps4 likely directly contributes to the ESCRT-filament remodeling required to bring membrane close enough for productive fusion [40–43]. While there is clear evidence that Chm7 binds directly to Snf7 [16], and is required for Snf7 and additional “downstream” ESCRTs (including Vps4) to be recruited to the nuclear envelope [18,21,22,24], the precise choreography remains mysterious. Furthermore, Chm7/CHMP7 has a putative microtubule interacting and transport domain (MIT)-interacting motif (MIM) domain [37], which may bind directly to Vps4 as well. As a further complication, this putative MIM domain also precisely overlaps with the NES of Chm7/CHMP7 [18,33], suggesting that direct binding to Xpo1/Crm1 might curtail polymer formation and/or Vps4 recruitment.
Regardless of the impact of Xpo1/Crm1, in both the yeast and mammalian systems, it seems most plausible that a Chm7-Heh1/CHMP7-LEM2 system would be most useful at closing small (<100 nm) holes. There are several data to support this assertion, including the observation of membrane necks of ~50 nm in diameter that are formed at the nuclear envelope upon Chm7 hyperactivation [18], the formation of ~50 nm diameter CHMP7-LEM2 polymers in vitro [39], and the demonstration that ESCRTs are found in ~30–50 nm holes at the reforming nuclear envelope [23]. A challenge with being definitive here, however, is the lack of experimental strategies capable of precisely driving a hole in the nuclear envelope with a defined diameter. Indeed, mechanical (or laser)-induced perturbations are currently the state-of-the art experimental strategies and likely lead to variable and large (hundreds of nanometer to micron scale) ruptures [25,26,28]. It is unlikely that the CHMP7-LEM2 system would, at least on its own, be capable of repairing this scale of disruption. Furthermore, there is also new published evidence [29] and in preprint [33] that in scenarios in which there is a catastrophic rupture of, for example, the nuclear envelope-like membranes of micronuclei [44], there is a hyperstimulation of the CHMP7-LEM2 system that may be deleterious [29,33](Figure 1E). In fact, it is proposed that the aberrant membrane morphologies driven by LEM2-CHMP7 on the surface of the micronuclear DNA may directly contribute to DNA damage and may be an input to chromothripsis [33]. While challenging to definitively prove, the concept that the over-stimulation of this surveillance system may be deleterious to cell viability can be found in multiple model systems [16,21]. Though the ultimate cause of cell death remains to be fully understood, it seems clear that the CHMP7-LEM2 interaction needs to be tightly regulated in order to remain productive. The latter idea is extremely relevant during an open mitosis, when mechanisms must exist to prevent CHMP7 and LEM2 from aberrantly interacting. Early clues as to the nature of this regulation may come from proteins like CC2DB1 that help modulate CHMP7 function at the end of mitosis [20], but almost certainly also will require post-translational modifications to one or both proteins.
Do large nuclear ruptures trigger a nuclear envelope-reassembly program?
In a model in which CHMP7-LEM2 only acts at small nuclear envelope holes like nuclear pores, additional mechanisms must be in play to repair larger ruptures. Consistent with this idea, recent work has uncovered a key role for Barrier-to-Autointegration Factor (BAF) in repairing mechanically-induced ruptures in mammalian cell culture [28]. While CHMP7 is recruited to mechanically-induced rupture sites, it is in fact dispensable for the efficient repair of these ruptures [28]. Most interestingly, the exposure of genomic DNA to the cytosol triggers the recruitment of an unphosphorylated cytosolic pool of BAF that coats the surface of the exposed chromatin [28](Figure 2B). This pool is distinct from BAF localized within the nucleus, which is constitutively phosphorylated [45]. Thus, there is a clear conceptual parallel to Chm7 in that there is a dedicated cytosolic form of BAF that is ultimately responsible for sensing the exposure of nuclear contents. It should also be noted that budding yeast do not have a BAF orthologue, leading them to perhaps rely more heavily on ESCRT machinery for nuclear envelope surveillance/repair. This BAF recruitment is essential to repair ruptures as it likely forms a surface competent to recruit membrane with integral INM proteins rich in other LEM domain proteins (Table 1; Figure 2C). It may also be possible that the recruitment and chromatin-coating of BAF provides a stopgap to rapidly restore nuclear compartmentalization in lieu of a kinetically slower membrane repair mechanism. Consistent with this idea, BAF has been shown to form a cross-linked network that establishes a diffusion barrier important for nuclear envelope reformation at the end of mitosis [46]. Direct testing of these ideas will require the development of more sophisticated tools to monitor membrane sealing, which so far rely on the indirect measure of the re-accumulation of nuclear localization signal (NLS)-containing reporters.
Figure 2. Model for repair of large nuclear envelope ruptures.
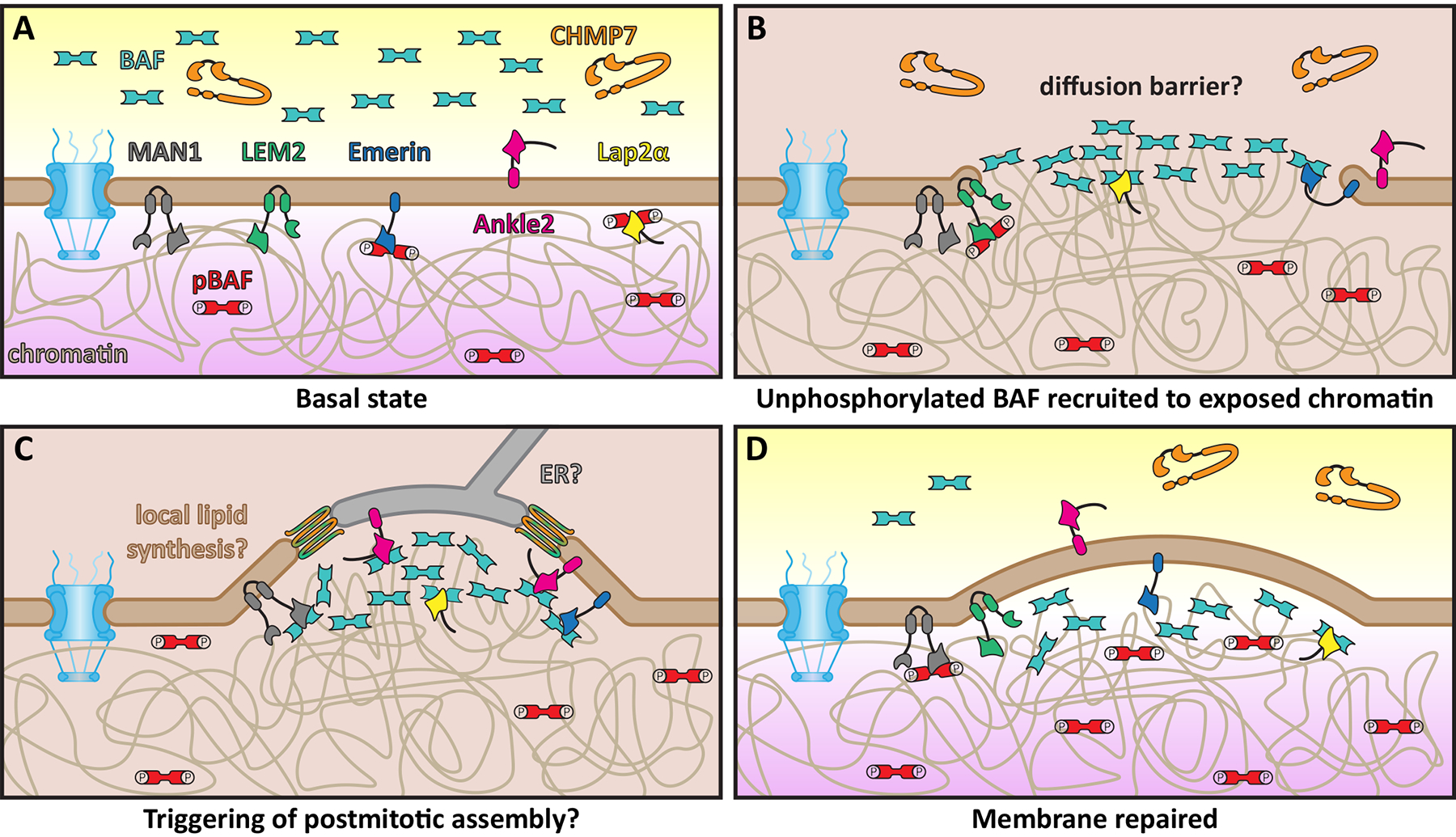
A: Schematic of basal/unperturbed state of mammalian nuclear envelope, which is surveilled by CHMP7 (orange) and unphosphorylated BAF (teal). Key proteins involved with surveillance and repair including integral INM LEM proteins MAN1 (grey), LEM2 (green), Emerin (blue), Ankle2 (pink), and Lap2α (yellow) are also shown. Phosphorylated BAF (pBAF, red) is specifically found in the nucleus, bound to LEM domain proteins and chromatin (beige). B: Following nuclear envelope rupture, cytosolic, unphosphorylated BAF binds to exposed chromatin, possibly forming a diffusion barrier. C: LEM domain proteins are recruited to sites of rupture by BAF coating the exposed chromatin. Membrane for repair may be donated from the ER and/or local synthesis. ESCRTs (orange/green) are also recruited to this site, likely to seal small holes in the reforming nuclear membranes, analogous to post-mitotic nuclear envelope reformation. D: The nuclear envelope is repaired and nuclear-cytoplasmic compartmentalization is reestablished. In all panels: nuclear pore complex, blue; outer and inner nuclear membranes, brown; cytoplasm, yellow; nucleoplasm, purple.
BAF’s mechanism of recruitment to exposed chromatin at rupture sites bears a striking resemblance to the sequence of events that leads to nuclear envelope reformation at the end of mitosis. In this process, BAF also acts as an early-acting factor [47,48] that coats the chromatin surface [46,49]. Further, ESCRTs are also not essential but nonetheless help to make the process more efficient, likely by sealing small holes in terminal steps [23,24](Figure 2C). It will be interesting to more thoroughly assess the commonalities/differences between the order of assembly of distinct nuclear envelope factors at chromatin exposed by rupture and that exposed during mitotic nuclear envelope reformation. A key question, for example, would be whether the biochemical components that form the “core” and “non-core” regions, as defined in post-mitotic nuclear envelope reformation [50], show any preference for being recruited to an interphase rupture site. Not only will this be important to understand a nuclear envelope repair mechanism, but it may also inform the robustness of the repaired domain and whether it might be susceptible to future rupture events. In the context of a question: could a single stochastic nuclear rupture event predispose a cell nucleus to future ruptures? The answer to this may very well be “yes”, as evidence that a single rupture may permanently weaken the nuclear envelope can be gleaned from studying micronuclei disruption: Micronuclei formed without “non-core” membranes are much more likely to irreversibly rupture than counterparts that contained a normal core and non-core load [51]. Thus, this micronuclei story is a cautionary tale for the re-sealing of nuclear envelopes both at the end of mitosis but also after a rupture event, as it suggests there is a discrete ordering of recruitment/assembly of specific nuclear envelope proteins critical for establishing a mechanically stable nuclear envelope domain refractory to perturbation.
Is the future in the membrane itself?
In addition to new protein components, the lipid composition of the nuclear membranes is also emerging as a critical aspect of not only nuclear envelope stability, but also repair. For example, the hyperactivation of Chm7 results in dramatic membrane deformation including intranuclear invaginations emanating from the INM [18,33], which may in fact include the delivery of newly synthesized phospholipids as a response to the triggering of this futile repair mechanism (Figure 1E). Consistent with this idea and in a more physiological setting, work in preprint has uncovered synthetic genetic relationships between important regulators of lipid synthesis and CHMP7 in C. elegans, suggesting an interdependency between ESCRT-mediated nuclear envelope sealing at the end of meiosis and lipid synthesis [52]. Analogously, losses of nuclear integrity in fission yeast lacking HEH1/LEM2 can be suppressed by overexpressing enzymes required for long-chain fatty acids [53]. These works build on a long history of genetic relationships uncovered between lipid synthesis pathways and nuclear envelope structure and function [54–63], which has culminated recently with demonstrations of how the INM is a site of lipid metabolism capable of giving rise to nuclear lipid droplets [64–66]. Further evidence supports that this local metabolism, if not properly regulated, leads to disruptions in nuclear integrity [64].
The evidence that membrane composition impacts both nuclear envelope integrity but also repair leads to a broader point: There must be a fine balance that allows cells to achieve robust nuclear mechanics capable of preventing aberrant rupture, while simultaneously allowing sufficient dynamicity for productive membrane remodeling essential for growth, metabolism, and homeostasis over both short and long terms [67]. Thus, a priority going forward must be to develop more precise tools capable of monitoring the origin of the nuclear membranes, their flow and biophysical properties (e.g. tension) both at steady-state and upon nuclear envelope remodeling in physiological and pathological scenarios. Only then will we be able to more thoroughly investigate emerging concepts in this field, such as recently published work that Heh1/Lem2 may also directly control membrane flow in a way that impacts nuclear envelope surface area [68]. While this mechanism remains obscure, it nonetheless suggests that proteins critical for maintaining and repairing the nuclear envelope might also directly impact the properties of the lipid bilayers they are embedded in, perhaps in ways that can be directly modulated. An understanding of how nuclear membrane repair pathways leverage these yet to be defined control mechanisms will provide exciting new insight into how the nuclear envelope membrane system maintains nuclear identity.
Acknowledgments
We thank D.J. Thaller for critical comments on the manuscript and the following National Institutes of Health grants for support: R01 GM105672; R01 HL124402; R21 AG058033; R21 CA231269.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lusk CP, King MC: The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol 2017, 44:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatch E, Hetzer M: Breaching the nuclear envelope in development and disease. J Cell Biol 2014, 205:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatch EM: Nuclear envelope rupture: little holes, big openings. Curr Opin Cell Biol 2018, 52:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah P, Wolf K, Lammerding J: Bursting the bubble - nuclear envelope rupture as a path to genomic instability? Trends Cell Biol 2017, 27:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houthaeve G, Robijns J, Braeckmans K, De Vos WH: Bypassing border control: nuclear envelope rupture in disease. Physiology (Bethesda) 2018, 33:39–49. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F: ESCRT machinery is required for plasma membrane repair. Science 2014, 343:1247136. [DOI] [PubMed] [Google Scholar]
- 7.Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI: Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 2018, 360:eaar5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, Jaiswal JK: Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun 2014, 5:5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radulovic M, Schink KO, Wenzel EM, Nahse V, Bongiovanni A, Lafont F, Stenmark H: ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J 2018, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anding AL, Baehrecke EH: Cleaning house: selective autophagy of organelles. Dev Cell 2017, 41:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ungricht R, Kutay U: Mechanisms and functions of nuclear envelope remodelling. Nat Rev Mol Cell Biol 2017, 18:229–245. [DOI] [PubMed] [Google Scholar]
- 12.De Magistris P, Antonin W: The dynamic nature of the nuclear envelope. Curr Biol 2018, 28:R487–R497. [DOI] [PubMed] [Google Scholar]
- 13.Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, et al. : Autophagy mediates degradation of nuclear lamina. Nature 2015, 527:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, Nakatogawa H: Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 2015, 522:359–362. [DOI] [PubMed] [Google Scholar]
- 15.Roberts P, Moshitch-Moshkovitz S, Kvam E, O’Toole E, Winey M, Goldfarb DS: Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol Biol Cell 2003, 14:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster BM, Thaller DJ, Jager J, Ochmann SE, Borah S, Lusk CP: Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing. EMBO J 2016, 35:2447–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster BM, Colombi P, Jager J, Lusk CP: Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 2014, 159:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.••.Thaller DJ, Allegretti M, Borah S, Ronchi P, Beck M, Lusk CP: An ESCRT-LEM protein surveillance system is poised to directly monitor the nuclear envelope and nuclear transport system. Elife 2019, 8:e45284. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work in budding yeast shows that Chm7 contains NESs (as does CHMP7, see 33) and is thus spatially segregated from Heh1/LEM2. Exposure of the WH domain of Heh1 can recruit and activate Chm7 leading to membrane remodeling. Thus, the surveillance system is poised to directly respond to perturbations of the nuclear envelope and nuclear transport system.
- 19.Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, Ellisman MH, Hetzer MW: Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J Cell Biol 2019, 218:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.•.Ventimiglia LN, Cuesta-Geijo MA, Martinelli N, Caballe A, Macheboeuf P, Miguet N, Parnham IM, Olmos Y, Carlton JG, Weissenhorn W, et al. : CC2D1B coordinates ESCRT-III activity during the mitotic reformation of the nuclear envelope. Dev Cell 2018, 47:547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduces a new regulatory factor that controls ESCRT-III function during nuclear envelope reassembly.
- 21.Gu M, LaJoie D, Chen OS, von Appen A, Ladinsky MS, Redd MJ, Nikolova L, Bjorkman PJ, Sundquist WI, Ullman KS, et al. : LEM2 recruits CHMP7 for ESCRT-mediated nuclear envelope closure in fission yeast and human cells. Proc Natl Acad Sci U S A 2017, 114:E2166–E2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olmos Y, Perdrix-Rosell A, Carlton JG: Membrane binding by CHMP7 coordinates ESCRT-III-dependent nuclear envelope reformation. Curr Biol 2016, 26:2635–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG: ESCRT-III controls nuclear envelope reformation. Nature 2015, 522:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H: Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 2015, 522:231–235. [DOI] [PubMed] [Google Scholar]
- 25.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J: Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, et al. : ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016, 352:359–362. [DOI] [PubMed] [Google Scholar]
- 27.Penfield L, Wysolmerski B, Mauro M, Farhadifar R, Martinez MA, Biggs R, Wu HY, Broberg C, Needleman D, Bahmanyar S: Dynein-pulling forces counteract lamin-mediated nuclear stability during nuclear envelope repair. Mol Biol Cell 2018, 29:852–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.••.Halfmann CT, Sears RM, Katiyar A, Busselman BW, Aman LK, Zhang Q, O’Bryan CS, Angelini TE, Lele TP, Roux KJ: Repair of nuclear ruptures requires barrier-to-autointegration factor. J Cell Biol 2019, 218:2136–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduces a key role for a specific pool of unphosphorylated BAF in the recognition of exposed chromatin during mechanically induced nuclear rupture. BAF is also required for membrane repair by recruiting many LEM domain proteins.
- 29.•.Willan J, Cleasby AJ, Flores-Rodriguez N, Stefani F, Rinaldo C, Pisciottani A, Grant E, Woodman P, Bryant HE, Ciani B: ESCRT-III is necessary for the integrity of the nuclear envelope in micronuclei but is aberrant at ruptured micronuclear envelopes generating damage. Oncogenesis 2019, 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]; With (33) demonstrates that CHMP7 hyperactivation might directly contribute to DNA damage at micronuclei.
- 30.Timney BL, Tetenbaum-Novatt J, Agate DS, Williams R, Zhang W, Chait BT, Rout MP: Simple kinetic relationships and nonspecific competition govern nuclear import rates in vivo. J Cell Biol 2006, 175:579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popken P, Ghavami A, Onck PR, Poolman B, Veenhoff LM: Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex. Mol Biol Cell 2015, 26:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King MC, Lusk CP, Blobel G: Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature 2006, 442:1003–1007. [DOI] [PubMed] [Google Scholar]
- 33.••.Vietri M, Schultz SW, Bellanger A, Jones CM, Raiborg C, Skarpen E, Pedurupillay CRJ, Kip E, Timmer R, Jain A, et al. : Unrestrained ESCRT-III drives chromosome fragmentation and micronuclear catastrophe. bioRxiv 2019:517011. [DOI] [PubMed] [Google Scholar]; Demonstrates (with 18) that CHMP7 has an NES. Unregulated binding of CHMP7 with LEM2 leads to aberrant membrane remodeling and might directly contribute to DNA damage either in the nucleus, or in micronuclei as a potential input to chromothrypsis.
- 34.Thaller DJ, Lusk CP: Fantastic nuclear envelope herniations and where to find them. Biochemical Society Transactions 2018, 46:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough J, Frost A, Sundquist WI: Structures, functions, and dynamics of ESCRT-III/Vps4 membrane remodeling and fission complexes. Annu Rev Cell Dev Biol 2018, 34:85–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoneberg J, Lee IH, Iwasa JH, Hurley JH: Reverse-topology membrane scission by the ESCRT proteins. Nat Rev Mol Cell Biol 2017, 18:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer I, Brune T, Preiss R, Kolling R: Evidence for a nonendosomal function of the Saccharomyces cerevisiae ESCRT-III-like protein Chm7. Genetics 2015, 201:1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caputo S, Couprie J, Duband-Goulet I, Konde E, Lin F, Braud S, Gondry M, Gilquin B, Worman HJ, Zinn-Justin S: The carboxyl-terminal nucleoplasmic region of MAN1 exhibits a DNA binding winged helix domain. J Biol Chem 2006, 281:18208–18215. [DOI] [PubMed] [Google Scholar]
- 39.••.von Appen A, LaJoie D, Johnson IE, Trnka M, Pick SM, Burlingame AL, Ullman KS, Frost A: A role for liquid-liquid phase separation in ESCRT-mediated nuclear envelope reformation. bioRxiv 2019:577460. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that LEM2 can induce conformational changes in CHMP7, which might underlie its activation. Also show the formation of a CHMP7-LEM2 copolymer in vitro.
- 40.Adell MAY, Migliano SM, Upadhyayula S, Bykov YS, Sprenger S, Pakdel M, Vogel GF, Jih G, Skillern W, Behrouzi R, et al. : Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. Elife 2017, 6:e31652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoneberg J, Pavlin MR, Yan S, Righini M, Lee IH, Carlson LA, Bahrami AH, Goldman DH, Ren X, Hummer G, et al. : ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science 2018, 362:1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mierzwa BE, Chiaruttini N, Redondo-Morata L, von Filseck JM, Konig J, Larios J, Poser I, Muller-Reichert T, Scheuring S, Roux A, et al. : Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat Cell Biol 2017, 19:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfitzner A-K, Mercier V, Roux A: Vps4 triggers sequential subunit exchange in ESCRT-III polymers that drives membrane constriction and fission. bioRxiv 2019:718080. [Google Scholar]
- 44.Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW: Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols RJ, Wiebe MS, Traktman P: The vaccinia-related kinases phosphorylate the N’ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol Biol Cell 2006, 17:2451–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samwer M, Schneider MWG, Hoefler R, Schmalhorst PS, Jude JG, Zuber J, Gerlich DW: DNA cross-bridging shapes a single nucleus from a set of mitotic chromosomes. Cell 2017, 170:956–972 e923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorjanacz M, Klerkx EP, Galy V, Santarella R, Lopez-Iglesias C, Askjaer P, Mattaj IW: Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J 2007, 26:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL, Hiraoka Y: BAF is required for emerin assembly into the reforming nuclear envelope. J Cell Sci 2001, 114:4575–4585. [DOI] [PubMed] [Google Scholar]
- 49.Haraguchi T, Kojidani T, Koujin T, Shimi T, Osakada H, Mori C, Yamamoto A, Hiraoka Y: Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J Cell Sci 2008, 121:2540–2554. [DOI] [PubMed] [Google Scholar]
- 50.LaJoie D, Ullman KS: Coordinated events of nuclear assembly. Curr Opin Cell Biol 2017, 46:39–45. [DOI] [PubMed] [Google Scholar]
- 51.•.Liu S, Kwon M, Mannino M, Yang N, Renda F, Khodjakov A, Pellman D: Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018, 561:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study on micronuclei suggests that the underlying molecular composition of the nuclear envelope membranes could impact its susceptibility to nuclear rupture and its ability to repair.
- 52.Penfield L, Shankar R, Szentgyörgyi E, Laffitte A, Mauro M, Audhya A, Müller-Reichert T, Bahmanyar S: Local regulation of lipid synthesis controls ER sheet insertion into nuclear envelope holes to complete nuclear closure. bioRxiv 2019:757013. [Google Scholar]
- 53.Kinugasa Y, Hirano Y, Sawai M, Ohno Y, Shindo T, Asakawa H, Chikashige Y, Shibata S, Kihara A, Haraguchi T, et al. : The very-long-chain fatty acid elongase Elo2 rescues lethal defects associated with loss of the nuclear barrier function in fission yeast cells. J Cell Sci 2019, 132:jcs229021. [DOI] [PubMed] [Google Scholar]
- 54.Hodge CA, Choudhary V, Wolyniak MJ, Scarcelli JJ, Schneiter R, Cole CN: Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum. J Cell Sci 2010, 123:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos-Rosa H, Leung J, Grimsey N, Peak-Chew S, Siniossoglou S: The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J 2005, 24:1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han GS, O’Hara L, Carman GM, Siniossoglou S: An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J Biol Chem 2008, 283:20433–20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneiter R, Hitomi M, Ivessa AS, Fasch EV, Kohlwein SD, Tartakoff AM: A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol Cell Biol 1996, 16:7161–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mall M, Walter T, Gorjanacz M, Davidson IF, Nga Ly-Hartig TB, Ellenberg J, Mattaj IW: Mitotic lamin disassembly is triggered by lipid-mediated signaling. J Cell Biol 2012, 198:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorjanacz M, Mattaj IW: Lipin is required for efficient breakdown of the nuclear envelope in Caenorhabditis elegans. J Cell Sci 2009, 122:1963–1969. [DOI] [PubMed] [Google Scholar]
- 60.Golden A, Liu J, Cohen-Fix O: Inactivation of the C. elegans lipin homolog leads to ER disorganization and to defects in the breakdown and reassembly of the nuclear envelope. J Cell Sci 2009, 122:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahmanyar S, Biggs R, Schuh AL, Desai A, Muller-Reichert T, Audhya A, Dixon JE, Oegema K: Spatial control of phospholipid flux restricts endoplasmic reticulum sheet formation to allow nuclear envelope breakdown. Genes Dev 2014, 28:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siniossoglou S, Santos-Rosa H, Rappsilber J, Mann M, Hurt E: A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J 1998, 17:6449–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters AD, Amoateng K, Wang R, Chen JH, McDermott G, Larabell CA, Gadal O, Cohen-Fix O: Nuclear envelope expansion in budding yeast is independent of cell growth and does not determine nuclear volume. Mol Biol Cell 2019, 30:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbosa AD, Lim K, Mari M, Edgar JR, Gal L, Sterk P, Jenkins BJ, Koulman A, Savage DB, Schuldiner M, et al. : Compartmentalized synthesis of triacylglycerol at the inner nuclear membrane regulates nuclear organization. Dev Cell 2019, 50:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romanauska A, Kohler A: The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell 2018, 174:700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sołtysik K, Ohsaki Y, Tatematsu T, Cheng J, Fujimoto T: Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat Commun 2019, 10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King MC, Lusk CP: A model for coordinating nuclear mechanics and membrane remodeling to support nuclear integrity. Curr Opin Cell Biol 2016, 41:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.•.Kume K, Cantwell H, Burrell A, Nurse P: Nuclear membrane protein Lem2 regulates nuclear size through membrane flow. Nat Commun 2019, 10:1871. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduces the interesting concept that Lem2 might directly impact membrane flow in a way that contributes to nuclear scaling.
- 69.Gonzalez Y, Saito A, Sazer S: Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus 2012, 3:60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yam C, He Y, Zhang D, Chiam KH, Oliferenko S: Divergent strategies for controlling the nuclear membrane satisfy geometric constraints during nuclear division. Curr Biol 2011, 21:1314–1319. [DOI] [PubMed] [Google Scholar]
- 71.Pieper GH, Sprenger S, Teis D, Oliferenko S: ESCRT-III/Vps4 controls heterochromatin-nuclear envelope attachments. bioRxiv 2019:579805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asencio C, Davidson IF, Santarella-Mellwig R, Ly-Hartig TB, Mall M, Wallenfang MR, Mattaj IW, Gorjanacz M: Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell 2012, 150:122–135. [DOI] [PubMed] [Google Scholar]
- 73.Yam C, Gu Y, Oliferenko S: Partitioning and remodeling of the Schizosaccharomyces japonicus mitotic nucleus require chromosome tethers. Curr Biol 2013, 23:2303–2310. [DOI] [PubMed] [Google Scholar]


