Abstract
Background: We compared women's acceptability of urine and cervico-vaginal sample self-collection for high-risk (oncogenic) human papillomavirus (hrHPV) testing and assessed whether acceptability varied across racial/ethnic groups.
Methods: As part of a test accuracy study of urine-based hrHPV testing, we recruited a convenience sample of women 25–65 years of age at two colposcopy clinics in North Carolina between November 2016 and January 2019. After self-collection of urine and cervico-vaginal samples, women completed a questionnaire on the acceptability of the sample collection methods. We coded open-ended questions inductively. All results are presented stratified by racial/ethnic group.
Results: We included 410 women (119 Hispanic, 115 non-Hispanic Black, 154 non-Hispanic White, and 22 women with other racial identities). Most women (79%, 95% confidence interval [CI] = 76%–83%) had positive feelings about urine-based hrHPV testing. Women generally preferred urine (78%, 95% CI = 74%–82%) over cervico-vaginal self-collection (18%, 95% CI = 14%–22%), but the degree differed by racial/ethnic group, increasing from 75% in non-Hispanic Black to 82% in Hispanic women (p = 0.011). Most women reported at least one positive aspect of urine (89%) and cervico-vaginal self-collection (85%) for hrHPV testing with the most common positive aspect being easy sample collection, although 16% of women were concerned about performing the cervico-vaginal self-collection correctly.
Conclusions: Self-collection for hrHPV-based cervical cancer screening is highly acceptable to women across different racial/ethnic groups in the United States, and most women in our study would be more likely to attend future cervical cancer screening appointments if screening were urine based. Urine-based hrHPV testing is a promising approach to improve cervical cancer screening coverage.
Keywords: HPV testing, self-collection, urine, acceptability, cervical cancer screening
Introduction
Invasive cervical cancer (ICC) is among the most common cancers in women worldwide,1 although regular screening allows for detection and treatment of cervical lesions before malignant transformation occurs. In recent years, testing for high-risk (oncogenic) human papillomavirus (hrHPV) infection has emerged as a new cervical cancer screening method. The US Preventive Services Task Force (USPSTF) now endorses primary hrHPV testing or a combination of Pap and hrHPV testing (co-testing) every 5 years for cervical cancer screening of women at age 30–65 years.2
Not all population groups, however, benefit equally from cervical cancer screening programs. Within the United States, ICC incidence and mortality rates are higher among racial and ethnic minority women compared with White women.3 The vast majority of women who develop ICC have been underscreened.4–6 Lower socioeconomic status is associated with lower cervical cancer screening rates, partly because of resource-related barriers, including the cost of screening, inadequate transportation, and inflexible working hours.7,8 Other barriers to cervical cancer screening commonly experienced by racial and ethnic minority women include linguistic barriers, low health literacy, and lack of knowledge.9,10 Culturally tailored interventions led by community health workers have been used to improve cervical cancer screening rates among racial and ethnic minority women.11,12 However, some women remain reluctant to undergo a gynecological examination to obtain the cervical specimen required for Pap or hrHPV testing owing to fear of pain and embarrassment.8,13 High-risk HPV testing on self-collected specimens such as cervico-vaginal brushes or swabs and urine could be an attractive alternative for these women, given that urine and cervico-vaginal sample self-collection can reliably be done by women themselves in clinical settings or at home.14,15
It is increasingly recognized that self-collected samples are a feasible and accurate option to test for hrHPV or other sexually transmitted infections such as Chlamydia trachomatis or Neisseria gonorrhea.16–19 A recent meta-analysis found self-collected cervico-vaginal sampling for PCR-based hrHPV testing to be as sensitive as on provider-collected sampling for the detection of cervical intraepithelial neoplasia grade 2 or higher (CIN2+).20 Data on the accuracy of urine-based hrHPV testing are more limited, but several studies have found a reasonable sensitivity for detection of CIN2+.14,17,21–24 In comparison studies, women generally prefer self-collection to sample collection by a health care provider.25–29 Most acceptability studies to date have focused on self-collected cervico-vaginal brushes or swabs for hrHPV testing.26,30–32 The few studies that have compared acceptability of self-collection of urine and cervico-vaginal samples for hrHPV testing suggest that women might prefer urine-based hrHPV testing to self-collected cervico-vaginal brushes or swabs.28,29,33 However, none of these studies assessed whether preference for urine-based testing varied by racial and ethnic groups. As part of a study on test accuracy of urine-based hrHPV testing,34 we compared the acceptability of self-collection of urine and cervico-vaginal samples for hrHPV testing and assessed whether women's reactions to the sample collection methods differed across racial and ethnic groups.
Methods
Study population
Between November 2016 and January 2019, we recruited a convenience sample of women 25–65 years of age who attended colposcopy clinics at the University of North Carolina (UNC) Women's Hospital or Duke University Hospital. Women were eligible for participation in the study if they underwent colposcopy because of abnormal cytology results, infection with HPV subtypes 16 or 18, had a persistent infection with other hrHPV subtypes, or were treated for CIN2+. We also invited women to participate in the study who were both negative for intraepithelial lesion or malignancy (NILM) on cytology and positive for hrHPV subtypes other than 16 or 18. In this group, colposcopy was performed for study purposes and not as part of routine clinical care because current guidelines of the American Society for Colposcopy and Cervical Pathology (ASCCP) do not recommend immediate referral for colposcopy for these women.35
We identified potentially eligible women by reviewing electronic medical records and inviting them through phone or during a clinic visit to participate in the study. Women without a cervix and pregnant women were excluded; in addition, women who were referred to colposcopy for study purposes were excluded if they were taking anticoagulants. Written informed consent was obtained from all participants (including “research only” participants who underwent a nonindicated colposcopy), and the study was approved by the Institutional Review Boards of UNC and Duke University.
Study procedures
Participating women received comprehensive verbal and written information concerning the study procedures (including sample self-collection) in English or Spanish, based upon their preference. Women provided two urine samples, one sample from the initial stream (∼20 mL) and a second from the midstream (up to 100 mL), and they self-collected a cervico-vaginal swab using a Viba brush (Rovers Medical Devices BV, The Netherlands). Women inserted the brush to the top of the vaginal canal and rotated it five times before they removed the brush and released the brush head into a preservative liquid-based cytology media (ThinPrep; Hologic, Inc., Bedford, MA). After self-collection of samples, women were handed a questionnaire capturing information on the acceptability of the different sample collection methods as well as demographics and medical history. Women then underwent a gynecological examination during which a health care provider collected a cervical scrapping for hrHPV testing using a brush-like device (Wallach Papette; Wallach Surgical Devices, Trumbull, CT) and performed colposcopy. Loop electrosurgical excision procedure was performed when clinically indicated. At the end of the visit, women received a gift card for their study participation.
Acceptability questionnaire
The acceptability questionnaire collected data on sociodemographics and medical history. Furthermore, it contained both closed-ended and open-ended questions concerning women's attitudes toward urine self-collection, cervico-vaginal self-collection, and provider collection of cervical samples, and concerns and suggestions for improvements. Overall, the questionnaire contained 36 items and required ∼15 minutes for completion. To assess overall attitude toward urine and cervico-vaginal self-collection methods, we asked whether women's feelings were “mostly positive,” “neutral,” or “mostly negative.” We also asked women which of the two self-collection methods they preferred. In open-ended questions, we asked what women liked and did not like about the two self-collection methods, what concerns they had about self-collection, and what suggestions they had to improve these methods. Furthermore, we made a post hoc addition to ask the last 333 women which type of sample collection they would choose for future hrHPV testing, and whether they were more or less likely to attend their next recommended cervical cancer screening appointment if the hrHPV test was based on urine self-collection.
Statistical analyses
Women who participated in a study on test accuracy of urine-based hrHPV testing34 were eligible for inclusion in this analysis of the acceptability of sample self-collection for hrHPV-based cervical cancer screening. Women who completed the acceptability questionnaire and provided both urine and self-collected cervico-vaginal samples were included. We used descriptive statistics to assess sociodemographic and medical characteristics of included women. We coded responses to open-ended questions inductively and summarized emerging likes, dislikes, concerns, and suggestions regarding self-collection using descriptive statistics. For acceptability results, we report percentages of women giving a specific answer with 95% confidence intervals (CIs). All results were stratified by racial/ethnic group. We used Fisher's exact test to examine whether acceptability of self-sampling significantly differed by racial/ethnic group. Statistical significance was set at a level of p < 0.05. We used McNemar's test to assess whether the percentage of women having positive feelings about urine self-collection differed from the percentage of women having positive feelings about cervico-vaginal self-collection.
Results
Study population
Of 413 women eligible for the diagnostic test accuracy analysis,34 410 women completed the acceptability questionnaire and provided both urine and self-collected cervico-vaginal sample. The study population consisted of 119 Hispanic, 115 non-Hispanic Black, 154 non-Hispanic White, and 22 women with other racial identities. The median age was 37 years (interquartile range [IQR] = 31–46 years) and similar across racial/ethnic groups (Table 1). The majority of Hispanic women (n = 60, 53%) and non-Hispanic White women (n = 68, 45%) were married or lived with a partner, whereas most non-Hispanic Black women were single (n = 61, 56%). Overall, 25% of participants were college graduates (n = 98), but this percentage increased from 7% in Hispanic women to 39% in non-Hispanic White women. Among Hispanic women, 77% were without health insurance coverage, whereas only 21% of non-Hispanic Black and 25% of non-Hispanic White women were uninsured. The vast majority of non-Hispanic White (82%) and Black (61%) women were comfortable using tampons, compared with 26% of Hispanic women.
Table 1.
Baseline Characteristics of 410 Participating Women
| Total (N = 410) | Hispanic (n = 119) | Non-Hispanic black (n = 115) | Non-Hispanic white (n = 154) | Other* (n = 22) | |
|---|---|---|---|---|---|
| Patient group | |||||
| Colposcopy clinic | 341 (83) | 116 (97) | 82 (71) | 125 (81) | 18 (82) |
| Research only | 69 (17) | 3 (3) | 33 (29) | 29 (19) | 4 (18) |
| Age (years), median (IQR) | 37 (31–46) | 37 (32–43) | 36 (31–50) | 36 (29–47) | 39 (34–44) |
| Marital status | |||||
| Married/living with partner | 160 (40) | 60 (53) | 21 (19) | 68 (45) | 11 (50) |
| Divorced/separated/widowed | 98 (25) | 22 (25) | 27 (25) | 35 (26) | 3 (14) |
| Single | 138 (35) | 25 (22) | 61 (56) | 44 (29) | 8 (36) |
| Missing | 14 | 6 | 6 | 2 | 0 |
| Education | |||||
| Some high school or less | 68 (17) | 53 (47) | 8 (7) | 6 (4) | 1 (5) |
| High school graduate | 90 (23) | 28 (25) | 27 (25) | 29 (19) | 6 (27) |
| Some college | 139 (35) | 24 (21) | 51 (46) | 57 (38) | 7 (32) |
| College graduate | 98 (25) | 8 (7) | 24 (22) | 58 (39) | 8 (36) |
| Missing | 15 | 6 | 5 | 4 | 0 |
| Monthly income (USD), median (IQR) | 2080 (1400–4000) | 1500 (1200–2000) | 2092 (1530–3458) | 3000 (1833–7167) | 3750 (1760–8000) |
| Unemployed | 13 | 3 | 7 | 2 | 1 |
| Missing | 64 | 27 | 20 | 15 | 2 |
| Health insurance | |||||
| Private | 153 (38) | 20 (17) | 45 (39) | 79 (52) | 9 (41) |
| Medicaid/Medicare/TRICARE | 95 (23) | 7 (6) | 45 (40) | 35 (23) | 8 (36) |
| None | 160 (39) | 92 (77) | 24 (21) | 39 (25) | 5 (23) |
| Missing | 2 | 0 | 1 | 1 | 0 |
| Number of live births, median (IQR) | 2 (1–3) | 3 (2–4) | 2 (1–3) | 1 (0–2) | 2 (0–3) |
| Missing | 8 | 3 | 3 | 2 | 0 |
| Median number of sexual partners in past 3 months (range) | 1 (0–5) | 1 (0–2) | 1 (0–5) | 1 (0–3) | 1 (0–2) |
| Missing | 5 | 4 | 1 | 0 | 0 |
| Feelings toward using tampons | |||||
| Comfortable | 93 (60) | 10 (26) | 27 (61) | 51 (82) | 5 (42) |
| Neutral | 15 (10) | 5 (13) | 4 (9) | 3 (5) | 3 (25) |
| Uncomfortable | 21 (13) | 4 (11) | 8 (18) | 7 (11) | 2 (17) |
| Never used tampons | 27 (17) | 19 (50) | 5 (11) | 1 (2) | 2 (17) |
| Missing/question not present on survey | 254 | 81 | 71 | 92 | 10 |
| Current smoker | 92 (23) | 8 (7) | 31 (27) | 46 (30) | 7 (32) |
| Missing | 6 | 4 | 1 | 1 | 0 |
Results are presented as n (%) if not otherwise stated.
Includes Asian (10), American Indian/Alaskan Native (5), Native Hawaiian/Other Pacific (1), Black/Indian (1), Irish (1), Mediterranean (1), and not further specified (3).
IQR, interquartile range; USD, US Dollars.
Acceptability of urine and cervico-vaginal sample self-collection
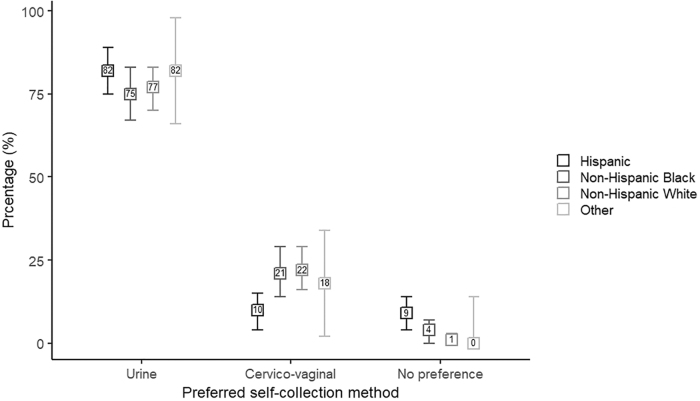
Most women (79%, 95% CI = 76%–83%) had positive feelings about urine self-collection for hrHPV testing (Table 2). The feelings toward urine self-collection did not significantly differ across racial/ethnic groups (p = 0.169) with 81% (95% CI = 74%–88%) of Hispanic women, 81% (95% CI = 74%–89%) of non-Hispanic Black women, 78% (95% CI = 72%–85%) of non-Hispanic White women, and 70% (95% CI = 50%–90%) of women with other racial identities reporting positive feelings. The percentage of women having positive feelings about cervico-vaginal brush self-collection (68%, 95% CI = 64%–73%) was significantly lower than for urine self-collection (79%, p < 0.001). Feelings about cervico-vaginal brush self-collection did not vary across racial/ethnic groups with 71% (95% CI = 63%–79%) of Hispanic women, 69% (95% CI = 60%–77%) of non-Hispanic Black women, 69% (95% CI = 61%–76%) of non-Hispanic White women, and 45% (95% CI = 25%–66%) of women with other racial identities reporting positive feelings (p = 0.213). Overall, most women preferred urine (78%, 95% CI = 74%–82%) over cervico-vaginal brush self-collection (18%, 95% CI = 14%–22%), but the degree to which women preferred urine self-collection differed by racial/ethnic group (p = 0.011). The preference for urine-based hrHPV testing ranged from 75% (95% CI = 67%–83%) among non-Hispanic Black and 77% (95% CI = 70%–83%) among non-Hispanic White to 82% in both Hispanic women (95% CI = 75%–89%) and women with other racial identities (95% CI = 66%–98%; Fig. 1).
Table 2.
Reported Acceptability of Sample Self-Collection for Human Papillomavirus-Based Cervical Cancer Screening
| Total (N = 410) | Hispanic (n = 119) | Non-Hispanic black (n = 115) | Non-Hispanic white (n = 154) | Other* (n = 22) | p-value† | |
|---|---|---|---|---|---|---|
| Overall feeling about urine self-collection | 0.169 | |||||
| Mostly positive | 79 (76%–83%) | 81 (74%–88%) | 81 (74%–89%) | 78 (72%–85%) | 70 (50%–90%) | |
| Neutral | 19 (15%–23%) | 16 (9%–23%) | 18 (11%–25%) | 22 (15%–28%) | 30 (10%–50%) | |
| Mostly negative | 1 (0%–2%) | 4 (0%–7%) | 1 (0%–3%) | 0 (0%–2%) | 0 (0%–15%) | |
| Missing (N) | 11 | 5 | 3 | 1 | 2 | |
| Overall feeling about brush self-collection | 0.212 | |||||
| Mostly positive | 68 (64%–73%) | 71 (63%–79%) | 69 (60%–77%) | 69 (61%–76%) | 45 (25%–66%) | |
| Neutral | 29 (25%–34%) | 26 (18%–34%) | 30 (21%–38%) | 29 (22%–37%) | 45 (25%–66%) | |
| Mostly negative | 2 (1%–4%) | 3 (0%–5%) | 2 (0%–4%) | 2 (0%–4%) | 9 (0%–21%) | |
| Missing (n) | 2 | 1 | 0 | 1 | 0 | |
| n = 333 | n = 108 | n = 90 | n = 115 | n = 20 | ||
| Preferred sampling method for future HPV testing | 0.029 | |||||
| Urine self-collection | 70 (64%–75%) | 70 (58%–79%) | 69 (58%–79%) | 73 (63%–80%) | 63 (39%–82%) | |
| Brush self-collection | 20 (15%–25%) | 14 (7%–24%) | 17 (10%–28%) | 25 (17%–34%) | 26 (11%–52%) | |
| Provider-based sampling | 10 (7%–14%) | 16 (9%–27%) | 13 (7%–23%) | 3 (1%–9%) | 11 (2%–36%) | |
| Missing (n) | 64 | 35 | 15 | 13 | 1 | |
| Likelihood of attending next cervical cancer screening, if test is urine based | 0.001 | |||||
| More likely | 78 (74%–83%) | 85 (77%–93%) | 85 (78%–93%) | 73 (65%–81%) | 55 (33%–77%) | |
| Neutral | 20 (15%–24%) | 13 (5%–20%) | 13 (6%–21%) | 27 (19%–35%) | 35 (14%–56%) | |
| Less likely | 2 (0%–3%) | 3 (0%–6%) | 1 (0%–3%) | 0 (0%–3%) | 10 (0%–23%) | |
| Missing (n) | 31 | 29 | 1 | 1 | 0 |
Numbers are percentages and 95% confidence intervals if not otherwise stated.
Includes Asian (10), American Indian/Alaskan Native (5), Native Hawaiian/Other Pacific (1), Black/Indian (1), Irish (1), Mediterranean (1), and not further specified (3).
†p-value for difference in responses across racial/ethnic groups based on Fisher's exact test.
FIG. 1.
Percentage of women with 95% confidence intervals preferring a certain self-collection method for high-risk human papillomavirus testing among 394 women with nonmissing responses, stratified by racial/ethnic group.
Among the 333 women asked, 70% (95% CI = 64%–75%) preferred urine self-collection for future hrHPV testing over brush self-collection (20%, 95% CI = 15%–25%) and provider-based sample collection (10%, 95% CI = 7%–14%). Across all racial/ethnic groups, urine self-collection was strongly preferred for future hrHPV testing, but the percentage of women favoring urine self-collection varied, ranging from 63% (95% CI = 39%–82%) among women with other racial identities to 73% (95% CI = 63%–80%) among non-Hispanic White women (p = 0.029). Among Hispanic and non-Hispanic Black women brush self-collection and provider-based sample collection were similarly popular for future hrHPV testing. In contrast, among non-Hispanic White women, brush self-collection (25%, 95% CI = 17%–34%) was clearly preferred over provider-based sample collection (3%, 95% CI = 1%–9%). The majority of Hispanic (85%, 95% CI = 77%–93%), non-Hispanic Black (85%, 95% CI = 78%–93%), and non-Hispanic White women (73%, 95% CI = 65%–81%) stated that they were more likely to attend the next cervical cancer screening appointment if it was based on urine self-collection. Only 2% of women reported that they would be less likely to attend cervical cancer screening if it was based on urine self-collection (Table 2).
The vast majority of women reported at least one positive aspect of urine (89%) and cervico-vaginal self-collection (85%) for hrHPV testing. Overall, the most frequently reported positive aspects were that urine and cervico-vaginal self-collection were easy to conduct (Table 3). However, whereas 64% of non-Hispanic White women stated that urine and cervico-vaginal self-collection were easy to do, this was less common among Hispanic women (37% for urine, 24% for cervico-vaginal self-collection). About half of participants reported no dislikes of the urine (54%) and the cervico-vaginal self-collection (47%) for hrHPV testing, with non-Hispanic Black women being the most likely to report no dislikes of the urine (63%) and the cervico-vaginal self-collection (57%). The most frequently reported dislikes of the urine self-collection were urine cup-related issues (18%) and the test being unhygienic (8%). Participant dislikes related to urine cups included “having to coordinate peeing in a small cup” and “trying to get it to the right [measurement] line.” More non-Hispanic White (15%) than Hispanic (3%) and non-Hispanic Black women (3%) felt that urine collection was unhygienic. The most frequently reported dislikes of the cervico-vaginal self-collection were that the sample collection was difficult to perform (19%) and uncomfortable (17%). Participants reported difficulties as “taking [the] brush off the stick’ and being “unsure if [the brush] was far enough inside.”
Table 3.
Reported Likes, Dislikes, Concerns, and Suggestions Concerning Sample Self-Collection for Human Papillomavirus-Based Cervical Cancer Screening
| Total (N = 410), n (%) | Hispanic (n = 119), n (%) | Non-Hispanic black (n = 115), n (%) | Non-Hispanic white (n = 154), n (%) | Other (n = 22), n (%) | |
|---|---|---|---|---|---|
| Likes1 | |||||
| Urine collection | |||||
| Easy to do | 215 (52) | 44 (37) | 62 (54) | 98 (64) | 11 (50) |
| Good sampling materials2 | 108 (26) | 34 (29) | 28 (25) | 39 (25) | 7 (32) |
| Quickly done | 44 (11) | 11 (9) | 13 (11) | 18 (12) | 2 (9) |
| General positive experience | 38 (9) | 17 (14) | 10 (9) | 9 (6) | 2 (9) |
| Nothing | 26 (6) | 1 (1) | 15 (13) | 10 (6) | 0 (0) |
| No response | 20 (5) | 4 (3) | 2 (2) | 13 (8) | 1 (5) |
| Brush self collection | |||||
| Easy to do | 186 (45) | 28 (24) | 51 (44) | 98 (64) | 9 (41) |
| Comfort | 63 (15) | 28 (24) | 19 (17) | 15 (10) | 1 (5) |
| Good sampling materials3 | 63 (15) | 11 (9) | 21 (18) | 27 (18) | 4 (18) |
| Privacy (Do it yourself) | 60 (15) | 19 (16) | 17 (15) | 19 (12) | 5 (23) |
| General positive experience | 32 (8) | 18 (15) | 7 (6) | 5 (3) | 2 (9) |
| Quickly done | 27 (7) | 6 (5) | 7 (6) | 13 (8) | 1 (5) |
| Nothing | 41 (10) | 14 (12) | 14 (12) | 10 (6) | 3 (14) |
| No response | 20 (5) | 10 (8) | 1 (1) | 8 (5) | 1 (5) |
| Dislikes1 | |||||
| Urine collection | |||||
| Cup-related issues4 | 74 (18) | 4 (3) | 23 (20) | 42 (27) | 5 (23) |
| Unhygienic/messy | 33 (8) | 3 (3) | 3 (3) | 23 (15) | 4 (18) |
| Urination-related issues5 | 20 (5) | 3 (3) | 6 (5) | 9 (6) | 2 (9) |
| Nothing | 222 (54) | 66 (55) | 73 (63) | 72 (47) | 11 (50) |
| No response | 69 (17) | 45 (38) | 9 (8) | 13 (8) | 2 (9) |
| Brush self collection | |||||
| Test difficult to perform | 77 (19) | 18 (15) | 22 (19) | 30 (19) | 7 (32) |
| Discomfort | 68 (17) | 24 (20) | 19 (17) | 21 (14) | 4 (18) |
| Poor sampling materials6 | 54 (13) | 9 (8) | 13 (11) | 25 (16) | 7 (32) |
| Nothing | 192 (47) | 42 (35) | 66 (57) | 77 (50) | 7 (32) |
| No response | 64 (16) | 37 (31) | 7 (6) | 17 (11) | 3 (14) |
| Concerns7 | |||||
| Urine collection | |||||
| Test result-related concerns8 | 22 (5) | 11 (9) | 3 (3) | 7 (5) | 1 (5) |
| Urination-related concerns5 | 10 (2) | 0 (0) | 5 (4) | 4 (3) | 1 (5) |
| Other concerns9 | 12 (3) | 5 (4) | 4 (3) | 3 (2) | 0 (0) |
| No concerns | 350 (85) | 93 (78) | 106 (92) | 134 (87) | 17 (77) |
| No response | 21 (5) | 12 (10) | 0 (0) | 6 (4) | 3 (14) |
| Brush self collection | |||||
| Performing sampling correctly | 64 (16) | 19 (16) | 15 (13) | 27 (18) | 3 (14) |
| Sampling material-related concerns10 | 26 (6) | 5 (4) | 5 (4) | 13 (8) | 3 (14) |
| Test result-related concerns11 | 15 (4) | 5 (4) | 4 (3) | 6 (4) | 0 (0) |
| Discomfort | 11 (3) | 5 (4) | 2 (2) | 2 (1) | 2 (9) |
| No concerns | 277 (68) | 70 (59) | 91 (79) | 103 (67) | 13 (59) |
| No response | 33 (8) | 17 (14) | 2 (2) | 12 (8) | 2 (9) |
| Suggestions7 | |||||
| Urine collection | |||||
| Improve urine collection process12 | 46 (11) | 3 (3) | 18 (16) | 23 (15) | 2 (9) |
| Improve urine cups13 | 20 (5) | 1 (1) | 5 (4) | 13 (8) | 1 (5) |
| Provide additional materials14 | 11 (3) | 2 (2) | 3 (3) | 5 (3) | 1 (5) |
| No suggestions | 268 (65) | 74 (62) | 85 (74) | 96 (62) | 13 (59) |
| No response | 63 (15) | 36 (30) | 5 (4) | 17 (11) | 5 (23) |
| Brush collection | |||||
| Improve the brush15 | 56 (14) | 12 (10) | 24 (21) | 18 (12) | 2 (9) |
| Improve instructions for use | 17 (4) | 7 (6) | 2 (2) | 7 (5) | 1 (5) |
| Improve liquid container16 | 15 (4) | 2 (2) | 2 (2) | 10 (6) | 1 (5) |
| No suggestions | 241 (59) | 66 (55) | 70 (61) | 94 (61) | 11 (50) |
| No response | 65 (16) | 28 (24) | 10 (9) | 21 (14) | 6 (27) |
All responses given by more than 5% of all participating women are shown.
Good sampling materials captures general positive responses about the aspects of the urine self-test, including the urine self-test being noninvasive and hygienic, being better than a regular pap smear, having clear directions for use, and requiring a small amount of urine to complete the test.
Good sampling materials captures the general positive responses about aspects of the brush self-test, including the brush self-test being noninvasive and hygienic, being better than a regular pap smear, and having clear directions for use.
Cup-related issues included: difficulty getting urine into the sample collection cup, difficulty measuring urine in the cup, dislike of using multiple sample collection cups, feeling that the sample collection cup was too small, and having issues switching between the two sample collection cups while giving a urine sample.
Urination-related issues and concerns included: difficulty producing the amount of urine required for the test and having to drink lots of water before giving the urine sample.
Poor sampling materials captures the general negative responses about aspects of the brush self-test, including the patients disliking sampling materials such as the gloves, the brush and the sample collection cup and believing the brush self-test was unhygienic.
All responses given by >2% of all participating women are shown.
For the urine self-test, test result-related concerns included: accuracy of the test results, privacy of test results, and fear of testing positive for HPV.
Other concerns included: hygiene of the urine test, safety of the urine test, wait times to take the test, and not having their name on sample collection materials.
Sampling material-related concerns included: accidentally dropping the test materials, accidental contamination of the sample, separating the brush from the sample collection stick, safety of the brush, hygiene of the brush, and the brush coming off the stick upon insertion into the vagina.
For the brush self-test, test result-related concerns included: accuracy of the test results, fear of testing positive for HPV, and delayed test results.
Suggestions to improve the urine collection process included: clearer instructions for using the test, reminding patients to not urinate before the test, requiring less urine, and taking off lids before giving the urine collection materials to patients.
Suggestions to improve the urine cups included: having clearer measurement lines on the cups and using a larger cup.
Suggestions to provide additional materials included: gloves, drinking water, urine funnel, and a tray cart or table in the bathroom.
Suggestions to improve the brush included: having a softer brush, making it easier to detach the brush head from the stick, having a shorter brush and insertion stick, placing a measurement marker on the stick, having a brush that does not need to be detached from the stick, and requiring less brush rotations.
Suggestions to improve the liquid container included: having a larger/wider liquid container, getting rid of the liquid container altogether, and using a different liquid.
Most participants reported no concerns related to the urine (85%) and the cervico-vaginal self-collection (68%), with non-Hispanic Black women being the most likely to report no concerns for both collection methods (Table 3). The most common concern about the cervico-vaginal self-collection was performing the test correctly (16%). Participant concerns about performing the test correctly included “ensuring brush tip does not get contaminated” and being unsure “if you collected enough specimen.” For the urine self-collection, some participants (11%) would like improvements to be made to the urine collection process, including using a “bigger collection cup” and having a “message prior to appointment [informing patients] to not urinate.” For the cervico-vaginal self-collection, some participants (14%) would like improvements made to the brush, including making the “brush a little softer” and having “a better way to remove the brush from the stick.”
Discussion
Among a racially and ethnically diverse group of 410 women recruited through colposcopy clinics in North Carolina, both urine and cervico-vaginal brush self-collection for hrHPV testing were found to be highly acceptable. However, more women had positive feelings about urine self-collection than about cervico-vaginal self-collection. Accordingly, when asked directly, most women preferred urine over cervico-vaginal self-collection, although the degree of preference varied by racial/ethnic group. Although three quarters of non-Hispanic Black and White women favored urine self-collection, this was true for more than four of five Hispanic women.
Our findings are in line with a recent systematic review and meta-analysis of 37 studies from 24 countries, which found a very high acceptability of sample self-collection for hrHPV testing—notably, urine-based hrHPV testing was not covered in this meta-analysis.32 As in our study, common reasons for preferring self-collection were ease of use, reduced embarrassment, privacy, and comfort. Both in the meta-analysis and in our study, people's uncertainty about performing the self-collection correctly was the most commonly reported reason for disliking self-collection of cervico-vaginal samples using a brush. Few studies to date have compared acceptability of urine self-collection to cervico-vaginal brush self-collection for hrHPV testing, but none of them compared results across racial/ethnic groups.14,28,29,33 A study on young screening nonattenders in Scotland receiving a self-collection kit in the postal mail found that women were slightly more likely to return urine samples than self-collected vaginal samples (odds ratio = 1.18, 95% CI = 1.00–1.38).33 However, in this study no questionnaires or interviews were used to further investigate acceptability of sample self-collection. Among colposcopy patients in Ontario, Canada, and North Carolina, urine and cervico-vaginal sample self-collection for hrHPV testing was highly acceptable, and as in our study, most women (>78%) preferred urine over other self-collection methods.14,28 The preference for urine-based hrHPV testing is further corroborated by a recent study among colposcopy patients from England.29 Nearly all women included in that study felt confident providing a urine sample for hrHPV testing, and the majority of them would be happy to provide only urine samples for hrHPV testing in the future.
To our knowledge, this is the first study to compare acceptability of different self-collection strategies for hrHPV-based cervical cancer screening across racial/ethnic groups. We found that among all racial/ethnic groups both urine and cervico-vaginal brush self-collection were highly acceptable; however, for future cervical cancer screening, significantly more Hispanic women (16%) than non-Hispanic White women (3%) still preferred provider-based sample collection (p = 0.029). This is in line with findings from a previous US study showing that non-Hispanic women were more likely to prefer self-collection over provider collection than Hispanic women.36 The same study also found higher education to be positively associated with preference for sample self-collection.36 In our study population, only 7% of Hispanic women, but 22% of non-Hispanic Black and 39% of non-Hispanic White women were college graduates. Thus, education level could partly explain why more Hispanic than non-Hispanic White women preferred provider-based sample collection for future hrHPV testing. When asked to choose a self-collection method, more Hispanic women (82%) favored urine self-collection compared with 75% of non-Hispanic Black and 77% of non-Hispanic White women. This might be related to the finding that 50% of Hispanic women in our study had never used tampons, whereas 89% of non-Hispanic Black and nearly all non-Hispanic White women had used tampons in the past. Women who have never used a tampon may be more nervous and reluctant to use a device to self-collect a cervico-vaginal sample than women who are used to inserting tampons and may therefore favor urine self-collection. A study among female adolescents found that tampon use in the past was positively associated with perceived comfort of cervico-vaginal sample self-collection.26
A main strength of our study is that we included a racially and ethnically diverse study population and compared acceptability of sample self-collection for hrHPV-based cervical cancer screening across different racial/ethnic groups. Furthermore, selection bias was reduced as almost all women who were otherwise eligible for inclusion in this study filled the acceptability questionnaire (only one woman did not and was excluded for that reason). A limitation of our study is that women's written responses tended to be brief and simple. Individual interviews or focus groups will be necessary to gain more in-depth insights into women's likes, dislikes, and concerns regarding urine-based hrHPV testing, and to further explore preferences for specific self-collection methods across racial/ethnic groups. Our study population consisted of a convenience sample of women at increased risk for cervical disease who either were scheduled for a colposcopy appointment owing to abnormal screening results or were willing to undergo colposcopy for study purposes. Thus, the attitudes toward sample self-collection observed in our study population might not be generalizable to a primary screening population. Future studies should assess acceptability of sample self-collection across different racial/ethnic groups among primary screening populations.
Recent studies have found urine-based hrHPV testing to be reasonably sensitive for the detection of high-grade cervical intraepithelial neoplasia.14,17,21–24 However, for a screening method to be effective, it has to be both diagnostically accurate and acceptable to the target population. Cervical cancer incidence rates in the United States are higher among ethnic and racial minorities compared with White women.3 We found sample self-collection for hrHPV testing to be highly acceptable among all racial/ethnic groups included in our study, and most women preferred urine to cervico-vaginal self-collection, although the degree of preference varied across racial/ethnic groups. Understanding acceptability of sample self-collection across different racial/ethnic and sociodemographic groups is important to be able to offer targeted screening approaches that are highly effective within a specific group, with the ultimate goal of reducing cervical cancer-related health disparities.
Conclusions
Urine-based hrHPV testing for cervical cancer screening is highly acceptable to women across different racial and ethnic groups in the United States, and most women included in our study stated that they would be more likely to attend future cervical cancer screening appointments if screening were urine based. Urine-based hrHPV testing is a promising approach to improve cervical cancer screening coverage among underscreened women.
Acknowledgments
The authors thank all women who participated in this research project. The authors also thank Johana Bravo, Elena DiRosa, and Sara Smith for the day-to-day coordination of this study including the recruitment of participants and data collection. Furthermore, the authors thank the UNC Center for AIDS Research HIV/STD Laboratory Core for processing, storage, and clinical trial support.
Authors' Contribution
J.S. Smith, L. Rahangdale, L.S. Romocki, and V. Sivaraman designed the study. L. Rahangdale, A. Knittel, J.W. Schmitt, K. Miele, A. Baker, and C. Edelman were involved in the patient recruitment and data collection. J.A.E. Nelson led the laboratory sample processing, storage and shipment for HPV testing. F.H. McGuire and S.A. Desai coded the open-ended questions. Y. Liu, Q. Li, F.H. McGuire, and E. Rohner performed the statistical analyses. E. Rohner, F.H. McGuire and J.S. Smith wrote the first draft of the manuscript. All authors read and commented on the manuscript and approved the final version.
IRB Status
Approved, UNC IRB# 15–2872 and Duke IRB# Pro00083075.
Author Disclosure Statement
J.S. Smith has received research grants, supply donations, and consultancies; served on paid advisory boards; and/or been a paid speaker for Arbor Vita, Becton Dickenson Corporation, Hologic, Rovers Medical Devices, and Trovagene in the past 5 years. Julie A.E. Nelson has received financial support from Hologic for work on a different study.
Funding Information
This research was supported by National Institutes of Health (NIH) grant U54 CA156733 to J.S. Smith and the University of North Carolina UNC at Chapel Hill Center for AIDS Research (CFAR), a National Institutes of Health (NIH)-funded program (P30 AI50410). E. Rohner was supported by a grant from the Swiss Cancer Research foundation (BIL KFS-4423-02-2018). Self-collection brushes were donated by Rovers.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–1953 [DOI] [PubMed] [Google Scholar]
- 2. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer. JAMA 2018;320:674. [DOI] [PubMed] [Google Scholar]
- 3. Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017;123:1044–1050 [DOI] [PubMed] [Google Scholar]
- 4. Andrae B, Kemetli L, Sparen P, et al. Screening-Preventable Cervical Cancer Risks: Evidence From a Nationwide Audit in Sweden. JNCI J Natl Cancer Inst 2008;100:622–629 [DOI] [PubMed] [Google Scholar]
- 5. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: Systematic review and meta-analysis. Prev Med (Baltim) 2007;45:93–106 [DOI] [PubMed] [Google Scholar]
- 6. Pruitt SL, Werner CL, Borton EK, et al. Cervical Cancer Burden and Opportunities for Prevention in a Safety-net Healthcare System. Cancer Epidemiol Biomarkers Prev 2018;27:1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ackerson K, Gretebeck K. Factors influencing cancer screening practices of underserved women. J Am Acad Nurse Pract 2007;19:591–601 [DOI] [PubMed] [Google Scholar]
- 8. Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Community Health 2017;42:770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan DNS, So WKW. A systematic review of the factors influencing ethnic minority women's cervical cancer screening behavior. Cancer Nurs 2017;40:E1–E30 [DOI] [PubMed] [Google Scholar]
- 10. Musselwhite LW, Oliveira CM, Kwaramba T, et al. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta Cytol 2016;60:518–526 [DOI] [PubMed] [Google Scholar]
- 11. Thompson B, Vilchis H, Moran C, Copeland W, Holte S, Duggan C. Increasing cervical cancer screening in the United States-Mexico Border Region. J Rural Heal 2014;30:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brien MJ, Halbert CH, Bixby R, Pimentel S, Shea JA. Community health worker intervention to decrease cervical cancer disparities in Hispanic women. J Gen Intern Med 2010;25:1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marlow LAV, Waller J, Wardle J. Barriers to cervical cancer screening among ethnic minority women: A qualitative study. J Fam Plan Reprod Heal Care 2015;41:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senkomago V, Des Marais AC, Rahangdale L, Vibat CRT, Erlander MG, Smith JS. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J Clin Virol 2016;74:26–31 [DOI] [PubMed] [Google Scholar]
- 15. Des Marais AC, Zhao Y, Hobbs MM, et al. Home self-collection by mail to test for human papillomavirus and sexually transmitted infections. Obstet Gynecol 2018;132:1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asciutto KC, Ernstson A, Forslund O, Borgfeldt C. Self-sampling with HPV mRNA analyses from vagina and urine compared with cervical samples. J Clin Virol 2018;101:69–73 [DOI] [PubMed] [Google Scholar]
- 17. Sahasrabuddhe VV, Gravitt PE, Dunn ST, et al. Evaluation of clinical performance of a novel urine-based HPV detection assay among women attending a colposcopy clinic. J Clin Virol 2014;60:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snijders PJF, Verhoef VMJ, Arbyn M, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer 2013;132:2223–2236 [DOI] [PubMed] [Google Scholar]
- 19. Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: A Systemic Review and Meta-Analysis. PLoS One 2015;10:e0132776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on Self-Sampling HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018;363:k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lorenzi AT, Fregnani JHT, Dockter J, et al. High-risk human papillomavirus detection in urine samples from a referral population with cervical biopsy-proven high-grade lesions. J Low Genit Tract Dis 2018;22:17–20 [DOI] [PubMed] [Google Scholar]
- 22. Leeman A, del Pino M, Molijn A, et al. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: Cross-sectional data from a triage population. BJOG An Int J Obstet Gynaecol 2017;124:1356–1363 [DOI] [PubMed] [Google Scholar]
- 23. Cuzick J, Cadman L, Ahmad AS, et al. Performance and diagnostic accuracy of a urine-based human papillomavirus assay in a referral population. Cancer Epidemiol Biomarkers Prev 2017;26:1053–1059 [DOI] [PubMed] [Google Scholar]
- 24. Bernal S, Palomares JC, Artura A, et al. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the Cobas 4800 HPV test. J Clin Virol 2014;61:548–552 [DOI] [PubMed] [Google Scholar]
- 25. Hoebe CJPA, Rademaker CW, Brouwers EEHG, ter Waarbeek HLG, van Bergen JEAM. Acceptability of Self-Taken Vaginal Swabs and First-Catch Urine Samples for the Diagnosis of Urogenital Chlamydia trachomatis and Neisseria gonorrhoeae With an Amplified DNA Assay in Young Women Attending a Public Health Sexually Transmitted Disease Clinic. Sex Transm Dis 2006;33:491–495 [DOI] [PubMed] [Google Scholar]
- 26. Kahn JA, Bernstein DI, Rosenthal SL, et al. Acceptability of human papillomavirus self testing in female adolescents. Sex Transm Infect 2005;81:408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serlin M, Shafer M-A, Tebb K, et al. What sexually transmitted disease screening method does the adolescent prefer? Adolescents' attitudes toward first-void urine, self-collected vaginal swab, and pelvic examination. Arch Pediatr Adolesc Med 2002;156:588–591 [DOI] [PubMed] [Google Scholar]
- 28. Sellors JW, Lorincz AT, Mahony JB, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 2000;163:513–518 [PMC free article] [PubMed] [Google Scholar]
- 29. Sargent A, Fletcher S, Bray K, Kitchener HC, Crosbie EJ. Cross-sectional study of HPV testing in self-sampled urine and comparison with matched vaginal and cervical samples in women attending colposcopy for the management of abnormal cervical screening. BMJ Open 2019;9:e025388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson C, Breithaupt L, Des Marais A, et al. Acceptability and ease of use of mailed HPV self-collection among infrequently screened women in North Carolina. Sex Transm Infect 2018;94:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbee L, Kobetz E, Menard J, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control 2010;21:421–431 [DOI] [PubMed] [Google Scholar]
- 32. Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex Transm Infect 2017;93:56–61 [DOI] [PubMed] [Google Scholar]
- 33. Sinka K, Lacey M, Robertson C, et al. Acceptability and response to a postal survey using self-taken samples for HPV vaccine impact monitoring. Sex Transm Infect 2011;87:548–552 [DOI] [PubMed] [Google Scholar]
- 34. Rohner E, Rahangdale L, Sanusi B, et al. Test accuracy of human papillomavirus in urine for detection of cervical intra-epithelial neoplasia. J Clin Microbiol 2020;58(3). pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 2012;62:147–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Womens Health (Larchmt) 2005;14:721–728 [DOI] [PubMed] [Google Scholar]