Abstract
Extended travel in deep space poses potential hazards to the reproductive function of female and male astronauts. These hazards include exposure to cosmic radiation, microgravity, increased gravity, psychological and physical stress, and circadian rhythm disruptions. This Review focuses on the effects of microgravity, hypergravity and cosmic radiation. Cosmic radiation contains protons and helium nuclei, as well as high charge and energy (HZE) particles. Studies performed on Earth in which rodents were exposed to experimentally generated HZE particles have demonstrated high sensitivity of ovarian follicles and spermatogenic cells to HZE particles. Exposure to microgravity during space flight and to simulated microgravity on Earth disrupts spermatogenesis and testicular testosterone synthesis in rodents, whereas the male reproductive system seems to adapt to exposure to moderate hypergravity. A few studies have investigated the effects of microgravity on female reproduction, and findings of disrupted oestrous cycling and in vitro follicle development are cause for concern. Many remaining data gaps need to be addressed, including the effects of microgravity, hypergravity and space radiation on the male and female reproductive tracts, hypothalamic–pituitary regulation of reproduction and prenatal development of the reproductive system, as well as the combined effects of the multiple reproductive hazards encountered in space.
Introduction
Although more than 530 men and women have travelled to space since 1961, all but the 24 men who orbited or landed on the Moon have been in low Earth orbit. In 2013, the US National Aeronautics and Space Administration (NASA) announced plans for a human mission to Mars; it is estimated that this mission will take 2–3 years. Space travel poses multiple potential hazards to reproductive health, including exposure to ionizing radiation, microgravity, hypergravity, psychological stress, physical stress and circadian rhythm disruption. Astronauts are exposed to microgravity, stress and disruption of circadian rhythms whether travelling in low Earth orbit or in deep space, with exposure to hypergravity occurring on launch and re-entry. However, low Earth orbit and deep space travel differ in terms of exposure to ionizing radiation. This article reviews the effects of space flight in low Earth orbit on reproductive endpoints in humans and experimental animals, as well as effects of simulated microgravity, hypergravity and experimentally generated space radiation on reproductive endpoints. Control of reproduction by circadian rhythms, effects of circadian rhythm disruption and effects of stress on reproductive endpoints are reviewed elsewhere1–4 and so are not discussed here.
Overview of space travel hazards
Space radiation.
On Earth, humans are primarily exposed to low linear energy transfer (LET) photon radiation (that is, X-rays and γ-rays with LET <10 keV/μm), whereas the radiation field in space contains electrons, protons (hydrogen nuclei), neutrons, alpha particles (helium nuclei) and heavy nuclei with very high energies (high charge and energy (HZE) particles) and LET greater than 10 keV/μm 5. Cosmic radiation includes galactic cosmic rays and solar particle events. Exposure to ionizing radiation during space travel cannot be avoided by shielding due to the high energies and secondary radiations produced when primary radiation interacts with materials5. On Earth, we are protected from cosmic radiation by the Earth’s magnetic field and atmospheric shield5. In low Earth orbit, astronauts are still protected by the Earth’s magnetic field; however, once outside the Earth’s magnetic field in deep space, space travellers are exposed to galactic cosmic rays and solar particle events. Solar particle events consist mostly of protons and electrons, with small amounts of helium and heavy ions, which are ejected from the Sun during solar eruptions. Galactic cosmic rays consist of approximately 85% protons, 14% alpha particles, 1% heavier nuclei and 2% electrons5; however, heavy nuclei make up 21% of the ionizing dose equivalent of galactic cosmic rays because the absorbed dose is proportional to the square of the charge5,6. During interplanetary travel, such as a Mars mission, astronauts will also be exposed to radiation belts containing charged particles trapped by the planet’s magnetic field5. The estimated cumulative dose of radiation from a 3-year long Mars mission is 0.4 Gy7.
About one-third of the DNA damage caused by ionizing radiation is estimated to be caused by direct ionization of cellular macromolecules, while the remaining two-thirds is due to generation of reactive oxygen species (ROS) via ionization of water8,9. Exposure to low LET radiation results in sparsely distributed ionization within cells, while high LET radiation exposure results in dense ionization10,11. If the HZE particle traverses a cell nucleus, this can cause complex, clustered DNA damage, which is not easily repaired by the cell12. Moreover, exposure to ionizing radiation causes biological changes within the cell that result in chronically increased generation of ROS12, which can result in oxidative stress (a state of imbalance between prooxidants and antioxidants within tissues). Oxidative stress has been mechanistically implicated in reproductive disorders, including premature ovarian failure and preeclampsia13–15. The biological effects of charged particles depend on radiation dose, dose rate, particle charge, LET (amount of energy transferred from the charged particle as it traverses a cell or tissue per unit of path length) and kinetic energy of the particle5. In this Review, LET is expressed in units of keV/μm and energy is expressed in units of MeV or GeV per atomic mass unit (u).
Microgravity and hypergravity.
Humans evolved in the 1 g gravitational field on Earth, whereas the space environment entails exposures to microgravity within spacecrafts (10−3–10−6 g), zero gravity in space and exposure to hypergravity during re-entry. Exposure to microgravity causes numerous physiological effects, including altered fluid and electrolyte balance, cardiovascular changes, decreased bone mineral density, muscle wasting, increased percentage body fat, insulin resistance, sensorimotor changes (including in vision and vestibular function), changes in lung capacity, increased glomerular filtration rate and decreased sweating13,16–20. Cardiovascular effects include decreased left ventricular volume, decreased blood pressure and increased prevalence of cardiac arrhythmias16. Examples of lung capacity changes include vital capacity decreases initially during microgravity, then returns to preflight values, while residual capacity is decreased throughout exposure to microgravity21. Increased oxidative stress has been implicated in some of the effects of microgravity, including the effects on female reproduction13.
As a result of the limited opportunities for experiments on-orbit in true microgravity, as well as confounding on-orbit exposures (such as radiation, circadian rhythm disruption, stress and high g-forces during re-entry), several methods have been developed to simulate microgravity. These fall predominantly into three categories: randomization of the direction of gravity over time (using clinostat or a random positioning machine); creating a counteracting force (magnetic levitation); hind-limb suspension22,23. The first two methods have been used with unicellular organisms, plants, Drosophila, zebrafish and mammalian cell cultures22. The hind-limb suspension model involves suspending rodents by the tail or in a whole-body harness in a 30° head down position, while in humans bed rest with a 6° head-down tilt has been used to simulate the major physiological effects of microgravity, including fluid shifts and lack of weight bearing23. Hypergravity has been simulated with acute centrifugation to model effects of launch and re-entry23. Long-term centrifugation has been paired with ground-based microgravity simulation to study the effects of gravity as a continuum from microgravity to hypergravity23. Centrifugation is also used in on-orbit experiments to provide normal gravity in the setting of exposure to other aspects of the space environment.
Female reproductive effects
The female reproductive system is regulated by a range of hormones (Box 1). The system can be affected by space flight in a range of ways, which will be discussed here.
Box 1 |. Regulation of female reproduction.
Folliculogenesis and the synthesis of sex steroids in the ovary are regulated by the gonadotropin hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH), which are secreted by the pituitary gland in response to gonadotropin releasing hormone (GnRH) from the hypothalamus109. GnRH, LH and FSH are in turn under feedback control by ovarian hormones109. The ovarian germ cells (called oocytes) are formed, enter meiosis, arrest in the first prophase of meiosis and become enclosed in somatic cells to form primordial follicles during prenatal (primates) or early postnatal (rodents) development in female mammals110. Therefore, female mammals are born with a finite complement of oocytes, which constitute the ovarian reserve. Recruitment of primordial follicles into the growing pool occurs throughout life in a gondadotropin-independent manner, while LH and FSH regulate the later stages of follicular development (secondary, antral and preovulatory)111,112. Antral and preovulatory follicles synthesize increasing amounts of oestradiol, which through negative feedback maintains low levels of LH and FSH secretion. Once oestradiol concentration surpasses a threshold, it becomes a positive stimulus, increasing GnRH release, which triggers the LH and FSH surges that cause ovulation1. The somatic granulosa and theca cells of the ovulated follicle then undergo a process called luteinization to become the corpus luteum, which synthesizes progesterone to prepare the uterus for implantation should conception occur113.
Effects of space flight on female reproduction.
A major strength of space flight experiments is that they expose animals or cultured cells and tissues to the multiple potential hazards actually experienced by humans during space travel, including microgravity, hypergravity, circadian rhythm disruption and stress. However, exposure to multiple potential hazards simultaneously is also a disadvantage of space flight experiments because it makes it difficult to sort out the relative contributions of the various exposures. In addition, space flight experiments have been conducted in low Earth orbit, where radiation exposures are low compared with in deep space. For these reasons, ground-based experiments will continue to be required to complement space flight experiments.
Female astronauts often suppress their menstrual cycles during space flight, particularly during longer missions, most commonly using combined oral contraceptives containing an oestrogen and a progestin24,25. Female astronauts often delay childbearing until they have completed several space flights, and the mean age at first space flight is 38 for female astronauts26. Therefore, it is not surprising that use of assisted reproductive technology (ART) is common in female astronauts after space flight due to advanced maternal age24. Success rates for ART in female astronauts reportedly do not differ from those for similarly aged women not exposed to space flight24. The same paper reported that the mean age of female astronauts who had pregnancies ending in live birth after space flight was 40 years, while the mean age for those whose pregnancies ended in spontaneous abortions was 41 years24; however, the numbers were too small to determine whether the risk of spontaneous abortions is increased after space flight. To our knowledge no studies have investigated other reproductive endpoints in female astronauts during or after flight.
Reproductive endpoints have been investigated after low Earth orbit in invertebrates, amphibians and fish. These studies showed that fertilization and embryonic development can occur in the space environment, but various subtle abnormalities have been noted in sea urchins (Lytechinus pictus), frogs (Xenopus laevis) and newts (Pleurodeles waltl)27–29. Abnormalities included decreased cortical granule discharge and fusion during fertilization in sea urchins and thicker blastocoel roof in frogs. Four medaka fish (Oryzias latipes) were the first vertebrates to successfully mate in space during a 15 day mission aboard a Space Shuttle; they laid 43 eggs, from which 8 fry hatched in space and another 30 fry hatched after landing30. Examination of the ovaries of fry hatched in space revealed normal numbers of germ cells, and fry hatched in space produced offspring after re-entry30.
On-orbit experiments that have investigated mammalian female reproduction are summarized in Table 1. Of note, duration of time in space in all of these studies was fairly short, lasting up to 15 days. To our knowledge only one study has investigated the effect of space flight on oestrous cycling. Oestrous cycling stopped in female mice during space flight and the ovaries lacked corpora lutea, which is consistent with cessation of oestrous cycles26.
Table 1:
Effects of space flight and simulated gravity on female reproduction
| Model and study details | Hormones* | Oestrous cycles | Ovarian or uterine phenotypes | Gestation | Pregnancy outcome | Ref |
|---|---|---|---|---|---|---|
| Space flight | ||||||
| Mice | ND | Acyclic | No ovulation and CL; ↓uterine oestrogen receptor expression |
ND | ND | 26 |
| Male and female rats allowed to mate during flight; females examined after flight for signs of pregnancy (COSMOS-1129 18.5 day flight) | ND | ND | ND | ND | 2 of 5 females showed signs of early gestation and subsequent pregnancy loss | 31 |
| Pregnant rats flown GD 13–18, some euthanized on landing, some allowed to give birth; vivarium and synchronous controls | ND | ND | Decreased placental weights in flight group | Lengthened labour in flight group | NS litter size on GD18 or at birth; NS fetal weight GD18; ↓birth weight; ↑ perinatal mortality |
32 |
| Pregnant rats, GD 9–20 in space, hemi-hysterectomy then parturition after return to Earth; synchronous and vivarium controls (NIH.R1 mission) | ↑FSH ↓pituitary LH NS LH, P4, pituitary FSH |
ND | NS ovarian, pituitary weights, # antral follicles, # corpora lutea | NS gestation length, maternal weight gain, duration of parturition; ↑lordosis contractions, but not vertical contractions during labour | NS litter size; ↓birth weight; ↑ perinatal mortality |
33–35 |
| Pregnant rats, GD 10–20 in space, parturition after return to Earth; synchronous and vivarium controls (NIH.R2 mission) | ND | ND | ↓myometrial connexin 43 at term; edematous myometrium on GD20, not postpartum; ↓myometrial volume between GD 20 and postpartum in flight rats only |
NS gestation length, maternal weight gain, duration of parturition, fetal mass at GD 20; ↑increase in lordosis contractions, but not vertical contractions during labour |
↓birth weight37; NS birth weight35 | 35–37 |
| Simulated microgravity | ||||||
| Rats, HLS for 38–42 days, euthanized on oestrus | ↓ E2 NS P4, LH, FSH |
↑cycle length; ↓oestrus; ↑diestrus |
NS ovarian and uterine weights | ND | ND | 44 |
| Simulated hypergravity | ||||||
| Rats, 1.5, 2.3, or 3.1g by centrifugation | ↑P4 during lengthened diestrus; ↑prolactin surge if hypergravity exposure >8 h during diestrus |
Lengthened diestrus for 10–16 days, followed by cycle normalization; lengthened diestrus inhibited by bromo-ergocriptine (inhibitor of prolactin) | ND | ND | ND | 49,51,52 |
| SD rats (2.5, 3.6 and 4.5g) and Swiss-Webster mice (2.5g) males and females acclimated to centrifugation 2 months before mating; pregnancy determined by female weight gain | ND | ND | ND | Dose-dependent decrease in pregnancy rates in rats | ↑ neonatal mortality (no surviving offspring in rats centrifuged continuously); neonatal mice survived continuous centrifugation | 47 |
| Male and female mice exposed to 1, 2.3, 2.6, 2.9, or 3.5 g 8 weeks before mating and through GD18 (females) | ND | ND | ND | NS resorptions | NS litter size; ↓ fetal weights and crown rump lengths; ↓ skeletal development (size and gross morphology of bones altered |
48 |
| Pregnant SD rats (primigravid and bigravid) 1, 1.5g by centrifugation GD11 to postpartum day 3. | ND | ND | ND | ND | ↓birth weight ↑ neonatal mortality |
46 |
| Pregnant SD rats 1g or 2g by centrifugation GD11-postpartum day 3. Pairfed (with 2g rats) and ad libitum controls at 1g | ND | ND | ND | ND | ↓birth weight ↑ neonatal mortality in 2g, but not in pairfed or ad libitum controls; ↓mammary gland glucose oxidation and lipogenesis in 2g |
53 |
All hormone concentrations are in serum unless otherwise noted.
Abbreviations: CL: corpora lutea; E2: estradiol; GD: gestational day; HLS: hind-limb suspension; NS: not statistically significant; ND: not determined; P4: progesterone
The first studies to examine the effects of space flight on mammalian mating and pregnancy were carried out in unmanned biosatellites. Male and female rats were flown for 18.5 days on the COSMOS-1129 biosatellite mission. The males and females were initially separated; the separator was removed during the mission, allowing the rats to mate, but mating was not monitored. After landing, it was reported that 2 of 5 female rats showed signs of early pregnancy with resorption, while it could not be determined whether the rats without signs of pregnancy had oestrous cycle disturbances with lack of ovulation or problems related to mating, fertilization or implantation31. Pregnant female rats launched on gestation day (GD) 13 and landed on GD 18 on the COSMOS-1514 biosatellite gained only 8% as much weight as vivarium controls and synchronous controls housed in a biosatellite mockup despite similar food consumption. However, on re-entry, the flight group demonstrated rapid catch up weight gain and similar litter sizes as ground controls, but decreased fetal and placental weights and delayed fetal skeletal development32. Of the five flight females that were allowed to give birth, four delivered live litters and one delivered a stillborn litter; all had lengthened labour32. Litters from flight mothers had increased mortality during the first postnatal week. Male and female pups that survived to adulthood were fertile, and the females delivered apparently normal offspring32.
In studies of the effects of space flight on pregnancy, Sprague Dawley (SD) rats were launched into orbit from the Kennedy Space Center on GD 9 (study NIH.R1) or GD 11 (NIH.R2) and they were landed on day GD 20. The NIH.R1 rats were anesthetized 3 h after landing for collection of one uterine horn, after which the rats were allowed to recover and deliver the pups in the other uterine horn vaginally. Dams were euthanized 3 h after delivery, and pups were cross-fostered to non-space flight dams33,34. Space flight between GD 9 and GD 20 (NIH.R1) did not alter maternal weight gain during pregnancy, duration of pregnancy, duration of parturition, litter size, ovarian or pituitary weights, numbers of healthy or atretic ovarian antral follicles, number of corpora lutea, serum concentrations of progesterone or LH or levels of FSH in the pituitary compared with synchronous flight controls (similar in all aspects except no flight), no surgery vivarium controls and unilateral hysterectomy vivarium controls33. Serum levels of FSH were increased compared with those of vivarium controls only and levels of LH in the pituitary were decreased compared with synchronous and vivarium hysterectomy controls33. Space flight litters had similar birth weights compared with all control groups when weights for female and male pups were analyzed separately34. During labour, the flight dams had about twice as many lordosis contractions, which occur before the birth of the first pup, than synchronous controls, while the number of vertical contractions, which occur just before the expulsion of the pup from the vagina, did not differ35.
In the NIH.R2 study35–37, 4 of 10 dams per group were euthanized 3h after landing on GD 20, while the remaining 6 were allowed to give birth (GD 22–23) and were euthanized 3h after delivery36,37. Fetal mass at GD 20 did not differ between the groups37. Maternal weight gain from GD 11 to GD 20 differed among the groups, with lower weight gain in the flight group than in the synchronous and no surgery vivarium controls (percent maternal weight gain for NIH.R2 dams was reported differently in two different publications: 34.1±1.6% and 46.5±1.9% increases in weight for flight and synchronous controls, respectively,37 and 23.8±1.0% and 28.6±1.0% increases for flight and synchronous controls, respectively35). For flight and synchronous controls, Burden and colleagues37 reported birth weights of 5.6±0.1 g and 6.2±0.1 g, respectively, and Ronca and Alberts35 reported birth weights of 5.9±0.4 g and 5.8±0.4 g, respectively. Burden and colleagues reported that the birth weights were statistically significantly lower for the flight group than for all three control groups37. Pregnancy duration, litter size at birth and maternal care of pups during parturition were not affected by space flight35,37.
The effects of space flight on labour contractions were nearly identical to those in the NIH.R1 study, with increased frequency of lordosis contractions and no effect on vertical contractions35 On the basis of videos of maternal behaviour during space flight in both studies the authors concluded that female rats exercised the external obliques (which are involved in vertical contractions) during rolling movements, but that the transverse abdominal muscles (involved in lordosis contractions), which are needed for postural control under gravity, were not exercised during flight. Histological analyses of abdominal muscles were consistent with these observations, displaying atrophy of the transverse abdominal muscles and hypertrophy of the external obliques38.
In Burden and colleagues’ study uterine histology on GD 20 from the flight group compared with histology from the synchronous control group (the other control groups were not examined) appeared normal, with the exception of oedema in the circular (inner) layer of myometrium in the flight group; however, this change was no longer apparent at 3h postpartum37. Myometrial muscle volume and nuclear density in both myometrial layers on GD 20 or postpartum were not statistically significantly different between the flight and synchronous control groups37. A statistically significant decrease was found in myometrial volume between GD 20 and postpartum in the flight group, but not in the synchronous control group, which the authors attributed to resolution of the oedema noted at GD 2037. Under normal conditions, the number of uterine myometrial gap junctions increases at term, which enables electrical coupling of myometrial cells that is important for normal labour contractions. Immunostaining for the major myometrial gap junction protein connexin 43 was decreased postpartum in the flight group compared with all control groups, while no effects were observed on the endometrial or myometrial gap junction protein connexin 26 immunostaining36.
Taken together, the results from the COSMOS-1514, NIH.R1 and NIH.R2 studies show that exposure to low Earth orbit during mid to late gestation does not cause major disruptions in fetal development or parturition. Inconsistent effects have been noted on maternal weight gain, overall duration of labour and neonatal weights33,35–37, while increased lordotic contractions during labour were consistently observed in both the NIH.R1 and NIH.R2 studies35. The increased number of lordotic contractions is associated with decreased uterine expression of connexin 43, which is important for synchronization and coordination of labour contractions, and with atrophy of the transverse abdominal muscles, which might be compensated for by the increased number of lordosis contractions. The COSMOS-1129 study showed that mammalian mating and pregnancy onset are possible in space, but causes of pregnancy loss were not identified. Studies with a longer duration of exposure to space flight are needed to investigate the effects of space flight on the entire process of ovarian folliculogenesis (which is estimated to take about 50 days in rodents and more than 6 months in humans39,40) to examine effects on oestrous cycling and hypothalamic–pituitary–ovarian axis function, as well as on fertilization, implantation and pregnancy.
Effects of simulated microgravity and hypergravity on female reproduction.
Several studies41–43 have examined the effects of simulated microgravity on cultured ovarian fragments, ovarian follicles or isolated luteal cells (Table 2), while to our knowledge only one study44 has tested the effects of simulated microgravity (using hind-limb suspension) on female reproductive endpoints (Table 1). Several studies have described the effects of simulated hypergravity on female reproductive endpoints (Table 1)45–53.
Table 2:
Effects on female reproductive endpoints in vitro
| Model | In vitro effects | Pregnancy outcome | Ref |
|---|---|---|---|
| RWV versus static controls, alginate encapsulated isolated cultured secondary follicles or ovarian fragments | Decreased proliferation of granulosa cells and oocyte GDF9 in fragments; growth of secondary follicle oocytes in alginate, but abnormal oocyte ultrastructure | ND | 41 |
| RCCS with high aspect ratio culture vessels versus static controls, mouse GV oocytes matured in vitro | Inhibited oocyte maturation, abnormal meiotic spindles with abnormal tubulin localization in both meiosis I and II | ND | 55 |
| Mouse oocytes and sperm IVF and cultured to blastocyst stage in 3D clinostat versus static 1g controls | Minimal effect fertilization rate NS development to 2-cell stage at 24h; ↓development to blastocyst at 96h |
↓Birth rate of implanted 2-cell embryos and blastocysts | 57 |
| RCCS with high aspect ratio culture vessels and rotation and static culture control groups; 2-cell (1.5 dpc), 8-cell (2.5 dpc), and morula or early blastocyst (3.5 dpc) embryos harvested from mice and cultured for 24 h in simulated microgravity, then for 48 h in static culture. Static culture control group | 2-cell and 8-cell arrested development at 24h and 70% and 100% death by 72h, respectively; 40% of 3.5 dpc embryos progressed to late blastocyst stage; ↑SAPK phosphorylation after 24h culture of 3.5 dpc embryos; rotational controls had similar but less severe effects |
ND | 58 |
| High aspect ratio vessel RCCS with spinner flask rotational controls or clinostat with static controls; Cultured luteal cells from corpora lutea of pregnant rats |
↓progesterone secretion both microgravity groups; ↑increased DNA damage, apoptosis, ↓active mitochondria in clinostat group |
ND | 42,43 |
Abbreviations: dpc: days post coitum; GV: germinal vesicle stage prior to completion of first meiotic division; IVF: in vitro fertilization; ND: not determined; NS: not statistically significant; RWV: rotating wall vessel; RCCS: rotary cell culture system
Normally cycling female SD rats underwent hind-limb suspension or were housed in the same cage type and allowed to ambulate freely for 38–42 days beginning at 77 days of age until euthanasia on the day of vaginal oestrus44. The rats were fed the AIN-93G purified diet or the Harlan Teklad 8728C diet, which contains phytoestrogen-rich soy meal. Phytoestrogens are compounds naturally present in soy meal that have oestrogenic and antioestrogenic activity54. Hind-limb suspension resulted in lengthened oestrous cycles characterised by more time spent in the diestrous stage and less time spent in the oestrous stage. In addition, hind-limb suspension was associated with decreased serum concentrations of oestradiol, no effect on serum or pituitary levels of LH and FSH or serum levels of progesterone, no effect on ovarian or uterine weights and decreased absolute pituitary weight. All of these effects were more pronounced or only occurred (that is, time in oestrus and pituitary weight) in the AIN-93G diet groups. As a result of other differences in the two diets in addition to the phytoestrogen content (such as higher caloric density and cornstarch content of the AIN-93G diet and different sources of protein), the effects of diet could not be definitively attributed to effects of the phytoestrogen in the diet containing soy meal.
Ovarian secondary follicle development in cultured half ovaries from 14-day old Institute of Cancer Research (ICR) mice was disrupted by exposure to microgravity simulated using a rotating wall vessel for 2 or 4 days. The authors reported decreased numbers of healthy secondary follicles per unit area, absent proliferation of granulosa cells in secondary follicles (detected using PCNA immunostaining) and absent immunostaining for the oocyte-secreted protein GDF9 in secondary follicles after 2 and 4 days of culture under simulated microgravity compared with normal gravity controls41. By contrast, isolated secondary follicles encapsulated in alginate survived culture in simulated microgravity for 4 days and had similar oocyte growth as encapsulated secondary follicles cultured in normal gravity. However, ultrastructural examination of oocytes from encapsulated secondary follicles cultured under microgravity revealed disruption of microvilli extending into the zona pellucida, vacuolated mitochondria, cytoplasmic vacuoles, and “dispersing” Golgi apparatus41. Unfortunately, the same endpoints were not examined in ovarian fragments and cultured follicles, no rotational control group was included and the number of replicates per group and other experimental details were not provided. Therefore, although the results of this study suggest that microgravity has an adverse effect on growth and development of secondary follicles, these results need to be confirmed in future studies.
The effects of simulated microgravity on maturation of oocytes were investigated using a rotary cell culture system with high aspect ratio culture vessels to reduce shear stress55. Oocytes were harvested at the germinal vesicle stage (which is the metaphase of the first meiotic division) from immature Kunming mice 48 h after injection of 7.5 IU equine chorionic gonadotropin to stimulate development of a cohort of preovulatory follicles. The oocytes were then cultured in a medium supplemented with FSH, LH and oestradiol for up to 16 h in simulated microgravity or at normal gravity in static culture (control group). No rotational control group was included to control for shear forces, turbulence and vibrations. Meiosis resumption, as assessed by breakdown of the oocyte nuclear envelope, did not differ between the two groups. However, the percentage of oocytes that reached metaphase of the second meiotic division, assessed as the presence of extrusion of the first polar body, was 73% under 1 g, but only 9% under microgravity. Moreover, 13% of oocytes cultured under microgravity showed cytoplasmic projections or blebbing, while these features were rare (<5%) in oocytes cultured under normal gravity. This finding under microgravity was related to abnormal meiotic spindle formation during both the first and second meiotic divisions, with abnormal localization of γ-tubulin (which is essential for spindle formation) around the chromosomes of oocytes cultured in simulated microgravity. In contrast to the abnormal organization of microtubules, microfilament organization and function, which are necessary for migration of the chromosomes and spindles to the periphery of the oocyte, were not disrupted under simulated microgravity55. Whether the oocytes would have been able to resume normal meiosis progression given more time is unknown. Resumption in mitosis has been reported after somatic cell microtubule disruption following 20 h in simulated microgravity, suggesting that perhaps similar resumption in meiosis would occur with more time56.
Fertilization of mouse oocytes occurred normally under microgravity simulated using a 3D clinostat; however, continued culture under microgravity resulted in delayed development to the blastocyst stage and fewer trophoectoderm cells in those embryos that reached the blastocyst stage compared with embryos at normal gravity57. The rate of live births from embryos developed in simulated microgravity and implanted at the expanded blastocyst stage into recipient females kept at normal gravity was less than half that of embryos developed under normal gravity57. Preimplantation development of mouse embryos has also been investigated using a rotary cell culture system with high aspect ratio culture vessels to simulate microgravity compared with static culture controls and rotation controls58. Embryos were harvested 1.5 (2-cell stage), 2.5 (8-cell stage) or 3.5 (morula or early blastocyst stage) days post coitum from MF-1 female mice that had been mated with C57BL/6J;SJL/J F1 males. The embryos were then cultured for 24 h in one of the three conditions, followed by an additional 48 h in static culture. The 2-cell and 8-cell stage embryos cultured in either simulated microgravity or rotation showed arrested development, and 70% and 100%, respectively, had died by 72 h in culture. By contrast, nearly 40% of morula or early blastocyst stage embryos cultured in simulated microgravity progressed to the expanded blastocyst stage by 48 h. Both simulated microgravity and rotational control morula or early blastocyst stage embryos had upregulation of SAPK (a stress-activated protein kinase) phosphorylation at Thr183/Thr185, a marker for activation of SAPK signalling, after 24 h of culture compared with static controls. All of these effects were similar, but less severe in rotation controls than in animals in simulated microgravity.
In other work, luteal cells from the corpora lutea of pregnant rats were cultured in microgravity simulated using a high aspect ratio vessel and compared with cells from spinner flask controls or in microgravity simulated using a clinostat with static controls. Luteal cells cultured in both microgravity models exhibited decreased progesterone secretion compared with their respective control cultures during up to 8 days of culture42,43. Luteal cells cultured in the clinostat model for 2 days had increased DNA damage measured by COMET assay, increased apoptosis and decreased numbers of active mitochondria compared with static controls42.
Exposure of female rats to hypergravity (1.5g and 2.3g) caused by centrifugation resulted in a lengthened diestrous stage of the oestrous cycle; the diestrous stage lasted 10–16 days (normal diestrous length is 1–2 days). Subsequently, oestrous cycles normalized despite continuation of hypergravity exposure51. As a result of the similarity of prolonged vaginal diestrus during microgravity with prolonged vaginal diestrus during pseudopregnancy in rodents (which is elicited by cervical stimulation that causes a surge of prolactin secretion), suppression of prolactin secretion was tested and was found to block the induction of lengthened diestrus by hypergravity52. Moreover, exposure to hypergravity for ≥8 h during the diestrous phase of the oestrous cycle, but not during other cycle stages, induced a surge of prolactin secretion49.
The effects of hypergravity on pregnancy in mice and rats adapted to centrifugation before mating (2.5g, 3.6g or 4.7g for rats and 2.5g for mice) and centrifuged continuously during gestation and after birth were first reported by Oyama and Platt47. Decreasing rates of pregnancy were observed in rats with increasing gravity, with no pregnancies in those exposed to 4.7g; no offspring survived the neonatal period with continuous centrifugation47. By contrast, neonatal mice reportedly survived centrifugation; however, comparisons with control 1g mice were not described47. Exposure of female and male mice to hypergravity before mating and through gestation decreased pregnancy rates up to 2.9g (with no pregnancies at 3.5g), decreased fetal size and altered skeletal development, with increased longest dimension of most bones measured at 2.3g, decreased longest dimension and perimeter of most bones at 2.6g and of all bones at 2.9g, compared with 1g controls48. No differences in mating ability or gestation length were observed in rats adapted to hypergravity (2.2g or 3.1g), but the number of fetuses and maternal care of pups were dose-dependently decreased compared with those of 1g controls50. Exposure of pregnant rats to hypergravity during the second half of gestation did not alter gestation length, duration of parturition (time from birth of first pup to birth of last pup), litter size or birth weights; however, the frequency of lordosis contractions during labour decreased by half45 and neonatal pup mortality was increased in rats exposed to hypergravity46,59.
The increased neonatal mortality observed in several studies in rats exposed to hypergravity does not seem to be caused by decreased maternal food intake and weight gain during hypergravity, as neonatal mortality was not increased in pair-fed control females53. Rather, decreased neonatal survival of pups born to dams exposed to hypergravity was associated with decreased glucose oxidation and lipogenesis in mammary glands, decreased size of adipocytes with decreased rate of lipolysis and an increased rate of glucose incorporation into lipids in the liver53. All of these changes would be predicted to adversely affect the ability of the dam to produce milk with adequate triglyceride and energy content.
Taken together the results of experiments conducted during space flight and those using simulated microgravity and hypergravity on Earth are consistent with a continuum of responses as gravity increases from nearly absent to several times Earth’s gravity. These responses include decreased pregnancy rates and neonatal offspring survival and an increasing number of lordosis contractions with increasing gravity. By contrast, exposure to either simulated microgravity or simulated hypergravity lengthened oestrous cycles due to lengthened diestrus, while space flight resulted in cessation of cycling, suggesting that other exposures, such as stress or circadian rhythm disruption, during space flight besides microgravity affect the hypothalamic–pituitary–ovarian axis.
Effects of space radiation on female reproduction.
Studies of the effects of space radiation on female reproduction have been ground-based, utilizing experimentally generated charged particles, one ion species at a time with high dose rates. Generating these particles requires highly specialized facilities, such as the accelerator-based NASA Space Radiation Research Laboratory at Brookhaven National Laboratory60.
High LET neutrons and HZE particles seem to be more damaging to the ovary than similar doses of low LET γ-rays. Studies have reported that 60% of ovarian follicles were depleted in C57BL6/CBA F1 hybrid mice at 24h after irradiation with 0.5 Gy γ-rays61 and that 90% of follicles were depleted 1 week after 0.6 Gy γ-rays in Swiss albino mice (both low LET)62. The studies summarized in this section show that the doses of charged oxygen and iron particles at which 50% of ovarian follicles are depleted are 0.05 Gy and 0.28 Gy, respectively, at 1 week after irradiation. Unfortunately, no studies have performed detailed dose-response analyses of follicle depletion by γ-rays at doses below 0.5 Gy.
In a study using high LET neutrons and low LET γ-rays, irradiation of 7-day old C57BL/6N mice with 1 Gy of 0.53 MeV neutrons induced apoptosis (assessed using a TUNEL assay) in about 15% and 35% of oocytes in ovaries at 6h and 12h after irradiation, respectively, and 1 Gy of 2.13 MeV neutrons induced apoptosis of 45% and 50% of oocytes at 6 h and 12 h after irradiation63. By contrast, 1 Gy γ-radiation resulted in 30% and 15% of oocytes undergoing apoptosis at 6h and 12 h, respectively63.
Irradiation of 3-month old C57BL/6J mice with 0, 0.05, 0.3 or 0.5 Gy high LET charged oxygen particles (LET 16.5 keV/μm and energy 600 MeV/n) induced dose-dependent increases in immunostaining for γH2AX (a marker of double strand DNA breaks), 4-HNE (a marker of oxidative lipid damage) and PUMA (a proapoptotic BH3-only BCL2 family protein) in oocytes and granulosa cells of follicles at various stages of development at 6h after irradiation64. Consistent with apoptotic destruction of immature ovarian follicles, a dose-dependent decrease in primordial follicles and primary follicles was seen at 1 week after irradiation, with no primordial follicles remaining in the ovaries of mice irradiated with 0.5 Gy and 72% of primordial follicles depleted after irradiation with 0.05 Gy. Charged iron particles were less potent destroyers of ovarian follicles than oxygen. By 1 week after irradiation of C57BL/6J mice with 0.05 Gy or 0.5 Gy high LET iron particles (LET 179 keV/μm and energy 600 MeV/n), primordial follicles were depleted by 10% and 80%, respectively65. This study demonstrated that γH2AX, oxidative lipid damage (levels of 4-HNE) and apoptosis (levels of activated caspase 3) were elevated at 6 h after irradiation and remained elevated in ovarian follicles at 1 week after charged iron particle irradiation65. Consistent with persistent oxidative damage and apoptosis, primordial follicles were depleted by 57% and 98% in mice 8 weeks after irradiation with 0.05 and 0.5 Gy of charged iron particles, respectively65. Serum concentrations of LH and FSH were statistically significantly elevated at 8 weeks after irradiation with 0.5 Gy charged iron or oxygen particles, which is consistent with what would be expected after loss of ovarian negative feedback due to depletion of follicles64,65. A proposed model for destruction of ovarian follicles by charged particle radiation, which is based on these results, is shown in Figure 1.
Figure 1: Proposed mechanisms of HZE particle radiation-induced destruction of ovarian follicles.
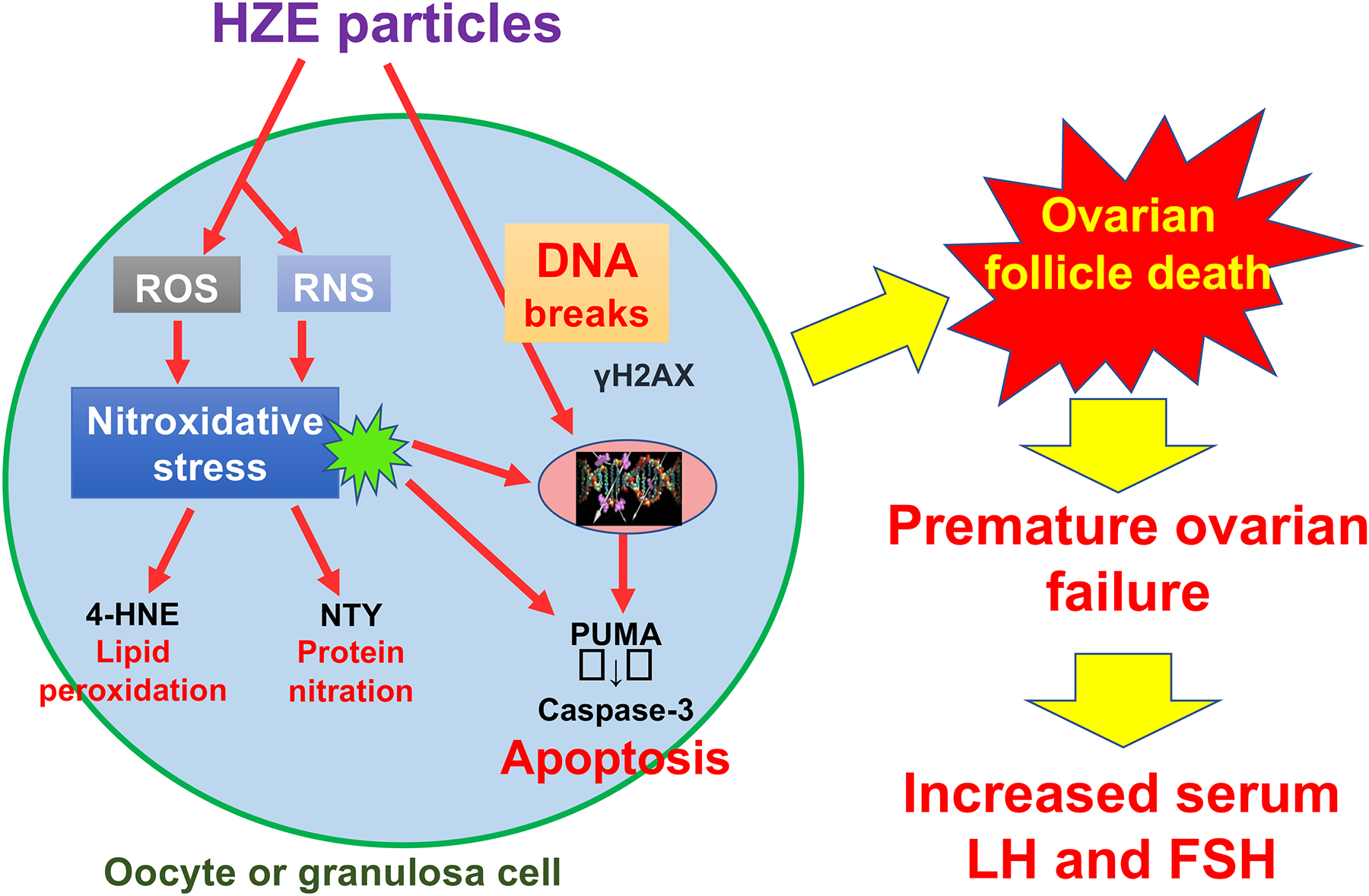
HZE particles such as oxygen and iron ions can directly ionize DNA causing DNA strand breaks (such as double strand breaks identified by γH2AX immunostaining). Irradiation with HZE particles also results in generation of reactive oxygen and nitrogen species (ROS and RNS), which react with cellular macromolecules causing nitroxidative damage to lipids (e.g. 4-hydroxynonenal, 4-HNE), proteins (e.g. nitrotyrosine, NTY) and DNA. These insults lead to activation of apoptosis by upregulation of proapoptotic BCL2 family proteins such as PUMA and ultimately activation of caspase 3 in oocytes and granulosa cells of ovarian follicles at all stages of folliculogenesis. Destruction of the irreplaceable primordial follicle pool causes premature ovarian failure with increased serum LH and FSH concentrations due to loss of ovarian negative feedback.
Numerous animal models in which ovarian follicles are depleted by exposure to radiation, chemicals, genetic alterations or other manipulations show increased risk of ovarian tumours66,67. Ovarian failure is also associated with ovarian cancer in humans, as the majority of ovarian cancers occur after menopause66,67. In mice irradiated with 0.5 Gy charged iron particles, the prevalence of epithelial ovarian tumours increased by about fourfold at 15 months after irradiation compared with non-irradiated mice68.
Decreased uterine size and uterine thickness on imaging and endometrial and myometrial atrophy on histology were observed in premenopausal women exposed to high doses (40–65 Gy) of low LET radiation as part of radiation therapy69. Uterine volume was decreased in women treated with low LET radiation (9–54 Gy) to the abdomen or pelvis before 15 years of age; the effect was more pronounced with younger age at irradiation70. Uterine blood flow was undetectable in three women, with greatly reduced uterine volume after prepubertal irradiation of the uterus; neither uterine volume nor blood flow was improved by high dose oestrogen therapy, which suggests that the effects of radiation on the prepubertal uterus are not reversible70. Indeed, a study of uterine responses to oestrogen replacement in women who received total body irradiation reported greater increases in uterine volume, endometrial thickness and uterine blood flow in women treated with radiation after puberty than in those irradiated before puberty71. Rats irradiated with 5 Gy of low LET γ-rays displayed flattening of the endometrial surfaces, depletion of deep uterine glands and fewer proliferating luminal and glandular epithelial cells than non-irradiated rats72. Women who have had cancer treated with radiotherapy to the pelvis or abdomen have reduced fecundity and an increased risk of adverse pregnancy outcomes such as miscarriage, preterm birth, perinatal death of the fetus/neonate and low birth weight even in the absence of premature ovarian failure, which is consistent with direct adverse effects of radiation on the uterus73.
Only a few studies have examined the effects of charged particle radiation on the uterus. The incidence of endometriosis in rhesus macaques was statistically significantly increased by exposure to proton (0.25–6.5 Gy; 32–2,300 MeV) or X-radiation compared to non-irradiated controls, with 73% of the cases occurring between 9 years and 17 years after irradiation74,75. The incidence after proton irradiation varied with energy and dose; the highest incidence of 73% occurred in macaques exposed to 3–12 Gy of mixed-energy protons, compared with 26% in controls. Irradiation with 4.5–7.0 Gy X-rays induced endometriosis in 71% of the irradiated macaques75, which suggests that proton radiation and X-radiation have similar potencies for this endpoint.
Induction of cell death by monoenergetic protons (1–10 Gy; LET 8.35 keV/μm and 4.86 MeV) and γ-rays (0.2–1.6 Gy) has been examined in cultured human endometrial carcinoma cells (HEC1B and AN3CA cells) 4 h after irradiation76. HEC1B cells underwent apoptosis (assessed by presence of PARP cleavage) after exposure to 1 Gy protons and 0.4 Gy γ-rays, but not after higher doses. On the basis of decreased cell survival, the authors of this study speculated that at higher doses the cells progressed directly to necrosis. The AN3CA cells (which are less differentiated than HEC1B cells) showed no evidence of apoptosis (lack of PARP cleavage) and underwent rapid necrosis after 10 Gy proton radiation and above 0.2 Gy γ-radiation. While the experiments included only three replicates per group and no statistical analyses were presented, these results are not consistent with greater potency of proton radiation than γ-radiation for these endpoints in uterine carcinoma cells.
In summary, in vivo studies demonstrate that exposure to HZE particles and neutrons induces oxidative damage, double strand DNA breaks and apoptosis in oocytes and granulosa cells of ovarian follicles in various stages of development, which results in dose-dependent depletion of the ovarian reserve and increased incidence of ovarian tumours. Data are lacking to determine the relative potencies of various HZE particles compared with γ-radiation or X-radiation in eliciting these ovarian effects. The effects of charged particles on the uterus and other parts of the female reproductive tract have hardly been studied; this is an important area for future research.
Male reproductive effects
The hypothalamic–pituitary–testis axis shares similarities with the hypothalamic–pituitary–ovary axis in that spermatogenesis and synthesis of sex steroids in the testis are regulated by the pituitary gonadotropin hormones, LH and FSH, which are secreted in response to GnRH from the hypothalamus. However, there are also some differences (Box 2).
Box 2 |. Regulation of male reproduction.
The hypothalamic–pituitary–testis axis has some similarities with the hypothalamic–pituitary–ovary axis. However, unlike the ovary, the testis contains diploid germline stem cells, which are capable of self-renewal, throughout life. These cells are called spermatogonial stem cells, and they are located between the basement membrane of the seminiferous tubules of the testis and the Sertoli cells. These stem cells can divide to generate more stem cells or differentiating spermatogonia that become primary spermatocytes upon entering meiosis, forming secondary spermatocytes after the first meiotic division and haploid spermatids after the second meiotic division, which differentiate into spermatozoa that are released into the tubule lumen83. Testosterone production by the Leydig cells of the testis, which are located in the interstitial spaces between seminiferous tubules, is stimulated by LH from the pituitary gland. Testosterone signalling via androgen receptors in the Sertoli cells is necessary for the progression through meiosis, and absence of testosterone causes arrest of spermatogenesis in early meiosis114. FSH signalling regulates genes that are important for optimal structure and function of the Sertoli cells115.
Effects of space flight on male reproduction.
Reproductive hormones have been measured in male astronauts during and after space missions of various durations (Table 3). Four male astronauts were studied before, during and after a 5-day space mission77. Serum, urine and salivary concentrations of testosterone decreased statistically significantly during flight compared with levels before the flight. By contrast, serum levels of LH increased, which is consistent with decreased testicular negative feedback77. Serum levels of testosterone remained lower than levels before the flight at 1 week after re-entry and returned to preflight levels at 2 weeks after re-entry in three of four men, while LH levels fell rapidly within 1 day after re-entry77. Fifteen male astronauts on International Space Station (ISS) missions lasting 48–215 days provided blood samples preflight, up to five times during flight and on landing day78. Serum levels of free, bioavailable (which includes free and albumin-bound) and total testosterone did not decrease during space flight, but decreased statistically significantly on landing day and returned to baseline levels by 15 days after landing. Similarly decreased serum levels of free and total testosterone were noted in astronauts after completion of 12–13 days of flight on the Space Shuttle78.
Table 3:
Effects of space flight and simulated altered gravity on male reproduction
| Model | Hormones * | Testis weight | Testis | Epididymis | Other | Ref |
|---|---|---|---|---|---|---|
| Space flight | ||||||
| Men, 5-day space flight, blood, urine, and saliva before, during, after flight | ↓T, ↑LH during flight ↓T 1week after flight T back to baseline in 3 of 4 men 15 days after flight |
ND | ND | ND | ND | 77 |
| Men, 48–215-day space flight, blood before, during, after | NS T during flight ↓T landing day NS 15 days after landing |
ND | ND | ND | ND | 78 |
| Wistar rats flown on COSMOS-605 biosatellite for 22 days | ↓T (cited in23) | ↓ (cited in23) | No effects on spermatogenic tissue or disorders in the spermatogenic process | ND | abstract only; article in Russian | 23,107 |
| Rats flown 18.5 days on COSMOS-1129 biosatellite and mated 1 or 2.5–3 mo after landing | ND | ND | ND | ND | Abstract only; article in Russian. Offspring of 1 month post flight mating pairs had delayed postnatal growth and development. Offspring of 2.5 month post flight mating pairs did not differ from controls. | 108 |
| Rats, 14-day space flight; simulated launch, vivarium, and HLS controls | ↓T and ↓testicular T on landing day in flight and HLS groups | ↓ in HLS and flight | ↓testicular sperm counts in HLS, NS flight; ↓spermatogonia in stage VI and VII seminiferous tubules in HLS and flight |
ND | ND | 79–81 |
| SD rats, 14-day flight on Space Shuttle STS-58 | ↑urine T and LH days 1–3 postflight | ND | ND | ND | ND | 97 |
| Mice, 91-day flight in Mouse Drawer System on Space Shuttle STS-128; vivarium controls and Mouse Drawer System controls performed after flight | ND | ND | Degenerated seminiferous epithelium | ↓↓epididymal sperm counts | 3/6 flight mice died; 0/6 grounds controls died | 82 |
| Simulated microgravity | ||||||
| Men, bed rest 60–90 days | NS T during bed rest; ↓T on reambulation |
ND | ND | ND | ND | 78 |
| Men, bed rest 14 days | NS T during bed rest | ND | ND | ND | ND | 87 |
| Bull sperm flown in unmanned rocket with about 320 sec exposure to microgravity | ND | ND | ND | NS % motile sperm; ↑% sperm with straight line motility and increased sperm velocity | ND | 93 |
| Human sperm, clinostat rotation with static and horizontal rotation controls; human sperm in parabolic flight (1g, microgravity, 2g) | Clinostat — NS epididymal sperm motility; Parabolic flight microgravity — ↓% motile epididymal sperm, ↓% progressive motility, ↓mean linearity of motility; Parabolic flight 2g — ↓% motile epididymal sperm |
94 | ||||
| Rats, 7–14 day HLS | ↓T ↓testicular T; ↑LH |
↓↓ | ↓↓testicular sperm counts; ↓seminiferous tubule diameter | ↓↓epididymal sperm counts | Testes not maintained intrascrotally | 79,80,88,89 |
| SD rats, 7-day HLS using tail suspension (exp 1 and 2) or whole body harness (exp 3) | ↓ T; ↓testicular T (measured exp 2 only); ↑LH (measured exp 1 and 2 only) |
NS | NS testicular sperm counts | NS epididymal sperm counts | Testes maintained intrascrotally by inguinal canal ligation or whole-body harness; rats suspended 2–6 hours post ligation; euthanasia by decapitation exp 1 and 2, anesthesia and cardiac puncture exp 3 | 88 |
| SD rats, 7-day HLS using tail suspension | ↓ T; NS testicular T; NS serum LH |
NS | NS testicular sperm counts | NS epididymal sperm counts | Testes maintained intrascrotally by inguinal ligation; rats suspended 7 days post ligation; euthanasia by CO2 or ether inhalation followed by decapitation | 89 |
| Fisher 344 rats, 6-week HLS using tail suspension | NS T, LH and FSH | ↓ (HLS, but not TO) | ↓↓testicular sperm counts (HLS); ↓testicular sperm counts (TO); ↓seminiferous tubule diameter and histology; (HLS, but not TO) | ↓↓epididymal sperm by histology; NS epididymal wt; (HLS, but not TO) |
Testes maintained intrascrotally by inguinal ligation; rats suspended 7 days post ligation; included ligated tail only (TO) harnessed but not suspended controls. | 91 |
| B6D2F1 mice, 7 day HLS using tail suspension with tail only controls | ↓ T | ↓ | ↑seminiferous tubules with disrupted spermatogenesis; ↑germ cell apoptosis | NS %motile epididymal sperm; ↓↓epididymal sperm path velocity, progressive velocity, track speed, and beat frequency | No mention of inguinal ligation to maintain intrascrotal testes | 95 |
| C57BL/6J mice, 30 day HLS using tail suspension with free running controls ± essential phospholipids supplementation | ND | ↓all HLS groups; NS differences among HLS groups | ND | ↓↓epididymal sperm concentration and motility; phospholipid supplemented groups only had recovery immediately and/or by 12h after HLS ended | No mention of inguinal ligation to maintain intrascrotal testes | 96 |
| Simulated hypergravity | ||||||
| SD rats, subjected to 2g by centrifugation for 14 days | ↑urine T days 1–8; ↓urine LH days 2–3 and 10–12 |
ND | ND | ND | ND | 97 |
| Long Evans rats subjected to 2.3 or 4.1 g by centrifugation for 52 days | ↓T and LH for duration of 4.1g; ↓T days 1 and 4, NS days 15 and later; NS LH at 2.3g |
NS 2d post centrifugation | ND | ND | NS seminal vesicle and prostate weights 2d post centrifugation | 99 |
| Rats subjected to 2g by centrifugation for 14 days | NS T after 14d centrifugation | NS | NS testicular histology; NS testicular spermatid counts | ND | ND | 98 |
Hormone concentrations are in serum unless otherwise specified
Abbreviations: HLS: hind-limb suspension; ; ND: not determined; NS: not statistically significant; T: testosterone
Several studies have reported on the effects of space flight on male reproductive endpoints in rodents (Table 3). Most of these studies involved short-term space flights of 7–22 days79–81, while one had 91 days of space flight82. Both serum and testicular concentrations of testosterone 8–11 h after landing were statistically significantly decreased to a similar extent in male Wistar rats flown for 14 days on the COSMOS 2044 unmanned space vehicle. Serum and testicular concentrations of testosterone were also decreased in hind-limb suspended controls compared with vivarium and simulated launch controls (which were exposed to vibrational and g-force stresses, but not microgravity). In addition, testes weights were decreased to a greater extent in hind-limb suspended controls than space flight rats79,80. Testicular sperm head counts were not statistically significantly decreased in space flight rats, while they were almost completely absent in hind-limb suspended rats (due to testicular warming)79. Numbers of spermatogonia in stage VI seminiferous tubule cross-sections were statistically significantly decreased by 5–10% in space flight testes from COSMOS 204480 and COSMOS 188781 compared with vivarium and simulated launch controls. Stage VI seminiferous tubule cross-sections in mice contain stem, proliferative and differentiating spermatogonia, pachytene primary spermatocytes, step 6 round spermatids and step 15 elongated spermatids83. Numbers of type A spermatogonia (which includes proliferative and differentiating spermatogonia) in stage VII seminiferous tubules were similarly decreased by 5–10% in flight rats versus vivarium and simulated launch controls from COSMOS 204480. Stage VII seminiferous tubule cross-sections contain stem, proliferative and differentiating spermatogonia, preleptotene and pachytene primary spermatocytes, step 7 round spermatids and step 16 elongated spermatids83. On the basis of limited radiation dosimetry performed on COSMOS 1887 and Space Shuttle flights, it was concluded that the decreased spermatogonia numbers could not be attributed entirely to radiation exposures84. As the spermatogonial stem cells are fairly resistant to radiation-induced cell death compared with proliferating spermatogonia, the effects on spermatogonial numbers observed with space flight are presumed to be reversible84.
Six male C57BL/10J mice (three wild-type and three transgenic pleiotropin overexpressing mice) were individually housed in a Mouse Drawer System, launched on the Space Shuttle Discovery and housed on the ISS, with a total of 91 days of space flight. There were two sets of ground controls which were carried out after the space flight: one wild-type and two transgenic mice were housed in the Mouse Drawer System and one wild-type mouse and two transgenic mice were housed in normal vivarium cages, with food, water and environmental conditions similar to those of the space flight mice82. Only one wild-type and two transgenic mice survived space flight, while all 6 ground controls survived. Testicular histomorphology of the mice exposed to space flight showed tubular degenerative changes, with some tubules containing only a few spermatogonia and no other germ cells, while others were less affected. In addition, inflammatory exudates were noted in the interstitial tissue of the space flight mice82. Epididymal sperm counts in space flight mice were decreased by about 90% compared with those of both control groups. Testicular mRNA transcripts for the androgen receptor and IL-1β were increased about threefold and 15-fold, respectively, in space flight mice compared with ground controls, while expression of LH and FSH receptors was not affected82.
The effects of space flight on sea urchin (Lytechinus pictus and Stronglyocentrotus pupuratus) sperm motility and phosphorylation of sperm flagellar proteins known to be involved in the initiation of motility were investigated 20 h after their launch on two Space Shuttle missions85. Sperm were activated to swim by dilution into artificial seawater, and activation was terminated at 0, 30 or 60 seconds by addition of an electrophoresis buffer. Western blotting was performed after return to Earth. Exposure to space flight accelerated the phosphorylation of FP130 (which is a phosphothreonine-containing protein) and a 32 kDa phosphoserine-containing protein, compared with ground controls at 1 g. Responses of sperm to the egg chemotactic protein speract were delayed in microgravity. Additional ground studies confirmed that vibration and acceleration similar to those experienced during launch did not statistically significantly alter sperm motility.
Freeze-dried spermatozoa samples from four different mouse strains have been flown to the ISS. 6 days after launch, they were stored at −95°C for 9 months and then returned to Earth; control samples from the same males were retained on Earth and also stored at −95°C for 9 months86. DNA damage to the spermatozoa was statistically significantly increased in space samples from three of the four strains as assessed by Comet assay86. Sperm were rehydrated and used for intracytoplasmic sperm injection. DNA damage in zygotes assessed by measuring γH2AX immunofluorescence intensity in male pronuclei was increased in one of the four mouse strains (BCF1). Two-cell stage embryos transferred to recipient females resulted in live offspring at similar rates as ground controls, but at lower rates than those fertilized with fresh spermatozoa from the same males86.
Effects of simulated microgravity and hypergravity on male reproduction.
Bed rest with a −6° head down tilt for 60–90 days78 or for 14 days87 did not affect serum concentrations of free or total testosterone in male volunteers; however, both levels declined after re-ambulation78 (Table 3). The authors attributed the post-bed rest change to fluid shifts.
Initial studies with the hind-limb suspension model of microgravity in male rats reported dramatic reductions in testis weights, testicular sperm head counts, epididymal sperm counts and serum and testicular fluid concentrations of testosterone after 7–14 days of hind-limb suspension79,80,88,89. Decreased levels of testicular homogenization-resistant sperm nuclei and epididymal sperm counts were subsequently shown to be largely due to elevation of testicular temperature due to intra-abdominal movement of the testes. When testes were maintained intrascrotally, either via a ligature around the scrotum88,89 or through use of a whole body harness88 rather than a tail harness, the effects of short-term hind-limb suspension on sperm production disappeared, but serum levels of testosterone remained decreased. Surprisingly, the results for testicular fluid concentrations of testosterone and serum levels of LH differed between the latter two studies88,89, despite them having used the same rat strain (SD), rodent chow, duration of caudal elevation and light–dark cycle. One study reported no change in serum levels of LH or testicular fluid concentrations of testosterone after testicular ligation89, while the other reported that testicular fluid concentrations of testosterone were decreased to about 35–45% of control levels and LH levels were elevated88. Differences in experimental design that might account for these disparate findings include duration of time after ligation surgery before suspension was started and differences in the method of euthanasia. Although intratesticular testosterone is required for normal spermatogenesis, effects on spermatogenesis typically do not occur until testosterone concentrations fall below 20–25% of control levels90.
In contrast to short-term exposure to caudal elevation, exposure of rats to hind-limb elevation with scrotal ligation for 6 weeks resulted in statistically significant decreases in testis weights, testicular homogenization-resistant sperm nuclei counts and seminiferous tubule diameters, no changes in serum concentrations of testosterone and LH, no changes in epididymal or seminal vesicle weights and absent or severely decreased numbers of epididymal sperm compared with free roaming controls91. Although ligation prevents the testes from moving out of the scrotum, testicular temperature might still be mildly increased in this model as the testes rest next to the body during hind-limb suspension. The authors speculated that long-term exposure to mildly increased temperatures might have affected spermatogenesis91. These findings of seminiferous tubule degeneration and decreased epididymal sperm counts are similar to those reported in mice flown on the Space Shuttle for 91 days82.
The effects of simulated microgravity on testis fragments from prepubertal Wistar rats immersed in culture medium in a rotating wall vessel for 3 days were compared with fragments from ground control rats (cultured in normal gravity conditions immersed in medium) and grid control rats (cultured in normal gravity conditions on floating metal grids)92. Culture on floating grids preserved tubular architecture, including tight junctions between Sertoli cells, and resulted in less tubular apoptosis than immersion culture; these parameters did not differ between simulated microgravity and ground control testis fragments. Testosterone release was increased in simulated microgravity compared with both ground control and grid control, while testosterone release upon LH stimulation was decreased in microgravity fragments compared with ground control fragments. An apparent increase in pyknotic Leydig cells without an increase in TUNEL-positive Leydig cells in simulated microgravity compared with both controls was seen, which the authors postulated was due to a non-apoptotic cell death mechanism involving increased plasma membrane permeability92.
At least four studies have examined the effects of microgravity on mammalian sperm motility. Bull sperm were flown in unmanned rockets in which weightless conditions lasted for about 360 seconds in the TEXUS 19 and 26 missions. Results from the two missions were consistent. The total percentage of motile sperm did not differ during microgravity compared with samples from 1 g controls, but the percentage with progressive motility and the straight-line velocity were increased during microgravity93. Sperm collected from 18 human volunteers were subjected to simulated microgravity in a clinostat with static and rotational controls (duration of exposure not reported) or to normal gravity, microgravity and 2 g hypergravity during 10 cycles of 100 seconds each of parabolic flight94. No statistically significant differences in any motility parameters were observed between clinostat rotation at 5 rpm or at 50 rpm and the controls. By contrast, percentage of motile sperm, percentage with progressive motility and mean linearity of motility saw a statistically significant decrease and parameters of speed did not change at microgravity compared with 1 g during parabolic flight. Percentage of motile sperm was the only parameter that was statistically significantly decreased at 2 g compared with 1 g94.
Two subsequent studies used hind-limb suspension to simulate microgravity in mice. Neither study described using scrotal ligation to prevent movement of the testes out of the scrotum95,96. Hind-limb suspension with a tail harness of C57BL/6J mice for 30 days with or without supplementation with essential phospholipids reportedly decreased the percentage of motile epididymal sperm by about fivefold immediately after cessation of suspension compared with free roaming controls96. Motility did not recover 12 h after cessation of suspension in unsupplemented mice, but mice supplemented with essential phospholipids had a complete recovery of sperm motility 12 h after cessation of suspension96. Epididymal sperm concentration was decreased by half immediately after and 12 h after cessation, while phospholipid supplementation prevented the decrease at both time points96. Testis weights were decreased by 27–36% in the hind-limb suspension groups compared with the unsupplemented control group, with no statistically significant differences between the supplemented and unsupplemented hind-limb suspended groups. Hind-limb suspension by tail harness of B6D2F1 mice for 7 days statistically significantly decreased testis weights by 5% and serum levels of testosterone by 70%, disrupted spermatogenesis in approximately 5% of seminiferous tubules and doubled the percentage of apoptotic germ cells compared with tail harness only controls95. While the percentage of motile sperm did not differ between groups, several motility parameters (such as path velocity, progressive velocity, track speed and beat frequency) were statistically significantly decreased in the hind-limb suspended mice95.
In rats, two studies have examined the effects of simulated hypergravity at 2g by centrifugation for 12 days on 24-h urine97 or for 14 days on serum concentrations of reproductive hormones98. Urine concentrations of testosterone were increased on days 1–8 of centrifugation, but did not differ from controls on days 9–12, while urine concentrations of LH were decreased on days 2, 3 and 10–1297. No changes in serum or testicular concentrations of testosterone, testes weights, testicular spermatid counts or testicular histology were observed after 14 days at 2 g98. Another study examined the effects of centrifugation at 2.3 g or 4.1 g for 52 days on male reproductive endpoints99. At 4.1 g, plasma levels of testosterone and LH were decreased throughout the study, including 2 days after centrifugation for testosterone. By contrast, at centrifugation at 2.3 g, testosterone levels were decreased transiently on days 1 and 4, and LH was not statistically significantly altered99. Weights of testes, prostates and seminal vesicles were not affected by exposure to hypergravity99. Differences in testosterone and LH responses to hypergravity among these studies might be due to the use of blood versus urine hormone measurements as daily urine collection provides an integrated measure of hormone secretion during the preceding 24 h, while blood concentrations will have greater variability due to episodic secretion of LH and testosterone.
Taken as a whole, the data from space flight and ground-based in vivo microgravity and hypergravity experiments support the idea that exposure to microgravity decreases serum levels of testosterone during short-term exposures, with adaptation occurring during longer exposures. Decreases in testis weights, testicular sperm counts, and epididymal sperm counts are observed with long-term, but not short-term exposures, of rats to simulated microgravity (hindlimb suspension with testes maintained scrotally). By contrast, the more limited data on exposure to hypergravity suggest that exposure up to 2.3 g has limited effects on serum concentrations of testosterone, male reproductive organ weights or testis histology, while exposure to 4.2 g suppressed both serum levels of testosterone and LH without affecting male reproductive organ weights. Contradictory effects on sperm motility have been observed in two studies of brief exposures of isolated mammalian sperm to microgravity.
Effects of space radiation on male reproduction.
Similar to the ovary, our understanding of the testicular effects of space radiation derives primarily from ground-based studies, utilizing experimentally generated HZE particles, one ion species at a time. Overall, these studies support the idea that high LET charged particle radiation is more damaging to the testis than low LET radiation. However, the reported relative biological effectiveness (RBE) compared with photon radiation varies considerably depending on the charged particle, LET, endpoints examined and experimental differences.
The RBEs for testis weight (as a proxy for spermatogonial killing) 28 days after irradiation with low LET helium (LET 1.6–6.0 keV/μm, 228 MeV/u) or high LET carbon (LET 11–105 keV/μm, 400 MeV/u), neon (LET 35–225 keV/μm, 420 MeV/u) and argon (LET 90–680 keV/μm, 570 MeV/u) HZE particles compared with low LET γ-rays from 60Co decay ranged from 1.13 for helium to 3.02 for argon in B6D2F1 mice100. The data showed quite different RBEs for different ions at similar LETs, which is consistent with effects of ion mass or charge independent of LET.
The effects on overall spermatogonia survival in stage VI and stage I seminiferous tubules 72 h after irradiation were compared in male B6D2F1 mice after irradiation with 0.01–1.00 Gy X-rays or helium (LET 1.6 keV/μm, 228 MeV/u), neon (LET 35 keV/μm, 420 MeV/u), argon (LET 90 keV/μm, 570 MeV/u) or iron (670 MeV/u) particles84,100. Analysis of stage VI tubule cross-sections has the advantage that all of the cell types present are easily identified morphologically; counts of stage VI tubules at 72 h provide information about radiosensitivity of stem, proliferative and early differentiating spermatogonia, while counts of stage I tubules provide information about radiosensitivity of spermatogonial stem cells and later differentiating intermediate and type B spermatogonia84. Helium, neon and argon were similarly potent to X-rays, with about 50% of spermatogonia depleted at doses of 0.3–0.4 Gy, while iron was more potent, with 62% of spermatogonia depleted at 0.1 Gy. Of the various spermatogonial developmental stages, the differentiated intermediate and type B spermatogonia were the most sensitive, with 50% depletion occurring at 0.2 Gy helium and 0.15 Gy argon. By contrast, type As spermatogonia (the spermatogonial stem cells) were the most resistant cells with minimal effects at doses below 1 Gy X-rays, 0.8 Gy helium and 0.3 Gy argon.
Reciprocal translocations in spermatocytes at diakinesis of metaphase as an indicator of spermatogonial stem cell sensitivity to radiation-induced genetic damage were enumerated 6 months after irradiation in CBAxC57BL/6J F1 mice and Wistar rats101. In mice, the RBEs compared with γ-rays from 60Co decay were 0.9 for protons (1.26 keV/μm, 50 MeV), 1.3 for protons (0.23 keV/μm, 9 GeV), 0.7 for helium (0.83 keV/μm, 4 GeV) and 1.3 for carbon ions (7.6 keV/μm, 4 GeV) at doses of 0.5–4.0 Gy and 6.6 for neutrons (40 keV/μm, 1.5 MeV) at doses of 0.15–2.00 Gy. In rats, the RBEs were 1.7 for protons and 1.3 for helium.
The mechanisms of charged particle-induced germ cell death in the testis have been examined after carbon102,103 and iron104,105 ion irradiation (Figure 2). 24 h after 2 Gy carbon irradiation (31.3 keV/μm, 200 MeV/u), testicular levels of the p53 and BAX proteins, germ cell apoptosis index and epididymal sperm DNA damage measured by sperm chromatin structure assay were increased in 10 week old Swiss Webster mice102. 7 days after irradiation with 0.5 Gy or 4 Gy carbon ions, testicular lipid oxidation increased and concentrations of the antioxidant glutathione decreased103. Proteomic analysis of testis homogenates identified proteins that differed by at least threefold among groups. Three molecular chaperone proteins and two metabolic enzymes were upregulated, while the antioxidant proteins glutathione peroxidase 4 and glutathione transferase mu7 and the cell cycle regulators PIN1 and Cyclin D1 were downregulated103.
Figure 2: Proposed mechanisms of HZE particle radiation-induced destruction of testicular germ cells.

HZE particles such as carbon and iron ions can directly ionize DNA causing DNA damage, which leads to increased levels of p53. Irradiation with HZE particles also results in generation of reactive oxygen and nitrogen species (ROS and RNS), which react with cellular macromolecules, including DNA. These insults lead to activation of apoptosis by upregulation of proapoptotic BCL2 family proteins such as BAX and ultimately activation of caspase 3 and apoptotic death of differentiating germ cells. Spermatogonial stem cells are relatively resistant to destruction by HZE irradiation, and therefore spermatogenesis eventually recovers. Oxidative damage also activates protective mechanisms such as upregulation of the transcription factor NRF2 and its target antioxidant genes, which might limit damage.
24 h after 0.5 Gy and 1 Gy of iron ion irradiation (600 MeV/u) of male KunMing mice, seminiferous tubules displayed disrupted basement membranes and thinning seminiferous epithelium, increased numbers of apoptotic cells (using annexin V to detect these cells), increased testicular protein levels of the proapoptotic proteins activated caspase 3, PARP1 and BAX, and also increased protein levels of the transcription factor NRF2 and its targets hemeoxygenase 1 and NAD(P)H quinone oxidoreductase 1104. Regarding PARP1, it was not specified whether PARP1 itself or products of its cleavage by caspases was increased.
Prepubertal 4-week old Swiss Webster male mice were euthanized 2, 5 or 8 weeks after 2 Gy of iron ion irradiation (31.3 keV/μm, 140 MeV/u). Epididymal sperm counts and sperm motility were statistically significantly decreased at 2 weeks, partially recovered at 5 weeks and fully recovered at 8 weeks, which is consistent with the known resistance of spermatogonial stem cells to charged particle radiation105. Seminiferous tubule morphology was severely disrupted at 2 weeks with degeneration of germ cells and increased numbers of apoptotic germ cells, mainly leptotene and pachytene spermatocytes, and epididymal sperm had statistically significantly decreased ATP content. Proteomic analyses of epididymal sperm extracts revealed 16 differentially expressed proteins, including downregulation of three mitochondrial proteins and two glycolytic enzymes, of which the glycolytic enzyme alpha enolase (Eno1) was involved in the most biological processes and was selected for further analysis. Immunofluorescence revealed that Eno1 localized to the sperm midpiece, and Eno1 mRNA and protein levels were downregulated in sperm of irradiated mice105.
Overall, the testes are sensitive to germ cell destruction by ionizing radiation. Spermatogonial stem cells are relatively radioresistant compared to subsequent, differentiated spermatogonial stages. In contrast to the ovaries, which lack germline stem cells in postnatal life, the surviving spermatogonial stem cells are able to replenish testicular germ cells after irradiation84,100. The RBE of high LET charged particle radiation compared to γ-radiation for decreasing sperm production varies with the specific endpoint measured, LET, ion mass, and charge, tending to be higher for particles with higher LET, charge, and mass. The mechanism of charged particle irradiation-induced testicular germ cell destruction involves direct ionization of DNA, generation of ROS with resulting oxidative damage to macromolecules, activation of p53, and induction of germ cell apoptosis102,103,104,105.
Conclusions
Travel in deep space poses multiple simultaneous hazards to human reproduction that are beginning to be understood. Exposure of male mice to microgravity in low Earth orbit or male rats to simulated microgravity causes seminiferous tubule degeneration and decreased epididymal sperm counts82,91. Only a few studies have investigated the effects of space flight or simulated microgravity on ovarian folliculogenesis, but the studies reported disruption of oestrous cycling26 and impaired in vitro follicle development41. Irradiation of female mice with HZE oxygen or iron particles at doses less than or similar to those that are expected to occur during a 3-year Mars mission induces oxidative stress, DNA damage and apoptosis in ovarian follicles, which results in depletion of the ovarian reserve and premature ovarian failure65,68. Unlike the ovary, the testes are better able to recover from germ cell depletion by HZE particle irradiation due to the radioresistance of spermatogonial stem cells81,84,100. However, experiments to date have used HZE particle beams composed of a single species that are not representative of space radiation. Future studies should examine the effects of mixed particle beams more representative of galactic cosmic rays or solar particle events on spermatogenesis and folliculogenesis. NASA has developed the capability to rapidly switch among charged particles to more closely simulate radiation exposures from galactic cosmic rays or solar particle events6, so such experiments are now possible.
Microgravity during space flight causes subtle abnormalities in fertilization and embryonic development in sea urchins and amphibians28,29,106. Mating, fertilization and hatching of medaka fish during orbit resulted in offspring with apparently normal ovaries and fertility30. Limited mammalian data suggest that space flight and microgravity adversely affect very early embryonic development, while development during the second half of gestation proceeds fairly normally. Fertilization of mouse oocytes seems to proceed normally in simulated microgravity57, but subsequent embryonic development is delayed or arrested, and rats mated during space flight showed evidence of early pregnancy loss31. Space flight from mid to late gestation increased the number of lordosis contractions during labour due to alterations in the abdominal musculature, with inconsistent (no or modest) effects on birth rate, litter size, birth weights and neonatal mortality32,33,35–37. By contrast, exposure to hypergravity decreased the number of labour contractions, birth weights and neonatal pup survival45–48,53.
Major gaps in knowledge exist regarding the effects of long duration space missions in low Earth orbit on mammalian reproduction. Undertaking studies to fill these gaps is important as they will examine the effects of the multiple simultaneous hazards to which human space travellers are exposed. At the same time, it is critical to continue to conduct ground-based studies to understand the effects of radiation typical of deep space and to tease out which of the multiple hazards present during space travel are of greatest concern for reproduction and how to mitigate those effects. Very little is known about the effects of space radiation or altered gravity on the male or female reproductive tracts, hypothalamic and pituitary regulation of reproduction, prenatal development of the reproductive system or potential transgenerational effects on reproduction. All of these are important areas for future study if humans are to undertake multi-year missions in deep space or colonize other planets.
Glossary
- Ionizing radiation
Radiation that carries sufficient energy to detach electrons from atoms or molecules, ionizing them.
- Microgravity
Gravity near zero-g; the condition in which people or objects appear to be weightless.
- Hypergravity
Conditions in which the force of gravity exceeds that on Earth’s surface, which is 1-g.
- Centrifugation
The application of centrifugal force to increase the effective gravitational force for experimental purposes.
- Vaginal oestrus
Vaginal cytology consisting of keratinized epithelial cells; this cytology characterizes oestrus, the day of the rodent estrous cycle when ovulation occurs.
- Secondary follicle
The next stage of follicular development after primary follicles; characterized by more than one layer of cuboidal granulosa cells and theca cell layers outside the granulosa cell layers.
- Zona pellucida
The layer of glycoprotein that surrounds the oocyte plasma membrane; during fertilization, the sperm binds specific proteins within the zona pellucida.
- Shear stress
The force acting on an object or surface parallel to the plane or slope in which it lies.
- Preovulatory follicles
Mature ovarian follicles capable of ovulating in response to an LH surge.
- Primordial follicles
Quiescent ovarian follicles that are arrested in the first meiotic prophase; characterized by an incomplete layer of squamous granulosa cells.
- Primary follicles
The next stage of follicular development after primordial follicles; characterized by a single layer of cuboidal granulosa cells or a layer of mixed squamous and cuboidal granulosa cells.
- Relative biological effectiveness
The ratio of absorbed dose of one type of radiation compared to the absorbed dose of another type of radiation that give an identical biological effect.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Endocrinology thanks F. Strollo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
REFERENCES
- 1.Evans MC & Anderson GM Integration of Circadian and Metabolic Control of Reproductive Function. Endocrinology 159, 3661–3673, doi: 10.1210/en.2018-00691 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Boden MJ, Varcoe TJ & Kennaway DJ Circadian regulation of reproduction: From gamete to offspring. Prog. Biophys. Mol. Biol 113, 387–397 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Sominsky L et al. Linking Stress and Infertility: A Novel Role for Ghrelin. Endocr. Rev 38, 432–467, doi: 10.1210/er.2016-1133 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Nargund VH Effects of psychological stress on male fertility. Nat Rev Urol 12, 373–382, doi: 10.1038/nrurol.2015.112 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Dietze G et al. Authors on behalf of the International Commission on Radiological Protection. Assessment of Radiation Exposure of Astronauts in Space. Ann. ICRP 42(4), 1–338 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Slaba TC et al. GCR Simulator Reference Field and a Spectral Approach for Laboratory Simulation NASA Technical Publication, NASA/TP-2015–218698 (NASA, Langley, VA, 2015). [Google Scholar]
- 7.Cucinotta FA & Durante M Cancer Risk from Exposure to Galactic Cosmic Rays: Implications for Space Exploration by Human Beings. Lancet Oncol. 7, 431–435 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Spitz DR, Azzam EI, Li JJ & Gius D Metabolic Oxidation/Reduction Reactions and Cellular Response to Ionizing Radiation: A Unifying Concept in Stress Response Biology. Cancer Metastasis Rev. 23, 311–322 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Dayal D, Martin SM, Limoli CL & Spitz DR Hydrogen Peroxide Mediates the Radiation-Induced Mutator Phenotype in Mammalian Cells. Biochem. J 413, 185–191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sridharan DM, L.J. C, Whalen MK, Cucinotta FA & Pluth JM Defining the Biological Effectiveness of Components of High-LET Track Structure. Radiat. Res 184, 105–119 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Tokuyama Y, Furusawa Y, Ide H, Yasui A & Terato H Role of Isolated and Clustered DNA Damage and the Post-Irradiating Repair Process in the Effects of Heavy Ion Beam Irradiation. J. Radiat. Res. (Tokyo) 56, 446–455, doi: 10.1093/jrr/rru122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridharan DM et al. Understanding Cancer Development Processes after HZE-Particle Exposure: Role of ROS, DNA Damage Repair and Inflammation. Radiat. Res 183, 1–26 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Steller JG, Alberts JR & Ronca AE Oxidative Stress as Cause, Consequence, or Biomarker of Altered Female Reproduction and Development in the Space Environment. Int. J. Mol. Sci 19, 3729, 3721–3726, doi: 10.3390/ijms19123729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aitken RJ & Roman SD in Molecular Mechanisms in Spermatogenesis (ed Cheng CY) Ch. 9, 154–171 (Landes Bioscience and Springer Science+Business Media, 2008). [Google Scholar]
- 15.Devine PJ, Perreault SD & Luderer U Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity. Biol. Reprod 86, 1–10, doi: 10.1095/biolreprod.111.095224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Wang H & Liu Z Effects of real and simulated weightlessness on the cardiac and peripheral vascular functions of humans: A review. Int. J. Occup. Med. Environ. Health 28, 793–802, doi: 10.13075/ijomeh.1896.00301 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Nishimura N & Kawai Y Adaptation to microgravity, deconditioning, and countermeasures. J. Physiol. Sci 67, 271–281, doi: 10.1007/s12576-016-0514-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ade CJ, Broxterman RM & Barstow TJ VO(2max) and Microgravity Exposure: Convective versus Diffusive O(2) Transport. Med. Sci. Sports Exerc 47, 1351–1361, doi: 10.1249/mss.0000000000000557 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Bergouignan A et al. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. NPJ Microgravity 2, 16029, doi: 10.1038/npjmgrav.2016.29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernikos J & Schneider VS Space, Gravity and the Physiology of Aging: Parallel or Convergent Disciplines? Gerontology 56, 157–166 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Prisk GK Microgravity and the respiratory system. Eur. Respir. J 43, 1459–1471, doi: 10.1183/09031936.00001414 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Herranz R et al. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13, 1–17, doi: 10.1089/ast.2012.0876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tou J, Ronca A, Grindeland R & Wade C Models to study gravitational biology of Mammalian reproduction. Biol. Reprod 67, 1681–1687 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Jennings RT & Baker ES Gynecological and Reproductive Issues for Women in Space: A Review. Obstet. Gynecol. Surv 55, 109–116 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Jain V & Wotring VE Medically Induced Amenorrhea in Female Astronauts. NPJ Microgravity 2, 16008, doi: 10.1038/npjmgrav.2016.8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronca AE et al. Effects of Sex and Gender on Adaptations to Space: Reproductive Health. J. Womens Health 23, 967–974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schatten G, Simerly C & Schatten H Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc. Natl. Acad. Sci. U. S. A 82, 4152–4156 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza KA, Black SD & Wassersug RJ Amphibian development in the virtual absence of gravity. Proc. Natl. Acad. Sci. U. S. A 92, 1975–1978 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aimar C et al. Microgravity and hypergravity effects on fertilization of the salamander Pleurodeles waltl (urodele amphibian). Biol. Reprod 63, 551–558 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Ijiri K Development of space-fertilized eggs and formation of primordial germ cells in the embryos of Medaka fish. Adv. Space Res 21, 1155–1158 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Serova LV & Denisova LA The effect of weightlessness on the reproductive function of mammals. Physiologist 25, S9–12 (1982). [PubMed] [Google Scholar]
- 32.Serova LV, Denisova LA, Makeeva VF, Chelnaya NA & Pustynnikova AM The Effect of Microgravity on the Prenatal Development of Mammals. Physiologist 27, S107–S110 (1984). [Google Scholar]
- 33.Burden HW et al. Effects of space flight on ovarian-hypophyseal function in postpartum rats. J. Reprod. Fertil 109, 193–197 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Wong AM, De Santis MJIP & Science B Rat gestation during space flight: Outcomes for dams and their offspring born after return to earth. Integr. Physiol. Behav. Sci 32, 322–342, doi: 10.1007/bf02688630 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Ronca AE & Alberts JR Physiology of a microgravity environment selected contribution: effects of spaceflight during pregnancy on labor and birth at 1 G. J. Appl. Physiol. (1985) 89, 849–854; discussion 848, doi: 10.1152/jappl.2000.89.2.849 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Burden HW, Zary J & Alberts JR Effects of space flight on the immunohistochemical demonstration of connexin 26 and connexin 43 in the postpartum uterus of rats. J. Reprod. Fertil 116, 229–234 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Burden HW, Poole MC, Zary J, Jeansonne B & Alberts JR The effects of space flight during gestation on rat uterine smooth muscle. Journal of Gravitational Physiology 5, 23–29 (1998). [PubMed] [Google Scholar]
- 38.Fejtek M & Wassersug R Effects of laparotomy, cage type, gestation period and spaceflight on abdominal muscles of pregnant rodents. J. Exp. Zool 284, 252–264 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Hirshfield AN Overview of Ovarian Follicular Development: Considerations for the Toxicologist. Environ. Mol. Mutagen 29, 10–15 (1997). [PubMed] [Google Scholar]
- 40.Gougeon A Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses. Endocr. Rev 17, 121–155 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Zhang S et al. Simulated Microgravity Using a Rotary Culture System Compromises the In Vitro Development of Mouse Preantral Follicles. PLoS One 11, e0151062, doi: 10.1371/journal.pone.0151062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Bhat GK & Sridaran R Clinostat rotation induces apoptosis in luteal cells of the pregnant rat. Biol. Reprod 66, 770–777 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Bhat GK, Yang H & Sridaran R Simulated conditions of microgravity suppress progesterone production by luteal cells of the pregnant rat. Journal of Gravitational Physiology 8, 57–66 (2001). [PubMed] [Google Scholar]
- 44.Tou JC, Grindeland RE & Wade CE Effects of diet and exposure to hindlimb suspension on estrous cycling in Sprague-Dawley rats. Am. J. Physiol 286, E425–433, doi: 10.1152/ajpendo.00287.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Ronca AE, Baer LA & Wade CE Hypergravity effects on pregnancy and parturition. J Gravit Physiol 9, P203–204 (2002). [PubMed] [Google Scholar]
- 46.Ronca AE, Baer LA, Daunton NG & Wade CE Maternal reproductive experience enhances early postnatal outcome following gestation and birth of rats in hypergravity. Biol. Reprod 65, 805–813 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Oyama J & Platt WT Reproduction and Growth of Mice and Rats under Conditions of Simulated Increased Gravity. Am. J. Physiol 212, 164–166 (1967). [DOI] [PubMed] [Google Scholar]
- 48.Moore J & Duke J Effect of chronic centrifugation on mouse breeding pairs and their offspring. Physiologist 31, S120–121 (1988). [PubMed] [Google Scholar]
- 49.Megory E & Oyama J Hypergravity induced prolactin surge in female rats. Aviat. Space Environ. Med 56, 415–418 (1985). [PubMed] [Google Scholar]
- 50.Megory E & Oyama J Hypergravity effects on litter size, nursing activity, prolactin, TSH, T3, and T4 in the rat. Aviat. Space Environ. Med 55, 1129–1135 (1984). [PubMed] [Google Scholar]
- 51.Megory E, Konikoff F, Ishay JS & Lelyveld J Hypergravity: its effect on the estrous cycle and hormonal levels in female rats. Life Sci. Space Res 17, 213–218 (1979). [DOI] [PubMed] [Google Scholar]
- 52.Megory E & Ishay JS Hypergravity induced prolonged diestrous in the rat can be prevented by bromergocryptine or by previous exposure to the same conditions - a “memory” effect. Life Sci. 27, 1503–1507 (1980). [DOI] [PubMed] [Google Scholar]
- 53.Lintault LM et al. In a hypergravity environment neonatal survival is adversely affected by alterations in dam tissue metabolism rather than reduced food intake. J. Appl. Physiol. (1985) 102, 2186–2193, doi: 10.1152/japplphysiol.01015.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Wang H et al. Variation in Commercial Rodent Diets Induces Disparate Molecular and Physiological Changes in the Mouse Uterus. Proc. Natl. Acad. Sci. U. S. A 102, 9960–9965 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu C et al. Simulated Microgravity Compromises Mouse Oocyte Maturation by Disrupting Meiotic Spindle Organization and Inducing Cytoplasmic Blebbing. PLoS One 6, e22214, doi: 10.1371/journal.pone.0022214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uva BM et al. Morpho-functional alterations in testicular and nervous cells submitted to modelled microgravity. J. Endocrinol. Invest 28, 84–91 (2005). [PubMed] [Google Scholar]
- 57.Wakayama S et al. Detrimental effects of microgravity on mouse preimplantation development in vitro. PLoS One 4, e6753, doi: 10.1371/journal.pone.0006753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y et al. A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK. Reprod. Sci 16, 947–959, doi: 10.1177/1933719109337544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronca AE Studies toward birth and early mammalian development in space. Adv. Space Res 32, 1483–1490, doi: 10.1016/s0273-1177(03)90385-1 (2003). [DOI] [PubMed] [Google Scholar]
- 60.La Tessa C, Sivertz M, Chiang I-H, Lowenstein D & Rusek A Overview of the NASA Space Radiation Laboratory. Life Sci. Space Res 11, 18–23 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Pesty A, Doussau M, Lahaye J-B & Lefèvre B Whole-Body or Isolated Ovary 60Co Irradiation: Effects on in Vivo and in Vitro Folliculogenesis and Oocyte Maturation. Reprod. Toxicol 29, 93–98 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Mathur S, Nandchahal K & Bhartiya HC Radioprotection by MPG of Mice Ovaries Exposed to Sublethal Gamma Radiation Doses at Different Postnatal Ages. Acta Oncol. 30, 981–983 (1991). [DOI] [PubMed] [Google Scholar]
- 63.Nitta Y & Hoshi M Relationship between Oocyte Apoptosis and Ovarian Tumors Induced by High and Low LET Radiations in Mice. Int. J. Radiat. Biol 79, 241–250 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Mishra B, Ripperdan R, Ortiz L & Luderer U Very Low Doses of Heavy Oxygen Ion Radiation Induce Premature Ovarian Failure. Reproduction 154, 123–133, doi: 10.1530/REP-17-0101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra B, Ortiz L & Luderer U Charged Iron Particles, Typical of Space Radiation, Destroy Ovarian Follicles. Hum. Reprod 31, 1816–1826, doi: 10.1093/humrep/dew126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderhyden BC Loss of Ovarian Function and the Risk of Ovarian Cancer. Cell Tissue Res. 322, 117–124 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Salehi F, Dunfield L, Phillips KP, Krewski D & Vanderhyden BC Risk Factors for Ovarian Cancer: An Overview with Emphasis on Hormonal Factors. J. Toxicol. Environ. Health. B. Crit. Rev 11, 301–321 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Mishra B, Lawson GW, Ripperdan R, Ortiz L & Luderer U Charged-Iron-Particles Found in Galactic Cosmic Rays are Potent Inducers of Epithelial Ovarian Tumors. Radiat. Res 190, 142–150, doi: 10.1667/RR15028.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrivé L et al. Radiation-induced uterine changes: MR imaging. Radiology 170, 55–58, doi: 10.1148/radiology.170.1.2909120 (1989). [DOI] [PubMed] [Google Scholar]
- 70.Larsen EC et al. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstetetricia Gynecologica Scandinavica 83, 96–102 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Bath LE et al. Ovarian and uterine characteristics after total body irradiation in childhood and adolescence: response to sex steroid replacement. Br. J. Obstet. Gynaecol 106, 1265–1272 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Carabajal E et al. Radioprotective potential of histamine on rat small intestine and uterus. Eur. J. Histochem 56, e48, doi: 10.4081/ejh.2012.e48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Critchley HOD & Wallace WHB Safety of Pregnancy during and after Cancer Treatment. J. Natl. Cancer Inst. Monogr 34, 64–68 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Wood DH, Yochmowitz MG, Hardy KA & Salmon YL Animal studies of life shortening and cancer risk from space radiation. Adv. Space Res 6, 275–283 (1986). [DOI] [PubMed] [Google Scholar]
- 75.Fanton JW & Golden JG Radiation-induced endometriosis in Macaca mulatta. Radiat. Res 126, 141–146 (1991). [PubMed] [Google Scholar]
- 76.Palumbo G et al. Effect of space radiation on expression of apoptosis-related genes in endometrial cells: a preliminary study. Physica Medica 17 Suppl 1, 241–246 (2001). [PubMed] [Google Scholar]
- 77.Strollo F et al. The Effect of Microgravity on Testicular Androgen Secretion. Aviat. Space Environ. Med 69, 133–136 (1998). [PubMed] [Google Scholar]
- 78.Smith SM, Heer M, Wang Z, Huntoon CL & Zwart SR Long-duration space flight and bed rest effects on testosterone and other steroids. Journal of Clinical Endocrinology Metabolism 97, 270–278, doi: 10.1210/jc.2011-2233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merrill AH Jr., Wang E, Mullins RE, Grindeland RE & Popova IA Analyses of plasma for metabolic and hormonal changes in rats flown aboard COSMOS 2044. Journal of applied physiology (Bethesda, MD: 1985) 73, 132s–135s, doi: 10.1152/jappl.1992.73.2.S132 (1992). [DOI] [PubMed] [Google Scholar]
- 80.Amann RP et al. Effects of microgravity or simulated launch on testicular function in rats. Journal of Applied Physiology (Bethesda, MD: 1985) 73, 174s–185s, doi: 10.1152/jappl.1992.73.2.S174 (1992). [DOI] [PubMed] [Google Scholar]
- 81.Sapp WJ et al. Effects of spaceflight on the spermatogonial population of rat seminiferous epithelium. FASEB J. 4, 101–104 (1990). [DOI] [PubMed] [Google Scholar]
- 82.Masini MA et al. The impact of long-term exposure to space environment on adult mammalian organisms: a study on mouse thyroid and testis. PLoS One 7, e35418, doi: 10.1371/journal.pone.0035418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russell LD, Ettlin RA, Hikim APS & Clegg ED Histological and Histopathological Evaluation of the Testis. (Cache River Press, 1990). [Google Scholar]
- 84.Sapp WJ et al. Comparative study of spermatogonial survival after x-ray exposure, high LET (HZE) irradiation or spaceflight. Adv. Space Res 12, 179–189 (1992). [DOI] [PubMed] [Google Scholar]
- 85.Tash JS & Bracho GE Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J. 13 Suppl, S43–54 (1999). [DOI] [PubMed] [Google Scholar]
- 86.Wakayama S et al. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc. Natl. Acad. Sci. U. S. A 114, 5988–5993, doi: 10.1073/pnas.1701425114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrando AA, Lane HW, Stuart CA, Davis-Street J & Wolfe RR Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am. J. Physiol 270, E627–633, doi: 10.1152/ajpendo.1996.270.4.E627 (1996). [DOI] [PubMed] [Google Scholar]
- 88.Deaver DR et al. Effects of caudal elevation on testicular function in rats. Separation of effects on spermatogenesis and steroidogenesis. J. Androl 13, 224–231 (1992). [PubMed] [Google Scholar]
- 89.Hadley JA, Hall JC, O’Brien A & Ball R Effects of a simulated microgravity model on cell structure and function in rat testis and epididymis. Journal of Applied Physiology (Bethesday, MD: 1985) 72, 748–759, doi: 10.1152/jappl.1992.72.2.748 (1992). [DOI] [PubMed] [Google Scholar]
- 90.Zirkin BR, Santulli R, Awoniyi CA & Ewing LL Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043–3049, doi: 10.1210/endo-124-6-3043 (1989). [DOI] [PubMed] [Google Scholar]
- 91.Tash JS, Johnson DC & Enders GC Long-term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. Journal of Applied Physiology (Bethesda, MD: 1985) 92, 1191–1198, doi: 10.1152/japplphysiol.00931.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Ricci G, Esposito R, Catizone A & Galdieri M Direct effects of microgravity on testicular function: analysis of hystological, molecular and physiologic parameters. J. Endocrinol. Invest 31, 229–237, doi: 10.1007/bf03345595 (2008). [DOI] [PubMed] [Google Scholar]
- 93.Engelmann U, Krassnigg F & Schill WB Sperm motility under conditions of weightlessness. J. Androl 13, 433–436 (1992). [PubMed] [Google Scholar]
- 94.Ikeuchi T et al. Human sperm motility in a microgravity environment. Reprod. Med. Biol 4, 161–168, doi: 10.1111/j.1447-0578.2005.00092.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamiya H et al. Effect of simulated microgravity on testosterone and sperm motility in mice. J. Androl 24, 885–890 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Usik MA & Ogneva IV Cytoskeleton Structure in Mouse Sperm and Testes After 30 Days of Hindlimb Unloading and 12 Hours of Recovery. Cell. Physiol. Biochem 51, 375–392, doi: 10.1159/000495235 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Ortiz RM, Wade CE & Morey-Holton E Urinary excretion of LH and testosterone from male rats during exposure to increased gravity: post-spaceflight and centrifugation. Proc. Soc. Exp. Biol. Med 225, 98–102 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Veeramachaneni DN, Deaver DR & Amann RP Hypergravity does not affect testicular function. Aviat. Space Environ. Med 69, A49–52 (1998). [PubMed] [Google Scholar]
- 99.Gray GD, Smith ER, Damassa DA & Davidson JM Effects of centrifugation stress on pituitary-gonadal function in male rats. J. Appl. Physiol 48, 1–5, doi: 10.1152/jappl.1980.48.1.1 (1980). [DOI] [PubMed] [Google Scholar]
- 100.Alpen EL & Powers-Risius P The Relative Biological Effectiveness of High-Z, High-LET Charged Particles for Spermatogonial Killing. Radiat. Res 88, 132–143 (1981). [PubMed] [Google Scholar]
- 101.Vaglenov A, Fedorenko B & Kaltenboeck B RBE and Genetic Susceptibility of Mouse and Rat Spermatogonial Stem Cells to Protons, Heavy Charged Particles, and 1.5 MeV Neutrons. Adv. Space Res 39, 1093–1101 (2007). [Google Scholar]
- 102.Li HY et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed Environ Sci 26 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Li H et al. Proteomic Analysis of Testis for Mice Exposed to Carbon Ion Radiation. Mutat. Res 755, 148–155 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Zhao Q et al. 56Fe ion irradiation induced apoptosis through Nrf2 pathway in mouse testis. Life Sci. 157, 32–37 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Li H et al. Comparative proteomics reveals the underlying toxicological mechanism of low sperm motility induced by iron ion radiation in mice. Reprod. Toxicol 65, 148–158 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Schatten H et al. Effects of Spaceflight Conditions on Fertillization and Embryogenesis in the Sea Urchin Lytechinus Pictus. Cell Biol. Int 23, 407–415 (1999). [DOI] [PubMed] [Google Scholar]
- 107.Plakhuta-Plakutina GI, Serova LV, Dreval AA & Tarabrin SB [Effect of 22-day space flight factors on the state of the sex glands and reproductive capacity of rats]. Kosm. Biol. Aviakosm. Med 10, 40–47 (1976). [PubMed] [Google Scholar]
- 108.Serova LV, Denisova LA, Apanasenko ZI, Kuznetsova MA & Meizerov ES [Reproductive function of the male rat after a flight on the Kosmos-1129 biosatellite]. Kosm. Biol. Aviakosm. Med 16, 62–65 (1982). [PubMed] [Google Scholar]
- 109.Padmanabhan V, Puttabyatappa M & Cardoso R in Female Reproduction Vol. 2 Encyclopedia of Reproduction (eds Spencer T & Flaws J) 121–129 (Elsevier, 2018). [Google Scholar]
- 110.Pepling ME From Primordial Germ Cell to Primordial Follicle: Mammalian Female Germ Cell Development. Genesis 44, 622–632 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Juengel JL & McNatty KP The Role of Proteins of the Transforming Growth Factor-β Superfamily in the Intraovarian Regulation of Follicular Development. Hum. Reprod. Update 11, 144–161 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Zheng W, Nagaraju G, Liu Z & Liu K Functional Roles of the Phosphatidylinositol 3-Kinases (PI3Ks) Signaling in the Mammalian Ovary. Mol. Cell. Endocrinol 356, 24–30 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Hennebold JD in Female Reproduction Vol. 2 Encyclopedia of Reproduction (eds Flaws JA & Spencer TE) 99–105 (Academic Press, 2018). [Google Scholar]
- 114.Abel MH et al. Spermatogenesis and Sertoli Cell Activity in Mice Lacking Sertoli Cell Receptors for Follicle-Stimulating Hormone. Endocrinology 149, 3279–3285 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oduwole OO, Peltoketo H & Huhtaniemi IT Role of Follicle-Stimulating Hormone in Spermatogenesis. Frontiers in Endocrinology (Lausanne) 9, 763, doi: 10.3389/fendo.2018.00763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


