Abstract
Background
Healthcare workers (HCWs) are especially vulnerable to infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Aim
The aim of this study was to describe the epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19) among HCWs from February 24th to April 30th, 2020, in a hospital in Madrid, Spain.
Methods
This was a retrospective cohort study. Cumulative COVID-19 incidence was calculated for all HCWs and categorized according to presumed level of COVID-19 exposure (high, medium, and low).
Findings
Among 1911 HCWs, 213 (11.1%) had COVID-19 during the study period. Cases increased gradually from March 8th, peaking on March 17th and declining thereafter. The peak of cases among HCWs was reached 14 days before the peak in admitted COVID-19 cases in the hospital. There were no significant differences in the proportion of COVID-19 cases according to level of occupational exposure (P = 0.123). There were five departments and two professions in which >20% of the workers had confirmed COVID-19. Temporal clusters were identified in three of these departments and one profession, with most of the cases occurring over a period of less than five days. The prevalence of comorbidities was low and 91.5% of patients had mild or moderate symptoms. Eleven patients were admitted to the hospital and one patient needed intensive care. None of the patients died. The median time of sick leave was 20 (interquartile range: 15–26) days.
Conclusion
The results suggest that HCW–HCW transmission accounted for part of the cases. In spite of a low prevalence of comorbidities and a mild clinical course in most cases, COVID-19 caused long periods of sick leave.
Keywords: Health personnel, COVID-19, Hospital infections, Infection control
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causal agent of coronavirus disease 2019 (COVID-19) [1]. It is estimated that by May 25th, 2020, COVID-19 had caused 5,304,772 infections and 342,029 deaths [2]. The first COVID-19 case was declared in Spain on January 31st, 2020, and since then, Spain has been one of the countries most affected by the pandemic [2,3].
Healthcare workers (HCWs) are especially vulnerable to infection by SARS-CoV-2. In the first published series of 138 patients from Wuhan, China, 29% of the cases were HCWs [1]. The proportion of HCWs was much lower (3.8%) in a later case series from China including 72,314 COVID-19 cases [4]. In Spain, 22% of all the COVID-19 cases have been among HCWs, and in Italy, 20% of responding HCWs were infected [5,6].
Very few studies have focused on SARS-CoV-2 infection among HCWs. Moreover, the scarce data available hitherto have focused on the proportion of HCWs infected but have not sufficiently described epidemiological and clinical characteristics of the affected workers.
There are important implications of COVID-19 among HCWs. First, there are consequences for their health: in the previously mentioned series from China, 14.8% of the cases among HCWs were classified as severe or critical and five of the patients died [4]. Second, infected HCWs could also transmit the infection to vulnerable patients if they are not properly and swiftly isolated. Third, high rates of infection among HCWs could cause problems due to understaffing in the health system. Fourth, the workers may transmit the infection to close family contacts, other HCWs, and the community [7,8].
Due to the important implications of COVID-19 among HCWs, and the lack of detailed information published about this issue, it is important to better characterize its epidemiology and clinical characteristics in order to inform decision-makers on appropriate prevention and management strategies. Therefore, we designed a retrospective cohort study whose aim was to describe the epidemiological and clinical characteristics of SARS-CoV-2 infection among HCWs in a hospital in Madrid, Spain.
Methods
Study subjects
The study was performed in Hospital Universitario Infanta Sofía, a public tertiary hospital in Madrid, Spain. All HCWs working in the hospital between February 24th and April 30th, 2020, were included. Workers from the laboratory, radiology department, security services, maintenance services, kitchen facilities, cleaning workers and porters were also excluded as they are contracted by external companies and are not employed by the hospital.
Variables and data collection
All data were gathered retrospectively. The number of HCWs according to their profession and department for the study period was obtained from the human resources department. Data for COVID-19 cases were extracted from the clinical records of the occupational health department and recorded in an anonymized database.
During the study period, HCWs experiencing symptoms consistent with probable COVID-19 were instructed to present at the occupational health outpatient clinic, where they were managed according to the hospital protocol: a nasopharyngeal swab was collected and analysed with polymerase chain reaction (PCR) for SARS-CoV-2. For symptomatic HCWs with negative PCR, this test was repeated after 48–72 h if symptoms persisted.
According to the hospital protocol, HCWs with positive SARS-CoV-2 PCR remained on sick leave until a negative follow-up PCR was obtained. Follow-up PCRs among patients with a previous positive PCR were obtained between days 2 and 5 after symptom resolution and repeated thereafter until a negative PCR result was obtained. Workers were allowed to return to work when they fulfilled two criteria: symptoms had resolved, and they had a negative follow-up PCR.
A case of COVID-19 was defined as any HCW presenting to the occupational health outpatient clinic with symptoms consistent with COVID-19 and with positive SARS-CoV-2 PCR. The date of the case was defined as the date of presentation to the occupational health outpatient clinic. The following variables were obtained from the clinical records: age, sex, profession (head of department (i.e. a physician in charge of a medical or surgical department), physician, nurse supervisor, nurse/auxiliary nurse, other), department, date of presentation at the outpatient clinic, date of symptom onset, duration of sick leave, admission to hospital, presence of comorbidities (arterial hypertension, diabetes mellitus, current tobacco consumption, cardiovascular disease, and chronic obstructive bronchopulmonary disease or asthma). Nine patients had no information on the day of symptom onset: for these patients, the date of symptom onset was assumed to be the date of presentation to the outpatient clinic. Clinical course was divided in the following categories: mild symptoms (including myalgia, ageusia, anosmia, headache, sore throat, cough, or temperature <38°C, plus no need to stay in bed), moderate symptoms (including fever ≥38°C, or need to stay in bed, with or without any of the mild symptoms), and unilateral or bilateral infiltrates in chest X-ray.
HCWs were stratified in three categories according to their presumed level of occupational exposure to COVID-19 cases: high risk (HCWs with usual contact with COVID-19 patients: accident and emergency, internal medicine, intensive care, and pneumology departments), moderate risk (HCWs with occasional contact with COVID-19 patients: other medical and surgical departments not included in the high- or low-risk groups), and low risk (including administrative workers, social workers, hospital management, and pharmacy, pathology, and preventive medicine departments). For nurses working in the hospital wards and haemodialysis unit, the department was not available and therefore they were included in a separate category for the purpose of risk of occupational exposure.
In addition to these variables, information was collected about the training in COVID-19 prevention and in use of personal protective equipment (PPE) in the hospital during the study period. Total numbers of notified COVID-19 cases confirmed by PCR were obtained from the Madrid Autonomous Region website [9].
Statistical analysis
COVID-19 cumulative incidence during the study period was calculated for all HCWs, stratified by presumed level of occupational exposure, and stratified by department and profession. For departments and professions with more than 10 HCWs, of whom more than 20% were diagnosed with COVID-19, date of presentation was analysed in detail to detect possible transmission clusters. Descriptive analyses were carried out using frequency distributions or median and interquartile range (IQR), as appropriate. The proportions of HCWs acquiring COVID-19 among the three categories according to risk of occupational exposure were compared with χ2-test. Statistical analyses were carried out with Stata version 13.0 (Stata Corp., College Station, TX, USA).
Ethics
The study was approved by the Ethics Committee of Hospital Universitario ‘La Paz’ (PI-4133). Informed consent was not required.
Results
By April 30th, 2020, there were 1911 HCWs in our hospital who fulfilled inclusion criteria. Among these, 652 presented to the occupational health outpatient clinic with symptoms consistent with COVID-19 and were tested with at least one PCR during the study period. A total of 213 workers, accounting for 11.1% of all HCWs included, had microbiological confirmation of COVID-19. Median age was 42 years (IQR: 34–52), and 171 (80.3%) cases were women.
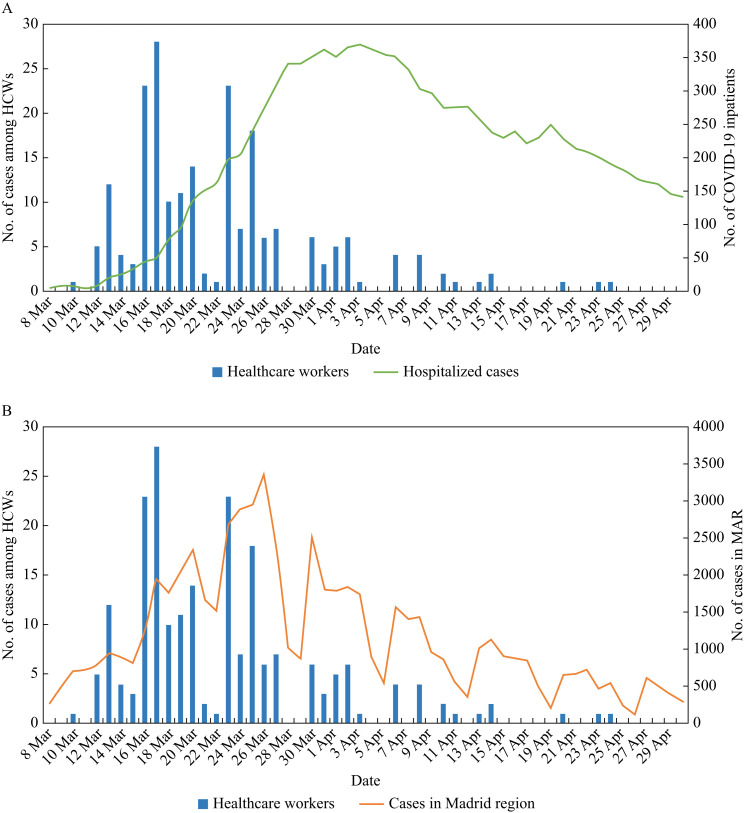
Figure 1 shows the number of confirmed cases among HCWs per day, along with the number of patients admitted in the hospital with COVID-19 (A) and the total number of cases notified in the Madrid Autonomous Region (B) during the study period. The first case among HCWs was diagnosed on March 8th and cases increased gradually, reaching a peak on March 17th and declining thereafter. The peak of cases among HCWs was reached 14 days before the peak in admitted COVID-19 cases in the hospital, which occurred on March 31st (Figure 1A), and 9 days before the peak in the notified cases in the Madrid Autonomous Region, which occurred on March 26th (Figure 1B). As expected, cases among HCWs very rarely presented during weekends.
Figure 1.
Number of healthcare workers with confirmed COVID-19 by day of presentation to the clinic (blue bars). The number of patients with COVID-19 who were admitted to the hospital for the study period (A, green line) and the number of microbiologically confirmed reported cases in the Madrid Autonomous Region (MAR) (B, orange line) are also shown. Weekend days (Saturday and Sunday) are March 7th–8th, 14th–15th, 21st–22nd and 28th–29th, and April 4th–5th, 11th–12th, 18th–19th, 25th–26th. One patient with COVID-19 who stayed in the hospital from February 24th to 28th is not shown in (A). HCWs, healthcare workers.
The median delay between the date of symptom onset and the date of presentation to the outpatient clinic was 2 days (IQR: 1–5). The first date of symptom onset was February 17th and then increased gradually, reaching a peak on March 16th and gradually declining thereafter.
The proportion of cases among HCWs according to level of occupational exposure is shown in Table I . There were no significant differences in the proportion of COVID-19 cases according to level of occupational exposure (high, medium, or low) (P = 0.123). There were five departments of more than 10 HCWs in which more than 20% of the workers had confirmed COVID-19: dermatology (4/15, 26.7%), pneumology (4/13, 30.8%), neurology (5/14, 35.7%), oncology (3/11, 27.3%), and paediatrics (10/26, 38.5%).
Table I.
Proportion of COVID-19 cases according to level of occupational exposure
| Level of exposure | Department | COVID-19 cases | Total no. of HCWs |
|---|---|---|---|
| High | Medical COVID-19 areasa | 9 (18.7%) | 48 |
| Intensive care unit | 13 (5.7%) | 226 | |
| Emergency department | 30 (10.1%) | 298 | |
| Total high exposure level | 52 (9.1%) | 572 | |
| Medium | Outpatient clinic | 25 (14.1%) | 177 |
| Gynaecology/obstetrics | 4 (5.4%) | 74 | |
| Daycare hospital | 4 (10.5%) | 38 | |
| Medical no COVID-19 areasb | 37 (16.1%) | 229 | |
| Surgical | 24 (9.9%) | 243 | |
| Total medium exposure level | 94 (12.3%) | 761 | |
| Low | Pathology | 1 (6.2%) | 16 |
| Pharmacy | 3 (10.0%) | 30 | |
| Administrative, management, and preventive medicine departments, and social workers | 5 (8.5%) | 59 | |
| Total low exposure level | 9 (8.6%) | 105 | |
| Nurses working in hospital wards and haemodialysis unit | 58 (12.6%) | 460 | |
| Total | 213 (11.2%) | 1898 |
Includes internal medicine and pneumology departments.
Includes all medical departments except those classified as high risk.
The proportions of cases according to profession are shown in Table II . There were two professions in which more than 20% of the workers had confirmed COVID-19: medical heads of department (23.1% with COVID-19) and nurse supervisors (37.5%).
Table II.
Proportion of COVID-19 cases according to profession
| Work category | COVID-19 cases | Total no. of HCWs |
|---|---|---|
| Head of department | 9 (23.1%) | 39 |
| Physician | 64 (13.0%) | 491 |
| Nurse supervisor | 9 (37.5%) | 24 |
| Nurse/auxiliary nurse | 126 (10.2%) | 1231 |
| Othera | 5 (4.4%) | 113 |
| Total | 213 (11.2%) | 1898 |
Other includes: administrative workers, hospital management, physiotherapists, occupational therapists, midwives, social workers, technicians.
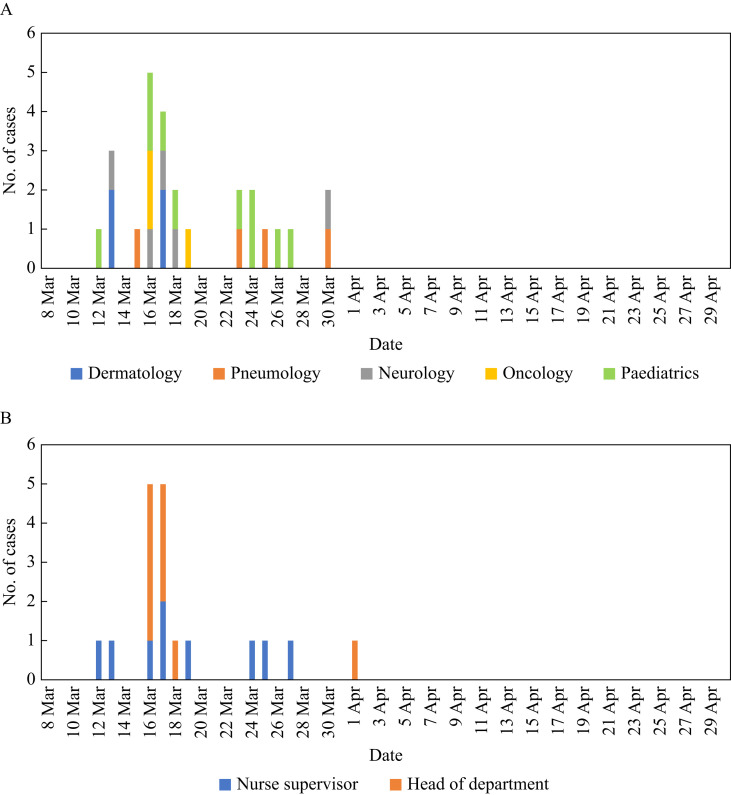
The number of cases according to date of presentation for the departments and professions in which more than 20% of the HCWs had COVID-19 are shown in Figure 2 A and B, respectively. The cases in the oncology, dermatology and neurology departments seemed to be clustered in time, with all cases presenting in a period of 3 and 4 days in the oncology and dermatology departments, and four out of five cases presenting in a period of 5 days in the neurology department. According to work category, there also seemed to be a temporal cluster among heads of department, with eight out of nine cases presenting over a period of 3 days (Figure 2A and B).
Figure 2.
Number of cases according to date of presentation for the departments (A) and professions (B) with more than 10 HCWs and in which more than 20% of the HCWs had COVID-19.
Regarding comorbidities, 10 workers (4.75%) had arterial hypertension, nine (4.2%) had asthma or chronic obstructive bronchopulmonary disease, seven (3.3%) were active smokers, four (1.9%) had cardiovascular disease, and one worker had diabetes mellitus.
Regarding the clinical course, 144 (67.6%) had mild symptoms, 51 (23.9%) had moderate symptoms, seven (3.3%) had unilateral lung infiltrates in chest X-ray, and 11 (5.2%) had bilateral lung infiltrates. In addition, two patients with unilateral lung infiltrates also had thromboembolic disease: one patient had deep venous thrombosis and the other had pulmonary embolism. The majority of cases were managed in their home, but 11 cases (5.2%) needed admission to the hospital for a median of 4 days (IQR: 4–10). One of the patients with bilateral pneumonia needed ICU admission and orotracheal intubation because of severe respiratory failure. None of the patients died.
Among the 213 workers, 25 were still on sick leave at the end of the study period. The median time of sick leave among the 188 HCWs who had returned to work was 20 days (IQR: 15–26). After symptom resolution, the median number of PCRs performed per HCW was 1 (IQR: 1–2), and the median time to obtain a negative PCR result (and therefore to allow the HCW to return to work) was 5 days (IQR: 1–7).
Training in infection control practices and use of PPE was provided by the occupational health department from February 20th to March 13th for the intensive care unit, hospitalization wards, surgical rooms, daycare hospital, obstetric ward, nurse supervisors, and the internal medicine, anaesthesia, general surgery and emergency departments. In addition one training session was provided in the pharmacy department on April 22nd. There were a total of 23 training sessions involving a total of 298 (15.6%) HCWs.
Discussion
This study has analysed the epidemiology of SARS-CoV-2 infection among HCWs in a public hospital in Spain during the height of the COVID-19 epidemic. In all, 11.1% of the HCWs had microbiologically confirmed COVID-19. The peak of cases preceded the peak of admitted patients with COVID-19, with a lag of two weeks. The risk of COVID-19 did not differ significantly among three groups with different levels of occupational exposure to COVID-19 patients, and a few clusters were detected in specific departments and professions: these facts suggest that a considerable proportion of the transmissions occurred from HCW to HCW, rather than from patient to HCW. Infected workers had low prevalence of comorbidities and the clinical course was mild in most cases; nevertheless, COVID-19 caused long periods of sick leave.
The sources of HCW infection include patients with COVID-19, other HCWs, and the community. During the COVID-19 pandemic several reports have identified factors increasing the risk of patient–HCW exposure – mainly excessive workload, shortage of PPE, lack of training in infection control measures, or use of PPE that does not fulfil safety requirements [[10], [11], [12]]. Also, HCWs may become infected outside their workplace if there is ongoing transmission in the community (community–HCW) [13]. However, it has been pointed out that there is potential for transmission between HCWs, with potential occasions such as clinical meetings, clinical handovers, lunchbreaks, and shared use of small work spaces [14]. Nevertheless, the transmission of COVID-19 between workers has not been sufficiently studied: we are only aware of a study in Singapore that identified a cluster of intrahospital HCW–HCW transmission, and an anecdotal recent report from newspapers that described an outbreak in Madrid, where five HCWs acquired COVID-19 after a lunch that took place in a hospital with more than 25 participants, who were not wearing masks during the event [8,15].
In our study, the fact that cases peaked well before the peak in admitted COVID-19 patients and the peak of diagnosed cases in the community, the lack of differences in the risk of infection among groups with different levels of exposure to COVID-19, and the identification of several temporal clusters among certain groups suggest that transmission among HCWs accounts for part of the cases. It is possible that several HCWs became infected during the first days (either from undiagnosed COVID-19 cases in the hospital, or from the community) and then in turn transmitted the infection to other HCWs. Three departments (oncology, dermatology, and neurology) had most of the cases diagnosed in a short period of time, suggesting transmission in shared facilities or clinical meetings. There was also a temporal cluster among the heads of department, and a high proportion of the nurse supervisors affected, although the latter were not temporally related. These two groups were having meetings every day as well as doing regular night shifts, and it is possible that there had been transmissions during the meetings or through fomites in the staff rooms or the emergency phone.
In our hospital, workers were instructed to wear adequate PPE when contacting patients with confirmed or suspected COVID-19. However, at the time of the study the use of face masks was neither recommended by health authorities for the general population (their compulsory use was only instituted for public transport on May 3rd and closed spaces on May 20th) nor specifically recommended for HCWs who were not having contact with patients [[16], [17], [18]]. During the period of February 20th to March 13th, training about COVID-19 transmission and PPE was provided to HCWs by the occupational health department. However, the training focused on the contact with COVID-19 patients and did not specifically recommend wearing face masks when not interacting with patients. Until approximately March 13th to 16th, HCWs did not systematically wear face masks when being in contact with other HCWs in the hospital facilities. After noting an increase in cases, most departments started systematically wearing face masks at all times in the hospital; shared meals during the night shifts were stopped, instructing workers to keep a distance of ≥2 m in the refectory; and ward rooms were disinfected on March 13th and 16th. We cannot precisely determine the influence of these measures on COVID-19 transmission in the hospital, but we presume that they were effective since the cases in HCWs started declining after March 17th despite an increasing number of patients admitted to the hospital with COVID-19. It is unlikely that better training on the use of PPE could explain the decrease in cases among HCWs, as this training was not continued after March 13th (except for one additional session in the pharmacy department on April 22nd).
The prevalence of comorbidities was low and the clinical course was mild in most of the cases; our HCWs were in general healthier and had a less severe clinical course than the series published in the general population and can probably be explained by a healthy worker effect, as well as a younger age. Also, the Spanish Ministry of Health recommended that HCWs with vulnerability to COVID-19 complications (such as HCWs aged >60 years, pregnant women, and those with certain comorbidities) who could not be relocated to avoid COVID-19 exposure should be preventively kept away from work: this applied to 58 HCWs in our hospital [1,4,19]. Nevertheless, some cases did experience severe complications such as bilateral pneumonia, thromboembolic disease, or severe respiratory failure. In addition, cases had to stay away from work for a long time, for a median time of 20 days; this long sick leave, along with the workers preventively kept away from work, in a time of increasing healthcare demands due to the pandemic may have important effects due to understaffing, which entails an increase in workload and may produce additional stress on the already overworked staff that remains in the hospital [20].
Our study has two main limitations. First, not all HCWs in the hospital were systematically tested, but tests were limited to symptomatic HCWs presenting to the occupational health outpatient clinic. Thus, we could have missed a proportion of workers with asymptomatic infection. Second, the only available diagnostic test during the study period was SARS-CoV-2 PCR in nasopharyngeal swabs, which can have false negatives even in symptomatic cases: a recent review found a sensitivity of 76% (95% CI: 59–94) [21]. Therefore, our study has probably underestimated the percentage of affected HCWs.
During the COVID-19 pandemic, health services had the challenge of ensuring sufficient PPEs and that these were adjusted to quality standards. Spanish health institutions had difficulties in providing adequate PPE to all healthcare workers during these times and we cannot exclude that some transmissions could be attributed to lack of adequate PPEs or their suboptimal use. However, our results suggest that HCW–HCW transmission accounted for at least part of the COVID-19 cases found in our hospital: this transmission route is rarely mentioned in other studies [8]. We believe that this possibility should be further explored in other settings, and suggest preventive measures such as the systematic use of face masks at all times in the workplace, ensuring adequate working spaces to avoid overcrowding, and keeping a safety distance in meetings, clinical handouts, and work meals that could reduce the transmission of COVID-19 in healthcare settings. Also, surveillance of potential clusters could potentially be useful for contact tracing and prompt identification of transmissions [8].
In conclusion, in one public hospital in Spain, 11.1% of the HCWs had symptoms consistent with COVID-19 and microbiological confirmation of infection. The facts that cases among HCWs peaked two weeks before the peak of admitted patients, that the risk of COVID-19 was similar among three groups with different levels of occupational exposure, and that several temporal clusters were detected in specific departments and professions, suggest that HCW–HCW transmission accounted for part of the cases. In spite of a low prevalence of comorbidities and a mild clinical course in most cases, severe complications were diagnosed in some HCWs, and COVID-19 caused long periods of sick leave.
Acknowledgements
We thank all the nurses, physicians and occupational risk prevention technicians that took care of the HCWs in the occupational health outpatient clinic during the study.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2020. Coronavirus disease 2019. Situation report-126. [Google Scholar]
- 3.Spiteri G., Fielding J., Diercke M., Campese C., Enouf V., Gaymard A. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Equipo COVID-19 . 2020. RENAVE/CNE/CNM (ISCIII). Informe sobre la situación de COVID-19 en personal sanitario en España. [Google Scholar]
- 6.Editorial COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y., Trevathan E., Qian Z., Li Y., Li J., Xiao W. Asymptomatic SARS-CoV-2 infection in household contacts of a healthcare provider, Wuhan, China. Emerg Infect Dis. 2020;26(8) doi: 10.3201/eid2608.201016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Goh J.Q., Yeo D.W.T. Containment of COVID-19 cases among healthcare workers: the role of surveillance, early detection, and outbreak management. Infect Control Hosp Epidemiol. 2020 May 11:1–7. doi: 10.1017/ice.2020.219. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consejería de Sanidad. Comunidad de Madrid. Informe diario de situación COVID-19. 04/06/2020. June 4th, 2020.
- 10.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17 doi: 10.1093/cid/ciaa287. ciaa287 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godderis L., Boone A., Bakusic J. COVID-19: a new work-related disease threatening healthcare workers. Occup Med (Lond) 2020;70:315–316. doi: 10.1093/occmed/kqaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellizzi S., Fiamma M., Arru L., Farina G., Manca A. COVID-19: the daunting experience of healthcare workers in Sardinia, Italy. Infect Control Hosp Epidemiol. 2020 Apr 20:1–2. doi: 10.1017/ice.2020.149. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reusken C.B., Buiting A., Bleeker-Rovers C., Diederen B., Hooiveld M., Friesema I. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(12) doi: 10.2807/1560-7917.ES.2020.25.12.2000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belingheri M., Paladino M.E., Riva M.A. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105:353. doi: 10.1016/j.jhin.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Güell O. El país; 2020. Un almuerzo de despedida celebrado en el Gregorio Marañón causa un brote de coronavirus en el hospital. [Google Scholar]
- 16.Oficial del Estado Boletín. May 3 2020. Orden TMA/384/2020, de 3 de mayo, por la que se dictan instrucciones sobre la utilización de mascarillas en los distintos medios de transporte y se fijan requisitos para garantizar una movilidad segura de conformidad con el plan para la transición hacia una nueva normalidad. [Google Scholar]
- 17.Oficial del Estado Boletín. May 20 2020. Orden SND/422/2020, de 19 de mayo, por la que se regulan las condiciones para el uso obligatorio de mascarilla durante la situación de crisis sanitaria ocasionada por el COVID-19. [Google Scholar]
- 18.Subdirección General de Sanidad Ambiental y Salud Laboral. Dirección General de Salud Pública CeIPdSLdlCdSPdC. Procedimiento de actuación para los Servicios de Prevención de Riesgos Laborales frente a la exposición al nuevo coronavirus (SARS-CoV-2). 5 de marzo de 2020.
- 19.Subdirección General de Sanidad Ambiental y Salud Laboral. Dirección General de Salud Pública CeIPdSLdlCdSPdC. Procedimiento de actuación para los Servicios de Prevención de Riesgos Laborales frente a la exposición al SARS-CoV-2. 30 de marzo de 2020.
- 20.Ehrlich H., McKenney M., Elkbuli A. Protecting our healthcare workers during the COVID-19 pandemic. Am J Emerg Med. 2020;38:1527–1528. doi: 10.1016/j.ajem.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson K.E., Caliendo A.M., Arias C.A., Englund J.A., Lee M.J., Loeb M. 2020. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]