Abstract
Nitrous acid (HONO) is an important precursor of hydroxyl radical (OH) in the atmosphere. It is also toxic to human health. In this work, HONO concentrations were measured in Shijiazhuang using a Monitor for AeRosols and Gases in ambient Air (MARGA) from December 15, 2019 to March 15, 2020, which covered the heavy air pollution season, the Chinese New Year (CNY) vocation and the Corona Virus Disease-19 (COVID-19) lockdown period. During & after CNY overlapping COVID-19 lockdown, the air quality was significantly improved because of both the emission reduction and the increase in diffusion ability of air masses. The mean HONO concentration was 2.43 ± 1.08 ppbv before CNY, while it decreased to 1.53 ± 1.16 ppbv during CNY and 0.97 ± 0.76 ppbv after CNY. The lockdown during & after CNY reduced ~31% of ambient HONO along with ~62% of NO and ~36% of NO2 compared with those before CNY after the improvement of diffusion ability had been taken into consideration. Heterogeneous reaction of NO2 on ground surface dominated the nocturnal HONO sources, followed by heterogeneous reaction on aerosol surface, vehicle emission, reaction between NO and OH and emission from soil on pollution days throughout the observation. Except for elevated soil emission, other nighttime HONO sources and sinks decreased significantly during & after CNY. The relative importance of heterogeneous reaction of NO2 on surfaces further increased because of both the decrease in vehicle emission and the increase in the heterogeneous conversion kinetics from NO2 to HONO during & after CNY.
Keywords: Nitrous acid, Concentrations and sources, Anthropogenic activities, COVID-19, Heterogeneous conversion
Graphical abstract
Highlights
-
•
High concentration of HONO was observed from 0.08 to 12.67 ppbv in Shijiazhuang.
-
•
Heterogeneous conversion of NO2 to HONO dominated the nighttime HONO source.
-
•
The lockdown during & after CNY reduced ~31% of ambient HONO.
-
•
Heterogeneous conversion kinetics from NO2 to HONO increased during & after CNY.
-
•
The T and aerosol properties promoted conversion of NO2 to HONO during & after CNY.
1. Introduction
Nitrous acid (HONO) is a crucial atmospheric species because it is the vital precursor of hydroxyl radical (OH) (Alicke et al., 2003; Ren et al., 2006; Spataro and Ianniello, 2014), which is able to clean up primary air pollutants and produce secondary air pollutants in the atmosphere. Photolysis of H2O2, HCHO, O3 and HONO and the reaction between NO and HO2 are the major sources of OH radical in the atmosphere (Alicke et al., 2003; Volkamer et al., 2010), whereas photolysis of HONO is the dominant primary OH source in the early morning when other OH sources are still weak (Alicke et al., 2003; Spataro et al., 2013; Tan et al., 2018; Tang et al., 2015; Volkamer et al., 2010). Photolysis of HONO accounts for average up to 60% of OH production in the boundary layer in some cases (Alicke et al., 2003; Elshorbany et al., 2009; Tan et al., 2018). It has been found that HONO is capable of promoting secondary aerosols (Czader et al., 2015; Liu et al., 2020; Xing et al., 2019; Zhang et al., 2019a; Zhang et al., 2019c) and ozone formation (Zhang et al., 2019a). In addition, exposures to high concentration of HONO may damage mucous membranes and result into the respiratory system of asthmatics (Ohyama et al., 2019; Ohyama et al., 2010; Rasmussen et al., 1995). HONO is also a precursor of the mutagenic and carcinogenic nitrosamines via reaction with secondary and tertiary amines (Pitts et al., 1978; Sleiman et al., 2010). Therefore, it is important to investigate the atmospheric HONO from the point view of both atmospheric chemistry and toxicology, in particular, in the regions suffering from heavy air pollution.
Atmospheric HONO has been investigated since the late 1970s (Perner and Platt, 1979). Field experiments were carried out at different environments worldwide in the last few decades (Acker et al., 2006; Crilley et al., 2016; Lee et al., 2016; Levy et al., 2014; Michoud et al., 2014; Spataro and Ianniello, 2014; Zhang et al., 2012a). The HONO concentrations varied from a few pptv in remote areas (Beine et al., 2001; Gu et al., 2020; Honrath et al., 2002; Liao et al., 2006; Spataro et al., 2017; Zhang et al., 2012a; Zhou et al., 2001) to several ppbv in polluted urban environment (Crilley et al., 2016; Hu et al., 2002; Spataro et al., 2013; Zhang et al., 2019e). In China, the concentration and budget of atmospheric HONO have also been extensively studied (Spataro et al., 2013), such as in Beijing (Hendrick et al., 2014; Hu et al., 2002; Spataro et al., 2013; Wang et al., 2017; Zhang et al., 2019e), Shanghai (Wang et al., 2013; Zhang et al., 2019b), Guangzhou (Hu et al., 2002; Su et al., 2008a), Hongkong (Xu et al., 2015), Ji'nan (Li et al., 2018) and Xi'an (Huang et al., 2017). Overall, the HONO concentrations were higher in China than those observed in Europe and America. At the present time, direct emissions from combustion and soil, homogeneous reaction between NO and OH radical, heterogeneous reaction of NO2 on aerosol and ground surfaces and photolysis reactions of nitrates have been identified as the major sources of atmospheric HONO (Spataro and Ianniello, 2014), whereas their relative contributions depend on the location and the season. For example, heterogeneous reaction of NO2 was proposed to be an important HONO source in the night (Wang et al., 2017; Zhang et al., 2019c) and even in daytime in Beijing-Tianjin-Hebei (BTH) (Zhang et al., 2019c), but it was unimportant compared with the unknown sources and homogeneous reaction between NO and OH in Ji'nan (Li et al., 2018) or compared with traffic emission on haze days in Beijing (Zhang et al., 2019e). At the same time, the traffic emission was found to be an unimportant HONO source during nighttime in BTH (Zhang et al., 2019c), while it could contribute ~50% to HONO sources on haze days in Beijing (Liu et al., 2020; Meng et al., 2019; Zhang et al., 2019e). In addition, an unknown daytime HONO source, which is highly correlated with light intensity (Lee et al., 2016; Michoud et al., 2014), was frequently observed at various places. More and more studies proposed that it might be associated with photo-enhanced conversion of NO2 (Michoud et al., 2014; Su et al., 2008b) and photolysis of surface nitrate or particulate nitrates although an uncertainty may reduce their importance (Liu et al., 2019b). These results mean that the study about atmospheric HONO budget is still far from closed, which requires a significant effort on both the HONO measurement and the determination of related kinetic parameters for its production pathways (Liu et al., 2019b).
Shijiazhuang is one of the cities suffering from heavy air pollution in China (Qin et al., 2017; Tan et al., 2019; Xie et al., 2019; Zhang et al., 2020). Fine particulate matter (PM) pollution is more serious in Shijiazhuang than the neighboring areas such as Beijing (Cheng et al., 2019; Qin et al., 2017; Zhang et al., 2019). However, the studies on air pollution regarding both fine particles and HONO are very limited in Shijiazhuang. To the best of our knowledge, there is no publication on the concentration and the source studies of atmospheric HONO in Shijiazhuang. Therefore, it is meaningful to investigate the HONO sources for understanding the complex atmospheric chemistry in Shijiazhuang. In particular, during the Chinese New Year (CNY) in 2020 overlapping the Corona Virus Disease-19 (COVID-19) epidemic, the COVID-19 lockdown led to significant reduction of traffic and industry emissions. This provides us a unique opportunity to understand the influence of anthropogenic activities on atmospheric HONO concentrations and confirm the relative importance among different sources. In this work, we performed continuous field observations of HONO and other air pollutants at an urban station in Shijiazhuang from December 15, 2019 to March 15, 2020. The changes of HONO concentrations and the sources have been discussed during the COVID-19 lockdown compared with that before CNY.
2. Material and methods
2.1. Field measurements
Field measurements were performed at Hebei Atmospheric Supersite, which is located in the campus of Shijiazhuang University (Lat. 38.0281° and Lon. 114.6070°). The observation station (Fig. S1) is on a rooftop of the main teaching building (5 floors, ~23 m above the surface) which is around 250 m from the Zhujiang road. It is a typical urban observation station surrounded by traffic and residential emissions.
Mass concentration of PM2.5 was measured by a beta attenuation mass monitor (BAM-1020, Met One Instruments) with a smart heater (Model BX-830, Met One Instruments Inc., USA) to control the RH of the incoming air to 35% and a PM2.5 inlet (URG) to cut off the particles with diameter larger than 2.5 μm. Water-soluble ions (Cl−, NO3 −, SO4 2−, Na+, NH4 +, K+, Mg2+, and Ca2+) in PM2.5 and gas pollutants (HCl, HONO, HNO3, SO2 and NH3) were measured using an analyzer for Monitoring AeRrosols and Gases in ambient Air (MARGA, ADI 2080, Applikon Analytical B.V.) with 1 h of time resolution. Trace gases including NOx, SO2, CO and O3 were measured with the corresponding analyzer (Thermo Scientific, 42i, 43i, 48i and 49i). Meteorological parameters including temperature, pressure, relative humidity (RH), wind speed and direction were measured using a weather station (WXT 520, Vaisala). Planetary boundary layer (PBL) height was measured using a Doppler Lidar (EV-Lidar-CAM, Everise Technology Ltd.). Particles size distribution from 10 to 760 nm was measured with a scanning mobility particle sizer (SMPS, TSI), which is consist of a differential mobility analyzer (DMA 3938, TSI) and a condensation particle counter (CPC 3776, TSI). The particles from 500 to 10,000 nm were measured by an APS (3321, TSI). OC and EC were measured according to the National Institute for Occupational Safety and Health (NIOSH) protocol using an OC/EC analyzer (Model 4, Sunset).
The MARGA was externally calibrated using anionic solutions (Cl−, Br−, NO3 −, SO4 2−) and cationic solutions (Li+, Na+, K+, Mg2+ and Ca2+) seasonally. Internal calibration was also hourly carried out using LiBr standard solutions. The detection limit of Cl−, NO3 −, SO4 2−, Na+, NH4 +, K+, Mg2+, and Ca2+ were 0.01, 0.05, 0.04, 0.05, 0.05, 0.09, 0.06 and 0.09 μg m−3, respectively. All of the instruments for trace gas measurements were calibrated using the corresponding standard gases weekly. The detection limits are 0.05, 0.05, 40, and 0.5 ppbv for NOx, SO2, CO and O3, respectively. External calibration was performed biweekly for the OC/EC analyzer using sucrose solutions.
2.2. HONO budget calculation
The major sources of ambient HONO include direct emission from soil (E soil) (Meusel et al., 2018; Oswald et al., 2015) and vehicle exhaust (E vehicle) (Trinh et al., 2017), homogeneous reaction between NO and OH (P NO-OH) in the atmosphere, photolysis of nitrate (P nitrate) (Bao et al., 2018), heterogeneous reaction of NO2 on aerosol surface (P aerosol) (Liu et al., 2015) and ground surface (P ground) (Li et al., 2018; Liu et al., 2019b; Wang et al., 2017). Photolysis of HNO3 and nitrophenol were usually not considered in source budget analysis because they were believed as minor HONO sources (Lee et al., 2016). The sinks of HONO include photolysis (L photolysis), the homogeneous reaction with OH radical (L HONO-OH), dry deposition (L deposition) (Liu et al., 2019b) and vertical and horizontal transport (T trans) (Soergel et al., 2011). Thus, the HONO budget can be calculated by,
| (1) |
where is the observed change rate of HONO mixing ratios (ppbv h−1), P unknown is the production rate of HONO from the unknown sources.
In our previous work (Liu et al., 2020), the calculation methods for these terms have been discussed in detail. Briefly, the emission rate of HONO (E HONO, ppbv h−1) from soil and vehicle were calculated based on the emission flux (F HONO, g m−2 s−1) and the PBL height (H, m) according to the following equation,
| (2) |
where, α is the conversion factor (), M is the molecular weight (g mol−1), T is the temperature (K) and P is the atmospheric pressure (Pa). For vehicle emission,
| (3) |
where, EI HONO is the emission inventory of HONO (g s−1), A is the urban area of Shijiazhuang (496 km2, measured based on Google map), EI NOx,vehicle is the emission inventory of NOx from vehicle exhaust (0.160 Gg day−1 in Shijiazhuang before CNY) (Qi et al., 2017). The daily mean EI NOx,vehicle was further converted into hourly mean emission inventory based on the hourly mean traffic index (www.nitrafficindex.com, Fig. S2A) before CNY. During & after CNY, the hourly mean emission inventory of NOx was further calculated according to its reduction ratio from traffic emission (62% which will be discussed in Section 3.1). The NOx emission inventories are shown in Fig. S2B and C. The emission ratio of HONO to NOx (1.26%) was estimated using a low limit correlation method (Li et al., 2012). This value is very close to those derived values in Hongkong (1.2 ± 0.4%, (Xu et al., 2015) and 1.23 ± 0.35% (Liang et al., 2017)), Guangzhou (1.0%) (Li et al., 2012) and Beijing (1.17%, 1.3% and 1.41%) (Liu et al., 2020; Meng et al., 2019; Zhang et al., 2019e). The F HONO, soil was calculated using the temperature-dependent emission flux of HONO from grassland with 35–45% of water content (Oswald et al., 2013). Homogeneous reaction between OH and NO was calculated based on the measured NO concentrations and the estimated OH concentrations in the light of second-order reaction. The second-order reaction rate constant (k NO-OH) is 7.2 × 10−12 cm3 molecule−1 s−1 (Li et al., 2012). The daytime OH concentration was estimated according to (Li et al., 2018),
| (4) |
where, J O1D and J NO2 are the photolysis frequency of O3 and NO2 (s−1), respectively, c OH and c NO2 are the concentration of OH and NO2 (molecules cm−3), respectively. The J O1D, J NO2 and J HONO (photolysis frequency of HONO) were calculated using the hourly mean solar zenith angle, the longitude and latitude of the observation station under clear sky condition using a box model, then calibrated according to the measured UV light intensity (Liu et al., 2020). The nighttime OH concentration was assumed to be 1.0 × 105 molecules cm−3 (Li et al., 2012; Tan et al., 2018). The calculated J values and OH concentrations were shown in Fig. S3. Production rate of HONO from photolysis of nitrate was calculated based the measured nitrate concentrations and the photolysis frequency of nitrate (J nitrate). The mean J nitrate value of 8.24 × 10−5 s−1 (Bao et al., 2018) was normalized to the measured UV light intensity in this work. Heterogeneous conversion of NO2 on aerosol and ground surfaces was calculated according to (Huang et al., 2017; Li et al., 2018),
| (5) |
| (6) |
where, k het is the quasi first-order reaction rate constant for heterogeneous conversion from NO2 to HONO (s−1), c HONO,corr is the corrected HONO concentration after the HONO emitted from vehicle exhaust has been subtracted (ppbv), is the mean NO2 concentration from t 1 to t 2. The calculated nighttime k het was 0.016 ± 0.006 h−1 from December 15, 2019 to March 15, 2020, which is comparable with those derived in urban environment such as Guangzhou (0.016 h−1), Milan (0.012 h−1) and Kathmandu (0.014 h−1), and higher than that in Ji'nan (0.0068 ± 0.0045 h−1) (Li et al., 2018; Xu et al., 2015). Interestingly, the nighttime k het before CNY was 0.012 ± 0.005 h−1, which was significantly lower than that during and after CNY (0.017 ± 0.003 h−1, P < 0.05). Therefore, the production rates of HONO from heterogeneous reaction in different periods were calculated using the corresponding k het. The contributions of aerosol and ground surfaces to heterogeneous conversion from NO2 to HONO were further calculated based on the measured surface area to volume ratio of aerosol (S a, 0.0017 ± 0.0013 m−1) and the estimated ground surface to volume ratio (S g, 0.0043 ± 0.0018 m−1) according to,
| (7) |
where 2.2 is a roughness of urban ground surface (Li et al., 2018). It should be pointed out that direct emission from soil should also be considered in Eq. (6). However, we did not consider it similar to the previous work (Huang et al., 2017; Li et al., 2018) because the ambient concentration of HONO contributed by soil could not be calculated like that from vehicle emission using NOx as a reference. This might result into some uncertainty for calculating the k het and will be discussed in Section 3.3.
The loss rates of HONO by photolysis (L photolysis), homogeneous reaction with OH radicals (L HONO-OH) and dry deposition (L deposition) (Liu et al., 2019b) were calculated according to the following equations.
| (8) |
| (9) |
| (10) |
where, J HONO is the photolysis rate of HONO (s−1), k HONO-OH is the second-order reaction rate constant between HONO and OH (6 × 10−12 cm3 molecule−1 s−1) (Atkinson et al., 2004), and v d is the dry deposition rate of HONO (0.001 m s−1) (Han et al., 2017). The vertical and horizontal transport was estimate according to Eq. (11),
| (11) |
where k dilution is a dilution rate (0.23 h−1, including both vertical and horizontal transport) (Dillon et al., 2002), c HONO and c HONO,background is the HONO concentration at the observation site and background site, respectively (Dillon et al., 2002). In this work, the lowest nighttime HONO concentration was taken as the c HONO,background.
3. Results and discussion
3.1. Overview of the air quality during observation
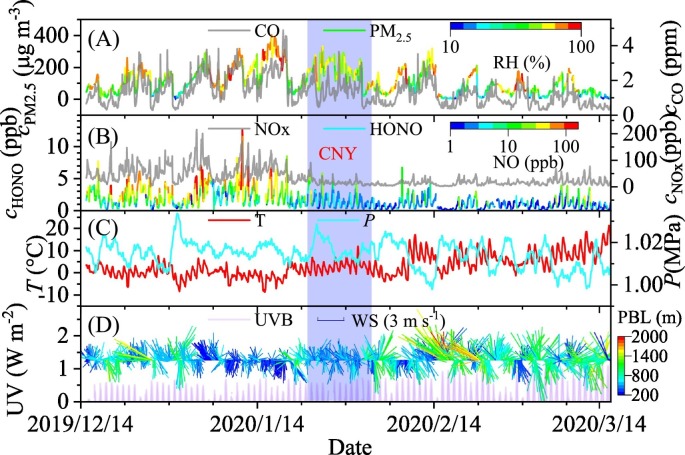
Fig. 1 shows the time series of selected parameters including the concentrations of PM2.5, CO, NOx and HONO (Fig. 1A and B) and the meteorological parameters such as temperature, pressure, wind speed, wind direction and PBL height (Fig. 1C and D) during the observation. The CNY vacation from January 23 to February 2 was highlighted by the light purple column. The temperature increased gradually and the pressure varied from 998 to 1034 hPa during the whole observation (Fig. 1C). Before and during CNY, the frequency of stagnant weather conditions characterized by low wind speed and low PBL height was higher, while the UV light intensity was lower than those after CNY (Fig. 1D). Five light precipitations occurred on December 15, 2019, February 14 and 15, 24 and March 8, 2020. These datasets will be ruled out in following discussion.
Fig. 1.
The time series of (A) PM2.5 and CO concentrations, (B) HONO and NOx concentrations, (C) temperature and pressure and (D) wind direction, wind speed and ultraviolet light intensity from December 15, 2019 to March 15, 2020. The concentrations of PM2.5 and HONO are colored by RH and NO concentration, respectively. The wind direction and wind speed are colored by planetary boundary layer (PBL) height. The highlighted period is during Chinese New Year (CNY).
Six pollution events before CNY, one during CNY and another six after CNY overlapping the COVID-19 lockdown were identified according to the concentration of PM2.5 (Fig. 1A). After strong wind (regardless of wind direction) accompanied with relatively high PBL height cleaning up the air masses (Fig. 1D), a pollution episode gradually built up with a typical duration of 3– 5 days. Interestingly, the PM2.5 concentration well kept pace of the reciprocal of the PBL height (Fig. S4A), in particular, before CNY. This means that the sources of PM2.5 should be quite stable and the mass loading of fine PM should be greatly determined by the diffusion ability of air masses in Shijiazhuang. As shown in Fig. 1A, the high PM2.5 concentration usually coincided with the high RH (> 50%) which is favorable of heterogeneous conversion of gas phase precursors (Hodas et al., 2014; Wang et al., 2016; Wu et al., 2019). In addition, the PM2.5 evolved synchronously with CO, especially, before CNY. These results mean that both secondary production and primary emissions contribute to PM2.5 accumulation in Shijiazhuang.
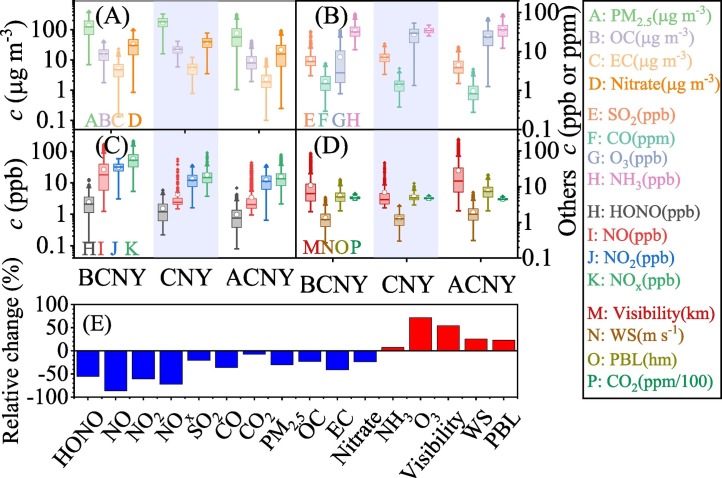
Before CNY, the hourly mean PM2.5 concentration varied from 7 to 403 μg m−3 with a mean value of 137.9 ± 85.8 μg m−3 (Fig. 2A). 70.3% of hourly PM2.5 concentration was higher than the daily mean air quality standard of China (75 μg m−3) before CNY. This indicates the serious air pollution in Shijiazhuang when compared with other cities, such as Beijing and Tianjin (Fu et al., 2014). During CNY, a pollution event occurred continuously from January 22 to February 1. Although the maximum PM2.5 concentration during CNY was lower than that before CNY, the mean PM2.5 concentration (173.9 ± 72.7 μg m−3) was higher than that before CNY because of the long-term favorable metrological conditions for pollutants accumulation including high RH (Fig. 1A), low wind speed and low PBL height (Fig. 1D). After CNY, pollution event still happened frequently. However, both the mass concentration of PM2.5 and the frequency of pollution event decreased significantly because of both the reduction of primary emission (indicated by CO, Fig. 1A) and the increase in wind speed and PBL height (Fig. 1D). The mean concentration of PM2.5 was 76.7 ± 61.4 μg m−3, and 36.1% of hourly mean PM2.5 concentration were higher than 75 μg m−3 after CNY.
Fig. 2.
(A)–(D) Changes of pollutants concentration and meteorological parameters before CNY (BCNY), during CNY and after CNY (ACNY) and (E) relative changes of pollutants and meteorological parameters during & after CNY compared with that before CNY.
From the point view of chemical composition, the fraction of both nitrate and sulfate increased during and after CNY compared with that before CNY (Fig. S5). The fractions of secondary inorganic aerosol (SIA, including NO3 −, SO4 2− and NH4 +) increased significantly from 48.6 ± 13.8% to 53.4 ± 13.6% (P < 0.05). The mass ratio of nitrate to sulfate decreased from 1.78 ± 0.78 before CNY to 1.38 ± 0.48 during CNY and then recovered to 1.88 ± 1.08 after CNY (Fig. S4A). This implies that reduction of traffic emission should be prominent during CNY, while both traffic and industry sectors contribute to the emission reduction after CNY overlapping COVID-19 lockdown. In addition, higher conversion ratios of nitrate (NOR) were observed during (0.44 ± 0.11) and after CNY (0.29 ± 0.17) compared with that before CNY (0.17 ± 0.10) (Fig. S6). The above results mean that air quality was greatly improved, while chemical conversion of the precursors to PM was slightly enhanced during and after CNY overlapping COVID-19 lockdown when compared with the counterpart.
The relative change of other pollutants and meteorological parameters including OC, EC, nitrate, SO2, CO, O3, NH3, HONO, NO, NO2, NOx, visibility, wind speed, PBL height and CO2 are shown in Fig. 2. The absolute concentrations of PM2.5 components and SO2 increased significantly during CNY compared with that before CNY, while followed by a decease after CNY. For example, the mean concentration of OC, EC, nitrate and SO2 were 22.2 ± 7.7, 5.4 ± 2.5, 37.7 ± 17.5 μg m−3 and 7.2 ± 2.4 ppbv during CNY, respectively, while the corresponding mean values were 16.1 ± 7.9, 5.1 ± 3.1, 32.7 ± 22.2 μg m−3 and 6.4 ± 3.1 ppbv before CNY and 9.8 ± 6.6, 2.3 ± 1.7, 21.5 ± 24.9 μg m−3 and 4.5 ± 2.3 ppbv after CNY. The concentration of CO and CO2 decreased from 1.7 ± 0.9 and 0.49 ± 0.04 ppm before CNY to 1.4 ± 0.6 and 0.47 ± 0.03 during CNY, and further decreased to 1.0 ± 0.5 and 0.45 ± 0.02 ppm after CNY. The concentrations of NOx well followed PM2.5 before CNY, while it slightly increased in the pollution events during and after CNY (Fig. 1). The mean NOx concentrations were 58.0 ± 33.7, 17.7 ± 12.4 and 15.7 ± 10.4 ppbv before, during and after CNY, respectively. As a more effective tracer of traffic emission, the mean NO concentration decreased from 26.3 ± 26.2 ppbv before CNY to 4.2 ± 6.6 ppbv during CNY and to 3.4 ± 4.4 ppbv after CNY. The concentration of NH3 was almost constant with the mean concentration of 19.6 ± 10.0, 19.2 ± 5.6, 19.9 ± 11.4 ppbv, respectively, during the three periods. However, the concentration of O3 increased significantly during CNY (27.5 ± 24.4 ppbv) and after CNY (24.4 ± 13.4 ppbv) compared with that before CNY (7.2 ± 7.9 ppbv) due to increase in UV light (Fig. 1D). The above results indicate that emission reduction from traffic sector is prominent during CNY and after CNY, while industry sector might further contribute to the emission reduction after CNY overlapping the COVID-19 lockdown in Shijiazhuang. To simplify the discussion, the datasets during and after CNY will be combined and marked as during & after CNY in the following section because both periods experienced reduction of anthropogenic activities.
During & after CNY, the reduction ratios of NO, NO2, NOx, SO2, CO, CO2, PM2.5, OC, EC and nitrate were 86.4%, 60.5%, 72.2%, 20.6%, 36.3%, 7.7%, 29.8%, 23.1%, 41.0% and 23.9%, respectively, compared with those before CNY (Fig. 2E). When the background concentrations of CO (235 ppbv) (Kim et al., 2010) and CO2 (413 ppm, www.esrl.noaa.gov) were taken into consideration, reduction fraction of CO and CO2 were 42.2% and 47.1%, respectively. At the same time, the concentration of NH3 and O3 increased 7.2% and 71.4%, respectively, during & after CNY compared with before CNY. The visibility increased 54.3% due to the improvement of air quality. The diffusion ability of air masses also increased obviously (~24.1%). For example, the wind speed increased 25.5% and the PBL height increased 22.7% during & after CNY compared with those before CNY. When all of these measured sulfur species (SO2 and sulfate), nitrogen species (NO, NO2, HNO3, HONO and nitrate) and carbon species (CO, CO2, OC and EC) were taken into consideration, the reduction ratios of total measured sulfur, nitrogen and carbon were 22.7%, 62.8% and 40.8%, respectively, during & after CNY compared with before CNY. This means that ~40% of total nitrogen and ~20% of total carbon reduction might be related to decrease of anthropogenic activities, while reduction of total sulfur might be mainly related to improvement of diffusion ability during & after CNY overlapping COVID-19 lockdown. According to the emission inventory of Hebei province (MEIC v1.3) (Li et al., 2017), transportation sector accounted for 32.6%, 2.6% and 9.1% of NOx, SO2 and carbon species emission in 2016, respectively, while the corresponding contribution from industry sector were 51.5%, 72.3% and 61.1%. If we assume that NO measured at our observation site is mainly from traffic emission and the emission profile of NOx in Shijiazhuang is the same as that in Hebei province, the lockdown during & after CNY overlapping COVID-19 may reduce ~62% (=86.4%-24.1%) of NOx from traffic emissions and ~ 22% of NOx from industry emissions after the improvement of diffusion ability has been considered.
3.2. Influence of COVID-19 lockdown on HONO concentration
As shown in Fig. 1B, elevated HONO concentrations were frequently observed during the observation. HONO well followed the PM2.5 pollution events before CNY. Increase of HONO concentration was still discernable in each PM2.5 pollution event during & after CNY although it was significantly lower than that before CNY. Throughout the observation, the HONO concentration ranged from 0.08 to 12.67 ppbv, with a mean value of 1.64 ± 1.41 ppbv. The maximum hourly value of 12.67 ppbv was recorded in the morning of January 11, 2020. The mean HONO concentration was 2.43 ± 1.08 ppbv before CNY, which was significantly higher than that during CNY (1.53 ± 1.16 ppbv) and after CNY (0.97 ± 0.76 ppbv). 49.7%, 22.4% and 9.1% of hourly mean values exceeding 2.0 ppbv before, during and after CNY, respectively. The nighttime mean HONO concentration was 1.96 ± 1.32 ppbv, compared with that in daytime (1.26 ± 1.43 ppbv) throughout the observation. Before CNY, their corresponding nighttime and daytime mean values were 2.84 ± 1.36 and 1.93 ± 1.76 ppbv, while they were 2.84 ± 1.08 and 1.09 ± 1.10 ppbv during CNY, and 1.19 ± 0.69 and 0.71 ± 0.76 ppbv after CNY.
The mean HONO concentrations before CNY was higher than those reported in foreign cities (Acker et al., 2006; Crilley et al., 2016; Lee et al., 2016; Levy et al., 2014; Michoud et al., 2014; Shon et al., 2007) and several Chinese cities such as Guangzhou(Hu et al., 2002), Hongkong (Xu et al., 2015), Ji'nan (Li et al., 2018) and Xi'an (Huang et al., 2017) as summarized in Table 1 , while it was close to these previous observations performed in Beijing (Zhang et al., 2019e) and Shanghai (Cui et al., 2018). Even at noontime (11:00–13:00), the mean HONO concentration was 1.13 ± 1.21 ppbv throughout the observation, and 1.83 ± 1.52, 0.86 ± 0.51, 0.56 ± 0.46 ppbv before, during and after CNY, respectively. Before CNY, 66.7% of the noontime HONO concentrations were higher than 1.00 ppbv, which was the highest level compared with that ever reported in the urban atmospheres. The high HONO concentration observed in this work indicates the intensive HONO sources and potentially strong atmospheric oxidizing capacity in Shijiazhuang. The lockdown during & after CNY overlapping COVID-19 reduced ~55% of HONO concentration, which is ranked the top three pollutants mostly affected by the lockdown along with NO and NO2 (Fig. 2E). The reduction ratio was ~31% after the improvement of diffusion ability of air masses was subtracted.
Table 1.
HONO concentrations measured in urban environment.
| Observation duration | Location | Instrument | cHONO (ppbv) (median) | References |
|---|---|---|---|---|
| 2001.5–6 | Rome, Italy | DOAS | <2 | (Acker et al., 2006) |
| 2009.4–5 | Houston, USA | ID-CIMS | <1.5(0.19) | (Levy et al., 2014) |
| 2009.7, 2010.1–2 | Paris, France | LOPAP | 0.01–0.5, 0.01–1.7 | (Michoud et al., 2014) |
| 2014.6 | York, UK | LOPAP | 0.33–1.15 | (Crilley et al., 2016) |
| 2014.7–8 | London, UK | LOPAP | 0.2–1.8 | (Lee et al., 2016) |
| 2003.1–2 | Kathmandu, Nepal | DOAS | 0.15–7.45(1.7) | (Yu et al., 2009) |
| 2004.5–2004.6 | Soul, South Korea | WD-IC | 1.8 ± 0.4(1.3) | (Shon et al., 2007) |
| 2000.7, 11 | Guangzhou, China | GAC | 1.0–2.7 | (Hu et al., 2002) |
| 2007.8 | Beijing, China | WD-IC | 1.45 ± 0.58(1.47) | (Spataro et al., 2013) |
| 2011.5, 11 | Hongkong, China | LOPAP | 0.35 ± 0.30, 0.93 ± 0.67 | (Xu et al., 2015) |
| 2014.2–3 | Beijing, China | LOPAP | 0.28–3.24 | (Hou et al., 2016) |
| 2015.7–8 | Xi'an, China | LOPAP | 1.12 ± 0.97 | (Huang et al., 2017) |
| 2006.8–9 | Beijing, China | LOPAP, GAC | 0.034–3.69 | (Yang et al., 2014) |
| 2015.9–10, 2016.1, 4–5, 6–7 | Beijing, China | AIM-IC | 2.27 ± 1.82; 1.05 ± 89;1.05 ± 0.95; 1.38 ± 0.90 | (Wang et al., 2017) |
| 2016.5 | Shanghai, China | LOPAP | 0.48–5.84(2.31) | (Cui et al., 2018) |
| 2016.12 | Beijing, China | LOPAP | 3.5 ± 2.7 | (Zhang et al., 2019e) |
| 2015.9–2016.8 | Jinan, China | LOPAP | 1.15 ± 1.07 | (Li et al., 2018) |
| 2019.12.15–2020.1.22 | Shijiazhuang, China | MARGA | 2.43 ± 1.08(2.10) | This work |
| 2019.1.23–2020.2.2 | 1.53 ± 1.16(1.20) | This work | ||
| 2019.2.3–2020.2.22 | 0.97 ± 0.76(0.75) | This work |
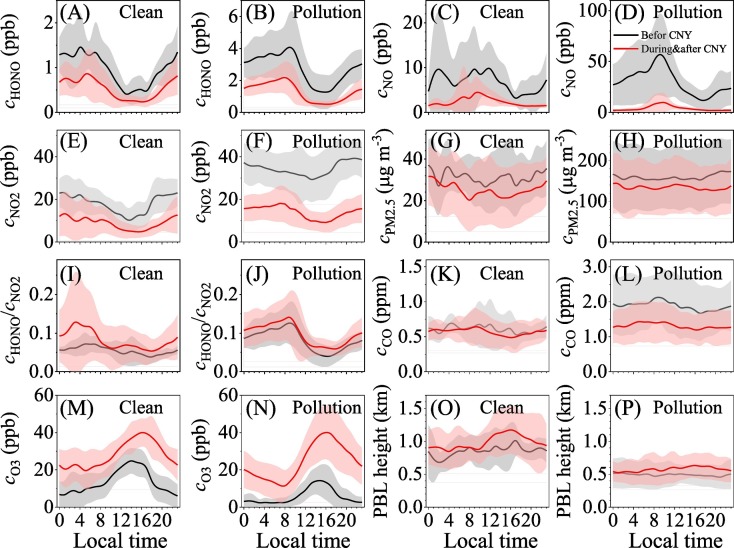
Fig. 3 shows the diurnal curves of HONO, NO, NO2, PM2.5, HONO/NO2 ratio, CO, O3 and PBL height on clean days (c PM2.5 < 50 μg m−3) and pollution days (c PM2.5 ≥ 50 μg m−3 and RH < 90%). The black and red lines are the diurnal curves before CNY and during & after CNY, respectively. The shadows indicate the standard errors (±1σ). Overall, the nighttime concentrations of HONO, NO, NO2, HONO/NO2 ratio and CO were higher than the corresponding daytime values regardless of the pollution level and the period, while O3 showed an opposite trend due to increase in UV light after sunrise. PM2.5 showed relative flat diurnal curves on pollution days although its nighttime concentrations were slightly higher than the daytime concentrations on clean days, in particular, during & after CNY. The PBL height also showed relative flat diurnal curves before CNY, while the slightly elevated PBL heights in daytime were discernable during & after CNY.
Fig. 3.
Diurnal curves of (A) and (B) HONO, (C) and (D) NO, (E) and (F) NO2, (G) and (H) PM2.5, (I) and (J) HONO/NO2 ratio, (K) and (L) CO, (M) and (N) O3, (O) and (P) PBL height on clean days (cPM2.5 < 50 μg m−3) and pollution days (cPM2.5 ≥ 50 μg m−3 and RH < 90%). The black lines are the diurnal curves before CNY and the red ones are during & after CNY. The shadows indicate standard errors (±1σ). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The diurnal variation of HONO before CNY was similar to NO, while this similarity disappeared during & after CNY. Their similar nighttime profiles suggest that vehicle emissions may pose a significant effect on the measured HONO level (Li et al., 2018) before CNY, while other processes such as secondary conversion should become prominent during & after CNY. This was consistent with the fact that high concentration of HONO before CNY was highly correlated with NO concentration (Fig. 1B), while it was well correlated with the HONO/NO2 ratio during the whole observation (Fig. S4D). The HONO/NO2 ratio also showed similar diurnal curve of HONO on pollution days, which implies secondary conversion of NO2 should play an important role in the observed HONO levels. Besides the first peak of HONO/NO2 in the morning, which is determined by the complex effect among evolution of boundary layer, traffic emission and the photolysis loss of HONO, a second peak of HONO/NO2 at around noontime (13:00–14:00) during & after CNY was observable on both clean and pollution days. This implies that additional HONO sources may be related to the solar radiation intensity (Lee et al., 2016; Li et al., 2018).
As shown in Fig. 3, diurnal curves of HONO, NO and NO2 during & after CNY were significantly below those before CNY on both clean days and pollution days. It did so for CO only on pollution days. The diurnal curves of O3 during & after CNY were significantly above those before CNY. These results further confirmed the reduction of HONO, NO, NO2 and CO, but the increase of O3 during & after CNY overlapping COVID-19 lockdown. However, the diurnal variations of PM2.5 during & after CNY were only slightly below that before CNY. This might be related to the weak increase in PBL height during & after CNY compared with that before CNY (Fig. 3O and P). It should be noted that the mean nighttime and daytime HONO/NO2 ratio were 0.095 ± 0.051 and 0.075 ± 0.053, respectively, throughout the observation. These values are in the range of the reported HONO/NO2 in literatures (Elshorbany et al., 2009; Huang et al., 2017; Li et al., 2018; Liu et al., 2014; Tong et al., 2015), but at a high level end. Interestingly, the HONO/NO2 ratio on both clean days and polluted days during & after CNY were slightly higher than the counterpart. This implies that chemical conversion from NO2 to HONO should be more effective during & after CNY compared with that before CNY. These results also mean that the relative contribution of HONO sources should have changed in different periods.
3.3. Influence of COVID-19 lockdown on HONO sources on pollution days
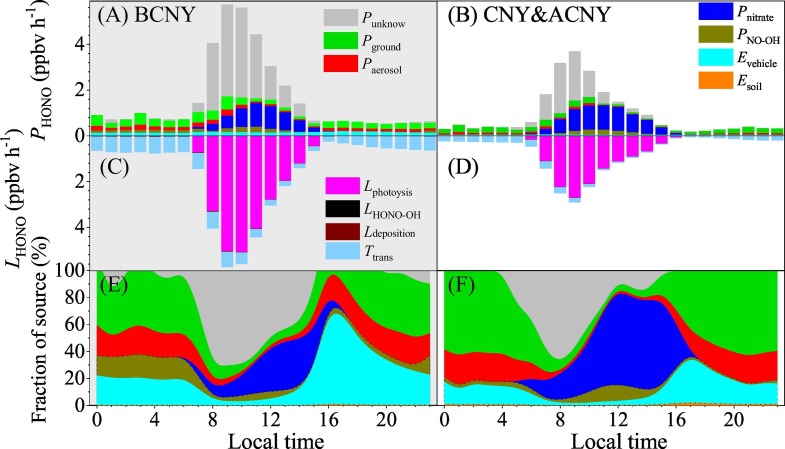
Fig. 4 compared the budget of HONO before CNY with those during & after CNY on pollution days (with PM2.5 concentration > 50 μg m−3 and RH <90%). Table S1 summarized the mean intensities of these sources and sinks. Overall, the nighttime sources were comparable with the sinks in different periods, while significant underestimation of the sources presented in the daytime. Besides the possible unknown HONO sources, a possible reason might be related to the variation of PBL height. When we calculating the HONO sources, heterogeneous conversion from NO2 to HONO and emissions from vehicle and soil were dependent on PBL height according to Eqs. (2), (7). If these sources were more sensitive to surface HONO concentration (Fig. S4B) than NO2, the increase in PBL height in daytime should underestimate their contribution to HONO sources. On the other hand, OH concentration was estimated based on J O1D and J NO2. Although the calculated OH concentrations were comparable with that observed in Beijing (Tan et al., 2018), the uncertainty of OH concentration might also contribute the underestimation of HONO sources. In addition, light-enhanced heterogeneous reactions on both aerosol (Liu et al., 2014) and ground surfaces (Liu et al., 2019) have not been considered in this work. This might lead to the observed underestimation. Actually, the contribution of photolysis of nitrate became prominent at noontime, in particular, during & after CNY. This decreased the fraction of the unknown HONO sources. In the following section, we will mainly concentrate on the nighttime HONO sources. However, the unknown sources will also be taken into consideration when we calculating the relative contributions if the total sinks are over the total sources.
Fig. 4.
HONO budget before CNY and during & after CNY on polluted days with PM2.5 concentration > 50 μg m−3 and RH <90%.
In the night, heterogeneous reaction of NO2 on ground surface was the largest HONO source, followed by heterogeneous reaction on aerosol surface, vehicle emission, homogeneous reaction between NO and OH and soil emission in the whole observation period (Fig. 4). Emission from soil was a minor HONO source during our observation due to the low temperature. E soil varied from 0.00044 to 0.0091 ppbv h−1, with a mean value of 0.0026 ± 0.0014 ppbv h−1 throughout the observation. It was slightly higher during & after CNY (0.0031 ± 0.0016 ppbv h−1) than that before CNY (0.0020 ± 0.0008 ppbv h−1) because of the increase in temperature (Fig. 1C). The nighttime E soil were 0.0020 ± 0.0007 ppbv h−1 before CNY and 0.0030 ± 0.0015 ppbv h−1 during & after CNY, respectively. The corresponding relative contributions to nighttime HONO sources were (0.27 ± 0.08) % and (0.83 ± 0.36) %. Direct emission from vehicle exhaust was an important HONO source. E vehicle ranged from 0.015 to 0.45 ppbv h−1, with a mean value of 0.099 ± 0.078 ppbv h−1 throughout the observation. It decreased from 0.16 ± 0.081 ppbv h−1 before CNY to 0.052 ± 0.022 ppbv h−1 during & after CNY due to the reduction of vehicle emission. The corresponding nighttime values were 0.16 ± 0.078 and 0.051 ± 0.021 ppbv h−1, which contributed 22.2 ± 6.7% (BCNY) and 14.7 ± 5.9% (CNY&ACNY) to HONO sources. These relative contributions were close to that in Ji'nan (12%–21%) (Li et al., 2018), while they were much lower than that in Beijing (~50%) (Liu et al., 2020; Meng et al., 2019; Zhang et al., 2019e). This can be well explained by the fact that the vehicle population in Shijiazhuang is much smaller than that in Beijing (http://www.stats.gov.cn). Because the NO concentrations were very high before CNY (Fig. 1), homogeneous reaction between NO and OH played an important role in HONO source in the night. The nighttime P NO-OH was 0.076 ± 0.091 ppbv h−1 before CNY, while it decreased to 0.0092 ± 0.014 ppbv h−1 during & after CNY. Their corresponding fractions in the nighttime HONO sources were 10.8 ± 6.0% and 2.2 ± 1.1%. Compared with above sources, heterogeneous conversion of NO2 on aerosol and ground surfaces were the most important nighttime HONO source in Shijiazhuang. This is consistent with the high HONO/NO2 as shown in Fig. 3. The nighttime P aerosol were 0.14 ± 0.08 ppbv h−1 before CNY, which was only second to the P ground (0.30 ± 0.12 ppbv h−1). During & after CNY, they were 0.069 ± 0.055 ppbv h−1 (P aerosol) and 0.20 ± 0.12 ppbv h−1 (P ground), respectively. Thus, heterogeneous reaction of NO2 on aerosol surface contributed 19.0 ± 3.9% to the nighttime HONO sources before CNY. It increased to 21.2 ± 5.7% during & after CNY due to the decrease of E vehicle and P NO-OH. The relative contribution of ground surface increased from 38.9 ± 7.8% before CNY to 48.0 ± 13.1% during & after CNY. Photolysis was the major loss of HONO in the daytime. Before CNY, it was 2.65 ± 2.77 ppbv h−1, while it decreased to 1.59 ± 1.86 ppbv h−1 during & after CNY. In the night, vertical and horizontal transport dominated the sinks of HONO, with a mean value of 0.60 ± 0.31 ppbv h−1 before CNY and 0.26 ± 0.19 ppbv h−1 during & after CNY.
Except for E soil (increased 50%), the reduction ratio of all other sources and sinks ranged from 33% to 88% in the night during & after CNY when compared with that before CNY as shown in Table S1. In the daytime, besides E soil, P nitrate and L HONO-OH increased 18% and 67%, respectively, whereas other sources and sinks decreased from 40% to 68%. The increase of E soil was related to increase in temperature, while it was related to increase in irradiation intensity for P nitrate and increase in OH concentrations for L HONO-OH. In the night, vehicle related sources (E vehicle and P NO-OH) were the mostly influenced sources, while heterogeneous conversion (in particular, on ground surface) was less affected by the reduction of anthropogenic activities during and after CNY overlapping COVID-19. Therefore, this led to an increased relative contribution of heterogeneous reaction on ground and aerosol surfaces in the night (Fig. 4). This is consistent with the good correlation between HONO concentrations and NO, NO2 and PM2.5 concentrations as shown in Fig. S7.
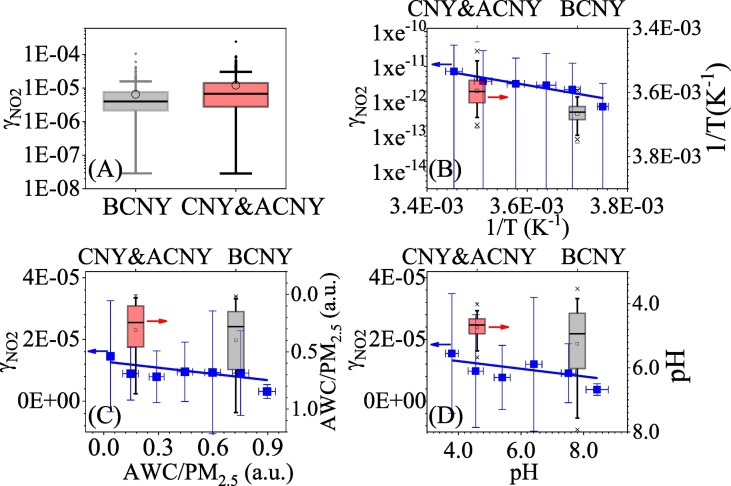
As pointed above, the k het during & after CNY (0.017 ± 0.003 h−1, P < 0.05) was significantly higher than that before CNY (0.012 ± 0.005 h−1). We further derived the uptake coefficient of NO2 (γNO2) on both aerosol and ground surfaces according to,
| (12) |
| (13) |
where, S is the total surface to volume ratio of ground and aerosols (S g + S a, m−1), ω is the mean velocity of NO2 molecules, R is the ideal gas constant, T is the temperature (K), M is the molecular weight of NO2 (kg mol−1). Fig. 5A compared the derived nighttime γNO2 before CNY with that during & after CNY. It was 6.43 ± 9.02 × 10−6 before CNY, which is significantly (P < 0.05) lower than 1.20 ± 1.76 × 10−5 during & after CNY. These values are larger than that derived in Ji'nan (1.4 ± 2.4 × 10−6) (Li et al., 2018) and the laboratory values (5 × 10−9 - 9.6 × 10−6) on different particles (Ndour et al., 2009; Underwood et al., 1999; Underwood et al., 2001). The elevated γNO2 means that reaction kinetic is favorable of heterogeneous conversion from NO2 to HONO during & after CNY although both NO2 and PM2.5 concentrations decreased compared with before CNY. As pointed in Section 2.2, direct emission of HONO from soil was not considered when we calculating the k het. This will lead to an additional uncertainty for the γNO2. However, this uncertainty should be small because the emission rate of HONO from vehicle (0.099 ± 0.078 ppbv h−1) was as ~38 times as that from soil (0.0026 ± 0.0014 ppbv h−1).
Fig. 5.
(A) The derived nighttime γNO2 before CNY and during & after CNY, the dependence of the γNO2 on (B) the temperature, (c) the ratio of AWC/PM2.5 and the aerosol pH. The box plots in (B)–(D) are the distribution of nighttime temperature, AWC/PM2.5 and aerosol pH before CNAY and during & after CNY.
As shown in Fig. 5B, the γNO2 is well negatively correlated with the reciprocal of temperature (lnγNO2 = −2.46 × 103/T-2.69, R = 0.92). This means the conversion from NO2 to HONO requires to overcome an apparent activation energy (20.5 kJ mol−1). Thus, the higher nighttime temperature (278.0 ± 4.1 K) during & after CNY was favorable of this reaction compared with that before CNY (272.7 ± 2.6 K). On the other hand, the γNO2 was negatively correlated with both the ratio of aerosol water content to PM2.5 mass concentration (AWC/PM2.5) and the aerosol acidity (pH), which were calculated using the ISORROPIA-II model with the gas phase and particle phase concentrations of the relevant species, the temperature and the RH as inputs in a forward mode under metastable conditions (Ding et al., 2019). The corresponding equation is γNO2 = 1.28 × 10−5-6.71 × 10−6 AWC/PM2.5 (R = 0.71) and γNO2 = 1.79 × 10−5-1.24 × 10−6 pH (R = 0.57) (Fig. 5C and D). Both the nocturnal AWC/PM2.5 ratio (0.31 ± 0.27) and aerosol pH (4.87 ± 0.89) during & after CNY were significantly lower than those values (0.35 ± 0.31 and 5.17 ± 1.19) before CNY. This can be explained by the higher SIA fraction (Fig. S3) and the lower RH during & after CNY. These results are well agreement with previous laboratory studies that water can competitively inhibit the uptake of NO2 (Liu et al., 2015; Zhang et al., 2012b) and particle phase acids can promote HONO formation from heterogeneous reaction of NO2 (Bao et al., 2018; Han et al., 2016). This means the variation of aerosol properties might be another reason lead to the elevated reactivity for heterogeneous conversion from NO2 to HONO during & after CNY. Thus, the increase in the relative contribution of heterogeneous reactions on ground and aerosol surfaces can be explained by both the effective heterogeneous conversion from NO2 to HONO and the reduction of direct emission from vehicle.
4. Conclusions
During & after CNY overlapping COVID-19 lockdown, great reduction of air pollutants emission was observed in Shijiazhuang. The reduction ratio of NO, NO2, NOx, SO2, CO, CO2, PM2.5, OC, EC and nitrate concentrations were 86.4%, 60.5%, 72.2%, 20.6%, 42.2%, 47.1%, 29.8%, 23.1%, 41.0% and 23.9%, respectively, compared with those before CNY. At the same time, the diffusion ability of air mass increased obviously as evident by the increase in wind speed (25.5%) and PBL height (22.7%) during & after CNY. The lockdown during & after CNY overlapping COVID-19 might reduce ~62% of NOx from traffic emissions after improvement of diffusion ability was taken into consideration.
The mean HONO concentration was 2.43 ± 1.08 ppbv before CNY, which was significantly higher than those during CNY (1.53 ± 1.16 ppbv) and after CNY (0.97 ± 0.76 ppbv). The lockdown during & after CNY overlapping COVID-19 reduced ~31% of ambient HONO compared with that before CNY after the improvement of diffusion ability was considered. Heterogeneous reaction of NO2 on ground surface was the largest nocturnal HONO source, followed by heterogeneous reaction on aerosol surface, vehicle emission, homogeneous reaction between NO and OH and emission from soil in the observation period. Except for emission from soil, most of the HONO sources and sinks decreased from 33% to 88% in the night during & after CNY when compared with that before CNY. The relative importance of heterogeneous reaction of NO2 on surfaces further increased during & after CNY because of both the decrease in vehicle emission and the increase in heterogeneous reaction kinetics compared with that before CNY. This work confirmed that reducing anthropogenic activities definitely reduced ambient HONO concentrations, while it nonlinearly responded to the reduction of anthropogenic activities.
CRediT authorship contribution statement
Yongchun Liu: Conceptualization, Methodology, Data curation, Writing - original draft. Shuangying Ni: Conceptualization, Methodology, Investigation, Writing - original draft. Tao Jiang: Investigation, Resources. Shubin Xing: Investigation, Resources, Writing - review & editing. Yusheng Zhang: Investigation, Data curation. Xiaolei Bao: Conceptualization, Methodology, Data curation, Investigation, Resources, Writing - original draft. Zeming Feng: Investigation, Data curation. Xiaolong Fan: Investigation, Data curation. Liang Zhang: Investigation, Data curation. Haibo Feng: Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This research was financially supported by the Ministry of Science and Technology of the People's Republic of China (2019YFC0214701), the National Natural Science Foundation of China (41877306), the Strategic Priority Research Program of Chinese Academy of Sciences and Beijing University of Chemical Technology.
Editor: Jianmin Chen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141025.
Appendix A. Supplementary data
Supplementary material
References
- Acker K., Febo A., Trick S., Perrino C., Bruno P., Wiesen P., Möller D., Wieprecht W., Auel R., Giusto M., Geyer A., Platt U., Allegrini I. Nitrous acid in the urban area of Rome. Atmos. Environ. 2006;40:3123–3133. [Google Scholar]
- Alicke B., Geyer A., Hofzumahaus A., Holland F., Konrad S., Patz H.W., Schafer J., Stutz J., Volz-Thomas A., Platt U. OH formation by HONO photolysis during the BERLIOZ experiment. J. Geophys. Res.-Atmos. 2003;108:17. [Google Scholar]
- Atkinson R., Baulch D.L., Cox R.A., Crowley J.N., Hampson R.F., Hynes R.G., Jenkin M.E., Rossi M.J., Troe J. Evaluated kinetic and photochemical data for atmospheric chemistry: volume I - gas phase reactions of Ox, HOx, NOx and SOx species. Atmos. Chem. Phys. 2004;4:1461–1738. [Google Scholar]
- Bao F., Li M., Zhang Y., Chen C., Zhao J. Photochemical aging of Beijing urban PM2.5: HONO production. Environ. Sci. Technol. 2018;52:6309–6316. doi: 10.1021/acs.est.8b00538. [DOI] [PubMed] [Google Scholar]
- Beine H.J., Allegrini I., Sparapani R., Ianniello A., Valentini F. Three years of springtime trace gas and particle measurements at Ny-Ålesund, Svalbard. Atmos. Environ. 2001;35:3645–3658. [Google Scholar]
- Cheng J., Su J., Cui T., Li X., Dong X., Sun F., Yang Y., Tong D., Zheng Y., Li Y., Li J., Zhang Q., He K. Dominant role of emission reduction in PM2.5 air quality improvement in Beijing during 2013–2017: a model-based decomposition analysis. Atmos. Chem. Phys. 2019;19:6125–6146. [Google Scholar]
- Crilley L.R., Kramer L., Pope F.D., Whalley L.K., Cryer D.R., Heard D.E., Lee J.D., Reed C., Bloss W.J. On the interpretation of in situ HONO observations via photochemical steady state. Faraday Discuss. 2016;189:191–212. doi: 10.1039/c5fd00224a. [DOI] [PubMed] [Google Scholar]
- Cui L., Li R., Zhang Y., Meng Y., Fu H., Chen J. An observational study of nitrous acid (HONO) in Shanghai, China: the aerosol impact on HONO formation during the haze episodes. Sci. Total Environ. 2018;630:1057–1070. doi: 10.1016/j.scitotenv.2018.02.063. [DOI] [PubMed] [Google Scholar]
- Czader B.H., Choi Y., Li X., Alvarez S., Lefer B. Impact of updated traffic emissions on HONO mixing ratios simulated for urban site in Houston, Texas. Atmos. Chem. Phys. 2015;15:1253–1263. [Google Scholar]
- Dillon M.B., Lamanna M.S., Schade G.W., Goldstein A.H., Cohen R.C. Chemical evolution of the Sacramento urban plume: transport and oxidation. J. Geophys. Res. Atmos. 2002;107:ACH 3–1-ACH 3-15. [Google Scholar]
- Ding J., Zhao P., Su J., Dong Q., Du X., Zhang Y. Aerosol pH and its driving factors in Beijing. Atmos. Chem. Phys. 2019;19:7939–7954. [Google Scholar]
- Elshorbany Y.F., Kurtenbach R., Wiesen P., Lissi E., Rubio M., Villena G., Gramsch E., Rickard A.R., Pilling M.J., Kleffmann J. Oxidation capacity of the city air of Santiago, Chile. Atmos. Chem. Phys. 2009;9:2257–2273. [Google Scholar]
- Fu G.Q., Xu W.Y., Yang R.F., Li J.B., Zhao C.S. The distribution and trends of fog and haze in the North China Plain over the past 30 years. Atmos. Chem. Phys. 2014;14:11949–11958. [Google Scholar]
- Gu R.R., Zheng P.G., Chen T.S., Dong C., Wang Y.N., Liu Y.M., Liu Y.H., Luo Y.Y., Han G.X., Wang X.F., Zhou X.H., Wang T., Wang W.X., Xue L.K. Atmospheric nitrous acid (HONO) at a rural coastal site in North China: seasonal variations and effects of biomass burning. Atmos. Environ. 2020;229:11. [Google Scholar]
- Han C., Yang W., Wu Q., Yang H., Xue X. Key role of pH in the photochemical conversion of NO2 to HONO on humic acid. Atmos. Environ. 2016;142:296–302. doi: 10.1021/acs.est.5b05101. [DOI] [PubMed] [Google Scholar]
- Han X., Zhang M.G., Skorokhod A., Kou X.X. Modeling dry deposition of reactive nitrogen in China with RAMS-CMAQ. Atmos. Environ. 2017;166:47–61. [Google Scholar]
- Hendrick F., Muller J.F., Clemer K., Wang P., De Maziere M., Fayt C., Gielen C., Hermans C., Ma J.Z., Pinardi G., Stavrakou T., Vlemmix T., Van Roozendael M. Four years of ground-based MAX-DOAS observations of HONO and NO2 in the Beijing area. Atmos. Chem. Phys. 2014;14:765–781. [Google Scholar]
- Hodas N., Sullivan A.P., Skog K., Keutsch F.N., Collett J.L., Decesari S., Facchini M.C., Carlton A.G., Laaksonen A., Turpin B.J. Aerosol liquid water driven by anthropogenic nitrate: implications for lifetimes of water-soluble organic gases and potential for secondary organic aerosol formation. Environ Sci Technol. 2014;48:11127–11136. doi: 10.1021/es5025096. [DOI] [PubMed] [Google Scholar]
- Honrath R.E., Lu Y., Peterson M.C., Dibb J.E., Arsenault M.A., Cullen N.J., Steffen K. Vertical fluxes of NOx, HONO, and HNO3 above the snowpack at Summit, Greenland. Atmos. Environ. 2002;36:2629–2640. [Google Scholar]
- Hou S., Tong S., Ge M., An J. Comparison of atmospheric nitrous acid during severe haze and clean periods in Beijing, China. Atmos. Environ. 2016;124:199–206. [Google Scholar]
- Hu M., Zhou F., Shao K., Zhang Y., Tang X., Slanina J. Diurnal variations of aerosol chemical compositions and related gaseous pollutants in Beijing and Guangzhou. J Environ Sci Health, Part A. 2002;37:479–488. doi: 10.1081/ese-120003229. [DOI] [PubMed] [Google Scholar]
- Huang R.-J., Yang L., Cao J., Wang Q., Tie X., Ho K.-F., Shen Z., Zhang R., Li G., Zhu C., Zhang N., Dai W., Zhou J., Liu S., Chen Y., Chen J., O’Dowd C.D. Concentration and sources of atmospheric nitrous acid (HONO) at an urban site in Western China. Sci. Total Environ. 2017;593:165–172. doi: 10.1016/j.scitotenv.2017.02.166. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Chung Y.S., Tans P.P. On the regional distributions of background carbon monoxide concentrations observed in East Asia during 1991–2008. Asia-Pacific J Atmos Sci. 2010;46:89–95. [Google Scholar]
- Lee J.D., Whalley L.K., Heard D.E., Stone D., Dunmore R.E., Hamilton J.F., Young D.E., Allan J.D., Laufs S., Kleffmann J. Detailed budget analysis of HONO in central London reveals a missing daytime source. Atmos. Chem. Phys. 2016;16:2747–2764. [Google Scholar]
- Levy M., Zhang R., Zheng J., Zhang A.L., Xu W., Gomez-Hernandez M., Wang Y., Olaguer E. Measurements of nitrous acid (HONO) using ion drift-chemical ionization mass spectrometry during the 2009 SHARP field campaign. Atmos. Environ. 2014;94:231–240. [Google Scholar]
- Li X., Brauers T., Häseler R., Bohn B., Fuchs H., Hofzumahaus A., Holland F., Lou S., Lu K.D., Rohrer F., Hu M., Zeng L.M., Zhang Y.H., Garland R.M., Su H., Nowak A., Wiedensohler A., Takegawa N., Shao M., Wahner A. Exploring the atmospheric chemistry of nitrous acid (HONO) at a rural site in Southern China. Atmos. Chem. Phys. 2012;12:1497–1513. [Google Scholar]
- Li M., Zhang Q., Kurokawa J.I., Woo J.H., He K., Lu Z., Ohara T., Song Y., Streets D.G., Carmichael G.R., Cheng Y., Hong C., Huo H., Jiang X., Kang S., Liu F., Su H., Zheng B. MIX: a mosaic Asian anthropogenic emission inventory under the international collaboration framework of the MICS-Asia and HTAP. Atmos. Chem. Phys. 2017;17:935–963. [Google Scholar]
- Li D., Xue L., Wen L., Wang X., Chen T., Mellouki A., Chen J., Wang W. Characteristics and sources of nitrous acid in an urban atmosphere of northern China: results from 1-yr continuous observations. Atmos. Environ. 2018;182:296–306. [Google Scholar]
- Liang Y., Zha Q., Wang W., Cui L., Lui K.H., Ho K.F., Wang Z., Lee S.-c., Wang T. Revisiting nitrous acid (HONO) emission from on-road vehicles: a tunnel study with a mixed fleet. J. Air Waste Manage. Assoc. 2017;67:797–805. doi: 10.1080/10962247.2017.1293573. [DOI] [PubMed] [Google Scholar]
- Liao W., Case A.T., Mastromarino J., Tan D., Dibb J.E. Observations of HONO by laser-induced fluorescence at the South Pole during ANTCI 2003. Geophys. Res. Lett. 2006;33 [Google Scholar]
- Liu Z., Wang Y., Costabile F., Amoroso A., Zhao C., Huey L.G., Stickel R., Liao J., Zhu T. Evidence of aerosols as a media for rapid daytime HONO production over China. Environ Sci Technol. 2014;48:14386–14391. doi: 10.1021/es504163z. [DOI] [PubMed] [Google Scholar]
- Liu Y., Han C., Ma J., Bao X., He H. Influence of relative humidity on heterogeneous kinetics of NO2 on kaolin and hematite. Phys. Chem. Chem. Phys. 2015;17:19424–19431. doi: 10.1039/c5cp02223a. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Mekic M., Jiang H., Zhou W., Loisel G., Song W., Wang X., Gligorovski S. Photoenhanced uptake of NO2 and HONO formation on real urban grime. Environ. Sci. Technol. Lett. 2019;6:413–417. [Google Scholar]
- Liu Y.H., Lu K.D., Li X., Dong H.B., Tan Z.F., Wang H.C., Zou Q., Wu Y.S., Zeng L.M., Hu M., Min K.E., Kecorius S., Wiedensohler A., Zhang Y.H. A comprehensive model test of the HONO sources constrained to field measurements at Rural North China Plain. Environ Sci Technol. 2019;53:3517–3525. doi: 10.1021/acs.est.8b06367. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Lian C., Yan C., Feng Z., Zheng F., Fan X., Chen Y., Wang W., Chu B., Wang Y., Cai J., Du W., Daellenbach K.R., Kangasluoma J., Bianchi F., Kujansuu J., Petäjä T., Wang X., Hu B., Wang Y., Ge M., He H., Kulmala M. The promotion effect of nitrous acid on aerosol formation in wintertime Beijing: possible contribution of traffic-related emission. Atmos Chem Phys Discuss. 2020;2020:1–43. [Google Scholar]
- Meng F., Qin M., Tang K., Duan J., Fang W., Liang S., Ye K., Xie P., Sun Y., Xie C., Ye C., Fu P., Liu J., Liu W. High resolution vertical distribution and sources of HONO and NO2 in the nocturnal boundary layer in urban Beijing, China. Atmos. Chem. Phys. Discuss. 2019;2019:1–34. [Google Scholar]
- Meusel H., Tamm A., Kuhn U., Wu D., Leifke A.L., Fiedler S., Ruckteschler N., Yordanova P., Lang-Yona N., Poehlker M., Lelieveld J., Hoffmann T., Poeschl U., Su H., Weber B., Cheng Y. Emission of nitrous acid from soil and biological soil crusts represents an important source of HONO in the remote atmosphere in Cyprus. Atmos. Chem. Phys. 2018;18:799–813. [Google Scholar]
- Michoud V., Colomb A., Borbon A., Miet K., Beekmann M., Camredon M., Aumont B., Perrier S., Zapf P., Siour G., Ait-Helal W., Afif C., Kukui A., Furger M., Dupont J.C., Haeffelin M., Doussin J.F. Study of the unknown HONO daytime source at a European suburban site during the MEGAPOLI summer and winter field campaigns. Atmos. Chem. Phys. 2014;14:2805–2822. [Google Scholar]
- Ndour M., Nicolas M., D’Anna B., Ka O., George C. Photoreactivity of NO2 on mineral dusts originating from different locations of the Sahara desert. Phys. Chem. Chem. Phys. 2009;11:1312–1319. doi: 10.1039/b806441e. [DOI] [PubMed] [Google Scholar]
- Ohyama M., Oka K., Adachi S., Takenaka N. Effects of nitrous acid exposure on pulmonary tissues in guinea pigs. Inhal. Toxicol. 2010;22:930–936. doi: 10.3109/08958378.2010.496476. [DOI] [PubMed] [Google Scholar]
- Ohyama M., Nakajima T., Minejima C., Azuma K., Oka K., Itano Y., Kudo S., Takenaka N. Association between indoor nitrous acid, outdoor nitrogen dioxide, and asthma attacks: results of a pilot study. Int. J. Environ. Health Res. 2019;29:632–642. doi: 10.1080/09603123.2018.1559924. [DOI] [PubMed] [Google Scholar]
- Oswald R., Behrendt T., Ermel M., Wu D., Su H., Cheng Y., Breuninger C., Moravek A., Mougin E., Delon C., Loubet B., Pommerening-Röser A., Sörgel M., Pöschl U., Hoffmann T., Andreae M.O., Meixner F.X., Trebs I. HONO emissions from soil Bacteria as a major source of atmospheric reactive nitrogen. Science. 2013;341:1233–1235. doi: 10.1126/science.1242266. [DOI] [PubMed] [Google Scholar]
- Oswald R., Ermel M., Hens K., Novelli A., Ouwersloot H.G., Paasonen P., Petäjä T., Sipilä M., Keronen P., Bäck J., Königstedt R., Hosaynali Beygi Z., Fischer H., Bohn B., Kubistin D., Harder H., Martinez M., Williams J., Hoffmann T., Trebs I., Sörgel M. A comparison of HONO budgets for two measurement heights at a field station within the boreal forest in Finland. Atmos. Chem. Phys. 2015;15:799–813. [Google Scholar]
- Perner D., Platt U. Detection of nitrous acid in the atmosphere by differential optical absorption. Geophys. Res. Lett. 1979;6:917–920. [Google Scholar]
- Pitts J.N., Grosjean D., Van Cauwenberghe K., Schmid J.P., Fitz D.R. Photooxidation of aliphatic amines under simulated atmospheric conditions: formation of nitrosamines, nitramines, amides, and photochemical oxidant. Environ Sci Technol. 1978;12:946–953. [Google Scholar]
- Qi J., Zheng B., Li M., Yu F., Chen C.C., Liu F., Zhou X.F., Yuan J., Zhang Q., He K.B. A high-resolution air pollutants emission inventory in 2013 for the Beijing-Tianjin-Hebei region, China. Atmos. Environ. 2017;170:156–168. [Google Scholar]
- Qin K., Wang L.Y., Wu L.X., Xu J., Rao L.L., Letu H., Shi T.W., Wang R.F. A campaign for investigating aerosol optical properties during winter hazes over Shijiazhuang, China. Atmos. Res. 2017;198:113–122. [Google Scholar]
- Rasmussen T.R., Brauer M., Kjaergaard S. Effects of nitrous acid exposure on human mucous membranes. Am. J. Respir. Crit. Care Med. 1995;151:1504–1511. doi: 10.1164/ajrccm.151.5.7735607. [DOI] [PubMed] [Google Scholar]
- Ren X., Brune W.H., Mao J., Mitchell M.J., Lesher R.L., Simpas J.B., Metcalf A.R., Schwab J.J., Cai C., Li Y., Demerjian K.L., Felton H.D., Boynton G., Adams A., Perry J., He Y., Zhou X., Hou J. Behavior of OH and HO2 in the winter atmosphere in New York City. Atmos. Environ. 2006;40:252–263. [Google Scholar]
- Shon Z.H., Lee G., Song S.K., Lee M., Han J., Lee D. Characteristics of reactive nitrogen compounds and other relevant trace gases in the atmosphere at urban and rural areas of Korea during May–June, 2004. J. Atmos. Chem. 2007;58:203–218. [Google Scholar]
- Sleiman M., Gundel L.A., Pankow J.F., Jacob P., 3rd, Singer B.C., Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soergel M., Regelin E., Bozem H., Diesch J.M., Drewnick F., Fischer H., Harder H., Held A., Hosaynali-Beygi Z., Martinez M., Zetzsch C. Quantification of the unknown HONO daytime source and its relation to NO2. Atmos. Chem. Phys. 2011;11:10433–10447. [Google Scholar]
- Spataro F., Ianniello A. Sources of atmospheric nitrous acid: state of the science, current research needs, and future prospects. J. Air Waste Manage. Assoc. 2014;64:1232–1250. doi: 10.1080/10962247.2014.952846. [DOI] [PubMed] [Google Scholar]
- Spataro F., Ianniello A., Esposito G., Allegrini I., Zhu T., Hu M. Occurrence of atmospheric nitrous acid in the urban area of Beijing (China) Sci. Total Environ. 2013;447:210–224. doi: 10.1016/j.scitotenv.2012.12.065. [DOI] [PubMed] [Google Scholar]
- Spataro F., Ianniello A., Salvatori R., Nardino M., Esposito G., Montagnoli M. Sources of atmospheric nitrous acid (HONO) in the European High Arctic. Rendiconti Lincei-Scienze Fisiche E Naturali. 2017;28:25–33. [Google Scholar]
- Su H., Cheng Y.F., Cheng P., Zhang Y.H., Dong S., Zeng L.M., Wang X., Slanina J., Shao M., Wiedensohler A. Observation of nighttime nitrous acid (HONO) formation at a non-urban site during PRIDE-PRD2004 in China. Atmos. Environ. 2008;42:6219–6232. [Google Scholar]
- Su H., Cheng Y.F., Shao M., Gao D.F., Yu Z.Y., Zeng L.M., Slanina J., Zhang Y.H., Wiedensohler A. Nitrous acid (HONO) and its daytime sources at a rural site during the 2004 PRIDE-PRD experiment in China. J. Geophys. Res.-Atmos. 2008;113 [Google Scholar]
- Tan Z., Rohrer F., Lu K., Ma X., Bohn B., Broch S., Dong H., Fuchs H., Gkatzelis G.I., Hofzumahaus A., Holland F., Li X., Liu Y., Liu Y., Novelli A., Shao M., Wang H., Wu Y., Zeng L., Hu M., Kiendler-Scharr A., Wahner A., Zhang Y. Wintertime photochemistry in Beijing: observations of ROx radical concentrations in the North China Plain during the BEST-ONE campaign. Atmos. Chem. Phys. 2018;18:12391–12411. [Google Scholar]
- Tan F.Z., Wang W.J., Qi S.F., Kan H.D., Yu X.P., Liu Y., Wu D.Y., Xu B., Meng F., Liu S.E. Air pollutants and outpatient visits for cardiovascular disease in a severe haze-fog city: Shijiazhuang, China. BMC Public Health. 2019;19:10. doi: 10.1186/s12889-019-7690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., An J., Wang F., Li Y., Qu Y., Chen Y., Lin J. Impacts of an unknown daytime HONO source on the mixing ratio and budget of HONO, and hydroxyl, hydroperoxyl, and organic peroxy radicals, in the coastal regions of China. Atmos. Chem. Phys. 2015;15:9381–9398. [Google Scholar]
- Tong S., Hou S., Zhang Y., Chu B., Liu Y., He H., Zhao P., Ge M. Comparisons of measured nitrous acid (HONO) concentrations in a pollution period at urban and suburban Beijing, in autumn of 2014. Sci. China Chem. 2015;58:1393–1402. [Google Scholar]
- Trinh H.T., Imanishi K., Morikawa T., Hagino H., Takenaka N. Gaseous nitrous acid (HONO) and nitrogen oxides (NOx) emission from gasoline and diesel vehicles under real-world driving test cycles. J. Air Waste Manage. Assoc. 2017;67:412–420. doi: 10.1080/10962247.2016.1240726. [DOI] [PubMed] [Google Scholar]
- Underwood G.M., Miller T.M., Grassian V.H. Transmission FT-IR and Knudsen cell study of the heterogeneous reactivity of gaseous nitrogen dioxide on mineral oxide particles. J. Phys. Chem. A. 1999;103:6184–6190. [Google Scholar]
- Underwood G.M., Song C.H., Phadnis M., Carmichael G.R., Grassian V.H. Heterogeneous reactions of NO2 and HNO3 on oxides and mineral dust: a combined laboratory and modeling study. J. Geophy. Res.- Atmos. 2001;106:18055–18066. [Google Scholar]
- Volkamer R., Sheehy P., Molina L.T., Molina M.J. Oxidative capacity of the Mexico City atmosphere – part 1: a radical source perspective. Atmos. Chem. Phys. 2010;10:6969–6991. [Google Scholar]
- Wang S., Zhou R., Zhao H., Wang Z., Chen L., Zhou B. Long-term observation of atmospheric nitrous acid (HONO) and its implication to local NO2 levels in Shanghai, China. Atmos. Environ. 2013;77:718–724. [Google Scholar]
- Wang G., Zhang R., Gomez M.E., Yang L., Zamora M.L., Hu M., Lin Y., Peng J., Guoc S., Meng J., Li J., Cheng C., Hu T., Ren Y., Wang Y., Gao J., Cao J., An Z., Zhou W., Li G., Wang J., Tian P., Marrero-Ortiz W., Secrest J., Du Z., Zheng J., Shang D., Zeng L., Shao M., Wang W., Huang Y., Wang Y., Zhu Y., Li Y., Hu J., Pan B., Cai L., Cheng Y., Ji Y., Zhang F., Rosenfeld D., Liss P.S., Duce R.A., Kolb C.E., Molina M.J. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. U. S. A. 2016;113:13630–13635. doi: 10.1073/pnas.1616540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang X., Guo J., Wang Z., Zhang M. Observation of nitrous acid (HONO) in Beijing, China: seasonal variation, nocturnal formation and daytime budget. Sci. Total Environ. 2017;587:350–359. doi: 10.1016/j.scitotenv.2017.02.159. [DOI] [PubMed] [Google Scholar]
- Wu L., Sun J., Zhang X., Zhang Y., Wang Y., Zhong J., Yang Y. Aqueous-phase reactions occurred in the PM2.5 cumulative explosive growth during the heavy pollution episode (HPE) in 2016 Beijing wintertime. Tellus Series B-Chem. Phys. Meteorol. 2019;71 [Google Scholar]
- Xie Y.Z., Liu Z.R., Wen T.X., Huang X.J., Liu J.Y., Tang G.Q., Yang Y., Li X.R., Shen R.R., Hu B., Wang Y.S. Characteristics of chemical composition and seasonal variations of PM2.5 in Shijiazhuang, China: impact of primary emissions and secondary formation. Sci. Total Environ. 2019;677:215–229. doi: 10.1016/j.scitotenv.2019.04.300. [DOI] [PubMed] [Google Scholar]
- Xing L., Wu J., Elser M., Tong S., Liu S., Li X., Liu L., Cao J., Zhou J., El-Haddad I., Huang R., Ge M., Tie X., Prévôt A.S.H., Li G. Wintertime secondary organic aerosol formation in Beijing–Tianjin–Hebei (BTH): contributions of HONO sources and heterogeneous reactions. Atmos. Chem. Phys. 2019;19:2343–2359. [Google Scholar]
- Xu Z., Wang T., Wu J., Xue L., Chan J., Zha Q., Zhou S., Louie P.K.K., Luk C.W.Y. Nitrous acid (HONO) in a polluted subtropical atmosphere: seasonal variability, direct vehicle emissions and heterogeneous production at ground surface. Atmos. Environ. 2015;106:100–109. [Google Scholar]
- Yang Q., Su H., Li X., Cheng Y., Lu K., Cheng P., Gu J., Guo S., Hu M., Zeng L., Zhu T., Zhang Y. Daytime HONO formation in the suburban area of the megacity Beijing, China. Sci China-Chem. 2014;57:1032–1042. [Google Scholar]
- Yu Y., Galle B., Panday A., Hodson E., Prinn R., Wang S. Observations of high rates of NO2-HONO conversion in the nocturnal atmospheric boundary layer in Kathmandu, Nepal. Atmos. Chem. Phys. 2009;9:6401–6415. [Google Scholar]
- Zhang Q., Zheng Y., Tong D., Shao M., Wang S., Zhang Y., Xu X., Wang J., He H., Liu W., Ding Y., Lei Y., Li J., Wang Z., Zhang X., Wang Y., Cheng J., Liu Y., Shi Q., Yan L., Geng G., Hong C., Li M., Liu F., Zheng B., Cao J., Ding A., Gao J., Fu Q., Huo J., Liu B., Liu Z., Yang F., He K., Hao J. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc. Natl. Acad. Sci. U. S. A. 2019;116:24463–24469. doi: 10.1073/pnas.1907956116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhou X., Bertman S., Tang D., Alaghmand M., Shepson P.B., Carroll M.A. Measurements of ambient HONO concentrations and vertical HONO flux above a northern Michigan forest canopy. Atmos. Chem. Phys. 2012;12:8285–8296. [Google Scholar]
- Zhang Z., Shang J., Zhu T., Li H., Zhao D., Liu Y., Ye C. Heterogeneous reaction of NO2 on the surface of montmorillonite particles. J. Environ. Sci. 2012;24:1753–1758. doi: 10.1016/s1001-0742(11)61014-0. [DOI] [PubMed] [Google Scholar]
- Zhang J., An J., Qu Y., Liu X., Chen Y. Impacts of potential HONO sources on the concentrations of oxidants and secondary organic aerosols in the Beijing-Tianjin-Hebei region of China. Sci. Total Environ. 2019;647:836–852. doi: 10.1016/j.scitotenv.2018.08.030. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen J., Xue C., Chen H., Zhang Q., Liu X., Mu Y., Guo Y., Wang D., Chen Y., Li J., Qu Y., An J. Impacts of six potential HONO sources on HOx budgets and SOA formation during a wintertime heavy haze period in the North China Plain. Sci. Total Environ. 2019;681:110–123. doi: 10.1016/j.scitotenv.2019.05.100. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Chen J.M., Xue C.Y., Chen H., Zhang Q., Liu X.G., Mu Y.J., Guo Y.T., Wang D.Y., Chen Y., Li J.L., Qu Y., An J.L. Impacts of six potential HONO sources on HOx budgets and SOA formation during a wintertime heavy haze period in the North China Plain. Sci. Total Environ. 2019;681:110–123. doi: 10.1016/j.scitotenv.2019.05.100. [DOI] [PubMed] [Google Scholar]
- Zhang W., Tong S., Ge M., An J., Shi Z., Hou S., Xia K., Qu Y., Zhang H., Chu B., Sun Y., He H. Variations and sources of nitrous acid (HONO) during a severe pollution episode in Beijing in winter 2016. Sci. Total Environ. 2019;648:253–262. doi: 10.1016/j.scitotenv.2018.08.133. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Liu B.S., Zhang Y.F., Li Y.F., Sun X.Y., Gu Y., Dai C.L., Li N., Song C.B., Dai Q.L., Han Y., Feng Y.C. A refined source apportionment study of atmospheric PM2.5 during winter heating period in Shijiazhuang, China, using a receptor model coupled with a source-oriented model. Atmos. Environ. 2020:222. [Google Scholar]
- Zhou X.L., Beine H.J., Honrath R.E., Fuentes J.D., Simpson W., Shepson P.B., Bottenheim J.W. Snowpack photochemical production of HONO: a major source of OH in the Arctic boundary layer in springtime. Geophys. Res. Lett. 2001;28:4087–4090. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material