Abstract
The novel coronavirus (SARS-CoV-2) is primarily a respiratory pathogen and its clinical manifestations are dominated by respiratory symptoms, the most severe of which is acute respiratory distress syndrome (ARDS). However, COVID-19 is increasingly recognized to cause an overwhelming inflammatory response and cytokine storm leading to end organ damage. End organ damage to heart is one of the most severe complications of COVID-19 that increases the risk of death. We proposed a two-fold mechanism responsible for causing acute coronary events in patients with COVID-19 infection: Cytokine storm leading to rapid onset formation of new coronary plaques along with destabilization of pre-existing plaques and direct myocardial injury secondary to acute systemic viral infection. A well-coordinated immune response is the first line innate immunity against a viral infection. However, an uncoordinated response and hypersecretion of cytokines and chemokines lead to immune related damage to the human body. Human Coronavirus (HCoV) infection causes infiltration of inflammatory cells that cause excessive production of cytokines, proteases, coagulation factors, oxygen radicals and vasoactive molecules causing endothelial damage, disruption of fibrous cap and initiation of formation of thrombus. Systemic viral infections also cause vasoconstriction leading to narrowing of vascular lumen and stimulation of platelet activation via shear stress. The resultant cytokine storm causes secretion of hypercoagulable tissue factor without consequential increase in counter-regulatory pathways such as AT-III, activated protein C and plasminogen activator type 1. Lastly, influx of CD4+ T-cells in cardiac vasculature results in an increased production of cytokines that stimulate smooth muscle cells to migrate into the intima and generate collagen and other fibrous products leading to advancement of fatty streaks to advanced atherosclerotic lesions. Direct myocardial damage and cytokine storm leading to destabilization of pre-existing plaques and accelerated formation of new plaques are the two instigating mechanisms for acute coronary syndromes in COVID-19.
Introduction
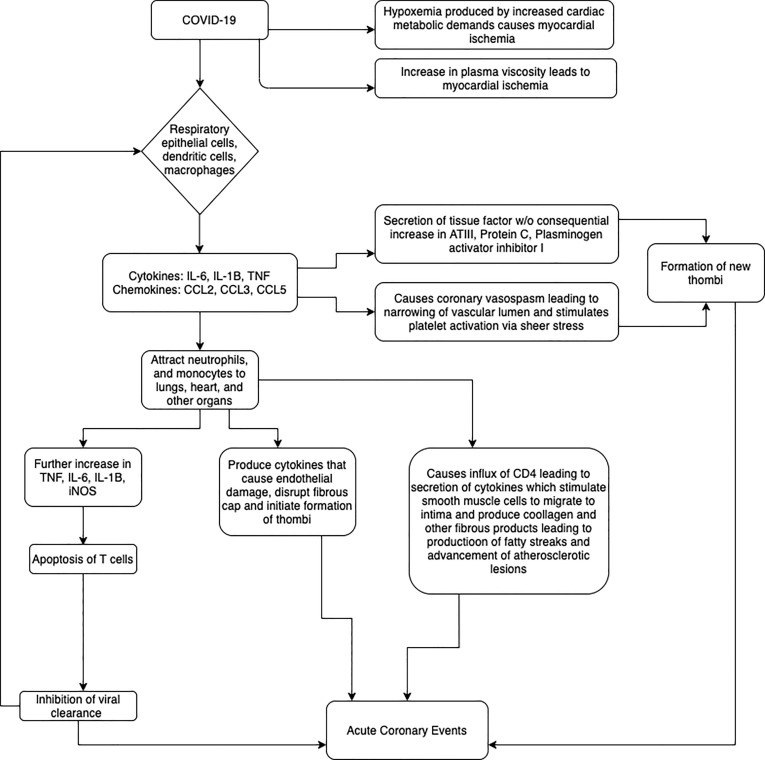
In December 2019, an outbreak of the novel coronavirus disease occurred in Wuhan, China that has subsequently been termed the Coronavirus Disease- 2019 (COVID-19). Like other coronaviruses, the novel coronavirus (SARS-CoV-2) is primarily a respiratory pathogen and its clinical manifestations are dominated by respiratory symptoms, the most severe of which is acute respiratory distress syndrome (ARDS). However, COVID-19 has been increasingly recognized to cause an overwhelming inflammatory response and cytokine storm subsequently resulting in multi-systemic end organ damage [1]. End organ damage to the heart has emerged as one of the most severe complications of COVID-19 that significantly increases the overall mortality in these patients [2]. Patients with pre-existing cardiac conditions have been observed to have a heightened cytokine storm leading to more significant cardiac injury and worse overall outcomes [3], [4]. Among the various cardiac manifestations of COVID-19 are acute myocardial injury (AMI), left ventricular systolic dysfunction, heart failure, arrhythmias and acute coronary events [5]. While there is a lack of medical literature regarding the association of acute coronary events in COVID-19 patients, data can be extrapolated from previous clinical and epidemiological studies in Influenza and acute inflammatory conditions [6], [7], [8], [9] that are also known to cause systemic inflammatory stress. Existing studies have proposed multiple pathophysiologic mechanisms for AMI in the setting of these viral infections. These include, inflammatory and increased shear stress causing plaque rupture leading to acute coronary event, aggravation of pre-existing coronary artery disease causing more severe cardiac injury and other direct and indirect causes of myocardial injuries (Fig. 1 ).
Fig. 1.
Possible mechanisms for causation of Acute Coronary Events in COVID-19. SARS-CoV-2 can cause acute coronary events either directly through direct myocardial injury or indirectly through cytokines and chemokines.
In this piece, we aim to further hypothesize and discuss possible pathophysiologic mechanisms for acute coronary events in COVID-19 infection.
Cytokine storm in systemic viral infections
It is well known that cytokines play an important role in the pathology of a systemic viral illness. A well-coordinated immune response is a part of the first line innate immunity against most viral infections. However, an uncoordinated response results in the hyper-secretion of cytokines and chemokines which further lead to immune related end-organ damage [12]. HCoV (human coronavirus) infection triggers the release of cytokines (IL-6, IL-1B and TNF) and chemokines (CCL-2, CCL-3, and CCL-5) from respiratory epithelial cells, dendritic cells (DCs) and macrophages [13], [14]. Rapidly increasing cytokines and chemokines attract inflammatory cells such as neutrophils and monocytes, causing excessive infiltration of inflammatory cells in lungs and other organs. Through a positive feedback mechanism, the rapid infiltration of monocytes into organs triggers the elevation of proinflammatory cytokines (TNF, IL-6, IL1B and iNOS) which subsequently intensifies the systemic inflammatory response. Additionally, these mononuclear macrophage-derived proinflammatory cytokines induce apoptosis of T cells, which further decreases the viral clearance and results in propagation of the disease [10]. Another consequence of rapid viral replication, inhibition of viral clearance and the over-abundance of inflammatory cytokines is the induction of apoptosis of end organ epithelial and endothelial cells through FAS-FAS ligand (FASL) or TRAIL-death receptor 5 (DR5) [11], [15], [16], [17]. These mechanisms lead to severe end organ damage including, but not limited to, Acute Respiratory Distress Syndrome (ARDS).
Similar to patients affected by other HCoV (SARS, and MERS), patients with COVID-19 have elevated levels of IL-2R and IL-6, which positively correlate with the overall severity of the disease2. In COVID-19, the severity of cytokine storm has been recognized an important factor in predicting the clinical course of extrapulmonary organ failure and mortality [18].
Hypothesis.
Respiratory viral infections have been known to be associated with an increased risk of cardiovascular morbidity and mortality [7]. Systemic inflammation caused by the viral infections plays a key role in the pathogenesis of acute coronary syndromes in these patients [19].
We hypothesize a bi-faced mechanism for the occurrence of acute coronary events in an acute systemic viral infection:
-
1.
Rapid new onset formation of coronary plaques along with acute plaque change (APC) in pre-existing plaques,
-
2.
Direct myocardial injury secondary to acute systemic viral infection.
Acute myocardial infarction in influenza and human coronavirus like infections is well studied [7]. Given the scarcity of information about COVID-19 and its stark similarity with respiratory viral syndromes, it is prudent to extrapolate information to better understand the pathophysiology behind cardiovascular complications of COVID-19.
Evaluation of hypothesis
Destabilization of preexisting plaques in acute systemic viral infections
Most acute coronary syndromes occur due to thrombotic complications at the site of atherosclerotic plaque formation. Disruption of the plaque surface exposes underlying tissue factors, collagen and phospholipids which further causes platelet adhesion and formation of an acute or subacute thrombus. Systemic inflammation after acute viral infections plays a central role in triggering plaque disruption and causation of acute coronary syndromes. When compared to patients with stable coronary artery disease, those with acute coronary syndrome have higher inflammatory markers such as C-reactive protein, procalcitonin, WBC and neutrophil myeloperoxidase across the entire coronary arterial network, in addition to the culprit lesion [20], [21], [22]. As mentioned above, respiratory viruses lead to an increase in infiltration of inflammatory cells (macrophages, neutrophils and T cells) that ultimately produce cytokines, proteases, coagulation factors, oxygen radicals and vasoactive molecules that cause endothelial damage, disrupt fibrous cap and initiate the formation of thrombi [23], [24].
Mechanisms causing direct myocardial injury
A pivotal step in the evolution of acute coronary syndrome is the activation of platelets and formation of platelet thrombi. Platelets can be directly activated by pathogens which can in-turn augment the inflammatory process [25], [26]. Acute infections such as influenza also cause coronary vasoconstriction and stimulate platelet activation via shear stress [27]. Cytokine storm induced by respiratory viral infections also leads to increased secretion of tissue factor without consequential increase in counter-regulatory pathways such as anti-thrombin III activity, activated protein C and plasminogen activator inhibitor type 1 [28], [29], [30], [31], [32]. This leads to the formation of new onset thrombi that can propagate in size causing myocardial ischemia. Furthermore, severe hypoxemia induced by respiratory viral infections, increased cardiac metabolic demands induced by the subsequent cytokine storm and the eventual decrease in mean arterial pressure owing to sepsis causes further development of myocardial ischemia [33]. There is some evidence suggesting presence of heightened plasma viscosity during fever [34], which further increases the chances of myocardial ischemia.
Accelerated formation of plaques in acute systemic viral infections
Atherosclerosis is an inflammatory condition that begins with endothelial injury. A variety of stimuli are known to cause endothelial injury and help accelerate plaque formation. The inflammatory cells including mononuclear monocytes that arise from systemic viral infections induced chemokines and cytokines play a pivotal role in formation of lipid-laden foam cells. These lipid laden foam cells are hallmarks of atheroma formation. Furthermore, influx of CD4 + T-cells results in an increased production of cytokines that stimulate smooth muscle cells to migrate to the intima and generate collagen and other fibrous products [35]. There is a complex interplay in downstream biochemical processes between various inflammatory cytokines and tissue cells leading to the evolution of fatty streak to advanced atherosclerotic lesions. These lesions, formed during an incident event of COVID-19 can be further propagated during subsequent systemic viral illnesses leading to acute coronary syndrome and contributing to increased cardiovascular disease burden.
Conclusion
Improved understanding of underlying mechanisms by which COVID-19 causes myocardial injury will help thousands of individuals currently infected by the SARS-CoV2 to take preventive measures and follow up more intensively to achieve early detection, prevention and treatment of subsequent atherosclerotic disease and new onset myocardial injury. While it is known that repeated influenza infections cause proportionally increased risk of MI, it is yet to be determined if repeated infection with SARS-CoV2 is associated with increased risk of MI. However, based on the knowledge of common mechanisms by which systemic viral infections cause multi-organ damage, it is rational to assume that re-infection with SARS-CoV2 and subsequent infection with other viruses will lead to super-added myocardial injury. Therefore, further research is needed to study in detail, the pathophysiologic mechanisms underlying this association and identify high risk groups that can possibly benefit from intensive cardiovascular screening and follow-up.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110125.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M., Miller C.C., Zarubaev V.V. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016;1(3):274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 9.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 10.Channappanavar R., Fehr A.R., Vijay R. Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson S., Maini M.K., Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res. 2015;35(4):252–264. doi: 10.1089/jir.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law H.K., Cheung C.Y., Ng H.Y. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau S.K.P., Lau C.C.Y., Chan K.H. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94(Pt 12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 15.Herold S., Steinmueller M., von Wulffen W. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205(13):3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Högner K., Wolff T., Pleschka S. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia [published correction appears in PLoS Pathog. 2016 Jun;12(6):e1005716] PLoS Pathog. 2013;9(2) doi: 10.1371/journal.ppat.1003188. e1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigue-Gervais I.G., Labbé K., Dagenais M. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe. 2014;15(1):23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Biasucci L.M., Leo M., De Maria G.L. Local and systemic mechanisms of plaque rupture. Angiology. 2008;59(2 Suppl):73S–76S. doi: 10.1177/0003319708319747. [DOI] [PubMed] [Google Scholar]
- 20.Mauriello A., Sangiorgi G., Fratoni S. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45:1585–1593. doi: 10.1016/j.jacc.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 21.Buffon A., Biasucci L.M., Liuzzo G., D'Onofrio G., Crea F., Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347:5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A., Shimada K., Sano T. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol. 2005;45:1594–1599. doi: 10.1016/j.jacc.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 23.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 24.Hansson G.K., Robertson A.K., Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald J.R., Foster T.J., Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 26.McNicol A., Israels S.J. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- 27.Lassila R., Badimon J.J., Vallabhajosula S., Badimon L. Dynamic monitoring of platelet deposition on severely damaged vessel wall in flowing blood: effects of different stenoses on thrombus growth. Arteriosclerosis. 1990;10:306–315. doi: 10.1161/01.atv.10.2.306. [DOI] [PubMed] [Google Scholar]
- 28.Gando S., Kameue T., Morimoto Y., Matsuda N., Hayakawa M., Kemmotsu O. Tissue factor production not balanced by tissue factor pathway inhibitor in sepsis promotes poor prognosis. Crit Care Med. 2002;30:1729–1734. doi: 10.1097/00003246-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Wilson R.F., Farag A., Mammen E.F., Fujii Y. Sepsis and antithrombin III, prekallikrein, and fibronectin levels in surgical patients. Am Surg. 1989;55:450–456. [PubMed] [Google Scholar]
- 30.Mesters R.M., Mannucci P.M., Coppola R., Keller T., Ostermann H., Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood. 1996;88:881–886. [PubMed] [Google Scholar]
- 31.Shorr A.F., Bernard G.R., Dhainaut J.F. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10:R92. doi: 10.1186/cc4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green J., Doughty L., Kaplan S.S., Sasser H., Carcillo J.A. The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost. 2002;87:218–223. [PubMed] [Google Scholar]
- 33.Madjid M., Naghavi M., Litovsky S., Casscells S.W. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 2003;108:2730–2736. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 34.Naghavi M., Barlas Z., Siadaty S., Naguib S., Madjid M., Casscells W. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation. 2000;102(25):3039–3045. doi: 10.1161/01.cir.102.25.3039. [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.