Abstract
Multivisceral transplantation is a life-saving treatment for many chronically ill patients with advanced abdominal pathologies. For such transplants, a complex arterial reconstruction is required, with numerous anastomoses on a composite donor graft and the native aorta. In these patients, anastomotic disruption or pseudoaneurysm formation, often in the setting of infection, are deadly complications. Open surgical repair is hazardous, because many of these patients have dense adhesions. Reported cases of disruption at the aortic anastomosis to date have resulted in patient demise. We report the case of a pediatric multivisceral transplant recipient with ruptured aortic pseudoaneurysm. He underwent an emergent endovascular parallel stent grafting technique, which successfully controlled bleeding and maintained graft perfusion.
Keywords: Aortic pseudoaneurysm, Endovascular, Multivisceral transplant, Stent graft
Arterial complications after multivisceral transplantation (MVTx) are immediately life threatening and thus require urgent repair. Arterial bleeding complications may occur in 2% to 5% of cases and can be quite variable, owing to the multitude of vascular reconstructions used for MVTx.1, 2, 3 Such complications often stem from bacterial or fungal infections of the composite arterial graft, leading to anastomotic pseudoaneurysm development or frank rupture. Open surgical approaches to repair are impeded by the extreme hostility of the abdomen in these patients and may not be feasible. When performed, results are poor with many patients succumbing to visceral graft loss or rehemorrhage.1,2 Pseudoaneurysms at the anastomosis between the native aorta and composite graft are particularly challenging, because simple repair may lead to graft thrombosis and loss of the visceral graft. Reported cases of ruptured pseudoaneurysms at this location have led to patient demise. Endovascular solutions are appealing because they avoid the treacherous dissection in the setting of uncontrolled aortic bleeding and still allow both exclusion of the pseudoaneurysm and uninterrupted transplant organ perfusion. This report describes a case of complete aortic anastomotic disruption successfully repaired with a parallel stent graft technique, thus controlling hemorrhage without sacrifice of the transplanted viscera. Informed consent for the use of case details and images was obtained.
Case report
A 10-year-old boy with complex medical history who had been hospitalized since undergoing a MVTx 3 months prior became hemodynamically unstable and profoundly anemic. He was found to have complete disruption of the transplant aortic conduit from the native abdominal aorta by computed tomography arteriogram (Fig 1, A-D). Vascular surgery was consulted to provide an endovascular solution.
Fig 1.
Computed tomography arteriogram performed at time of rupture. Axial slice demonstrates large pseudoaneurysm cavity with large hematoma. (A) The aortic conduit can be seen maintaining perfusion. Three-dimensional reconstruction (B) and maximum intensity projection reconstructions (C, anterior-posterior and D, lateral projections) of the abdomen show the disruption itself and the orientation of the aortic conduit. Red arrow, native aorta; red arrowheads, donor aortic conduit; white arrowheads, donor visceral branches.
The patient was born with gastroschisis, the management of which led to numerous enteric fistulas and bowel resections. He was left with a high jejunostomy and short gut physiology. The patient was maintained with total parenteral nutrition, but eventually developed total parenteral nutrition-related liver disease and it was very difficult to maintain intravenous (IV) access. He required multiple central venous interventions, including recanalization and stenting of the innominate vein to permit IV access. He became hospital-bound 3 months before transplantation owing to repeated episodes of variceal bleeding, severe edema, maintenance of IV access, and increasingly complex social structure. His transplantation included liver, small bowel, colon, pancreas, and stomach. The arterial conduit to the donor organs was constructed out of donor thoracic aorta, with the superior mesenteric arteries and celiac arteries reconstructed via a Carrel patch anastomosis to this aortic conduit. An end-to-side anastomosis connected the donor conduit to the native midinfrarenal aorta.
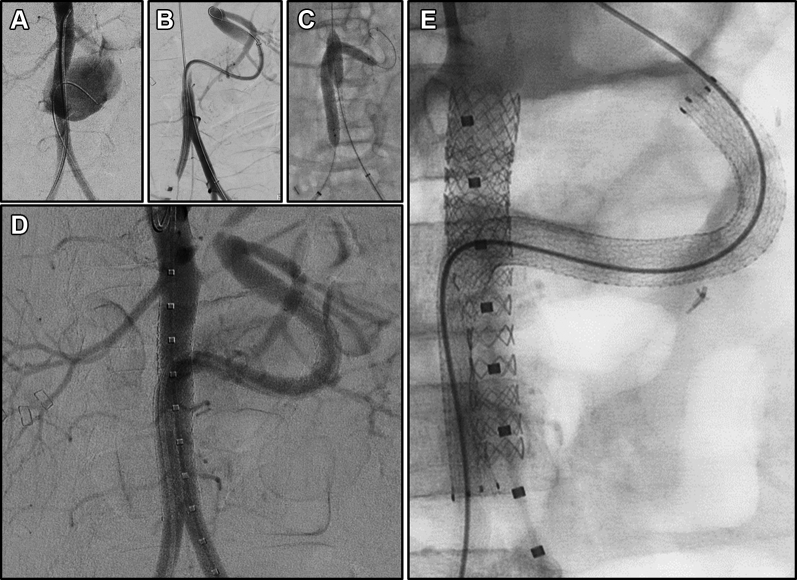
After MVTx, the patient suffered numerous complications, including an abdominal wound dehiscence and evisceration (repaired by debridement and closure with biological mesh), acute cellular rejection of the small bowel, renal failure requiring dialysis, sepsis, respiratory failure, and fungemia. A computed tomography arteriogram was obtained when he became hemodynamically unstable and severely anemic 3 months after MVTx. Given his complex abdominal anatomy, we designed an endovascular approach. In a hybrid operating room, we performed a bilateral common femoral artery exposure and systemic heparin was administered. Endovascularly, we accessed the pseudoaneurysm with an angled hydrophilic wire and catheter and successfully placed a catheter in the aortic conduit. This finding was confirmed with an angiogram, which revealed the patent donor celiac and superior mesenteric arteries at the end of the donor aortic conduit (Fig 2, B). An interventional sheath was advanced over a stiff wire into the donor conduit. An 8-mm balloon-expandable stent-graft (8 × 59 mm; Gore VBX, BXA085902A; W. L. Gore & Associates, Flagstaff, Ariz) was introduced into the aorta from the left and an 8 × 150 mm self-expanding stent-graft (Gore Viabahn, VBJ081502A) was inserted to the distal end of the conduit within the sheath from the right. The sheaths were retracted over the stents to prepare for deployment.
Fig 2.
Endovascular repair of aortic anastomotic disruption. (A) Bilateral femoral arterial access was obtained via cutdowns, and arteriogram was performed. (B) The conduit was cannulated from the right femoral artery, and a 6F sheath was placed over a stiff guide wire. An arteriogram with simultaneous injection from the both arterial sheaths was performed. (C) The Viabahn stent graft was positioned and deployed slowly. The VBX stent was inflated in the aorta before deployment of the native aortic segment of the Viabahn. Once the Viabahn had been fully deployed, the stents were re-angioplastied. The VBX was over-ballooned with a 12-mm balloon and extended with an additional 12-mm stent. (D) Final arteriogram shows flow from injection in proximal aorta. A slow leak is still identified via the gutter. (E) Final configuration of the stents.
The VBX and Viabahn stent grafts were deployed simultaneously. Care was taken to ensure that the VBX stent was fully expanded and held in place on its deployment balloon during Viabahn deployment through the native aorta. Both stent grafts needed to brace against one another to maintain position in the distal aorta, which was too large for either stent to achieve wall apposition alone. An 8-mm balloon was kept inflated within the VBX during postdeployment balloon dilation of the Viabahn stent. A larger VBX stent graft (11 × 29 mm; Gore VBX, BXA112901A) was placed immediately inferior to the renal arteries and overlapping the existing 8-mm VBX. This was dilated further with a 12-mm angioplasty balloon to create the proximal aortic seal (Fig 2, C). The final angiogram revealed a gutter leak from the distal aorta leading to contrast filling of the pseudoaneurysm. An additional simultaneous angioplasty with an 8-mm balloon in the Viabahn and a 7-mm balloon in the VBX was performed to mold the stent grafts in the distal aorta. The residual leak was sluggish (Fig 2, D) and was left without further intervention. The common femoral arteries were repaired primarily, and the small groin incisions were closed.
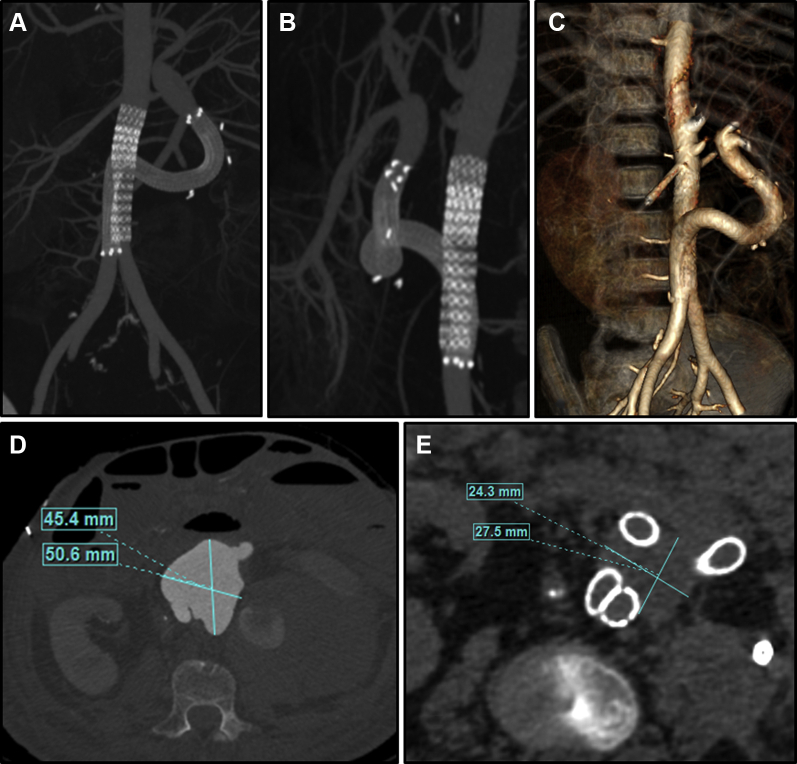
Immediately postoperatively, the patient became hemodynamically stable. On postoperative day 8, a computed tomography scan revealed a thrombosed pseudoaneurysm without residual leak, and patent stent grafts with flow to the donor viscera and preservation of the native aortic bifurcation. (Fig 3, A-C). The hematoma has continued to decrease in size at 3 months (Fig 3, D, E) and arterial duplex scan shows normal flow in the graft (Fig 4, C). He was started on indefinite antifungal therapy with caspofungin as well as broad-spectrum antibiotics. Although his aortic pseudoaneurysm remained controlled, the patient subsequently developed new worsening enterocutaneous fistulae and severe gastrointestinal bleeding. He was ultimately withdrawn from life support.
Fig 3.
Computed tomography arteriogram of completed stent at 2 weeks. (A, B) maximum intensity projection images and (C) three-dimensional reconstruction demonstrate patency of stent grafts without evidence of persistent leak. Active pseudoaneurysm chamber and hematoma decreased substantially in size (D vs E) with resolution of the pseudoaneurysm and no continued evidence of leak at 3 months. (E) Evolution of the fluid collection of the left flank which has been subsequently drained and found to be a fungal abscess (drain can be seen at right of image).
Fig 4.
Computed tomography and duplex imaging at 3 months after stent graft placement. (A) Three-dimensional reconstruction of stent graft configuration at 3 months demonstrates the flexible stent graft's accommodation to the anatomy as the hematoma continues to decompress. (B) Cross-section of the abdominal aorta inferior to the disruption show the interaction of the parallel stent grafts. Note the potential gutter spaces (arrows) between the stents. (C) Duplex of the liver allograft demonstrates normal arterial flow in the main hepatic artery at 3 months.
Discussion
MVTx is a life-saving therapy for patients with intestinal and liver failure and complex abdominal pathology. Although the use of MVTx is increasing, these are still relatively uncommon and challenging procedures with tremendous inherent variation. The arterial reconstructions performed for MVTx are variable but often include a donor thoracic aortic conduit sewn end-to-side to the native recipient infrarenal aorta.3 Aortic anastomotic disruptions associated with MVTx are rare but deadly complications. When occurring late, as in the presented case, they may be associated with bacterial or fungal infection. The few reported cases of aortic pseudoaneurysm rupture in the literature have resulted in mortality, likely owing to the complexity of the arterial reconstructions and the fragility of these patients.1,2,4 Similarly, mortality after arterial pseudoaneurysm in liver transplant patients range between 25% and 85%.5 Open surgical techniques for the control and repair of such catastrophes are difficult owing to hostility of the abdomen, often complicated by infection, scarring, and adhesions in an otherwise medically frail patient. Hybrid techniques using endovascular balloon occlusion to allow for open surgical repair have been advocated, but cases still require surgical exploration and have demonstrated delayed, fatal rebleeding events.1
Although endovascular solutions avoid abdominal exposure, the variability of the arterial anatomy in these patients poses unique challenges. A variety of endovascular techniques have been described to manage arterial pseudoaneurysms in the transplant setting, including covered stents, aortic stent grafts, and arterial embolization with agents such as coils, hydrogels, and thrombin. Although simple embolization may be a viable option for bleeding control when the pseudoaneurysm is saccular and originates from a smaller branch vessel, it is not an option when the bleeding involves the native aorta-conduit anastomosis as in our case. One report of an aortic stent graft deployment in the native aorta was effective for sealing a remnant stump rupture from a previous MVTx in one patient who already undergone successful retransplantation.1 This solution is attractive when the residual stump can be sealed off without risk to the functional viscera. Unfortunately, this is not a feasible approach in cases like ours, because it would result in a loss of graft organ perfusion. Available bifurcated aortic stent grafts are unlikely to accommodate the unique post-surgical anatomy of MVTx patients. One group reported the use of physician-modified endografts for treatment of various visceral transplant pseudoaneurysms.6 Here, a large aortic endograft must be carefully modified on the back table before deployment and cannulated intraoperatively to deploy a balloon-expandable endograft branch. This method is unlikely to work for a case such as ours, given the acuity of bleeding (with associated shortage of time to modify a device) and that anatomically, a long gap with a tortuous path needed to be traversed before sealing in the aortic conduit (Fig 2, B). Available balloon-deployable stent grafts required for such devices are generally neither flexible nor long enough without the use of multiple stents.
Therefore, we opted for a variation of parallel stent graft technique. The parallel stent graft technique (commonly referred to as snorkel or chimney stenting) has been used successfully in renal transplant recipients for pseudoaneurysms at the arterial anastomosis, an analogous problem to the one presented here.7,8 Parallel stent grafts have been more commonly evaluated in management of aortic aneurysms, especially near the aortic arch or visceral segments where dedicated fenestrated or branched grafts may be unavailable.9,10 Our technique, although analogous to the parallel stent grafts used in aortic aneurysm disease, has key differences. In an aortic aneurysm, the main aortic stent graft is a large diameter, self-expanding endograft that is oversized substantially to allow it to wrap around the relatively small parallel stent grafts. Given the anatomic constraints in our pediatric patient, the two parallel stent grafts were similar in diameter. The resultant potential gutter spaces can be identified in Fig 4, B. These gutter spaces likely explain the persistent flow into the pseudoaneurysm identified on completion imaging at the time of repair (Fig 2, D) while fully anticoagulated. Despite these concerns, persistent endoleak occurs in only a minority of aortic aneurysm cases, with rates of 0%-20% at postoperative imaging.11 In the current case, our parallel graft length of more than 30 mm was likely adequate to create a seal in the aorta and may explain the resolving pseudoaneurysm at follow-up imaging.
We chose a balloon-expandable stent graft for the vertical native aortic stent. An advantage of this stent graft type is that, once deployed, its diameter can be further expanded using larger balloon angioplasty (within certain device-specific parameters). We therefore created a funnel-shaped endograft where the diameter was 12 mm proximally and 7 to 8 mm distally, running in parallel with the 8-mm self-expanding stent graft. To traverse the pseudoaneurysm and the aortic conduit graft, a self-expanding stent graft was chosen for its greater flexibility and resiliency to compressive forces. In this pediatric case, the combination of these two stent graft types was instrumental in creating a hemostatic seal. During deployment, it was crucial to keep the aortic stent held in position while expanded on its balloon to allow the parallel self-expanding stent graft to deploy and immediately have an opposing surface contact. Otherwise, the self-expanding stent, which is somewhat longitudinally compressed owing to the curved path, may spring inferiorly across the aortic bifurcation after being fully deployed. In an adult, a similar approach would likely have been feasible, but may have required use of larger available aortic self-expanding graft.
Conclusions
Late aortic anastomotic disruption complicating MVTx is a rare but deadly problem. Given the poor results of hybrid or open techniques, we argue that an endovascular approach using parallel stent grafts may be feasible and life saving. Long-term problems should be expected, particularly relating to persistent graft infection, which may ultimately require reintervention or stent graft explantation. Even without infection, in a pediatric patient one would expect that with bodily growth, the stent graft may need to be explanted in favor of a more definitive reconstruction. Despite the shortcomings, endovascular intervention should be considered as an initial approach in these very complicated cases.
Acknowledgments
The authors acknowledge Dr George Mazariegos and Dr Ajai Khanna from the Hillman Center for Pediatric Transplantation, the multivisceral transplant surgical and anesthesia teams, and the staff of the UPMC Children's Hospital of Pittsburgh surgical ICU that cared for this patient.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Amesur N.B., Zajko A.B., Costa G., Abu-Elmagd K.M. Combined surgical and interventional radiologic management strategies in patients with arterial pseudo-aneurysms after multivisceral transplantation. Transplantation. 2014;97:235–244. doi: 10.1097/TP.0b013e3182a9029a. [DOI] [PubMed] [Google Scholar]
- 2.Tzakis A.G., Kato T., Levi D.M., Defaria W., Selvaggi G., Weppler D. 100 multivisceral transplants at a single center. Ann Surg. 2005;242:480–490. doi: 10.1097/01.sla.0000183347.61361.7a. discussion: 91-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa G., Parekh N., Osman M., Armanyous S., Fujiki M., Abu-Elmagd K. Composite and multivisceral transplantation: nomenclature, surgical techniques, current practice, and long-term outcome. Surg Clin North Am. 2019;99:129–151. doi: 10.1016/j.suc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Calvo Pulido J., Manrique Municio M., Loinaz Segurola C., Justo Alonso I., Caso Maestro O., Garcia-Sesma A. Aortic graft mycotic pseudoaneurysm as a severe complication after multivisceral transplantation: a case report. Transplant Proc. 2016;48:539–542. doi: 10.1016/j.transproceed.2015.10.083. [DOI] [PubMed] [Google Scholar]
- 5.Volpin E., Pessaux P., Sauvanet A., Sibert A., Kianmanesh R., Durand F. Preservation of the arterial vascularisation after hepatic artery pseudoaneurysm following orthotopic liver transplantation: long-term results. Ann Transplant. 2014;19:346–352. doi: 10.12659/AOT.890473. [DOI] [PubMed] [Google Scholar]
- 6.Mafeld S., Logue J.A., Masson S., Thakkar R., Amer A., Wilson C. Treatment of visceral transplant pseudoaneurysms using physician-modified fenestrated stent grafts: initial experience. Cardiovasc Interv Radiol. 2019;42:920–926. doi: 10.1007/s00270-019-02168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glebova N.O., Brooke B.S., Desai N.M., Lum Y.W. Endovascular interventions for managing vascular complication of renal transplantation. Semin Vasc Surg. 2013;26:205–212. doi: 10.1053/j.semvascsurg.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Che H., Men C., Yang M., Zhang J., Chen P., Yong J. Endovascular repair of a transplant renal artery anastomotic pseudoaneurysm using the snorkel technique. J Vasc Surg. 2014;60:1052–1055. doi: 10.1016/j.jvs.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Moulakakis K.G., Mylonas S.N., Avgerinos E., Papapetrou A., Kakisis J.D., Brountzos E.N. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012;55:1497–1503. doi: 10.1016/j.jvs.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Patel R.P., Katsargyris A., Verhoeven E.L.G., Adam D.J., Hardman J.A. Endovascular aortic aneurysm repair with chimney and snorkel grafts: indications, techniques and results. Cardiovasc Interv Radiol. 2013;36:1443–1451. doi: 10.1007/s00270-013-0648-5. [DOI] [PubMed] [Google Scholar]
- 11.Tanious A., Lee J.T., Shames M. Snorkel endovascular abdominal aortic aneurysm repair versus fenestrated endovascular aneurysm repair: is it a competition? Semin Vasc Surg. 2016;29:68–73. doi: 10.1053/j.semvascsurg.2016.07.002. [DOI] [PubMed] [Google Scholar]