Abstract
Extracranial carotid artery dissection represents up to 22% of acute neurovascular disease in young patients. There are no specific guidelines regarding indication for endovascular management of carotid artery dissection with stenting and its complications. We describe three patients with carotid artery dissection and associated dissecting aneurysm who underwent endovascular stenting with the multilayer flow modulator. At 12-month follow-up, the dissecting aneurysms were resolved, and positive clinical outcome was achieved in all patients. Our results suggest that the multilayer flow modulator may be an alternative option for endovascular interventions in patients with carotid artery dissection and pseudoaneurysms because it favors laminar flow, and it may promote spontaneous healing of the wall by progressively reducing the vascular stress in the aneurysm wall. However, further studies are needed to confirm these findings.
Keywords: Internal carotid artery dissection, Stents, Dissecting aneurysm, Endovascular procedures
Extracranial carotid artery dissection represents 5% to 22% of acute neurovascular disease presentations in patients younger than 45 years.1 Data and guidelines regarding the optimal treatment of carotid artery dissection are lacking. Endovascular management with stenting has been recommended after failure of medical therapy or to treat secondary vascular complications, such as dissecting aneurysms, that may be symptomatic because of progression or enlargement, thromboembolism, or compromised flow.2 Here, we describe successful treatment of three patients with extracranial carotid artery dissection with dissecting aneurysms using the multilayer flow modulator (MFM [Cardiatis, Isnes, Belgium]; Fig 1), with positive angiographic and clinical outcome in all cases. After being informed, the patients consented to the procedure and the use of these data for publication.
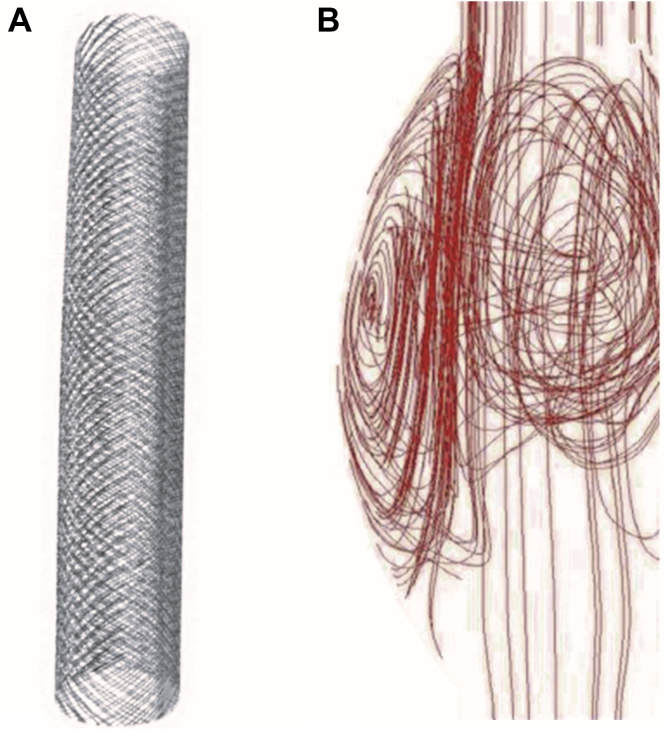
Fig 1.
A, Multilayer flow modulator (MFM), an uncovered, self-expanding stent with high radial force and flexibility constructed of braided fatigue- and corrosion-resistant cobalt alloy wire (Phynox). B, Graphics showing flow dynamics within stent and aneurysm sac.
Case reports
Patient 1
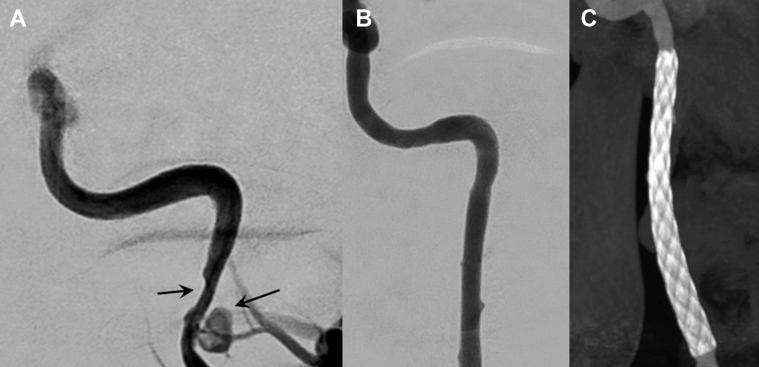
A 35-year-old man with no prior history of trauma, migraine, or vascular risk factors presented with a moderate but persistent headache that had begun the day before consultation; otherwise, the findings on neurologic examination were normal. Brain magnetic resonance imaging demonstrated a bilateral internal carotid artery (ICA) dissection with a right cervical ICA aneurysm (4 × 8 mm) and stenosis (∼50%) of the left ICA (Fig 2, A). The patient was started on aspirin 100 mg daily. Digital subtraction angiography (DSA) was performed 1 month later, confirming dissection of the right ICA with unresolved aneurysm (5 × 8 mm) at its distal-third section. The left ICA showed moderate stenosis (∼50%) for which medical management was deemed reasonable (Fig 2, B and C). The patient was started on clopidogrel 300 mg the day before the procedure. The right ICA was catheterized, and a 6- × 30-mm stent (CPMS 0630/110, MFM) was deployed at the site of the aneurysm, covering the lesion successfully (Figs 3 and 4).
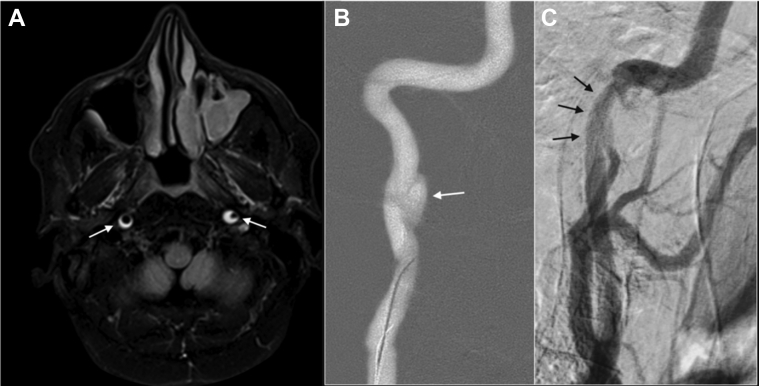
Fig 2.
Patient 1, preprocedure magnetic resonance imaging and angiography. A, Axial T1, fast spin-echo sequence with fat saturation showing two lesions of high signal intensity in the C1 segments of both extracranial internal carotid arteries (ICAs), indicative of intramural hematomas (arrows). B, Roadmap of conventional angiography with right anterior oblique projection showing a dissecting aneurysm of the right ICA (arrow). C, Digital subtraction angiography (DSA) showing stenosis of C1 segment and vertical portion of C2 in the left ICA (arrows).
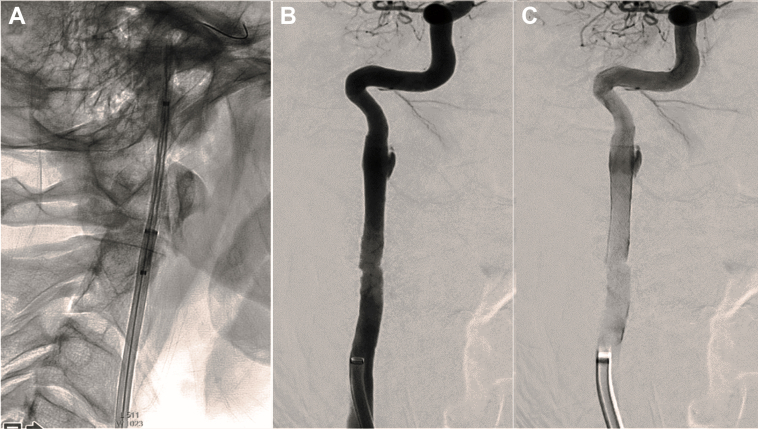
Fig 3.
Patient 1, intraprocedural and postprocedural angiography. A, Ascent of the stent toward the cervical portion of the internal carotid artery (ICA) visualized by radiopaque markers. B and C, After stent release, adequate patency of the right ICA is observed, with minimal stagnant opacification of the aneurysm through the stent.
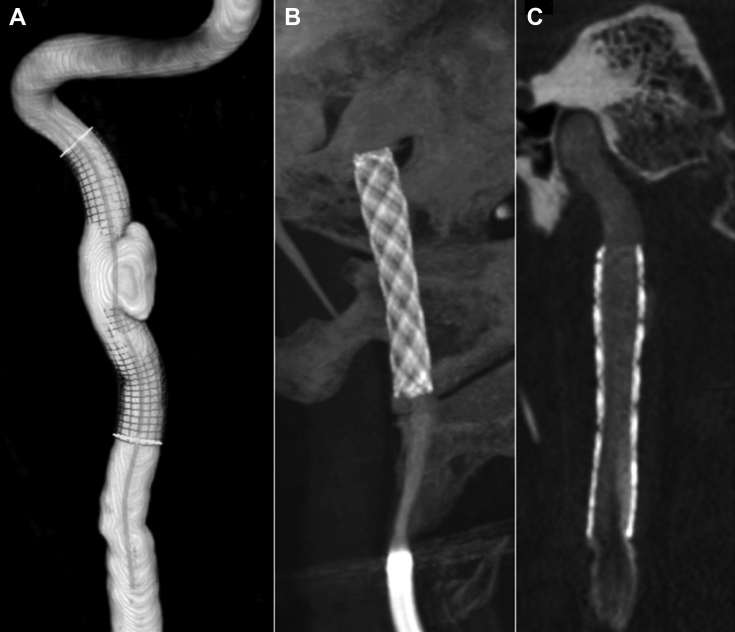
Fig 4.
Patient 1, preprocedure and postprocedure imaging. A, Three-dimensional reconstruction of the preprocedural angiography image shows the aneurysm and is useful for measuring the self-expandable stent. B and C, Postprocedure images with XperCT software, evaluating the location of the stent and proper attachment to the arterial walls, with minimal filling of the aneurysm sac.
Patient 2
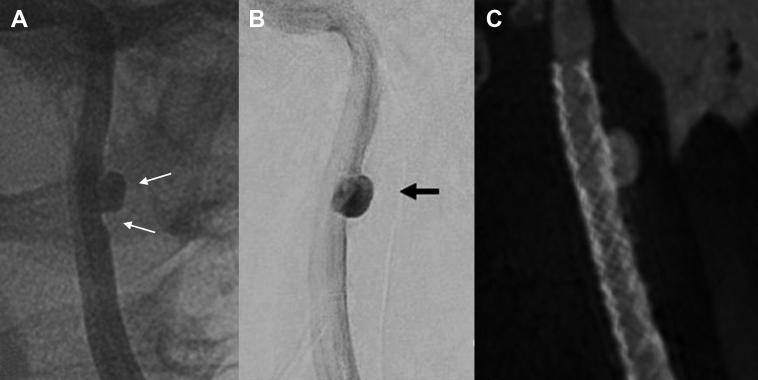
A 49-year-old woman presented with a moderate but persistent headache after minor trauma 2 weeks before consultation. She had no prior history of headaches or any other relevant medical history. The night before consultation, she had a transient left eye blindness of 15 seconds. Findings on urgent non-contrast-enhanced computed tomography (CT) were normal, but CT angiography revealed left ICA dissection and a 5- × 3.5-mm aneurysm on the lateral contour of the C1 segment with moderate stenosis. Treatment with aspirin 100 mg daily was initiated as the patient was monitored in the stroke unit. Endovascular management was discussed with the patient, who accepted treatment after considering the therapeutic options. Clopidogrel was then added to the antiplatelet regimen the day before the procedure at a dose of 300 mg. DSA depicted a left extracranial ICA dissection with a 6- × 4-mm aneurysm arising from a moderately stenotic segment (<50%) of its distal-third section. The left ICA was catheterized, and after the aneurysm was crossed with a 0.018-inch guidewire (SteelCore; Abbott, Abbott Park, Ill), the 6- × 40-mm MFM stent was placed, achieving optimal expansion within the vessel walls after balloon angioplasty, resulting in full coverage of the lesion (Fig 5).
Fig 5.
Patient 2, preprocedure and postprocedure imaging. A, Presurgical angiographic evaluation of the dissecting aneurysm and associated moderately stenotic segment. B, Absence of aneurysm filling after deployment of the stent. C, Postprocedural images obtained with XperCT software, evaluating stent placement and its optimal expansion.
Patient 3
A 28-year-old man, a smoker with no other relevant medical history, suffered a blunt left cervical trauma the week before consultation at the emergency department, where he presented with left-sided Horner syndrome. In particular, during the first evaluation, the patient showed a moderate ptosis of the left eyelid (3-mm droop) and a decrease in 40% to 50% of the left pupil diameter in comparison to the contralateral pupil. Findings on non-contrast-enhanced CT were normal, but CT angiography showed a left ICA dissection at the C1 segment causing moderate stenosis, with an associated small aneurysm. Aspirin 100 mg daily was initially started, and after 1 month, magnetic resonance angiography showed moderate aneurysm growth. Considering lesion enlargement and persistence of neurologic signs, endovascular management was proposed, and the patient consented. After initiation of clopidogrel 300 mg the day before the procedure, DSA showed an aneurysm of 5 × 4 mm in the posterolateral wall of the distal left extracranial ICA. A long introducer (Neuron Max 6F; Penumbra, Alameda, Calif) was placed in the left common carotid artery, then a 6- × 30-mm MFM stent was advanced over a 0.018-inch guidewire (SteelCore) and deployed at the lesion site, with a 5-mm balloon angioplasty for optimal wall apposition, resulting in technical success (Fig 6).
Fig 6.
Patient 3, preprocedure and postprocedure images. A, Digital subtraction angiography (DSA) showing the dissected internal carotid artery (ICA) segment with associated aneurysm and vessel wall irregularity (arrows). B, DSA showing stagnation within the aneurysm sac after treatment (arrow). C, Postprocedure images with XperCT software showing correct location of the stent in place, properly attached to the arterial walls, covering the neck of the aneurysm.
Results
There were no perioperative complications, and after all procedures, arterial patency was verified with XperCT Philips software (Philips, Best, The Netherlands), demonstrating adequate expansion and placement of the stents. All patients were followed up at 6 and 12 months with CT angiography or carotid ultrasound and neurologic evaluation. The three dissecting aneurysms had resolved with no ischemic or other adverse events in this time. Patients 1 and 2 had normal neurologic examination findings, whereas patient 3 showed improvement of Horner syndrome signs. In particular, at the 6-month follow-up examination, the patient presented with light ptosis (<2 mm) and normal diameter of the left pupil, and at 12 months, symptoms had completely resolved. All patients were maintained on dual antiplatelet therapy for 3 months (aspirin 100 mg and clopidogrel 75 mg) and then on aspirin (100 mg daily) for 6 months.
Discussion
We describe three cases in which extracranial ICA dissection with associated aneurysm was treated successfully with the MFM stent with positive angiographic and clinical outcomes. No patient experienced ischemic events, postprocedural in-stent stenosis, or complications. These findings suggest that stenting with the MFM may be a safe and effective alternative option to promote healing of extracranial carotid artery dissection in select cases.
Data regarding natural history of extracranial ICA dissection are scarce.3,4 Dissecting aneurysms are a rare complication of carotid artery dissection that may be present in 12% to 17% of cases5,6 and may lead to neurologic symptoms from hemodynamic compromise, enlargement, or thrombus formation.4
The most common presentation of an extracranial dissection is headache,7 usually head and neck pain along with partial Horner syndrome.8 The headache or neck pain probably results from the direct tear in the blood vessel wall. Because arteries have many nerve plexuses surrounding them, this may cause pain away from the actual site of the dissection.9 Oculosympathetic paresis (also referred to as partial Horner syndrome) is defined as ptosis and miosis without anhidrosis. This phenomenon is caused by ischemia or compression of sympathetic fibers that run from the ICA plexus. Almost a third of patients have partial Horner syndrome. Resolution of both symptoms has been commonly described after resolution of the vascular lesion.10
Carotid artery dissection constitutes a potential long-term risk for distal embolic stroke.11 Moreover, dissecting aneurysms may not heal and remain unresolved in 65% of cases.3 Treatment of carotid artery dissection and its complications is often empirical in the absence of data from randomized controlled trials.
In the acute phase of carotid artery dissection, medical therapy with antithrombotics is recommended to prevent primary or recurrent ischemic events.12 Endovascular treatment with stenting has been recommended after failure of medical therapy or to treat secondary vascular complications, such as aneurysms and severe stenosis.1,13 Moreover, endovascular therapy is also recommended in patients with neurologic symptoms, such as headache, Horner syndrome, or thromboembolic events.13, 14, 15
The effectiveness and safety of endovascular management in patients with carotid artery dissection have been repeatedly reported.2,16, 17, 18, 19, 20 A systematic review of 141 patients from 31 studies with extracranial dissection treated with endovascular management found a technical success rate of 99% and a procedural complication rate of 1.3%.11 A systematic review of endovascular stenting of extracranial carotid artery with aneurysms, which included 258 aneurysms (240 pseudoaneurysms and 18 true aneurysms), confirmed the technical feasibility of the procedure with a success rate of 92.8% and low complication rate (1.8%).21
The MFM is an uncovered, self-expanding stent with high radial force and flexibility constructed of braided fatigue- and corrosion-resistant cobalt alloy wire (Phynox), designed as a treatment strategy for peripheral aneurysm of the extremities or visceral arteries.22,23 The three-dimensional wire layering of the MFM alters blood flow in a way that supports the formation of organized stable-layered thrombus inside an aneurysm sac,24 and it has been successfully applied to favor repair of thoracoabdominal aortic aneurysm.22 In the presented cases, MFM was selected because of its high radial force, providing proper attachment of the stent to the carotid walls, and its capacity to restore laminar flow in the artery.
In particular, exploiting MFM's ability to modulate the formation of layered thrombus inside the aneurysm sac allows protection against risk of rupture; therefore, we expected to favor spontaneous repair of three carotid artery dissecting pseudoaneurysms. While preserving patency of the emergent branches,22 this stent may reduce wall stress and promote healing of the dissected artery through its flow-modulating effect.23 The multilayered structure allows effective endothelialization and protection of the injured vessel wall.
In all our cases, the lesions were located in the C1 segment of the carotid, close to the petrous intracranial segment, and therefore we deemed it reasonable not to use a distal carotid embolism protection filter because it would have conferred a higher risk of arterial injury and possible subsequent carotid occlusion or thromboembolic events.
As this was the first experience using this device in extracranial carotid dissection, we maintained periprocedural antithrombotic therapy according to the standard practice in the center, depending on the clinical characteristics of the patients. In all cases, dual antiplatelet therapy with aspirin and clopidogrel was initiated and maintained for 3 months, whereas single antiplatelet therapy with aspirin was prolonged for 6 months more, with complete resolution of the carotid lesions at follow-up and good clinical outcome with no adverse events.
We have experienced a good technical result with the MFM stent regarding expansion and arterial caliber. During the second and third procedures, we performed a gentle angioplasty to improve the correct stent wall apposition, undersizing the balloon diameter (1 mm below the maximum arterial diameter) to lower the risk of further vessel damage and obtaining resolution of the stenotic segments. The aim of this angioplasty was mainly to achieve proper stent accommodation to the artery to ensure that it would exert its main function of restoring laminar flow, keeping the pressure against the arterial wall as low as possible.
To our knowledge, only one previous case report by Baptista-Sincos et al13 described the use of MFM in a patient with carotid artery dissection. However, the authors needed adjunctive coiling to achieve occlusion of the sac, whereas we achieved complete resolution of pseudoaneurysms with the MFM stent alone. On the contrary, in our series, the aneurysms were smaller, and therefore we aimed for spontaneous healing of the aneurysm without adjunctive coiling, expecting that the MFM characteristics would support the formation of organized thrombus into the aneurysm sac and progressive resolution of the vascular lesion.
We considered endovascular treatment because of the concurrence of neurologic symptoms along with the presence of dissecting aneurysms in patients with carotid artery dissection, with the aim of preventing major complications in otherwise healthy young patients. We believe the MFM stent has an advantageous profile, favoring flow restoration and lesion healing in the presence of a fragile dissecting aneurysm vessel wall.
Conclusions
According to the evidence reported in our study, we suggest that MFM stents may be considered to treat extracranial ICA dissections complicated with pseudoaneurysm in patients who are clinically symptomatic (eg, Horner syndrome, transient ischemic attack) or show lesion persistence despite medical treatment. Importantly, these findings should be confirmed in larger trials with longer follow-up to test the possibility of carotid artery dissections with pseudoaneurysm as an indication for MFM stents, including imaging studies to ascertain stent integrity and patency.
Acknowledgments
The authors acknowledge the team of the Interventional Neuroradiology Section of the Vall d’Hebron Hospital (Barcelona, Spain) and Superior Medical Experts for editing support.
Footnotes
O.L. and C.P. contributed equally to this article.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Patel R.R., Adam R., Maldjian C., Lincoln C.M., Yuen A., Arneja A. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012;20:145–152. doi: 10.1097/CRD.0b013e318247cd15. [DOI] [PubMed] [Google Scholar]
- 2.Chen P.R., Edwards N.J., Sanzgiri A., Day A.L. Efficacy of a self-expandable porous stent as the sole curative treatment for extracranial carotid pseudoaneurysms. World Neurosurg. 2016;88:333–341. doi: 10.1016/j.wneu.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Guillon B., Brunereau L., Biousse V., Djouhri H., Lèvy C., Bousser M.G. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology. 1999;53:117–122. doi: 10.1212/wnl.53.1.117. [DOI] [PubMed] [Google Scholar]
- 4.Desfontaines P., Despland P.A. Dissection of the internal carotid artery: aetiology, symptomatology, clinical and neurosonological follow-up, and treatment in 60 consecutive cases. Acta Neurol Belg. 1995;95:226–234. [PubMed] [Google Scholar]
- 5.Debette S., Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8:668–678. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee V.H., Brown R.D., Jr., Mandrekar J.N., Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67:1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 7.Dziewas R., Konrad C., Drager B., Evers S., Besselmann M., Lüdemann P. Cervical artery dissection—clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179–1184. doi: 10.1007/s00415-003-0174-5. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh H.U. Headache in intracranial and cervical artery dissections. Curr Pain Headache Rep. 2016;20:8. doi: 10.1007/s11916-016-0544-1. [DOI] [PubMed] [Google Scholar]
- 9.Reeves A.G., Swenson R.S. Disorders of the nervous system: a primer. https://www.dartmouth.edu/∼dons/ Available at: Accessed May 30, 2019.
- 10.Rao A.S., Makaroun M.S., Marone L.K., Cho J.S., Rhee R., Chaer R.A. Long-term outcomes of internal carotid artery dissection. J Vasc Surg. 2011;54:370–374. doi: 10.1016/j.jvs.2011.02.059. discussion: 375. [DOI] [PubMed] [Google Scholar]
- 11.Pham M.H., Rahme R.J., Arnaout O., Hurley M.C., Bernstein R.A., Batjer H.H. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68:856–866. doi: 10.1227/NEU.0b013e318209ce03. discussion: 866. [DOI] [PubMed] [Google Scholar]
- 12.Brott T.G., Halperin J.L., Abbara S., Bacharach J.M., Barr J.D., Bush R.L. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 13.Baptista-Sincos A.P., Simplicio A.B., Sincos I.R., Leaderman A., Neto F.S., Moraes A. Flow-diverting stent in the treatment of cervical carotid dissection and pseudoaneurysm: review of literature and case report. Ann Vasc Surg. 2018;46:372–379. doi: 10.1016/j.avsg.2017.06.151. [DOI] [PubMed] [Google Scholar]
- 14.Ahlhelm F., Benz R.M., Ulmer S., Lyrer P., Stippich C., Engelter S. Endovascular treatment of cervical artery dissection: ten case reports and review of the literature. Interv Neurol. 2013;1:143–150. doi: 10.1159/000351687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Hassen W., Machet A., Edjlali-Goujon M., Legrand L., Ladoux A., Mellerio C. Imaging of cervical artery dissection. Diagn Interv Imaging. 2014;95:1151–1161. doi: 10.1016/j.diii.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Biggs K.L., Chiou A.C., Hagino R.T., Klucznik R.P. Endovascular repair of a spontaneous carotid artery dissection with carotid stent and coils. J Vasc Surg. 2004;40:170–173. doi: 10.1016/j.jvs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Oishi H., Yoshida K., Oyama M., Tsuji O., Sonobe M. Combination of stenting and coil embolization for carotid artery pseudoaneurysm causing symptomatic mass effect: case report. No Shinkei Geka. 2002;30:437–441. [PubMed] [Google Scholar]
- 18.Rahal J.P., Dandamudi V.S., Heller R.S., Safain M.G., Malek A.M. Use of concentric Solitaire stent to anchor Pipeline flow diverter constructs in treatment of shallow cervical carotid dissecting pseudoaneurysms. J Clin Neurosci. 2014;21:1024–1028. doi: 10.1016/j.jocn.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Redekop G., Marotta T., Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 20.Zeleňák K., Zeleňáková J., DeRiggo J., Kurča E., Kantorová E., Poláček H. Treatment of cervical internal carotid artery spontaneous dissection with pseudoaneurysm and unilateral lower cranial nerves palsy by two silk flow diverters. Cardiovasc Intervent Radiol. 2013;36:1147–1150. doi: 10.1007/s00270-012-0472-3. [DOI] [PubMed] [Google Scholar]
- 21.Li Z., Chang G., Yao C., Guo L., Liu Y., Wang M. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42:419–426. doi: 10.1016/j.ejvs.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Sultan S., Kavanagh E.P., Bonneau M., Kang C., Alves A., Hynes N.M. Kinetics of endothelialization of the multilayer flow modulator and single-layer arterial stents. Vascular. 2016;24:78–87. doi: 10.1177/1708538115585073. [DOI] [PubMed] [Google Scholar]
- 23.Calik E., Erkut B. Endovascular repair of a Stanford type A dissection with the Cardiatis multilayer flow modulator. Interact Cardiovasc Thorac Surg. 2019;28:321–323. doi: 10.1093/icvts/ivy241. [DOI] [PubMed] [Google Scholar]
- 24.Argenteri A., Bianchi G. Use of multilayer flow modulator in the treatment of aneurysms. Minerva Medica; Torino, Italy: 2012. Overview; pp. 1–7. [Google Scholar]