Abstract
Objective
To evaluate the effect of perfluorobutane microbubbles (Sonazoid®, GE Healthcare) on steam popping during radiofrequency (RF) ablation for treating hepatocellular carcinoma (HCC), and to assess whether popping affects treatment outcomes.
Materials and Methods
The institutional review board approved this retrospective study, which included 90 consecutive patients with single HCC, who received percutaneous RF ablation as the first-line treatment. The patients were divided into two groups, based on the presence or absence of the popping phenomenon, which was defined as an audible sound with a simultaneous sudden explosion within the ablation zone as detected via ultrasonography during the procedure. The factors contributing to the popping phenomenon were identified using multivariable logistic regression analysis. Local tumor progression (LTP) and disease-free survival (DFS) were assessed using the Kaplan-Meier method with the log-rank test for performing comparisons between the two groups.
Results
The overall incidence of the popping phenomenon was 25.8% (24/93). Sonazoid® was used in 1 patient (4.2%) in the popping group (n = 24), while it was used in 15 patients (21.7%) in the non-popping group (n = 69). Multivariable analysis revealed that the use of Sonazoid® was the only significant factor for absence of the popping phenomenon (odds ratio = 0.10, p = 0.048). There were no significant differences in cumulative LTP and DFS between the two groups (p = 0.479 and p = 0.424, respectively).
Conclusion
The use of Sonazoid® has a suppressive effect on the popping phenomenon during RF ablation in patients with HCC. However, the presence of the popping phenomenon may not affect clinical outcomes.
Keywords: Liver, Hepatocellular carcinoma, Radiofrequency ablation, Contrast media, Explosions
INTRODUCTION
Hepatocellular carcinoma (HCC) represents about 90% of primary liver cancers and its management constitutes a major global health problem despite advancement in the field (1). Although liver transplantation and surgical resection are important curative treatment modalities for HCC, radiofrequency (RF) ablation is the standard of care for patients with Barcelona-Clinic Liver Cancer (BCLC) stage 0 and A tumors, which are not suitable for surgery, owing to the scarcity of donor organs and limited hepatic reserve in patients with chronic liver disease (2).
However, several reports have raised concerns over the unexplained recurrence of aggressive tumor after RF ablation for HCC (3,4,5,6,7,8), which deserves special attention. Although the exact mechanism underlying this type of tumor recurrence remains unclear, intravascular tumor spread may be one of the causes of this serious complication. This could be the result of a sudden increase in the internal pressure of the ablated tissue, leading to the popping phenomenon with scattering of tumor cells around the ablation zone (9). Attempts have been made using modified ablation techniques with low (10) or multi-step incremental RF power (11) to minimize the occurrence of the popping phenomenon or the increase in the intra-tumor pressure during thermal ablation.
We observed an interesting effect during our recent experiences with RF ablation for HCC after perfluorobutane microbubble (Sonazoid®, GE Healthcare, Oslo, Norway)-enhanced ultrasonography (US) (12): the ablation zone created by RF ablation-mediated heating expands more smoothly, with fewer instances of the popping phenomenon with the use of Sonazoid®. Similarly, our experimental study (13) that used an in vivo rabbit liver model showed that the popping sound was barely perceivable (which is commonly perceived during conventional RF ablation) during RF ablation after Sonazoid® uptake. Therefore, we hypothesized that the use of Sonazoid® may suppress the popping phenomenon during RF ablation for HCC and may contribute to a reduction in unintended pressure-related complications (5,9). However, to date, there has been no study on the role of Sonazoid® beyond the conventional lesion characterization using contrast-enhanced US.
The aim of this retrospective study was to identify the relevant factors, including Sonazoid® use, which may be responsible for the popping phenomenon during percutaneous RF ablation for HCC. Moreover, we evaluated the association between the occurrence of steam popping and the clinical outcomes after treatment.
MATERIALS AND METHODS
Study Overview
We conducted a retrospective case-control study using the records from the database of patients with HCC treated at a tertiary academic cancer center. This study was approved by the responsible Institutional Review Board (approval number: SMC 2019-03-008) and the requirement for informed consent was waived. We followed the standardized terminology and reporting criteria for RF ablation provided by the International Working Group on Image-Guided Tumor Ablation (14).
Patients
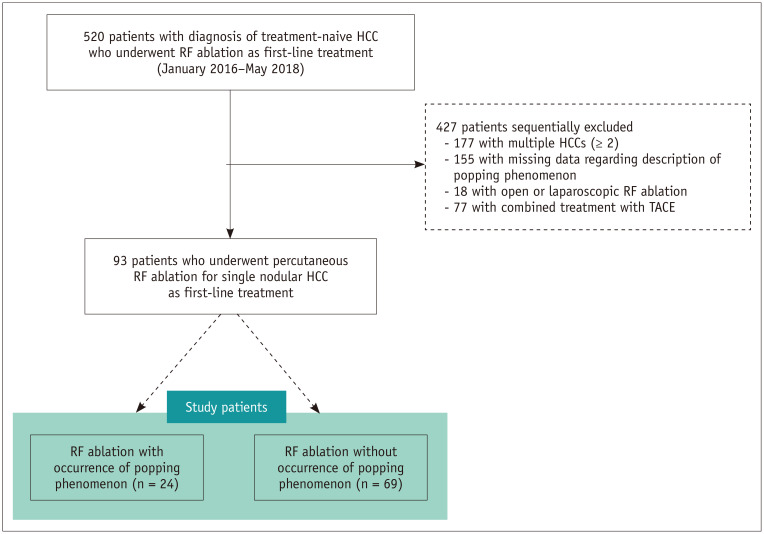
A total of 520 consecutive patients with treatment-naive HCCs, who underwent RF ablation as the first-line treatment between January 2016 and May 2018 at Samsung Medical Center, Sungkyunkwan University, Seoul, Korea, were included. Our institutional inclusion criteria for RF ablation procedures in patients with HCCs were [similar to those described in our previous studies (15,16)] as follows: 1) a single nodular HCC measuring < 3 cm or multinodular HCCs (≤ 3 in number, each measuring < 3 cm); 2) Child-Pugh class A or B; 3) absence of macrovascular invasion and extrahepatic metastasis during pretreatment imaging evaluation; and 4) normal prothrombin time and platelet count > 50000 cells/mL. Among these patients, 427 were excluded for the following reasons: 177 patients with multiple HCCs (≥ 2), 155 with missing data describing the popping phenomenon during RF ablation on electronic medical records (EMRs), 18 patients treated with the non-percutaneous approach such as open or laparoscopic RF ablation, and 77 patients who underwent combined treatment with transarterial chemoembolization (Fig. 1). Finally, 93 patients (69 men and 24 women; mean age, 61.0 years; mean tumor size, 1.5 cm) who underwent percutaneous RF ablation for single nodular HCC as first-line treatment were included.
Fig. 1. Flow diagram of patient selection process used in study.
HCC = hepatocellular carcinoma, RF = radiofrequency, TACE = transarterial chemoembolization
The information collected at baseline included patient-related data (age, sex, etiology of chronic liver disease, laboratory data, and survival data), procedure-related data (ablation time, number of overlapping ablations, type and gauge of RF electrode, use of Sonazoid®, or artificial ascites), and tumor-related data (tumor size and location, and serum α-fetoprotein levels).
Definition of the Popping Phenomenon
Although several previous studies have investigated the popping phenomenon during RF ablation (10,13,17,18), to the best of our knowledge, none have provided a standardized definition for it. In the current study, the popping phenomenon was defined as an audible popping sound with a simultaneous sudden explosion within the ablation zone around the active RF electrode tip, as identified by US by the primary operator holding the RF electrode during the entire session. Operators assessed the presence or absence of the popping phenomenon prospectively, while keeping a record of the procedure on the EMR system immediately after treatment. Subsequently, patients were classified into the popping group (n = 24) or the non-popping group (n = 69).
RF Ablation Procedures
All RF ablation procedures were performed percutaneously under US guidance (RS80A, Samsung Medison, Seoul, Korea or LOGIQ E9, GE Healthcare) by 1 of 3 radiologists on an inpatient basis (all with more than 7 years of experience in image-guided ablation). The detailed methodology of the RF ablation procedure was identical to that used in previous studies (15,16). We used commercially available RF electrodes with generators (VIVA Multi RF generator®, STARmed, Goyang, Korea or M-3004® System, RF Medical, Seoul, Korea) with a single internally cooled electrode (Proteus®, STARmed) or a single internally cooled wet electrode (Jet-Tip, RF Medical) or multiple internally cooled electrodes (Octopus®, STARmed), depending on tumor size and location, and equipment availability. The term wet electrode refers to a RF electrode that allows saline infusion into the target tissue to increase electrical and thermal conductance (19). Due to the known risk of aggressive intrasegmental recurrence after RF ablation for HCCs based on a previous study (4), we routinely used a multistep incremental increase in RF power, to avoid sudden increases in intra-tumor pressure (20) in the following manner: starting at 30–40 W for 1 minute and subsequently increasing the RF power by 5–10 W every minute up to 100–120 W.
The fusion imaging technique (S-Fusion, Samsung Medison or Volume Navigation, GE Healthcare) was used to avoid mistargeting the index tumor. Sonazoid® was used to enhance lesion conspicuity if the index tumor was not clearly visible even with fusion imaging (12). Artificial ascites were used if the risk of collateral thermal injury to an adjacent structure was expected during ablation, or if the sonic window needed enhancement for a better RF electrode path (21). The therapeutic objective of RF ablation for HCC was to obtain at least 0.5 cm of normal liver tissue surrounding the tumor as an ablative margin (14). After the procedure, the electrode path was cauterized to prevent bleeding and tumor seeding during electrode retraction. Contrast material-enhanced multiphase computed tomography (CT) scans were obtained immediately after RF ablation to determine the technical success of each treatment, and to assess any immediate major complications.
Follow-Up after Treatment
All patients underwent contrast-enhanced multiphase CT, chest radiography, and laboratory tests including serum α-fetoprotein level 1 month after initial treatment, every 3 months during the first 2 years, and every 4–6 months thereafter, to assess therapeutic outcomes and delayed complications (22). Gadoxetic acid-enhanced magnetic resonance imaging (MRI) was performed to further characterize an indeterminate hepatic lesion on follow-up CT. Chest CT, brain MRI, and whole-body bone scintigraphy were also performed if extrahepatic recurrence was suspected on the basis of clinical symptoms or unexplained elevation of tumor markers.
Outcomes
The primary outcome of the study was to identify the risk factors responsible for the occurrence of the popping phenomenon during RF ablation for HCC. The secondary outcomes included local tumor progression (LTP) rate, intrahepatic distant recurrence (IDR) rate, and disease-free survival (DFS) rate based on the presence or absence of the popping phenomenon. Moreover, the incidence of aggressive intrasegmental recurrence was analyzed for the two groups. LTP was defined as the new appearance of enhancing tumor tissue at the margin of the ablation zone on follow-up images. DFS was defined as the time interval in the follow-up period during which the patient did not experience LTP, IDR, extrahepatic recurrence, or death (22). Aggressive intrasegmental recurrence was defined as the simultaneous development of multiple nodular (at least 3) or infiltrative tumor recurrence in the treated hepatic segment during follow-up, based on a previous study (4). The observation time was defined as the interval between initial treatment and the last visit to the outpatient clinic or death before January 31, 2019.
Statistical Analysis
Continuous data were evaluated using the Mann-Whitney U tests for comparison of between the baseline and clinical variables of the two groups, because we assumed that our data did not approximate normal distribution, due to the relatively small sample size of the popping group. Categorical variables were analyzed using both the chi-squared and Fisher's exact tests. Risk factors for the popping phenomenon during RF ablation were assessed using logistic regression analysis. All variables in the univariate analyses were included in multivariate analyses because we hypothesized that all the procedure-related control variables and location of the tumor could influence the occurrence of the popping phenomenon. The possible risk factors analyzed included tumor size, type or size of RF electrode, total ablation time, number of overlapping ablations during the procedure, use of artificial ascites, use of Sonazoid®, and perivascular or subcapsular location. Moreover, the individual operator was entered into the multivariate model to minimize bias arising from the subjective measurement of the popping phenomenon. The variance inflation factor between the variables in the multivariate analyses was calculated for detecting multicollinearity. The value of any variable did not exceed 1.5. Cumulative LTP and IDR rates and DFS were estimated during the Kaplan-Meier method with the log-rank test. Statistical analyses were performed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
The baseline characteristics of all patients (n = 93) are presented in Table 1. All patients were classified as BCLC stage 0 (n = 66) or A (n = 27). The median size of the index tumor was 1.5 cm (range, 1.2–1.8 cm). Sonazoid® was used for contrast-enhanced US in 16 of 93 patients (17.2%), due to poor visibility of the index tumor on B-mode US imaging. The 3 radiologists observed the popping phenomenon during RF ablation in the following frequency: radiologist 1, 46.1%, 6 of 13 patients; radiologist 2, 23.3%, 14 of 60 patients; and radiologist 3, 20.0%, 4 of 20 patients. The overall incidence rate of the popping phenomenon was 25.8% (24/93). In the popping group, Sonazoid® was used in 1 patient (1/24, 4.2%), while it was used in 15 patients (15/69, 21.7%) in the non-popping group. There were no significant differences in the patients' clinical and tumor characteristics, or technical parameters during RF ablation between the two groups (p > 0.05) (Tables 1, 2).
Table 1. Baseline Clinical and Tumor Characteristics of Study Patients.
| Variable | Popping Group (n = 24) | Non-Popping Group (n = 69) | P |
|---|---|---|---|
| Age (years) | 59.5 (55.5–64.5) | 61.0 (54.0–68.0) | 0.503 |
| Number of men* | 17 (70.8) | 52 (75.4) | 0.662 |
| Underlying chronic liver disease* | 0.563 | ||
| Hepatitis B virus | 20 (83.3) | 49 (71.0) | |
| Hepatitis C virus | 1 (4.2) | 7 (10.1) | |
| Other | 3 (12.5) | 13 (18.9) | |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.9) | 0.8 (0.5–1.1) | 0.078 |
| Albumin (g/dL) | 4.3 (4.1–4.5) | 4.2 (3.9–4.5) | 0.499 |
| Prothrombin time (INR) | 1.1 (1.04–1.17) | 1.11 (1.05–1.23) | 0.484 |
| Child-Pugh class* | > 0.999 | ||
| Class A | 22 (91.7) | 62 (89.9) | |
| Class B | 2 (8.3) | 7 (10.1) | |
| Tumor size (cm) | 1.5 (1.3–2.0) | 1.5 (1.2–1.8) | 0.663 |
| Location of tumor* | |||
| Perivascular | 10 (41.7) | 31 (44.9) | 0.782 |
| Subcapsular | 10 (41.7) | 34 (49.3) | 0.520 |
| α-fetoprotein prior to treatment | 5.6 (3.1–19.3) | 5.9 (3.5–12.6) | 0.976 |
Unless indicated otherwise, data are medians with interquartile ranges in parentheses. *Data are number of patients, with percentages in parentheses. Continuous data were evaluated by using Mann-Whitney U tests. Categorical variables were analyzed by chi-squared tests or Fisher's exact tests. INR = international normalized ratio
Table 2. Technical Parameters of RF Ablation in Study Patients.
| Variable | Popping Group (n = 24) | Non-Popping Group (n = 69) | P |
|---|---|---|---|
| Size of RF electrode* | 0.636 | ||
| 15 gauge | 14 (58.3) | 44 (63.8) | |
| 17 gauge | 10 (41.7) | 25 (36.2) | |
| Type of RF electrode* | > 0.999 | ||
| Internally cooled type | 20 (83.3) | 58 (84.1) | |
| Internally cooled wet type | 4 (16.7) | 11 (15.9) | |
| Total ablation time (min) | 14.5 (8.5–23.5) | 15.0 (11.0–19.0) | 0.712 |
| Number of overlapping ablations | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.870 |
| Use of artificial ascites* | 5 (20.8) | 24 (34.8) | 0.203 |
| Use of Sonazoid® (GE Healthcare) for CEUS* | 1 (4.2) | 15 (21.7) | 0.061 |
Unless indicated otherwise, data are medians with interquartile ranges in parentheses. *Data are number of patients, with percentages in parentheses. Continuous data were evaluated by using Mann-Whitney U tests. Categorical variables were analyzed by chi-squared tests or Fisher's exact tests. CEUS = contrast-enhanced ultrasonography, RF = radiofrequency
Risk Factor Analysis for the Popping Phenomenon
Univariate analysis did not reveal any statistically significant risk factor for the popping phenomenon during RF ablation for treating HCC. Multivariate analysis revealed that the use of Sonazoid® was the only significant risk factor associated with the popping phenomenon (odds ratio = 0.10, 95% confidence interval = 0.01–0.98; p = 0.048) (Table 3).
Table 3. Risk Factor Analysis for Popping Phenomenon during RF Ablation for Hepatocellular Carcinoma.
| Variable | Popping Phenomenon | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Tumor size (cm) | 1.42 (0.47–4.31) | 0.538 | 3.50 (0.57–22.81) | 0.191 |
| Size of RF electrode | 0.636 | 0.557 | ||
| 15 gauge | 1 [reference] | 1 [reference] | ||
| 17 gauge | 1.26 (0.49–3.25) | 1.54 (0.37–6.46) | ||
| Type of RF electrode | 0.934 | 0.707 | ||
| Internally cooled type | 1 [reference] | 1 [reference] | ||
| Internally cooled wet type | 0.95 (0.27–3.32) | 0.68 (0.09–4.97) | ||
| Total ablation time (min) | 0.99 (0.93–1.06) | 0.873 | 1.02 (0.89–1.17) | 0.757 |
| Number of overlapping ablations [none] | 0.93 (0.66–1.31) | 0.672 | 0.76 (0.43–1.36) | 0.352 |
| Use of artificial ascites [none] | 0.49 (0.16–1.49) | 0.209 | 0.61 (0.17–2.20) | 0.452 |
| Use of Sonazoid® for CEUS [none] | 0.16 (0.02–1.26) | 0.081 | 0.10 (0.01–0.98) | 0.048 |
| Perivascular location [none] | 0.88 (0.34–2.24) | 0.782 | 0.57 (0.18–1.78) | 0.331 |
| Subcapsular location [none] | 0.74 (0.29–1.88) | 0.521 | 0.84 (0.26–2.66) | 0.763 |
| Operator | 0.206 | 0.107 | ||
| 1 | 1 [reference] | 1 [reference] | ||
| 2 | 0.36 (0.10–1.23) | 0.103 | 0.17 (0.03–1.13) | 0.067 |
| 3 | 0.29 (0.06–1.37) | 0.118 | 0.11 (0.01–1.00) | 0.053 |
Numbers in parentheses are 95% CIs. Logistic regression model was used. Reference category for each categorical variable is in square brackets in first column. CI = confidence interval
Clinical Follow-Up for Outcomes
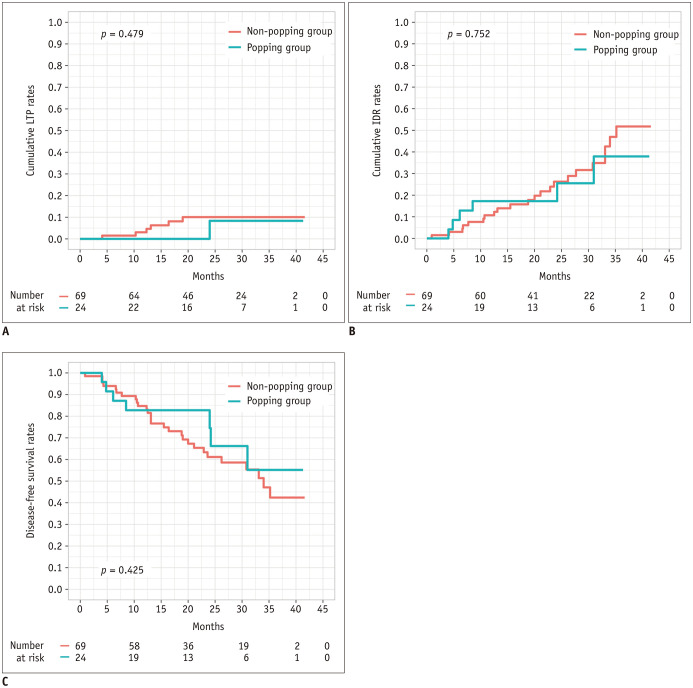
The median follow-up period of the study was 27.2 months (interquartile range: 17.8–34.0 months). At the time of censoring, LTP had developed in 6 of 69 (8.7%) patients in the non-popping group and in 1 of 24 (4.2%) patients in the popping group. The cumulative LTP rates at 1 and 3 years were 2.9% and 8.7%, respectively, in the popping group and 0% and 4.2% in the non-popping group, respectively and were not statistically significant (p = 0.479) (Fig. 2A). IDR was observed in 6 of 24 (25.0%) patients in the popping group and in 22 of 69 (31.9%) patients in the non-popping group. Cumulative IDR rates were not significantly different between the groups (25.0% vs. 37.5% at 1 year and 10.1% vs. 31.9% at 3 years, respectively; p = 0.752) (Fig. 2B). The DFS rates at 1 and 3 years were estimated to be 82.8% and 55.2%, respectively, in the popping group, and 87.8% and 65.4%, respectively, in the non-popping group, without a significant difference (p = 0.425) (Fig. 2C). Moreover, 2 cases of aggressive intrasegmental recurrence had developed in the non-popping group during follow-up (p = 0.405) (Fig. 3), while none were observed in the popping group.
Fig. 2. Types of recurrence and survival curves in patients from both groups.
A. Cumulative LTP rates and number of patients at risk. B. Cumulative IDR rates and number of patients at risk. C. Disease-free survival rates and number of patients at risk. These outcomes were estimated using Kaplan-Meier method with log-rank test. IDR = intrahepatic distant recurrence, LTP = local tumor progression
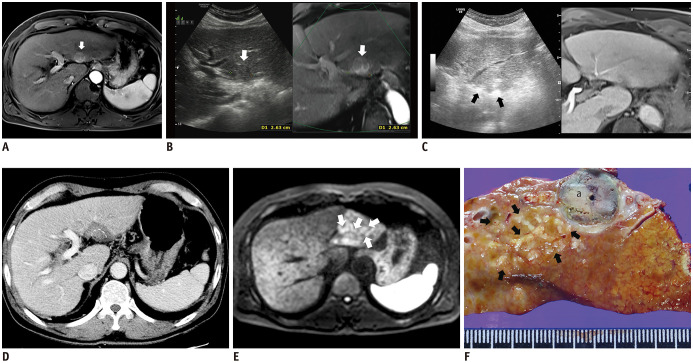
Fig. 3. Multiple nodular form of recurring aggressive intrasegmental HCC after RF ablation in absence of popping phenomenon.
A. Axial magnetic resonance imaging showing treatment-naive HCC measuring 2.6 cm in segment II of liver (white arrow) during arterial phase. B. On planning, axial fused MR/US image showing low echoic index tumor (white arrow). C. Oblique axial view of fused MR/US image showing two RF electrodes (black arrows) inserted in parallel into index tumor Sonazoid® (GE Healthcare) was not used and popping phenomenon was not observed during RF ablation. D. CT showing adequate circumferential ablative margins except for subcapsular portion, in posterior aspect of ablation zone (white dotted line) immediately after RF ablation. E. Diffusion-weighted magnetic resonance imaging obtained 7 months after RF ablation showing multiple small high-signal intensity nodules (white arrows), with subtle arterial enhancement and delayed wash-out (not shown), which developed simultaneously in peripheral area of ablation zone. F. Photograph of gross specimen after left lateral sectionectomy displays multiple nodular HCCs (more than 20, black arrows) in peripheral portion of segment II far from previous ablation zone (a). MR/US = magnetic resonance/ultrasonography
DISCUSSION
We found that the use of Sonazoid® has a suppressive effect on the steam popping phenomenon during RF ablation for the treatment of HCC in our study. However, the occurrence of steam popping during the procedure was not associated with the clinical outcomes after RF ablation.
The popping phenomenon during RF ablation for treating HCC was first reported by Livraghi et al. (23). A previous study investigating the “popping sound” during RF ablation showed (18) that an incidence of approximately 58% was relatively common. However, a relatively low incidence of 25.8% was observed in the current study. This discrepancy can be partly explained as follows. First, Sonazoid® was used exclusively during RF ablation in our study cohort (17.2%). Second, the mean tumor size in our cohort was relatively smaller than that in the previous study (1.5 cm vs. 2.6 cm, respectively). In general, the deposition of RF energy can be relatively higher for the treatment of large tumors, which may lead to a greater increase in intra-tumor pressure with an enhanced risk for the popping phenomenon. Our RF ablation protocol used the low-power technique with multistep increments in RF power, to prevent a rapid increase in intra-tumor pressure and portal endothelial damage, which was concurrent with previous in vivo (10) and ex vivo (11) experiments. However, even this modified technique could not fully prevent the popping phenomenon from occurring in our study.
Multivariate analysis revealed that Sonazoid® can suppress the popping phenomenon during RF ablation. This finding is consistent with our previous experimental in vivo study using a rabbit model (13). In that study, innumerable perfluorobutane microbubbles were observed around RF electrodes during ablation, which might have functioned like a pressure valve, releasing some of the built-up pressure and thus, suppressing an increase in intra-tissue pressure. Moreover, gas-containing microbubbles showed markedly low electrical conductivity, which was inversely related to tissue impedance. Iida et al. (17) reported that a sudden decrease in tissue impedance resulted in a rapid elevation in intra-tissue temperature, possibly leading to popping following the vaporization of intra-tissue fluid. Thus, the maintenance of relatively high tissue impedance by microbubbles during RF ablation seems to lower the frequency of popping events if Sonazoid® is used for RF ablation for the treatment of HCC. The use of Sonazoid® can theoretically decrease in the ablation zone due to an increase in the tissue impedance. Thus, we recommend that careful consideration be given to the RF ablation with Sonazoid®, owing to the risk of incomplete ablation caused by a decrease in RF energy delivery. However, the effect of Sonazoid® on ablation volume should be validated with prospective comparative studies.
Although most interventional radiologists are anecdotally aware of the occurrence of the popping phenomenon during RF ablation for HCC treatment, only few clinical studies have addressed its clinical effects (5,18,24). Moreover, these studies yielded conflicting results in terms of treatment outcomes. Angonese et al. (5) and Kotoh et al. (24) have suggested that the aggressive tumor recurrence after RF ablation for HCC may be caused by increased intratumoral pressure, consequently resulting in intravascular tumor spread. However, the popping phenomenon was not clearly defined in these two studies, rendering the comparison with the results of other studies difficult. In contrast, Fernandes et al. (18), who used a similar definition of the popping phenomenon to the one used in the current study, reported that the occurrence of a “popping sound” during RF ablation was not associated with an increased risk of early tumor recurrence or poor patient survival. In our study, LTP, IDR, DFS, and aggressive intrasegmental recurrence were not significantly affected by the occurrence of the popping phenomenon. This may indicate that the popping phenomenon may be a physical effect of thermal ablation and may not have a significant impact on the treatment results after all. However, considering an increased risk of peritoneal seeding due to rupture of the tumor (25) while treating subcapsular HCC using RF ablation, Sonazoid® could help to reduce this risk by suppressing the popping phenomenon, irrespective of its conventional role in contrast-enhanced US. Further research is needed to determine whether this newly identified role of Sonazoid® will ultimately improve the clinical outcomes of patients with HCC.
Our study has several limitations. First, although we used data from a prospective registry of an RF ablation database, and any potential bias resulting from subjective judgement of the popping phenomenon by each operator was adjusted for by using multivariate analysis, the subjective measurement of the popping phenomenon is an inherent limitation. Second, larger studies with more patients may be needed to validate the association between the popping phenomenon and aggressive intrasegmental recurrence, based on the considerably low incidence of aggressive intrasegmental recurrence after RF ablation in our cohort. This is crucial because aggressive intrasegmental recurrence could indicate transportal tumor spread due to a sudden increase in intratumoral pressure. Third, we were unable to measure the technical RF parameters related to impedance or RF power during the popping phenomenon in detail for each treatment. Finally, our posttreatment follow-up period was relatively short, which made it difficult to determine long-term safety following the occurrence of popping phenomena.
In conclusion, our study demonstrated that Sonazoid® significantly suppresses the occurrence of the popping phenomenon during RF ablation for the treatment of HCC. However, the occurrence of the popping phenomenon may not affect the clinical outcomes of RF ablation directly.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Mori Y, Tamai H, Shingaki N, Moribata K, Shiraki T, Deguchi H, et al. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3:509–515. doi: 10.1007/s12072-009-9131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276:274–285. doi: 10.1148/radiol.15141215. [DOI] [PubMed] [Google Scholar]
- 5.Angonese C, Baldan A, Cillo U, D'Alessandro A, De Antoni M, De Giorgio M, et al. Complications of radiofrequency thermal ablation in hepatocellular carcinoma: what about “explosive” spread? Gut. 2006;55:435–436. doi: 10.1136/gut.2005.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, et al. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 7.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332–335. doi: 10.1007/s10147-003-0328-6. [DOI] [PubMed] [Google Scholar]
- 8.Pua U. Rapid intra-hepatic dissemination of hepatocellular carcinoma with pulmonary metastases following combined loco-regional therapy. Korean J Radiol. 2013;14:640–642. doi: 10.3348/kjr.2013.14.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang TW, Lim HK, Cha DI. Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:95–101. doi: 10.3350/cmh.2017.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe J, Kim KW, Kim YI, Chung JW, Huh J, Park J, et al. Feasibility of a low-power radiofrequency ablation protocol to delay steam popping. J Vasc Interv Radiol. 2016;27:268–274. doi: 10.1016/j.jvir.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Kotoh K, Nakamuta M, Morizono S, Kohjima M, Arimura E, Fukushima M, et al. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005;25:542–547. doi: 10.1111/j.1478-3231.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 12.Min JH, Lim HK, Lim S, Kang TW, Song KD, Choi SY, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014;20:61–70. doi: 10.3350/cmh.2014.20.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min JH, Kim YS, Rhim H, Lee MW, Kang TW, Song KD, et al. Effect of parenchymal uptake of perfluorobutane microbubbles (Sonazoid®) on radiofrequency ablation of the liver: in vivo experimental study. Liver Int. 2016;36:1187–1195. doi: 10.1111/liv.13081. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70–78. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280:300–312. doi: 10.1148/radiol.2016151243. [DOI] [PubMed] [Google Scholar]
- 17.Iida H, Aihara T, Ikuta S, Yamanaka N. Effectiveness of impedance monitoring during radiofrequency ablation for predicting popping. World J Gastroenterol. 2012;18:5870–5878. doi: 10.3748/wjg.v18.i41.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes ML, Lin CC, Lin CJ, Chen WT, Lin SM. Prospective study of a ‘popping’ sound during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:237–244. doi: 10.1016/j.jvir.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel “cooled-wet” electrode for radiofrequency ablation. Eur Radiol. 2000;10:852–854. doi: 10.1007/s003300051018. [DOI] [PubMed] [Google Scholar]
- 20.Kotoh K, Morizono S, Kohjima M, Enjoji M, Sakai H, Nakamuta M. Evaluation of liver parenchymal pressure and portal endothelium damage during radio frequency ablation in an in vivo porcine model. Liver Int. 2005;25:1217–1223. doi: 10.1111/j.1478-3231.2005.01167.x. [DOI] [PubMed] [Google Scholar]
- 21.Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013;68:e641–e651. doi: 10.1016/j.crad.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection–propensity score analyses of long-term outcomes. Radiology. 2015;275:908–919. doi: 10.1148/radiol.15141483. [DOI] [PubMed] [Google Scholar]
- 23.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 24.Kotoh K, Enjoji M, Arimura E, Morizono S, Kohjima M, Sakai H, et al. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2005;11:6828–6832. doi: 10.3748/wjg.v11.i43.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019;11:227–237. doi: 10.4251/wjgo.v11.i3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]