Abstract
Objective
To assess the incremental prognostic value of coronary computed tomography angiography (CCTA) in comparison to a clinical risk model (Framingham risk score, FRS) and coronary artery calcium score (CACS) for future cardiac events in ischemic stroke patients without chest pain.
Materials and Methods
This retrospective study included 1418 patients with acute stroke who had no previous cardiac disease and underwent CCTA, including CACS. Stenosis degree and plaque types (high-risk, non-calcified, mixed, or calcified plaques) were assessed as CCTA variables. High-risk plaque was defined when at least two of the following characteristics were observed: low-density plaque, positive remodeling, spotty calcification, or napkin-ring sign. We compared the incremental prognostic value of CCTA for major adverse cardiovascular events (MACE) over CACS and FRS.
Results
The prevalence of any plaque and obstructive coronary artery disease (CAD) (stenosis ≥ 50%) were 70.7% and 30.2%, respectively. During the median follow-up period of 48 months, 108 patients (7.6%) experienced MACE. Increasing FRS, CACS, and stenosis degree were positively associated with MACE (all p < 0.05). Patients with high-risk plaque type showed the highest incidence of MACE, followed by non-calcified, mixed, and calcified plaque, respectively (log-rank p < 0.001). Among the prediction models for MACE, adding stenosis degree to FRS showed better discrimination and risk reclassification compared to FRS or the FRS + CACS model (all p < 0.05). Furthermore, incorporating plaque type in the prediction model significantly improved reclassification (integrated discrimination improvement, 0.08; p = 0.023) and showed the highest discrimination index (C-statistics, 0.85). However, the addition of CACS on CCTA with FRS did not add to the prediction ability for MACE (p > 0.05).
Conclusion
Assessment of stenosis degree and plaque type using CCTA provided additional prognostic value over CACS and FRS to risk stratify stroke patients without prior history of CAD better.
Keywords: Coronary computed tomography angiography; Coronary artery calcium scoring; Stroke; Plaque, atherosclerotic; Coronary stenosis
INTRODUCTION
In stroke survivors, cardiac events are the most common cause of death in long-term survivors after first-ever stroke and the cause of greater medical costs (1,2,3). Prevalence of asymptomatic coronary artery disease (CAD) is also significant in patients with stroke (4,5,6). Therefore, the evaluation of occult CAD and the identification of prognostic factors for cardiac events may alter patients' prognosis.
Although the Stroke Council and the Council on Clinical Cardiology of the American Heart Association (AHA) and American Stroke Association recommend noninvasive testing for CAD in patients with significant carotid disease and high CAD risk scores based on Framingham algorithms, there remains the question of how and which of the remaining stroke patients should be screened (7).
With the advancements in non-invasive imaging techniques, coronary artery calcium score (CACS) and coronary computed tomography angiography (CCTA) have been widely adopted for the evaluation of CAD. Recently, several studies have reported that CCTA has incremental prognostic value over CACS because CCTA can evaluate not only the degree of stenosis but also coronary plaque characteristics (8,9,10). However, there is a paucity of data regarding the potential role of CCTA to screen patients with ischemic stroke. Therefore, our study aimed to evaluate the prevalence of subclinical CAD in acute stroke patients without known cardiac disease or chest pain and assess the incremental prognostic value of CCTA in comparison to clinical risk factors and CACS.
MATERIALS AND METHODS
Study Population
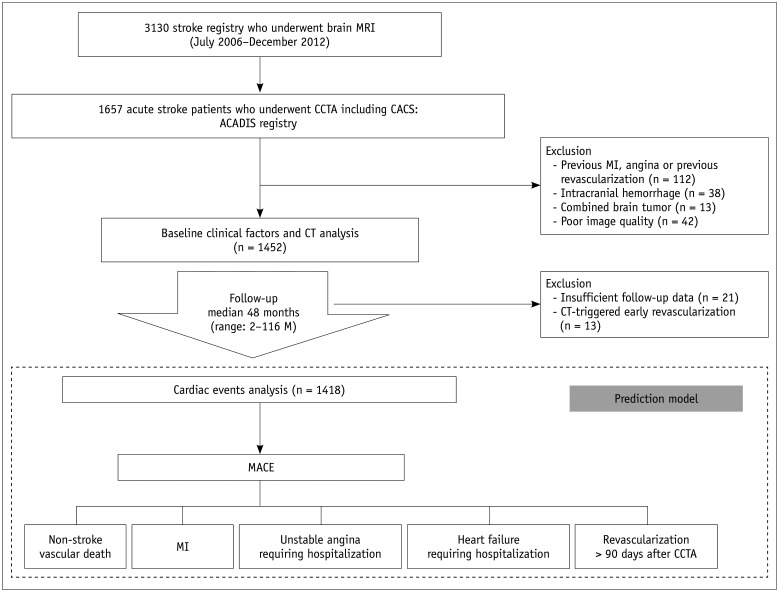
The Assessment of Coronary Artery Disease in Stroke Patients study is a longitudinal retrospective observational cohort study that evaluates CAD in stroke patients without previous cardiac disease or chest pain. From the stroke registry which consists of patients who had ischemic stroke and underwent brain magnetic resonance imaging (MRI) (n = 3130) at Seoul National University Bundang Hospital between July 2006 and December 2012, we retrospectively selected 1657 patients who met the following inclusion criteria: 1) patients who were diagnosed with ischemic stroke using brain MRI when they had an acute focal neurological deficit and 2) patients who underwent CCTA including CACS within 3 months after acute stroke.CCTA was performed if patients presented with at least one of the following: 1) significant stenosis (≥ 50%) in the intracranial or extracranial arteries on imaging such as carotid Doppler, CT angiography, or MR angiography; 2) ≥ 1 risk factors for CAD, such as hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, and central obesity; and 3) older age (men, > 45 years; women, > 55 years) (11,12). We excluded patients with the following characteristics: 1) patients with history of myocardial infarction (MI) and angina or patients who previously underwent percutaneous coronary intervention (PCI) or bypass grafting (n = 112); 2) patients with intracranial hemorrhage including intracerebral, subdural, or subarachnoid hemorrhage (n = 38); 3) patients with brain tumor (n = 13); or 4) patients with poor CCTA image quality (n = 42) (Fig. 1). Additionally, 34 patients were excluded due to missed follow-up (n = 21) or CT-triggered PCI (n = 13) to prevent CT-related bias. Finally, 1418 patients were enrolled. This study was approved by the local Institutional Review Board, and all patients provided written informed consent for CAD evaluation using cardiac CCTA.
Fig. 1. Flowchart of study design.
ACADIS = Assessment of Coronary Artery Disease in Stroke Patients, CACS = coronary artery calcium score, CCTA = coronary computed tomography angiography, MACE = major adverse cardiovascular events, MI = myocardial infarction
CCTA Data Acquisition
A 64-multidetector row CT scanner (Brilliance 64, Philips Medical Systems) was used with the following parameters: collimation, 64 × 0.625 mm; rotation time, 420 msec; tube voltage, 100 kV or 120 kV; and tube current, 800 mA. Prior to contrast injection, CACS was performed using a prospective electrocardiographically (ECG) triggered acquisition technique with 120-kV tube voltage, 55-mAs tube current, and 2.5-mm scan thickness. Agatston score was calculated using a threshold of 130 Hounsfield units (HU). Patients who had a heart rate > 70 beats per minute received an intravenous injection of 10 mg of esmolol and 0.6 mg of sublingual nitroglycerin as premedications unless contraindicated. To minimize radiation dose, CCTA technique was selected based on heart rate and body mass index (BMI). Based on the BMI, 120 kV was applied in patients with BMI ≥ 25 kg/m2, and 100 kV was applied in patients with BMI < 25 kg/m2. Images were acquired using retrospective ECG gating with tube current modulation or prospective ECG triggering based on the heart rate, with 70 beats per minute as the threshold.
Clinical Risk Factors
Basic demographic data were acquired from the electrical medical record. Past medical history of MI, angina, hypertension, stroke, and diabetes mellitus, family history of premature coronary heart disease (CHD) (CHD in male first-degree relatives aged less than 55 years, CHD in female first-degree relatives aged less than 65 years), and smoking were systematically obtained by personal interviews. Body weight, height, and blood pressure were also measured during their visit. Total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, and fasting plasma glucose levels were measured with blood sampling obtained after a 12-hour fast. Framingham risk score (FRS) was calculated to estimate the 10-year risk of CAD (13).
Image Analysis
All scans were evaluated independently by two experienced radiologists (with 12 and 6 years' clinical experience, respectively). After performing an independent evaluation, a consensus interpretation was achieved to establish a final CCTA diagnosis based on the additional reconstruction or review of thin-section data. We analyzed stenosis degree and plaque types according to the 16-segment model based on the AHA classification.
Stenosis degree was evaluated using a 4-point grading scale as follows: none, 1–49%, 50–69%, and ≥ 70%. Each patient was classified into one of the four groups according to the most severe segment. Stenosis more than 50% was defined as obstructive CAD.
Plaque types were classified as follows: 1) calcified plaque, defined as plaque having calcification (≥ 130 HU) in more than 50% of the entire volume; 2) mixed plaque, plaque having calcification in < 50% of the entire volume; 3) non-calcified plaque, plaque having solely soft-tissue density; and 4) high-risk plaque, plaque possessing at least two of the following characteristics: low-density with the lowest pixel < 30 HU within each plaque, positive arterial remodeling with remodeling index ≥ 1.1, a napkin-ring sign (characterized by low intraplaque attenuation surrounded by a higher attenuation rim), or spotty calcification < 3 mm in length (14). We hypothesized that the risk of an event would be greatest in high-risk plaque, followed by non-calcified, mixed, and calcified plaques. Patients were stratified into the four plaque types based on highest risk. For example, a patient with both high-risk plaque and non-calcified plaque would be classified in the high-risk plaque group.
Follow-Up and End Point of the Study
During the median follow-up period of 48 months (range, 2–116 months), clinical data were acquired by reviewing patients' medical records or telephone contact with enrolled individuals with trained personnel. The primary end point of this study was non-stroke major adverse cardiovascular events (MACE), which was a composite of the following: 1) non-stroke vascular death; death from a cardiac cause such as acute MI, arrhythmia, or heart failure; and other cardiovascular death, which included any sudden death, including unobserved and unexpected death unless proven otherwise by autopsy (15,16), 2) MI according to the 4th universal criteria (17), 3) unstable angina (UA) that required hospital stay, 4) revascularization therapy ≥ 90 days after CCTA and referral due to new symptoms or an abnormal functional stress test (late revascularization), and 5) heart failure requiring hospitalization (18). Late revascularization was determined by subsequent diagnostic tests (i.e., single-photon emission computed tomography, stress-induced echocardiography) when subjects were asked regarding the occurrence of new chest pain.
Statistical Analyses
Baseline clinical data and CCTA findings were compared between the group with and without events using a chi-squared test for categorical variables and an independent t test for continuous variables.
The Kaplan-Meier survival analysis was used to evaluate the cumulative survival based on stenosis degree and plaque types. Univariate and multivariate Cox proportional hazards regression analysis was used to assess significant associations between baseline FRS, CACS, and CT variables including stenosis degree and plaque types and the risk of MACE.
To determine the incremental prognostic value of CCTA variables compared to the FRS and CACS, we developed six prediction models that assessed the associations between the potential predictors and MACE using a Cox proportional hazards regression as follows: Model A, clinical risk factors (FRS); Model B, FRS + CACS; Model C, FRS + stenosis degree; Model D, FRS + stenosis degree + CACS; Model E, model C + plaque type; and Model F, Model D + plaque type. The Harrell's C-index was determined for each model. Considering that established categories did not exist for the expected rates of MACE in the study population, patient reclassification ability of each model was assessed using the integrated discrimination improvement (IDI) index. The absolute IDI is presented using p values.
For all tests, p value < 0.05 was considered as statistically significant. All statistical analyses were performed using a statistical package R 2.10.1 (R Foundation for Statistical Computing) and Statistical Analysis System (SAS) version 9.3 (SAS Institute, Inc.).
RESULTS
Study Population and Outcome Results
In 1418 patients, 50 experienced mortality regardless of the cause. Therefore, 16 patients with non-cardiovascular death (6, cancer-related death; 5, respiratory diseases including pneumonia or interstitial lung disease; 4, recurrent stroke-related death; and 1, trauma-related death) were excluded from MACE. Ultimately, MACE was observed in 108 patients (7.6%) (non-stroke vascular death [n = 34], MI [n = 17], UA [n = 12], late revascularization [n = 34], and heart failure requiring hospitalization [n = 11]).
Table 1 summarizes the clinical and CT findings according to the presence or absence of events. Older age, male sex, hypertension, diabetes, family history of premature CHD, and symptomatic carotid artery were more frequently observed in the event group than in the non-event group. The mean FRS and mean CACS were significantly higher in the event group than those in the non-event group (both p < 0.001). Among patients with a “zero” CACS (n = 487), 73 patients (15.0%) had non-calcified plaque or high-risk plaque, and ten events (2.1%) (2, non-stroke vascular death; 2, MI; 1, UA; 2, heart failure requiring hospitalization; and 3, revascularization) were observed. Nine of these events were observed in the setting of high-risk plaque (n = 6) and non-calcified plaque (n = 3).
Table 1. Comparison of Baseline Clinical Risk Factors and CT Findings and Major Adverse Cardiovascular Events.
| Variables | Total (n = 1418) | Event (n = 108) | Non-Event (n = 1310) | P |
|---|---|---|---|---|
| Clinical risk factors | ||||
| Age (years) | 68.0 ± 12.2 | 72.4 ± 10.8 | 67.6 ± 12.2 | < 0.001* |
| Male sex | 875 (61.7) | 82 (75.9) | 793 (60.5) | 0.001* |
| Body mass index (kg/m2) | 23.9 ± 3.4 | 24.0 ± 3.3 | 23.3 ± 3.8 | 0.054 |
| Hypertension | 810 (57.1) | 73 (67.6) | 737 (56.3) | 0.033* |
| Diabetes | 345 (24.3) | 37 (34.3) | 308 (23.5) | 0.020* |
| Hypercholesterolemia | 317 (22.4) | 26 (24.1) | 291 (22.2) | 0.720 |
| Current smoker | 354 (25.0) | 35 (32.4) | 319 (24.4) | 0.084 |
| Family history of stroke | 237 (16.7) | 20 (18.5) | 217 (16.6) | 0.688 |
| Family history of premature CHD | 80 (5.6) | 15 (13.9) | 65 (5.0) | 0.001* |
| Atrial fibrillation | 149 (10.5) | 17 (15.7) | 132 (10.1) | 0.075 |
| Symptomatic carotid artery disease | 166 (11.7) | 21 (19.4) | 145 (11.1) | 0.019* |
| Initial NHSS | 4.6 ± 5.5 | 6.7 ± 7.0 | 4.4 ± 5.4 | 0.002* |
| Total cholesterol | 127.4 ± 79.0 | 128.3 ± 79.7 | 116.9 ± 69.2 | 0.157 |
| HDL-cholesterol | 45.2 ± 10.9 | 44.6 ± 11.4 | 45.2 ± 10.9 | 0.592 |
| LDL-cholesterol | 101.2 ± 32.0 | 101.6 ± 32.1 | 96.1 ± 29.8 | 0.096 |
| FRS | 14.2 ± 9.1 | 18.7 ± 7.7 | 13.8 ± 9.1 | < 0.001* |
| Low | 492 (34.7) | 12 (11.1) | 480 (36.6) | < 0.001* |
| Intermediate | 587 (41.4) | 58 (53.7) | 529 (40.4) | 0.008* |
| High | 339 (23.9) | 38 (35.2) | 301 (23.0) | 0.007* |
| Medication | ||||
| Statin | 345 (24.3) | 34 (31.5) | 311 (23.7) | 0.103 |
| ACE-inhibitor or ARB | 775 (54.7) | 68 (63.0) | 707 (54.0) | 0.107 |
| β-blocker | 192 (13.5) | 21 (19.4) | 171 (13.1) | 0.080 |
| Aspirin | 798 (56.3) | 71 (65.7) | 727 (55.5) | 0.055 |
| CACS | ||||
| Total score | 243.4 ± 546.0 | 614.6 ± 912.1 | 212.5 ± 491.6 | < 0.001* |
| 0 | 487 (34.3) | 10 (9.3) | 477 (36.4) | < 0.001* |
| 0.1–100 | 421 (29.7) | 30 (27.8) | 391 (29.8) | 0.742 |
| 100.1–400 | 269 (19.0) | 25 (23.1) | 244 (18.6) | 0.253 |
| > 400 | 241 (17.0) | 43 (39.8) | 198 (15.1) | < 0.001* |
| CCTA | ||||
| Stenosis degree | ||||
| None | 416 (29.3) | 1 (0.9) | 415 (31.7) | < 0.001* |
| 1–49% | 574 (40.5) | 24 (22.2) | 550 (42.0) | < 0.001* |
| 50–69% | 227 (16.0) | 29 (26.9) | 198 (15.1) | 0.003* |
| ≥ 70% | 201 (14.2) | 54 (50.0) | 147 (11.2) | < 0.001* |
| Plaque type | ||||
| Calcified plaque | 358 (25.2) | 18 (16.7) | 340 (26.0) | 0.805 |
| Mixed plaque | 323 (22.8) | 30 (27.8) | 293 (22.4) | 0.232 |
| Non-calcified plaque | 237 (16.7) | 35 (32.4) | 202 (15.4) | < 0.001* |
| High-risk plaque | 84 (5.9) | 24 (22.2) | 60 (4.6) | < 0.001* |
Data are presented as mean ± standard deviation or n (%). *p < 0.05. ACE = angiotensin-converting enzyme, ARB = angiotensin II receptor blocker, CACS = coronary artery calcium score, CCTA = coronary computed tomography angiography, CHD = coronary heart disease, FRS = Framingham risk score, HDL = high-density lipoprotein, LDL = low density lipoprotein, NIHSS = National Institutes of Health Stroke Scale
Regarding CCTA analysis, 1002 patients (70.7%) had at least one plaque and 428 patients (30.2%) had obstructive CAD. The obstructive CAD was significantly associated with MACE (p < 0.05). Regarding plaque analysis, the prevalence of calcified plaque and mixed plaque was not significantly different between the two groups, whereas those of non-calcified plaque and high-risk plaque were significantly higher in the event group than in the non-event group (both p < 0.001). The prevalence of events was highest in patients with high-risk plaque (28.6%, 24/84) and successively decreased for non-calcified plaque (14.8%, 35/237), mixed plaque (9.3%, 30/323), and calcified plaque (5.0%, 18/358). The mean radiation exposures of CCTA at 100 kVp and 120 kVp were 1.7 ± 0.4 mSv and 2.3 ± 0.4 mSv and 6.2 ± 0.7 mSv and 8.2 ± 0.8 mSv by prospective and retrospective ECG gating, respectively.
Clinical and CT Variables associated with Cardiovascular Events
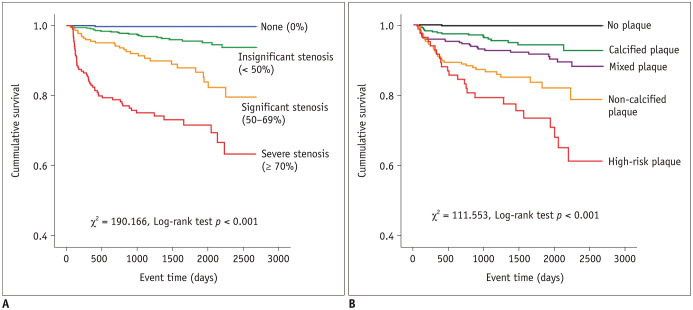
In a univariate Cox regression analysis, FRS, CACS, and CT-related stenosis degree were all positively associated with events (Table 2). The hazard ratios (HRs) of intermediate- and high-risk FRS were 4 and 5 times that of low-risk FRS. After the adjustment of the baseline clinical factors including age, sex, hypertension, diabetes, current smoking, BMI, atrial fibrillation, and family history of premature CHD, the HR of CACS > 400 was the highest (HR = 7.1), followed by the cohort of CACS 100.1–400 (HR = 3.6) and the cohort of CACS 0.1–100 (HR = 3.0) (Table 2). Stenosis degree showed a positive association with event occurrence (all p < 0.05). HRs of stenosis with ≥ 70% and 50–69% were 113.2 and 45.9, respectively (Fig. 2). Regarding plaque types, the risk of events was highest for high-risk plaque (HR = 80.3), followed by non-calcified plaque (HR = 53.8), mixed plaque (HR = 26.2), and calcified plaque (HR = 17.5). The Kaplan-Meier curves showed that cumulative events increased significantly with the extent of stenosis degree and plaque type (all log-rank test, p < 0.001) (Fig. 2).
Table 2. Univariate and Multivariate Cox Regression Analyses Predicting Coronary Heart Events with FRS, CACS, and CCTA Variables.
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| FRS | ||||||
| Low | 1 | (Ref) | < 0.001* | |||
| Intermediate | 4.1 | 2.2–7.7 | < 0.001* | |||
| High | 4.9 | 2.5–9.3 | < 0.001* | |||
| CACS | ||||||
| 0 | 1 | (Ref) | < 0.001* | 1 | (Ref) | < 0.001* |
| 0.1–100 | 3.5 | 1.7–7.2 | 0.001* | 3.0 | 1.5–6.3 | 0.003* |
| 100.1–400 | 5.0 | 2.4–10.5 | < 0.001* | 3.6 | 1.7–7.7 | 0.001* |
| > 400 | 10.3 | 5.2–20.6 | < 0.001* | 7.1 | 3.4–14.7 | < 0.001* |
| CCTA | ||||||
| Stenosis degree | ||||||
| None | 1 | (Ref) | < 0.001* | 1 | (Ref) | < 0.001* |
| 1–49% | 17.2 | 2.3–127.4 | 0.005* | 14.9 | 2.0–110.9 | 0.008* |
| 50–69% | 60.2 | 8.2–442.0 | < 0.001* | 45.9 | 6.2–342.0 | < 0.001* |
| ≥ 70% | 150.6 | 20.8–1089.4 | < 0.001* | 113.2 | 15.4–834.6 | < 0.001* |
| Plaque type | ||||||
| No plaque | 1 | (Ref) | < 0.001* | 1 | (Ref) | < 0.001* |
| Calcified plaque | 23.3 | 3.1–174.6 | 0.002* | 17.5 | 2.3–131.8 | 0.001* |
| Mixed plaque | 39.1 | 5.3–286.7 | < 0.001* | 26.2 | 3.5–194.2 | 0.001* |
| Non-calcified plaque | 74.5 | 10.2–544.1 | < 0.001* | 53.8 | 7.3–396.3 | < 0.001* |
| High-risk plaque | 125.4 | 16.9–927.5 | < 0.001* | 80.3 | 10.7–601.9 | < 0.001* |
Multivariate analysis was calculated after adjustment of FRS including baseline clinical risk factors. *p < 0.05. CI = confidence interval, HR = hazard ratio, Ref = reference
Fig. 2. Kaplan-Meier survival curves of MACE stratified by CCTA features.
A. Segment-based stenosis categories stratified into none, 1–49%, 50–69%, and ≥ 70% luminal stenosis. Of note, half of MACE was observed during first year when stenosis was ≥ 70%. B. Plaque type categories stratified into no plaque, calcified plaque, mixed plaque, non-calcified plaque, and high-risk plaque.
Various Predicting Models and Incremental Prognostic Value of CCTA
Table 3 summarizes the prediction models, which were constructed with covariates of the clinical and CCTA variables, and their comparison. The reclassification ability of the prediction model was significantly better when CACS was added to FRS (Model B: IDI, 0.19; 95% confidence interval [CI], 0.09–0.28; p < 0.001) compared with FRS alone (Model A). Adding CCTA-related stenosis degree instead of CACS (Model C) led to better improvement of reclassification ability compared with Model B (IDI, 1.43; 95% CI, 1.24–1.63; p < 0.001). However, the addition of CACS into the prediction model (Model D) did not show better incremental reclassification ability compared with Model C (IDI, 0.01; 95% CI, −0.02–0.04; p = 0.431). The incorporation of plaque type into Model C (Model E) showed the highest discrimination index (C-statistics, 0.85) and significantly better reclassification ability compared with Model B (IDI, 1.51; 95% CI, 1.31–1.71; p < 0.001) and Model C (IDI, 0.08; 95% CI, 0.01–0.14; p = 0.023). However, the addition of CACS into the prediction model (Model F) did not show better reclassification ability for MACE than Model E (IDI, 0.06; 95% CI, −0.01–0.12; p = 0.067).
Table 3. Effect of Variables on Model Prediction Accuracy and Risk Reclassification.
| Model | Included Variable | IDI Index (95% CI) | P | Model Prediction (C-Index) |
|---|---|---|---|---|
| Model A | FRS | - | - | 0.66 |
| Model B | FRS + CACS | 0.19 (0.09–0.28) vs. Model A | < 0.001* | 0.72 |
| Model C | FRS + stenosis degree | 1.62 (1.42–1.83) vs. Model A | < 0.001* | 0.83 |
| 1.43 (1.24–1.63) vs. Model B | < 0.001* | |||
| Model D | FRS + stenosis degree + CACS | 1.63 (1.43–1.83) vs. Model A | < 0.001* | 0.83 |
| 1.45 (1.26–1.63) vs. Model B | < 0.001* | |||
| 0.01 (−0.02–0.04) vs. Model C | 0.431 | |||
| Model E | FRS + stenosis degree + plaque type | 1.69 (1.50–1.89) vs. Model A | < 0.001* | 0.85 |
| 1.51 (1.31–1.71) vs. Model B | < 0.001* | |||
| 0.08 (0.01–0.14) vs. Model C | 0.023* | |||
| 0.06 (−0.02–0.14) vs. Model D | 0.117 | |||
| Model F | FRS + stenosis degree + plaque type + CACS | 1.76 (1.56–1.95) vs. Model A | < 0.001* | 0.85 |
| 1.57 (1.39–1.75) vs. Model B | < 0.001* | |||
| 0.14 (0.05–0.23) vs. Model C | 0.003* | |||
| 0.13 (0.04–0.21) vs. Model D | 0.004* | |||
| 0.06 (−0.01–0.12) vs. Model E | 0.067 |
*p < 0.05. IDI = integrated discrimination improvement
DISCUSSION
The major finding of this study is that the assessment of stenosis degree and plaque type with the use of CCTA provides additional prognostic value over CACS and FRS to risk stratify stroke patients without prior history of CAD, better.
The prevalence of CAD is substantial in stroke patients, even in the absence of known CHD, because CAD and stroke share similar risk factors. In an autopsy series of fatal stroke, approximately 80% of patients were found to have coronary plaque and 37.5% had obstructive CAD (4). A recent study using invasive coronary angiography has reported an overall CAD prevalence of 61.9% and obstructive CAD prevalence of 25.7% in ischemic stroke patients with no known CHD (6). Studies investigating stroke patients for CAD using CCTA are being reported with the prevalence of obstructive CAD ranging from 18% to 48% (5,19,20). These results are similar with the result of our study that the prevalence of obstructive CAD and subclinical atherosclerosis were 30.2% and 70.7%, respectively.
Whether stroke patients should be investigated for asymptomatic CAD remains controversial. Previous studies found that the 10-year risk of non-stroke vascular event is projected to be as close to 20% (16,21). Therefore, the National Cholesterol Education Program-Adult III recommendation recognizes stroke of carotid origin and carotid atherosclerosis as “CHD risk equivalents” (22). In our study, the 7.6% rate of non-stroke MACE during the median follow-up of 48 months yields a similar annual risk of 2%. This relatively high prevalence suggests the need for the risk stratification to detect and prevent CAD in stroke patients. The FRS is a simple tool used to estimate the 10-year risk for CHD and is considered valuable in stroke patients (23). However, the FRS may inaccurately estimate the risk of MACE because clinical factors underpinning the FRS are variable, are affected by confounding factors, and thus are not easily quantified on a numeric scale (24). CACS predicts CHD independently and provides better prognostic value compared to clinical risk factors (25). However, CACS underestimates the risk of patients with non-calcified plaque because stenosis degree and plaque characteristics are not represented in CACS (26). The prevalence of non-calcified plaque may be higher in high-risk patients than in low-risk patients (26,27). In our cohort, the prevalence of non-calcified plaque including high-risk plaque were 22.6% in all patients and 15.0% in patients with “zero” CACS. Altogether, 73 patients with plaque showed zero CACS, and 9 developed MACE (12.3%). Therefore, the prevalence of MACE is not negligible even in patients with zero CACS, particularly when non-calcified plaque or high-risk plaque is observed.
Beyond CACS, CCTA-defined severity of CAD has shown improved prognostic value for CHD (8,9,10). Hur et al. (20) reported that the incremental prognostic value of CCTA-defined stenosis degree in stroke patients, and the incidence rate of cardiac events was 8.2% during a median follow-up period of 409 days. Compared to that study, the present study has the following strengths: it includes a large number of ischemic stroke patients during a relatively long-term observational study (median, 4 years). We believe that intermediate- to long-term follow-up is required for the effective management of CHD because the risk of MI increases continuously in the period beyond 2 years after a stroke (28). Moreover, our study investigates the incremental value of plaque composition in stroke patients. Recently, several studies on plaque features assessed by CCTA associated with cardiac events have been reported, but these studies included only non-stroke patients with suspected CAD (29,30). Our study shows that plaque component is also important as much as stenosis degree for risk stratification in post-stroke patients.
Furthermore, we suggest that CCTA in the absence of CACS may be a sufficient evaluation, considering that the addition of CACS to the CCTA variables did not improve the reclassification ability in our models and that CACS requires an additional pre-contrast scan. Recently, the introduction of various ultra-low-dose CCTA techniques has reduced radiation dose of CCTA to < 1 mSv (31). In view of the incremental prognostic value of CCTA and comparable radiation dose of CCTA to CACS, our results suggest that CCTA has a potential role for occult CAD screening in asymptomatic stroke patients, although further study assessing its cost-effectiveness, radiation hazard, and requirement of contrast media is needed.
Our study has several limitations. First, it was derived from a single-center study registry performed with a selected group of patients with acute ischemic stroke who underwent CCTA because of vascular risk factors. Furthermore, this study does not reflect on whether CCTA screening improves the outcome of cardiac events. Hence, future large randomized trials should be conducted to evaluate the influence of CCTA screening on treatment and to determine optimal treatment strategies in patients with ischemic stroke. Second, acute ischemic stroke is a heterogeneous disease with different etiologies. Therefore, the prognostic value of CCTA may be different depending on the etiologies of stroke (32). However, these categories were not considered in this study. Third, we considered death by unknown cause to be cardiac death because autopsy was not performed in each patient.
In conclusion, our study demonstrated that the assessment of stenosis degree and plaque type using CCTA provides incremental prognostic value over CACS and FRS and is valuable for risk stratification in stroke patients without a prior history of CHD.
Footnotes
This work was supported by grant No. 12-2013-024 from the SNUBH Research Fund and the National Research Foundation grant NRF-2010-0023504 funded by the Korea government (MEST).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. 2000;31:2080–2086. doi: 10.1161/01.str.31.9.2080. [DOI] [PubMed] [Google Scholar]
- 2.Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CS, Gorelick PB, Ye X, Harley C, Goldberg GA. Additional stroke-related and non-stroke-related cardiovascular costs and hospitalizations in managed-care patients after ischemic stroke. Stroke. 2009;40:1425–1432. doi: 10.1161/STROKEAHA.108.534354. [DOI] [PubMed] [Google Scholar]
- 4.Gongora-Rivera F, Labreuche J, Jaramillo A, Steg PG, Hauw JJ, Amarenco P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke. 2007;38:1203–1210. doi: 10.1161/01.STR.0000260091.13729.96. [DOI] [PubMed] [Google Scholar]
- 5.Seo WK, Yong HS, Koh SB, Suh SI, Kim JH, Yu SW, et al. Correlation of coronary artery atherosclerosis with atherosclerosis of the intracranial cerebral artery and the extracranial carotid artery. Eur Neurol. 2008;59:292–298. doi: 10.1159/000121418. [DOI] [PubMed] [Google Scholar]
- 6.Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011;42:22–29. doi: 10.1161/STROKEAHA.110.584086. [DOI] [PubMed] [Google Scholar]
- 7.Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation. 2003;108:1278–1290. doi: 10.1161/01.CIR.0000090444.87006.CF. [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (COronary CT Angiography Evaluation For Clinical Outcomes: an InteRnational Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 9.Hadamitzky M, Täubert S, Deseive S, Byrne RA, Martinoff S, Schömig A, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34:3277–3285. doi: 10.1093/eurheartj/eht293. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi N, Nabavi V, Hajsadeghi F, Flores F, French WJ, Mao SS, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107:10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Calvet D, Song D, Yoo J, Turc G, Sablayrolles JL, Choi BW, et al. Predicting asymptomatic coronary artery disease in patients with ischemic stroke and transient ischemic attack: the PRECORIS score. Stroke. 2014;45:82–86. doi: 10.1161/STROKEAHA.113.003414. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SS, Nam HS, Heo JH, Kim YD, Lee SK, Han KH, et al. Ischemic stroke: measurement of intracranial artery calcifications can improve prediction of asymptomatic coronary artery disease. Radiology. 2013;268:842–849. doi: 10.1148/radiol.13122417. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 14.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11:390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 15.Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke. 2013;44:1505–1511. doi: 10.1161/STROKEAHA.111.000142. [DOI] [PubMed] [Google Scholar]
- 16.Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005;36:2748–2755. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 18.Hadamitzky M, Meyer T, Hein F, Bischoff B, Martinoff S, Schömig A, et al. Prognostic value of coronary computed tomographic angiography in asymptomatic patients. Am J Cardiol. 2010;105:1746–1751. doi: 10.1016/j.amjcard.2010.01.354. [DOI] [PubMed] [Google Scholar]
- 19.Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121:1623–1629. doi: 10.1161/CIRCULATIONAHA.109.906958. [DOI] [PubMed] [Google Scholar]
- 20.Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis. 2015;238:271–277. doi: 10.1016/j.atherosclerosis.2014.10.102. [DOI] [PubMed] [Google Scholar]
- 21.Steg PG, Bhatt DL, Wilson PW, D'Agostino R, Sr, Ohman EM, Röther J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Towfighi A, Markovic D, Ovbiagele B. Utility of Framingham coronary heart disease risk score for predicting cardiac risk after stroke. Stroke. 2012;43:2942–2947. doi: 10.1161/STROKEAHA.112.668319. [DOI] [PubMed] [Google Scholar]
- 24.Schlendorf KH, Nasir K, Blumenthal RS. Limitations of the Framingham risk score are now much clearer. Prev Med. 2009;48:115–116. doi: 10.1016/j.ypmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99:472–475. doi: 10.1016/j.amjcard.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 27.van Werkhoven JM, de Boer SM, Schuijf JD, Cademartiri F, Maffei E, Jukema JW, et al. Impact of clinical presentation and pretest likelihood on the relation between calcium score and computed tomographic coronary angiography. Am J Cardiol. 2010;106:1675–1679. doi: 10.1016/j.amjcard.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke. 2000;31:615–621. doi: 10.1161/01.str.31.3.615. [DOI] [PubMed] [Google Scholar]
- 29.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litmanovich DE, Tack DM, Shahrzad M, Bankier AA. Dose reduction in cardiothoracic CT: review of currently available methods. Radiographics. 2014;34:1469–1489. doi: 10.1148/rg.346140084. [DOI] [PubMed] [Google Scholar]
- 32.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]