Abstract
Objective
The present study aimed to investigate whether quantitative dual-energy computed tomography (DECT) parameters offer an incremental risk stratification benefit over the CT ventricular diameter ratio in patients with acute pulmonary embolism (PE) by using propensity score analysis.
Materials and Methods
This study was conducted on 480 patients with acute PE who underwent CT pulmonary angiography (CTPA) or DECT pulmonary angiography (DE CT-PA). This propensity-matched study population included 240 patients with acute PE each in the CTPA and DECT groups. Altogether, 260 (54.1%) patients were men, and the mean age was 64.9 years (64.9 ± 13.5 years). The primary endpoint was all-cause death within 30 days. The Cox proportional hazards regression model was used to identify associations between CT parameters and outcomes and to identify potential predictors. Concordance (C) statistics were used to compare the prognoses between the two groups.
Results
In both CTPA and DECT groups, right to left ventricle diameter ratio ≥ 1 was associated with an increased risk of all-cause death within 30 days (hazard ratio: 3.707, p < 0.001 and 5.573, p < 0.001, respectively). However, C-statistics showed no statistically significant difference between the CTPA and DECT groups for predicting death within 30 days (C-statistics: 0.759 vs. 0.819, p = 0.117).
Conclusion
Quantitative measurement of lung perfusion defect volume by DECT had no added benefit over CT ventricular diameter ratio for predicting all-cause death within 30 days.
Keywords: Acute pulmonary embolism, Dual-energy computed tomography (DECT), Ventricular diameter, Lung perfusion
INTRODUCTION
Risk stratification is important in patients with acute pulmonary embolism (PE) because optimal management, monitoring, and therapeutic strategies depend on the prognosis (1,2). Many computed tomography (CT) parameters have been proposed as potential predictors of PE severity and clinical outcome. Currently, the CT ventricular diameter (VD) ratio is a well-established and widely used prognostic indicator in patients with acute PE. A previous meta-analysis demonstrated that the quantitative CT parameter of right to left ventricle diameter ratio greater than 1 showed the strongest predictive ability and most robust evidence for an adverse clinical outcome in patients with acute PE (3).
Dual-energy computed tomography (DECT) has been used in the diagnosis and evaluation of PE, and recent studies have shown that quantitative parameters of DECT are helpful in predicting the clinical outcome of patients with PE (4,5,6,7,8,9,10,11,12,13). A previous study demonstrated that DECT perfusion imaging could display pulmonary perfusion defects with good agreement to scintigraphic findings (5). Several studies have described the functional relevance of perfusion defects (PDs) detected on DECT, and studies have shown that the extent of PDs measured with DECT correlates with an adverse clinical outcome in patients with PE (9,10). Studies demonstrating the clinical utility of PDs using DECT have been published, but there is little evidence for the additional risk stratification benefit of the CT VD ratio in patients with acute PE (8,9,10,11).
The purpose of the present study was to investigate whether quantitative DECT parameters offer incremental risk stratification benefits over the CT VD ratio in patients with acute PE by using a propensity score analysis.
MATERIALS AND METHODS
Patient Population
This single-center, propensity score-matched study compared the predictive value of quantitative DECT parameters and CT VD ratio in patients with acute PE. Institutional Review Board approval was obtained, and the requirement for informed consent was waived for this retrospective propensity score-matched study.
All consecutive patients who underwent CT pulmonary angiography (CTPA) or DECT pulmonary angiography (DE CT-PA) and were suspected to have acute PE between January 2015 and December 2017 were considered potentially eligible for this analysis. Among 3419 patients (CTPA group, n = 2045, DECT group, n = 1374), the following patients were excluded: patients with negative CT results (n = 2486), patients who did not clinically or radiologically meet the criteria for acute PE (n = 47), those in whom DECT or CT was performed as a follow-up CT examination after receiving anticoagulation therapy (n = 105), and those for whom CT image quality was insufficient or CT image data were not available (n = 21). Finally, 484 patients (23.6%) who were diagnosed with acute PE by CTPA and 276 patients (20.1%) who were diagnosed with acute PE by DECT were recruited for the present study (Fig. 1).
Fig. 1. Flowchart of patient selection.
CTPA = CT pulmonary angiography, DECT = dual-energy CT, DE CT-PA = DECT pulmonary angiography, PE = pulmonary embolism
Patient clinical information, including age, sex, and medical history (hypertension, diabetes mellitus, smoking, heart disease [including congenital heart disease, coronary artery disease, myocardial infarction, valvular heart disease, heart failure, arrhythmia and cardiomyopathy], chronic obstructive pulmonary disease [COPD], pneumonia, history of cancer, history of deep vein thrombosis [DVT]), was recorded based on patient medical records.
To reduce potential selection bias related to the use of a non-randomized cohort to generate two groups (CTPA and DECT groups) with comparable characteristics, propensity score–matched analyses were performed (14). The following variables were used to develop the propensity score and create a well-matched control group: age, sex, hypertension, diabetes mellitus, smoking, heart disease, COPD, pneumonia, history of cancer, and history of DVT. The balance of covariates between the groups was assessed by the absolute standardized mean difference before and after the matching procedure. An absolute standardized mean difference of 0.1 or less indicates balanced covariates between the two groups (15).
The propensity-matched study population included 240 patients with acute PE in the CTPA group and 240 patients with acute PE in the DECT group. Altogether, 260 (54.1%) were men, and the mean age was 64.9 years (64.9 ± 13.5).
CT Examination
CTPA was performed for all participants by using a 64- or 128-channel CT system (Revolution EVO, GE Healthcare, Chicago, IL, USA or Somatom Definition AS, Siemens Healthineers, Forchheim, Germany), and DE CT-PA was performed for all participants by using a dual-source CT system (Somatom Definition Flash, Siemens Healthineers). All patients received 50–90 mL of iopamidol (370 mg/mL iodine, Pamiray 370, Dongkook Pharmaceutical, Seoul, Korea) via an antecubital vein at 4 mL/s by a power injector. Following contrast injection, 30 mL of saline was administered. During the scan, patients held their breath on inspiration. Pulmonary trunk attenuation was tracked by a bolus-tracking technique. Image acquisition was triggered manually once attenuation in the pulmonary trunk reached 100 Hounsfield units (HU). Radiation exposure was estimated from the dose-length product (DLP). The calculated mean radiation dose was 5.2 mSv (DLP range, 189–903 mGy*cm) based on the scan range and patient body weight.
Image Analysis
A radiologist with over 10 years of experience in chest CT analysis analyzed the CT data; the radiologist was blinded to patient identities and clinical histories. All scans were processed and read using a dedicated workstation equipped with dual-energy post-processing software (Syngo MMWP VE36A, Siemens Healthineers). The weighted average image was approximately 120 kV and was automatically generated from a combination of the 140-kV and 100-kV data used for DE CT-PA. Color-coded iodine maps were merged with the corresponding CT angiographic images with soft tissue settings to create fusion images, allowing simultaneous depiction of occluded PAs and lung perfusion.
For quantitative analysis, maximal diameters of the right and left ventricles (RV and LV) were measured on transverse sections by identifying the maximal distance between the ventricular endocardium and the interventricular septum perpendicular to the long axis of the heart (Fig. 2). RV/LV diameter ratios were calculated by dividing the maximum diameters of the RV and LV. PD volume was analyzed and quantified from iodine maps by using dedicated Volume analysis software (version VE36A, Siemens Healthineers). PD attenuation values were measured automatically from −1 to −1024 HU in HU (Fig. 3). Total lung volume was analyzed by Lung Parenchyma Analysis (Syngo InSpace, Siemens Healthineers) and measured automatically by determining the sum of values from 1024 to 1 HU and from −1 to −1024 HU. The trachea and bronchus were excluded by a semiautomatic segmentation technique. The PDs values measured on iodine maps were carefully reviewed and compared to CT findings. PDs related to lung parenchymal abnormalities (e.g., infiltration, effusion, or emphysema) were manually excluded. The relative perfusion defect volume (RelPD%) was calculated as follows: RelPD% = PD volume / total lung volume x 100. To assess the inter-observer agreement for quantitative measurements, 50 of 240 patients in the DECT group were randomly selected and an independent reviewer with over 5 years of experience in chest CT analysis measured the PD volume and ventricular ratios.
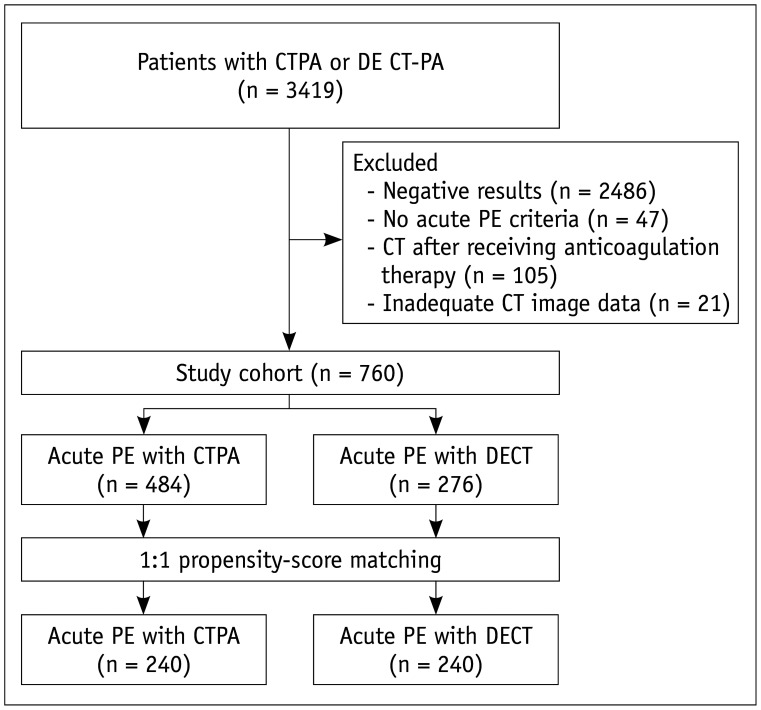
Fig. 2. Measurement of ventricular diameter ratio in 45-year-old woman with acute pulmonary embolism.
Axial CT image shows measurement of maximum diameters (black arrows) of right and left ventricles. Ventricular diameter is maximal distance between ventricular endocardium and interventricular septum perpendicular to long axis of heart. White arrows indicate pulmonary embolism.
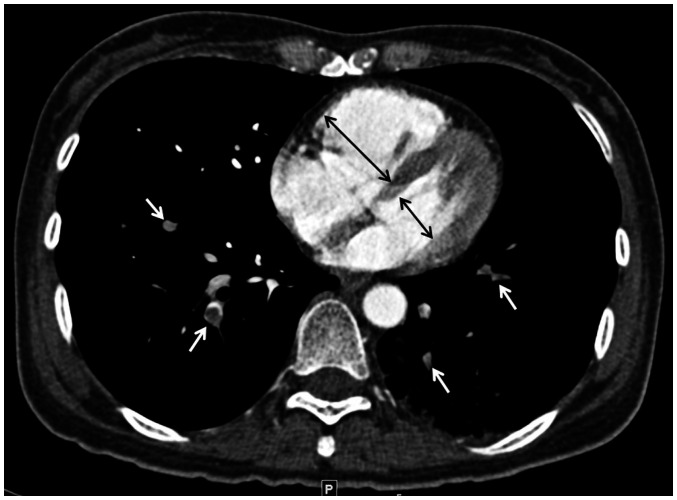
Fig. 3. 68-year-old woman with acute pulmonary embolism.
A. Iodine map generated on axial CT image with dedicated software shows pulmonary embolism (arrows) and perfusion defects in both lungs. B. Map obtained with volume analysis software shows perfusion defect volume, measured from −1024 to −1 HU, of 261.46 cm3 and relative perfusion defect volume of 11.4%.
Clinical Outcome
Clinical outcome data were obtained via a review of the electronic medical records or by telephone contact from a dedicated research nurse who was blinded to the CT results. The primary endpoints of the present study were death within 30 days from any cause. Patient death status was ascertained by querying the National Health Insurance Corporation.
Statistical Analysis
An analytic sample was created using propensity score-based matching to correct for differences in patient characteristics in the two groups. Propensity score matching was conducted in a 1:1 ratio by nearest neighbor matching. The adequacy of the propensity model was confirmed by checking the covariate balance before and after matching.
Comparisons between the CTPA and DECT groups were performed. The differences between categorical variables were analyzed by chi-squared test or Fisher's exact test. The differences between continuous variables were analyzed by the Shapiro–Wilk test or Mann–Whitney U test. A Cox proportional hazards regression model was used to identify associations between CT parameters and outcomes and to identify potential predictors. Only variables with p values less than 0.20 in univariate analyses were added to the final multivariate models to prevent model over-fitting. From the Cox proportional hazards model, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Concordance (C) statistics were used to compare the predictive prognosis between the two groups. Inter-observer agreement was tested using intraclass correlation coefficients (ICCs). A p value < 0.05 was considered statistically significant. All statistical analyses were performed using R (version 3.2.2., R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline Clinical and CT Characteristics
Baseline characteristics of the two groups are shown in Table 1. Both groups were matched for baseline variables, and no significant differences were observed for any of the baseline comparisons.
Table 1. Patient Clinical Characteristics before and after Propensity Score Matching.
| Characteristics | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| CTPA Group (n = 484) | DECT Group (n = 276) | Standardized Difference | CTPA Group (n = 240) | DECT Group (n = 240) | Standardized Difference | |
| Age (years) | 67.8 ± 15.4 | 64.6 ± 13.9 | −0.227 | 64.3 ± 15.8 | 65.4 ± 13.5 | 0.079 |
| Sex (female) | 268 (55.4) | 150 (54.3) | −0.021 | 128 (52.9) | 132 (54.5) | 0.033 |
| Clinical condition | ||||||
| Hypertension | 213 (44.0) | 137 (49.6) | 0.112 | 118 (48.8) | 118 (48.8) | 0 |
| Diabetes mellitus | 88 (18.2) | 66 (23.9) | 0.134 | 53 (21.9) | 54 (22.3) | 0.009 |
| Smoking* | 117 (24.2) | 57 (20.7) | −0.086 | 55 (22.7) | 53 (21.9) | −0.020 |
| Heart disease† | 26 (5.4) | 29 (10.5) | 0.167 | 16 (6.6) | 21 (8.7) | 0.067 |
| COPD | 30 (6.2) | 20 (7.2) | 0.040 | 19 (7.9) | 19 (7.9) | 0 |
| Pneumonia | 63 (13.0) | 38 (13.8) | 0.021 | 33 (13.6) | 33 (13.6) | 0 |
| Cancer | 206 (42.6) | 160 (58.0) | 0.311 | 126 (52.1) | 131 (54.1) | 0.041 |
| DVT | 57 (11.8) | 77 (27.9) | 0.358 | 48 (19.8) | 48 (19.8) | 0 |
Values are presented as mean ± standard deviation or patient number with (%). *Current or former smoker, †Heart disease includes congenital heart disease, coronary artery disease, myocardial infarction, valvular heart disease, heart failure, arrhythmia and cardiomyopathy. COPD = chronic obstructive pulmonary disease, CTPA = CT pulmonary angiography, DECT = dual-energy CT, DVT = deep vein thrombosis
In the CTPA group, patients who died showed a higher prevalence of pneumonia and cancer (all p < 0.05). In the DECT group, patients who died showed a higher prevalence of pneumonia, cancer, and DVT (all p < 0.05). Other clinical characteristics were not significantly different between patients who survived and those who died (Table 2).
Table 2. Baseline Characteristics of Matched Study Population according to Mortality Status.
| Characteristics | CTPA Group | DECT Group | ||||
|---|---|---|---|---|---|---|
| Survivor (n = 207) | Death (n = 35) | P | Survivor (n = 197) | Death (n = 45) | P | |
| Age (years) | 65 (58, 78) | 67 (55, 75) | 0.115 | 69 (57, 74) | 70 (62, 74) | 0.148 |
| Sex (female) | 107 (51.7) | 21 (60.0) | 0.362 | 111 (56.3) | 21 (46.7) | 0.239 |
| Clinical condition | ||||||
| Hypertension | 98 (47.3) | 20 (57.1) | 0.283 | 91 (46.2) | 27 (60.0) | 0.094 |
| Diabetes mellitus | 44 (21.3) | 9 (25.7) | 0.555 | 42 (21.3) | 12 (26.7) | 0.437 |
| Smoking* | 47 (22.7) | 8 (22.9) | 0.984 | 42 (21.3) | 11 (24.4) | 0.647 |
| Heart disease† | 13 (6.3) | 3 (8.6) | 0.613 | 17 (8.6) | 4 (8.9) | 0.956 |
| COPD | 14 (6.8) | 5 (14.3) | 0.126 | 13 (6.6) | 6 (13.3) | 0.129 |
| Pneumonia | 24 (11.6) | 9 (25.7) | 0.024 | 18 (9.1) | 15 (33.3) | < 0.001 |
| Cancer | 99 (47.8) | 27 (77.1) | 0.001 | 96 (48.7) | 35 (77.8) | 0.001 |
| DVT | 44 (21.3) | 4 (11.4) | 0.175 | 45 (22.8) | 3 (6.7) | 0.014 |
| Treatment | ||||||
| Anticoagulants | 177 (85.5) | 26 (74.2) | 0.150 | 159 (80.7) | 33 (73.3) | 0.367 |
| Thrombolytic treatment | 6 (2.8) | 2 (5.7) | 0.703 | 5 (2.5) | 2 (4.4) | 0.845 |
| Inferior vena cava filter | 22 (10.6) | 0 | 0.088 | 23 (11.6) | 1 (2.2) | 0.102 |
| CT measurement | ||||||
| Ventricular diameter ratio, median | 0.97 (0.87, 1.26) | 1.10 (0.98, 1.24) | < 0.001 | 0.94 (0.87, 1.01) | 1.09 (0.99, 1.32) | < 0.001 |
| Ventricular diameter ratio (≥ 1) | 89 (43.0) | 26 (74.3) | < 0.001 | 53 (26.9) | 33 (73.3) | < 0.001 |
| Relative perfusion defect volume (%), median | - | - | - | 7.73 (5.04, 12.49) | 10.21 (6.88, 13.87) | < 0.001 |
Values are presented as median value (1st quantile, 3rd quantile) or patient number (%). *Current or former smoker, †Heart disease includes congenital heart disease, coronary artery disease, myocardial infarction, valvular heart disease, heart failure, arrhythmia and cardiomyopathy.
In both groups, VD ratios (1.10 vs. 0.97; p < 0.001 and 1.09 vs. 0.94; p < 0.001) were significantly higher in the death group than in the survival group. In the DECT group, the RelPD% (10.21% vs. 7.73%; p < 0.001) was also significantly higher in the death group than in the survival group (Table 2).
Clinical and CT Variables Associated with Outcome
During the median follow-up period of 133 days (interquartile range: 35–401 days), there were 35 deaths within 30 days from any cause in the CTPA group and 45 deaths within 30 days from any cause in the DECT group.
In univariate analysis using a Cox hazards regression model in the CTPA group, pneumonia and cancer were predictors of all-cause death within 30 days (all, p < 0.05) (Table 3). Patients with a larger VD ratio (≥ 1 vs. < 1) had a significantly higher risk of death within 30 days (p = 0.001) (Table 3). In the DECT group, univariate analysis using a Cox hazards regression model revealed that pneumonia, cancer, and DVT were predictors of all-cause death within 30 days (all, p < 0.05). Patients with a larger VD ratio (≥ 1 vs. < 1) and a larger PD volume had a significantly higher risk of death within 30 days (all p < 0.001) (Table 3).
Table 3. Univariate Analysis Using Cox Proportional Hazards Regression Model.
| Characteristics | CTPA Group | DECT Group | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.009 | 0.987–1.032 | 0.417 | 1.024 | 0.998–1.049 | 0.065 |
| Sex (female) | 1.317 | 0.670–2.590 | 0.424 | 0.672 | 0.374–1.207 | 0.283 |
| Hypertension | 1.442 | 0.738–2.817 | 0.283 | 1.616 | 0.890–2.934 | 0.115 |
| Diabetes mellitus | 1.277 | 0.598–2.726 | 0.526 | 1.289 | 0.666–2.496 | 0.451 |
| Smoking* | 0.992 | 0.451–2.183 | 0.983 | 1.180 | 0.598–2.330 | 0.632 |
| Heart disease† | 1.387 | 0.425–4.531 | 0.587 | 1.256 | 0.450–3.510 | 0.663 |
| COPD | 2.082 | 0.807–5.366 | 0.129 | 2.177 | 0.921–5.144 | 0.076 |
| Pneumonia | 2.554 | 1.196–5.451 | 0.015 | 3.949 | 2.120–7.357 | < 0.001 |
| Cancer | 3.329 | 1.512–7.329 | 0.002 | 3.413 | 1.689–6.895 | < 0.001 |
| DVT | 0.502 | 0.177–1.423 | 0.195 | 0.260 | 0.081–0.840 | 0.024 |
| Anticoagulants | 1.787 | 0.774–4.072 | 0.278 | 2.104 | 0.813–5.062 | 0.181 |
| Thrombolytic treatment | 0.518 | 0.321–0.947 | 0.186 | 0.584 | 0.238–1.142 | 0.238 |
| Inferior vena cava filter | 2.031 | 0.781–9.712 | 0.142 | 2.579 | 0.744–10.164 | 0.115 |
| VD ratio (≥ 1) | 3.407 | 1.596–7.270 | 0.001 | 7.471 | 3.593–15.534 | < 0.001 |
| RelPD% | - | - | - | 1.065 | 1.013–1.120 | 0.012 |
*Current or former smoker, †Heart disease includes congenital heart disease, coronary artery disease, myocardial infarction, valvular heart disease, heart failure, arrhythmia and cardiomyopathy. Dash (−) indicates no patient. CI = confidence interval, HR = hazard ratio, RelPD% = relative perfusion defect volume, VD = ventricular diameter
In multivariate analysis using a Cox hazards regression model adjusted for age, pneumonia, cancer, and VD ratio (≥ 1) were associated with an increased risk of death within 30 days. In the CTPA group, pneumonia, cancer, and VD ratio (≥ 1) (HR, 3.707; 95% CI, 1.730–7.941; p < 0.001) were associated with an increased risk of death within 30 days. In the DECT group, pneumonia, cancer, VD ratio (≥ 1) (HR, 5.573; 95% CI, 2.758–11.261; p < 0.001) and RelPD% (HR, 1.038; 95% CI, 1.005–1.072; p = 0.022) were associated with an increased risk of death within 30 days. The C-statistics showed no statistically significant difference between the CTPA and DECT groups for predicting death within 30 days (C-statistics: 0.759 vs. 0.819, p = 0.117) (Table 4).
Table 4. Multivariate Analysis Using Cox Proportional Hazards Regression Model.
| Characteristics | CTPA Group | DECT Group | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.002 | 0.961–1.024 | 0.817 | 1.032 | 1.004–1.065 | 0.260 |
| COPD | 2.064 | 0.793–5.370 | 0.137 | 1.578 | 0.624–3.908 | 0.342 |
| Pneumonia | 2.092 | 0.971–4.509 | 0.049 | 2.178 | 1.102–4.288 | 0.025 |
| Cancer | 3.111 | 1.380–7.013 | 0.006 | 3.972 | 1.936–8.147 | < 0.001 |
| DVT | 0.739 | 0.255–2.141 | 0.577 | 0.513 | 0.153–1.718 | 0.282 |
| VD ratio (≥ 1 vs. < 1) | 3.707 | 1.730–7.941 | < 0.001 | 5.573 | 2.758–11.261 | < 0.001 |
| RelPD% | - | - | - | 1.038 | 1.005–1.072 | 0.022 |
| Concordance-index | 0.759 | 0.819 | ||||
Dash (−) indicates no patient.
There was excellent inter-observer agreement between the two radiologists in the measurement parameters of DECT. The ICCs for RelPD% and VD ratios were 0.88 (95% CI: 0.81–0.97) and 0.93 (95% CI: 0.89–0.99), respectively.
DISCUSSION
This study was designed to investigate whether quantitative DECT parameters provide incremental risk stratification benefits over the CT VD ratio in patients with acute PE by using a propensity score analysis. Based on this study, quantitative measurement of lung PD volume by DECT offered no added benefit over CT VD ratio for predicting all-cause death within 30 days.
Risk stratification for patients with acute PE is important to establish appropriate treatment and management. CT parameters have emerged as prognostic markers to assess the severity of hemodynamic compromise from acute PE and identify patients at heightened risk for fatal or nonfatal adverse events, thus guiding clinical management (3,16,17). For clinical purposes, the RV/LV diameter ratio measured on CT shows the strongest predictive value across all endpoints and provides the most robust evidence for adverse clinical outcomes in patients with acute PE. Many studies have supported that RV dysfunction assessed on CT was associated with an increased risk of early complications, including all-cause death and PE-related serious adverse events (16,17,18,19,20,21,22). In addition, previous meta-analyses have demonstrated that increased RV/LV diameter ratio is the strongest predictor of adverse clinical outcomes in patients with acute PE (3,21,22). This measurement is a simple quantitative value that can be easily measured in axial or 4-chamber images using CT. Our results are in agreement with those of previous studies. In our study, right ventricular dysfunction was assessed by CT by using two-dimensional axial transverse images. According to our study, right ventricular dysfunction on CT was an independent predictor of all-cause death within 30 days.
DECT has been proposed as a new imaging technique for detecting PE (11,12). A unique feature of DECT is that it allows differentiation of materials based on their energy absorption (4,23). Thus, DECT allows simultaneous assessment of pulmonary vasculature and parenchymal iodine distribution (5,6,24). In the lung, the pattern of iodine enhancement on DECT has been shown to correspond to lung blood volume on planar scintigraphy (5). Several studies have reported that the quantitative values of lung PDs on DECT correlated with right ventricular dysfunction and adverse clinical outcomes (8,9,10,11). A previous study reported that the extent of lung PDs on DECT correlated well with right ventricular dysfunction on CT and death (9). Another study demonstrated that of all evaluated CT parameters, the PD volume measured by DECT showed the highest predictive power for detecting an adverse clinical outcome (10). Conversely, our previous study revealed that lung PDs quantified on DECT had no added benefit in predicting death within 30 days or for predicting PE-related death (11). Based on previous studies, quantitative DECT parameters have potential for use as prognostic makers in acute PE. However, the value of quantitative DECT parameters for prognosis and risk stratification in acute PE is controversial. The heterogeneity of study groups, definitions, and outcomes prohibits consensus on the prognostic performance of DECT.
We conducted a propensity score-matched study to compare the predictive value of quantitative DECT parameters and CT VD ratio in patients with acute PE. Propensity score adjustment is a method of balancing the distribution of biases and confounders between groups, thereby increasing between-group comparability. Propensity score analysis is increasingly being applied as a statistical method in observational studies (14). We constructed two models to evaluate the added value of DECT parameters (lung PD volume) in predicting all-cause death within 30 days. Although PDs measured on DECT were associated with an increased risk of death within 30 days, C-statistics showed no statistically significant difference between the two groups (CTPA group and DECT group) in predictive prognosis with respect to predicting death in patients with acute PE. These results suggest that DECT parameters (lung PD) had no added benefit over the simple quantified CT value of VD ratio for predicting death within 30 days in patients with acute PE.
Lung perfusion imaging is based on quantification of tissue enhancement at serial time points following contrast administration. Previous studies have demonstrated that the extent of PDs, identified by perfusion scintigraphy, correlated with clinical outcomes in patients with PE (25,26). Consequently, the extent of PDs quantified on DECT is potentially predictive of hemodynamic changes in acute PE. Thus, these are emerging as imaging biomarkers for risk stratification. However, there are several issues regarding quantitative measurements in DECT. First, DECT scans are usually obtained at a single time-point, so DECT provides an iodine distribution map of the lung microcirculation at a given time point (27). Therefore, quantitative DECT parameters can vary according to different clinical settings and with different imaging protocols. Second, there is no standardized analytical method for lung PDs using DECT in terms of HU threshold and analytical software. In addition, additional time is required to analyze lung perfusion using special software.
Our study has certain limitations. First, this study was conducted at a single center with a modest sample size. In addition, the retrospective nature of this study may be associated with a selection bias. However, we conducted a propensity score-matched study to balance the distribution of biases and confounders between groups. Second, the imaging protocol and analytical method for lung perfusion may have significantly influenced the results. Currently, there is no standardized analytical method for assessing lung PDs using DECT in terms of HU threshold and analytical software.
In conclusion, an increased RV/LV diameter ratio was associated with increased risk of all-cause death within 30 days in patients with acute PE. However, quantitative measurement of lung PD volume by DECT offered no added benefit over CT VD ratio in predicting all-cause death within 30 days. Our present data failed to provide an additional benefit of functional lung assessment on DECT for predicting future death in patients with PE. Future large trials with much longer follow-up periods must be performed to estimate the potential influence of DECT findings on treatment strategies and optimize the management and outcome of patients with acute PE.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Konstantinides S, Goldhaber SZ. Pulmonary embolism: risk assessment and management. Eur Heart J. 2012;33:3014–3022. doi: 10.1093/eurheartj/ehs258. [DOI] [PubMed] [Google Scholar]
- 2.Remy-Jardin M, Pistolesi M, Goodman LR, Gefter WB, Gottschalk A, Mayo JR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology. 2007;245:315–329. doi: 10.1148/radiol.2452070397. [DOI] [PubMed] [Google Scholar]
- 3.Meinel FG, Nance JW, Jr, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128:747–759.e2. doi: 10.1016/j.amjmed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510–1517. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- 5.Thieme SF, Becker CR, Hacker M, Nikolaou K, Reiser MF, Johnson TR. Dual energy CT for the assessment of lung perfusion—Correlation to scintigraphy. Eur J Radiol. 2008;68:369–374. doi: 10.1016/j.ejrad.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Thieme SF, Johnson TR, Lee C, McWilliams J, Becker CR, Reiser MF, et al. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol. 2009;193:144–149. doi: 10.2214/AJR.08.1653. [DOI] [PubMed] [Google Scholar]
- 7.Ferda J, Ferdová E, Mírka H, Baxa J, Bednářová A, Flohr T, et al. Pulmonary imaging using dual-energy CT, a role of the assessment of iodine and air distribution. Eur J Radiol. 2011;77:287–293. doi: 10.1016/j.ejrad.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Chae EJ, Seo JB, Jang YM, Krauss B, Lee CW, Lee HJ, et al. Dual-energy CT for assessment of the severity of acute pulmonary embolism: pulmonary perfusion defect score compared with CT angiographic obstruction score and right ventricular/left ventricular diameter ratio. AJR Am J Roentgenol. 2010;194:604–610. doi: 10.2214/AJR.09.2681. [DOI] [PubMed] [Google Scholar]
- 9.Bauer RW, Frellesen C, Renker M, Schell B, Lehnert T, Ackermann H, et al. Dual energy CT pulmonary blood volume assessment in acute pulmonary embolism–Correlation with D-dimer level, right heart strain and clinical outcome. Eur Radiol. 2011;21:1914–1921. doi: 10.1007/s00330-011-2135-1. [DOI] [PubMed] [Google Scholar]
- 10.Apfaltrer P, Bachmann V, Meyer M, Henzler T, Barraza JM, Gruettner J, et al. Prognostic value of perfusion defect volume at dual energy CTA in patients with pulmonary embolism: correlation with CTA obstruction scores, CT parameters of right ventricular dysfunction and adverse clinical outcome. Eur J Radiol. 2012;81:3592–3597. doi: 10.1016/j.ejrad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Im DJ, Hur J, Han KH, Lee HJ, Kim YJ, Kwon W, et al. Acute pulmonary embolism: retrospective cohort study of the predictive value of perfusion defect volume measured with dual-energy CT. AJR Am J Roentgenol. 2017;209:1015–1022. doi: 10.2214/AJR.17.17815. [DOI] [PubMed] [Google Scholar]
- 12.Weidman EK, Plodkowski AJ, Halpenny DF, Hayes SA, Perez-Johnston R, Zheng J, et al. Dual-energy CT angiography for detection of pulmonary emboli: incremental benefit of iodine maps. Radiology. 2018;289:546–553. doi: 10.1148/radiol.2018180594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leithner D, Wichmann JL, Vogl TJ, Trommer J, Martin SS, Scholtz JE, et al. Virtual monoenergetic imaging and iodine perfusion maps improve diagnostic accuracy of dual-energy computed tomography pulmonary angiography with suboptimal contrast attenuation. Invest Radiol. 2017;52:659–665. doi: 10.1097/RLI.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 14.Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. 2015;16:286–296. doi: 10.3348/kjr.2015.16.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang DK, Thilo C, Schoepf UJ, Barraza JM, Jr, Nance JW, Jr, Bastarrika G, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–849. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283–293. doi: 10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–3280. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 19.Lu MT, Cai T, Ersoy H, Whitmore AG, Quiroz R, Goldhaber SZ, et al. Interval increase in right-left ventricular diameter ratios at CT as a predictor of 30-day mortality after acute pulmonary embolism: initial experience. Radiology. 2008;246:281–287. doi: 10.1148/radiol.2461062004. [DOI] [PubMed] [Google Scholar]
- 20.Choi KJ, Cha SI, Shin KM, Lim J, Yoo SS, Lee J, et al. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thromb Res. 2014;133:182–186. doi: 10.1016/j.thromres.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Trujillo-Santos J, den Exter PL, Gómez V, Del Castillo H, Moreno C, van der Hulle T, et al. Computed tomography-assessed right ventricular dysfunction and risk stratification of patients with acute non-massive pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11:1823–1832. doi: 10.1111/jth.12393. [DOI] [PubMed] [Google Scholar]
- 22.Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J. 2014;43:1678–1690. doi: 10.1183/09031936.00147813. [DOI] [PubMed] [Google Scholar]
- 23.Goo HW, Goo JM. Dual-energy CT: new horizon in medical imaging. Korean J Radiol. 2017;18:555–569. doi: 10.3348/kjr.2017.18.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EY, Seo JB, Oh SY, Lee CW, Hwang HJ, Lee SM, et al. Assessment of perfusion pattern and extent of perfusion defect on dual-energy CT angiography: correlations between the causes of pulmonary hypertension and vascular parameters. Korean J Radiol. 2014;15:286–294. doi: 10.3348/kjr.2014.15.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006;85:253–262. doi: 10.1097/01.md.0000236952.87590.c8. [DOI] [PubMed] [Google Scholar]
- 26.Azarian R, Wartski M, Collignon MA, Parent F, Hervé P, Sors H, et al. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J Nucl Med. 1997;38:980–983. [PubMed] [Google Scholar]
- 27.Hoey ET, Mirsadraee S, Pepke-Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol. 2011;196:524–532. doi: 10.2214/AJR.10.4842. [DOI] [PubMed] [Google Scholar]